Abstract
A PCR-based assay (Mrnif) targeting the nifH gene of Methanobrevibacter ruminantium was developed to detect fecal pollution from domesticated ruminants in environmental water samples. The assay produced the expected amplification product only when the reaction mixture contained DNA extracted from M. ruminantium culture, bovine (80%), sheep (100%), and goat (75%) feces, and water samples from a bovine waste lagoon (100%) and a creek contaminated with bovine lagoon waste (100%). The assay appears to be specific and sensitive and can distinguish between domesticated- and nondomesticated-ruminant fecal pollution in environmental samples.
Agricultural waste is the dominant source of fecal pollution in lakes and rivers and contributes a third of the fecal pollution in estuaries (20). Much of the waste originates from domesticated-ruminant industries, such as the cattle (grazing animals and confined-animal feeding operations) and sheep industries, which are estimated to produce 40 and 86 kg of waste per 1,000 kg of animal weight per day, respectively (7). Fecal pollution from agricultural industries carried by storm runoff during heavy rain events can adversely affect the health of watersheds due to eutrophication, sediment loading, and introduction of microorganisms pathogenic to humans (7, 22). The task of assigning the source of fecal pollution entering a water body as agriculturally derived is complicated by a lack of methods distinguishing between fecal pollution from domesticated ruminants and nondomesticated ruminants (e.g., elk and deer).
Several methods have focused on the identification of ruminant versus human contamination (2, 8, 12), but there is currently no established method for differentiating between domesticated- and nondomesticated-ruminant feces for microbial source tracking, even though sheep farms and cattle operations constitute significant sources of fecal contamination in surface waters (7). Intestinal methanogens are host specific and have potential as animal-specific markers of fecal pollution (17, 18). Methanogens constitute 0.5 to 3% of the total bacterial population in ruminants (10) and are usually present in high numbers (106 to 108 methanogens/g of wet feces). Methanobrevibacter ruminantium, a dominant ruminant methanogen, exhibits properties that suggest potential as a useful indicator of fecal pollution. It is known to inhabit the rumen only (9), is present in concentrations as high as 106 to 108·ml−1 rumen fluid (14), and has strict nutrient requirements (1) that prevent environmental aftergrowth. The goal of this study was to develop a PCR-based assay to detect the nifH gene of Methanobrevibacter ruminantium in surface waters and to determine the specificity of the assay for fecal pollution from domesticated ruminants.
Primers were designed for the nifH gene sequence of Methanobrevibacter ruminantium (GenBank accession no. AB019137) using the DNAStar PrimerSelect program (v. 5.0). The Mrnif primers (Mrnif-f, 5′-AATATTGCAGCAGCTTACAGTGAA-3′; Mrnif-r, 5′-TGAAAATCCTCCGCAGACC-3′) produced an amplicon of 336 bp (Fig. 1), and BLAST searches of the primer sequences showed homology to sequences within the Methanobrevibacter ruminantium nifH gene only. No significant similarity was seen with other microbial sequences in searchable databases.
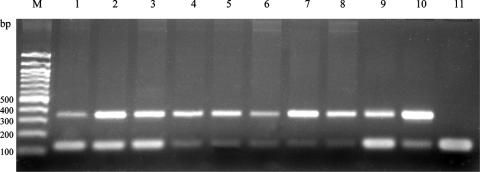
FIG. 1.
Mrnif amplification of DNA from the feces of sheep (samples S10, S11, S12, S13, and S17) with different concentrations of the IAC. The expected product for Mrnif is 336 bp; that for the IAC is 120 bp. The positive control is M. ruminantium plus 10−8 μM with 10−9 μM IAC. Lanes: M, 100-bp ladder; 1, S12 plus 10−9 μM IAC; 2, S13 plus 10−9 μM IAC; 3, S17 plus 10−9 μM IAC; 4, S10 plus 10−10 μM IAC; 5, S11 plus 10−10 μM IAC; 6, S12 plus 10−10 μM IAC; 7, S13 plus 10−10 IAC; 8, S17 plus 10−10 μM IAC; 9, M. ruminantium plus 10−9 μM IAC; 10, M. ruminantium plus 10−10 μM IAC; 11, negative control (IAC with no template DNA added).
The Mrnif primer pair was tested against a variety of bacterial, fecal, and environmental samples to determine specificity for domesticated ruminants. Additionally, a pair of universal bacterial primers (PRBA338f, 5′-ACTCCTACGGGAGGCAGCAG-3′; PRUN518r, 5′-ATTACCGCGGCTGCTGG-3′) targeting the V3 region of the 16S rRNA gene was used to verify the presence of amplifiable bacterial DNA in each sample (11). All samples tested using the PRBA338f and PRUN518r primers amplified the expected product, suggesting that all samples contained bacterial DNA.
Because DNA extracted from environmental samples may contain PCR inhibitors (6), an internal amplification control (IAC) was included in each reaction as a control to prevent false negative results. The IAC, with the sequence 5′-TACAGTAGCTAATATTGCAGCAGCTTACAGTGAAGACAATAAGAAAGTCATGGTTATTGGCTGCCTTGAAGAGGAATTTGATGTAATCTTATATGATGTTCTTGGAGATGTGGTCTGCGGAGGATTTTCAGTTCCTCTAA-3′, was designed as reported previously (18) using the M. ruminantium nifH gene to yield a 120-bp PCR product. Amplification of the IAC used the same forward and reverse primers as the Mrnif assay. To identify the appropriate concentration of the IAC for the Mrnif assay, serial dilutions of the IAC (100 μM to 10−12 μM) were tested with DNA extracted from the feces of cows, sheep, and goats in various concentrations, from 50 ng to 0.01 ng in 20-μl Mrnif PCR assays (described below). The concentration of IAC chosen for the Mrnif assays was 10−9 μM.
PCR was carried out in 20-μl amplification reaction mixtures containing 1× PCR buffer, 0.1% bovine serum albumin, 200 μM deoxynucleoside triphosphate, 1 U Taq polymerase, 0.5 μM of each primer, 10−9 μM IAC, and various concentrations of the DNA template. The cycling conditions for the Mrnif assay include an initial denaturation for 2 min at 92°C and 30 cycles of denaturation for 30 s at 92°C, annealing for 15 s at 62°C, and elongation for 30 s at 72°C. A final elongation was performed for 6 min at 72°C. The positive controls for the PCR were FTA card extracts (17) of Methanobrevibacter ruminantium cultures (OCM 146); the negative controls included master mix with IAC but no other DNA template.
The specificity of the Mrnif primer pair was assayed using DNA from a wide variety of bacteria and methanogens. These included 15 species of Methanobrevibacter, 12 additional methanogen genera, and 19 known bacterial cultures as described previously (17, 18). Whole-cell PCR was conducted on 477 unknown bacterial isolates cultured from brain heart infusion plus 0.02% NaN2 and eosin-methylene blue agar as described previously (18). Amplification using the Mrnif primer pair yielded a product of the expected size only with DNA extracted from M. ruminantium culture; no amplification was observed with bacteria or other methanogens.
To verify the identities of the amplification products, those amplified from DNA extracted from cow, sheep, and goat fecal samples (three each) using the Mrnif primers were cloned into the pGEM-T vector using pGEM-T vector system II (Promega, Madison, WI) as previously described (18). A total of 26 clones were sequenced, including at least 2 clones from each fecal sample. Plasmids were purified using a Zyppy plasmid mini prep II kit (Zymo Research) and sequenced commercially by Macrogen USA using the T7 promoter primer. Each of the sequences was translated using the ExPASy translate tool (4) and compared to that of the translated M. ruminantium nifH gene (GenBank accession no. AB019137) using the bl2seq local alignment tool with the BLOSUM80 matrix (16). All 26 sequences were more than 90% identical to the published sequence, suggesting that the product amplified using the Mrnif assay is the nifH gene of M. ruminantium.
To determine the cell detection limit for the Mrnif assay, a culture of M. ruminantium (OCM 146) was pelleted at 12,000 × g, resuspended in 1 ml of sterile phosphate-buffered saline, enumerated using a Petroff-Hausser counting chamber (Hausser Scientific, Horsham, PA), added to 500 ml of filter-sterilized marine water, and diluted in 10-fold increments. The samples were filtered and processed, and the DNA was extracted as previously described (17). A negative control contained filtered marine water with no M. ruminantium cells added. The extracted DNA was amplified in 20-μl Mrnif PCR assays as described above. A lower detection limit of 90 cells in 500 ml was established, suggesting sensitivity of the method.
A comparison for determining the specificity of the Mrnif assay for domesticated-ruminant feces was conducted between the Mrnif primers developed in this study and a previously developed primer pair, CF-128/Bac708r (2). The CF-128f/Bac708r primers amplify a fragment of the 16S rRNA gene in the Bacteroides-Prevotella group, and the assay has been proposed as a ruminant-specific gene marker for microbial source tracking (2). PCR was conducted for both of the primer pairs, using the reaction conditions listed above for the Mrnif assay.
Fecal samples, samples from a bovine waste lagoon and a creek contaminated with bovine lagoon waste, and sewage samples were collected and processed as reported previously (18). Various concentrations of extracted DNA (50, 75, and 100 ng) were used as the template in the PCR assays for both the Mrnif and CF-128f/Bac708r primer pairs.
The proportions of samples from bovine and sheep feces, the bovine waste lagoon, and the contaminated creek amplified using the Mrnif and CF-128/Bac708r primer pairs were comparable (Tables 1 and 2). Samples that were successfully amplified using the Mrnif and CF-128f/Bac708r assays produced products with the use of all three template amounts (50, 75, and 100 ng template DNA). The CF-128/Bac708r primers (2) produced amplicons of the expected sizes in DNA extracted from 80% of cow fecal samples, 100% of sheep, deer, and goat samples, and 4% of human fecal samples (Table 1). By contrast, the Mrnif primers amplified only DNA from domesticated-ruminant fecal samples (80% of cow, 100% of sheep, and 75% of goat samples); no amplification was observed in 24 deer and 50 human fecal samples (Table 2). Both primer pairs amplified DNA extracted from the bovine waste lagoon and the contaminated creek water samples, but neither primer pair amplified products in environmental bacteria or DNA extracted from sewer samples (Table 2). The comparison showed that the Mrnif assay developed in this study was specific for fecal pollution from domesticated ruminants while the CF-128/Bac708r primer amplified DNA extracted from both domesticated- and nondomesticated-ruminant feces.
TABLE 1.
Fecal samples tested for Mrnif assay specificity in comparison to CF128f/Bac708r primer specificity
| Source of fecal sample | No. of samples amplified with indicated primer pair/total no. of samples
|
|
|---|---|---|
| Mrnif PCR | CF-128/Bac708r | |
| Cow | 40/50 | 40/50 |
| Sheep | 24/24 | 24/24 |
| Goat | 18/24 | 24/24 |
| Deer | 0/24 | 24/24 |
| Human | 0/50 | 2/50 |
| Swine | 0/25 | 0/25 |
| Horse | 0/20 | 0/20 |
| Rat | 0/20 | 0/24 |
| Chicken | 0/24 | 0/24 |
| Dog | 0/24 | 0/24 |
TABLE 2.
Environmental samples tested for Mrnif and CF128f/Bac708r primer specificity
| Sample group and source | Total no. of samples tested | No. of samples amplified with indicated primer pair
|
|
|---|---|---|---|
| Mrnif | CF-128f/Bac708r | ||
| Expected | |||
| Bovine waste lagoon | 2 | 2 | 2 |
| Bovine lagoon waste-contaminated creek | 2 | 2 | 2 |
| Not expected | |||
| Swine waste lagoon | |||
| Surface | 3 | 0 | NAa |
| Sludge | 1 | 0 | NA |
| Anaerobic layer | 3 | 0 | NA |
| Sewer | 22 | 0 | 0 |
| Coastal water and creek | 111 | 0 | NA |
| Coastal sediment | 17 | 0 | NA |
| Environmental bacteria | 477 | 0 | 0 |
| Fluvial water | 10 | 0 | NA |
NA, not applicable.
A possible reason for this difference is the host specificity of the target indicator organism. The primers CF-128/Bac708r amplify a fragment of the 16S rRNA gene in the Bacteroides-Prevotella group, which is widely distributed in the intestinal tracts of humans and animals. Many uncultured Bacteroides/Prevotella strains are unique to the rumen, although there is little diversity observed between domestic and wild ruminants (3). Alternatively, methanogens tend to occupy host-specific niches due to intestinal system differences (rumen versus monogastric) and can be considered host specific (17). Dietary differences among host species may affect microbial communities, leading to greater methanogen diversity between domesticated and nondomesticated ruminants. In a study of methanogens present in the rumens of sheep fed different diets (grazing diet versus hay-fed diet), a higher level of methanogen diversity was observed in grazing sheep than in hay-fed sheep, with different phylotypes dominating the community in animals fed a restricted diet (23). High-fiber diets have also been correlated with higher methane production in different animals (21), which again indicates that diet affects the composition of methanogen communities in animals. A study investigating the colonization of young lambs showed that the Methanobrevibacter community is the first to develop and remains stable throughout the growth and dietary changes of the lambs (13). The lack of Mrnif amplification in DNA extracted from deer feces indicates either that nondomesticated deer lack M. ruminantium in comparison to levels in domesticated ruminants, such as cows, sheep, and goats, or that the Mrnif assay developed in this study may target specific strains of M. ruminantium present only in domesticated ruminants.
Both the Mrnif and CF-128f/Bac708r assays amplified products in only 80% of samples of DNA extracted from bovine feces. The Mrnif assay also amplified only 75% of goat fecal DNA extracts. The bovine samples were collected from different areas in Mississippi, and the goat samples were collected at two different farms in Mississippi. The absence of Methanobrevibacter ruminantium in 20% of the cow and 25% of the goat fecal samples may be due to age, diet, and health differences in individual animals as well as differential antibiotic use among farms (15). Because the goat industry is not classified as a predominant agricultural industry in the United States (19), the lack of the expected product in 25% of goat fecal samples should not detract from the usefulness of the Mrnif method for microbial source tracking.
Realistically, regulators are more concerned with identifying contamination from composite samples, such as those from sewage overflow or animal waste lagoons, rather than those from individual animals. Therefore, the detection of the Mrnif marker in a dairy waste lagoon but not sewage or swine waste lagoon samples indicates specificity for domesticated-ruminant contamination. Further, a similar study showed the utility of using a molecular marker detecting only a small population of individual humans but a high percentage of sewers, indicating the importance of testing composite samples rather than individual fecal samples (17).
Detection of M. ruminantium in samples from a creek 1/4 mi from a bovine waste lagoon spillover event indicates the potential usefulness of the Mrnif assay in tracing the source of domesticated-ruminant fecal contamination. Various amounts of total DNA extracted from the bovine waste lagoon (130, 100, 50, 20, 10, 5, and 1 ng) and an adjacent creek (80, 20, 10, 5, and 1 ng) were tested to determine the limit of detection of the Mrnif marker in contaminated surface water. The lower detection limit in DNA extracted from cow, sheep, and goat feces was established by testing 50, 20, 10, 5, 1, 0.1, and 0.01 ng and 1.0 pg fecal DNA in 20-μl PCRs. The level of detection for the Mrnif assay in the creek was comparable to that of detection in the lagoon water itself (10 ng total DNA), showing the sensitivity of the method. The detection limits of the assay for DNA extracted from cow feces (0.1 ng), sheep and goat feces (1.0 ng), and the bovine waste lagoon and creek contaminated with bovine waste (10 ng) correspond to a lower cell detection limit of 90 cells in a 500-ml sample volume. These detection limits are comparable to previously published limits of detection for a methanogen-specific sewage primer pair (17).
Because methanogens are known to reside in marine and fluvial water and sediments (5), nonpolluted water and sediment samples were collected from the nearshore zone of the Mississippi Sound and feeder creeks over an 8-month period (18) and tested with 10, 25, and 50 ng total DNA using the Mrnif primer pair. No amplification was observed in DNA extracted from 111 coastal water and creek samples, 17 sediment samples, and 10 fluvial water samples, indicating that Methanobrevibacter ruminantium is not a normal inhabitant of the marine or fluvial environment. No environmental sediment samples were positive for the nifH gene of M. ruminantium, indicating that this organism does not reside in marine sediments, and no sediment methanogens were amplified with these primers.
The Mrnif assay developed in this study is a specific, sensitive, and rapid method for detecting fecal pollution from domesticated ruminants in surface waters and is the first assay that targets methanogens as indicators of domesticated-ruminant fecal pollution. Addition of an IAC for PCR enhanced the reliability of the assay for environmental testing by distinguishing true from false negative results, a common problem with PCR amplification of DNA isolated from environmental sources (6). With the development of other host-specific fecal indicators, this assay can be used in conjunction with those for sewage (17) and swine fecal pollution (18) to distinguish among multiple sources of fecal pollution in complex watersheds. Further research is needed to study the survivability of Methanobrevibacter ruminantium in the environment, the usefulness of the assay across a wider geographic range, and the sensitivity of the assay compared to that of other regulatory indicator methods.
Nucleotide sequence accession numbers.
Sequences obtained in this study have been submitted to GenBank (accession no. EU099627 to EU099652).
Acknowledgments
This work was supported by the National Oceanic and Atmospheric Administration Oceans and Human Health Program under grant NA04OAR4600216 and by the U.S. Environmental Protection Agency, Gulf of Mexico Program Office, through grants MX964295 and MX964012.
Footnotes
Published ahead of print on 7 September 2007.
REFERENCES
- 1.Balch, W. E., G. E. Fox, L. J. agrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernhard, A. E., and K. G. Field. 2000. PCR assay to discriminate human and ruminant feces on the basis of host differences in Bacteroides-Prevotella genes encoding 16S rRNA. Appl. Environ. Microbiol. 66:4571-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dick, L. R., A. E. Bernhard, T. J. Brodeur, J. W. Santo Domingo, J. M. Simpson, S. P. Walters, and K. G. Field. 2005. Host distributions of uncultivated fecal Bacteroidales bacteria reveal genetic markers for fecal source identification. Appl. Environ. Microbiol. 71:3184-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ExPASy Proteomics Server. 1999. Translate tool. http://expasy.org/tools/dna.html.
- 5.Friedrich, M. W. 2005. Methyl-coenzyme M reductase gene: unique functional markers for methanogenic and anaerobic methane-oxidizing Archaea. Methods Enzymol. 397:428-442. [DOI] [PubMed] [Google Scholar]
- 6.Hoorfar, J., B. Malorny, A. Abdulmawjood, N. Cook, M. Wagner, and P. Fach. 2004. Practical considerations in design of internal amplification controls for diagnostic PCR assays. J. Clin. Microbiol. 42:1863-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubbard, R. K., G. L. Newton, and G. M. Hill. 2004. Water quality and the grazing animal. J. Anim. Sci. 82(Suppl. E):E255-E263. [DOI] [PubMed] [Google Scholar]
- 8.Khatib, L. A., Y. L. Tsai, and B. H. Olson. 2002. A biomarker for the identification of cattle fecal pollution in water using the LTIIa toxin gene from enterotoxigenic Escherichia coli. Appl. Microbiol. Biotechnol. 59:97-104. [DOI] [PubMed] [Google Scholar]
- 9.Lai, M. C., C. C. Lin, P. H. Yu, Y. F. Huang, and S. C. Chen. 2004. Methanocalculus chunghsingensis sp. nov., isolated from an estuary and a marine fishpond in Taiwan. Int. J. Syst. Evol. Microbiol. 54:183-189. [DOI] [PubMed] [Google Scholar]
- 10.Lin, C., L. Raskin, and D. A. Stahl. 1997. Microbial community structure in gastrointestinal tracts of domesticated animals: comparative analyses using rRNA-targeted oligonucleotide probes. FEMS Microbiol. Ecol. 22:281-294. [Google Scholar]
- 11.Øvreås, L., L. Forney, F. L. Daae, and V. Torsvik. 1997. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 63:3367-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanks, O. C., J. W. Santo Domingo, R. Lamendella, C. A. Kelty, and J. E. Graham. 2006. Competitive metagenomic DNA hybridization identifies host-specific microbial genetic markers in cow fecal samples. Appl. Environ. Microbiol. 72:4054-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skillman, L. C., P. N. Evans, G. E. Naylor, B. Morvan, G. N. Jarvis, and K. N. Joblin. 2004. 16S ribosomal DNA-directed PCR primers for ruminal methanogens and identification of methanogens colonising young lambs. Anaerobe 10:277-285. [DOI] [PubMed] [Google Scholar]
- 14.Smith, P. H., and R. E. Hungate. 1958. Isolation and characterization of Methanobacterium ruminantium n. sp. J. Bacteriol. 75:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tajima, K., T. Nagamine, H. Matsui, M. Nakamura, and R. I. Aminov. 2001. Phylogenetic analysis of archaeal 16S rRNA libraries from the rumen suggests the existence of a novel group of archaea not associated with known methanogens. FEMS Microbiol. Lett. 200:67-72. [DOI] [PubMed] [Google Scholar]
- 16.Tatusova, T. A., and T. L. Madden. 1999. Blast 2 sequences—a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174:247-250. [DOI] [PubMed] [Google Scholar]
- 17.Ufnar, J. A., S. Wang, J. M. Christiansen, H. Yampara-Iquise, C. A. Carson, and R. D. Ellender. 2006. Detection of the nifH gene of Methanobrevibacter smithii: a potential tool to identify sewage pollution in recreational waters. J. Appl. Microbiol. 101:44-52. [DOI] [PubMed] [Google Scholar]
- 18.Ufnar, J. A., D. F. Ufnar, S. Y. Wang, and R. D. Ellender. 2007. Development of a swine-specific fecal pollution marker based on host differences in methanogen mcrA genes. Appl. Environ. Microbiol. 73:5209-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.USDA. 2002. Census of agriculture. http://www.agcensus.usda.gov/.
- 20.USEPA. 1986. Ambient water quality criteria for bacteria—1986. http://www.epa.gov/waterscience/beaches/files/1986crit.pdf.
- 21.Whitford, M. F., R. M. Teather, and R. J. Forster. 2001. Phylogenetic analysis of methanogens from the bovine rumen. BMC Microbiol. 1:5. http://www.biomedcentral.com/1471-2180/1/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wing, S., S. Freedman, and L. Band. 2002. The potential impact of flooding on confined animal feeding operations in eastern North Carolina. Environ. Health Perspect. 110:387-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright, A. D. G., A. J. Williams, B. Winder, C. T. Christophersen, S. L. Rodgers, and K. D. Smith. 2004. Molecular diversity of rumen methanogens from sheep in Western Australia. Appl. Environ. Microbiol. 70:1263-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]