Abstract
Shewanella oneidensis MR-1 is a gram-negative facultative anaerobe capable of utilizing a broad range of electron acceptors, including several solid substrates. S. oneidensis MR-1 can reduce Mn(IV) and Fe(III) oxides and can produce current in microbial fuel cells. The mechanisms that are employed by S. oneidensis MR-1 to execute these processes have not yet been fully elucidated. Several different S. oneidensis MR-1 deletion mutants were generated and tested for current production and metal oxide reduction. The results showed that a few key cytochromes play a role in all of the processes but that their degrees of participation in each process are very different. Overall, these data suggest a very complex picture of electron transfer to solid and soluble substrates by S. oneidensis MR-1.
The ability of certain bacteria to reduce metal oxides has been explored in depth over the last 20 years. Microbes capable of such activity, called dissimilatory metal-reducing bacteria (DMRB), are of great interest to the science and engineering community due to their roles in various geobiological phenomena and for possible use in bioremediation and biotechnology applications. Organisms that have been a focus of interest in recent years include DMRB in genera such as Clostridium (43), Geobacter (7, 19), Aeromonas (44), Rhodoferax (11), Desulfobulbus (18), and Shewanella (10, 21). All of these DMRB have also been shown to produce current in microbial fuel cell (MFC) systems (6, 27). This study focuses on the electron transfer mechanisms employed by Shewanella oneidensis MR-1, a strain that was chosen as a model organism based on its metabolic versatility (37), its sequenced and annotated genome (16), and its genetic accessibility with regard to both generation and complementation of mutants (15).
MR-1 and other members of the Shewanella genus were originally studied because of their ability to couple the oxidation of organic compounds and H2 to the reduction of manganese and iron oxides (1, 26, 28, 29, 37, 38, 46, 52). The mechanisms responsible for metal oxide reduction are not fully understood, but it is clear that a number of genes are involved (2, 3, 13, 15, 17, 32, 35, 40-42, 45, 53). These include the mtrA, mtrB, mtrC (also known as omcB), omcA, and cymA genes. mtrA encodes a periplasmic decaheme c-type cytochrome involved in Mn(IV) and Fe(III) reduction (2-5, 45). mtrB encodes an outer membrane (OM) protein of 679 amino acids and may play a role in the binding of metals during reduction (3) and/or may be required for the proper localization and insertion of OM cytochromes involved in direct electron transfer (35). mtrC and omcA encode OM decaheme c-type cytochromes that have been hypothesized to serve as terminal Mn(IV) (4, 33, 36, 39, 40) and Fe(III) (4, 15) reductases and to be involved in electron transfer to electrodes in MFCs (15). cymA encodes a cytoplasmic membrane-bound, tetraheme c-type cytochrome that is involved in mediating electron flow from the cytoplasm to several terminal reductases (including OM c-type cytochromes) during anaerobic respiration (34, 41, 48).
The cymA and mtr genes described above represent only a small fraction of the part of the MR-1 genome that may be involved in energy metabolism. Forty-two possible c-type cytochrome genes have been identified in the MR-1 genome (16, 24, 32), and only a few of these have been characterized. In addition, some regulatory and protein secretion genes, as well as hydrogenase and genes associated with type IV pilin production, have been implicated in energy metabolism (15, 47, 50, 51). For example, Thompson et al. (50) suggested that the fur (ferric uptake regulator) gene product is a global regulator that positively controls genes involved in electron transport. Additionally, Wan et al. (51) proposed that omcA is a direct target of fur modulon activation. Saffarini et al. (47) identified crp, which encodes the cyclic AMP receptor protein, as a global regulator of anaerobic respiration, including Fe(III) and Mn(IV) reduction. An analysis of MR-1's twin-arginine translocation machinery suggests that this system plays a key role in the secretion of a number of redox proteins to the periplasm, some of which are either directly or indirectly involved in metal reduction (14).
The diverse energy metabolism of MR-1 is clearly governed by a number of elements; however, the evidence thus far only offers a glimpse into the larger picture of energy metabolism. Questions still remain as to which gene products are utilized for electron transfer to solid substrates and whether these gene products are universally utilized for different types of metal oxide reduction and electron transfer to MFC electrodes.
The mechanism(s) of electron transfer to solids by MR-1 was investigated using the wild-type (WT) strain and mutants deficient in c-type cytochromes and protein secretion systems. The evaluation was based on the ability of each mutant to (i) produce current in an MFC system (10, 23), (ii) reduce Mn(IV) oxides, and (iii) reduce solid-phase iron(III) oxides. In addition, two hydrogenase mutants (carrying hyaB:pDS3.1 and hydA:pDS3.1), three regulatory mutants (carrying tatC:pDS3.1, fur:pKNOCK and crp:Tn5), an OM protein mutant (carrying ompW:pDS3.1), and a quorum-sensing mutant (carrying luxS:pDS3.1) were evaluated for current production in an MFC. These data represent the first identification of gene products specifically related to current production in strain MR-1 and strongly suggest that strain MR-1 features multiple pathways for electron transfer to different substrates.
MATERIALS AND METHODS
MR-1 WT, cytochrome deletion mutants and their complements, protein secretion and regulatory mutants. (i) Culture and growth conditions.
A list of the mutants used in this study is provided in Table 1. The WT, cytochrome mutant, and protein secretion mutant cultures were grown aerobically in batch culture using a defined medium containing 18 mM lactate as the sole carbon source, 50 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid], 7.5 mM NaOH, 28 mM NH4Cl, 1.3 mM KCl, 4.3 mM NaH2PO4·H2O, 100 mM NaCl, and 10 ml/liter each of vitamin solution (20), amino acid solution, and trace mineral stock solution. The amino acid solution (pH 7.0) contained: 2 g/liter l-glutamic acid, 2 g/liter l-arginine, and 2 g/liter dl-serine. The trace mineral solution (pH 7.0) contained 78.49 μM C6H9NO3, 121.71 μM MgSO4·7H2O, 29.58 μM MnSO4·H2O, 171.12 μM NaCl, 3.60 μM FeSO4·7H2O, 6.80 μM CaCl2·2H2O, 4.20 μM CoCl2·6H2O, 9.54 μM ZnCl2, 0.40 μM CuSO4·5H2O, 0.21 μM AlK(SO4)2·12H2O, 1.62 μM H3BO3, 1.03 μM Na2MoO4·2H2O, 1.01 μM NiCl2·6H2O, and 0.76 μM Na2WO4·2H2O. Cultures were grown at 30°C and agitated at rate of 140 rpm until the late stationary phase was achieved.
TABLE 1.
Summary of the evaluated set of MR-1 cytochrome mutants, complements, and selected transport protein mutants
| Functional category | Open reading frame targeted | Gene product deleted (gene) | Figure keya |
|---|---|---|---|
| Energy metabolism: electron transport | SO0479 | Octaheme cytochrome c; involved with the sulfur cycle | 2 |
| SO0610 | Ubiquinol-cytochrome c reductase (petC) | 3 | |
| SO0714 | Periplasmic monoheme cytochrome c4; involved in sulfite oxidation | 4 | |
| SO0716 | Periplasmic monoheme cytochrome c (sorB); involved in sulfite oxidation | 5 | |
| SO0717 | Periplasmic monoheme cytochrome c4; involved in sulfite oxidation | 6 | |
| SO0845 | Diheme cytochrome c (napB); involved in nitrate reduction | 7 | |
| SO0939 | Split-soret diheme cytochrome c | 8 | |
| SO0970 | Fumarate reductase tetraheme cytochrome c (fccA) | 9 | |
| SO1413 | Split tetraheme flavocytochrome c | 10 | |
| SO1421 | Fumarate reductase flavoprotein subunit (ifcA1) | 11 | |
| SO1427 | Periplasmic decaheme cytochrome c, involved in DMSO reduction (dmsC) | 12 | |
| SO1659 | OmcA-like decaheme cytochrome c | 13 | |
| SO1776 | OM protein precursor (mtrB), involved in metal oxide reduction | 14 | |
| SO1777 | Periplasmic decaheme cytochrome c (mtrA), involved in metal oxide reduction | 15 | |
| SO1778/SO1779 | Decaheme cytochrome c complex (omcA mtrC), involved in metal oxide reduction | 16 | |
| SO1780 | OM decaheme cytochrome c (mtrF) | 17 | |
| SO1782 | Periplasmic decaheme cytochrome c (mtrD) | 18 | |
| SO2098 | Quinone-reactive Ni/Fe-hydrogenase, large subunit (hyaB) | ||
| SO2361 | Cytochrome c oxidase, cbb3 type, subunit III (ccoP) | 19 | |
| SO2363 | Cytochrome c oxidase, cbb3 type, subunit II (ccoO) | 20 | |
| SO2727 | Small tetraheme cytochrome c (cctA) | 21 | |
| SO2930 | Cytochrome c with carbohydrate binding domain, involved with the sulfur cycle | 22 | |
| SO2931 | Cytochrome c lipoprotein, involved with the sulfur cycle | 23 | |
| SO3300 | Split tetraheme flavocytochrome c | 24 | |
| SO3420 | Monoheme cytochrome c | 25 | |
| SO3920 | Periplasmic Fe hydrogenase, small subunit (hydA) | ||
| SO3980 | Cytochrome c552 nitrite reductase (nrfA), involved with nitrite reduction | 26 | |
| SO4047 | SoxA-like diheme c, involved with the sulfur cycle | 27 | |
| SO4048 | Diheme c4 | 28 | |
| SO4142 | Monoheme cytochrome c | 29 | |
| SO4144 | Octaheme cytochrome c, involved in tetrathionate reduction | 30 | |
| SO4360 | MtrA-like decaheme cytochrome c | 31 | |
| SO4484 | Monoheme cytochrome c (shp) | 32 | |
| SO4485 | Diheme cytochrome c | 33 | |
| SO4572 | Triheme cytochrome c | 34 | |
| SO4591 | Tetraheme cytochrome c (cymA), involved in anaerobic respiration (except trimethylamine oxide) | 35 | |
| SO4606 | Diheme cytochrome c oxidase, subunit II (cyoA), involved in aerobic respiration | 36 | |
| SO4666 | Cytochrome c (cytcB) | 37 | |
| Protein secretion | SO0166 | General secretion protein (gspG) | 38 |
| SO0169 | General secretion protein (gspD) | 39 | |
| SO0414 | Type 4 prepilin-like proteins leader peptide processing enzyme (pilD) | 40 | |
| Protein transport | SO4204 | Secretion-independent periplasmic translocation protein (tatC) | |
| Quorum sensing | SO1101 | Autoinducer-2 production protein (luxS) | |
| Unknown | SO1673 | OM protein (ompW), putative | |
| Regulatory: DNA interactions | SO1937 | Ferric uptake regulation protein (fur) | |
| SO0624 | Catabolic gene activator (crp) |
See Fig. S1 in the supplemental material.
A different growth strategy was utilized for MR-1 insertional mutants, including the hyaB:pDS3.1, hydA:pDS3.1, luxS:pDS3.1, fur:pKNOCK, crp:Tn5, ompW:pDS3.1, and tatC:pDS3.1 mutants. These mutants (and the WT) were grown anaerobically using a phosphate-buffered basal media containing lactate (30 mM) as an electron donor, fumarate (60 mM) as an electron acceptor, and 1 g/liter yeast extract (pH 7.0) (22). The cells were washed twice in a phosphate buffer (50 mM PO4, 100 mM NaCl, pH 7.0) and injected into the MFC anode compartment.
The final optical density at 600 nm of every culture (for each growth method) was measured and used to calculate an experimental dilution of an optical density at 600 nm of 0.4. The appropriate volume of cells was then injected into each experimental setup such that approximately 2 × 109 cells/ml were present for every evaluation.
(ii) Mutagenesis.
S. oneidensis MR-1 mutants were constructed either by allele replacement using a two-step homologous recombination method as described by Marshall et al. (30) and Gorby et al. (15) or by a cre-lox recombination method described by Marx and Lidstrom (31) (for details, see the supplemental material). Additionally, complemented strains of the mtrA, mtrB, mtrC, and omcA in-frame deletion mutants were constructed using the cloning vector pBBR1MCS-5. The genes mtrA, mtrB, and mtrC were constitutively expressed under control of the lacZ promoter, while the expression of omcA was controlled by its natural promoter.
Mutants deficient in type IV prepilin peptidase (PilD; SO0166) and type II secretion system proteins (GspG and GspD) were generated using minihimarRB-1 transposon mutagenesis as described by Bouhenni et al. (8) and Beliaev and Saffarini (3, 15, 47). The ferric uptake regulation mutant, SO1937:pKNOCK (fur:pKNOCK), was created by integrating the suicide plasmid pKNOCK-Kr into the chromosomal fur locus as described by Thompson et al. (50) and Yost et al. (53). The cyclic AMP receptor protein insertional mutant, SO0624:Tn5 (crp:Tn5), was constructed using a Tn5 insertion in crp as described by Saffarini et al. (47). The suicide plasmid pDS3.1 (30, 51) was used to construct the insertional mutants SO2098:pDS3.1 (hyaB:pDS3.1), SO3920:pDS3.1 (hydA:pDS3.1), SO1101:pDS3.1 (luxS:pDS3.1), SO1673:pDS3.1 (ompW:pDS3.1), and SO4204:pDS3.1 (tatC:pDS3.1).
MFC experiments.
Current production was observed using dual-compartment MFCs of the type shown in Fig. 1. The MFCs were assembled using proton-exchange membranes (Nafion 424; DuPont) and electrodes constructed from graphite felt (GF-S6-06; Electrolytica) bonded to platinum wire (0.3 mm; Alfa-Aesar) (15). The cathode electrodes were electroplated with a platinum catalyst over the entire surface area to drive the oxygen reduction reaction, while the anode electrodes were exposed to MR-1 (the catalyst for lactate oxidation). A sodium-PIPES buffer (100 mM NaCl, 50 mM PIPES, pH 7.0) was used in both anode and cathode compartments as the diluting solution for bacteria. Each compartment had a working volume of 25 ml. Anaerobic conditions were maintained at the anode by continuously flushing the compartment with sterile filtered N2 gas at a rate of 20 ml/min. Aerobic conditions were maintained at the cathode by continuously flushing the compartment with air at a rate of 40 ml/min. Each bacterial strain was evaluated in triplicate, and cell voltage (V) was recorded every 5 minutes across a 10-Ω resistor (R) by a high-impedance digital multimeter (model 2700; Keithley Instruments). Baseline cell voltages were collected under two conditions: (i) buffer only at the anode and (ii) buffer and bacteria at the anode but no additional lactate. Each baseline voltage was monitored for 1 hour prior to changing the anodic condition. Lactate (2 mM) was added to each MFC system after the bacteria were added and a baseline voltage was observed. Two more lactate additions occurred thereafter, depending on when the cell voltage dropped to baseline levels (approximately every 24 h). MFCs were operated in batch after the addition of bacteria (no solution exchanges) over a period of 3 days. A lactate concentration of 2 mM was chosen based on previous experiments demonstrating that a higher concentration of lactate (i.e., 20 mM) yields higher charge, but the maximum current density remained the same.
FIG. 1.

Dual-compartment fuel cell diagram. Bacteria are inoculated into the anode compartment, attach to the graphite felt electrode, and begin transferring electrons. Electrons are conducted through the anode electrode and across the external circuit to the cathode electrode. The cathode electrode is graphite felt that has been electroplated with platinum, the catalyst driving the reduction of oxygen to water. The anode and cathode compartments are physically separated by a proton-conductive membrane that facilitates the transfer of protons from the anode to the cathode, completing the cell reaction. The cell voltage (V) is measured across a 10-Ω resistance (R), and current is calculated as I = V/R.
Current (I) was calculated according to I = V/R, and maximum current densities were calculated using the maximum current values that remained constant for approximately 4 hours (corresponding to each lactate feeding) divided by the total apparent surface area of the electrode (20 cm2).
Metal oxide reduction experiments. (i) Manganese(IV) oxide.
Manganese(IV) oxide reduction was investigated using autoclaved, anaerobic δ-manganese oxide (δMnO2) that was prepared as described by Burdige and Nealson (9). Duplicate manganese oxide reduction experiments were conducted in anaerobic serum bottles using 20 mM lactate, 300 μM δMnO2, sodium-PIPES buffer (described above), and an appropriate volume of bacterial cell suspension. Experimental controls were prepared with (i) cells and δMnO2 but without lactate and (ii) lactate and δMnO2 but without cells. A colorimetric assay with Leucoberbelin blue (LBB) (25) was used to quantify the amount of Mn(IV) present in each bottle immediately after inoculation and after 48 hours of exposure to bacteria. Briefly, a 0.1-ml volume of subsample was added to 0.9 ml of LBB solution and then incubated for 15 min in the dark. Color changes in the LBB solution were measured at a fixed wavelength of 690 nm. Mn(IV) concentrations were determined based on a standard curve generated from known concentrations of KMnO4. The percentage of Mn(IV) depletion was calculated based on measured Mn(IV) concentrations after 24 and 48 hours of exposure.
(ii) Solid-phase iron(III) oxide.
Solid ferric oxide reduction was investigated using autoclaved anaerobic hydrous ferric oxide (HFO), prepared according to the protocol by Cornell and Schwertmann (12). This form of HFO is not stabilized by silica and becomes more crystalline with time; therefore, all strains were evaluated within a period of 3 days to ensure comparable results between strains. X-ray diffraction spectra showed that the solid-phase iron was a combination of goethite, hematite, and nanoparticles of HFO, which we refer to as the HFO mixture (HFOM). Triplicate HFOM reduction experiments were performed in sterile, anaerobic serum bottles. Each bottle contained 20 mM of HFOM as the sole electron acceptor, 20 mM of lactate as the sole electron donor, and the sodium-PIPES buffer (described above). Experimental controls were prepared with (i) cells and HFOM but without lactate and (ii) lactate and HFOM but without cells. The production of Fe(II) was monitored using a colorimetric ferrozine assay (49). Briefly, a subsample from each bottle was acidified and added to the ferrozine solution. Color changes in the ferrozine solution were measured at a fixed wavelength of 562 nm. Fe(II) concentrations were determined based on a standard curve generated from known concentrations of FeCl2.
Scanning electron microscopy (SEM) sample preparation.
Selected MFC electrodes were removed at the end of the experiment, and subsamples were fixed and ethanol dehydrated according to the protocol outlined by Gorby et al. (15).
RESULTS
MFC experiments.
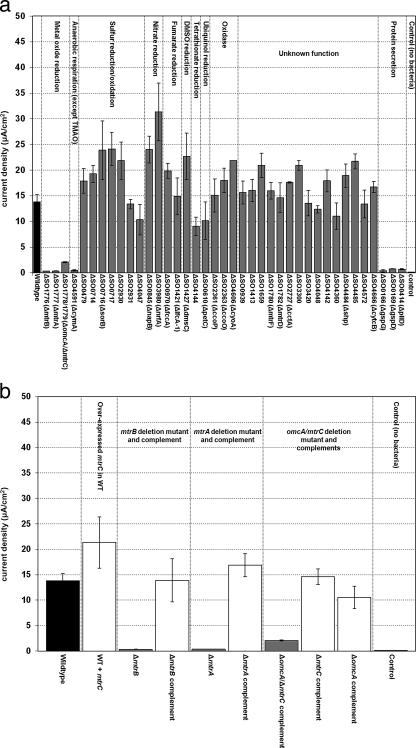
The average current densities obtained for S. oneidensis MR-1 WT and mutants are shown in Fig. 2a and b. It is apparent from Fig. 2a that only a few of the deletion mutants were significantly diminished in their abilities to produce current in an MFC system relative to the WT. Specifically, the mutants lacking mtrA, mtrB, omcA mtrC, and cymA generated less than 20% of the current produced by the WT. For example, the ΔmtrA mutant produced 0.35 ± 0.05 μA/cm2, compared to 13.8 ± 1.37 μA/cm2 (382 ± 76.7 μW/m2 or 3.03 × 104 ± 6.08 × 103 μW/m3 given the entire volume of the nonconductive chamber) produced by the WT. However, the complemented strains of the mtrA, mtrB, and omcA mtrC deletion mutants had full recovery or enhanced current-producing abilities; i.e., the complemented mtrA and mtrB mutants produced 13.9 ± 4.23 μA/cm2 and 16.9 ± 2.27 μA/cm2, respectively. Additionally, a 35% increase in current production (relative to the WT) was observed when the mtrC cytochrome was overexpressed in the WT strain (21.3 ± 5.02 μA/cm2) (Fig. 2b).
FIG. 2.
Current density values for MR-1 WT and cytochrome deletion and protein secretion mutants (a) and MR-1 WT and selected cytochrome mutants with their complementations (b). Averages and standard deviations were obtained using the peak current density values corresponding to each lactate injection for triplicate experiments. Maximum current density was determined to be the highest level of current density that remained constant for at least 3 hours. Averages and standard deviations of the maximum current density were calculated using these data values (between 100 and 200 data points were utilized).
Several cytochrome deletion mutants also showed higher current values than the WT strain (at least 20% higher) (Fig. 2a). These are the ΔSO0714, ΔSO0716 (ΔsorB), and ΔSO0717 (monoheme cytochromes predicted to be involved in sulfite oxidation); ΔSO0845 (diheme c-type cytochrome predicted to be involved in nitrate reduction, ΔnapB); ΔSO1427 (periplasmic decaheme c-type cytochrome predicted to be involved in dimethyl sulfoxide (DMSO) reduction, ΔdmsC); ΔSO2930 (c-type cytochrome with carbohydrate binding domain predicted to be involved with the sulfur cycle); and ΔSO3980 (cytochrome reductase predicted to be involved with nitrite reduction, ΔnrfA) mutants.
The cytochrome mutants were not the only group deficient in current production relative to the WT strain (Fig. 2a). The mutants deficient in type IV prepilin peptidase or type II secretion (ΔpilD, ΔgspD, and ΔgspG mutants) and three other mutants (fur:pKNOCK, crp:Tn5, and tatC:pDS3.1 mutants) were all severely limited in current-producing abilities, showing less than 20% of WT values (data not shown).
A selected number of MFC electrodes were examined by SEM to evaluate the distribution of cells on the electrode surfaces after current data were collected. It is apparent from these images (Fig. 3a to c) that the electrode exposed to the WT strain featured much greater surface coverage (with intact cells and what appears to be a developing biofilm) than that exposed to the ΔpilD or ΔomcA ΔmtrC mutant.
FIG. 3.
SEM images of graphite anode fibers used during the MFC evaluations of the MR-1 ΔpilD mutant (a), ΔomcA ΔmtrC mutant (b), and WT (c).
Metal oxide reduction experiments. (i) Manganese(IV) oxide.
The average percentages of Mn(IV) oxide reduced by the MR-1 WT, cytochrome and protein secretion mutants, and cytochrome-complemented strains were investigated. As with current production, the ΔmtrA, ΔmtrB, and ΔcymA mutants were limited in their ability to reduce Mn(IV) oxide relative to the WT strain (Fig. 4). For example, results after 24 hours show no Mn(IV) oxide reduction by the ΔmtrA or ΔmtrB mutant (Fig. 4). However, after 48 hours (data not shown), the ΔmtrB mutant reduced 17.3% ± 2.39% of the starting concentration of Mn(IV) oxide, while the WT reduced 36.7% ± 0.39%. The ΔgspD, ΔgspG, and ΔpilD mutants were also affected in their ability to reduce Mn(IV) oxide, although not as significantly as the ΔmtrA and ΔmtrB mutants. Unlike the results obtained for current production, the ΔomcA ΔmtrC complex mutant was able to reduce Mn(IV) oxides after 24 hours (29.9% ± 0.66%), and then after 48 hours the reduction exceeded that by the WT (43.8% ± 0.30%). Several strains also exhibited higher Mn(IV) oxide reduction than the WT, including all of the cytochrome mutants involved with sulfur redox reactions (ΔSO0479, ΔSO0714, ΔsorB, ΔSO0717, ΔSO2930, ΔSO2931, and ΔSO4047 mutants), nitrate reduction (ΔnapB and ΔnrfA), DMSO reduction (ΔdmsC mutant), and tetrathionate reduction (ΔSO4144 mutant) and many mutants that have unknown functions in MR-1's energy metabolism (ΔcctA, ΔSO3420, ΔSO4048, ΔSO4360, Δshp, ΔSO4485, and ΔcytcB mutants). The ΔmtrA and ΔmtrB complemented mutants were able to reduce Mn(IV) oxides as well as the WT. However, the ΔomcA ΔmtrC mutant showed an ability to reduce Mn(IV) oxide equal to that of the WT regardless of complementation (data not shown). The deletion of the omcA and mtrC genes did not appear to have an effect on Mn(IV) oxide reduction in MR-1.
FIG. 4.
Average percentage of Mn(IV) oxide reduced after 24 hours of exposure to MR-1 WT and cytochrome deletion and protein secretion mutants. Error bars indicate standard deviations.
(ii) Solid-phase Fe(III) oxide.
The average concentrations of Fe(II) resulting from the reduction of HFOM by the MR-1 WT and mutants are shown in Fig. 5. These data show that the ΔmtrA, ΔmtrB, ΔomcA ΔmtrC, ΔcymA, ΔSO4144, ΔSO4572 (a triheme c-type cytochrome), ΔgspG, ΔgspD, and ΔpilD mutants were limited in HFOM reduction relative to the WT. For example, the ΔomcA ΔmtrC mutant produced 68.4 ± 5.08 μM and the WT produced 170 ± 32.7 μM of Fe(II) after 24 hours. Data collected after 48 hours showed a slight increase in Fe(II) production from the ΔomcA ΔmtrC mutant (98.7 ± 22.7 μM); however, this amount was much less than that from the WT (245 ± 12.6 μM).
FIG. 5.
Average Fe(II) concentration resulting from solid Fe oxide (HFOM) reduction after 24 hours of exposure to MR-1 WT and cytochrome deletion and protein secretion mutants. Averages and standard deviations were calculated based on the measured Fe(II) concentrations from triplicate experiments.
Other mutants, such as the ΔnapB (345 ± 68.0 μM), ΔifcA1 (380 ± 42.8 μM), ΔmtrF (368 ± 0.00 μM), ΔmtrD (362 ± 22.7 μM), and ΔSO3420 (492 ± 25.2 μM) mutants, had even greater capacity to reduce HFOM than the WT. For example, the concentration of Fe(II) produced by the ΔifcA1 and ΔmtrF mutants was two to three times higher than that produced by the WT after 24 hours. The ΔnapB mutant was also enhanced in its ability to produce current in an MFC but had decreased Mn(IV)-reducing ability. The ΔifcA1 mutant is deficient in the ability to produce a tetraheme c-type flavocytochrome, the ΔmtrF mutant lacks the ability to encode an OM decaheme c-type cytochrome, the ΔmtrD lacks the ability to encode a periplasmic decaheme c-type cytochrome, and the ΔSO3420 mutant is deficient in the ability to produce a monoheme cytochrome c′.
The ΔmtrA, ΔmtrB, and ΔomcA ΔmtrC complemented strains had a full recovery of HFOM-reducing ability relative to the WT strain (data not shown).
DISCUSSION
The results from our investigations do not substantiate a working hypothesis that MR-1 utilizes the same set of gene products to transfer electrons to different solid substrates. From these data it is evident that the electron transport system in MR-1 is complex, with much functional redundancy. There are some gene products that participate in electron transfer to several solid substrates, including MFC electrodes, Mn(IV) oxides, and solid Fe(III) oxides; however, the overall picture of how specific gene products participate in electron transfer to these substrates is not yet clear. For example, the ΔmtrA, ΔmtrB, ΔomcA ΔmtrC, ΔcymA, ΔgspG, and ΔpilD mutants were all limited in their abilities to produce current and reduce HFOM relative to the WT strain. However, the ΔomcA ΔmtrC mutant was not limited in Mn(IV) oxide reduction, implying that the ΔomcA ΔmtrC genes are perhaps not directly involved with solid-phase Mn(IV) oxide reduction. The trends in the solid substrate data (Fig. 2a, 4, and 5) suggesting that the ΔomcA ΔmtrC mutant is not as limited in the ability to transfer electrons to solids as the ΔmtrA and ΔmtrB mutants. This is especially apparent in the Mn(IV) oxide reduction data, which show that after 48 hours, the ΔomcA ΔmtrC mutant has reduced 43.8% ± 0.30% of the starting concentration of Mn(IV), compared to 1.65% ± 2.20% for the ΔmtrA mutant, 17.3% ± 2.93% for the ΔmtrB mutant, and 39.1% ± 0.57% for the WT strain.
Additionally, the ΔomcA ΔmtrC mutant shows higher concentrations of Fe(II) (approximately two times higher) from the reduction of HFOM after 24 (Fig. 5) and 48 (data not shown) hours, relative to the ΔmtrA and ΔmtrB mutants. Additionally, higher values of current production (approximately five times higher) were observed for the ΔomcA ΔmtrC mutant relative to the ΔmtrA and ΔmtrB mutants (Fig. 2a) during the experimental duration. Higher Mn(IV) and Fe(III) reduction rates of an mtrC mutant, relative to mutants lacking only mtrA or mtrB, have also been reported by Beliaev et al. (4).
The data presented here suggest that the omcA and mtrC gene products are not the only OM cytochromes serving as terminal reductases for Mn(IV) or Fe(III). Furthermore, the solid metal oxide reduction data taken together with the current production data (which demonstrated limitation patterns similar to those for the mtr and omc mutants) indicate that MR-1 features more than one pathway for electron transfer to solids.
The ΔcymA and ΔgspD mutants show the same patterns as the ΔmtrB and ΔmtrA; i.e., they are limited in current production, HFOM reduction, and Mn(IV) oxide reduction. These results are in agreement with those of Myers and Myers (34), who suggested that the cymA cytochrome is a necessary component in the electron transport chain that is common to fumarate, nitrate, Fe(III), and Mn(IV) oxides.
Interestingly, the two mutants deficient in type II protein secretion, the ΔgspD and ΔgspG mutant, yielded different results from each other. Relative to the ΔgspD mutant, the ΔgspG mutant showed a limited ability to reduce HFOM and Mn(IV) oxide and to produce current in an MFC.
Another surprising result revealed by this data set was the number of cytochrome deletion mutants that were able to exceed the WT levels of current production and HFOM and Mn(IV) oxide reduction. There are many possibilities for why this might occur, including the redirection of electron flow to an alternate terminal reductase(s) in the occurrence of a “roadblock” to a desired pathway or reductase. It may also be the case that the cytochromes themselves are negative regulators of the transcription and/or translation of factors involved with these processes. Furthermore, an alternative possibility is that these mutants are enhanced in their abilities to form biofilms and therefore yield higher current production and metal oxide reduction rates due to the presence of more bacteria at the surface.
The production of current in an MFC is directly linked to the ability of the bacteria to oxidize a substrate and transfer electrons resulting from this oxidation to the anode electrode. The current production results for each evaluated mutant showed that only 5 cytochrome deletion mutants, out of the 36 tested, were severely limited in current-producing ability relative to the WT. These five mutants produced less than one-quarter of the current density demonstrated by the WT and include the ΔmtrA, ΔmtrB, complex ΔomcA ΔmtrC, and ΔcymA mutants (Fig. 2a). Both type II protein secretion mutants and the pilD mutant were also severely limited in current production, as previously discussed. Figure 3 shows three different MFC electrodes that were exposed to (i) the ΔpilD mutant, (ii) the ΔomcA ΔmtrC mutant, and (iii) the WT for 3 days with periodic lactate additions. SEM images of the electrodes after MFC evaluation showed the ΔpilD cells looking stressed, and apparently limited in their ability to successfully colonize the electrode (Fig. 3a) relative to the MR-1 WT (Fig. 3c). The ΔomcA ΔmtrC mutant was able to form a monolayer of cells on the electrode surface, and the cells appeared to be in good condition. These images suggest that two mechanisms may play a role in current production: (i) the ability to produce certain OM proteins for electron transport and (ii) the ability to colonize the electrode surface. Presently, work is being done to determine which mechanism contributes most significantly to these results.
The decrease in current production and Fe(III) oxide reduction by the deletion mutants could be attributed to a loss of cell viability; i.e., the mutants are dead and therefore cannot transfer electrons during either process. However, additional experiments (data not presented) showed that the mtrA mutant [which is unable to reduce Fe(III) oxide and produce current] showed no loss in viability as judged by CFU during the 24-h time course experiment. The results from this experiment also correlate with the surface attachment ability of MR-1 and its mutants. For example, the ΔmtrA mutant [and the other deletion mutants showing limited abilities to reduce Fe(III) oxide and produce current] may have been unable to facilitate electron transfer because of an inability to produce OM proteins that interact with solid substrates. These results further indicate that electron transfer to solid substrates by MR-1 is directly related to physical interactions between the substrate surface and cells (or conductive cell appendages) (15).
The regulatory fur:pKNOCK and crp:Tn5 mutants were limited to below 20% of the current density values obtained for the MR-1 WT. These genes were previously implicated as influencing the regulation of metal oxide reduction and are here implicated as contributors to current production. The tatC:pDS3.1 mutant was also limited in current-producing abilities.
Notably, a putative OM protein, ΔSO1673 (ompW:pDS3.1), was also moderately affected in current density, showing only about 60% of the WT current density values.
As a whole, this is the first set of current densities obtained from MFCs that can be directly related via specific mutants to microbial physiology. Additionally, this is the first data set associating specific MR-1 gene products with current production in an MFC. Data from these MR-1 mutant evaluations also indicate that the gene products associated with current production are not necessarily identical with those involved in Mn(IV) and Fe(III) oxide reduction. Instead, these data suggest that several different pathways, and perhaps mechanisms, may be employed for current production and metal oxide reduction. Ultimately, the different patterns of metal oxide reduction and current production indicate a very complicated picture of electron flow via MR-1 cytochromes.
Supplementary Material
Acknowledgments
We thank Surya Prakash and Federico A. Viva for guidance and assistance in electroplating the cathode electrodes, Prithiviraj Chellamuthu for his assistance in collecting Mn(IV) reduction data, Alicia Thompson (USC Center for Electron Microscopy) and Bruce Arey (Pacific Northwest National Laboratory) for producing SEM imagery, and Everett Salas for preparing solid iron and manganese oxides and collecting XRD spectra.
This work was supported by the DOE Shewanella Federation program, award no. 58486720, and the AFOSR MURI program, award no. FA9550-06-1-0292.
We declare no competing financial interests.
Footnotes
Published ahead of print on 20 July 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Arnold, R. G., M. R. Hoffmann, T. J. Dichristina, and F. W. Picardal. 1990. Regulation of dissimilatory Fe(III) reduction activity in Shewanella putrefaciens. Appl. Environ. Microbiol. 56:2811-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beliaev, A. S., D. M. Klingeman, J. A. Klappenbach, L. Wu, M. F. Romine, J. M. Tiedje, K. H. Nealson, J. K. Fredrickson, and J. Zhou. 2005. Global transcriptome analysis of Shewanella oneidensis MR-1 exposed to different terminal electron acceptors. J. Bacteriol. 187:7138-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beliaev, A. S., and D. A. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beliaev, A. S., D. A. Saffarini, J. L. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. [DOI] [PubMed] [Google Scholar]
- 5.Bencheikh-Latmani, R., S. M. Williams, L. Haucke, C. S. Criddle, L. Wu, J. Zhou, and B. M. Tebo. 2005. Global transcriptional profiling of Shewanella oneidensis MR-1 during Cr(VI) and U(VI) reduction. Appl. Environ. Microbiol. 71:7453-7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 7.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouhenni, R., A. Gehrke, and D. Saffarini. 2005. Identification of genes involved in cytochrome c biogenesis in Shewanella oneidensis, using a modified mariner transposon. Appl. Environ. Microbiol. 71:4935-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdige, D. J., and K. H. Nealson. 1985. Microbial manganese reduction by enrichment cultures from coastal marine sediments. Appl. Environ. Microbiol. 50:491-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, I. S., H. Moon, O. Bretschger, J. K. Jang, H. I. Park, K. H. Nealson, and B. H. Kim. 2006. Electrochemically active bacteria (EAB) and mediator-less microbial fuel cells. J. Microbiol. Biotechnol. 16:163-177. [Google Scholar]
- 11.Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229. [DOI] [PubMed] [Google Scholar]
- 12.Cornell, R. M., and U. Schwertmann. 1996. Ferrihydrite, p. 491. In B. Bock (ed.), VCH, Weinheim, Germany. The iron oxides: structure, properties, reactions, occurence and uses.
- 13.DiChristina, T. J., C. M. Moore, and C. A. Haller. 2002. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene J. Bacteriol. 184:142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredrickson, J. K., A. S. Beliaev, W. Cannon, Y. Gorby, M. Lipton, P. Liu, M. F. Romine, R. Smith, and H. Trease. 2004. Global and physiological responses to substrate shifts in continuous and controlled batch cultures of Shewanella oneidensis MR-1. GTL: Contractor-Grantee Workshop II, abstr. 20. U. S. Department of Energy, Washington, DC.
- 15.Gorby, Y. A., S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. I. Ishii, B. Logan, K. H. Nealson, and J. K. Fredrickson. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 103:11358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson, and C. M. Fraser. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotech. 20:1118. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez, M. E., and D. K. Newman. 2001. Extracellular electron transfer. Cell. Mol. Life Sci. 58:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes, D. E., D. R. Bond, and D. R. Lovley. 2004. Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl. Environ. Microbiol. 70:1234-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes, D. E., S. K. Chaudhuri, K. P. Nevin, T. Mehta, B. A. Methe, A. Liu, J. E. Ward, T. L. Woodard, J. Webster, and D. R. Lovley. 2006. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ. Microbiol. 8:1805-1815. [DOI] [PubMed] [Google Scholar]
- 20.Kieft, T. L., J. K. Fredrickson, T. C. Onstott, Y. A. Gorby, H. M. Kostandarithes, T. J. Bailey, D. W. Kennedy, S. W. Li, A. E. Plymale, C. M. Spadoni, and M. S. Gray. 1999. Dissimilatory reduction of Fe(III) and other electron acceptors by a Thermus isolate. Appl. Environ. Microbiol. 65:1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, B. H., H. J. Kim, M. S. Hyun, and D. H. Park. 1999. Direct electrode reaction of Fe(III)-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9:127-131. [Google Scholar]
- 22.Kim, G. T., M. S. Hyun, I. S. Chang, H. J. Kim, H. S. Park, B. H. Kim, S. D. Kim, J. W. T. Wimpenny, and A. J. Weightman. 2005. Dissimilatory Fe(III) reduction by an electrochemically active lactic acid bacterium phylogenetically related to Enterococcus gallinarum isolated from submerged soil. J. Appl. Microbiol. 99:978-987. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim, and B. H. Kim. 2002. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 30:145. [Google Scholar]
- 24.Kolker, E., A. F. Picone, M. Y. Galperin, M. F. Romine, R. Higdon, K. S. Makarova, N. Kolker, G. A. Anderson, X. Qiu, K. J. Auberry, G. Babnigg, A. S. Beliaev, P. Edlefsen, D. A. Elias, Y. A. Gorby, T. Holzman, J. A. Klappenbach, K. T. Konstantinidis, M. L. Land, M. S. Lipton, L.-A. McCue, M. Monroe, L. Pasa-Tolic, G. Pinchuk, S. Purvine, M. H. Serres, S. Tsapin, B. A. Zakrajsek, W. Zhu, J. Zhou, F. W. Larimer, C. E. Lawrence, M. Riley, F. R. Collart, J. R. Yates, III, R. D. Smith, C. S. Giometti, K. H. Nealson, J. K. Fredrickson, and J. M. Tiedje. 2005. Global profiling of Shewanella oneidensis MR-1: expression of hypothetical genes and improved functional annotations. Proc. Natl. Acad. Sci. USA 102:2099-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krumbein, W. E., and H. J. Altmann. 1973. A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgolander Wiss. Meeresunters 25:347-356. [Google Scholar]
- 26.Lies, D. P., M. E. Hernandez, A. Kappler, R. E. Mielke, J. A. Gralnick, and D. K. Newman. 2005. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl. Environ. Microbiol. 71:4414-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logan, B. E., B. Hamelers, R. Rozendal, U. Schroder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete, and K. Rabaey. 2006. Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 40:5181-5192. [DOI] [PubMed] [Google Scholar]
- 28.Lovley, D. R., E. J. P. Phillips, and D. J. Lonergan. 1989. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl. Environ. Microbiol. 55:700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luu, Y.-S., and J. A. Ramsay. 2003. Microbial mechanisms of accessing insoluble Fe(III) as an energy source. World J. Microbiol. Biotechnol. 19:215-225. [Google Scholar]
- 30.Marshall, M. J., A. S. Beliaev, A. C. Dohnalkova, D. W. Kennedy, L. Shi, Z. Wang, M. I. Boyanov, B. Lai, K. M. Kemner, J. S. McLean, S. B. Reed, D. E. Culley, V. L. Bailey, C. J. Simonson, D. A. Saffarini, M. F. Romine, J. M. Zachara, and J. K. Fredrickson. 2006. c-type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol. 4:1324-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marx, C. J., and M. E. Lidstrom. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. BioTechniques 33:1062-1067. [DOI] [PubMed] [Google Scholar]
- 32.Meyer, T. E., A. S. Tsapin, I. Vandenberghe, L. De Smet, D. Frishman, K. H. Nealson, M. A. Cusanovich, and J. J. Van Beeumen. 2004. Identification of 42 possible cytochrome c genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes. J. Integrat. Biol. 8:57-77. [DOI] [PubMed] [Google Scholar]
- 33.Myers, C. R., and J. M. Myers. 2003. Cell surface exposure of the outer membrane cytochromes of Shewanella oneidensis MR-1. Lett. Appl. Microbiol. 37:254-258. [DOI] [PubMed] [Google Scholar]
- 34.Myers, C. R., and J. M. Myers. 1997. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J. Bacteriol. 179:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers, C. R., and J. M. Myers. 2002. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl. Environ. Microbiol. 68:5585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers, C. R., and J. M. Myers. 2004. The outer membrane cytochromes of Shewanella oneidensis MR-1 are lipoproteins. Lett. Appl. Microbiol. 39:466-470. [DOI] [PubMed] [Google Scholar]
- 37.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 38.Myers, C. R., and K. H. Nealson. 1990. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J. Bacteriol. 172:6232-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers, J. M., and C. R. Myers. 2003. Overlapping role of the outer membrane cytochromes of Shewanella oneidensis MR-1 in the reduction of manganese(IV) oxide. Lett. Appl. Microbiol. 37:21-25. [DOI] [PubMed] [Google Scholar]
- 40.Myers, J. M., and C. R. Myers. 2001. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl. Environ. Microbiol. 67:260-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers, J. M., and C. R. Myers. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J. Bacteriol. 182:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nealson, K. H., and D. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48:311-343. [DOI] [PubMed] [Google Scholar]
- 43.Park, H. S., B. H. Kim, H. S. Kim, H. J. Kim, G. T. Kim, M. Kim, I. S. Chang, Y. K. Park, and H. I. Chang. 2001. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7:297. [Google Scholar]
- 44.Pham, C. A., S. J. Jung, N. T. Phung, J. Lee, I. S. Chang, B. H. Kim, H. Yi, and J. Chun. 2003. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 223:129. [DOI] [PubMed] [Google Scholar]
- 45.Pitts, K. E., P. S. Dobbin, F. Reyes-Ramirez, A. J. Thomson, D. J. Richardson, and H. E. Seward. 2003. Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA: expression in Escherichia coli confers the ability to reduce soluble Fe(III) chelates. J. Biol. Chem. 278:27758-27765. [DOI] [PubMed] [Google Scholar]
- 46.Ruebush, S. S., S. L. Brantley, and M. Tien. 2006. Reduction of soluble and insoluble iron forms by membrane fractions of Shewanella oneidensis grown under aerobic and anaerobic conditions. Appl. Environ. Microbiol. 72:2925-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saffarini, D. A., R. Schultz, and A. Beliaev. 2003. Involvement of cyclic AMP (cAMP) and cAMP receptor protein in anaerobic respiration of Shewanella oneidensis. J. Bacteriol. 185:3668-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwalb, C., S. K. Chapman, and G. A. Reid. 2002. The membrane-bound tetrahaem c-type cytochrome CymA interacts directly with the soluble fumarate reductase in Shewanella. Biochem. Soc. Trans. 30:658-662. [DOI] [PubMed] [Google Scholar]
- 49.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 50.Thompson, D. K., A. S. Beliaev, C. S. Giometti, S. L. Tollaksen, T. Khare, D. P. Lies, K. H. Nealson, H. Lim, J. Yates III, C. C. Brandt, J. M. Tiedje, and J. Zhou. 2002. Transcriptional and proteomic analysis of a ferric uptake regulator (Fur) mutant of Shewanella oneidensis: possible involvement of Fur in energy metabolism, transcriptional regulation, and oxidative stress. Appl. Environ. Microbiol. 68:881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan, X.-F., N. C. VerBerkmoes, L. A. McCue, D. Stanek, H. Connelly, L. J. Hauser, L. Wu, X. Liu, T. Yan, A. Leaphart, R. L. Hettich, J. Zhou, and D. K. Thompson. 2004. Transcriptomic and proteomic characterization of the Fur modulon in the metal-reducing bacterium Shewanella oneidensis. J. Bacteriol. 186:8385-8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber, K. A., L. A. Achenbach, and J. D. Coates. 2006. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4:752-764. [DOI] [PubMed] [Google Scholar]
- 53.Yost, C., L. Hauser, F. Larimer, D. Thompson, A. Beliaev, J. Zhou, Y. Xu, and D. Xu. 2003. A computational study of Shewanella oneidensis MR-1: structural prediction and functional inference of hypothetical proteins. J. Integrat. Biol. 7:177-191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.