Abstract
Climatic factors and on-farm management practices were evaluated for their association with the concentrations (cyst/liter) and instantaneous loads (cysts/second) of Giardia duodenalis in storm-based runoff from dairy lots and other high-cattle-use areas on five coastal California farms over two storm seasons. Direct fluorescent antibody analysis was used to quantitate cysts in 350 storm runoff samples. G. duodenalis was detected on all five dairy farms, with fluxes of 1 to 14,000 cysts/liter observed in 16% of samples. Cysts were detected in 41% of runoff samples collected near cattle less than 2 months old, compared to 10% of runoff samples collected near cattle over 6 months old. Furthermore, the concentrations and instantaneous loads of cysts were ≥65 and ≥79 times greater, respectively, in runoff from sites housing young calves than in sites housing other age classes of animals. Factors associated with environmental loading of G. duodenalis included cattle age, cattle stocking number, and precipitation but not lot area, land slope, or cattle density. Vegetated buffer strips were found to significantly reduce waterborne cysts in storm runoff: each additional meter of vegetated buffer placed below high-cattle-use areas was associated with reductions in the concentration and instantaneous load of cysts by factors of 0.86 and 0.79 (−0.07 and −0.10 log10/m), respectively. Straw mulch, seed application, scraping of manure, and cattle exclusion did not significantly affect the concentration or load of G. duodenalis cysts. The study findings suggest that vegetated buffer strips, especially when placed near dairy calf areas, should help reduce the environmental loading of these fecal protozoa discharging from dairy farms.
Numerous best management practices (BMPs) exist to minimize the occurrence of waterborne zoonotic agents being transported from agricultural watersheds to downstream communities (25). For example, maintaining adequately wide vegetative buffers downslope from on-farm accumulations of fecal material has been shown to reduce the fluxes of waterborne Cryptosporidium parvum oocysts and fecal Escherichia coli discharged off agricultural landscapes during overland flow or pasture runoff conditions (3, 28). Water quality benefits generated by installing on-farm BMPs can be further enhanced by targeting on-farm land use practices that generate the majority of the fecal contamination. Hence, water quality monitoring efforts on agricultural watersheds need to be conducted on a spatial scale that corresponds with the spatial scale on which landowners make management decisions, allowing landowners to target specific land use practices (e.g., pasture, corral, barn, manure lagoon) that generate large amounts of fecal contamination (16).
In the Tomales Bay watershed, along the central coast of California, Lewis et al. (16) observed large fluxes of fecal coliforms in excess of 108 CFU/hectare/s discharging from dairy lots and other high-cattle-use areas during precipitation events that generated overland flow from these agricultural sites. These large fluxes of fecal coliforms suggest that enteric pathogens shed by infected cattle may also be present in runoff from similar high-cattle-use areas, given the abilities of bacterial and protozoal microorganisms shed in bovine feces to be eluted from fresh fecal matrices and entrained in overland flow during precipitation (2, 3, 5, 8, 27, 28). Given the common occurrence of Giardia duodenalis in dairy cattle herds (21, 22, 30, 31), the apparent low infectious dose of this protozoal parasite for healthy human volunteers (24), the fact that Tomales Bay is a major shellfish growing region in central California, and the abilities of oysters and other bivalves to concentrate Giardia cysts from the water column (10), we revisited a subset of these original dairies monitored by Lewis et al. (16) to (i) identify climatic and on-farm risk factors associated with high concentrations of cysts or high instantaneous loads (cysts/second) of G. duodenalis in storm-based runoff from these high-cattle-use areas and (ii) evaluate the efficacies of selected BMPs to reduce the concentration or instantaneous load of G. duodenalis in storm runoff compared to that in adjacent control sites. In addition to vegetated buffers, additional BMPs that were evaluated included straw mulching, seeding within the dairy lots, removing manure via scraping, and excluding winter cattle.
MATERIALS AND METHODS
Study location.
The Tomales Bay watershed is located approximately 64 kilometers north of San Francisco, CA, and encompasses approximately 559 square kilometers divided among three main tributaries: Lagunitas, Olema, and Walker creeks (9). The bay itself is approximately 19 kilometers long and less than 1.6 kilometers wide. The average bay depth is 3.7 m, with a maximum depth of 18.6 m. The region's Mediterranean climate results in cool wet winters and dry hot summers, with annual cumulative precipitation ranging from 600 to 1,300 mm (9, 15). Commercial production of oysters occurs on approximately 280 ha of leased bay tidal lands, which creates food safety concerns regarding the potential occurrence of waterborne zoonoses in the tributaries discharging into the bay.
Five dairy farms were selected based on their voluntary participation and location within the Tomales Bay region of California. Although we monitored water quality from a variety of land use practices throughout the dairy complex, we focused our efforts on locations that received large amounts of cattle use (e.g., corrals, lots) because of the high rates of fecal coliforms discharged from these areas during the 1999-2000 and 2000-2001 water years (16). These high-use areas had an average slope of 9° (range, 1° to 26°) and an average of 104 animals/ha (range, 5 to 309). In addition, these high-cattle-use areas resulted in soil surfaces with little to no vegetation, thereby increasing the likelihood of high levels of fecal microorganisms discharging from the site when overland flow conditions occurred (29). Identifying effective BMPs for areas that were at high risk for discharging high rates of fecal coliform was a priority for the project.
Dairy cattle lots and BMP evaluation.
Fifty-seven cattle lots stratified across the five dairies were enrolled the study. For each lot, we recorded the acreage, number of cattle present at the time of sampling, and percent slope. We assigned soil hydrologic conductivity based upon soil hydrologic group (15). A set of BMPs that were either already in practice by one or more dairy farms or newly installed for this project were evaluated. These included modifying the density of cattle or completely excluding cattle from the lot during the duration of the rainfall season, using a tractor to scrape and remove the accumulated layer of manure from the lot prior to the onset of winter rains, and channeling runoff from the lot through vegetative buffer strips as a means to reduce the load of waterborne microbial contaminants (2, 3, 8, 27, 29). Building upon these preexisting practices, we implemented vegetative surface treatments in October, prior to the onset of winter rains, for a subset of the dairy lots. This vegetative surface treatment involved adding 5.4 metric tons/hectare straw across the lot in order to provide cover during early winter storms combined with seeding the lot with 112 kg/hectare of annual barley (Hordeum vulgare) and 28 kg/hectare rye grasses (Lolium multiflorum) to provide ground cover during later winter storms after the straw had decomposed.
Storm water collection and protozoal quantification.
Following each rainstorm during the 2002-2003 and 2003-2004 water years, surface runoff water samples were collected from the different dairy lots on a rotating basis so that each dairy was visited several times per water year, ranging from early in the season (first flush events) to late spring (final flush events). Instantaneous flow at each sampling site and time point was measured with either a Global Waters flow meter (Global Waters Inc., Gold River, CA) or the area-velocity method and multiplied by 0.8 to adjust for surface interactions (18). Water samples were shipped on ice within 72 h of collection to the University of California—Davis for laboratory analysis. To each 500-ml water sample, 125 μl 0.2% Tween 80 and 25 ml of 50 mM morpholinepropanesulfonic acid (MOPS) sodium extraction solution were added. Samples were hand mixed and decanted into two 250-ml centrifuge bottles. Bottles were agitated for 5 min on a hand-wrist shaker at speed 7. The bottles were then centrifuged at 1,100 × g for 10 min. Supernatant was aspirated to leave a 1:1 pellet/liquid ratio.
For G. duodenalis quantitation, the pellet was resuspended in the residual supernatant and a 10-μl aliquot was dried overnight onto a three-well Merifluor slide (Meridian Bioscience Inc., Cincinnati, OH). Direct immunofluorescent microscopy (DFA) was conducted according to the manufacturer's instructions. All slides were read by the same microscopist. Given that the goal of our project was to identify climate and on-farm risk factors associated with waterborne transport of all forms of G. duodenalis discharging from high-cattle-use areas on diary farms, no effort was made to distinguish between different genotypes or assemblages of G. duodenalis.
Adjustment of Giardia cyst concentration for percent recovery.
G. duodenalis (synonymous with Giardia lamblia and Giardia intestinalis) cysts were obtained from calf fecal samples submitted to the Veterinary Medical Teaching Hospital in Davis, CA. Using 1× phosphate-buffered saline, calf feces were washed sequentially through no. 40, 100, and 200 mesh strainers and purified by sucrose flotation (1). Cyst concentrations were determined from the mean of eight hemacytometer counts and confirmed with DFA enumeration. Cyst suspensions were stored at 4°C and used within 1 month of collection.
Estimation of percent recovery was designed to mimic the range of field conditions experienced from the first to the last flush of the storm season and to contain a known number of G. duodenalis cysts so that the recovery efficiency of the analytical method for enumerating cysts could be measured. Soil samples from the dairy lots and cattle feces were added to negative-runoff water samples in order to generate a set of water samples containing either 1, 100, 500, or 700 nephelometric turbidity units. A dose of approximately 10, 50, 200, 500, or 1,500 G. duodenalis cysts was added to 500 ml water and refrigerated overnight before processing. Four replicate sample examinations were conducted for each dose and turbidity combination. All samples were processed as described above for the field samples.
Statistical analyses.
Percent recovery for the immunofluorescent microscopy procedure was estimated by fitting a negative-binomial-regression model to the observed number of cysts, with the number of spiked or expected cysts functioning as the offset variable (3, 11). The observed concentration of G. duodenalis cysts was then adjusted for the percent recovery for the DFA procedure to estimate the number of cysts/liter in runoff water below each dairy lot. These adjusted concentrations were then used to calculate the instantaneous parasite load discharging off each lot (cyst/s) (16) by multiplying the adjusted cyst concentration by the rate of instantaneous flow (liters/s) discharging from a dairy lot as follows: instantaneous load (cysts/s) = cyst concentration (cysts/liter) × runoff flow rate (liters/s).
Negative binomial regression was used to identify climatic and on-farm management factors associated with high concentrations of cysts or high instantaneous loads of G. duodenalis cysts in storm-based runoff from these high-cattle-use areas (11). Cyst concentrations and instantaneous loads functioned as the outcome variables, with the results for each dairy lot set as a group effect to adjust the P values for repeated sampling at the same sites. A forward-stepping approach was used to develop the multivariate regression model, with P values of <0.05 set as the criterion for inclusion of the variable in the final model.
RESULTS
Over the 2-year study, a total of 350 storm water runoff samples were processed from the five dairy farms between November 2002 and March 2004. Overall, one or more G. duodenalis cysts were detected in 16% of runoff samples discharging from high-cattle-use areas. The highest prevalence was in runoff discharging from locations housing calves under 2 months of age, with 41% of these runoff samples positive for G. duodenalis cysts, followed by 23% for runoff samples collected from locations housing calves 2 to 6 months in age and a prevalence of 10% in runoff samples discharging from locations housing calves over 6 months old and adult cows.
The results of the water spiking experiments for estimating the percent recovery for the DFA method are shown in Table 1. The overall percent recovery for enumerating G. duodenalis cysts was 27.6% (95% confidence interval [CI], 21.6 to 35.1%). This value was used to adjust the raw cyst counts by a factor of 3.62 prior to constructing the negative-binomial-regression models.
TABLE 1.
Percent recovery of quantitative immunofluorescent microscopy for enumeration of Giardia cysts in runoff water samples
| Stormwater turbidity (NTU)a | No. of cysts added per 500 ml runoff waterb | Mean no. (range) of cysts detectedc | Mean % recovery (range) |
|---|---|---|---|
| 1 | 10 | 2 (1-3) | 20 (10-30) |
| 50 | 11 (9-12) | 22 (18-24) | |
| 200 | 55 (28-108) | 27 (14-54) | |
| 100 | 10 | 0 | 0 |
| 50 | 0.3 (0-1) | 50 (0-200) | |
| 500 | 2 (1-3) | 43 (20-72) | |
| 500 | 50 | 0 | 0 |
| 500 | 0.3 (0-1) | 20 (0-80) | |
| 1,500 | 2.5 (2-3) | 57 (40-80) | |
| 700 | 50 | 0 | 0 |
| 500 | 0.3 (0-1) | 40 (0-160) | |
| 1,500 | 0.5 (0-1) | 23 (0-53) |
NTU, nephelometric turbidity units.
Four replicates per dose.
Number of Giardia cysts present in a 10-μl smear.
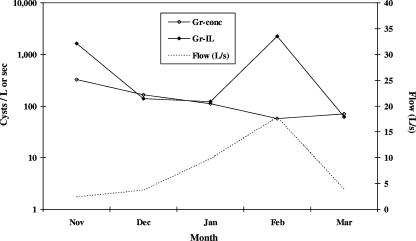
The adjusted parasite concentration for each field sample, multiplied by the surface-adjusted rate of instantaneous flow from the sample site, approximated the instantaneous load or flux of G. duodenalis (cysts/s) in runoff during the storm event. Figure 1 shows the mean instantaneous-flow rate (liters/s), G. duodenalis concentration (cysts/liter), and instantaneous load (cysts/s) for high-cattle-use areas during storm runoff conditions. The mean concentrations of cysts (with standard deviations in parentheses) were 327 (1,079), 164 (1,300), 112 (844), 57.5 (383), and 70.6 (257) cysts/liter for the months of November, December, January, February, and March, respectively. The mean instantaneous loads of cysts (with standard deviations in parentheses) were 1,619 (7,642), 139 (912), 121 (860), 2,253 (23,040), and 62 (224) cysts/s for the months of November, December, January, February, and March, respectively. Despite the flushing effect in the concentration of cysts as the storm season progresses from November to March, the instantaneous load of G. duodenalis reached a peak in February concomitant with peak runoff flow.
FIG. 1.
Mean concentrations (Gr-conc) and instantaneous loads (Gr-IL) of Giardia cysts discharging from dairy cattle high-use areas during runoff conditions, stratified by sampling month for 2002 to 2004, Tomales Bay, CA.
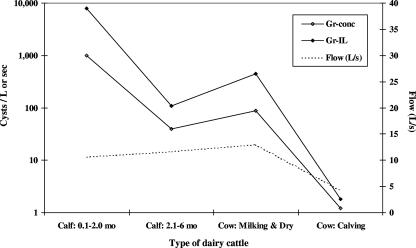
Figure 2 shows the mean G. duodenalis cyst concentrations and instantaneous loads in storm water runoff, stratified by cattle type (<2 months old, 2 to 6 months old, milking/dry, or calving). Runoff discharging from locations with calves under 2 months of age had the highest concentration of cysts (989 cysts/liter) and instantaneous loads (7,908 cysts/s), followed by locations with calves 2 to 6 months of age (38.9 cysts/liter, 109 cysts/s) or milking/dry cows (86.9 cysts/liter, 450 cysts/s), while runoff from locations with cows located in the calving pen had the lowest mean cyst levels (1.2 cysts/liter, 1.8 cysts/s).
FIG. 2.
Mean concentrations (Gr-conc) and instantaneous loads (Gr-IL) of Giardia cysts discharging from high-use dairy cattle areas during runoff conditions, stratified by cattle type for 2002 to 2004, Tomales Bay, CA.
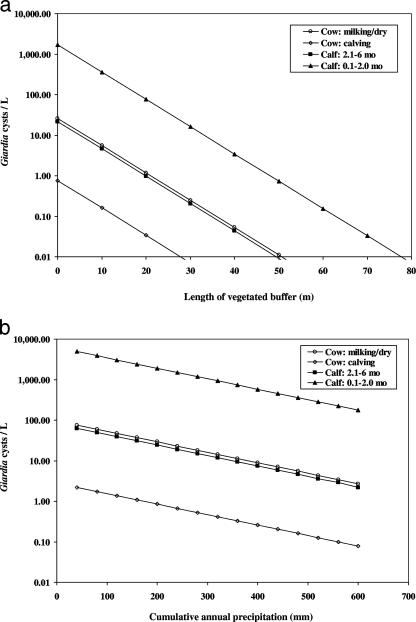
Age of dairy cattle, length of vegetated buffer, and cumulative annual precipitation were significantly associated with concentration of cysts in runoff (Table 2). Given that these three variables were in the regression model, additional factors, such as percent slope, area of lot, cattle density, and 24-hour precipitation, and additional BMPs, such as straw mulching, seeding, removal of manure via scraping, and winter exclusion of cattle, were not associated with cyst concentration. Model coefficients need to be exponentiated for interpretation. For example, when the concentration of cysts in runoff from sites housing milking or dry cows was used as the referent condition, the mean concentration of cysts was increased by a factor of ∼65 in runoff from sites housing calves of <2 months of age (e4.17 = 64.7), reduced by a factor of 0.03 in runoff from sites housing periparturient cows (e−3.54 = 0.03), and approximately similar for sites housing calves between 2 and 6 months of age. The interpretation of continuous variables is as follows: for each additional meter of vegetated buffer placed below high-cattle-use areas, the concentration of G. duodenalis cysts was reduced by a factor of 0.86 (e−0.15 × 1.0 = 0.86). For each additional 10 mm of cumulative annual precipitation, the concentration of G. duodenalis cysts was reduced by a factor of 0.94 (e−0.006 × 10 = 0.94). Figure 3a and b displays the association between these various host, management, and climatic factors and the predicted concentration of cysts in runoff from high-use cattle areas.
TABLE 2.
Negative-binomial-regression model for the association between cattle type, beneficial management practices, and precipitation and concentration of Giardia spp. (cysts/liter) discharging from dairy cattle high-use areas during runoff conditions from 2002 to 2004 in Tomales Bay, CA
| Risk factor | Coefficient | 95% CIb | P valueb |
|---|---|---|---|
| Type of dairy cattle | |||
| Cow, milking or drya | 0.0 | ||
| Cow, calving | −3.54 | (−4.85, −2.23) | <0.001 |
| Calf, 2.1 to 6.0 mo of age | −0.18 | (−1.59, 1.23) | 0.80 |
| Calf: 0.1 to 2.0 mo of age | 4.18 | (2.50, 5.85) | <0.001 |
| Length of vegetated buffer (m) | −0.15 | (−0.22, −0.09) | <0.001 |
| Cumulative annual precipitation (mm) | −0.006 | (−0.009, −0.003) | <0.001 |
| Constant or intercept term for the model | 5.43 | (4.42, 6.44) | <0.001 |
Referent condition for the categorical variable, type of dairy cattle.
Adjusted for potential lack of independence due to repeated sampling of high-use areas across storms.
FIG. 3.
Predicted Giardia concentrations (cysts/liter) in storm runoff from high-use dairy cattle areas as a function of cattle type and either length of vegetated buffer (a) or cumulative annual precipitation (b). Continuous variables in the background are set at their mean values to generate model predictions.
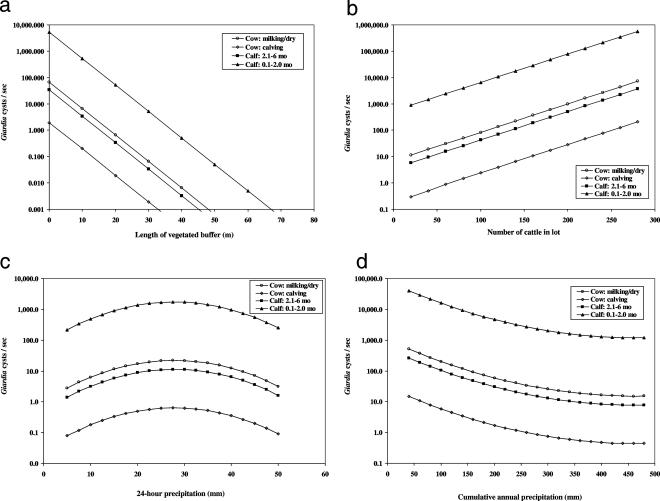
Age of dairy cattle, length of vegetated buffer, number of cattle, and 24-hour and cumulative annual precipitation were significantly associated with instantaneous load of cysts (cysts/s) discharging from high-use cattle areas (Table 3). Using the number of cysts discharging from sites housing milking or dry cows as the referent condition, the instantaneous load of cysts was increased by a factor 79 in runoff from sites housing calves of <2 months of age (e4.37 = 79), reduced by a factor of 0.03 in runoff from sites housing periparturient cows (e−3.54 = 0.029), and approximately similar for sites housing calves 2 to 6 months old. For each additional meter of vegetated buffer placed below high-use cattle areas, the instantaneous load of G. duodenalis cysts was reduced by a factor of 0.79 (e−0.23 × 1.0 = 0.79) (Fig. 4a). For each reduction in which 10 head of cattle were placed in a lot prior to or during the rainfall season, the instantaneous load of G. duodenalis cysts was decreased by a factor of 0.78 (e0.025 × −10 = 0.78) (Fig. 4b). For each additional 5 mm of 24-hour precipitation, the instantaneous loads of cysts discharging from these dairy sites increased by factors of 1.2 to 2.2 for precipitation totals ranging from 5 to 25 mm, but once these rainfall amounts exceeded 30 mm, the number of cysts discharging from these sites began to decline (Fig. 4c). For each additional 10 mm of cumulative annual precipitation, the instantaneous load of G. duodenalis cysts was reduced by a factor of 0.85 during early flush events and then declined to a factor of just 0.95 to 1.0 near the end of the rainfall season (Fig. 4d).
TABLE 3.
Negative-binomial-regression model for the association between cattle type, beneficial management practices, and precipitation and instantaneous load of Giardia spp. (cysts/s) discharging from dairy cattle high-use areas during runoff conditions from 2002 to 2004 in Tomales Bay, CA
| Risk factor | Coefficient | 95% CIb | P valueb |
|---|---|---|---|
| Type of dairy cattle | |||
| Cow, milking or drya | 0.0 | ||
| Cow, calving | −3.54 | (−4.63, −2.46) | <0.001 |
| Calf, 2.1 to 6.0 mo of age | −0.67 | (−1.97, 0.64) | 0.32 |
| Calf, 0.1 to 2.0 mo of age | 4.37 | (2.87, 5.87) | <0.001 |
| Length of vegetated buffer (m) | −0.23 | (−0.30, −0.17) | <0.001 |
| No. of cattle in lot | 0.02 | (0.009, 0.04) | 0.002 |
| 24-hour precipitation (mm) | 0.22 | (0.08, 0.37) | 0.003 |
| 24-hour precipitation2 (mm2) | −0.004 | (−0.007, −0.001) | 0.009 |
| Cumulative annual precipitation (mm) | −0.02 | (−0.03, −0.006) | 0.004 |
| Cumulative annual precipitation2 (mm2) | 0.00002 | (0.000001, 0.00003) | 0.04 |
| Constant or intercept term for the model | 4.37 | (2.23, 6.51) | <0.001 |
Referent condition for the categorical variable, type of dairy cattle.
Adjusted for potential lack of independence due to repeated sampling of high-use areas across storms.
FIG. 4.
Predicted instantaneous loads of Giardia (cysts/s) in storm runoff from high-use dairy cattle areas as a function of cattle type and either length of vegetated buffer (a), number of cattle in lot (b), 24-h precipitation (c), or cumulative annual precipitation (d). Continuous variables in the background are set at their mean values to generate model predictions.
DISCUSSION
Fluxes of 1 to ∼14,000 G. duodenalis cysts/liter were observed in 16% of samples from rainfall-associated runoff discharging from dairy lots and other high-cattle-use areas in the Tomales Bay watershed during the 2002-2003 and 2003-2004 rainfall years. Consistent with other work regarding waterborne C. parvum (4), the prevalence and mean concentration of cysts were highest early in the rainfall-runoff season (Fig. 1), resulting in a negative association between cumulative precipitation and concentration of G. duodenalis cysts (Fig. 3b). It is possible, though, that these high concentrations of waterborne G. duodenalis cysts occurring during November contained disproportionate numbers of inactivated cysts, given the warmer and drier conditions of September and October. The instantaneous loads of G. duodenalis cysts also showed a seasonal flushing effect (Fig. 1 and 4d), except during February, when the velocity of runoff reached a maximum from these high-cattle-use areas.
Consistent with fecal surveys that observe higher prevalences of G. duodenalis infection among young calves than among older cattle (31), both the concentrations and instantaneous loads of G. duodenalis cysts were substantially higher in runoff from sites housing young calves than in runoff from sites housing older cattle (Fig. 2). The results from the regression models in Tables 2 and 3 substantiate this finding in that the concentrations and instantaneous loads of cysts were at least 65 and 79 times greater, respectively, in runoff from sites housing young calves than in runoff from sites housing other age classes of animals. This suggests that beneficial management practices designed to reduce the concentrations and/or instantaneous loads of cysts discharging from high-cattle-use areas should likely focus their initial efforts on young-calf-rearing locations, early-season first-flush events, and curbing the peak runoff rates that tend to occur in February for this coastal region of California.
The age-stratified results shown in Fig. 2 need to be interpreted with caution, given that differences in waterborne cyst concentrations and instantaneous loads may not be the sole result of differences in fecal shedding by young versus older animals (12, 22, 30, 31). Young calves, dairy heifers, and lactating and periparturient cattle are often housed on substantially different types of surfaces (wooden hutch, straw bedding, dry manure, etc.), each with differing amounts of vegetative cover and infiltration capacities. Differences in percentages of grass cover (8, 29) and residual dry vegetative matter (28) were significantly associated with numbers of C. parvum oocysts in runoff. The observation in this study that runoff from sites housing periparturient cattle contained the lowest levels of waterborne cysts may not be the result of reduced fecal shedding of this parasite in this group of cattle, as has been observed in previous research on livestock fecal shedding of G. duodenalis cysts (6, 22, 32), but may instead result from the typically large amounts of straw placed on top of the calving pen for hygienic reasons. Furthermore, while the mean velocities of runoff were similar (10.6 to 13 liters/s) for sites housing calves and lactating and dry cows (Fig. 2), they were less than half this rate for the periparturient pens, most likely the result of these calving areas typically being at least partially covered by a roofing structure and thereby reducing the amount of precipitation reaching the pen surface.
Vegetated buffers were associated with reductions in both the concentrations and instantaneous loads of cysts in rainfall-associated runoff discharging from dairy lots and other high-cattle-use areas in the Tomales Bay watershed during the 2002-2003 and 2003-2004 rainfall years. For each additional meter of vegetated buffer placed below high-cattle-use areas, the concentration and instantaneous load of G. duodenalis cysts were reduced by factors of 0.86 and 0.79, or 0.07 and 0.10 log10 reductions per meter, respectively. Although direct comparisons to previous work on the abilities of vegetated buffers to retain or filter waterborne protozoa from agricultural runoff are difficult due to such factors as different buffer designs, different protozoa species (G. duodenalis compared to C. parvum), and different methods for calculating buffer efficiency, this value is substantially less than the usual ≥1.0 log10 reduction (reduction by a factor of 0.10) in the total number of C. parvum oocysts per meter of vegetated buffer as observed by Atwill et al. (2, 3), Davies et al. (8), Tate et al. (27), and Trask et al. (29). Much of this previous work has been conducted with idealized, simulated buffers or soil box designs where channelized flow was reduced or eliminated by repacking and leveling of the soil profile. The vegetated buffers observed in our current study with coastal dairies were often vegetated channels or ditches that conveyed rainfall-associated runoff away from the animal production facility. This channelized design of a vegetated buffer is not optimal and likely had a negative impact on buffer efficiency compared to experimental buffers that create more-sheet-flow-like conditions; nevertheless, even channelized vegetated buffers represent a significant on-farm beneficial management practice for microbial water quality compared to nonbuffered runoff.
Reducing the number of cattle from a high-cattle-use area was associated with a reduction in the instantaneous load of G. duodenalis cysts in rainfall-associated runoff (Fig. 4b). The finding that cattle stocking number is a risk factor for G. duodenalis detection in storm runoff is consistent with the results of livestock infectious disease ecology studies in some cases (14, 26) but not others (7, 13). Interestingly, the water quality benefits associated with high-cattle-use areas containing 10 fewer head of cattle (e0.025 × −10 = 0.78-fold reduction) were about the same as having an additional meter of vegetated buffer below these high-cattle-use locations (e−0.23 × 1.0 = 0.79-fold reduction). Given the substantial negative economic impact incurred by reducing the number of adult dairy cattle on a dairy operation, it is likely that installation of vegetated buffers will generate greater acceptance among the agricultural community when water quality programs are based on voluntary compliance.
The water spiking trials were necessary to assess the water processing recovery efficiency in order to adjust the raw cyst counts from the field samples to estimate the true parasite concentration in each sample. The overall percent recovery of 27% for G. duodenalis cysts by DFA analysis is similar to what has been found in other G. duodenalis and Cryptosporidium studies (19, 23). The Pereira et al. (23) study reported a mean percent recovery of 28% to 34% when oocysts were spiked into fecal samples and analyzed by DFA. The Nieminski et al. (19) study found 12% to 49% cyst recovery in spiked water samples. If increased assay sensitivity is desired for future studies, addition of an immunomagnetic separation concentration step before DFA analysis could be used because immunomagnetic separation has been shown to increase sensitivity by 1 to 2 log10 over DFA alone in cattle feces and bivalve tissues (17, 23).
In conclusion, the investigation of farm factors associated with protozoal loading in storm runoff and the BMP evaluations for high-use cattle areas have improved our understanding of the environmental ecology and control of Giardia on coastal California farms. The BMPs evaluated in this study represent practical on-farm solutions for improving water quality and decreasing pathogen loading in storm runoff. Although we are unaware of evidence that shellfish is a common source of food-borne giardiasis in humans, it may be prudent to minimize the concentration of waterborne G. duodenalis when such runoff discharges into shellfish growing waters. Using a systems approach (16, 20) to evaluate the flow, behavior, and control of microbes and nutrients from specific farm loading units will help farmers and regulatory agencies prioritize their management efforts.
Acknowledgments
This research was supported in part by the National Sea Grant College Program under NOAA grant NA06RG0142, project R/CZ-180, through the California Sea Grant College Program and in part by the California State Resources Agency. Additional support was received from the University of California—Davis Center for Food Animal Health.
We thank the University of California Cooperative Extension and the Tomales Bay Agricultural Group for participating in this study.
Footnotes
Published ahead of print on 14 September 2007.
REFERENCES
- 1.Arrowood, M. J., and C. R. Sterling. 1987. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 73:314-319. [PubMed] [Google Scholar]
- 2.Atwill, E. R., L. Hou, B. M. Karle, T. Harter, K. W. Tate, and R. A. Dahlgren. 2002. Transport of Cryptosporidium parvum oocysts through vegetated buffer strips and estimated filtration efficiency. Appl. Environ. Microbiol. 68:5517-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atwill, E. R., K. W. Tate, M. D. G. C. Pereira, J. Bartolome, and G. Nader. 2006. Efficacy of natural grassland buffers for removal of Cryptosporidium parvum in rangeland runoff. J. Food Prot. 69:177-184. [DOI] [PubMed] [Google Scholar]
- 4.Bodley-Tickell, A. T., S. E. Kitchen, and A. P. Sturdee. 2002. Occurrence of Cryptosporidium in agricultural surface waters during an annual farming cycle in lowland UK. Water Res. 36:1880-1886. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, S. A., and J. Schijven. 2002. Release of Cryptosporidium and Giardia from dairy calf manure: impact of solution salinity. Environ. Sci. Technol. 36:3916-3923. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Hermida, J. A., A. Delafosse, I. Pors, E. Ares-Mazas, and C. Chartier. 2005. Giardia duodenalis and Cryptosporidium parvum infections in adult goats and their implications for neonatal kids. Vet. Rec. 157:623-627. [DOI] [PubMed] [Google Scholar]
- 7.Charlier, J., E. Claerebout, E. DeMuelenaere, and J. Vercruysse. 2005. Associations between dairy herd management factors and bulk tank milk antibody levels against Ostertagia ostertagi. Vet. Parasitol. 133:91-100. [DOI] [PubMed] [Google Scholar]
- 8.Davies, C. M., C. M. Ferguson, C. Kaucner, M. Krough, N. Altavilla, D. A. Deere, and N. J. Ashbolt. 2004. Dispersion and transport of Cryptosporidium oocysts from fecal pats under simulated rainfall events. Appl. Environ. Microbiol. 70:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer, D. T., S. V. Smith, and R. R. Churchill. 1996. Simulation of a century of runoff across the Tomales Bay watershed, Marin County, California. J. Hydrol. 186:253-273. [Google Scholar]
- 10.Graczyk, T. K., A. S. Girouard, L. Tamang, S. P. Nappier, and K. J. Schwab. 2006. Recovery, bioaccumulation, and inactivation of human waterborne pathogens by the Chesapeake Bay nonnative oyster, Crassostrea areakensis. Appl. Environ. Microbiol. 72:3390-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardin, J., and J. Hilbe. 2001. Generalized linear models and extensions, p. 139-158. Stata Press, College Station, TX.
- 12.Huetink, R. E. C., J. W. B. van der Giessen, J. P. T. M. Noordhuizen, and H. W. Ploeger. 2001. Epidemiology of Cryptosporidium spp. and Giardia duodenalis on a dairy farm. Vet. Parasitol. 102:53-67. [DOI] [PubMed] [Google Scholar]
- 13.Hutchison, M. L., L. Walters, S. M. Avery, F. Munro, and A. Moore. 2005. Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl. Environ. Microbiol. 71:1231-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, P. W., P. Collins, G. T. Brown, and M. M. Aitken. 1983. Salmonella saint-paul infection in two dairy herds. J. Hyg. 91:243-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwagi, J. H. 1985. Soil survey of Marin County, California. USDA Soil Conservation Service in cooperation in with USDA Department of Interior, National Park Service, and University of California Agricultural Experiment Station, Davis, CA.
- 16.Lewis, D. J., E. R. Atwill, M. S. Lennox, L. Hous, B. Karle, and K. W. Tate. 2005. Linking on-farm dairy management practices to storm-flow fecal coliform loading for California coastal watersheds. J. Environ. Monit. Assess. 107:407-425. [DOI] [PubMed] [Google Scholar]
- 17.Miller, W. A., E. R. Atwill, I. A. Gardner, M. A. Miller, H. M. Fritz, R. P. Hedrick, A. C. Melli, N. M. Barnes, and P. A. Conrad. 2005. Clams (Corbicula fluminea) as bioindicators of fecal contamination with Cryptosporidium and Giardia spp. in freshwater ecosystems in California. Int. J. Parasitol. 35:673-684. [DOI] [PubMed] [Google Scholar]
- 18.Mosley, M. P., and A. I. McKercher. 1993. Streamflow, p. 8.1-8.39. In D. R. Maidment (ed.), Handbook of hydrology. McGraw Hill, Inc., New York, NY.
- 19.Nieminski, E. C., F. W. Schaefer III, and J. E. Ongerth. 1995. Comparison of two methods for detection of Giardia cysts and Cryptosporidium oocysts in water. Appl. Environ. Microbiol. 61:1714-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oborn, I., A. Modin-Edman, H. Bengtsson, G. M. Gustafson, E. Salomon, S. I. Nilsson, J. Homqvist, S. Jonsson, and H. Sverdrup. 2005. A systems approach to assess farm-scale nutrient and trace element dynamics: a case study at the Ojebyn dairy farm. Ambio 34:301-310. [DOI] [PubMed] [Google Scholar]
- 21.O'Handley, R. M., M. E. Olson, D. Frasaer, P. Adams, and R. C. Thompson. 2000. Prevalence and genotypic characterization of Giardia in dairy calves from Western Australia and Western Canada. Vet. Parasitol. 90:193-2000. [DOI] [PubMed] [Google Scholar]
- 22.Olson, M. E., R. M. O'Handley, B. J. Ralston, T. A. McAllilster, and R. C. A. Thompson. 2004. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 20:185-191. [DOI] [PubMed] [Google Scholar]
- 23.Pereira, M. D. G., E. R. Atwill, and T. Jones. 1999. Comparison of sensitivity of immunofluorescent microscopy to that of a combination of immunofluorescent microscopy and immunomagnetic separation for detection of Cryptosporidium parvum oocysts in adult bovine feces. Appl. Environ. Microbiol. 65:3236-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rendtorff, R. C. 1954. The experimental transmission of human intestinal protozoan parasites. II. Giardia lamblia cysts given in capsules. Am. J. Hyg. 59:209-220. [DOI] [PubMed] [Google Scholar]
- 25.Rosen, B. H., R. Croft, E. R. Atwill, S. Wade, and S. Stehman. 2000. Waterborne pathogens in agricultural watersheds: technical note 2. Watershed Science Institute, University of Vermont, Natural Resource Conservation Service, USDA, Burlington, VT.
- 26.Sevi, A., S. Massa, G. Annicchiarico, S. Dell'Aquila, and A. Muscio. 1999. Effect of stocking density on ewes’ milk yield, udder health, and microenvironment. J. Dairy Res. 66:489-499. [DOI] [PubMed] [Google Scholar]
- 27.Tate, K. W., M. D. G. C. Pereira, and E. R. Atwill. 2004. Efficacy of vegetated buffer strips for retaining Cryptosporidium parvum. J. Enivron. Qual. 33:2243-2251. [DOI] [PubMed] [Google Scholar]
- 28.Tate, K. W., E. R. Atwill, J. W. Bartolome, and G. Nader. 2006. Significant Escherichia coli attenuation by vegetative buffers on annual grasslands. J. Environ. Qual. 35:795-805. [DOI] [PubMed] [Google Scholar]
- 29.Trask, J. R., P. K. Kalita, M. S. Kuhllenschmidt, R. D. Smith, and T. L. Funk. 2004. Overland and near-surface transport of Cryptosporidium parvum from vegetated and nonvegetated surfaces. J. Environ. Qual. 33:984-993. [DOI] [PubMed] [Google Scholar]
- 30.Trout, J. M., M. Santin, E. Greiner, and R. Fayer. 2004. Prevalence of Giardia duodenalis genotypes in pre-weaned dairy calves. Vet. Parasitol. 124:179-186. [DOI] [PubMed] [Google Scholar]
- 31.Wade, S. E., H. O. Mohammed, and S. L. Schaaf. 2000. Epidemiologic study of Giardia sp. infection in dairy cattle in southeastern New York State. Vet. Parasitol. 89:11-21. [DOI] [PubMed] [Google Scholar]
- 32.Xiao, L., R. P. Herd, and K. E. McClure. 1994. Periparturient rise in the excretion of Giardia sp. cysts and Cryptosporidium parvum oocysts as a source of infection for lambs. J. Parasitol. 80:55-59. [PubMed] [Google Scholar]