Abstract
Lactobacillus casei ATCC 393 was selected as an antigen delivery vehicle for mucosal immunization against porcine parvovirus (PPV) infection. A 64-kDa fragment of PPV major protective antigen VP2 protein was used as the parvovirus antigen model. A recombinant Lactobacillus expressing VP2 protein was constructed with plasmid pPG611.1, where expression and localization of the VP2 protein from recombinant Lc393-rPPV-VP2 was detected via sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and immunofluorescence. Both local mucosal and systemic immune responses against PPV were induced in BALB/c mice immunized orally with the recombinant Lactobacillus expressing VP2 protein. The induced antibodies demonstrated neutralizing effects on PPV infection. These data indicated that the use of recombinant lactobacilli could be a valuable strategy for future vaccine development of PPV.
Porcine parvovirus (PPV), characterized as a member of the autonomous parvoviruses, is a major cause of reproductive failure in swine, resulting in early embryonic death, fetal death, stillbirths, and delayed return to estrus (3, 12, 13, 22), and the virus causes serious losses for pig producers. The molecular features of PPV are similar to those of other autonomous parvoviruses. PPV is a small, nonenveloped virus, and the virion contains a 5-kb, linear, minus-polarity, single-stranded DNA genome (25), which is encapsidated within a simple icosahedron protein coat composed of three structural polypeptides (VP1, VP2, and VP3, with masses of 84, 64, and 60 kDa, respectively) (24). The VP2 protein encompasses major antigenic domains of PPV and could induce PPV-neutralizing antibodies for the neutralization of PPV infection (26, 32, 38). Therefore, the VP2 protein plays a major role in PPV diagnosis and immune prophylaxis.
The mucosa tissues are particularly important for protection against diseases, such as those caused by viral, bacterial, and parasitic pathogens which invade the mucosal system (18, 39). Vaccines administered by parenteral routes generally fail to stimulate mucosal immune responses. Mucosal immunization has proven to be an effective approach against the colonization of pathogens and their further spread to the systemic circulation (7, 20, 23). Therefore, it is necessary to develop efficient and safe antigen vectors that will be able to trigger mucosal and systemic immune responses. One promising approach relies on the use of live bacterial vehicles (21). The potentiality of recombinant lactic acid bacteria to deliver heterologous antigens to the mucosal immune system has been investigated during the last decade (10, 11, 19, 28, 30, 31, 37). This approach offers a number of advantages (such as noninvasiveness and the possibility of eliciting both mucosal and systemic immune responses) over the traditional parenteral vaccination. In addition, lactobacilli have been used in a large variety of industrial food fermentation and preservation processes, are known for their beneficial effects on the health of humans and animals, and are considered “generally regarded as safe” microorganisms. Lactobacilli represent an original alternative to the use of attenuated pathogenic bacterial carriers, such as Salmonella, Vibrio, and Mycobacterium (6, 16, 34). Lactobacillus strains have been used as hosts to express many bacterial and viral antigens and have been proven to elicit immune responses after being used for inoculation by oral administration ( 10, 11, 19, 31, 37), which makes them attractive candidates from a pharmaceutical standpoint as agents for the delivery of antigens to the mucosa in particular vaccines. Furthermore, lactobacilli could survive transit through the upper gastrointestinal tract and colonize the intestinal tracts (1, 17). In addition, lactobacilli have shown intrinsic adjuvant activity (27, 29).
In this study, the potentiality of using L. casei 393 to express heterologous parvovirus protein and its ability to act as an antigen delivery carrier for oral vaccination were analyzed. The 64-kDa fragment of PPV capsid protein VP2 encompasses the major antigenic domains critical for neutralization, and a cell surface expression system, pPG611.1, was used in this study. The immunogenicity of the recombinant Lc393-rPPV-VP2 (rLc393-rPPV-VP2) was analyzed by post-intragastric administration of live bacteria to the BALB/c mice. This is the first report on the cloning and expression of PPV antigen in Lactobacillus. Our data have indicated that intragastric inoculation of rLc393-rPPV-VP2 could induce specific immune responses against PPV.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
Lactobacillus casei ATCC 393 (a kind gift of Jos Seegers, NIZO, The Netherlands) was grown anaerobically in MRS broth (Sigma) at 37°C without shaking. To analyze protein expression, rLc393-rPPV-VP2 was grown in basal MRS medium (10 g peptone, 8 g beef extract, 4 g yeast extract, 2 g potassium phosphate, 5 g sodium acetate, 1 ml Tween 80, 2 g diammonium citrate, 0.2 g magnesium sulfate, and 0.05 g manganese sulfate per liter) supplemented with 2% xylose. The plating of bacteria used in this study was performed on MRS medium with 1.5% agar. The antibiotic concentration used for the selection of lactobacilli transformants was 10 μg/ml of chloromycetin (Cm; Sigma).
Labeling of lactobacilli with fluorescence dye cFDA-SE.
L. casei 393 was labeled with 5′-(and 6′)-carboxyfluorescein diacetate succinimidyl ester (cFDA-SE; Molecular Probes, Sigma), a nonfluorescent membrane permeative ester that nonspecific prokaryotic and eukaryotic intracellular esterase converts to a fluorescent derivative that in turn is then linked covalently to intracellular protein via the probe's succinimidyl group (40). In brief, a 100-μM stock solution of cFDA-SE was prepared by first being dissolved in dimethyl sulfoxide (20 μl) and then being diluted further in ethanol (1 ml to reagent grade). This solution was filter sterilized before being aliquoted and stored at −20°C. L. casei 393 was grown overnight at 37°C in MRS broth. The bacterial cultures were centrifuged at 4,000 × g for 10 min, and the pellets were washed twice in sterile phosphate-buffered saline (PBS) and adjusted to a concentration of 1010 CFU/ml before being labeled with cFDA-SE at 37°C for 25 min. Fluorescence labeling was terminated by pelleting the bacteria. The labeled bacteria were washed twice in sterile PBS to remove excess cFDA-SE and resuspended in sterile PBS. The flow cytometry profile showed that about 99.5% of the cells were labeled.
Adhesion study of animals.
Seven-week-old BALB/c mice were obtained from the Laboratory Animal Center, Harbin Medical University of China. Fifteen mice were dosed with approximately 109 cFDA-SE-labeled lactobacilli by oral administration. Another group of 15 mice were fed orally with sterile PBS as a control. Groups of three mice each were sacrificed on days 1, 2, 4, 6, and 7, and then the duodenum, jejunum, ileum, and colon were extracted from each mouse. Individual sections were cut longitudinally, and any visible residual food particles or fecal material was removed from the intestine before being examined for the presence of adhering cFDA-SE-labeled lactobacilli. This examination was performed by adding 150 μl of PBS to every 1.0 cm of tissue and dislodging microbes from the mucosal surfaces of the tissues. Cell extracts were fixed with formaldehyde (0.75%, vol/vol) prior to flow cytometry analysis (17).
Bile tolerance of lactobacilli.
One-milliliter cultures of L. casei 393 grown in MRS medium (optical density at 600 nm of 1.0) were pelleted and reinoculated in 10 ml basal MRS medium supplemented with 0.05%, 0.1%, 0.2%, 0.3%, and 0.4% of bile (wt/vol) and grown at 37°C for 8 h without shaking, and we enumerated the surviving bacteria at 2-h intervals via the plate method.
Construction of VP2 expression plasmid.
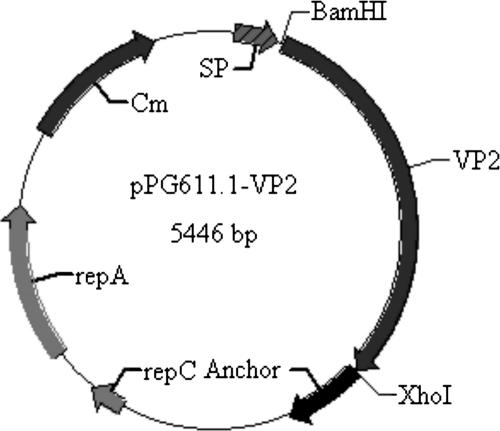
All DNA manipulations were performed according to standard procedures (36). The plasmid pPG611.1 used in this study is a kind of cell surface expression vector containing an SPUsp secretion signal peptide and an anchor domain structure (a gift of Jos Seegers, NIZO, The Netherlands). A 1.75-kb fragment of the gene encoding PPV antigen VP2 was obtained from the PPV genome by PCR. The oligonucleotides were 5′-CGAGGATCCTATGGTTCACTGGTTCGACGACCGCGAG-3′ (upper), containing a BamHI site (underlined), and 5′-AGCTTCTCGAGCCATGCTACCTGATTAACCGAGTAACTG-3′ (lower), containing an XhoI site (underlined). PCR amplification conditions were as follows: 95°C for 5 min; 30 cycles of 94°C for 1 min, 55°C for 1.2 min, and 72°C for 2 min; and 72°C for 10 min for final extension. The PCR product was digested by BamHI and XhoI and inserted into the corresponding sites of pPG611.1, giving rise to pPG611.1-VP2 (Fig. 1).
FIG. 1.
Map of pPG611.1-VP2, including the Cm resistance determinant, repA and repC replication elements, signal peptide (SP) obtained from the lactococcal Usp45 protein containing the xylose operon promoter, VP2 gene obtained from PPV genome DNA by PCR, and the cell wall anchor motif obtained from Streptococcus pyogenes M6 protein.
Generation of Lc393 transformants expressing PPV VP2 protein.
Electroporation was carried out according to the method described previously by Josson et al. (14), with some modifications. In brief, 10 μl recombinant plasmid DNA was added to 150 μl L. casei 393, mixed gently, and placed at 4°C for 5 min. The mixer was subjected to a single electric pulse (25 μF and 2,500 V/cm), resuspended in MRS medium without antibiotic, and incubated anaerobically at 37°C for 2 h. Recombinant strains were selected on MRS agar medium containing 10 μg/ml Cm. The presence and integrity of the constructions carried by the transformants were checked by extraction of recombinant plasmid DNA, followed by restriction analysis and sequencing.
Segregational and structural stability of pPG611.1-VP2.
To assess the segregational stability of pPG611.1-VP2 in L. casei 393, the recombinant strain was grown in MRS medium at 37°C without Cm. Serial subcultures were performed by diluting (1:100) the culture in fresh broth without antibiotics and growing it until mid-exponential phase (optical density at 600 nm of 1.0). After 70 generations, the percentage of Cm-resistant colonies was determined by plating 100-μl culture dilutions onto MRS plates without Cm and onto MRS plates supplemented with 10 μg/ml Cm. To determine the structural stability of recombinant plasmid after 70 generations, several colonies were picked up from the MRS plates at random and analyzed by colony PCR using forward and reverse primers to detect the integrity of the VP2 gene in the recombinant plasmid.
Protein expression, Western blotting, and immunofluorescence analysis.
To analyze the expression and localization of the VP2 fusion protein, overnight cultures of rLc393-rPPV-VP2 grown in basal MRS medium supplemented with xylose were collected by centrifugation at 12,000 × g for 10 min. The bacterial pellets were washed twice with sterile 50 mM Tris-Cl, pH 8.0, and lysed in a bead beater (Biospec, Bartlesville, OK) by vigorous shaking. The lysates were centrifuged at 15,000 × g for 10 min, and the supernatant was maintained at −20°C for further analysis. In a parallel experiment, the cells were treated with 10 mg/ml of protease K (Sigma) at 37°C for 1 h, washed three times to remove the protease, and then treated with lysozyme. The bacterial protein extracts and supernatant were examined using 10% SDS-PAGE. For Western blotting analysis, proteins were electrotransferred onto a nitrocellulose membrane and the immunoblots were developed using mouse anti-PPV-VP2 serum (the antiserum obtained from BALB/c mice immunized with virus-like particles of PPV VP2 protein). Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (Sigma) diluted 1:2,000 was used, and visualization of immunolabeled bands was then carried out by using the chemiluminescent substrate reagent (Pierce) according to the manufacturer's instructions.
Immunofluorescence was also used to analyze cell surface display of VP2 protein: 1-ml overnight cultures of rLc393-rPPV-VP2 were pelleted and washed twice with sterile PBS. The pellets were resuspended in PBS containing mouse anti-PPV-VP2 serum and incubated at 37°C for 2 h. The cells were centrifuged and washed three times with sterile PBS to remove noncombination antibodies. Then the pellets were incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma) containing 1% Evans blue (Pierce) at 37°C for 2 h. After being washed three times with sterile PBS, the pellets were resuspended in sterile PBS and laid on a glass slide. Confocal microscopy was used to visualize the fluorescence reaction on the cell surface of rLc393-rPPV-VP2. Wild-type L. casei 393 was used as a control.
Immunization.
rLc393-rPPV-VP2 was cultured and centrifuged. Cell pellets were washed once with sterile PBS and resuspended in PBS to a concentration of about 1 × 1010 CFU/ml. The vaccine group of mice received a dose of 1 × 109 cells of rLc393-rPPV-VP2. The control mice were immunized with an equivalent dose of Lc393-pPG611.1. The sham control group received 0.1-ml doses of PBS. All of the mice were immunized via oral administration. The immune protocol was administered on three consecutive days (days 0, 1, and 2). A booster immunization was given at days 14, 15, and 16, and a second booster was given at days 28, 29 and 30 (4).
ELISA of serum and intestine mucus.
Sera were prepared and stored at −20°C until required. The intestinal mucus of mice was extracted as described previously (8, 33). Polystyrene microtiter plates were coated overnight at 4°C with the PPV propagated on swine testicular (ST) cells, and the cultures of ST cells were used as a negative control antigen. Enzyme-linked immunosorbent assay (ELISA) plates were washed three times in PBS containing 1% Tween 20 and then saturated with PBS containing 5% skim milk at 37°C for 2 h. Serum or intestinal lavage fluid was used as a primary antibody diluted 10-fold in PBS containing 1% bovine serum albumin. After being incubated at 37°C for 1 h, the plates were washed three times with PBS containing 1% Tween 20. Bound antibodies were detected by using horseradish peroxidase-conjugated goat anti-mouse IgA or IgG (Sigma), followed by color development by using o-phenylenediamine dihydrochloride (Sigma) as the substrate. The absorbance was measured at 490 nm.
Neutralization ability of the induced antibodies.
Intestinal fluids and serum samples obtained from the mice immunized with Lc393-rPPV-VP2 were evaluated using a plaque reduction assay to determine the neutralization ability. Lavage fluids and sera obtained from the mice fed with a nonexpressor strain or PBS were used as negative controls. Fifty microliters of samples in twofold serial dilutions (from 1:2 to 1:512) was prepared in a 96-cell plate. PPV adjusted to 200 50% tissue culture infective doses in 50 μl of virus diluent (10% concentrated Hanks balanced salt solution, 0.1% bovine serum albumin, pH 7.4) was added to the cell plate containing serially diluted serum or intestinal lavage fluid. The antibody and virus mixture was mixed and incubated at 37°C for 1 h. Then 100 μl of ST cells (used for virus infection) was added to the antibody-virus mixture and incubated in a 5% CO2 incubator at 37°C for 5 days. The overlay medium was then discarded, after which the wells were washed three times with sterile PBS, pH 7.4, and stained with 1% crystal violet solution. Differences in the number of plaques formed between treatments were examined for the level of significance by Student's t test after analysis of variance.
RESULTS
Colonization ability of L. casei 393.
The colonization ability of L. casei 393 in the intestinal tracts was determined via oral administration of cFDA-SE-labeled lactobacilli to mice and isolation of the different regions of the intestine from such mice by post-orogastric intubation. Flow cytometric analysis of cell extracts (Table 1) indicated that the lactobacilli were able to survive and adhere to different regions of the intestinal tract. Adhesion was most prominent in the ileum, with 8.97 × 106 ± 0.76 × 106 cells being detected on day 1. By day 7, the amounts of L. casei 393 that remained adhered to the intestinal mucosa were 39.38, 71.30, 76.59, and 11.79% of that on the first day in the duodenum, jejunum, ileum, and colon, respectively (data not shown).
TABLE 1.
Amounts of cFDA-SE-labeled L. casei 393 isolated from different intestinal tracts
| Day | No. of cells on indicated part of the intestinal tract (avg ± SD)
|
|||
|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Colon | |
| 1 | 7.44E06 ± 0.75E06 | 8.71E06 ± 0.69E06 | 8.97E06 ± 0.76E06 | 8.45E06 ± 0.87E06 |
| 2 | 6.76E06 ± 0.41E06 | 7.62E06 ± 0.72E06 | 7.87E06 ± 0.78E06 | 6.15E06 ± 0.47E06 |
| 4 | 5.17E06 ± 0.51E06 | 7.33E06 ± 0.66E06 | 7.31E06 ± 0.64E06 | 5.54E06 ± 0.49E06 |
| 6 | 3.13E06 ± 0.29E06 | 6.57E06 ± 0.54E06 | 6.93E06 ± 0.62E06 | 1.31E06 ± 0.18E06 |
| 7 | 2.23E06 ± 0.17E06 | 6.21E06 ± 0.57E06 | 6.87E06 ± 0.59E06 | 9.96E06 ± 1.02E05 |
Bile tolerance of L. casei 393.
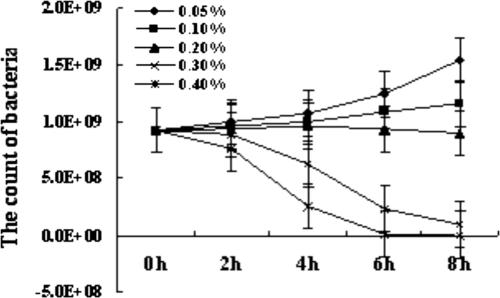
Bile tolerance is a selection criterion for probiotics (35). In normal intestines, the bile concentration ranges from 0.03% to 0.3% (5, 9). The bile tolerance of L. casei 393 indicated that the lactobacilli have a good property of tolerance of 0.05% to 0.2% bile. In 0.3% and 0.4% bile, lactobacilli could survive for 8 h and 4 h, respectively (Fig. 2).
FIG. 2.
The bile tolerance of L. casei 393, from using the plate method to enumerate the amounts of lactobacilli that survived in different concentrations of bile. Results were mean values of amounts of bacteria ± standard errors (error bars) of the means.
Cell surface expression of PPV antigen VP2.
The gene encoding VP2 protein was amplified and cloned as a BamHI-XhoI fragment into the corresponding site of expression plasmid pPG611.1, generating pPG611.1-VP2. The recombinant plasmid pPG611.1-VP2 was electroporated into L. casei 393. The segregational and structural stability of pPG611.1-VP2 in L. casei 393 was analyzed after 70 generations of subcultures in MRS broth without Cmr selection. The results indicated that the recombinant plasmid had good stability properties, with no loss of the Cm resistance phenotype or any structural rearrangement (data not shown).
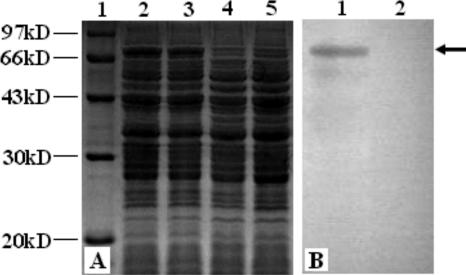
rLc393-rPPV-VP2 was grown overnight in basal MRS medium supplemented with either xylose or glucose. The lysates of the cells were analyzed by SDS-PAGE and Western blotting using mouse anti-PPV-VP2 serum. Coomassie blue gel staining showed that a 74-kDa fusion protein was expressed in lysates of rLc393-rPPV-VP2 induced by xylose (Fig. 3A, lanes 2 and 3), but it was not expressed when the same cells were grown in glucose (Fig. 3A, lane 4) or when wild-type L. casei 393 was grown in xylose (Fig. 3A, lane 5). An immunoreactive band was detected (Fig. 3B, lane 1) via Western blotting in a position similar to that observed via SDS-PAGE (Fig. 3A), and expression of the band was repressed when rLc393-rPPV-VP2 was grown in MRS medium containing glucose (Fig. 3B, lane 2). As the negative control, wild-type L. casei 393 did not display the corresponding immunoreactive band (data not shown). These results show that the xylose promoter from L. casei could efficiently induce the expression of PPV heterologous protein. However, the expression of VP2 protein was repressed by glucose.
FIG. 3.
Expression of VP2 protein in rLc393-rPPV-VP2. Total cell lysates were analyzed by SDS-PAGE and Western blotting with specific antiserum. (A) Coomassie blue gel staining shows the expression of a 74-kDa fusion protein in lysates from rLc393-rPPV-VP2 induced by xylose (lanes 2 and 3) but not by glucose (lane 4), or wild-type L. casei 393 was grown in xylose (lane 5). Lane 1, molecular mass marker. (B) An immunoreactive band was detected (lane 1) in a position similar to that observed via SDS-PAGE, as shown in panel A. No immunoreactive bands were observed for cell lysates from recombinant cells induced by glucose (lane 2).
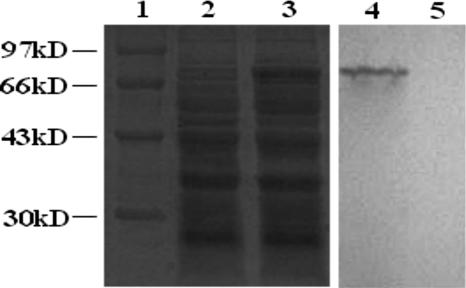
The VP2 protein could not be detected in the supernatant of overnight cultures of rLc393-rPPV-VP2 induced by xylose, even after the use of a 50-fold concentration in a Millipore Ultrafree-15 column (data not shown). The same cultures were incubated with lysozyme, which could release cell-anchored proteins, and the supernatant was analyzed by Western blotting. As can be observed in Fig. 4, the induced rLc393-rPPV-VP2 was treated with lysozyme and an approximately 74-kDa protein was released into the supernatant (Fig. 4, lane 2); this protein could not be detected in the supernatant of cells treated only with protease (Fig. 4, lane 4). Treatment with protease previous to lysozyme gives a single band of about 50 kDa (Fig. 4, lane 3). None of bands was observed in supernatant from untreated cells (data not shown).
FIG. 4.
Localization analysis of VP2 expression from Lc393-rPPV-VP2 by Western blotting. Lane 1, total lysates of xylose-inducible cells expressing VP2 fusion; lane 2, supernatant of the same cells treated with lysozyme; lane 3, supernatant of cells treated with protease, followed by lysozyme; lane 4, supernatant of cells treated with only protease.
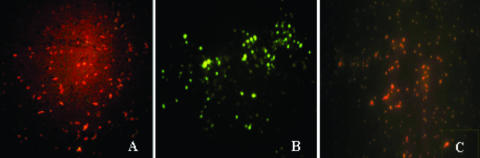
To confirm the cell surface display of the VP2 fusion protein, immunofluorescence analysis was used. Overnight cultures of rLc393-rPPV-VP2 expressing VP2 were grown in basal MRS medium supplemented with either xylose or glucose. The immunofluorescence was developed with the mouse anti-VP2 serum and fluorescein isothiocyanate fluorescence-conjugated goat anti-mouse IgG. The results indicated that there was green-yellow fluorescence on the cell surface of rLc393-rPPV-VP2 grown in xylose (Fig. 5B), but not that of rLc393-rPPV-VP2 grown in glucose, where the bacteria were dyed red by Evans blue (Fig. 5A). Wild-type L. casei 393 also did not display an immunofluorescence reaction, and the bacteria were dyed red by Evans blue (Fig. 5C).
FIG. 5.
The immunofluorescence reaction of VP2 protein on the cell surface of recombinant Lc393-rPPV-VP2. (A) When rLc393-rPPV-VP2 was grown in MRS medium containing glucose, no fluorescence appeared on the cell surfaces, and the bacteria were dyed red by Evans blue. (B) When rLc393-rPPV-VP2 was induced by xylose, there was a green-yellow fluorescence reaction on the surface of the bacteria. (C) When wild-type L. casei 393 was induced by xylose, the result of immunofluorescence was negative, and the bacteria were dyed red by Evans blue.
The results of localization analysis of VP2 protein expressed from the rLc393-rPPV-VP2 by Western blotting and immunofluorescence suggested that VP2 protein could be exposed outside the cell wall, and part appeared to be embedded in the cell wall.
Furthermore, whether the rLc393-rPPV-VP2 subcultured for 70 generations, without Cmr selection, could still express the VP2 antigen was determined. The result shows that the recombinant lactobacilli could still express the protein of interest (Fig. 6).
FIG. 6.
Expression and identification of VP2 protein in rLc393-rPPV-VP2 subcultured for 70 generations, without Cmr selection. Lane 1, molecular mass marker. Lane 3, Coomassie blue gel staining shows that a 74-kDa fusion protein was expressed from rLc393-rPPV-VP2 subcultured for 70 generations, without Cm selection, induced by xylose but not glucose, in a position similar to that observed via SDS-PAGE, as shown in Fig. 3A (lane 2). Lane 4, an immunoreactive band of protein expressed by rLc393-rPPV-VP2 subcultured for 70 generations was detected in the same position as that in lane 3. No immunoreactive bands were observed in the cell lysates from the recombinant cells induced by glucose (lane 5).
Immune responses of recombinant lactobacilli induced by intragastric immunization.
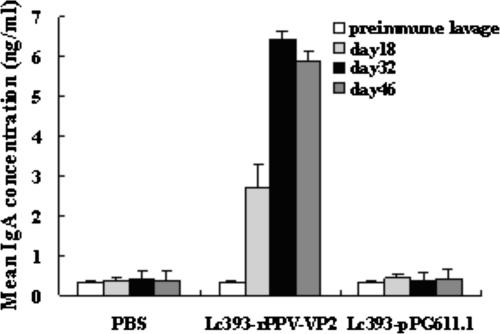
The mucosal immune response was studied by measuring the anti-PPV-VP2 IgA response in intestinal lavage fluids post-intragastric immunization. The concentration of mucosal IgA antibody against PPV-VP2 was determined via ELISA by using the PPV cultures as coating antigen, and the culture of ST cells was used as a negative control antigen. As the results show, there was no substantial difference in mucosal IgA level between the experimental and control groups prior to intragastric immunization, while oral immunization of recombinant Lc393-rPPV-VP2 elicited an antigen-specific mucosal IgA response. After the second booster, a high level of anti-PPV-VP2 IgA was obtained in the intestinal lavage fluids (Fig. 7) of mice immunized with rLc393-rPPV-VP2, whereas no anti-PPV-VP2 antibody was observed in the control groups of mice that received Lc393-pPG611.1 or PBS and for whom the ST cell culture was used as a negative control antigen (data not shown).
FIG. 7.
Anti-VP2-specific IgA levels in intestinal lavage fluid after intragastric immunization. The mice received three consecutive doses of 109 Lc393-rPPV-VP2 bacilli, three times at 2-week intervals. Control mice received 109 Lc393-pPG611.1 bacilli, while the negative control group received PBS. Intestinal lavage fluids collected on days 18, 32, and 46 after the first immunization were analyzed via ELISA by using PPV as the coating antigen. Bars represent the IgA titers ± standard errors of the means in each group.
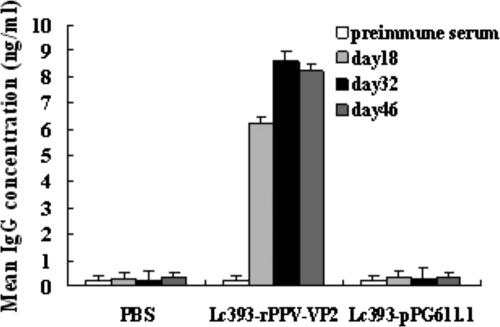
Likewise, the concentration of anti-PPV-VP2-specific IgG from immunized mice was also determined. After the first booster, a prompter and stronger level of anti-PPV-VP2-specific serum IgG was elicited from mice that were administered rLc393-rPPV-VP2 orally compared to the mucosal IgA level induced in the mice. No significant elicitation of anti-PPV-VP2 antibodies was observed in the control groups that received Lc393-pPG611.1 or PBS (Fig. 8) and for whom the ST cell culture was used as a negative control antigen (data not shown).
FIG. 8.
Anti-PPV-VP2 serum IgG response induced after intragastric immunization with rLc393-rPPV-VP2. Sera from groups of mice immunized orally with 109 rLc393-rPPV-VP2 and equivalent doses of Lc393-pPG611.1 and negative control sera from mice that received PBS were analyzed for the presence of anti-PPV-VP2-specific IgG by ELISA, using the PPV as the coating antigen. Bars represent the IgG titers ± standard errors of the means in each group.
Taken together, these results show that the rLc393-rPPV-VP2 generated in this study was able to elicit both PPV-specific systemic and mucosal antibody responses via oral administration.
Plaque reduction assay.
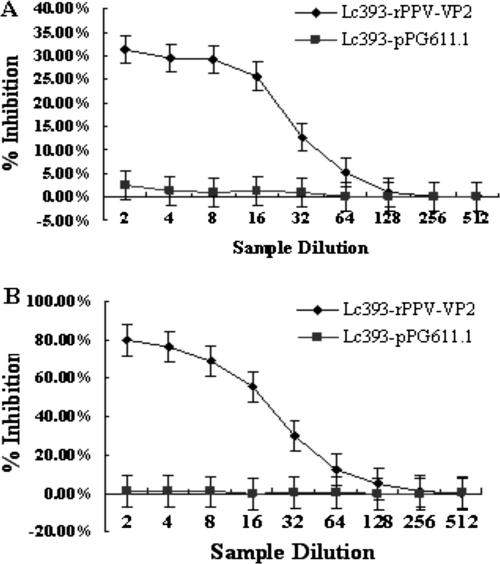
Plaque reduction assays were performed to further detect whether the antibody responses were specific against PPV VP2 protein. Results demonstrated that the presence of anti-PPV-VP2 IgA or IgG in the culture medium conferred statistically significant neutralizing effects (P < 0.05) on PPV infection (Fig. 9). A nearly 77.2% ± 1.27% reduction in the number of plaques was observed consistently when plaque reduction assays were carried out using two- to eightfold-diluted sera, and a 30.6% ± 1.11% reduction of plaques was observed by using two- to eightfold-diluted intestinal lavage fluids, which were obtained from the mice immunized with rLc393-rPPV-VP2. This inhibitory effect decreased gradually on further dilutions and reached a level similar to that of the buffer control or the control nonexpressor strain at dilutions of 1:128 and 1:256 of intestinal lavage fluid and sera, respectively.
FIG. 9.
Inhibition of viral plaque formation by (A) intestinal lavage fluids and (B) sera prepared from mice immunized with rLc393-rPPV-VP2. The maximum reduction in the number of plaques, expressed as a percentage of plaques obtained for the negative control samples, by using (A) intestinal lavage fluids or (B) sera collected from mice fed with Lc393-rPPV-VP2, was 31.2% ± 1.09% or 80.5% ± 1.13%, respectively. Results are mean values and standard errors (error bars) of triplicates.
DISCUSSION
With many pathogens, the initial infection occurs mainly at the mucosa tissues of the intestines. Therefore, it is important to develop vaccines that elicit protective immune responses to prevent the infection and replication of the pathogens at the mucosa via mucosal immunization. Furthermore, vaccines that can also induce specific immunity in the systemic lymphoid tissues are highly desirable, for most viral infections that gain entrance through the mucosal surfaces will become systemic. A great deal of research is being explored for oral application focused on the development of adequate mucosal vaccines with various vaccine delivery systems. In contrast, among the available approaches to stimulating efficient mucosal responses, using lactobacilli as carriers to deliver vaccine antigens probably constitutes one of the most successful strategies (21).
The use of lactic acid bacteria as a live vehicle for the delivery of antigens for mucosal immunization or other therapeutic molecules has been proposed. However, only a few systems have been described previously as using L. casei strains as a carrier for expressing heterologous viral antigens in a form that can be presented to and processed by the immune system of the mammalian host (10, 30, 31, 37). In this study, we engineered, for the first time, the use of L. casei 393 to express the PPV VP2 protein, which was without its antigenic properties being compromised and could be recognized by specific antiserum. Furthermore, the recombinant plasmid pPG611.1-VP2 in L. casei 393 has good segregational and structural stability without showing any structural rearrangement.
IgA is the predominant antibody at the mucosal surface, as it is produced locally at a level that exceeds that of all of other immunoglobulins (2, 15). Therefore, an efficient PPV oral vaccine will have to induce a specific mucosal IgA response. We evaluated the immunogenicity of rLc393-rPPV-VP2 by using BALB/c mice as an animal model. rLc393-rPPV-VP2 was shown to be able to elicit both mucosal and systemic immune responses after intragastric administration. The oral administration regimen used in this study consisted of three sets of three successive daily doses of the rLc393-rPPV-VP2 used as the experimental vaccine. This protocol was adapted from the procedure of Challacombe (4), who found that this pattern of immunization was effective consistently when particulate oral vaccines were used to immunize mice. Three successive daily doses of recombinant bacteria were required to ensure that a systemic antibody response to PPV could be elicited in all mice that received the recombinant strain intragastrically.
In order to confirm the efficacy of the induced antibodies in inhibiting the virus, we determined whether intestinal lavage fluids and sera could inhibit the infection of ST cells in a plaque reduction neutralization assay. Serum and intestinal samples collected from mice immunized with rLc393-rPPV-VP2 demonstrated statistically significant inhibition. For a delivery system, being able to adhere to and colonize the intestinal tract is important and desirable. In this study, we investigated the colonization potentiality of L. casei 393 in mouse intestines by using cFDA-SE. It was shown that 7.44 × 106 to 8.97 × 106 of the L. casei 393 cells fed orally to mice were able to adhere to the intestinal tracts of the mice, whereas the percentage of L. casei 393 that remained in the intestinal tract after the initial attachment as of day 1 varied from 11.79% to 76.59%. In particular, the amounts of L. casei 393 that remained adhered were maintained at high percentages of 71.3 to 87.49% and 76.59 to 87.74% in the jejunum and ileum, respectively. The recombinant strain generated in this study also demonstrated adherence and colonization abilities similar to those of its native counterpart (data not shown). Bile tolerance is a selection criterion for probiotics (35). The lactobacilli remained visible for 8 h after being incubated in MRS medium containing bile a concentration of 0.3% (wt/vol). The delivery system of L. casei 393 used in this study is able to adhere to and colonize intestinal tracts and to tolerate bile, which makes it, from a pharmaceutical standpoint, an attractive candidate as an agent for the delivery of antigens to the mucosa in particular vaccines.
In the present study, L. casei 393 was demonstrated to be able to survive transit in the upper gastrointestinal tract and was able to express and secrete heterologous parvovirus protein that induced specific immune responses against the antigen within the murine intestine milieu when administrated orally. Our research is under way to confirm the immune protection of the recombinant lactobacillus expressing VP2 protein for pigs, and the mechanism of how the antigen was able to transude into the intravascular compartment to induce systemic immune responses will also be investigated further. Nevertheless, the probiotic effects and harmless nature of L. casei 393 make it more appropriate as an oral vaccine carrier to deliver heterologous antigens for oral vaccines.
Acknowledgments
This work was supported by grant 30371074 from the National Natural Sciences Funds of China.
We thank NIZO food research for providing plasmid pPG611.1 and the bacterial strain L. casei ATCC 393.
Footnotes
Published ahead of print on 7 September 2007.
REFERENCES
- 1.Alander, M., R. Satokari, R. Korpela, M. Saxelin, T. Vilpponen-Salmela, and T. Mattila-Sandholm. 1999. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandtzaeg, P. 1994. Distribution and characteristics of mucosal immunoglobulin-producing cells, p. 251-279. In O. L. Pearay and H. Metecky (ed.), Handbook of mucosal immunology. Academic Press, Boston, MA.
- 3.Carwright, S. F., and R. A. Huck. 1967. Viruses isolated in association with herd infertility, abortions and stillbirths in pigs. Vet. Rec. 81:196-197. [Google Scholar]
- 4.Challacombe, S. J. 1983. Salivary antibodies and systemic tolerance in mice after oral immunization with bacterial antigens. Ann. N. Y. Acad. Sci. 409:177-193. [DOI] [PubMed] [Google Scholar]
- 5.Chang, Y. H., J. K. Kim, and H. J. Kim. 2001. Selection of a potential probiotic Lactobacillus strain and subsequent in vivo studies. Antonie Leeuwenhoek 80:193-199. [DOI] [PubMed] [Google Scholar]
- 6.Chatfield, S. N., I. G. Charles, and A. J. Makoff. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus toxin vaccine. Bio/Technology 10:888-892. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H. 2000. Recent advances in mucosal vaccine development. J. Control. Release 67:117-128. [DOI] [PubMed] [Google Scholar]
- 8.Elson, C. O., W. Ealding, and J. Lefkowiz. 1984. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretion. J. Immunol. Methods. 67:101-108. [DOI] [PubMed] [Google Scholar]
- 9.Gilliland, S. E., T. E. Staley, and I. Push. 1981. Importance of bile tolerance of Lactobacillus acidophilus used as a dietary adjunct. J. Dairy Sci. 67:3045-3051. [DOI] [PubMed] [Google Scholar]
- 10.Grangette, C., H. Muller-Alouf, D. Goudercourt, M. C. Geoffroy, M. Turneer, and A. Mercenier. 2001. Mucosal immune responses and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect. Immun. 69:1547-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho, P. S., J. Kwang, and Y. K. Lee. 2005. Intragastric administration of Lactobacillus casei expressing transmissible gastroenteritis coronavirus spike glycoprotein induced specific antibody production. Vaccine 23:1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo, H. S., C. R. Donaldson-Wood, R. H. Johnson, and R. S. F. Campbell. 1976. Observations of the pathogenesis of porcine parvovirus infection. Arch. Virol. 51:123-129. [DOI] [PubMed] [Google Scholar]
- 13.Joo, H. S., and R. H. Johnson. 1976. Porcine parvovirus: a review. Vet. Bull. 46:653-660. [Google Scholar]
- 14.Josson, K., T. Scheirlinck, F. Michiels, C. Platteeuw, P. Stanssens, and H. Joos. 1989. Characterization of a gram-positive broad-host-range plasmid isolated from Lactobacillus hilgardii. Plasmid 21:9-21. [DOI] [PubMed] [Google Scholar]
- 15.Kilian, M., and M. W. Russel. 1994. Functions of mucosal immunoglobulins, p. 127-143. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. R. McGhee, and J. Bienenstock (ed.), Handbook of mucosal immunology. Academic Press, San Diego, CA.
- 16.Kremer, L., L. Dupré, G. Riveau, A. Capron, and C. Locht. 1998. Systemic and mucosal immune responses after intranasal administration of recombinant Mycobacterium bovis Bacillus Calmette-Guérin expressing glutathione S-transferase from Schistosoma haematobium. Infect. Immun. 66:5669-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, Y. K., P. S. Ho, C. S. Low, H. Arvilommi, and S. Salminen. 2004. Permanent colonization by Lactobacillus casei is hindered by the low rate of cell division in mouse gut. Appl. Environ. Microbiol. 70:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.London, S. D. 1994. Cytotoxic lymphocytes in mucosal effector sites, p. 325-336. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. R. McGhee, and J. Bienenstock (ed.), Handbook of mucosal immunology. Academic Press, San Diego, CA.
- 19.Maassen, C. B. M., J. D. Laman, and M. J. Heijne den Bak-Glashouwer. 1999. Instruments for oral disease-intervention strategies: recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine 17:2117-2128. [DOI] [PubMed] [Google Scholar]
- 20.McGhee, J. R., J. Mestecky, M. T. Dertzbaugh, J. K. Eldridge, M. Hirasawa, and H. Kiyono. 1992. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 10:75-88. [DOI] [PubMed] [Google Scholar]
- 21.Medina, E., and C. A. Guzman. 2001. Use of live bacterial vaccine vectors for antigen delivery: potential and limitation. Vaccine 19:1573-1580. [DOI] [PubMed] [Google Scholar]
- 22.Mengeling, W. L., and R. C. Cutlip. 1975. Pathogenesis of in utero infection: experimental infection of five-week-old porcine fetuses with porcine parvovirus. Am. J. Vet. Res. 36:1173-1177. [PubMed] [Google Scholar]
- 23.Mestecky, J. 1987. The common mucosal immune system and current strategies for induction of immune responses in external secretion. J. Clin. Immunol. 7:265-276. [DOI] [PubMed] [Google Scholar]
- 24.Molitor, T. W., H. S. Joo, and M. S. Collett. 1983. Porcine parvovirus: virus purification and structural and antigenic properties of virion polypeptides. J. Virol. 45:842-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molitor, T. W., H. S. Joo, and M. S. Collett. 1984. Porcine parvovirus DNA: characterization of genomic and replicative form DNA of two virus isolates. Virology 137:241-254. [DOI] [PubMed] [Google Scholar]
- 26.Molitor, T. W., and H. S. Joo. 1990. Clinical and pathological features of porcine parvovirus-related disease and its diagnosis, p. 135-150. In P. Tijssen (ed.), Handbook of parvoviruses. CRC Press, Boca Raton, FL.
- 27.Ogawa, T., Y. Asai, K. Yasuda, and H. Sakamoto. 2005. Oral immunoadjuvant activity of a new symbiotic Lactobacillus casei subsp. casei in conjunction with dextran in BALB/c mice. Nutr. Res. 25:295-304. [Google Scholar]
- 28.Oliveiria, M. L. S., V. Monedero, E. N. Miyaji, L. C. C. Leite, P. L. Ho, and G. Perez-Martinez. 2003. Expression of Streptococcus pneumoniae antigens, PsaA (pneumococcal surface antigen A) and PspA (pneumococcal surface protein A) by Lactobacillus casei. FEMS Microbiol. Lett. 227:25-31. [DOI] [PubMed] [Google Scholar]
- 29.Perdigon, G., R. Fuller, and R. Raya. 2001. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 1:27-42. [PubMed] [Google Scholar]
- 30.Pouwels, P. H., R. J. Leer, and W. J. Boersma. 1996. The potential of Lactobacillus as a carrier for oral immunization: development and preliminary characterization of vector systems for targeted delivery of antigen. Bio/Technology 44:183-192. [DOI] [PubMed] [Google Scholar]
- 31.Reveneau, N., M. C. Geoffroy, C. Locht, P. Chagnaud, and A. Mercenier. 2002. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 20:1769-1777. [DOI] [PubMed] [Google Scholar]
- 32.Ridpath, J. F., and W. L. Mengeling. 1988. Uptake of porcine parvovirus into host and nonhost cells suggests host specificity is determined by intracellular factors. Virus Res. 10:17-28. [DOI] [PubMed] [Google Scholar]
- 33.Robinson, K., L. M. Chamberlain, K. M. Schofield, J. M. Wells, and R. W. F. Le Page. 1997. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 15:653-657. [DOI] [PubMed] [Google Scholar]
- 34.Ryan, E. T., J. R. Butterton, R. N. Smith, P. A. Carroll, T. I. Crean, and S. B. Calderwood. 1997. Protective immunity against Clostridium difficile toxin A induced by oral immunization with a live, attenuated Vibrio cholerae vector strain. Infect. Immun. 65:2941-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salminen, S., M. Playne, and Y. K. Lee. 2004. Successful probiotic lactobacilli: human studies on probiotic efficacy, p. 13-32. In C. Shortt (ed.), Functional dairy products. Technomic, Lancaster, United Kingdom.
- 36.Sambrook J., E. F. Fritisch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Scheppler, L., M. Vogel, A. W. Zuercher, M. Zuercher, J. E. Germond, S. M. Miescher, and B. M. Stadler. 2002. Recombinant Lactobacillus johnsonii as a mucosal vaccine delivery vehicle. Vaccine 20:2913-2920. [DOI] [PubMed] [Google Scholar]
- 38.Sedlik, C., M. Saron, J. Sarraseca, I. Casal, and C. Leclerc. 1997. Recombinant parvovirus-like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc. Natl. Acad. Sci. USA 94:7503-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Underdown, B. J., and J. Mestecky. 1994. Mucosal immunoglobulin, p. 79-98. In P. Ogra, J. Mestecky, M. E. Lamm, W. Strober, and J. R. Mcghee (ed.), Handbook of mucosal immunology. Academic Press, Boston, MA.
- 40.Weston, S. A., and C. R. Parish. 1990. New fluorescent dyes for lymphocyte migration studies: analysis by flow cytometry and fluorescent microscopy. J. Immunol. Methods 133:87-97. [DOI] [PubMed] [Google Scholar]