Abstract
Pathological studies demonstrated that the salivary gland hypertrophy virus of houseflies (MdSGHV) shuts down reproduction in infected females. The mechanism that underlay the disruption of reproduction functioned on several levels. Females infected at the previtellogenic stage did not produce eggs, reflecting a block in the gonadotropic cycle. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis of hemolymph samples demonstrated that MdSGHV infection reduced the levels of both the female-specific hexamerin and egg yolk proteins. Furthermore, reverse transcriptase quantitative real-time PCR data demonstrated that infection blocked hexamerin and yolk protein gene transcription. When females were allowed to develop eggs prior to infection (postvitellogenic stage), the outcome of mating attempts depended upon when mating took place. If egg-containing, virus-infected females were mated within 24 h of infection, they copulated and deposited a single batch of fertilized eggs. However, if mating was delayed for a longer period, the egg-containing females refused to copulate with healthy males. Both of these results suggested that a virus-induced signal influenced the central nervous system, shutting down female receptivity and egg production. All experiments demonstrated that MdSGHV-infected males did not display azoospermia and were fertile. Both healthy females mated with infected males, and the resulting F1 progeny were free of salivary gland hypertrophy symptoms, which suggests that the virus is not sexually or vertically transmitted.
In the early 1990s, an insect virus was detected and isolated from hypertrophied salivary glands of male and female houseflies, Musca domestica L., in Florida. The virus was described initially as a nonoccluded, enveloped, rod-shaped, double-stranded DNA virus (7). Feeding bioassays demonstrated that the virus could be transmitted per os to healthy adult houseflies and that infection with the virus was responsible for the salivary gland hypertrophy (SGH) symptoms. In these experiments, 95% of the female houseflies with symptoms of SGH showed no sign of ovarian development (7). A similar virus causing symptoms of SGH has been reported in the narcissus bulb fly, Merodon equestris (Fabricius) (3), and in various species of tsetse flies, Glossina spp. (3, 11). Comparisons of the different virus-host fly systems have demonstrated that the different viruses have several morphological and pathological properties in common (3, 7, 8, 11, 15, 16, 19). Electron microscopic observation of virus particles either in thin sections of hypertrophied salivary glands or from sucrose density gradient-purified, negatively stained preparations showed enveloped bacilliform virions. In all three fly groups, the virus replicates in the salivary gland tissue of male and female flies. Additionally, infected adults do not exhibit any external disease symptoms, and morphogenesis occurs in the nuclei, resulting in nuclear SGH.
The most thoroughly studied SGH viruses (SGHVs) are those associated with the tsetse flies, a host complex in which viral infection significantly inhibits reproduction. In Glossina morsitans Westwood, virus infection causing SGH resulted in male sterility (25) and suppressed female reproduction (24). Over 60 years ago, the symptoms of hypertrophied salivary glands were detected in the feral tsetse flies Glossina pallidipes Austen and G. morsitans (4, 29). A viral infection was later identified as the cause of not only salivary gland enlargement (11) but also testicular degeneration and ovarian abnormalities (9, 12, 24, 25). Studies on the natural incidence of the tsetse SGHV have indicated that the infection rates in all species are low; 0.4 to 5% of field-collected flies displayed enlarged salivary glands (8, 11, 12, 18, 20, 21). In laboratory colonies of G. morsitans, the incidence of symptomatic SGH has ranged from 1.1% (14) to 4% (15). Importantly, diagnostic PCR demonstrated that the tsetse SGHV can exist in an asymptomatic state in host flies (1); insect colonies displaying low levels (5%) of overt symptoms produced 100% tsetse SGHV-positive results in the diagnostic PCR. The tsetse SGHV was capable of infecting the male testes, causing azoospermia or oligospermia (11). Venereal transmission between infected and healthy tsetse flies has been reported (13).
We have shown that M. domestica SGHV (MdSGHV) is transmitted per os (7) and impacts female fecundity. The objectives of this study were to assess the effects of MdSGHV on the reproductive fitness and mating success of adult houseflies and to determine if the virus can be sexually or vertically transmitted.
MATERIALS AND METHODS
Insects.
Housefly pupae were obtained from the “Orlando Normal” colony of insecticide-susceptible flies maintained at the Center for Medical, Agricultural and Veterinary Entomology, USDA-ARS, in Gainesville, FL. Immature flies were raised using standard rearing methods and larval medium for this colony (10). Pupae were transferred to 30-cm/side cubic wire screen cages containing a plastic cup with deionized water and several Styrofoam chips to prevent emerging flies from drowning. Pupae were maintained under constant conditions (26°C, 12 h of light and 12 h of darkness, 40% relative humidity). Less than 16 h postemergence, adults were cold immobilized and separated according to sex. The sexed, virgin flies were placed in separate cages containing deionized water and a food substrate. Unless stated otherwise, the term food refers to a mixture of powdered milk, sugar, and powdered egg at a ratio of 6:6:1 (by volume). Flies were maintained under constant conditions (26°C, 12 h of light and 12 h of darkness, 40% relative humidity) until they reached the appropriate age for injection of MdSGHV.
Preparation of crude MdSGHV inoculum.
Intact salivary glands displaying hypertrophy were removed from an adult housefly and stored in 50 μl of saline solution (0.85% NaCl) at −35°C. Frozen glands were thawed, homogenized, diluted 1/10 in saline solution, and centrifuged at 400 × g for 3 min to pellet debris. The supernatant was filter sterilized by centrifugation in Ultrafree MC Millipore microcentrifuge tubes with a low-protein-binding Durapore membrane, 0.45-μm filter unit (Millipore, Billerica, MA) at 13,400 × g for 10 min. The filtrate, containing 1 infectious gland pair equivalent (IGE), was used for housefly injection.
Injection of ovarian extract into healthy flies.
One and 2 weeks after injection of sterile saline and an MdSGHV inoculum, healthy and MdSGHV-infected females, respectively, were carefully dissected in ice-cold saline solution, ensuring that salivary gland tissue was not disrupted during dissection. Ovaries were removed, and each pair of ovaries was rinsed eight times in saline prior to separate storage in 50 μl of saline solution at −35°C. Ovaries were obtained from postvitellogenic, healthy females (n = 2), from postvitellogenic, virus-infected females (n = 2), and from previtellogenic, virus-infected females (n = 4); one-half of the samples were dissected from flies 1 week after the initial injection, and one-half of the samples were dissected 2 weeks after the initial injection. To examine if injection of ovarian extract caused MdSGHV infection in healthy M. domestica adults, ovaries were thawed, homogenized, and filter sterilized as described above for the preparation of crude MdSGHV inoculum. Crude ovarian extracts at a concentration of 2 infectious ovary pair equivalents (IOE) per ml were then injected into the prothoraxes of 1-day-old females and males (2.5 μl per fly). Ten females and 10 males were inoculated with each extract. Groups of 20 treated flies were transferred to 16-oz plastic cups sealed with fine mesh gauze, provided with water and food ad libitum, and maintained under constant conditions (26°C, 12 h of light and 12 h of darkness, 40% relative humidity). After 7 days, all flies were cold immobilized and dissected to record SGH symptoms.
Virus quantitation.
Two approaches were used to determine the numbers of infectious viral copies in different tissues of viremic houseflies. For in vivo endpoint titration, serial 10-fold dilutions of filter-sterilized crude MdSGHV inoculum were injected into the hemocoels of healthy M. domestica females to examine the concentration-dependent infectivity of viral preparations obtained from hypertrophied salivary glands and from ovaries of MdSGHV-infected females. Stock mixtures containing 2 IGE/ml and 2 IOE/ml, respectively, were prepared as described above. Ten 1-day-old females were injected with each mixture (2.5 μl per fly). Treated insects were maintained under constant conditions (26°C, 12 h of light and 12 h of darkness, 40% relative humidity) and provided with water and food ad libitum. After 7 days, all flies were cold immobilized and dissected to record SGH symptoms. Three replicate assays were conducted.
For quantitation of MdSGHV DNA, salivary glands (n = 3) and ovaries (n = 3) obtained from MdSGHV-infected female houseflies were subjected to quantitative real-time PCR (qRT-PCR) using the iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA) with SYBR green product tagging. DNA was extracted using a Masterpure yeast DNA purification kit (Epicenter Technologies, Madison, WI) by following the manufacturer's protocol. A sample of purified genomic MdSGHV DNA (11 ng/μl with an estimated 8 × 107 viral copies per μl) was used as a standard, and serial 10-fold dilutions of this sample were included in the PCR template to create a standard curve. Nanopure water was used for all dilutions and served as a negative control during DNA amplification. The MdSGHV-specific qRT-PCR primers designed from the C10 open reading frame included forward primer qC10aF and reverse primer qC10aR (Table 1). qRT-PCR was performed using initial denaturation at 95°C for 10 min, followed by 40 cycles of 30 s at 95°C, 30 s at 60°C, and 30 s at 72°C, and one cycle of extension at 72°C for 10 min; the procedure was finalized with a melting curve obtained by gradually increasing the temperature at a rate of 0.1°C/s up to 95°C. Threshold cycle (Ct) values were obtained for data analysis. Based on the genome size of MdSGHV (unpublished data), a standard curve was created by plotting the Ct values against the viral copy numbers of each standard dilution. The quantities of MdSGHV DNA in tissue DNA samples were inferred from the regression line (correlation coefficient [R2], ≥0.998). Each dilution of standard DNA and each tissue DNA sample was run in triplicate wells, and the qRT-PCR was repeated three times.
TABLE 1.
Primer pairs used to conduct qRT-PCR
| Primer | Sequence |
|---|---|
| qC10aF | 5′-AGAGTTTGGGCCCCATTTAC |
| qC10aR | 5′-GTCGACTACTCGGCTCATATTG |
| MdP450F | 5′-ATATGCGGAGGCTGTAATGG |
| MdP450R | 5′-AGCTTGTTGTCATGCTCACG |
| MdG3PDHF | 5′-GTTGAAGGTCTCATGACCACTG |
| MdG3PDHR | 5′-AGCCATACCAGTGAGTTTACCG |
| YO2F | 5′-CTACTTTGCCGAATCCGTTG |
| YO2R | 5′-TGGTGATGATGCCCATGTAG |
| Hex2F | 5′-GCTGGAAACTTCCCTGAGTATC |
| Hex2R | 5′-CAGGAGTAGCAACGTCAACAAG |
Production of infected flies.
To produce same-age cohorts of infected houseflies for mating experiments, filter-sterilized virus suspensions obtained from homogenized hypertrophied salivary glands (2 IGE/ml) were injected into the prothoraxes of adult virgin flies (2.5 μl per fly). Symptomatic flies were detected initially after 2 days (23%), and by 4 days postinjection 100% of the injected adults expressed SGH symptoms consisting of grossly enlarged and discolored salivary glands. Below, the term “early-stage infection” is used to indicate insects that were used for experiments 1 day after injection, whereas the term “late-stage infection” indicates insects that were used three or more days after injection. One group of females, used 2 days after injection, is referred to as “mid-stage infection” flies. Healthy control flies were injected with only sterile saline solution.
Mating experiments.
Each mating arena consisted of a 2-liter Rubbermaid plastic jar that was sealed with a sleeve of cotton cloth which allowed introduction and removal of flies. In each mating arena, 20 virgin females and 25 virgin males were introduced. If fewer insects were available, a constant ratio of females to males of 1:1.25 was used. The different mating combinations included healthy females with healthy males, healthy females with virus-infected males (early- and late-stage infection), and virus-infected females (early-, mid-, and late-stage infection) with healthy males. Virus-infected females were injected either 1 day after emergence as young, previtellogenic flies or 5 to 7 days after emergence as mature, postvitellogenic flies. Flies in the mating arenas were observed for 2.5 h. Copulating couples were collected in separate plastic vials to record successful mating events. For each replicate, one or two groups of five mated females were transferred to egg-laying chambers. A subsample of male partners of these females was collected and transferred to 16-oz plastic cups sealed with fine-mesh gauze. These males were provided with water and food ad libitum and maintained under constant conditions (26°C, 12 h of light and 12 h of darkness, 40% relative humidity) for 5 to 7 days, after which they were cold immobilized and dissected to record SGH symptoms. If no copulation occurred in the mating arenas within 2.5 h, groups of five virgin females were transferred to egg-laying chambers. Subsamples of remaining couples and single flies from mating arenas were cold immobilized and dissected.
Egg collection and female mortality.
Groups of females were maintained in egg-laying chambers under constant conditions (26°C, 12 h of light and 12 h of darkness, 40% relative humidity) for up to 3 weeks. Each egg-laying chamber consisted of a 2-liter Rubbermaid plastic jar that was sealed with a sleeve of cotton cloth, in which females were provided with water and food ad libitum. In addition, an egg-laying substrate consisting of a moistened cotton pouch (5 by 5 cm) filled with spent larval rearing medium was introduced for 5 h every other day over a period of 21 days. Spent larval rearing medium was obtained from the Center for Medical, Agricultural and Veterinary Entomology, USDA-ARS, in Gainesville, FL. After the allotted 5 h, each egg-laying substrate was removed, the eggs were counted, and a subsample of up to 100 eggs was transferred onto a moist sheet of cotton cloth (5 by 5 cm). This cloth was placed on top of a moistened 1-cm layer of larval substrate (a 1:1 mixture of wheat bran and corn meal) in a 16-oz plastic cup sealed with fine-mesh gauze. The eggs were then incubated under constant conditions (26°C, 12 h of light and 12 h of darkness, 80% relative humidity), and larval hatching was determined after 24 h by removing the cloth and counting the number of unhatched eggs. Larvae were maintained under constant conditions (26°C, 12 h of light and 12 h of darkness, 80% relative humidity) and were reared to adulthood. Subsamples of the F1 generation flies were dissected 5 to 7 days after eclosion and examined for symptoms of SGH. Female mortality in the egg-laying chambers was recorded every other day, and dead females were removed from each chamber and dissected to record SGH symptoms and ovarian development. At the end of each 3-week period, all females were cold immobilized and dissected.
Hemolymph analyses.
To examine the impact of MdSGHV infection on the abundance of female-specific hexamerin (Hex-F) and egg yolk protein (YP), hemolymph samples were collected from M. domestica females using modified methods of Capurro et al. (5, 6). Upon emergence, adult female houseflies were deprived of food but not water for 24 h. Starved females were then injected with a filter-sterilized gland preparation originating from either a healthy fly (control treatment) or a virus-infected fly expressing late-stage SGH (virus treatment) as described above. Following injection (i.e., 1 day after eclosion), control-injected females were provided with either a protein-free diet (sugar) or a protein-containing diet (a mixture of sugar, powdered milk, and powdered egg at a ratio of 6:6:1). Virus-injected females were provided with the protein-containing diet. Females that received different treatments were maintained in groups of 10 in 16-oz plastic cups sealed with fine-mesh gauze under constant conditions (26°C, 12 h of light and 12 h of darkness, 40% relative humidity). Initially, hemolymph was collected from 1-day-old, starved females (i.e., flies that were provided with water only). After injection, hemolymph was collected from sugar-fed females and from protein-fed females for three consecutive days (2-, 3-, and 4-day-old flies). Hemolymph was withdrawn from cold-immobilized females by cutting off the hind legs and using a 5-μl capillary. Four hundred nanoliters of hemolymph from up to five flies was transferred into a clean microcentrifuge tube on ice. After addition of 5 μl of sample buffer (62.5 mM Tris-HCl, 10% [vol/vol] glycerol, 2% [wt/vol] sodium dodecyl sulfate [SDS], 5% [vol/vol] 2-β-mercaptoethanol, 0.00125% [wt/vol] bromophenol blue) and 4.6 μl of water, samples were stored at −20°C until they were analyzed. Two replicate samples were collected per day per treatment (control-injected flies on sugar, control-injected flies on protein, and virus-injected flies on protein). For comparison of the protein profiles of control and virus-infected females, samples were thawed and subjected to SDS-polyacrylamide gel electrophoresis using 4% stacking, 10% running polyacrylamide gel slabs. Molecular weight markers were purchased from Bio-Rad (Hercules, CA). Electrophoresis was conducted at a constant voltage of 100 V/cm until the bromophenol blue reached the bottom of the gels. Gels were stained with 0.1% (wt/vol) Coomassie blue R-250 in fixative (acetic acid-methanol-water, 10:50:40 [vol/vol/vol]) and photographed using a digital imaging system (Bio-Rad, Hercules, CA). After bleeding, sampled flies were placed individually into microcentrifuge tubes containing TRI reagent (Sigma, St. Louis, MO) and frozen at −80°C for follow-up reverse transcriptase qRT-PCR experiments.
The presence of selected proteins (adult Hex-F and YP) in the hemolymph samples was examined by using Western blots. Hemolymph samples collected from healthy and virus-infected females at 2 and 3 days postinjection were electrophoresed on SDS gel slabs and transferred to Immobilon P membranes (Millipore, Billerica, MA). Blots were blocked using 3% bovine serum albumin in phosphate-buffered saline (PBS) and probed with either anti-Hex-F or anti-YP rabbit polyclonal antibodies (1/200 dilution) prepared in 1% powdered milk in PBS. After incubation for 2 to 3 h at 21°C, the blots were washed in PBS, incubated in anti-rabbit polyvalent alkaline phosphatase conjugate (Sigma, St. Louis, MO) (diluted 1:1,000 in PBS-bovine serum albumin) for 1 h, washed, and stained in 5-bromo-4-chloro-3-indolylphosphate with 1 mg nitroblue tetrazolium in Tris-HCl buffer (pH 9.0) containing 1 mM MgC12.
Reverse transcriptase qRT-PCR of female-specific proteins.
After collection of hemolymph and diagnostic dissection, whole flies were submerged in TRI reagent (Sigma, St. Louis, MO) and stored at −80°C. For RNA isolation, these samples were thawed, and RNA was purified by following the manufacturer's instructions. Extracted RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI), and cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. Using the MIT Primer 3 program, primer sets were designed from the available sequences of the M. domestica female adult hexamerin (Hex2), yolk protein (YO2), P450 (MdP450), and glyceraldehyde-3-phosphodehydrogenase (MdG3PDH) genes (Table 1). The MdP450 and MdG3PDH genes were tested as potential housekeeping genes for normalizing the differences found among the cDNA samples. qRT-PCR was performed under the conditions described above. Using average Ct values, it was found that the P450 transcript was invariant among the cDNA preparations, whereas both fly age and infection altered the transcription of glyceraldehyde-3-phosphodehydrogenase. In light of these preliminary findings, P450 was used to normalize the expression of both Hex2 and YO2 in these reactions using REST software (22).
Gut protease activity.
To investigate whether MdSGHV infection hinders the ability of adult houseflies to ingest and digest protein, an azocasein feeding assay was conducted as follows. One-day-old females and males were injected with saline solution and with crude MdSGHV inoculum as described above to obtain cohorts of healthy and virus-infected flies, respectively. These fly cohorts were maintained in 20-cm/side cubic wire screen cages under constant conditions (26°C, 12 h of light and 12 h of darkness, 40% relative humidity) and provided with water and food ad libitum. After 6 days, the food source was removed from the cages to starve the insects. Twenty-four hours later, a substrate consisting of a 1:9 (wt/wt) mixture of powdered azocasein (Sigma, St. Louis, MO) and regular food was added, and the flies were allowed to feed for 24 h. Flies were then cold immobilized and dissected in ice-cold phosphate buffer (pH 7). For each sex and treatment, 10 guts were collected in 100 μl of phosphate buffer. A total of two samples per sex and treatment were stored at −80°C until they were used. Three replicates of this feeding assay were conducted. To process collected gut samples, each sample was thawed, homogenized, sonicated for 30 s, and divided into two subsamples (subsamples 1 and 2). To examine differences in the amount of azocasein ingested, subsample 1 was mixed with 150 μl of 500 mM NaOH, incubated at 37°C for 10 min, and centrifuged at 735 × g for 3 min. The supernatant was transferred to a clean well of a 96-well plate. To examine differences in the amount of azocasein digested (i.e., the amount of p-nitroaniline released), subsample 2 was centrifuged at 735 × g for 3 min, and the supernatant was mixed with 50 μl of 5% trichloroacetic acid and centrifuged at 9,300 × g for 10 min. The resulting supernatant was transferred to a clean well of a 96-well plate containing 150 μl of 500 mM NaOH. Emission was determined at 440 nm using a μQuant plate reader interfaced with KC4 software (BIO-TEK Instruments Inc., Winooski, VT). Samples from unfed females and males were used to normalize emission values. Standard curves generated using dilution series of azocasein and p-nitroaniline were used to calculate the amount of azocasein (μg) ingested and the amount of p-nitroaniline (ng) released during digestion per fly.
Statistics.
Statistical analyses were conducted using the SAS System for Windows (27). The average copulation ratios (i.e., the number of copulating couples per female in an arena) were compared by logistic regression using the SAS genmod procedure (proc genmod) and the SAS least-square means statement (lsmeans) (17). Comparisons of cumulative female mortality over time for healthy and virus-infected females, as well as egg hatch ratios for egg batches laid by females that received different treatments, were also subjected to logistic regression using proc genmod and lsmeans. Cumulative numbers of eggs were evaluated by analysis of variance using the SAS procedure for mixed linear models (proc mixed), and means were separated using lsmeans (23, 30). Emission values from the azocasein feeding assay were subjected to analysis of variance using the SAS procedure for general linear models (proc glm) and lsmeans (23, 30). Infection rates within a set of experiments were analyzed using proc genmod and lsmeans (17). The data were expressed as means ± standard errors.
RESULTS
Virus quantitation.
qRT-PCR revealed that 1.6 × 1010 ± 0.1 × 1010 (n = 3) and 3.4 × 108 ± 0.1 × 108 (n = 3) viral copies were present in the salivary glands and ovaries, respectively, of an MdSGHV-infected female housefly. Consequently, healthy flies that were injected with a stock solution of crude salivary gland inoculum and healthy flies that were injected with a crude ovarian inoculum were challenged with ∼7.8 × 107 ± 0.3 × 107 and ∼1.7 × 106 ± 0.1 × 106 virus particles, respectively. In order to determine the endpoint of viral infectivity, serial 10-fold dilutions of crude salivary gland or crude ovarian extracts were injected into healthy M. domestica females. Within 7 days, injection of crude salivary gland inoculum (stock concentration, 2 IGE/ml) diluted 106-fold resulted in 100% infection in groups of treated flies (Table 2). Flies injected with this dilution were challenged with an estimated 78 viral copies. Further dilution resulted in decreasing infection rates, and a 109-fold dilution did not produce any SGH symptoms (Table 2). When females were injected with crude ovarian inoculum (stock concentration, 2 IOE/ml), a 100-fold dilution resulted in 100% infection in the challenged flies within 7 days (Table 2). These females were injected with an estimated 16,800 viral copies. Higher dilutions produced decreasing infection rates, and no infection was found in females injected with a 105-fold dilution of crude ovarian extract, which contained an estimated 17 viral copies (Table 2).
TABLE 2.
Infection of M. domestica females 7 days after injection of infectious salivary gland or ovarian extracts at different concentrations
| Origin of MdSGHV-inoculum | Dilutiona | No. of viral copies injectedb | % Infectionc |
|---|---|---|---|
| Salivary glands | 104 | 7,822 | 100 ± 0 a |
| 105 | 782 | 100 ± 0 a | |
| 106 | 78 | 100 ± 0 a | |
| 107 | 8 | 77 ± 12 b | |
| 108 | 1 | 17 ± 12 c | |
| 109 | 0 | 0 ± 0 d | |
| Control | 0 | 0 ± 0 d | |
| Ovaries | 101 | 168,000 | 100 ± 0 a |
| 102 | 16,800 | 100 ± 0 a | |
| 103 | 1,680 | 73 ± 12 b | |
| 104 | 168 | 31 ± 11 c | |
| 105 | 17 | 0 ± 0 d | |
| Control | 0 | 0 ± 0 d |
Stock solutions contained 2 IGE per ml or 2 IOE per ml. Control females were injected with sterile saline solution alone.
Numbers of viral copies per gland pair and per ovary pair were determined by qRT-PCR.
Three replicate assays with 10 females per concentration were conducted. Values followed by different letters within a treatment series were significantly different (P ≤ 0.002, χ2 ≥ 9.56, df = 1; SAS proc genmod and lsmeans).
Injection of ovarian extracts into healthy flies.
Crude ovarian extracts prepared from MdSGHV-infected females were highly infectious when they were injected into the hemocoels of female and male houseflies. Within 7 days after injection, all flies (n = 120) showed symptoms of late-stage SGH, and the ovarian development of all females (n = 60) was halted at the previtellogenic stage. In contrast, none of the flies (n = 40) that were injected with control ovarian extracts originating from healthy females showed SGH symptoms, and all females (n = 20) had fully developed ovaries containing mature eggs. No mortality occurred in these experiments.
Effects of MdSGHV on houseflies.
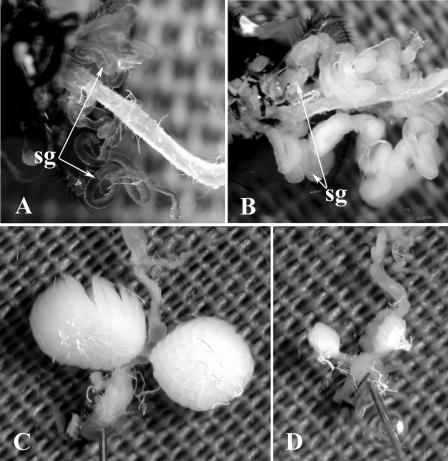
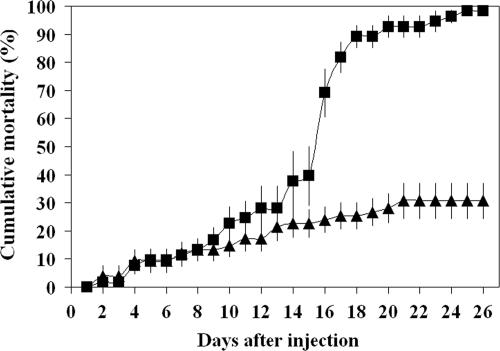
All saline-injected control flies had healthy, transparent salivary glands (Fig. 1A), whereas 100% of the virus-injected adults expressed SGH symptoms within 5 days after injection of the stock inoculum (Fig. 1B). When mature, postvitellogenic females (5 to 7 days old) were injected with MdSGHV and dissected 5 days after injection, they contained mature ovaries with fully developed eggs (Fig. 1C). When young, previtellogenic females (1 to 3 days old) were injected with MdSGHV, no ovarian development was seen after 5 days (Fig. 1D), a time when healthy control females (injected with sterile saline only) had fully developed ovaries with mature eggs (not shown). No morphological aberrations were observed in female accessory glands and spermathecae (data not shown). The mortality of virus-infected females significantly increased 16 days after injection compared with healthy control females (P < 0.0001, χ2 ≥ 20.63, df = 1; SAS proc genmod and lsmeans) (Fig. 2). Between 16 and 26 days after injection, the mortality rate increased from 69% ± 9% to 98% ± 2% (n = 11) and from 24% ± 5% to 31% ± 6% (n = 15) in groups of virus-infected and healthy females, respectively. Unlike the results for females, the testes of MdSGHV-infected male houseflies were normally developed and contained viable sperm, and no morphological aberrations were observed in the male ejaculatory duct.
FIG. 1.
Salivary glands (sg) and ovaries dissected from 7-day-old healthy or MdSGHV-infected M. domestica females. (A) Transparent salivary glands and digestive tract of a healthy female. (B) Grossly enlarged milky salivary glands of a virus-infected female. (C) Fully developed ovaries of a virus-infected female infected as a mature, postvitellogenic fly. (D) Undeveloped ovaries of a virus-infected female infected as young, previtellogenic fly.
FIG. 2.
Cumulative mortality of M. domestica females after injection of a saline solution (healthy) (▴) or MdSGHV inoculum (virus infected) (▪). The mortality rate of virus-infected females (n = 54) increased significantly at 16 days postinjection (P < 0.0001, χ2 ≥ 20.63, df = 1; SAS proc genmod and lsmeans) compared with healthy females (n = 72).
Mating behavior.
Healthy control males avidly attempted to copulate with both healthy and virus-infected females of all ages (Table 3). With the exception of 1 of 31 group combinations with healthy males, males aggressively approached females, and flies began mating within 10 to 20 min after recovery from cold immobilization. After a successful strike, each male landed on the dorsal thorax of the female, forced her wings into a horizontal position, and stroked her head with his forelegs. He then moved toward the rear of the female, grasped the ventral side of her abdomen with his hind legs, and positioned the aedeagus beneath her ovipositor, waiting for her to respond. At an early stage of infection (24 h after injection), the majority of the males were avid and copulated readily with healthy females, whereas at a late stage of infection (3 to 4 days after injection), viremic males showed reduced avidity and made only slow attempts to mate with healthy females (Table 3). As shown in Table 3, healthy females were responsive to mating attempts from both healthy and virus-infected males, regardless of the stage of male infection. After the sequence of male courtship behavior described above, a female readily extended her ovipositor into the genital opening of the male, which resulted in successful copulation. Young, previtellogenic females and mature, postvitellogenic females copulated readily when they were allowed to mate at an early stage of infection (24 h after injection) (Table 3). When allowed to mate at a late stage of infection (3 to 4 days after injection), infected females in both cohorts did not copulate with healthy males (Table 3). The only exception was 1 of 70 young females at a late stage of infection that copulated with a healthy male (Table 3). Healthy males performed normal mating rituals with infected females, but the females did not extend their ovipositors into the male genital opening. Males often approached the same female multiple times. At times they repeated their courtship rituals, stroking the female's head, extending her wings, and mounting her before releasing her.
TABLE 3.
Copulation and mating behavior of M. domestica in mating arenas with different combinations consisting of healthy and MdSGHV-infected females and malesa
| Combinationb
|
% Copulation (avg ± SE)c | Mating behaviord
|
||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Healthy (262) | Healthy (309) | 87 ± 3 a | Responsive | Avid |
| Healthy (118) | Infected for 24 h (149) | 63 ± 15 b | Responsive | Avid (5), slow (2) |
| Healthy (60) | Infected for 72 h (67) | 28 ± 8 c | Responsive | Slow |
| Infected for 24 h, postvitellogenic (74) | Healthy (93) | 90 ± 10 a | Responsive | Avid |
| Infected for 48 h, postvitellogenic (20) | Healthy (20) | 20 ± 0 ce | Slow response | Avid |
| Infected for 72 h, postvitellogenic (65) | Healthy (82) | 0 ± 0 d | No response | Avid |
| Infected for 24 h, previtellogenic (70) | Healthy (75) | 57 ± 14 bc | Responsive | Avid (2), slow (1) |
| Infected for 72 h, previtellogenic (70) | Healthy (67) | 1 ± 1 f | No response | Avid |
The group size varied from 5 to 57 flies per mating arena, with a female-to-male ratio of 1:1 or 1:1.25. Flies were observed for 2.5 h.
The numbers in parentheses are the total numbers of females and males used in the combinations.
The percent copulation for each group was calculated by dividing the number of couples by the number of females. Different letters after the values indicate significant differences (P ≤ 0.0233, χ2 ≥ 5.15, df = 1; SAS proc genmod and lsmeans).
The numbers in parentheses are the numbers of groups in which the behavior was observed.
The percentage of copulation was highest for mating combinations consisting of healthy females and healthy males (87% ± 3%) and of mature females with early-stage infection and healthy males (90% ± 10%) (Table 3). Significantly lower copulation rates were found when healthy females were paired with males at an early stage of infection (63% ± 15%) or with males at a late stage of infection (28% ± 8%) (P < 0.0001, χ2 ≥ 20.59, df = 1; SAS proc genmod and lsmeans), which was accompanied by a reduction in male avidity (Table 3). The percentage of copulation between healthy males and mature, virus-infected females was highest when females were allowed to mate at an early stage of infection (90% ± 10%) and was significantly reduced to 20% ± 0% and 0% ± 0% when females were allowed to mate at mid and late stages of infection, respectively (P < 0.0001, χ2 = 27.10, df = 1; SAS proc genmod and lsmeans). Reduced copulation rates were accompanied by a reduction in female responsiveness to male mating attempts (Table 3). For mating between healthy males and young, virus-infected females there was a significant decline in the percentage of copulation from 57% ± 14% with young females at an early stage of infection to 1% ± 1% with young females at a late stage of infection (P < 0.0001, χ2 = 14.95, df = 1; SAS proc genmod and lsmeans), again accompanied by a reduction in female responsiveness (Table 3).
Oviposition and egg hatch.
Healthy males that copulated with healthy and virus-infected females transferred viable sperm. Similarly, infected males at early and late stages of infection produced viable offspring when they were mated with healthy females. Healthy control females of all ages and in all mating combinations deposited multiple viable egg batches over a period of 21 days. In general, at the late stage of infection viremic females did not copulate (Table 3) and subsequently did not oviposit. The only exception was a single late-stage infected female that copulated but did not deposit eggs. Subsequent dissection revealed that this female had undeveloped ovaries but contained sperm in the spermathecae. Mature, virus-infected females that copulated within 24 to 48 h after injection (early to mid stage of infection) deposited one viable egg batch within 5 days after mating. The average oviposition rate for these mature, virus-infected females in a 3-week observation period was 99 ± 13 eggs per female and was significantly lower than the oviposition rates for healthy females that were fertilized by healthy or virus-infected males (P < 0.0001, t = 6.86, df = 399; SAS proc mixed and lsmeans). Healthy females deposited an average of 363 ± 28 eggs per female in a period of 3 weeks.
Subsamples of up to 100 eggs from groups of egg-laying females were transferred to larval rearing medium, and egg hatch was observed for all egg batches laid by healthy and virus-infected females. The percentage of eggs that hatched for batches deposited by virus-infected females (74% ± 10%; n = 675) was significantly lower than the percentage of eggs that hatched for egg batches originating from healthy females (91% ± 2%; n = 4,717) (P < 0.0001, χ2 = 153.55, df = 1; SAS proc genmod and lsmeans).
Vertical transmission.
No vertical transmission of MdSGHV was observed. Dissection of F1 adults at 5 to 7 days posteclosion revealed no symptoms of SGH. A total of 690 F1 adults were dissected, of which 135 originated from matings with virus-infected females and 555 originated from matings with virus-infected males.
Impact of viral infection on vitellogenesis.
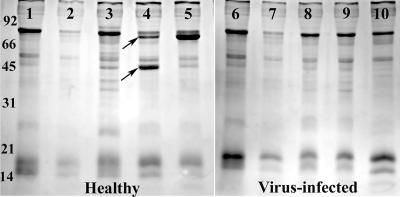
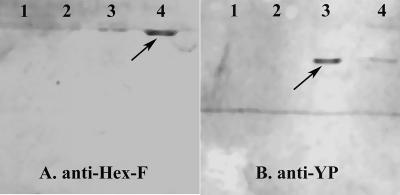
Examination of hemolymph from healthy females and MdSGHV-infected females after they were fed a protein-containing food source demonstrated that both Hex-F and YP were not produced in MdSGHV-infected flies (Fig. 3). At the time of injection, the healthy and infected females lacked profiles for both of the target proteins (Fig. 3, lanes 1 and 6). Similarly, the hemolymph profiles obtained for healthy flies fed only sucrose for 2 days were identical (Fig. 3, lanes 2 and 7). The hemolymph profiles of healthy females provided with a protein-containing diet showed that both Hex-F and YP were synthesized (Fig. 3, lanes 3 to 5). The synthesis of Hex-F (molecular mass, ∼70 kDa) was detected initially at 48 h after the flies fed on the protein diet; by 72 h after feeding, Hex-F was the major band detected in the hemolymph samples. Transient production of a second peptide presumed to be the YP (molecular mass, ∼44 kDa) was detected in the hemolymph samples of protein-fed healthy females. In these flies, the level of YP was greatest at 48 h after feeding (Fig. 3, lane 4) and decreased at 72 h after feeding (Fig. 3, lane 5). Neither the Hex-F band nor the YP band was detected in the samples collected from the MdSGHV-infected females (Fig. 3, lanes 8 to 10). The presence and relative levels of Hex-F and YP in the hemolymph samples from healthy and viremic flies were confirmed on Western blots that were probed with rabbit anti-Hex-F and anti-YP polyclonal antibodies (Fig. 4).
FIG. 3.
SDS-polyacrylamide gel electrophoresis gel of hemolymph samples collected from adult female houseflies. Lanes 1, 2, 6, and 7 contained samples from flies at the previtellogenic stage; lanes 1 and 6 contained hemolymph samples from newly eclosed females, and lanes 2 and 7 contained hemolymph samples from healthy females that were provided with only sugar for 3 days. Lanes 3, 4, and 5 contained samples from healthy flies provided with both sugar and protein collected after 1, 2, and 3 days, respectively. In lanes 3 to 5, note the presence of the adult female ∼70-kDa Hex-F (upper arrow) and the ∼45-kDa YP (lower arrow). Lanes 8, 9, and 10 contained hemolymph samples from virus-infected females that were also provided with both sugar and protein, which were collected after 1, 2, and 3 days, respectively. Note the lack of detectable Hex-F and YP bands in the virus-infected hemolymph.
FIG. 4.
Western blots of hemolymph samples that were probed with anti-Hex-F (A) and anti-YP (B) polyclonal antibodies. Lanes 1 and 2 contained samples derived from virus-infected females collected 2 and 3 days after infection, respectively. Lanes 3 and 4 contained samples from healthy females collected after 2 and 3 days, respectively.
Selected healthy and infected insects that were sampled for the hemolymph analysis were placed in TRI reagent and frozen at −80°C for reverse transcriptase qRT-PCR experiments. The results demonstrated that both the relative abundance of the YP transcripts and the relative abundance of the Hex-F transcripts were significantly decreased by viral replication. At 2 and 3 days postinfection, viremic females contained 2,144- and 1,596-fold fewer YP transcripts, respectively, and 1,120- and 94-fold fewer Hex-F transcripts, respectively, than healthy females. From these results, it appears that infection with MdSGHV down-regulates transcription of female-specific proteins associated with the gonadotropic cycle.
Gut protease activity.
An azocasein feeding assay was conducted to examine if MdSGHV-infection inhibited the ability of houseflies to ingest and digest a protein-containing food source. Both healthy and MdSGHV-infected male and female flies ingested the azocasein-laced food (Table 4). Analysis of consumption data demonstrated that in a 24-h feeding period virus-infected females ingested more food than healthy male and female flies. However, based on the levels of p-nitroaniline released in the gut lumen relative to the amount of azocasein ingested, both male and female healthy flies had significantly more proteolytic activity than their infected counterparts (P < 0.0001, F = 21.03, df = 3; SAS proc glm and lsmeans) (Table 4).
TABLE 4.
Amount of azocasein ingested and amount of p-nitroaniline released by healthy and MdSGHV-infected M. domestica females and males during a 24-h feeding assaya
| MdSGHV infection | Sex | Amt of azocasein ingested (μg/fly) (avg ± SE)b | Amt of p-nitroaniline released (ng) (avg ± SE)b
|
|
|---|---|---|---|---|
| Per fly | Per μg of azocasein ingested | |||
| No | Female | 75 ± 6 ab | 908 ± 1 a | 12.4 ± 1.5 a |
| Male | 60 ± 4 b | 1,002 ± 17 a | 17.0 ± 3.0 b | |
| Yes | Female | 98 ± 13 a | 667 ± 0 b | 7.4 ± 2.8 c |
| Male | 80 ± 6 ab | 631 ± 17 b | 7.8 ± 1.6 c | |
A total of six samples consisting of 10 guts per sample were collected for each infection state and sex.
Values followed by different letters in each column were significantly different (P ≤ 0.0337, F ≥ 3.53, df = 3; SAS proc glm and lsmeans).
DISCUSSION
Our results demonstrated that the MdSGHV selectively infects and causes detectable cytopathology in the salivary glands of both male and female houseflies. No other tissues exhibited the nuclear hypertrophy that characterized infected salivary glands. Under optimum laboratory conditions, the life span of virus-infected female houseflies was significantly reduced at 16 days after virus acquisition by injection. Infection with MdSGHV did not noticeably hinder the ability of female and male houseflies to feed or fly. In fact, the only overt symptom of MdSGHV infection was its impact on mating behavior and reproduction.
Successful mating for Musca spp. involves a complex and rigidly structured sequence of behavioral steps that maintain barriers to hybridization among closely related members of this genus (28). We observed that healthy males readily attempted to copulate with virus-infected females at both early and late stages of infection, indicating that infection did not affect the attractiveness of female flies to males. These results were not surprising, given that housefly males initiate courtship behavior with male flies, females of other species, and even inanimate objects (28). However, infection with MdSGHV had adverse effects on the outcome of mating attempts. At the stage when SGH is expressed, infected females (regardless of ovarian condition) refused to copulate with healthy males. Virus infection also affected the reproductive behavior of male houseflies. At a late stage of infection, the avidity and aggressiveness of attempts to copulate with healthy females were reduced. Gross morphology comparison of testes, accessory reproductive glands, and the ejaculatory duct dissected from healthy and virus-infected males did not show any differences, and virus-infected males were able to inseminate healthy females with viable sperm. In contrast, SGHV infection in male tsetse flies resulted in evident infection of the accessory reproductive glands and in a failure to produce complete spermatophores; SGHV-infected tsetse flies generally failed to transfer sperm to females (25).
Mature females that were mated soon after infection were able to mate and deposit eggs from the first gonadotropic cycle successfully. However, these females had significantly reduced fecundity after they deposited their first batch of eggs and stopped laying eggs within 1 week after copulation. Dissection of flies at this time revealed grossly enlarged salivary glands and undeveloped ovaries. Our results demonstrate that this was the result of the virus preventing yolk deposition in subsequent gonadotropic cycles. In females that were infected at an early age the ovaries never developed. Tsetse flies infected with SGHV also showed reduced fecundity but via different mechanisms. In Glossina morsitans centralis (Machado), the milk glands of infected females were infected and exhibited severe degeneration, especially in the neurosecretory cells lining the lumen of the gland (26). The ovarioles of infected G. pallidipes also showed necrosis and degeneration of the germaria (12).
There were no indications that the MdSGHV was sexually transmitted between mating partners or vertically transmitted to the next generation. It should be noted that the testable matings were restricted to mating crosses between infected males and healthy females; infected females displaying SGH did not mate. Additionally, female flies infected within 24 h after emergence did not produce detectable levels of Hex-F and YP. Preliminary work demonstrated that MdSGHV infection blocked transcription of the genes coding for these proteins. In healthy females, ingestion of a protein meal stimulates a pulse of ecdysone that triggers the synthesis of female-specific proteins that are involved in the gonadotropic cycle (2, 6). As determined in an azocasein feeding assay, virus-infected females and males of M. domestica were able to ingest and digest a protein-containing food substrate. Digestion was reduced in infected flies compared with healthy flies. Whether the reduction in proteolytic activity in infected females could account for down-regulation of vitellogenesis is unclear and requires additional examination. Alternatively, MdSGHV may cause a latent infection in the ovary. Crude ovarian homogenates were highly infectious when they were injected into healthy flies, and qRT-PCR results demonstrated that high numbers of viral copies (approximately 3 × 108 copies) were present in ovarian tissue of MdSGHV-infected houseflies. However, preliminary histological examination of ovarian tissues from infected flies did not reveal any apparent signs of viral infection (unpublished data). When the infectivity of MdSGHV originating from ovarian tissue of infected houseflies and the infectivity of MdSGHV originating from salivary glands of infected houseflies were compared, 200-fold more viral copies were necessary to produce 100% infection in groups of flies injected with crude ovarian extract.
In summary, the MdSGHV acts as a biological sterilizing agent in that it reduces the intrinsic rate of increase in infected houseflies. The MdSGHV possesses several unique properties, including suppression of ovarian development in young flies and in flies that become infected during stages of early egg maturation. If a female fly receives the virus postvitellogenesis, she may still mate, but her fecundity is significantly reduced as the virus blocks subsequent gonadotropic cycles. Postvitellogenic females that host the virus for more than a few days do not mate at all. Transmission appears to be horizontal via cofeeding of infected and uninfected adult flies. These properties suggest that the virus could be used as a population management tool, especially if it is deployed early in the fly season when populations are increasing. It remains to be seen whether an effective “attract and infect” delivery system can be developed by incorporating viral inoculum into known fly attractants.
Acknowledgments
We thank Natalie van Hoose and Jessica Noling for technical assistance, M. Capurro (University of San Paulo, Brazil) for generously providing rabbit polyclonal antibodies, and two anonymous reviewers for their critical comments on earlier drafts of the manuscript.
Footnotes
Published ahead of print on 7 September 2007.
REFERENCES
- 1.Abd-Alla, A., H. Bossin, F. Cousserans, A. Parker, M. Bergoin, and A. Robinson. 2007. Development of a non-destructive PCR method for detection of the salivary gland hypertrophy virus (SGHV) in tsetse flies. J. Virol. Methods 139:143-149. [DOI] [PubMed] [Google Scholar]
- 2.Adams, T. S., and J. W. Gerst. 1992. Interaction between diet and hormones on vitellogenin levels in the housefly, Musca domestica. Invertebr. Reprod. Dev. 21:91-98. [Google Scholar]
- 3.Amargier, A., J. P. Lyon, C. Vago, G. Meynadier, and J. C. Veyrunes. 1979. Mise en evidence et purification d'un virus dans la proliferation monstreuse glandulaire d'insectes. Etude sur Merodon equestris (Diptera, Syrphidae). C. R. Acad. Sci. Ser. D 289:481-484. [PubMed] [Google Scholar]
- 4.Burtt, E. 1945. Hypertrophied salivary glands in Glossina: evidence that G. pallidipes with this abnormality is peculiarly suited to trypanosome infection. Ann. Trop. Med. Parasitol. 39:11-13. [Google Scholar]
- 5.Capurro, M. D. L., O. Marinotti, C. S. Farah, A. A. James, and A. G. de Blanchi. 1997. The nonvitellogenic female protein of Musca domestica is an adult-specific hexamerin. Insect Mol. Biol. 6:97-104. [DOI] [PubMed] [Google Scholar]
- 6.Capurro, M. D. L., C. K. Moreira-Ferro, O. Marinotti, A. A. James, and A. G. de Bianchi. 2000. Expression patterns of the larval and adult hexamerin genes of Musca domestica. Insect Mol. Biol. 9:169-177. [DOI] [PubMed] [Google Scholar]
- 7.Coler, R. R., D. G. Boucias, J. H. Frank, J. E. Maruniak, A. Garcia-Canedo, and J. C. Pendland. 1993. Characterization and description of a virus causing salivary gland hyperplasia in the housefly, Musca domestica. Med. Vet. Entomol. 7:275-282. [DOI] [PubMed] [Google Scholar]
- 8.Ellis, D. S., and I. Maudlin. 1987. Salivary gland hyperplasia in wild caught tsetse from Zimbabwe. Entomol. Exp. Appl. 45:167-173. [Google Scholar]
- 9.Feldmann, U., H. Barnor, and R. Acs. 1992. Abweichungen in der Reproduktion von Glossina morsitans submorsitans Newstead (Diptera: Glossinidae): Untersuchungen zur Bestimmung eines gestörten Geschlechterverhältnisses und zum Übertragungensweg von fertilitäts-reduzierenden Viren an die Nachkommen. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 8:248-251. [Google Scholar]
- 10.Hogsette, J. A. 1992. New diets for production of house flies and stable flies (Diptera: Muscidae) in the laboratory. J. Econ. Entomol. 85:2291-2294. [DOI] [PubMed] [Google Scholar]
- 11.Jaenson, T. G. T. 1978. Virus-like rods associated with salivary gland hyperplasia in tsetse, Glossina pallidipes. Trans. R. Soc. Trop. Med. Hyg. 72:234-238. [DOI] [PubMed] [Google Scholar]
- 12.Jura, W. G. Z. O., T. R. Odhiambo, L. H. Otieno, and N. O. Tabu. 1988. Gonadal lesions in virus-infected male and female tsetse, Glossina pallidipes (Diptera: Glossinidae). J. Invertebr. Pathol. 52:1-8. [DOI] [PubMed] [Google Scholar]
- 13.Jura, W. G. Z. O., L. H. Otieno, and M. Chimtawi. 1989. Ultrastructural evidence for trans-ovum transmission of the DNA virus of tsetse Glossina pallidipes (Diptera: Glossinidae). Curr. Microbiol. 18:1-4. [Google Scholar]
- 14.Jura, W. G. Z. O., J. Zdarek, and L. H. Otieno. 1993. A simple method for artificial infection of tsetse, Glossina morsitans morsitans, larvae with the DNA virus of G. pallidipes. Insect Sci. Appl. 14:383-387. [Google Scholar]
- 15.Kokwaro, E. D., M. Nyindo, and M. Chimtawi. 1990. Ultrastructural changes in salivary glands of tsetse, Glossina morsitans morsitans, infected with virus and rickettsia-like organisms. J. Invertebr. Pathol. 56:337-346. [DOI] [PubMed] [Google Scholar]
- 16.Kokwaro, E. D., L. H. Otieno, and M. Chimtawi. 1991. Salivary glands of the tsetse Glossina pallidipes Austen infected with Trypanosoma brucei and virus particles: ultrastructural study. Insect Sci. Appl. 12:661-669. [Google Scholar]
- 17.Neter, J., W. Wasserman, and M. H. Kutner. 1990. Applied linear statistical models: regression, analysis of variance, and experimental designs. Richard Irwin Inc., Homewood, IL.
- 18.Odindo, M. O. 1982. Incidence of salivary gland hypertrophy in field populations of the tsetse Glossina pallidipes on the south Kenyan coast. Insect Sci. Appl. 3:59-64. [Google Scholar]
- 19.Odindo, M. O., C. C. Payne, N. E. Crook, and P. Jarrett. 1986. Properties of a novel DNA virus from the tsetse fly, Glossina pallidipes. J. Gen. Virol. 67:527-536. [DOI] [PubMed] [Google Scholar]
- 20.Odindo, M. O., D. M. Sabwa, P. A. Amutalla, and W. A. Otieno. 1981. Preliminary tests on the transmission of virus-like particles to the tsetse Glossina pallidipes. Insect Sci. Appl. 2:219-221. [Google Scholar]
- 21.Otieno, L. H., E. D. Kokwaro, M. Chimtawi, and P. Onyango. 1980. Prevalence of enlarged salivary glands in wild populations of Glossina pallidipes in Kenya, with a note on the ultrastructure of the affected organ. J. Invertebr. Pathol. 36:113-118. [Google Scholar]
- 22.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao, P. V. 1998. Statistical research methods in the life sciences. Duxbury Press, Pacific Grove, CA.
- 24.Sang, R. C., W. G. Z. O. Jura, L. H. Otieno, and R. W. Mwangi. 1998. The effects of a DNA virus infection on the reproductive potential of female tsetse flies, Glossina morsitans centralis and Glossina morsitans morsitans (Diptera: Glossinidae). Mem. Inst. Oswaldo Cruz 93:861-864. [DOI] [PubMed] [Google Scholar]
- 25.Sang, R. C., W. G. Z. O. Jura, L. H. Otieno, R. W. Mwangi, and P. Ogaja. 1999. The effects of a tsetse DNA virus infection on the functions of the male accessory reproductive gland in the host fly Glossina morsitans centralis (Diptera; Glossinidae). Curr. Microbiol. 38:349-354. [DOI] [PubMed] [Google Scholar]
- 26.Sang, R. C., W. G. Z. O. Jura, L. H. Otieno, and P. Ogaja. 1996. Ultrastructural changes in the milk gland of tsetse Glossina morsitans centralis (Diptera; Glissinidae) female infected by a DNA virus. J. Invertebr. Pathol. 68:253-259. [DOI] [PubMed] [Google Scholar]
- 27.SAS. 1999. User's guide, version 8.0. SAS Institute, Cary, NC.
- 28.Tobin, E. N., and J. G. Stoffolano. 1973. The courtship of Musca species found in North America, 1. The house fly Musca domestica. Ann. Entomol. Soc. Am. 66:1249-1257. [Google Scholar]
- 29.Whitnall, A. M. B. 1934. The trypanosome infections of Glossina pallidipes in Umfolosi game reserve, Zuhuland. Onderstepoot J. Anim. Ind. 11:7-21. [Google Scholar]
- 30.Younger, M. S. 1998. SAS companion for P. V. Rao's statistical research methods in the life sciences. Duxbury Press, Pacific Grove, CA.