Abstract
A new method of respiration rate measurement based on oxygen luminescence quenching in sensor spots was evaluated for the first time for aquatic bacterial communities. The commonly used Winkler and Clark electrode methods to quantify oxygen concentration both require long incubation times, and the latter additionally causes signal drift due to oxygen consumption at the cathode. The sensor spots proved to be advantageous over those methods in terms of precise and quick oxygen measurements in natural bacterial communities, guaranteeing a respiration rate estimate during a time interval short enough to neglect variations in organism composition, abundance, and activity. Furthermore, no signal drift occurs during measurements, and respiration rate measurements are reliable even at low temperatures and low oxygen consumption rates. Both a natural bacterioplankton sample and a bacterial isolate from a eutrophic river were evaluated in order to optimize the new method for aquatic microorganisms. A minimum abundance of 2.2 × 106 respiring cells ml−1 of a bacterial isolate was sufficient to obtain a distinct oxygen depletion signal within 20 min at 20°C with the new oxygen sensor spot method. Thus, a culture of a bacterial isolate from a eutrophic river (OW 144; 20 × 106 respiring bacteria ml−1) decreased the oxygen saturation about 8% within 20 min. The natural bacterioplankton sample respired 2.8% from initially 94% oxygen-saturated water in 30 min. During the growth season in 2005, the planktonic community of a eutrophic river consumed between 0.7 and 15.6 μmol O2 liter−1 h−1. The contribution of bacterial respiration to the total plankton community oxygen consumption varied seasonally between 11 and 100%.
Bacteria play a key role in aquatic ecosystems (2). Using hydrolytic enzymes (28), they decompose organic material, e.g., dead phytoplankton and allochthonous particles, to oligo- and monomeric substances. These low-molecular-weight substrates can be taken up and mineralized to plant nutrients and carbon dioxide (49). Therefore, the influence of microbial activities on biogeochemical processes, in particular on the carbon cycle, is highly significant (10, 13, 27, 39).
In aquatic food webs, bacteria consume 20 to 80% of the carbon provided by phytoplankton primary production (43) as well as from other organic carbon sources, e.g., periphyton (44), macrophyte production, and allochthonous matter (50), and probably respire the main portion of the consumed carbon (2). Bacterial and plankton respiration rates are essential for calculating carbon budgets of aquatic ecosystems. Carbon budgets, in turn, are important for estimating organic matter export to and accumulation in sediments or the enrichment of the atmosphere with carbon dioxide (3, 7). An intense discussion is ongoing concerning the role of pelagic systems, in particular the world's oceans, in the global C budget. Whether they act more as sources of CO2 by respiration or more as carbon sinks by channeling organic carbon as bacterial biomass into food webs has not yet been resolved (9, 53). However, detailed carbon budgets are rare because, in many cases, bacterial respiration (BR) has not been measured directly (9), and if so, then it was done with long incubation times. Respiration of bacterioplankton alone is typically derived from bacterial production and bacterial growth efficiency (BGE) measurements, for which only a very few estimates exist (9). However, total community respiration and BR alone are both necessary in order to evaluate heterotrophic mineralization versus metabolic costs of autotrophic primary production. This relationship will give an estimation of the dominant carbon pathways in different aquatic ecosystems.
In their review on BGE, del Giorgio and Cole (8) reported BGEs varying from 1 to 80% and concluded that greater numbers and more precise estimations of BR rates are needed. Understanding the BGE, its regulation by environmental factors, and species-specific differences is largely limited by the scarcity and uncertainty of BR measurements. Methodological problems may have contributed to this lack of reliable data. Conventional methods to measure BR, e.g., those of Winkler (54) and Clark (6), are still widely used and have been enhanced for specific applicability in different habitats over the last years (e.g., see references 5, 23, 35, 37, and 38). However, the application of both standard techniques is limited due to several disadvantages. At low temperatures, oxygen saturation is very high, and small changes in oxygen concentration may be below the detection limit of electrodes. Thus, samples have to be incubated for many hours or even days, causing changes in community composition (19). Clark-type electrodes may drift during the measurements and consume oxygen. Furthermore, a constant flow of the sample against the electrode is essential to get a steady signal from the Clark electrode. However, this treatment may influence oxygen sediment profiles, harm cells, or disrupt the electrode's membrane by mechanical exposure to particles. In addition, long-term storage of electrodes may exacerbate their lack of reliability and cause damage (18).
Therefore, alternative methods are needed to sensitively measure oxygen concentrations without the potential problems outlined above. A promising approach is a new type of optical electrodes, the so-called opt(r)odes, introduced into microbial research by Klimant et al. (29). Optodes for oxygen, temperature, or pH profiles were successfully applied to invertebrates, e.g., sponges (18), to plant tissue, to sediments, and even to ice formation (16, 20, 32). The main advantages of optodes are that (i) they are used in noninvasive systems, (ii) no oxygen is consumed by the optode itself, (iii) measurements are possible over a temperature range wider than that for classical methods, (iv) short incubation times are sufficient to obtain reliable data, and (v) no mechanical stress, i.e., constant stirring of the samples, is imposed on bacteria.
The main aim of the present study was to estimate respiration rates of natural plankton samples in closed systems with volumes of only a few milliliters as rapidly as possible to minimize changes in community composition during measurements. Therefore, planar optodes, so-called sensor spots, were placed into a 3.5-ml temperature-controlled cuvette. First, respiration rates of a freshwater bacterial isolate were determined in order to evaluate the linearity of oxygen consumption with the lowest possible cell number. This could be assessed within 1 hour. The applicability, sensitivity, and handling of the sensor spot method were tested for natural bacterioplankton samples. Finally, total planktonic community respiration and BR rates were monitored over one season for a eutrophic river. This was done in an attempt to explain the low percentage of respiring bacteria despite a sufficient nutrient and organic substrate supply (14). These data help to investigate the role of bacteria in the eutrophic River Warnow and, more specifically, to investigate if and to what extent they mineralize the phytoplankton biomass and autochthonous and allochthonous organic matter.
MATERIALS AND METHODS
Study site and sampling.
The River Warnow is a slowly running lowland river in Mecklenburg-Vorpommern, Northeastern Germany. The Warnow drains a catchment area (3,224 km2) of predominantly agricultural land as well as many peat bogs and flows through several eutrophic lakes. Therefore, the river is heavily loaded with nutrients and dissolved and particulate organic matter (14). Although these conditions are favorable for highly active bacterioplankton to occur, the majority of bacteria were in fact inactive using the cell-specific respiration marker 5-cyano-2,3-ditolyltetrazolium chloride (CTC) (15).
Water samples were taken every 3 weeks in spring and early summer, 1 km upstream of the river's estuary. Samples of 10 liters were obtained with a polyethylene bucket from the upper 50 cm of the <2-m water column and transported to the laboratory within 30 min. Subsamples were stored at the in situ temperature in darkness until they were used for respiration rate measurements. Total community respiration (including that of heterotrophic and autotrophic pro- and eukaryotes) was measured three times at the in situ temperature for at least 50 min, and BR was estimated for three 1-μm filtrates (gauze; Hydrobios, Kiel, Germany). For complete comparability, measurements of both fractions were done in darkness to avoid algal photosynthesis in the whole-plankton sample.
If both total community respiration and BR did not decrease significantly, the samples were concentrated by filtration. Two-hundred-milliliter samples were filtered onto a RoTrac membrane filter (0.1 μm; Oxyphen GmbH, Germany), followed by resuspension in 10 ml original or 1 μm filtered biotope water. All replicates were derived from separately filtered and concentrated samples. Subsamples of 1 ml were preserved and stored for later counting (see below).
Respiration of a bacterial isolate.
The bacterial strain OW 144 was isolated in January 2004 from River Warnow bacterioplankton by using medium 1 (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ], Braunschweig, Germany). OW 144 is an aerobic, gram-negative, rod-shaped bacterium similar to Rhanella aquatilis m 46 (1,398 nucleotides; 99% 16S rRNA gene sequence similarity) (GenBank accession no. AY253921). This strain uses >20 monomer substrates, including glucose, fructose, alanine, and glutamic acid. It was cultivated in a minimal medium (medium 81 [DSMZ] modified with 5 mg liter−1 iron citrate), with 0.5% (mass/vol) glucose as the sole carbon source, at 20°C with continuous agitation. This isolate exhibited a growth rate of 0.19 h−1, with an exponential phase of 8 h. All cells were harvested and measured during exponential growth to guarantee high respiration rates, as shown by CTC-derived formazan crystal formation (Polysciences, Inc., method [40]).
To estimate the lowest possible bacterial abundance yielding a measurable respiration rate within 1 h, an exponentially growing culture of OW 144 was washed three times with sterile medium (6,000 rpm, 5 min, 20°C). This was followed by dilution in a geometrical series with minimal medium (plus 0.5% glucose), resulting in 10 defined subsamples of different cell numbers. The experiment was repeated three times, starting from growing cultures. Subsamples of 450 μl were preserved and stored for later counting (see below). Respiration was measured for at least 30 min at 20°C (see below). Constancy of the cell-specific respiration rate was checked for every single measurement, i.e., for all abundances.
The effects of different substrate concentrations on the OW 144 respiration rate were also determined. Cells were harvested during the exponential phase, centrifuged (6,000 rpm, 5 min, 20°C), and washed three times with a carbon-free salt medium based on 5 mM Tris buffer (31). The bacterial suspension was diluted to ca. 20 × 106 cells ml−1. Glucose (Roth) was added to result in final concentrations of 0, 50, and 200 μM. Respiration was measured in the starved (0 μM) and glucose-enriched samples for at least 30 min at 20°C.
Statistical analyses were performed with one-way analysis of variance to test for significant differences between treatments (P < 0.05) and for linear correlations, using SigmaStat, version 3.11 (Jandel Scientific). Since the most important prerequisite for oxygen consumption measurements is linear uptake with time (52), all samples without a significant linear uptake of oxygen were excluded from the regression analysis. These were mostly pure isolate cultures with abundances above 150 × 106 cells ml−1.
Bacterial numbers.
Bacterial isolates were fixed with formaldehyde at a final concentration of 3.7%, and natural samples were fixed at a final concentration of 1.85%. Suspensions of the bacterial isolate were counted in a Bürker chamber (depth, 0.1 mm), using an Olympus BH-2 (Plan Apo UV 400×; 0.85 numerical aperture) microscope. Cell suspensions were transferred to the chamber and investigated after a sedimentation time of 5 to 15 min. A minimum of 400 cells per chamber and three replicated subsamples were counted. The standard error of triplicates was <15%.
Bacteria from natural communities were filtered in triplicate onto Irgalan black-stained 0.2-μm Isopore polycarbonate membranes (Sigma Aldrich Co.) and stained with 4′,6′-diamidino-2-phenylindole (DAPI) (29 μM in phosphate buffer, pH 7.6; Roth, Karlsruhe, Germany) (36). Filters were embedded in immersion oil (Olympus) and mounted on slides, and >400 cells per filter were counted under an epifluorescence microscope at a magnification of ×1,000 (Olympus BX51; UV excitation filter U-MWU2). The standard error of triplicates was <9%. Respiring bacteria were estimated directly in unfixed subsamples and incubated with CTC at a final concentration of 5 mM for >4 h at in situ temperature. Bacteria were counted under an epifluorescence microscope at a magnification of ×1,000 (Olympus BX51; blue excitation filter U-MWB2). The standard error of triplicates was <10%.
Respiration rates.
Oxygen concentrations in the respiration experiment were measured with SP-PSt3-PSUP-YOP-D5 oxygen sensor spots (PreSens GmbH, Regensburg, Germany) (for further specifications, such as measuring range and reproducibility, see Table 1). Molecular oxygen quenches the luminescence of the inert metal porphyrine complex immobilized in an oxygen-permeable matrix. This process guarantees a high temporal resolution and a measurement without drift, oxygen consumption, or gas exchange between the incubation chamber and the environment.
TABLE 1.
Technical specifications for PSt3 oxygen sensors and the FIBOX 3 system (PreSens GmbH)
| Parameter | Value |
|---|---|
| Measuring range | |
| mg O2 liter−1 | 0-45 |
| mmol O2 | 0-1.4 |
| Resolution (μmol O2) | |
| 1% ± 0.05% air saturation | 2.83 ± 0.14 |
| 30% ± 0.1% air saturation | 85.0 ± 0.28 |
| 100% ± 0.5% air saturation | 283.1 ± 1.40 |
| Accuracy (% air saturation at 20°C) | ±1 |
| Limit of detection (% air saturation) | 0.15 (0.01 mg O2 liter−1) |
| Response time (s)a | |
| Gas phase | <10 |
| Aqueous solution (with optical | |
| isolation, not stirred) | <60 |
| Temperature range (°C) | −10 to +50 |
The response times given are related to the time intervals when 90% of the signal change had occurred.
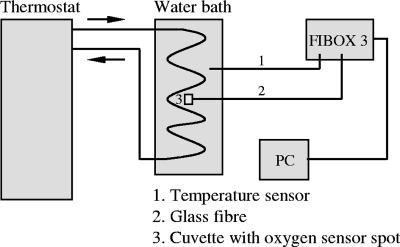
These sensor spots, also called planar optodes, were attached to the inner wall of a cuvette (3.5 ml, optical glass, autoclavable, and resistant to chemicals; Roth) with silicone glue. The cuvette was closed airtight by a polytetrafluorethylene cap. The photoluminescence lifetime of the luminophore within the sensor spot was measured by a fiber-optic oxygen meter (Fibox 3; PreSens GmbH) fixed to the outside of the cuvette opposite to the inside sensor spot. Excitation light (505 nm) was supplied by a glass fiber, which also transported the emitted fluorescence signal (>600 nm) back to the oxygen meter. Data were recorded using OxyView 3.51 software (PreSens GmbH). The sensor spot has a diameter of 0.5 cm and consists of an oxygen-permeable foil, in which the chemical luminescence reaction takes place, covered with an optical isolation layer against all ambient light. This layer prevents any chlorophyll excitation if a plankton sample is measured, which may otherwise lead to wrong oxygen values. The cuvette containing the sample was placed into a temperature-controlled water bath (±0.1 K) (DC10 and K10; Thermo Haake). A technical diagram of the equipment and setup is shown in Fig. 1.
FIG. 1.
Technical setup of oxygen measurement method.
The PreSens system was calibrated with a two-point calibration before each experiment. First, 0% oxygen saturation was defined by adding sodium dithionite to distilled water. Distilled water was then aerated for 20 min with ambient air and stirred for another 20 min to avoid oversaturation. Thus prepared, it was used to calibrate 100% oxygen saturation. Respiration data are expressed as oxygen concentrations (μmol O2 liter−1 h−1) or decreases of oxygen saturation (% unit of time−1). The unit describing the loss of saturation percentages is always normalized to a 100% physical saturation at the given temperature, although samples may have deviating initial saturations caused by previous respiration (87 to 111% for isolates) and photosynthesis (86 to 113% for plankton samples).
RESULTS
Method optimization and system check.
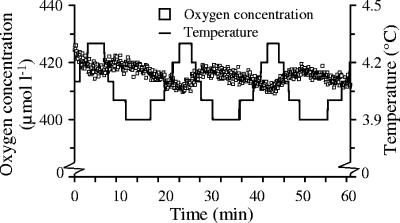
Temperature-induced changes in oxygen saturation will interfere with any respiration rate measurement of environmental samples. Thus, temperature control is essential when using optodes. In early experiments, oxygen concentrations changed by up to 10 μmol liter−1 (approximately ±2% oxygen saturation of 100% saturated sterile distilled water at 4°C) when the temperature varied about 0.5 K during measurements (Fig. 2). The fast-reacting temperature sensor and a respective mathematical correction for the temperature dependence of oxygen saturation obviously resulted in the observed oscillating oxygen concentrations at the on and off switches of the thermostat's heating unit. The response time of the sensor spot is dependent on its type, predominantly its matrix, and the type must be chosen carefully. However, the type used here is suitable for a broad temperature range and also works near the freezing point. This signal oscillation problem can be avoided by very tight temperature control (±0.1 K).
FIG. 2.
Oxygen concentration changes between 410 and 430 μmol liter−1 at fluctuating temperatures (4.1 ± 0.2°C) measured in sterile distilled water for 60 min. An oxygen concentration of 420 μmol liter−1 is equivalent to 103% oxygen saturation. Saturation ranged from 101 to 104%.
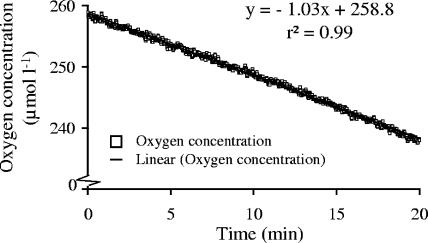
In sterile medium at 20°C, the oxygen concentration remained constant over at least 30 min, with an average of 278 μmol liter−1 ± 0.2% (Fig. 3). This mean is equivalent to 99% oxygen saturation. In comparison to the measured results, the oxygen concentration would have decreased by 125 μmol O2 liter−1 when the temperature increased from 4°C up to 20°C due to the lower solubility of oxygen at higher temperatures. In most repeated control experiments without organisms, oxygen concentrations varied only about 0.3% ± 0.2%, without any trend or drift. However, a steep decrease in oxygen concentration of >60 μmol liter−1 within the first 10 min of measurement occurred in enclosed control samples in several experiments. This may have been attributed to insufficient temperature adaptation of the samples. On the other hand, some physical properties of the sensor spot, which is already a fast-responding specimen, could have contributed to this phenomenon, but the dominating problems could not be singled out so far because they occurred only in field samples. Therefore, the initial 10-min section of the slope was excluded from the calculation when such a nonlinear decrease was observed.
FIG. 3.
Changes in oxygen concentration (μmol liter−1) in sterile minimal medium at 20.3°C as a function of time (min). The intersection point with the y axis was 278.5 μmol O2 liter−1, which is equivalent to 99% oxygen saturation at the given temperature.
Lowest abundance necessary for short-term respiration rate measurements.
Respiration rates of strain OW 144 were measured for 20 min at 20°C, with cell numbers ranging from 1 × 106 to 484 × 106 bacteria ml−1. Low abundances of <2 × 106 bacteria ml−1 did not result in any detectable oxygen decrease (data not shown). Very high bacterial abundances of >150 × 106 bacteria ml−1 led to a nonlinear oxygen decrease during the incubation time (data not shown).
In samples with approximately 20 × 106 respiring bacteria ml−1, the oxygen concentration decreased significantly and linearly (r2 = 0.99; P < 0.001), by 61.5 μmol O2 liter−1 h−1 (Fig. 4), which equals an 8% reduction within 20 min. Assuming no changes in cell number, bacteria at this density would have completely consumed the added carbon (0.5% glucose = 0.4 mM) within ca. 6.5 h. Considering a doubling time of 3.2 h, the exponential growth phase would last only 5.8 h, a time well within the standard incubation protocols for community respiration rate measurements.
FIG. 4.
Oxygen consumption (μmol liter−1) of strain OW 144 (16 × 106 cells ml−1) in minimal medium (0.5% glucose) within 20 min at 20°C.
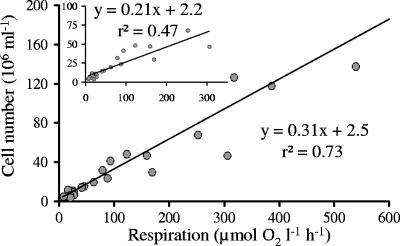
Bacterial numbers correlated significantly with the respiration rates of the samples (P < 0.001; r2 = 0.73; n = 10). Within short incubation times (<30 min), BR is theoretically detectable at an abundance of 2.2 × 106 bacteria ml−1 (Fig. 5, inset; see intersection point with the y axis). Cell-specific respiration at all dilution steps averaged 2.4 to 4 fmol O2 cell−1 h−1. For the investigation of bacterial strains, abundances should be below 150 × 106 cells ml−1 to avoid carbon limitation resulting in a nonlinear oxygen decrease. About 20 × 106 cells ml−1 are recommended, as this number is sufficient to measure a significant oxygen decrease within several minutes.
FIG. 5.
Respiration rate (μmol O2 liter−1 h−1) of a geometrically diluted bacterial culture (OW 144) cultivated in minimal medium (0.5% glucose) at 20°C (P < 0.001; r2 = 0.73). The insert shows the respiration rate of OW 144 at abundances below 100 × 106 cells ml−1 (P < 0.001; r2 = 0.47).
Substrate supply.
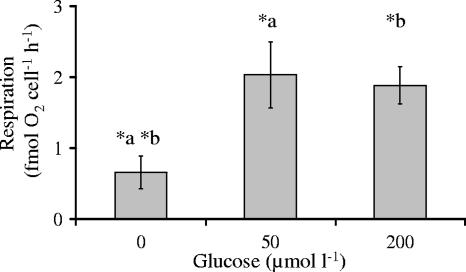
Natural assemblages often live under suboptimal and fast-changing conditions. Therefore, as an example, changes of substrate concentrations were investigated with respect to respiration rates. Bacteria without glucose as an external carbon source showed a low cell-specific respiration rate of 0.7 ± 0.2 fmol O2 cell−1 h−1 after approximately 1 h of starvation (Fig. 6). Incubation at external glucose concentrations of 50 and 200 μM resulted in significantly higher respiration rates, of 2 and 1.9 fmol O2 cell−1 h−1, respectively (t test; P < 0.01). Compared to these, at the standard substrate concentration of 400 μM (see above), respiration was slightly but not significantly enhanced. Thus, the maximum per-cell respiration rate seemed to be reached at 4 fmol O2 cell−1 h−1 for glucose.
FIG. 6.
Cell-specific respiration (fmol O2 cell−1 h−1) of isolate OW 144 at different glucose concentrations (μmol liter−1) at 20°C. Significant differences were observed between 0 μM glucose and 50 μM (*a) or 200 μM (*b) glucose (P < 0.01).
Respiration of natural communities.
The oxygen saturation in plankton from the River Warnow (sampled on 19 July 2005) in the warm summer of 2005 decreased 2.8%, from an initial value of 94%, within 30 min (Fig. 7). The total community respired 15.6 ± 2.3 μmol O2 liter−1 h−1, which is a significant decrease in oxygen. The bacterial fraction respired 15.6 ± 5.5 μmol O2 liter−1 h−1 and, hence, was responsible for 100% of planktonic community respiration. Bacterial abundance was 20.2 × 106 ± 1.9 × 106 ml−1, but only 9% (1.8 × 106 ± 0.1 × 106 ml−1) of bacteria were detected as respiring cells by the fluorescent dye CTC. There was no correlation between BR and the abundance of respiring bacteria (P = 0.55; rs = 0.25).
FIG. 7.
Oxygen concentration (μmol liter−1) of a bacterioplankton sample (20.2 × 106 ± 1.9 × 106 total cells and 1.8 × 106 ± 0.1 × 106 respiring cells ml−1) from the River Warnow at 21.6°C (P < 0.001; r2 = 0.88). In biotope water, 100% oxygen saturation equals 272.8 μmol O2 liter−1 at 21.6°C.
Calculating the cell-specific respiration of the total bacterial abundance, an “average” bacterium in this sample respired 0.8 fmol O2 h−1. This is comparable to the rate for the starving isolate OW 144 (Fig. 6). In contrast, calculating the cell-specific respiration for the highly active fraction (CTC+) only, each bacterium respired 8.7 fmol O2 h−1, which is more than 10 times higher than the OW 144 rate.
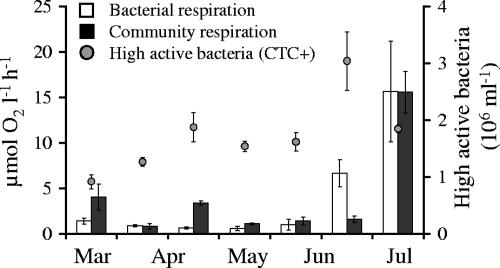
In spring and early summer 2005, the planktonic community respired between 0.7 and 15.6 μmol O2 liter−1 h−1. BR, as a percentage of total community respiration, varied between 11 and 100% (0.6 to 15.6 μmol O2 liter−1 h−1). The abundance of CTC-positive (highly active) bacteria ranged from 0.9 × 106 to 3 × 106 cells ml−1 (Fig. 8). Cell-specific respiration varied between 0.01 and 0.8 fmol O2 cell−1 h−1. On 28 June 2005, BR alone was significantly higher (four times) than the total planktonic respiration.
FIG. 8.
Community respiration and BR (μmol O2 liter−1 h−1) in the River Warnow during spring and early summer 2005, at temperatures ranging from 2.1 to 22.3°C. The second y axis shows the fraction of highly active (respiring) bacteria (106 ml−1).
DISCUSSION
Precise measurements of oxygen concentrations are required in aquatic microbial ecology for calculating rates of consumption and production. The method described here was applied successfully to aquatic bacteria. In addition to other advantages over the modified micro-Winkler method (e.g., see reference 33), planar optodes (Table 1) allow measurements at low temperatures. The above-mentioned method confirmed the correlation between oxygen saturation and temperature. In particular, the large effects of temperature variations near 0°C are problematic for obtaining representative measurements. Hence, temperature constancy during the assays is a prerequisite for accurate data recording.
A strong decrease in oxygen concentration occurred during the first 10 min of most measurements, which was probably caused by physical changes (e.g., temperature) in the samples. As mentioned before, oxygen solubility is temperature dependent. Therefore, it is wise to allow a period of adaptation (at least 10 min) before measurement.
The oxygen concentration of a sterile medium remained unchanged during the assay. The calculated variability of about 0.3% ± 0.2% was even below the resolution of 0.5% given by the manufacturer (PreSens GmbH). There was no measurable drift of the signal, which otherwise could have influenced the estimation of the respiration process, as documented for the oxygen consumption by Clark electrodes. In addition, Clark electrodes require calibrations before each experiment, which may cause a further drift in the signal. In contrast, the drift between calibrations for the oxygen sensor spot is negligible (29).
The data clearly indicate that only 2.2 × 106 respiring bacterial cells ml−1 are needed to obtain distinct oxygen depletion with the oxygen sensor spots. In an environmental sample, about 1.8 × 106 bacteria ml−1 were detected using the CTC method (40). Despite this small number of definitively respiring cells, respiration rates in plankton samples were also easily and accurately estimated with the new method. During measurements, the bacterial isolate was in the exponential phase, and most cells were alive and metabolically active. The number of CTC-positive cells measured in the environmental samples very likely seriously underestimated the portion of respiring cells. CTC is not always linearly reduced with increasing respiratory activity of bacteria (11) and is not visible in very small or low-activity cells. It is also contentious whether CTC diffuses into all cells equally (24). In addition, this dye is potentially toxic to bacteria (51) and hence represents a fluorochrome, restricted in its use to quantifying BR.
BR rates of natural samples are often converted from bacterial production (3H and 14C) and BGE measurements (8, 9). The measurement of gaseous 14C concentrations (25) or dissolved [3H]leucine (4) by chromatographic methods is scarcely used. To date, respiration is not often directly quantified, particularly for bacterial strains. Just recently, Arain et al. (1) applied oxygen sensor spots in microtiter plates to investigate the enzyme activity and respirometry of bacterial isolates. In contrast to the new application presented in this paper, they generally examined high bacterial abundances (0.2 × 108 to 8.6 × 108 CFU ml−1). Furthermore, Köster et al. (30) used respiration-based microbiosensors to quantify the available dissolved organic carbon respired by microorganisms. However, their optode-based sensor had a shelf life of only 2 weeks. Its sensitivity decreased over time, probably due to a changing physiological status of the immobilized cells. Although this microbiosensor measures respiration, it is not suited to estimate community respiration because it is more aimed at estimating available substrate concentrations. Oxygen-based respiration rate measurements within biofilms and sediments were also tested successfully (21, 22). The measuring technique was enhanced and adapted to biofilms as far as is now possible to visualize the oxygen distribution two-dimensionally, with the help of a charge-coupled-device camera. Thus, the contributions to overall respiration of so far less-investigated communities, such as periphyton, microbial mats, and other benthic communities, in several ecosystems are thus measurable in situ, resolving their microheterogeneous distribution. Additionally, (parts of) biofilms can be incubated in the cuvettes described here or in much larger cuvettes/vessels to measure their respiration under artificially altered conditions to estimate the dependency of respiration rates on abiotic factors.
Bacterial cell numbers of approximately 20 × 106 ml−1 in combination with only a 20-min measuring time are sufficient to obtain reliable respiration data. For larger cell numbers, oxygen decreased very fast and nonlinearly due to oxygen limitation of bacteria. This did not allow the evaluation of distinct oxygen consumption rates. Cell numbers of <2.2 × 106 ml−1 did not lead to any significant decrease in the measured signal over time because oxygen consumption appeared to be below the detection limit of the sensor spot. Nevertheless, the improved respiration method allows more precise calculations. The bacterial substrate utilization is measured at very short time intervals, during which any increase in oxygen consumption due to bacterial growth or variations in bacterial populations is negligible.
Only a small percentage of pelagic bacteria (10 to 20%) are metabolically active and respire carbon (e.g., see reference 12). The percentage of active bacteria in the River Warnow is much lower (15, 42), with a maximum of 4 × 106 bacteria ml−1 labeled as highly active by various methods, including the CTC method. This small number of active bacteria requires a longer measuring interval than that for individual isolates. Depending on the environmental conditions, the measuring time can be extended without changing populations. However, the main advantage of short measuring times is the stability of microbial communities with respect to constant abundances, activities, biomass, and species composition.
So far, respiration experiments using oxygen sensor spots have been performed only with freshwater bacterial isolates and bacterioplankton from a eutrophic river. Bacterial abundances in marine ecosystems are usually much lower, with <106 respiring bacteria ml−1 (e.g., see reference 42). Prolonging the measuring time would be one option for recording very low respiration rates, but the risk of community variations is increased. Another possibility would be to concentrate the bacteria (e.g., by filtration and centrifugation) in the water samples. However, it is important to guarantee that the concentration process does not interfere with bacterial activity and viability. Moreover, measuring the respiration of bacteria colonizing aggregates, e.g., marine snow, with the presented technique requires further improvements. Ploug and Jorgensen (34) established a system which allows more detailed studies of aggregate dynamics and activities under in situ conditions (26). A combination of both the oxygen sensor spot and the net-jet flow system seems to be promising for a noninvasive and non-oxygen-consuming measurement procedure.
Bacterial activity depends on inorganic nutrients, substrate concentration/availability, and grazing as well as on abiotic parameters, e.g., temperature. It is also highly species or even strain specific. An important and often addressed question is which part or portion of a bacterial community is responsible for a certain activity, in this case, respiration. If an absolute rate (of respiration) is related to the total community, the average cell-specific activity is clearly underestimated due to huge portions of dead or (temporarily) inactive fractions. Over the course of the seasons, different portions of intact and active cells addressed by different cellular properties dominated the viable part of the bacterial community in the River Warnow (15) and at least partly belonged to different bacterial strains (H. M. Freese, unpublished data). Hence, cell-specific respiration rates and the ratio of BR and community respiration may vary over a considerable magnitude. Cell-specific values of 2.4 to 4 fmol O2 cell−1 h−1 were calculated from the presented data, assuming that all bacteria are metabolically active. However, the majority of aquatic bacteria seemed to be inactive, or their specific activity was too low to be visualized (12, 45, 46; see above). Any calculation of cell-specific respiration based on CTC-positive cells typically leads to an overestimation because it is unclear how many cells fluoresce after dying or even die due to CTC toxicity (17). Nevertheless, calculating the cell-specific respiration only for the respiring active fraction, each bacterium respired 8.7 fmol O2 cell−1 h−1, which is much higher than the rate found for the log-phase cells of the investigated isolate.
Assuming that 30% of all cells in various aquatic ecosystems are active if investigated by microautoradiography (48), the average cell-specific respiration rate would amount to 2.6 fmol O2 cell−1 h−1. This is in good agreement with the values determined for isolate OW 144. Reliable and easily accessible methods are still urgently needed to estimate the active fraction of bacterioplankton (46) in order to attain precise cell-specific budgets. If, in contrast to the 9% CTC-positive bacteria found as the maximum percentage for the River Warnow, 30% of all cells would have been assumed to be active (the average from microautoradiography), almost 6 × 106 active bacteria ml−1 would participate in respiration. Assuming a cell-specific respiration rate of 2.6 fmol O2 cell−1 h−1, this portion of 6 × 106 active bacteria ml−1 could respire 15.6 μmol O2 liter−1 h−1, which is closely related to the measured value of 15.6 ± 5.6 μmol O2 liter−1 h−1 in the environment. Nevertheless, this rate still represents only one-quarter to one-half of that measured for the bacterial isolate OW 144, indicating again that CTC staining may seriously underestimate respiring bacteria and that other methods may also not address the active fraction properly. Although in June 2005 BR was four times higher than total planktonic respiration, these data can be explained by the observed aggregates in the water column at this date, which accumulated particularly highly active bacteria (47). Bacteria were washed out of the aggregates during filtering of water samples through 1-μm gauze for respiration rate measurements. Thus, highly active cells accumulated in the 1-μm fraction.
Dissolved organic carbon in riverine water usually consists of numerous mono-, di-, and oligomeric substrates, which are often poorly utilized by inhabiting bacteria. Large amounts of humic substances (40% of dissolved organic carbon), which are known to be degraded at very low rates, were detected (14) in the River Warnow. Therefore, a low percentage of highly active bacteria (4.4% on average) in combination with low growth rates and low hydrolytic enzyme activities (42) may well explain any delay in carbon decomposition.
To evaluate the experimental results, the new respiration method was compared to conventional procedures (Schumann et al. [41] studied freshwater and marine stations on the Baltic Coast, Germany, and Schwaerter et al. [43] studied eutrophic lakes in Denmark). Both sets of authors measured BR in eutrophic waters by using a Clark electrode and the Winkler method, respectively. Schumann et al. reported community respiration rates of 1.2 to 11.6 μg C liter−1 h−1 in winter, and Schwaerter et al. reported rates of 9.4 to 56.3 μg C liter−1 h−1 (25 to 150 μg O2 liter−1 h−1). Converting the field data presented in this paper, BR rates in the River Warnow accounted for 10.1 to 186.6 μg C liter−1 h−1 (0.6 to 15.6 μmol O2 liter−1 h−1). This corresponds well with the range of respiration rates measured before by the authors mentioned above. Schumann et al. (41) found cell-specific respiration rates of 0.01 to 0.09 fmol C bacterium−1 h−1 for the River Warnow. Cell-specific respiration using the oxygen sensor spot ranged from 0.01 to 0.06 fmol C bacterium−1 h−1 for the same river during winter and spring 2005. Thus, the optode technique provides very similar data to those obtained by the established Clark electrode method. However, the oxygen sensor spots represent a preferable alternative for measuring BR quickly and at small cell numbers without the methodological problems associated with long incubation times that lead to growth and successive changes in natural bacterioplankton communities.
Acknowledgments
We thank Christian Krause, PreSens GmbH, for technical assistance and helpful discussions about the technical equipment. We are also grateful to Kartika Senjarini for her extensive screening of substrate utilization of several bacterial isolates as well as for supplying the OW 144 strain.
This study was supported by grants from the Max-Buchner-Stiftung and the Ministry of Education, Science and Culture Mecklenburg, Vorpommern, Germany, to H. M. Freese.
Footnotes
Published ahead of print on 31 August 2007.
REFERENCES
- 1.Arain, S., G. T. John, C. Krause, J. Gerlach, O. S. Wolfbeis, and I. Klimant. 2006. Characterization of microtiter plates with integrated optical sensors for oxygen and pH, and their applications to enzyme activity screening, respirometry, and toxicological assays. Sens. Actuators B 113:639-648. [Google Scholar]
- 2.Azam, F., T. Fenchel, J. G. Field, J. S. Gray, L. A. Meyer Reil, and F. Thingstad. 1983. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10:257-263. [Google Scholar]
- 3.Bernardi, R., G. Giussani, M. Manca, and D. Ruggiu. 1990. Trophic status and the pelagic system in Lago Maggiore. Hydrobiologia 191:1-8. [Google Scholar]
- 4.Boyd, T. J., and A. F. Carlucci. 1994. Use of a H-3 labeled substrate to measure microbial biodegradation in marine waters. J. Microbiol. Methods 20:11-22. [Google Scholar]
- 5.Carignan, R., A. M. Blais, and C. Vis. 1998. Measurement of primary production and community respiration in oligotrophic lakes using the Winkler method. Can. J. Fish. Aquat. Sci. 55:1078-1084. [Google Scholar]
- 6.Clark, L. C. J. 1956. Monitor and control of blood and tissue oxygen tensions. Trans. Am. Soc. Artif. Intern. Organs 2:41-46. [Google Scholar]
- 7.Cole, J. J., and N. F. Caraco. 1998. Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6. Limnol. Oceanogr. 43:647-656. [Google Scholar]
- 8.del Giorgio, P. A., and J. J. Cole. 1998. Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 29:503-541. [Google Scholar]
- 9.del Giorgio, P. A., J. J. Cole, and A. Cimbleris. 1997. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 385:148-151. [Google Scholar]
- 10.del Giorgio, P. A., and C. M. Duarte. 2002. Respiration in the open ocean. Nature 420:379-384. [DOI] [PubMed] [Google Scholar]
- 11.del Giorgio, P. A., J. M. Gasol, D. Vaque, P. Mura, S. Agusti, and C. M. Duarte. 1996. Bacterioplankton community structure: protists control net production and the proportion of active bacteria in a coastal marine community. Limnol. Oceanogr. 41:1169-1179. [Google Scholar]
- 12.del Giorgio, P. A., and G. Scarborough. 1995. Increase in the proportion of metabolically active bacteria along gradients of enrichment in fresh-water and marine plankton—implications for estimates of bacterial-growth and production-rates. J. Plankton Res. 17:1905-1924. [Google Scholar]
- 13.Ducklow, H. W., D. A. Purdie, P. J. Williams, and J. M. Davies. 1986. Bacterioplankton: a sink for carbon in a coastal marine plankton community. Science 232:863-867. [DOI] [PubMed] [Google Scholar]
- 14.Freese, H. M., S. Görs, U. Karsten, and R. Schumann. 2007. Dissolved inorganic nutrients and organic substrates in the River Warnow (north-eastern Germany)—utilisation by bacterioplankton. Limnologica 37:264-277. [Google Scholar]
- 15.Freese, H. M., U. Karsten, and R. Schumann. 2006. Bacterial abundance, activity, and viability in the eutrophic River Warnow, Northeast Germany. Microb. Ecol. 51:117-127. [DOI] [PubMed] [Google Scholar]
- 16.Gansert, D., M. Burgdorf, and R. Losch. 2001. A novel approach to the in situ measurement of oxygen concentrations in the sapwood of woody plants. Plant Cell Environ. 24:1055-1064. [Google Scholar]
- 17.Gasol, J. M., and J. Aristegui. 2007. Cytometric evidence reconciling the toxicity and usefulness of CTC as a marker of bacterial activity. Aquat. Microb. Ecol. 46:71-83. [Google Scholar]
- 18.Gatti, S., T. Brey, W. Müller, H. Holst, and O. Heilmayer. 2002. Oxygen microoptodes: a new tool for oxygen measurements in aquatic animal ecology. Mar. Biol. 140:1075-1085. [Google Scholar]
- 19.Gattuso, J. P., S. Peduzzi, M. D. Pizay, and M. Tonolla. 2002. Changes in freshwater bacterial community composition during measurements of microbial and community respiration. J. Plankton Res. 24:1197-1206. [Google Scholar]
- 20.Glud, R. N., M. Kuhl, O. Kohls, and N. B. Ramsing. 1999. Heterogeneity of oxygen production and consumption in a photosynthetic microbial mat as studied by planar optodes. J. Phycol. 35:270-279. [Google Scholar]
- 21.Glud, R. N., C. M. Santegoeds, D. de Beer, O. Kohls, and N. B. Ramsing. 1998. Oxygen dynamics at the base of a biofilm studied with planar optodes. Aquat. Microb. Ecol. 14:223-233. [Google Scholar]
- 22.Glud, R. N., A. Tengberg, M. Kuhl, P. O. J. Hall, I. Klimant, and G. Host. 2001. An in situ instrument for planar O-2 optode measurements at benthic interfaces. Limnol. Oceanogr. 46:2073-2080. [Google Scholar]
- 23.Glud, R. N., F. Wenzhofer, A. Tengberg, M. Middelboe, K. Oguri, and H. Kitazato. 2005. Distribution of oxygen in surface sediments from central Sagami Bay, Japan: in situ measurements by microelectrodes and planar optodes. Deep-Sea Res. I 52:1974-1987. [Google Scholar]
- 24.Hatzinger, P. B., P. Palmer, R. L. Smith, C. T. Penarrieta, and T. Yoshinari. 2003. Applicability of tetrazolium salts for the measurement of respiratory activity and viability of groundwater bacteria. J. Microbiol. Methods 52:47-58. [DOI] [PubMed] [Google Scholar]
- 25.Hobbie, J. E., and C. C. Crawford. 1969. Respiration corrections for bacterial uptake of dissolved organic compound in natural waters. Limnol. Oceanogr. 14:528-532. [Google Scholar]
- 26.Kiorboe, T., K. Tang, H.-P. Grossart, and H. Ploug. 2003. Dynamics of microbial communities on marine snow aggregates: colonization, growth, detachment, and grazing mortality of attached bacteria. Appl. Environ. Microbiol. 69:3036-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirchman, D. L. 1997. Carbon cycle—microbial breathing lessons. Nature 385:121-122. [Google Scholar]
- 28.Kirchman, D. L., A. I. Dittel, S. Findlay, and D. Fischer. 2004. Changes in bacterial activity and community structure in response to dissolved organic matter in the Hudson River, New York. Aquat. Microb. Ecol. 35:243-257. [Google Scholar]
- 29.Klimant, I., V. Meyer, and M. Kühl. 1995. Fiber-optic oxygen microsensors, a new tool in aquatic biology. Limnol. Oceanogr. 40:1159-1165. [Google Scholar]
- 30.Köster, M., C. G. Gliesche, and R. Wardenga. 2006. Microbiosensors for measurement of microbially available dissolved organic carbon: sensor characteristics and preliminary environmental application. Appl. Environ. Microbiol. 72:7063-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez, J., and F. Azam. 1993. Periplasmic aminopeptidase and alkaline phosphatase activities in a marine bacterium: implications for substrate processing in the sea. Mar. Ecol. Prog. Ser. 92:89-97. [Google Scholar]
- 32.Mock, T., G. S. Dieckmann, C. Haas, A. Krell, J. L. Tison, A. L. Belem, S. Papadimitriou, and D. N. Thomas. 2002. Micro-optodes in sea ice: a new approach to investigate oxygen dynamics during sea ice formation. Aquat. Microb. Ecol. 29:297-306. [Google Scholar]
- 33.Peck, L. S., and R. F. Uglow. 1990. Two methods for the assessment of the oxygen content of small volumes of seawater. J. Exp. Mar. Biol. Ecol. 141:53-62. [Google Scholar]
- 34.Ploug, H., and B. B. Jorgensen. 1999. A net-jet flow system for mass transfer and microsensor studies of sinking aggregates. Mar. Ecol. Prog. Ser. 176:279-290. [Google Scholar]
- 35.Pomeroy, L. R., J. E. Sheldon, and W. M. Sheldon, Jr. 1994. Changes in bacterial numbers and leucine assimilation during estimations of microbial respiratory rates in seawater by the precision Winkler method. Appl. Environ. Microbiol. 60:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 37.Reinthaler, T., K. Bakker, R. Manuels, J. van Ooijen, and G. J. Herndl. 2006. Fully automated spectrophotometric approach to determine oxygen concentrations in seawater via continuous-flow analysis. Limnol. Oceanogr. Methods 4:358-366. [Google Scholar]
- 38.Revsbech, N. P. 1989. An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 34:474-478. [Google Scholar]
- 39.Rivkin, R. B., and L. Legendre. 2001. Biogenic carbon cycling in the upper ocean: effects of microbial respiration. Science 291:2398-2400. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez, G. G., D. Phipps, K. Ishiguro, and H. F. Ridgway. 1992. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl. Environ. Microbiol. 58:1801-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schumann, R., T. Rieling, S. Görs, A. Hammer, U. Selig, and U. Schiewer. 2003. Viability of bacteria from different aquatic habitats. I. Environmental conditions and productivity. Aquat. Microb. Ecol. 32:121-135. [Google Scholar]
- 42.Schumann, R., U. Schiewer, U. Karsten, and T. Rieling. 2003. Viability of bacteria from different aquatic habitats. II. Cellular fluorescent markers for membrane integrity and metabolic activity. Aquat. Microb. Ecol. 32:137-150. [Google Scholar]
- 43.Schwaerter, S., M. Soendergaard, B. Riemann, and L. Moeller Jensen. 1988. Respiration in eutrophic lakes: the contribution of bacterioplankton and bacterial growth yield. J. Plankton Res. 10:515-531. [Google Scholar]
- 44.Scott, J. T., and R. D. Doyle. 2006. Coupled photosynthesis and heterotrophic bacterial biomass production in a nutrient-limited wetland periphyton mat. Aquat. Microb. Ecol. 45:69-77. [Google Scholar]
- 45.Sherr, B. F., P. del Giorgio, and E. B. Sherr. 1999. Estimating abundance and single-cell characteristics of respiring bacteria via the redox dye CTC. Aquat. Microb. Ecol. 18:117-131. [Google Scholar]
- 46.Sherr, B. F., E. B. Sherr, and J. McDaniel. 1992. Effect of protistan grazing on the frequency of dividing cells in bacterioplankton assemblages. Appl. Environ. Microbiol. 58:2381-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherr, E. B., B. F. Sherr, and C. T. Sigmon. 1999. Activity of marine bacteria under incubated and in situ conditions. Aquat. Microb. Ecol. 20:213-223. [Google Scholar]
- 48.Smith, E. M., and P. A. Del Giorgio. 2003. Low fractions of active bacteria in natural aquatic communities? Aquat. Microb. Ecol. 31:203-208. [Google Scholar]
- 49.Tranvik, L. J. 1990. Bacterioplankton growth on fractions of dissolved organic carbon of different molecular weights from humic and clear waters. Appl. Environ. Microbiol. 56:1672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tranvik, L. J. 1992. Allochthonous dissolved organic-matter as an energy-source for pelagic bacteria and the concept of the microbial loop. Hydrobiologia 229:107-114. [Google Scholar]
- 51.Ullrich, S., B. Karrasch, H. Hoppe, K. Jeskulke, and M. Mehrens. 1996. Toxic effects on bacterial metabolism of the redox dye 5-cyano-2,3-ditolyl tetrazolium chloride. Appl. Environ. Microbiol. 62:4587-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams, P. J. L. 1981. Microbial contribution to overall marine plankton metabolism—direct measurements of respiration. Oceanol. Acta 4:359-364. [Google Scholar]
- 53.Williams, P. J. L. 1998. The balance of plankton respiration and photosynthesis in the open oceans. Nature 394:55-57. [Google Scholar]
- 54.Winkler, L. W. 1888. The determination of dissolved oxygen in water. Ber. Dtsch. Chem. Ges. 21:2834-2846. [Google Scholar]