Abstract
Many virulence genes in plant bacterial pathogens are coordinately regulated by “global” regulatory genes. Conducting DNA microarray analysis of bacterial mutants of such genes, compared with the wild type, can help to refine the list of genes that may contribute to virulence in bacterial pathogens. The regulatory gene algU, with roles in stress response and regulation of the biosynthesis of the exopolysaccharide alginate in Pseudomonas aeruginosa and many other bacteria, has been extensively studied. The role of algU in Xylella fastidiosa, the cause of Pierce's disease of grapevines, was analyzed by mutation and whole-genome microarray analysis to define its involvement in aggregation, biofilm formation, and virulence. In this study, an algU::nptII mutant had reduced cell-cell aggregation, attachment, and biofilm formation and lower virulence in grapevines. Microarray analysis showed that 42 genes had significantly lower expression in the algU::nptII mutant than in the wild type. Among these are several genes that could contribute to cell aggregation and biofilm formation, as well as other physiological processes such as virulence, competition, and survival.
Xylella fastidiosa is a xylem-limited, gram-negative, plant-pathogenic bacterium that is transmitted by insects such as the glassy-winged sharpshooter, Homalodisca coagulata (48). Strains of X. fastidiosa cause many diseases, including citrus variegated chlorosis, leaf scorch of live oak, pear leaf scorch, and Pierce's disease (PD) of grape (10, 46), which threatens the grapevine industry in the United States. The characteristic symptoms of PD are scorched leaves, matchstick (petioles attached to the cane after the scorched leaf blades have abscised), and green islands (patches of green tissue surrounded by brown tissue on infected canes). Eventually, the fruit desiccates, vine cordons die back, and diseased grapevines can die 2 to 3 years after infection (47). Genetic resistance to X. fastidiosa is not available in most commercial wine grape varieties, although several relatives of grape, such as Vitis tiliifolia, can harbor high populations of X. fastidiosa without typical symptoms of PD (16, 31). Currently, the control of insect vectors is the main management strategy for PD. Successful biocontrol of insect vectors depends on the insects' ecology and field environmental conditions. The identification of genetic factors that enable X. fastidiosa to express PD symptoms could lead to new disease management strategies, including new targets for disruption of the disease process.
Although the mechanisms of X. fastidiosa pathogenicity are not completely understood, the major symptoms of most of the diseases caused by this pathogen are similar to water stress, probably resulting from blockage of the xylem transport system (37). Previous studies have shown that X. fastidiosa is embedded in an extracellular translucent extracellular polysaccharide (EPS)-matrix biofilm within xylem vessels (44). These two observations suggest that X. fastidiosa cells can form bacterial aggregates (biofilm-like colonies) containing EPSs that occlude the xylem vessels, resulting in blockage of water transport and causing PD symptoms.
Sigma factors control virulence and pathogenicity factors in various bacterial pathogens in response to different environmental conditions (13, 52). algU encodes an alternate sigma factor, AlgU, that is highly conserved in gram-negative bacteria (17, 30) and confers tolerance to osmotic, oxidative, and heat stresses. Its role in the regulation of the biosynthesis of the EPS alginate has been extensively studied in the human pathogen Pseudomonas aeruginosa and the plant pathogen P. syringae (17, 30). Alginate may play a role in biofilm-related phenomena, including contribution to adhesion and antibiotic resistance in P. aeruginosa (17). Alginate is also involved in colonization by and dissemination of the plant pathogen P. syringae in planta (60). Although a homolog of algU is present in the genome of X. fastidiosa (53), its role in X. fastidiosa is unknown. In this study, we analyzed the effect of an insertional mutation in the algU gene of X. fastidiosa, performed whole-genome microarray analysis of gene expression in the mutant, and identified genes whose expression is controlled by algU.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All of the bacterial strains and plasmids used in this work are listed in Table 1. For growth profile, aggregation, adhesion, colony morphology determination, and biofilm formation, bacterial strains were cultured on PD3 medium (12) supplemented with 0.8% Gelrite instead of agar. After 7 days at 28°C, cells were harvested with a scraper (Fisher Scientific, CA), washed and resuspended in 1 ml of PD3 broth, and adjusted to an optical density at 600 nm (OD600) of 0.10. Bacterial cells used for pathogenicity tests were cultured for 5 days at 28°C on PW (27) Gelrite medium, harvested, and adjusted to the same OD600 as above with sterile water. When required, antibiotics were added as follows: ampicillin, 100 μg/ml; kanamycin, 10 μg/ml. All strains were stored in PD3 broth with 15% glycerol at −80°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Escherichia coli DH5α | DH1 F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 | N. T. Keen |
| Xylella fastidiosa | Wild-type X. fastidiosa A05 | 10 |
| X. fastidiosa algU::nptII | Tn5 insertional mutation in algU homologue of X. fastidiosa A05 | This work |
| Plasmids | ||
| pGEM-T Easy | Apr; cloning vector | Promega |
| pUC129 | Apr; cloning vector | New England BioLabs |
| pUC1284 | Apr; 0.891-kb fragment including algU ORF cloned into pUC129 | This work |
| pUC12841 | Apr Kmr; Tn5 insertion within algU ORF in pUC1284 | This work |
Construction of an algU::nptII mutant.
A 0.891-kb region of X. fastidiosa genomic DNA including the algU (PD1284) open reading frame (ORF) was amplified with Vent polymerase (New England BioLabs, Ipswich, MA) with primers algUP1 and algUP2 (see Table S1 in the supplemental material) and cloned into the SmaI site of pUC129 (Table 1) to make pUC1284. The PCR fragment in pUC1284 was sequenced to confirm the presence of an intact algU ORF. pUC1284 DNA was mutagenized with the Tn5 transposon with the EZ::TN <KAN-2> insertion kit (Epicentre Biotechnologies, Madison, WI) as outlined by the manufacturer. A plasmid with a Tn5 insertion within the algU ORF in pUC1284 was named pUC12841. Tn5 insertions in pUC12841 were precisely mapped by sequencing with transposon primers Kan-2 FP-1 or Kan-2 RP-1 (see Table S1 in the supplemental material).
X. fastidiosa electrocompetent cells of strain A05 isolated from Temecula (10) were prepared according to previously published procedures (18). One to two micrograms of pUC12841 DNA in a volume of 5 μl was electroporated into the cells in a 0.1-cm-gap cuvette at 1.8 kV, 200 Ω, and a capacitance of 25 μF in a GenePulser (Bio-Rad, Hercules, CA) with time constants of about 4 ms. The electrocompetent cells alone and PD3 broth with no bacterial cells served as negative controls. Electroporated cells were grown for 24 h in PD3 broth with shaking and plated on PD3 Gelrite medium supplemented with 10 μg/ml kanamycin as previously described to select for replacement of wild-type algU with algU::nptII by homologous recombination (20).
Genomic DNA extraction and confirmation of the algU::nptII mutant.
Wild-type X. fastidiosa or the algU::nptII mutant strain was cultured in 50 ml PD3 broth at 28°C for 7 to 10 days with or without antibiotics. The genomic DNAs were extracted with a MasterPure DNA purification kit (Epicentre Biotechnologies). The insertion of the construct into the genome of the algU::nptII mutant was confirmed by PCR with primers M13For/Rev and algUORF P1/P2, respectively (see Table S1 in the supplemental material). A. 0.891-kb fragment from the wild type and a 2.1-kb fragment from the mutant were cut from the gel, cloned into pGEM-T Easy (Promega, Madison, WI) (Table 1), sequenced, and compared with X. fastidiosa genomic sequences or Tn5 transposon sequences with Vector NTI (Invitrogen, CA), respectively. The Tn5 insertion within the algU ORF of algU::nptII genomic DNA was determined by sequencing with transposon primers Kan-2 FP-1 or Kan-2 RP-1 (see Table S1 in the supplemental material).
Colony morphology, growth curves, surface attachment, and cell aggregation.
The colony morphologies of the X. fastidiosa wild-type and algU::nptII mutant strains were analyzed after 10 to 14 days of growth at 28°C by plating 100 μl of 0.10 OD600 cell suspensions on PD3 Gelrite plates. In vitro growth curves were determined in 3 ml of PD3 broth after 3 to 21 days of growth at 28°C. Because of the aggregation of the cells in broth, immediately after inoculation and 3, 6, 9, 12, 15, 18, and 21 days later, the cells were dispersed by repeated pipetting or vortexing. Cell concentration was determined by measuring the OD600. For cell aggregation analysis, strains were grown in 25 ml of PD3 broth in petri dishes and incubated at 28°C without shaking for 4 days. After growth, the content of each petri dish was pipetted into a tube and the cells were dispersed by vortexing or pipetting and adjusted to an OD600 of 0.10. Two hundred microliters of each culture was then subcultured into eight tubes, each with 25 ml of fresh PD3 broth. Tubes were allowed to stand in the incubator without shaking. Three days after incubation, the tubes were vortexed and the OD540 (ODt) was measured. The concentration of bacterial cells was also measured by determining the OD600. These tubes were kept without shaking for 1 h to allow the bacterial cells to clump and settle. The OD540 of supernatants of the tubes (ODs) was again measured. The relative percentage of cell aggregation was measured by using the following formula: % aggregation = (ODt − ODs)/(ODt × 100) (5). This procedure was repeated for the remaining seven tubes of each culture at 6, 9, 12, 15, 18, 21, and 24 days after the initial incubation.
Biofilm formation.
X. fastidiosa wild-type and algU::nptII mutant cells were cultured in PD3 broth and incubated at 28°C without shaking for 4 to 6 days. Bacterial cells were collected and adjusted to an OD600 of 0.10. One-hundred-fifty-microliter aliquots of each culture were added to wells of 96-well microtiter plates. The negative control consisted of PD3 broth without bacteria. Plates were incubated at 28°C without shaking. At 3, 6, 9, 12, and 15 days after incubation, biofilm formation on the wall of the wells was determined by a crystal violet staining method (33). Each treatment had three replications, and the data were averaged.
LPS gel analysis.
Lipopolysaccharide (LPS) fractions were prepared by a mini-phenol-water extraction technique as described by Guihabert et al. (19). Twenty microliters of dissolved LPS in polyacrylamide gel electrophoresis (PAGE) sample buffer (0.3% Tris base, 0.2% glycerol, 0.05% bromophenol blue) was loaded and separated by deoxycholic acid-PAGE with 18% acrylamide in the bilayer stacking gel. Gels were silver stained and stored in water (28).
Tolerance of the algU::nptII mutant to desiccation stress in vitro.
The sensitivity of wild-type X. fastidiosa and the algU::nptII mutant to desiccation on filters was assessed by a modification of the procedure described by Ophir and Gutnick (43). Seven- to 10-day-old cultures were collected and adjusted to an OD600 of 0.10 with sterile distilled water and serially diluted to 1 ×10 4 CFU/ml. One milliliter of each dilution was vacuum filtered onto Millipore filters (no. HAWP04700; pore size, 0.25 μm; diameter, 3.5 cm). The filters were placed in petri dishes at 25°C for slow drying. At 0, 2, 4, 6, 8, 10, 12, and 14 days, filters were placed onto PD3 agar plates and incubated at 28°C for 3 weeks. Filters without dilutions, incubated for the same period of time, served as controls. The number of colonies on each filter was recorded. Each treatment consisted of five filters and was repeated three times.
Susceptibility to oxidative stress.
Sensitivity to hydrogen peroxide (H2O2) or sodium hypochlorite (NaOCl) was examined as described by Martin et al. (38). Millipore filter disks (diameter, 6 mm) were soaked with 10 μl of H2O2 (3 or 12%, vol/vol) or NaOCl (3 or 6%, vol/vol) and placed on PD3 Gelrite plates on which 100 μl of 7-day-old cultures of wild-type X. fastidiosa or the algU::nptII mutant were spread with a glass rod. The diameters of the inhibition zones surrounding the impregnated disks were measured after 14 to 21 days of incubation at 28°C. Three disks were placed in each treatment, each treatment was repeated three times, and the results were averaged.
Pathogenicity assays on grapes.
Wild-type X. fastidiosa and the algU::nptII mutant were grown on PW Gelrite medium for 5 days at 28°C, suspended in sterile deionized water, and adjusted to an OD600 of 0.10. Five to 10 20-μl drops of each suspension were used to inoculate 5 to 10 canes on plants of Vitis vinifera var. Pinot Noir by a needle inoculation procedure as previously described (24). Water inoculation served as a negative control. The inoculated vines were kept on the benches in a greenhouse with 75% humidity. The vines were observed for symptom development approximately every 2 weeks for 5 months after inoculation. The symptoms were rated on a visual scale of 0 to 5 as described before (19). Briefly, 0 represented healthy grape vines without scorched leaves (water control) and 5 represented plants with all leaves with heavy scorching or numerous matchsticks. The final disease index was an average of 10 independent replications for each X. fastidiosa strain.
Recovery and determination of populations of X. fastidiosa from inoculated grapes.
To recover and confirm the bacteria in inoculated grapes, 12 weeks after inoculation, petiole tissues (2 to 3 cm) from each vine inoculated with either X. fastidiosa wild-type or algU::nptII mutant cells were harvested at the inoculated points, as well as 25 cm and 50 cm above the inoculation points. Tissues were washed once with deionized water containing Tween 20; surface sterilized for 1 min in 20% commercial bleach, 1 min in 2% sodium hypochlorite, and 1 min in 70% ethanol; and rinsed three times in sterile deionized water. The samples were ground in 100 μl of sterile deionized water and cultured on PD3 and PW Gelrite media with or without kanamycin. After incubation for 21 days at 28°C, the identity of X. fastidiosa cells on PD3 Gelrite plates was confirmed by PCR with primers specific for wild-type X. fastidiosa, i.e., algUORFP1/P2 and tapBPD1993P1/P2 (see Table S1 in the supplemental material) (data not shown).
To determine the bacterial populations 16 weeks after inoculation, 2- to 3-cm petiole tissues of each vine inoculated with the X. fastidiosa wild-type and mutant strains were harvested and treated as described above. Tissues were tested by enzyme-linked immunosorbent assay (ELISA) with a PathoScreenXF kit according to the manufacturer's (Agdia Inc., IN) instructions. The antibodies used in the Agdia ELISA system are a mixture of polyclonal antibodies raised to whole cells of three serologically distinct isolates of X. fastidiosa (Agdia Inc.). The PD3-cultured X. fastidiosa wild-type and algU::nptII mutant cells were resuspended in phosphate-buffered saline (PBS; Agdia Inc.) and used to confirm that the ELISA worked equally well for quantifying the wild-type and mutant populations. Developed plates were measured at 650 nm with a SpectraMax microplate reader via SoftMaxPro (version 3.1.2; Molecular Devices Corp., CA). Bacterial populations were calculated via OD650 determination in comparison to the positive control (purified X. fastidiosa cell PBS suspension).
SEM.
X. fastidiosa wild-type and mutant cells in grapevine xylem were examined by scanning electron microscopy (SEM) (57). Petiole samples were collected above the inoculation points from symptomatic grapevines 12 weeks after inoculation. Preparation and observation of the samples by SEM were carried out in the University of California Riverside Central Facility for Advanced Microscopy and Microanalysis. Petioles of five leaves from symptomatic grapevines were cross-sectioned with a fine razor blade and immersed for 24 h in a modified Karnovsky solution. Samples were washed twice with ultrapure water for 30 min, and sections were transferred to a 1% aqueous solution of osmium tetroxide and incubated for 24 h at 4°C. Samples were subsequently dehydrated for 20 min each in an alcohol solution series (20, 30, 40, 50, 60, 70, 80, 90, and 100%), and the solvents were removed by vacuum (critical point dried) (57). The samples were mounted according to the manufacturer's instructions and observed with a Philips XL30 ESEM-FEG electron microscope. All images were recorded at a working distance of 9 mm according to the standard procedure with an accelerating voltage of 20 kV.
RNA isolation, quantification, and RT-PCR.
A modified hot-phenol RNA preparation procedure was used to extract total RNA from X. fastidiosa wild-type and mutant strains (7, 32). Bacterial cultures were incubated in 50 ml of PD3 broth at 28°C for 5 days under constant agitation. After the hot-phenol extraction, RNA was suspended in RNase-free distilled H2O and DNase treated with the Turbo DNA-free DNase (2 U/μl) (Ambion, TX). To ensure that the RNA preparation was DNA free, an aliquot of 1 μl of RNA (500 ng/μl) was then used to amplify the ORF of algU with algUORFP1/P2 primers (see Table S1 in the supplemental material). The quality of isolated RNAs was determined by denaturing RNA formaldehyde gel electrophoresis (7). The expression of algU was analyzed by reverse transcription (RT)-PCR with the AccessQuick RT-PCR system by following the manufacturer's (Promega) instructions.
Microarray hybridizations and microarray data analysis.
The gene expression profiles of wild-type X. fastidiosa and the algU::nptII mutant were analyzed with a NimbleGen prokaryotic gene expression array (NimbleGen System Inc., WI). DNA microarray chips were designed with 24-mer oligonucleotides according to the available X. fastidiosa genomic sequences. The expression levels of RNAs were averaged from three technical replications in a single hybridization experiment. The raw data were analyzed with the ArrayStar FirstLight. The expression levels of 2,188 genes under treatment (algU::nptII) and control (wild type) were analyzed (21). The hybridization signal intensity obtained from the wild-type or mutant RNA was normalized according to the total signal strength. The normalized hybridization signals were log plot analyzed for reliability (21) and were statistically analyzed by Student's t test (P < 0.001) for differential expression. The normalized signal intensity of the mutant was divided by that of the wild type to calculate the mutant/wild-type (M/W) ratio. M/W ratios obtained from individual hybridization experiments were averaged to give the final M/W ratio. Genes having ≥1.5 or ≤0.66 final M/W ratios were selected as mutated gene up-regulated or mutated gene down-regulated, respectively.
Validation of microarray data.
To validate the differential expression data obtained in microarray analysis, RT-PCR and PCR experiments were performed with specific primers designed to amplify an internal region of the ORF of each target gene (see Table S1 in the supplemental material). Several up-regulated and potential virulence-related genes were chosen, and primers were designed for their ORFs according to the X. fastidiosa Temecula1 genome sequences. cDNA was amplified from stored DNase-cleaned RNAs with the AccessQuick RT-PCR system by following the instructions of the manufacturer (Promega). The amplification conditions used were 45 min at 45°C for RT; 35 cycles of 2 min at 55°C for initial denaturation, 1 min at 55°C for annealing, and 2 min at 72°C for extension; and a final extension of 10 min at 72°C. Five microliters of the reaction mixture was run in agarose gels, and products were stained with ethidium bromide.
RESULTS
Confirmation of the mutational insertion and physiological properties of an algU::nptII mutant.
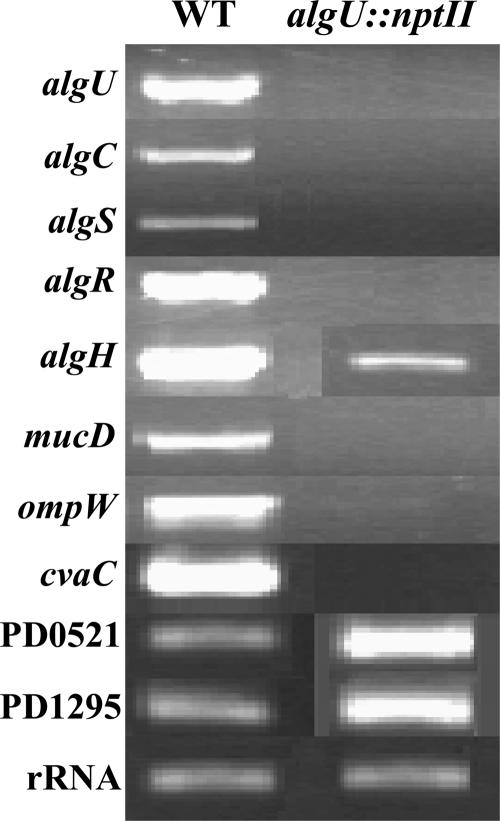
The algU::nptII mutant had the Tn5 transposon inserted in the algU ORF 79 bp downstream from the ATG start codon. After streaking five to eight times on PD3 Gelrite medium with 10 μg/ml kanamycin, the mutant still grew well, indicating that the mutant had stable genetic characteristics. cDNAs amplified by RT-PCR with algUORFP1/P2 (see Table S1 in the supplemental material) showed that there was no expression of algU within the algU::nptII mutant cells, while strong expression was detected in wild-type cells (Fig. 1). The in vitro growth curves of the wild-type and algU::nptII strains over 21 days were similar (data not shown). In PD3 broth, the wild-type strain formed large aggregates whereas the algU::nptII mutant grew in less aggregated clumps (Fig. 2A). An OD assay was used to quantify the effect of the algU::nptII mutation on cell-to-cell aggregation and showed that the percentage of aggregated cells of algU::nptII was significantly lower than that of the wild type (Fig. 2B).
FIG. 1.
RT-PCR of genes differentially expressed between wild-type (WT) X. fastidiosa and the algU::nptII mutant. rRNAs were detected in the algU::nptII mutant and the wild type in this RT-PCR condition. The algU, algC, algS, algR, algH, mucD, ompW, and cvaC (PD0216) RNAs were decreased in the mutant compared to the wild type, and the PD0521 and PD1295 RNAs were slightly increased in the algU::nptII mutant.
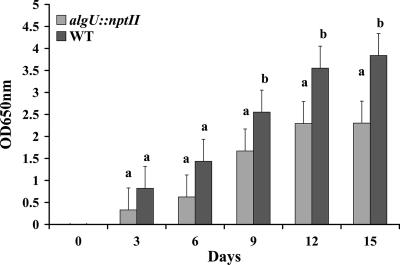
FIG. 2.
Cell-to-cell aggregation of wild-type X. fastidiosa and the algU::nptII mutant. (A) Cell-to-cell aggregations of the algU::nptII mutant (left) and wild-type X. fastidiosa (right) in PD3 broth in petri dishes. (B) Quantitative assessment of cell-to-cell aggregation of wild-type (WT) X. fastidiosa or the algU::nptII mutant by an OD assay as previously described (5). Three replicates were used in each experiment. For each assay time, different letters indicate significant differences (Student's t test, P < 0.05) between the wild type and the mutant.
Cells of the wild type attached to the surface of the flasks and formed wide rings, while the algU::nptII mutant cells attached to the surface formed lighter rings (data not shown). This indicated that the mutant had a reduced surface attachment ability, resulting in reduced biofilm formation. The ability of the algU::nptII mutant to form biofilm was investigated further by a crystal violet staining method. The wild type formed more biofilm in PD3 medium than did the mutant (Fig. 3). Deoxycholate-PAGE analysis showed that there was no significant alteration in the purified LPS profile of the algU::nptII mutant grown in vitro compared with the LPS profile of the wild type (data not shown).
FIG. 3.
Analysis of biofilm formation of wild-type (WT) X. fastidiosa and the algU::nptII mutant. Three replicates were used in each experiment. For each assay time, different letters indicate significant differences (Student's t test, P < 0.05) between the wild type and the mutant.
Tolerance to oxidative and desiccation stresses.
When wild-type X. fastidiosa and the algU::nptII mutant were exposed to desiccation stress in vitro in petri dishes, the survival rate of the mutant differed significantly from that of the wild type (Table 2), indicating that the mutant died off faster than the wild type. No significant differences in tolerance to oxidative stress (sensitivity to hydrogen peroxide or sodium hypochlorite) were observed between the mutant and wild-type strains (data not shown).
TABLE 2.
Tolerance of wild-type X. fastidiosa and the algU::nptII mutant to desiccation stress in vitro
| Genotype | Survival rate (%) of X. fastidiosa cells after drying on filtersa for:
|
||||
|---|---|---|---|---|---|
| 0 days | 2 days | 4 days | 6 days | 8 days | |
| Wild type | 100 | 51.4§ | 7.9§ | 0.7¶ | 0¶ |
| algU::nptII | 100 | 17.6¶ | 0.7¶ | 0¶ | 0¶ |
Data are averages of three independent replications for each treatment. Five filters per dilution were used in each treatment. For each assay date, different symbols indicate a significant difference (Student's t test, P < 0.05) between the wild-type and mutant survival rates. The data for days 10 to 14 were all 0 and are not shown.
Pathogenicity tests and recovery from infected plants.
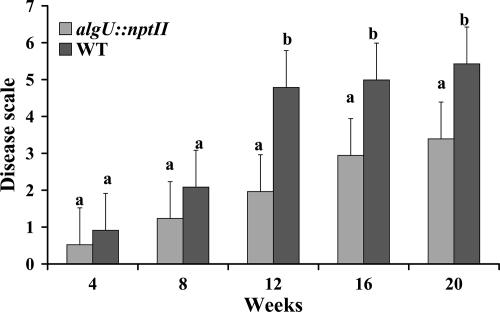
Grapevines inoculated with the algU::nptII mutant showed significantly less severe disease symptoms 12 to 20 weeks after inoculation than those inoculated with the wild type (Fig. 4). Water-inoculated control grapevines did not show any PD symptoms. All diseased grapevines were positive, and the asymptomatic water control grapevines were negative for the presence of X. fastidiosa when the plants were examined by ELISA. X. fastidiosa wild-type and algU::nptII mutant cells were also examined by SEM in grapevine xylem vessels 12 weeks after inoculation (data not shown), confirming the successful survival of cells of wild-type X. fastidiosa and the algU::nptII mutant in grapevine xylem vessels.
FIG. 4.
PD progression in grapevines inoculated with wild-type X. fastidiosa and the algU::nptII mutant. Disease severity was based on a visual disease scale of 0 to 5 and was assessed 4, 8, 12, 16, and 20 weeks after inoculation (19). The data are an average of 10 independent replications. For each time point, different letters indicate significant differences (Student's t test, P < 0.05) between the wild type and the mutant.
Bacteria were reisolated from macerated inoculated grapevine petioles on PD3 and PW Gelrite media. The bacterial genotypes were confirmed as wild-type X. fastidiosa or the algU::nptII mutant by PCR amplification with primers algUORFP1/P2 and tapBPD1993P1/P2 (see Table S1 in the supplemental material) (data not shown).
To gain further understanding of the mechanisms that could explain why the algU::nptII mutant had reduced virulence, bacterial populations and bacterial movement in infected grapevines were estimated from ELISAs. The ELISA showed no cross-reaction with any healthy tissue tested. Preliminary experiments showed that the ELISA used to quantify the X. fastidiosa populations worked equally well for wild-type and mutant cultures. Bacterial populations at inoculation points and at 25 cm and 50 cm above inoculation points were estimated from ELISAs by comparing the OD650 with that of the positive control X. fastidiosa with known concentrations (Table 3). An OD650 of the X. fastidiosa positive control (purified X. fastidiosa cells in a PBS suspension) of 1 represented approximately 1 ×104 CFU/ml. The average bacterial populations were calculated by comparing their OD650 values to that of the positive control and dividing by the average weight of 2- to 3-cm sampled petioles. There were no X. fastidiosa cells detected in the asymptomatic water-inoculated control grapevines. The cell population of the algU::nptII mutant was less than that of the wild type at 25 cm and 50 cm above inoculation points (Table 3). The actual populations could have been larger than we reported, since those were calculated on the basis of X. fastidiosa cultures in PBS rather than plant sap. Plant sap could lower ELISA detection. However, it is the relative difference between the wild type and the mutant that is significant (Table 3). These data suggest that the mutated algU gene may affect the growth and possibly the movement of X. fastidiosa inside the xylem, resulting in reduced pathogenicity.
TABLE 3.
Bacterial populations in grapevines 16 weeks after inoculation
| Strain A05 genotype | Population (106 CFU/g of tissue)a
|
||
|---|---|---|---|
| At inoculated point | Above inoculated points
|
||
| 25 cm | 50 cm | ||
| Wild type | 9.087 ± 2.6§ | 0.609 ± 0.18§ | 0.646 ± 0.28§ |
| algU::nptII | 1.267 ± 2.1¶ | 0.067 ± 0.013¶ | 0.063 ± 0.031¶ |
Data are averages of 10 independent replications for each treatment. Each treatment had five samples. For each assay point, different symbols indicate a significant difference (Student's t test, P < 0.05) between the wild-type and mutant populations. The data for asymptomatic water control were all 0 and are not shown.
DNA microarray analysis of gene expression in vitro.
The expression levels of 2,188 genes were monitored in the wild-type and algU::nptII mutant strains. The expression levels of RNAs were averaged from three technical replications in a single hybridization experiment. The normalized hybridization signals formed a linear pattern after the log plot analysis, indicating that the hybridization signals were stable, repeatable, and reliable (data not shown). rRNA of X. fastidiosa was detected in wild-type X. fastidiosa and the algU::nptII mutant (Fig. 1), indicating that this RT-PCR condition was reliable. Differential expression of algU, algC, algS, algR, algH, mucD, ompW, cvaC (PD0216), PD0521, and PD1295 was validated by RT-PCR (Fig. 1). Forty-three genes were differentially expressed in the algU::nptII strain compared to the wild type (Table 4). One gene (PD1926), predicated to encode a fimbrial protein, was shown to be negatively regulated by algU in the X. fastidiosa wild type. The other 42 differentially expressed genes appear to be positively regulated by algU in the X. fastidiosa wild type, including the following: genes predicted to function in macromolecular metabolism, intermediary metabolism, cell structure, and cellular processes; mobile gene elements; genes that encode conserved and hypothetical proteins; ORFs with undefined functions; and pathogenicity, virulence, and adaptation genes, according to the functional groups (Table 4).
TABLE 4.
Genes differentially expressed in the X. fastidiosa algU::nptII mutant in vitro, organized by functional groups
| Functional group and gened | ORF | Description | M/W ratioa,b | Expression in mutantc |
|---|---|---|---|---|
| Macromolecule metabolism | ||||
| Protein metabolism/degradation | ||||
| clpS | PD0664 | ATP-dependent Clp protease adaptor; posttranslational modification | 0.448 | Lower |
| clpA | PD0665 | ATP-dependent Clp protease subunit; posttranslational modification | 0.485 | Lower |
| clpB | PD1685 | ATP-dependent Clp protease subunit; posttranslational modification | 0.421 | Lower |
| mucD | PD1286 | Periplasmic protease | 0.616 | Lower |
| Protein metabolism/chaperones | ||||
| grpE | PD1371 | Heat shock protein GrpE | 0.40 | Lower |
| dnaK | PD1370 | Heat shock protein Hsp70; cochaperones are DnaJ and GrpE | 0.378 | Lower |
| RNA metabolism/ribosomal proteins | ||||
| rpmH | PD2123 | 50S ribosomal protein L34; unknown function | 0.449 | Lower |
| rplW | PD0439 | 50S ribosomal protein L23; unknown function | 0.499 | Lower |
| rplP | PD0444 | 50S ribosomal protein L16; unknown function | 0.426 | Lower |
| rpmC | PD0445 | 50S ribosomal protein L29; unknown function | 0.479 | Lower |
| rplN | PD0447 | 50S ribosomal protein L14; unknown function | 0.477 | Lower |
| rpsN | PD0450 | 30S ribosomal protein S14; unknown function | 0.244 | Lower |
| rpsH | PD0451 | 30S ribosomal protein S8; unknown function | 0.256 | Lower |
| rplF | PD0452 | 50S ribosomal protein L6; unknown function | 0.323 | Lower |
| rpsE | PD0454 | 30S ribosomal protein S5; unknown function | 0.437 | Lower |
| rpmD | PD0455 | 50S ribosomal protein L30; unknown function | 0.348 | Lower |
| rpsM | PD0458 | 30S ribosomal protein S13; unknown function | 0.394 | Lower |
| rpmB | PD0488 | 50S ribosomal protein L28; unknown function | 0.436 | Lower |
| rpmG | PD0489 | 50S ribosomal protein L33; unknown function | 0.353 | Lower |
| rpmE | PD0749 | 50S ribosomal protein L31; unknown function | 0.350 | Lower |
| Intermediary metabolism | ||||
| Energy metabolism, carbon/tricarboxylic acid cycle | ||||
| gltA | PD0750 | Citrate synthase; energy production and conversion | 0.496 | Lower |
| Regulatory functions/sigma factors and other regulatory components | ||||
| csrA | PD0095 | RsmA homologue; regulates the production of virulence determinants | 0.403 | Lower |
| Regulatory functions/two-component systems | ||||
| algR | PD1153 | Two-component system; regulatory protein | 0.617 | Lower |
| Regulatory functions/activators/repressors | ||||
| algH | PD1276 | Transcriptional regulator | 0.652 | Lower |
| Cell structures | ||||
| Surface structures | ||||
| PD1926 | Fimbrial protein, pilus assembly protein | 2.478 | Higher | |
| Membrane components/inner membrane | ||||
| algC | PD0120 | Phosphomannomutase | 0.3 | Lower |
| Membrane components/outer membrane | ||||
| mopB | PD1709 | Outer membrane protein | 0.479 | Lower |
| ompW | PD1807 | Outer membrane protein | 0.391 | Lower |
| Cellular processes | ||||
| Transport/protein, peptide secretion | ||||
| secB | PD1065 | Type II secretion system, preprotein translocase | 0.409 | Lower |
| Transport/carbohydrates, organic acids, alcohols | ||||
| algS | PD0347 | Sugar ABC transporter ATP-binding protein | 0.41 | Lower |
| Transport/cations | ||||
| bfr | PD1672 | Bacterioferritin; ferritin-like proteins | 0.178 | Lower |
| Mobile genetic elements | ||||
| Phage-related functions and prophages | ||||
| hfq | PD0066 | Host factor-I protein; ubiquitous RNA-binding protein Hfq | 0.32 | Lower |
| Pathogenicity, virulence, and adaptation | ||||
| Toxin production and detoxification | ||||
| cvaCe | PD0216 | Colicin V precursor; antibacterial polypeptide toxin | 0.40 | Lower |
| hspA | PD1280 | Heat shock protein (Hsp) | 0.469 | Lower |
| Hypothetical/conserved hypothetical proteins | ||||
| NDf | PD0159 | Unknown | 0.479 | Lower |
| ND | PD0521 | Unknown | 0.439 | Lower |
| ND | PD1354 | Unknown | 0.392 | Lower |
| ND | PD0968 | Unknown | 0.495 | Lower |
| ND | PD1028 | Unknown | 0.425 | Lower |
| ND | PD1058 | Putative transcriptional regulatory protein | 0.484 | Lower |
| ND | PD1295 | Putative integral membrane protein; involved in cell shape determination | 0.469 | Lower |
| ND | PD1668 | Putative integral membrane protein; involved in cell shape determination | 0.413 | Lower |
| ORFs with undefined category | ||||
| ND | PD1667 | HesB-like protein; unknown function | 0.462 | Lower |
The hybridization signal intensity (mean of three technical replicates) obtained with the mutant was divided by that obtained with the wild type to obtain the M/W ratio.
The normalized hybridization signals for those genes between the wild type and mutant are all statistically significantly different as analyzed by Student's t test (P < 0.001).
Genes having >1.5 or <0.66 final M/W ratios were designated as having higher or lower expression in the mutant, respectively.
Genes were detected on the basis of X. fastidiosa Temecula1 genomic sequences at the NCBI website.
Currently annotated as colicin V precursor.
ND, no designation.
DISCUSSION
An X. fastidiosa algU::nptII mutant did not differ in growth rate in vitro compared to the wild type, but it had reduced aggregation and attachment to surfaces, suggesting that algU may play roles in the synthesis of proteins and other molecules which are related to attachment. The algU::nptII mutant had reduced biofilm formation and reduced virulence on grapevine, suggesting that algU may play a role in biofilm and virulence. The mechanism of resistance of grapevines to infection includes the restriction of X. fastidiosa to fewer xylem vessels (15, 41). Decreased vessel-to-vessel movement of cells inside the xylem results in a delay of systemic infection and disease development (19, 25). The populations of the algU::nptII mutant at 25 cm and 50 cm above the inoculation points were reduced compared to those of the wild type (Table 3) but were also significantly smaller at the inoculation points. Thus, we cannot determine from these data specifically whether the algU::nptII mutant had decreased vessel-to-vessel movement inside the xylem. In vitro desiccation stress tolerance in plant pathogens has been correlated with resistance to dehydration in vivo and an increased ability to form microbial biofilms (22, 51). Wild-type X. fastidiosa tolerated desiccation stress for about 2 to 3 days, while the algU::nptII mutant did not tolerate desiccation as well as the wild type. The mechanism of toleration to desiccation related to algU in X. fastidiosa remains to be determined.
AlgU is a member of a family of alternative sigma factors, σE (rpoE), which are only distantly related to σ70 (49, 50). Environmental stresses induce the transcription of algU in P. aeruginosa (52), and virulence or persistence factors are controlled by σE (AlgU) (13). X. fastidiosa is exposed to a range of variable stress factors inside the xylem of plants (1), such as changes in osmolarity, availability of nutrients, and agents generating reactive oxygen intermediates. Gene expression profiles of the algU::nptII mutant of X. fastidiosa compared to those of the wild type via microarray analysis revealed that algU regulates various factors that could contribute to survival under the environmental conditions present in the xylem.
Although several of the P. aeruginosa alginate genes (algA, algD, algG, algF, algI, and algJ) were not found in the X. fastidiosa genome (53), other genes involved in alginate biogenesis in P. aeruginosa, such as mucD (PD1286), algR (PD1153), algH(PD1276), algC(PD0120), and algS (PD0347), are present and had decreased expression in the algU::nptII mutant of X. fastidiosa. In P. aeruginosa, the algC gene encodes a bifunctional enzyme that is involved in alginate production (phosphomannomutase activity) and LPS production (phosphoglucomutase activity) (11). Thus, the function of alginate homolog genes in X. fastidiosa may be involved not in alginate biosynthesis but rather in the synthesis of LPS or another form of EPS, either of which could play a role in biofilm formation and cell attachment. However, the purified LPS profile of the algU::nptII mutant grown in vitro were not significantly altered compared with that of the LPS from the wild type by the assay used. The mechanism by which algU regulates the synthesis of other EPSs or LPS in X. fastidiosa remains to be determined.
Previous studies showed that unique structural components of bacterial cells, such as the cell wall, outer membrane proteins, or actively secreted proteins, may be associated with bacterial pathogenicity or suppressing host defenses (4, 42, 45). In X. fastidiosa, expression of the outer membrane proteins MopB and OmpW appears to be positively regulated by AlgU. Since SecB is also positively regulated by AlgU, the secretion of other proteins by the type II, sec-dependent secretion system may be affected by AlgU.
A single gene that encodes a predicted fimbrial protein (PD1926) was shown to be negatively regulated by algU. PD1926 is located in the gene cluster PD1922 to PD1928 (58), which includes homologs of PilD (PD1922), PilC (PD1923), PilA (PD1924), PilB (PD1927), PilR (PD1928), and PilS (PD1929), which are thought to function in the biogenesis and twitching motility of type IV pili in P. aeruginosa (26, 39). Mutations in pilA, pilB, and pilR of X. fastidiosa resulted in a twitching-minus phenotype (34, 40). It is predicted that PD1926 is a gene involved in the formation or function of type IV pili of X. fastidiosa, but its specific contribution is not known. Since algU appears to negatively regulate PD1926, the mutation in algU might be predicted to enhance its role in the twitching phenotype. We did not measure twitching motility directly, but the algU mutant had reduced populations at distances away from the inoculation points of grapevines, which is not consistent with enhanced motility (40). However, the algU mutant achieved significantly smaller populations in grapevine overall, even at the inoculation points, so the effects on motility were not necessarily apparent in our assays. The algU mutant also had reduced cell-cell aggregation, attachment, and biofilm formation. If PD1926 is involved in the formation of type IV pili and has enhanced expression in the algU mutant, then the observed adherence phenotype of the mutant is consistent with the recent finding that mutants of X. fastidiosa lacking type IV pili had enhanced cell-cell aggregation and biofilm formation, which appears to be primarily the role of the shorter type I pili (34). The long type IV pili may partially mask the adhesion functions of shorter type I pili.
Several genes that encode ribosomal protein subunits were shown to be positively regulated by algU in this study, indicating that algU may also be involved in regulating the normal physiological metabolism of X. fastidiosa. Genes involved in physiological metabolism under stress, such as heat shock protein genes cplS, clpA, clpB, dnaK, grpE, and hspA; iron storage and detoxification gene bfr; and the energy-producing citrate synthase gene gltA, had decreased expression in the algU::nptII mutant. Plant pathogenic bacteria probably regulate heat shock proteins and iron acquisition mechanisms to help them adapt to the harsh environmental conditions present within hosts (59). Cellular homeostasis of iron is essential for preventing iron toxicity in eukaryotes and most prokaryotes. Bacterioferritin is one of three types of ferritin-like proteins in bacteria (55). Bacterioferritin might be involved indirectly in the resistance to redox stress in P. aeruginosa (35). bfr (PD0066) of X. fastidiosa was positively regulated by algU and is predicted to encode a bacterioferritin that may play a role in the acquisition of iron or protection against oxidative stress. In this study, there were no significant differences in sensitivity to oxidative stress between wild-type X. fastidiosa and the algU::nptII mutant, so bfr may be more likely to function in iron acquisition than in oxidative stress in this pathogen.
cvaC (PD0216), which encodes a colicin V precursor protein, was identified as positively regulated by AlgU in X. fastidiosa in this study. The colicin V precursor is an antibacterial polypeptide toxin that acts against closely related sensitive bacteria (23, 54). It was reported that the expression of cvaC was detected in the pathogenic condition but not in the nonpathogenic condition of X. fastidiosa (14). Previous studies showed that there are diverse endophytic bacterial populations inside the xylem of plants (3, 9); thus, it is predicted that successful colonization of xylem of grapevine by X. fastidiosa may depend on the ability of X. fastidiosa to compete with other indigenous microbes for essential nutrients (2). AlgU may play a role such competition in X. fastidiosa by regulation of cvaC.
RsmA is a homolog of CsrA, which is an RNA-binding protein of Escherichia coli. Mutation of csrA in E. coli resulted in enhanced biofilm formation (29). In a previous study, an rsmA mutant of X. fastidiosa also formed more biofilm compared with the wild type (8), suggesting that RsmA represses biofilm formation. RsmB is an untranslated RNA molecule that antagonizes RsmA activity in E. coli (36). rsmB (PD1761) is present in X. fastidiosa Temecula1 (53); thus, there may be the RsmA/RsmB posttranscriptional regulation system in X. fastidiosa. In this study, DNA microarray analysis showed that the expression of rsmA and another gene, hfq, that encodes an RNA-binding protein was lower in the algU::nptII mutant than in the wild type, indicating that rsmA and hfq are positively regulated by the alternative sigma factor AlgU in X. fastidiosa. A decline in RsmA expression is expected to exert a positive effect on the production of biofilm, but the algU::nptII mutant had a reduced ability to form biofilm.
Hfq, also called host factor I, is an abundant RNA-binding protein and can be involved in the translational regulation of target mRNAs by regulating the stability of RNAs (36). In P. aeruginosa PAO1, Hfq may indirectly affect quorum sensing (QS) and biofilm formation by regulating the RsmA/RsmY system (6, 56). Hfq binds to and stabilizes RsmY RNA. The stabilized RsmY RNA then binds to and inactivates RsmA, which would release the negative effect of RsmA on the expression of QS and biofilm formation (56). The absence of Hfq affects the expression of QS and biofilm formation in the reverse way (56).
RsmA (PD1208), RsmB (PD1761), and Hfq (PD0066) are present in X. fastidiosa Temecula1 (53). In this study, the expression of rsmA and hfq was lower in the algU::nptII mutant, while the expression of rsmB was not significantly different. Hfq may be involved in regulating the RsmA/RsmB system of X. fastidiosa, as in the RsmA/RsmY system of P. aeruginosa. Mutation of algU caused lower expression of hfq, which would result in reduced stabilization of rsmB RNA and a lack of inactivation of RsmA by Hfq-stabilized rsmB RNA. There would be a lower level of RsmA in the algU::nptII mutant, but it could be more active than if Hfq were expressed at normal levels. This could help to explain the decreased biofilm formation in the algU::nptII mutant. Thus, it is predicted that the biofilm formation in X. fastidiosa is regulated by algU through a complex Hfq/rsmB/rsmA-mediated system.
The X. fastidiosa algU::nptII mutant was affected in the expression of many physiological metabolism genes, acid resistance genes, and membrane-permeating protein genes, which may contribute to maintaining normal physiological metabolism and adaptation to the poor nutrient conditions of the xylem. The specific roles of these genes in xylem colonization should be investigated through mutagenesis and functional assays.
Supplementary Material
Acknowledgments
This project was supported by grants from the California Department of Food and Agriculture (CDFA) and the University of California Agriculture Experiment Station.
Footnotes
Published ahead of print on 7 September 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alves, G., T. Ameglio, A. Guilliot, P. Fleurat-Lessard, A. Lacointe, S. Sakr, G. Petel, and J.-L. Julien. 2004. Winter variation in xylem sap pH of walnut trees: involvement of plasma membrane H+-ATPase of vessel-associated cells. Tree Physiol. 24:99-105. [DOI] [PubMed] [Google Scholar]
- 2.Araújo, W. L., J. Marcon, W. Maccheroni, Jr., J. D. van Elsas, J. W. L. van Vuurde, and J. L. Azevedo. 2002. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl. Environ. Microbiol. 68:4906-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell, C. R., G. A. Dickie, W. L. G. Harvey, and J. Chan. 1995. Endophytic bacteria in grapevine. Can. J. Microbiol. 41:46-53. [Google Scholar]
- 4.Bruening, G., E. L. Civerolo, Y. Lee, J. M. Buzayan, P. A. Feldstein, and E. Re. 2005. A major outer membrane protein of Xylella fastidiosa induces chlorosis in Chenopodium quinoa. Phytopathology 95:S14. [Google Scholar]
- 5.Burdman, S., E. Jurkevitch, M. E. Soria-Diaz, A. M. G. Serrano, and Y. Okon. 2000. Extracellular polysaccharide composition of Azospirillum brasilense and its relation with cell aggregation. FEMS Microbiol. Lett. 189:259-264. [DOI] [PubMed] [Google Scholar]
- 6.Burrowes, E., C. Baysse, C. Adams, and F. O'Gara. 2006. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152:405-418. [DOI] [PubMed] [Google Scholar]
- 7.Chuang, S. E., D. L. Daniels, and F. R. Blattner. 1993. Global regulation of gene expression in Escherichia coli. J. Bacteriol. 175:2026-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooksey, D. A. 2004. DNA microarray and mutational analysis to identify virulence genes in Xylella fastidiosa, p. 174-177. In Proceedings of the 2004 Pierce's Disease Research Symposium. California Department of Food and Agriculture, San Diego.
- 9.Cooksey, D. A., and J. Borneman. 2005. Culture-independent analysis of endophytic microbial communities in grapevine in relation to Pierce's disease, p. 155-158. In Proceedings of the 2005 Pierce's Disease Research Symposium. California Department of Food and Agriculture, San Diego.
- 10.Costa, H. S., E. Raetz, T. R. Pinckard, C. Gispert, R. Hernandez-Martinez, C. K. Dumenyo, and D. A. Cooksey. 2004. Plant hosts of Xylella fastidiosa in and near southern California vineyards. Plant Dis. 88:1255-1261. [DOI] [PubMed] [Google Scholar]
- 11.Coyne, M. J., K. S. Russell, C. L. Coyle, and J. B. Goldberg. 1994. The Pseudomonas aeruginosa algC gene encodes phosphoglucomutase, required for the synthesis of a complete lipopolysaccharide core. J. Bacteriol. 176:3500-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, M. J., W. J. French, and N. W. Schaad. 1981. Axenic culture of the bacteria associated with phony peach disease of peach and plum leaf scald. Curr. Microbiol. 6:309-314. [Google Scholar]
- 13.Deretic, V., M. J. Schurr, J. C. Boucher, and D. W. Martin. 1994. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J. Bacteriol. 176:2773-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza, A. A., M. A. Takita, E. O. Pereira, H. D. Coletta, and M. A. Machado. 2005. Expression of pathogenicity-related genes of Xylella fastidiosa in vitro and in planta. Curr. Microbiol. 50:223-228. [DOI] [PubMed] [Google Scholar]
- 15.Feil, H., W. S. Feil, and A. H. Purcell. 2003. Effects of date of inoculation on the within-plant movement of Xylella fastidiosa and persistence of Pierce's disease within field grapevines. Phytopathology 93:244-251. [DOI] [PubMed] [Google Scholar]
- 16.Gilchrist, D., and J. E. Lincoln. 2006. Resistance to Pierce's disease by transgenic expression of plant-derived anti-apoptotic genes, p. 272-275. In Proceedings of the 2006 Pierce's Disease Research Symposium. California Department of Food and Agriculture, San Diego.
- 17.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilhabert, M. R., L. M. Hoffman, D. A. Mills, and B. C. Kirkpatrick. 2001. Transposon mutagenesis of Xylella fastidiosa by electroporation of Tn5 synaptic complexes. Mol. Plant-Microbe Interact. 14:701-706. [DOI] [PubMed] [Google Scholar]
- 19.Guilhabert, M. R., and B. C. Kirkpatrick. 2005. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to Xylella fastidiosa biofilm maturation and colonization and attenuate virulence. Mol. Plant-Microbe Interact. 18:856-868. [DOI] [PubMed] [Google Scholar]
- 20.Guilhabert, M. R., and B. C. Kirkpatrick. 2003. Transformation of Xylella fastidiosa with broad host range RSF1010 derivative plasmids. Mol. Plant Pathol. 4:279-285. [DOI] [PubMed] [Google Scholar]
- 21.Gusnanto, A., A. Ploner, and Y. Pawitan. 2005. Fold-change estimation of differentially expressed genes using mixture mixed-model. Stat. Appl. Genet. Mol. Biol. 4:Article 26. [DOI] [PubMed] [Google Scholar]
- 22.Habermann, G., E. C. Machado, J. D. Rodrigues, and C. L. Medina. 2003. CO2 assimilation, photosynthetic light response curves, and water relations of ‘Pera’ sweet orange plants infected with Xylella fastidiosa. Braz. J. Plant Physiol. 15:79-87. [Google Scholar]
- 23.Håvarstein, L. S., H. Holo, and I. F. Nes. 1994. The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by gram-positive bacteria. Microbiology 140:2383-2389. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Martinez, R., H. S. Costa, C. K. Dumenyo, and D. A. Cooksey. 2006. Differentiation of strains of Xylella fastidiosa infecting grape, almonds and oleander using a multiprimer PCR assay. Plant Dis. 90:1382-1388. [DOI] [PubMed] [Google Scholar]
- 25.Hill, B. L., and A. H. Purcell. 1995. Multiplication and movement of Xylella fastidiosa within grapevine and four other plants. Phytopathology 85:1368-1372. [Google Scholar]
- 26.Hobbs, M., E. S. R. Collie, P. D. Free, S. P. Livingston, and J. S. Mattick. 1993. PilS and PilR, a two-component transcriptional regulatory system controlling expression of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 72:669-682. [DOI] [PubMed] [Google Scholar]
- 27.Huang, Q., and J. L. Sherald. 2004. Isolation and phylogenetic analysis of Xylella fastidiosa from its invasive alternative host, porcelain berry. Curr. Microbiol. 48:73-76. [DOI] [PubMed] [Google Scholar]
- 28.Inzana, T. J., and M. A. Apicella. 1999. Use of a bilayer stacking gel to improve resolution of lipopolysaccharides and lipooligosaccharides in polyacrylamide gels. Electrophoresis 20:462-465. [DOI] [PubMed] [Google Scholar]
- 29.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keith, R. C., L. M. W. Keith, G. Hernandez-Guzman, S. R. Uppalapati, and C. L. Bender. 2003. Alginate gene expression by Pseudomonas syringae pv. tomato DC3000 in host and non-host plants. Microbiology 149:1127-1138. [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick, B., E. A. Weber, P. C. Andersen, A. H. Purcell, and M. A. Walker. 2001. Biological, cultural, and chemical management of Pierce's disease, p. 52-57. In Proceedings of the 2001 Pierce's Disease Research Symposium. California Department of Food and Agriculture, San Diego.
- 32.Kustu, S., E. Santero, J. Keener, D. Popham, and D. Weiss. 1989. Expression of σ54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol. Rev. 53:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leite, B., P. C. Andersen, and M. L. Ishida. 2004. Colony aggregation and biofilm formation in xylem chemistry-based media for Xylella fastidiosa. FEMS Microbiol. Lett. 230:283-290. [DOI] [PubMed] [Google Scholar]
- 34.Li, Y., G. Hao, C. D. Galvani, Y. Meng, L. De la Fuente, H. C. Hoch, and T. J. Burr. 2007. Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell-cell aggregation. Microbiology 153:719-726. [DOI] [PubMed] [Google Scholar]
- 35.Ma, J. F., U. A. Ochsner, M. G. Klotz, V. K. Nanayakkara, M. L. Howell, Z. Johnson, J. E. Posey, M. L. Vasil, J. J. Monaco, and D. J. Hassett. 1999. Bacterioferritin A modulates catalase A (KatA) activity and resistance to hydrogen peroxide in Pseudomonas aeruginosa. J. Bacteriol. 181:3730-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majdalani, N., C. K. Vanderpool, and S. Gottesman. 2005. Bacterial small RNA regulators. Crit. Rev. Biochem. Mol. Biol. 40:93-113. [DOI] [PubMed] [Google Scholar]
- 37.Marques, L. L. R., G. P. Manfio, D. M. Reid, H. Ceri, and M. E. Olson. 2001. Xylella fastidiosa are biofilm-forming phytopathogenic bacteria. Phytopathology 91:S58. [Google Scholar]
- 38.Martin, D. W., M. J. Schurr, H. Yu, and V. Deretic. 1994. Analysis of promoters controlled by the putative sigma factor AlgU regulating conversion to mucoidy in Pseudomonas aeruginosa: relationship to σE and stress response. J. Bacteriol. 176:6688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289-314. [DOI] [PubMed] [Google Scholar]
- 40.Meng, Y., Y. Li, C. D. Galvani, G. Hao, J. N. Turner, T. J. Burr, and H. C. Hoch. 2005. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 187:5560-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mollenhauer, H. H., and D. L. Hopkins. 1976. Xylem morphology of Pierce's disease-infected grapevines with different levels of tolerance. Physiol. Plant Pathol. 9:95-100. [Google Scholar]
- 42.Nandi, B., R. K. Nandy, S. Mukhopadhyay, G. B. Nair, T. Shimada, and A. C. Ghose. 2000. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J. Clin. Microbiol. 38:4145-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ophir, T., and D. L. Gutnick. 1994. A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl. Environ. Microbiol. 60:740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osiro, D., L. A. Colnago, A. M. Otoboni, E. G. Lemos, A. A. de Souza, H. D. Coletta Filho, and M. A. Machado. 2004. A kinetic model for Xylella fastidiosa adhesion, biofilm formation, and virulence. FEMS Microbiol. Lett. 236:313-318. [DOI] [PubMed] [Google Scholar]
- 45.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purcell, A. H. 1997. Xylella fastidiosa, a regional problem or global threat? J. Plant Pathol. 79:99-105. [Google Scholar]
- 47.Purcell, A. H., and D. L. Hopkins. 1996. Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 34:131-151. [DOI] [PubMed] [Google Scholar]
- 48.Purcell, A. H., and S. R. Saunders. 1999. Fate of Pierce's disease strains of Xylella fastidiosa in common riparian plants in California. Plant Dis. 83:825-830. [DOI] [PubMed] [Google Scholar]
- 49.Raina, S., D. Missiakas, and C. Georgopoulos. 1995. The rpoE gene encoding the σE (σ24) heat shock sigma factor of Escherichia coli. EMBO J. 14:1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouvière, P. E., A. De Las Penas, J. Mecsas, C. Z. Lu, K. E. Rudd, and C. A. Gross. 1995. RpoE, the gene encoding the second heat-shock sigma factor, σE, in Escherichia coli. EMBO J. 14:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnider-Keel, U., K. B. Lejbolle, E. Baehler, D. Haas, and C. Keel. 2001. The sigma factor AlgU (AlgT) controls exopolysaccharide production and tolerance towards desiccation and osmotic stress in the biocontrol agent Pseudomonas fluorescens CHA0. Appl. Environ. Microbiol. 67:5683-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schurrr, M. J., H. Yu, J. C. Boucher, N. S. Hibler, and V. Deretic. 1995. Multiple promoters and induction by heat shock of the gene encoding the alternative sigma factor AlgU (σE) which controls mucoidy in cystic fibrosis isolates of Pseudomonas aeruginosa. J. Bacteriol. 177:5670-5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson, A. J. G., F. C. Reinach, P. Arruda, et al. 2000. The genome sequence of the plant pathogen Xylella fastidiosa. Nature 406:151-157. [DOI] [PubMed] [Google Scholar]
- 54.Skvirsky, R. C., S. Reginald, and X. Shen. 1995. Topology analysis of the colicin V export protein CvaA in Escherichia coli. J. Bacteriol. 177:6153-6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith, J. L. 2004. The physiological role of ferritin-like compounds in bacteria. Crit. Rev. Microbiol. 30:173-185. [DOI] [PubMed] [Google Scholar]
- 56.Sonnleitner, E., M. Schuster, T. Sorger-Domenigg, E. P. Greenberg, and U. Blaesi. 2006. Hfq-dependent alterations of the transcriptome profile and effects on quorum sensing in Pseudomonas aeruginosa. Mol. Microbiol. 59:1542-1558. [DOI] [PubMed] [Google Scholar]
- 57.Tyson, G. E., B. J. Stojanovic, R. F. Kuklinski, T. J. Divittorio, and M. L. Sullivan. 1985. Scanning electron microscopy of Pierce's disease bacterium in petiolar xylem of grape leaves. Phytopathology 75:264-269. [Google Scholar]
- 58.Van Sluys, M. A., M. C. de Oliveira, C. B. Monteiro-Vitorello, C. Y. Miyaki, L. R. Furlan, et al. 2003. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 185:1018-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vierke, G., A. Engelmann, C. Hebbeln, and M. Thomm. 2003. A novel archaeal transcriptional regulator of heat shock response. J. Biol. Chem. 278:18-26. [DOI] [PubMed] [Google Scholar]
- 60.Yu, J., A. Penaloza-Vazquez, A. M. Chakrabarty, and C. L. Bender. 1999. Involvement of the exopolysaccharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Mol. Microbiol. 33:712-720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.