Abstract
Environmental strain Bacillus amyloliquefaciens FZB42 differs from the domesticated model organism of the same genus, Bacillus subtilis 168, in its ability to promote plant growth and suppress plant-pathogenic organisms present in the rhizosphere. This behavior is exerted mainly through the production of several nonribosomal cyclic lipopeptides and polyketides, which exhibit a broad range of action against phytopathogenic bacteria, fungi, and nematodes. Here, we provide evidence that the synthesis of the main antifungal agent of B. amyloliquefaciens FZB42, bacillomycin D, is regulated in multiple layers. Expression of the bacillomycin D operon (bmy) is dependent on a single σA-dependent promoter, Pbmy and is favored in its natural host by the small regulatory protein DegQ. The global regulators DegU and ComA are required for the full transcriptional activation of bmy. DegU retains a key role since it binds directly to two sites located upstream of the bacillomycin D promoter. Moreover, both DegU and a transmembrane protein of unknown function, YczE, act on a later level of gene expression, exerting their posttranscriptional effects in a hitherto-unknown manner.
Plant growth-promoting Bacillus amyloliquefaciens strain FZB42 is applied as a biocontrol agent, as it suppresses soilborne plant-pathogenic organisms. Its antagonistic function is attributed mainly to its ability to produce several secondary metabolites with antibacterial and antifungal activities (5). We have previously reported that FZB42 is a producer of the antibacterial polyketides bacillaene, difficidin/oxydifficidin (6), and a third polyketide which has been identified recently as macrolactin (5). Three cyclic lipopeptides are also produced by B. amyloliquefaciens FZB42 (referred to hereafter as FZB42): surfactin, fengycin, and bacillomycin D. Bacillomycin D has been shown to exert the main antifungal activity (20).
Bacillomycin D belongs to the iturin family of lipopeptides, with members such as iturin A and mycosubtilin. It is a cyclic heptapeptide with a β-amino fatty acid moiety and is synthesized nonribosomally according to the multicarrier thiotemplate mechanism (40). The bmy operon (37.2 kb) directs the biosynthesis of bacillomycin D and consists of four genes (bmyD, bmyA, bmyB, and bmyC) without orthologues in Bacillus subtilis 168. Several studies have elucidated the physicochemical and biological properties of peptides that belong to the iturin group (3, 25, 34). The chemical principles for the biosynthesis of iturin-like compounds are also largely characterized (12, 44). In contrast, little is known about the regulatory mechanisms that control the expression of the iturin-like lipopeptides (13).
Nonribosomal peptide synthetases require posttranslational modification to be functionally active (14). In bacilli, this is achieved by the sfp gene, which encodes a 4′-phosphopantetheinyl transferase and posttranslationally converts inactive apoenzyme peptide synthetases to their active holoenzyme forms (14, 18). Strains containing intact synthetases but a dysfunctional sfp gene, such as B. subtilis 168, which contains intact srf and fen operons but has a frameshift mutation in the sfp gene (27), are unable to produce the antibiotics. However, when B. subtilis 168 is complemented with a functional 4′-phosphopantetheinyl transferase, its antibiotic production is restored (30, 45).
It has also been reported that increased expression of the pleiotropic regulator DegQ in B. subtilis 168 (1) enhances the antibiotic production (45, 46). Interestingly, most of the natural Bacillus isolates that express peptide antibiotics show significantly elevated degQ expression compared to that of B. subtilis 168, due to the fact that the degQ promoter has a more σA consensus-like −10 hexamer in those strains (TACACT instead of CACACT) (50). However, whether DegQ directly influences the transcriptional regulation of the antibiotic operons or controls the expression of a posttranscriptional regulator involved in the antibiotic synthesis (for example, Sfp) has not been clarified.
Here, we have developed a genetic tool kit for FZB42 to decipher the complex regulatory network that controls the synthesis of bacillomycin D. 5′ deletion analysis of the bmyD promoter region and primer extension show that a single σA promoter, Pbmy, is responsible for the expression of the bmy operon, and additional upstream DNA cis-acting elements are necessary for the maximal activation of Pbmy. DegU is shown to directly bind to two distinct A/T-rich sites within these DNA cis elements. Finally, DegU and YczE posttranscriptionally regulate expression of bacillomycin D.
MATERIALS AND METHODS
Strains, plasmids, growth conditions, and DNA transformation.
The strains and the plasmids used in this study are listed in Table 1. Luria-Bertani (LB) medium and Difco medium 3 (purchased from BD) were used for the cultivation of bacteria. For antibiotic production and matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometric (MS) analysis, single colonies of FZB42 and its derivatives were incubated overnight in 10 ml LB with shaking (210 rpm) at 37°C. Landy medium (23) was inoculated with 1% of FZB42 and cultivated at 37°C and 210 rpm. At an optical density at 600 nm of 0.4 to 0.5, 0.6 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added. After a further 36 h of shaking, the supernatant was gathered for MALDI-TOF measurements. Thirty microliters of supernatant was used per hole for the agar diffusion inhibition test, using a growing culture of Saccharomyces cerevisiae as the indicator strain.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| MO1099 | JH642 MLSr amyE::erm trpC2 pheA1 | 15 |
| AK4 | MO1099 amyE::PbmyD−342bp-lacZ (Cmr) | This work |
| AK5 | MO1099 amyE::PbmyD−125bp-lacZ (Cmr) | This work |
| AK6 | MO1099 amyE::PbmyD−62bp-lacZ (Cmr) | This work |
| AK7 | MO1099 amyE::PbmyD+29bp-lacZ (Cmr) | This work |
| AK60 | AK4 with pAK64 (Kmr Phleor) | This work |
| AK61 | AK5 with pAK64 (Kmr Phleor) | This work |
| B. amyloliquefaciens | ||
| FZB42 | Wild type, producer of lipopeptides and polyketides | 19 |
| AK9 | FZB42 amyE::PbmyD−342bp-lacZ (Cmr) | This work |
| AK10 | FZB42 amyE::PbmyD−125bp-lacZ (Cmr) | This work |
| AK11 | FZB42 amyE::PbmyD−62bp-lacZ (Cmr) | This work |
| AK12 | FZB42 amyE::PbmyD+29bp-lacZ (Cmr) | This work |
| AK16 | FZB42 amyE::PbmyD−342bp-lacZ (Kmr) | This work |
| AK17 | FZB42 amyE::PbmyD−125bp-lacZ (Kmr) | This work |
| AK22 | AK16 comA::Emr | This work |
| AK23 | AK17 comA::Emr | This work |
| AK26 | AK16 yczE::Emr | This work |
| AK27 | AK17 yczE::Emr | This work |
| AK32 | AK16 degU::Emr | This work |
| AK33 | AK17 degU::Emr | This work |
| AK58 | FZB42 degU::Emr with pAK64 (Kmr Phleor) | This work |
| CH4 | FZB42 yczE::Emr | This work |
| CH23 | FZB42 comA::Emr | This work |
| CH41 | FZB42 PbmyD400bp::Pspac--lacI (Cmr) | This work |
| CH42 | CH4 yczE::Emr PbmyD400bp::Pspac-lacI (Cmr) | This work |
| CH43 | TF1 degU::Emr PbmyD400bp::Pspac-lacI (Cmr) | This work |
| TF1 | FZB42 degU::Emr | This work |
| S. cerevisiae | Indicator strain in agar diffusion assay | Laboratory stock |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 gyrA96 thi-1 relA1 | Laboratory stock |
| AK38 | DH5α(pREP4,pAK54) | This work |
| Plasmids | ||
| pDG148 | E. coli and B. subtilis shuttle vector, IPTG-inducible Pspac promoter, Apr Kmr Phleor | 42 |
| pDG268 | pBR322 derivative with promoterless lacZ, integration vector for recombination into B. subtilis amyE, Apr Cmr | 2 |
| pQE60 | Expression vector, IPTG-inducible promoter, His6-Taq, Apr | QIAGEN |
| pREP4 | Repressor plasmid carrying lacI, Kmr | QIAGEN |
| pECE73 | Cmr→Kmr exchange vector, Apr | 41 |
| pECE149 | LacI-bearing vector plasmid | BGSC |
| pUCcmR | Exchange of Ampr by Cmr cassette in pUC18 | This work |
| pAK5 | pDG268 carrying a fragment of bmyD from bp −342 to +184a | This work |
| pAK6 | pDG268 carrying a fragment of bmyD from bp −125 to +184 | This work |
| pAK7 | pDG268 carrying a fragment of bmyD from bp −62 to +184 | This work |
| pAK8 | pDG268 carrying a fragment of bmyD from bp +29 to +184 | This work |
| pAK9 | B. amyloliquefaciens FZB42 integration vector amyE::lacZ, Apr Cmr, pDG268 derivative | This work |
| pAK16 | pAK9 carrying a fragment of bmyD from bp −342 to +184 | This work |
| pAK17 | pAK9 carrying a fragment of bmyD from bp −125 to +184 | This work |
| pAK18 | pAK9 carrying a fragment of bmyD from bp −62 to +184 | This work |
| pAK19 | pAK9 carrying a fragment of bmyD from bp +29 to +184 | This work |
| pAK54 | pQE60 carrying degU of B. amyloliquefaciens FZB42 | This work |
| pAK64 | pDG148 carrying a 208-bp fragment of degQ | This work |
| pCH41A | pUCcmR carrying lacI (Cmr) | This work |
| pCH41B | pCH41A lacI PspacbmyD″ (Cmr) | This work |
| pCH41C | PCH41B bmyD′lacI PspacbmyD″ (Cmr) | This work |
The positions are relative to the bmy transcriptional start.
DNA transformation in B. subtilis MO1099 was performed as described previously (38). The media and buffers used for DNA transformation in FZB42 were the same as those described by Kunst and Rapoport (22), whereas competent cells were prepared using a previously described protocol (19, 20), which is based on the fact that FZB42 is competent in late exponential phase.
Primer extension analysis.
Total RNA was isolated from FZB42 cells grown in Difco medium, using the Nucleo Spin RNA L kit (Macherey Nagel). For the primer extension analysis, 40 μg of total RNA was mixed with 0.15 μM 5′-γ-32P-labeled primer (see Table S1 in the supplemental material for the two primers used in this study) at 70°C for 5 min. Then, 4 μl 5× reverse transcriptase buffer, 2 μl deoxynucleoside triphosphates (10 mM each), 1 μl RNase inhibitor (40 units), and 1 μl reverse transcriptase (200 units; Fermentas) were added to the mixture, and incubation was continued for 1 h at 42°C. The reaction was stopped by adding loading buffer. The samples were subsequently separated on a denaturating polyacrylamide sequencing gel (8 M urea, 7% acrylamide). Dideoxynucleotide sequencing reactions were done using the same 5′-γ-32P-labeled primer and the Thermo Sequenase cycle sequencing kit (USB) according to the manufacturer's instructions.
Construction of bmyD::lacZ transcriptional fusions in B. subtilis MO1099 and B. amyloliquefaciens FZB42.
Vector pAK9 (Table 1) is derived from the B. subtilis 168 integration vector pDG268 (2) and was constructed to enable us to integrate single-copy transcriptional fusions at the amyE locus in the FZB42 chromosome via double-crossover recombination. The highly homologous (89% on the nucleotide level) 5′ end of the B. subtilis amyE gene in pDG268 was kept intact, whereas the less homologous (81%) 3′ end of the B. subtilis amyE gene in pDG268 was replaced by the corresponding fragment of the amyE gene from FZB42.
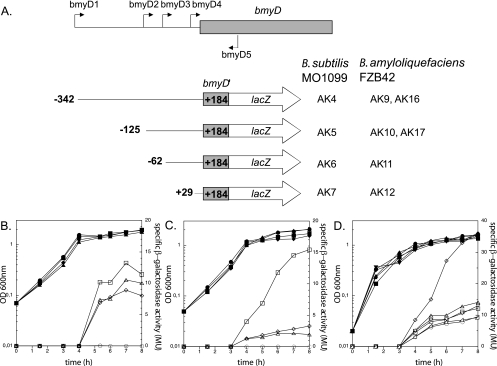
EcoRI/BamHI DNA fragments of the bmyD promoter region of FZB42, consisting of a common downstream end and variable upstream ends, were amplified by PCR using the primers bmyD1 to bmyD5 (these and the other primers used are listed in Table S1 in the supplemental material). The fragments were cloned into the B. subtilis integration vector pDG268 (2) and into its FZB42 counterpart pAK9, in each case upstream of the promoterless lacZ gene (Table 1). The derivative plasmids of pDG268 and pAK9 were linearized with XhoI and transformed into competent cells of B. subtilis MO1099 and FZB42, respectively. The correct chromosomal integration of the transcriptional fusions at the amyE locus was verified by PCR and Southern hybridization. (For a schematic representation of the fusions obtained, see Fig. 2A.)
FIG. 2.
Mapping of the bmyD promoter region by 5′ deletions. (A) Schematic representation of the different bmyD::lacZ fusions used in this study. The filled arrows indicate the primers used for generating the various reporter fusions (bmyD1 to -D5), whereas the 5′ and 3′ end termini of the bmyD::lacZ fusions are denoted with their nucleotide position relative to the transcriptional start. The derivative B. subtilis MO1099 and FZB42 strains that carry the respective fusions are shown on the right (see Table 1). (B and C) Expression of the series of bmyD::lacZ fusions, with different 5′ end termini, in B. subtilis MO1099 (B) and in FZB42 (C). Cells were grown in Difco medium at 37°C, and optical densities (OD) (closed symbols) and β-galactosidase activities (open symbols) were determined during growth. Squares, strains AK4/AK9; diamonds, strains AK5/AK10; triangles, strains AK6/AK11; circles, strains AK7/AK12. (D) Effect of DegQ on the expression patterns of the various bmyD::lacZ fusions in B. subtilis MO1099. Squares, strain AK4 without plasmid; circles, strain AK5 without plasmid; diamonds, strain AK60 (AK4+pAK64) with plasmid induced; triangles facing down, strain AK60 with plasmid uninduced; triangles facing up, strain AK61 (AK5+pAK64) with plasmid induced; rectangle-triangles, strain AK61 with plasmid uninduced.
Construction of plasmids and generation of B. amyloliquefaciens FZB42 mutant strains.
The comA mutant strain was constructed by amplifying a 2,092-bp PCR product with primers coma-1/2 and cloning in pGEM-T. To construct CH23, a central 104-bp fragment was removed from the comA gene with KspaI and Eco72I, and the Emr gene was inserted as a selective marker.
The lacI gene was prepared from plasmid ECE149 (BGSC) using restriction enzymes SmiI and BamHI and cloned into the BamHI- and Eco47III-digested plasmid pUCcmr to generate plasmid pCH41A.
The Pspac promoter was amplified with primers pspac-1-BamHI and pspac-2 using the plasmid pMUTIN-GFP+ (BGSC) as a template. The 5′part of the bmyD gene cluster was amplified with primers bmyDL-1 and bmyDL-2-sacI using FZB42 chromosomal DNA as a template. Both PCR fragments were fused together by the splicing by overlapping extension (SOE) method using pspac-1-BamHI and bmyDL-2-sacI as additional primers. The SOE-created fragment was digested with SacI and BamHI and inserted into the plasmid pCh41A, yielding pCH41B. To allow double-crossover homologous recombination, the further upstream promoter flanking region was amplified with primers bmyDR-1-HindIII and bmyDR-2 from FZB42 chromosomal DNA and inserted into pCH41B to create pCH41C. Integrative plasmid pCH41C was linearized with ScaI and transformed into FZB42 to create the mutant CH41 strain harboring the IPTG-inducible Pspac promoter. Chromosomal DNAs from the mutant strains TF1 (degU) and CH4 (yczE) were used in transformation of CH41, yielding CH42 and CH43.
To generate plasmid pAK64, a 0.2-kb DNA region encompassing the degQ coding region was amplified by PCR using the primer pair degQ1/2 and subsequently cloned into the HindIII/SphI-digested replication vector pDG148 under control of an IPTG-inducible promoter. Plasmid pAK64 was then transformed into AK4, AK5, and TF1, resulting in strains AK60, AK61, and AK58, respectively.
β-Galactosidase assay.
Specific β-galactosidase activity was determined from growing liquid cultures in Difco medium as described by Cutting and Van der Horn (7). β-Galactosidase was assayed using o-nitrophenyl-β-d-galactopyranoside as the substrate and is reported in Miller units (26).
MALDI-TOF MS analysis.
The wild-type and mutant strains were grown in Landy medium for 24 h. Lipopeptide products of FZB42 were identified in extracts of lyophilized culture filtrates by MALDI-TOF MS as previously described (20). α-Cyano-4-hydroxycinnamic acid was used as the matrix.
Purification of DegU.
A C-terminal His6 tag fusion to DegU was constructed using the commercially available plasmid pQE60 (QIAGEN). The B. amyloliquefaciens FZB42 degU gene was PCR amplified using the primers degU60a/b and was further cloned into an NcoI/BglII-digested pQE60 (QIAGEN) downstream of an IPTG-inducible promoter. The resulting plasmid, pAK54, was cotransformed with pREP4 (QIAGEN) into Escherichia coli DH5α competent cells, yielding AK38. To isolate DegU-His6, AK38 was grown and cells were harvested as previously described (17). The cells were then lysed and the protein was purified using the Protino Ni-1000 kit, according to the manufacturer's instructions (Macherey Nagel).
Electrophoretic mobility shift assay (EMSA).
A 450-bp DNA fragment harboring the bmyD promoter region between bp −342 and +108 relative to the translational start was amplified using primers bmyD1 and 5′-γ-32P-labeled rev1. The fragment was incubated at 37°C for 20 min with different amounts of purified DegU in binding buffer [20 mM Tris-HCl (pH 8), 100 mM KCl, 5 mM MgCl2, 0.5 mM dithiothreitol, 10% glycerol, 0.05% Nonidet P-40, 0.05 mg/ml bovine serum albumin, and 0.05 mg/ml poly(dI-dC) as a competitive nonspecific DNA]. The reaction mixtures were then separated on nondenaturating gels (8% polyacrylamide) in 1× Tris-borate-EDTA buffer at 60 V.
DNase I footprinting assay.
DNase I footprinting experiments were performed as described previously (48). A DNA fragment carrying the extended version of the bmyD promoter (450 bp; obtained by PCR amplification using primers bmyD1 and rev1) or the supercoiled plasmid pAK16 (carrying the same DNA fragment) was incubated in binding buffer with different amounts of DegU protein (0/0.8 and 1.6 μM) for 20 min at 37°C. Complexes were then treated with DNase I (0.6 μg/ml; Sigma) for 20 seconds, and the reaction was stopped by addition of nonspecific DNA (salmon sperm; 1.75 ng/μl) and rapid chilling on ice. Primer extension followed, with 5′-γ-32P-labeled bmyD1 and 5′-γ-32P-labeled rev1 for the template strand and for the nontemplate strand, respectively (denaturing temperature, 94°C [30 s]; annealing temperature, 58°C [30 s]; extension temperature, 72°C [30 s] [23 cycles]). The samples were then separated on 8 M urea-7% polyacrylamide sequencing gels and visualized using the Phosphorimager 445SI (Molecular Dynamics).
Nucleotide sequence accession numbers.
The nucleotide sequence accession numbers of two contigs containing the surfactin region together with the yczE gene (AJ575417) and the bacillomycin D operon (AJ575642) of FZB42 have been published previously (30). The accession numbers of the other gene sequences used in this study are as follows: degU, AM494075; and degQ-comP-comA, AM494074. They were derived from the complete genome sequence of FZB42, which is available under GenBank accession number CP000560.
RESULTS
Identification of the transcriptional start site of the bmy operon.
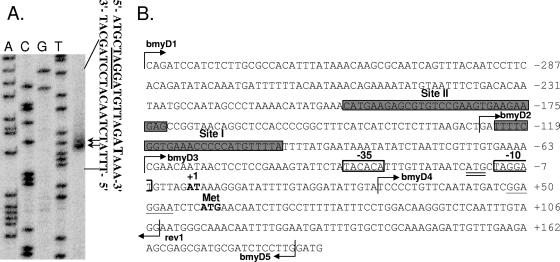
Primer extension analysis revealed two adjacent transcriptional start sites for the bmy operon: an adenine (A) nucleotide and a thymine (T) nucleotide, located 58 and 57 bp upstream of the gene's translation initiation codon (Fig. 1A). Sequences resembling the consensus of the −10 and −35 elements of the housekeeping sigma factor σA were found upstream of the mapped transcriptional start site (Fig. 1B). −35 (TATACA) and −10 (TAGGAT) hexamers could be identified in the proper distances from the transcriptional start and displayed 4/6 matches to the corresponding consensus sequences of σA. The spacer between them is 18 bp long and contains an extended −10 region directly upstream of the −10 hexamer, i.e., CATGc (the underlined nucleotides match the consensus, TRTGn [49]). Therefore, the promoter of bmyD (Pbmy) seems to be recognized and utilized by the vegetative sigma factor, despite the fact that bmy shows maximal expression during stationary phase (see below). Even though extensive attempts were made to identify additional transcriptional start sites upstream of the Pbmy, such sites were not found (data not shown).
FIG. 1.
Mapping of the transcriptional start of the bmy operon by primer extension analysis. (A) Primer extension was performed using the 5′-32P-end-labeled primer rev1. The first four lanes result from dideoxynucleotide sequencing reactions. Arrows indicate the positions of the two adjacent bmyD transcriptional starts. The respective DNA sequences are shown on the right. The transcriptional starts are indicated by larger letters, whereas the putative −10 element is underlined. (B) The bmyD promoter region. The positions of the two adjacent transcriptional starts (boldface, +1), the translational start (boldface, Met), the putative ribosome binding site (underlined), the −35 and −10 hexamers (boxes), and the extended −10 region (double underlined) are indicated. The 5′ end sites of the oligonucleotides used for primer extension (rev1) and for construction of reporter fusions (bmyD1 to -D5) are marked by arrows. The two DegU binding sites (sites I and II) are shaded.
DegQ is responsible for differences in bmy expression in B. subtilis MO1099 and B. amyloliquefaciens FZB42.
In order to monitor the transcriptional regulation of bacillomycin D, four reporter fusions of the bmy promoter region (Fig. 2A) to lacZ were generated. A series of nested fragments with a common downstream end (bp +184 relative to the transcriptional start) and variable upstream ends (bp −342, −125, −62, and +29 relative to the transcriptional start) were fused to a promoterless lacZ gene in pDG268 and pAK9 (see Materials and Methods). The transcriptional fusions were introduced as single copies in the chromosome of the heterologous host B. subtilis MO1099 and the homologous host FZB42, and their expression was determined throughout the growth cycle.
In B. subtilis MO1099, all four strains carrying the bmyD::lacZ reporter fusions were silent during the logarithmic phase (Fig. 2B). Upon entry into stationary phase, the transcriptional activity of AK4, AK5, and AK6 increased and reached its maximum after a further 3 hours. AK7 remained silent and did not show any β-galactosidase activity, since it lacked the entire promoter. The fact that the fusions of AK6 and AK5 showed no difference in their expression pattern throughout the whole growth cycle suggested that no additional trans-activating factor binds to the region between bp −125 and −62. AK4 exhibited slightly but reproducibly higher activity than the other two strains (AK6 and AK5) during stationary phase, indicating that the region between bp −342 and −125 might carry additional cis-activating elements.
In FZB42, the overall expression pattern of the various fusions was similar to that observed in B. subtilis MO1099, but with a notable exception (Fig. 2C). The strain carrying the full-length upstream promoter region, AK9, exhibited four- to fivefold more β-galactosidase activity in stationary phase than the strains harboring the shorter fusions (AK10 and AK11). This difference in expression levels between AK9 and AK10/AK11 is considerably higher than that observed for the corresponding strains of B. subtilis MO1099 (Fig. 2B), underlining the importance of this DNA upstream region for the full transcriptional activation of the bmy operon in its natural host.
Transcription of degQ in FZB42 is driven by the stronger σA promoter version, whereas B. subtilis MO1099, a derivative of the strain 168, carries the “defective” degQ promoter version. To test whether DegQ is responsible for the differential bmy expression patterns in the two strains, pAK64 (where degQ is expressed from an IPTG-inducible promoter [Table 1]) was transformed into AK4 and AK5 (Fig. 2A). As presented in Fig. 2D, the presence of uninduced pAK64 did not alter bmy expression from the two strains. Upon induction of pAK64, though, a significant 2.5-fold increase in the activity of AK4 could be observed. Since DegQ is present in higher levels in FZB42 than in B. subtilis MO1099, DegQ accounts, at least partially, for the different bmy expression in the two host strains. This is the first direct piece of evidence that DegQ controls the transcription of peptide antibiotics and not a subsequent step in their production. In the case of bacillomycin D, this effect seems to be dependent on a far-upstream region of the promoter. Since that DegQ is not a DNA binding protein, the effect on bacillomycin D should be mediated in an indirect manner, possibly through modulating the activity of another transcriptional regulator(s).
The global transcriptional regulators DegU and ComA control the production of bacillomycin D.
In order to obtain further information concerning the transcriptional regulation of bacillomycin D, a series of mutations in transcriptional regulatory proteins of FZB42 were generated. The mutations were then introduced into strains AK9 and AK10 (AK11 was excluded from this analysis, since it displays the same pattern of gene expression as AK10 [Fig. 2C]), and the β-galactosidase activities of the mutant strains were monitored along the growth curve and compared to that of the wild-type strain. Using this approach, several proteins involved in the regulation of the bmy operon were identified (Fig. 3A and data not shown).
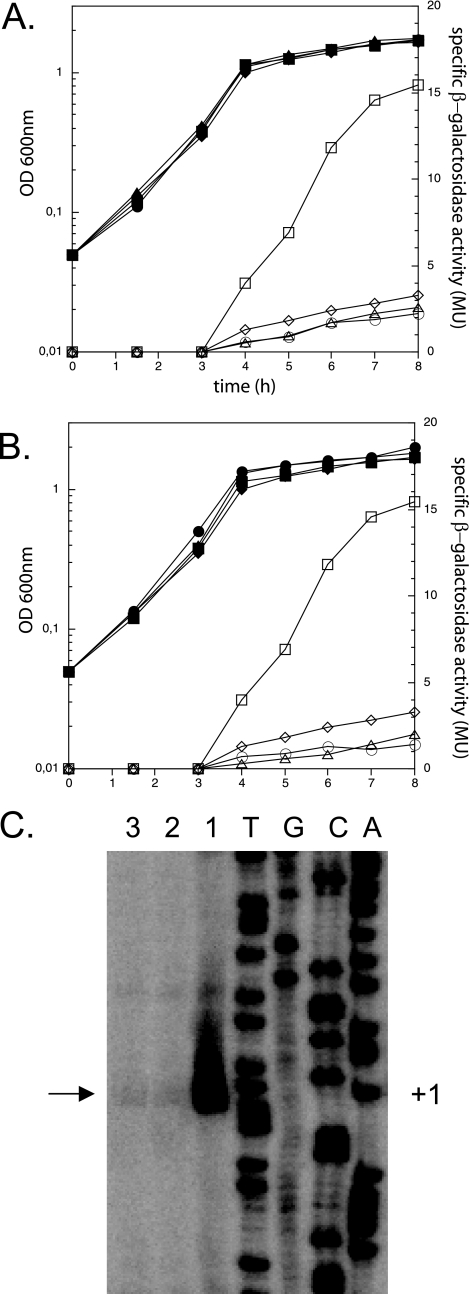
FIG. 3.
DegU and ComA control the expression of bacillomycin D. (A and B) Strains AK9 and AK10, carrying the two bmyD::lacZ fusions with the longest upstream regions (see also Fig. 2A), and their degU (A) and comA (B) mutant derivatives were grown in Difco medium at 37°C, and optical densities (OD) (closed symbols) and β-galactosidase activities (open symbols) were determined during growth. Squares, AK9; diamonds, AK10; triangles, mutant derivatives of AK9 (AK32 in panel A and AK22 in panel B); circles, mutant derivatives of AK10 (AK33 in panel A and AK23 in panel B). (C) Effects of degU and comA mutations on the activity of the bmy operon promoter (Pbmy). The intensities of the obtained transcripts indicate the effects exerted by the respective mutations on the activity of the Pbmy promoter. 1, FZB42; 2, TF1 (degU); 3, CH23 (comA). +1 and the adjacent arrow indicate the transcriptional start.
Two two-component response regulator proteins were found to be essential for full activation of bacillomycin D expression: DegU, which is known to control the expression of degradative enzymes (21, 28) and to be involved in the initiation of competence (17), and ComA, a regulator of late competence genes (16) and surfactin production (35, 36). Inactivation of the genes coding for each of these proteins resulted in severely impaired promoter activity during stationary phase, especially in strains harboring the whole promoter region (AK9 derivatives). bmy transcription was three- to fourfold lower in the degU and comA mutant derivatives of AK9 than in the parental strain and was similar to that of the wild-type strain AK10, which carries the shorter promoter region. Furthermore, in the absence of DegU or ComA, the activities of AK10 derivatives were also reduced in comparison to that of the parental strain, albeit to a much smaller extent (Fig. 3A and B).
Therefore, DegU and ComA are both required for the full activation of the bmyD promoter, and their effects seem to be exerted mostly through the DNA region located between bp −342 and −125 upstream of the bmyD transcriptional start. Note that despite the severity of the reported effects, the transcription of bmyD is not completely silenced in any of those mutants, implying that the σA-dependent promoter retains basic activity even in the absence of the two regulators.
To verify that the effects of DegU and ComA on transcription of the bmy operon were exerted via the unique housekeeping promoter of the identified operon, we performed additional primer extension experiments using wild-type FZB42 and its derivative mutant strains (Fig. 3C). Consistent with the results obtained from the lacZ reporter fusions, the two mutant strains exhibited significantly lower bmy transcript levels than the parental strain. This finding confirms the fact that cells lacking those regulators are still able to show basal expression of the σA-dependent Pbmy.
DegU binds at two sites in the bacillomycin D promoter region.
The actions of DegU and DegQ are strongly interconnected. DegU stimulates the expression of degQ, but DegQ needs DegU in order to regulate sacB (29). While both proteins were found to be important for optimal bmy expression, which of the two proteins exerted a direct effect on Pbmy? Inducing DegQ production in a degU mutant strain did not relieve bacillomycin D expression or production, as demonstrated by primer extension and MALDI-TOF MS (see Fig. S1 in the supplemental material). This means that the effect of DegU on bmy expression is epistatic to that of DegQ and that the latter needs the former in order to exert its role.
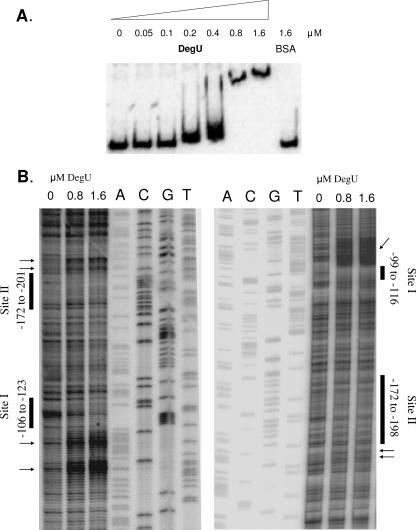
To examine whether the bmy promoter is a direct target of DegU, gel retardation EMSAs of a 450-bp DNA fragment harboring the region between bp −342 and +108 relative to the transcriptional start were performed using increasing concentrations of the response regulator. As seen in Fig. 4A, the bmyD promoter contains specific binding sites for DegU. The DNA fragment was slightly shifted with 0.2 and 0.4 μM unphosphorylated DegU, in a manner analogous to that for the DegU binding to the comK promoter (17). The mobility of the 450-bp DNA fragment changed dramatically upon incubation with higher protein concentrations (0.8 and 1.6 μM), indicating the presence of more than one DegU binding site at the promoter region of bmy. The observed shifts are DegU specific, since no DNA fragment migration was observed upon incubation of the DNA fragment with the same amounts of bovine serum albumin (Fig. 4A).
FIG. 4.
DegU binds directly to the bmy promoter. (A) Gel retardation mobility shift assays of a 32P-labeled bmyD promoter fragment using increasing concentrations of DegU, as indicated. The DNA fragment used harbors regions between bp −342 and +108 relative to the transcriptional start. (B) DNase I footprinting of DegU at the bmy promoter region. The DNase I digestion patterns were obtained using primer extension in order to be able to monitor both linear and supercoiled DNA; results were very similar with both types of DNA, and the results with supercoiled DNA are shown. The footprints of the coding and noncoding strands in the presence of increasing concentrations of DegU can be seen in the left and right panels, respectively. The protected and hypersensitive regions are marked with bars and arrows, respectively. The sequence reactions of the appropriate DNA strands were used as size markers.
Since DegU affects the expression of both the shorter and the longer versions of the bmyD::lacZ fusions (present in strains AK9 and AK10 [Fig. 3A]), we performed EMSA with two smaller DNA fragments. Fragment D1 encompassed the region between bp −342 and −126 relative to the transcriptional start, which is present only in AK9, while fragment D2 encompassed the DNA region between bp −125 and 108 bp, which is present in both AK9 and AK10. EMSA experiments with fragments D1 and D2 and increasing concentrations of the response regulator confirmed that unphosphorylated DegU directly binds to both DNA regions, with similar affinities (data not shown).
The exact locations of the DegU binding sites at the bmy promoter region were determined by DNase I footprinting. The footprinting patterns obtained with the coding and the noncoding strands led to similar conclusions. In the presence of DegU, a region spanning from bp −123 to −106 on the coding strand was protected. The protected area was followed by an extended region of hypersensitive sites ranging from bp −103 to −85. A second protected area with adjacent hypersensitive sites was identified between bp −201 and −172 on the coding strand. Consistently, the noncoding strand contained protected areas between bp −116 and −99 and bp −172 and −198 upon DegU addition. Hypersensitive sites could be observed in the region between bp −98 and −66 on the same strand (Fig. 4B).
Posttranscriptional control of bacillomycin D synthesis.
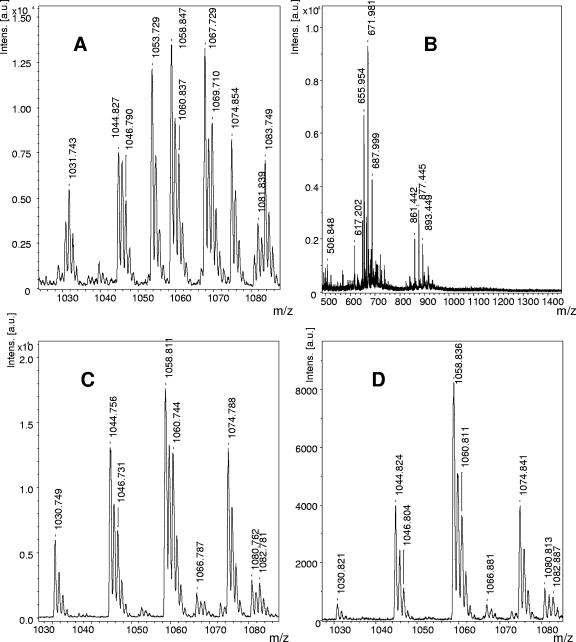
The assessment of the effects of the regulatory proteins DegU and ComA and of a yczE mutation on bacillomycin D synthesis was monitored by MALDI-TOF MS, which is an efficient and fast method to obtain qualitative and half-quantitative results for lipopeptides production in crude extracts in a minimum of time. As the reference, an MS of lipopeptide formation by the wild-type strain FZB42 is shown in Fig. 5A. Its main product is bacillomycin D, as judged from the intensities of the mass peaks at m/z 1031.7, 1053.7, 1067.7, 1069.7, 1081.7, and 1083.7, which can be attributed to sodium and potassium adducts of isoforms with fatty acid side chains of 14 to 16 carbon atoms. They are found together with those of surfactins in the mass range of m/z 1030 to 1090. The surfactin peaks appear at m/z 1030.8, 1044.8, 1046.8, 1058.8, 1060.8, and 1074.8. They are of lower intensity than the bacillomycin D peaks and correspond to surfactin species with fatty acid side chains of 13 to 15 carbon atoms. The peaks found at m/z 1463.8, 1477.8, 1485.8, 1487.8, 1499.8, 1501.8, 1513.8, 1515.8, 1527.8, and 1529.8, indicating C15 to C17 fengycins, show the lowest intensities of the lipopeptide products. In the MALDI-TOF MS of the wild-type strain (see Fig. S2A in the supplemental material), their intensities were approximately 10-fold lower than those of bacillomycin D. In the MS of the comA mutant CH23, the intensities of the bacillomycin D peaks were strongly reduced, showing a size similar to those of the fengycin peaks, which are slightly higher than those in the spectrum of the wild-type strain. It can be estimated that in CH23, bacillomycin D formation was lowered to about 10 to 20% of that in the wild-type corroborating our previous findings on the influence of ComA on bmy D expression. Apparently, deletion of comA in FZB42 severely impaired but did not silence transcription of the bmy operon. Pbmy retained its basal expression at the transcription level. In addition, in agreement with previous results (42), the comA mutant strain is also deficient in surfactin production (see Fig. S2B in the supplemental material).
FIG. 5.
MALDI-TOF MS of the lipopeptide products in culture filtrate extracts of the wild-type B. amyloliquefaciens FZB42 (A) and of the mutants CH3 (sfp) (B), TF1 (degU) (C), and CH4 (yczE) (D). C14-16 bacillomycin D-specific mass peaks at m/z 1031.7, 1053.7, 1067.7, 1069.7, 1081.7, and 1083.7 were present only in the supernatant of the wild type (A), while the mutant strains did not produce this antibiotic. The TF1 degU (C) and the CH4 yczE (D) mutant strains were still able to produce C13-15 surfactins, as indicated by the remaining mass peaks between m/z 1030 and 1090.
Mutant CH3 bears a mutation in the 4′-phosphopantetheinyl transferase Sfp that results in a complete deficiency of lipopeptide production as deduced from the MS of its culture filtrate (Fig. 5B). Surprisingly, bacillomycin D completely disappeared in a culture filtrate of the DegU mutant strain TF1 (Fig. 5C), suggesting that the role of DegU in bacillomycin synthesis goes beyond the mere activation of the Pbmy promoter during transcription. To test whether this effect was associated with the export of this lipopeptide to the extracellular milieu, a sonicated cell extract of the degU strain was analyzed by MALDI-TOF MS. However, in this case bacillomycin D also could not be detected (data not shown), suggesting that DegU influences both transcription of the bmy operon and a posttranscriptional step prior to its export to the surrounding environment.
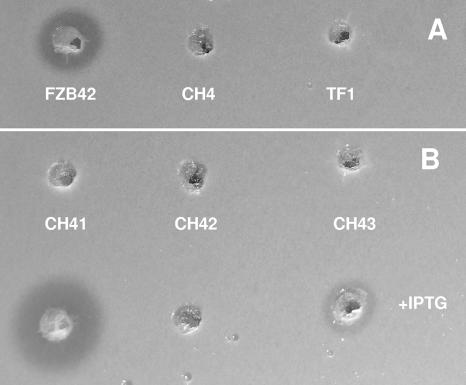
Surprisingly, the disruption of the yczE gene, which is located adjacent to sfp, also completely abolished the production of bacillomycin D, whereas surfactin and fengycin biosynthesis remained unimpaired (Fig. 5D). Previous results have shown that the fungicidal action exerted by bacillomycin D can be used to indicate production of bacillomycin D (20). While production of bacillomycin D by FZB42 was visible as a distinct zone of inhibition within a lawn of the baker's yeast Saccharomyces cerevisiae, no fungicide production was visible in the degU and yczE mutant strains TF1 and CH4, respectively (Fig. 6A). To test whether YczE exerts bacillomycin D synthesis on the transcriptional level, the yczE::Emr mutation was introduced into the PbmyD::lacZ fusion strains AK9 and AK10. The β-galactosidase activity was not influenced by the yczE mutation (see Fig. S3A in the supplemental material), suggesting that YczE acts posttranscriptionally on bacillomycin D synthesis. Since YczE encodes an integral membrane protein of 215 amino acids consisting of five predicted transmembrane spanning helices (http://www.cbs.dtu.dk/services/TMHMM/), its function could be implicated in the export of bacillomycin D. To test this hypothesis, a sonicated cell extract of the yczE mutant strain was analyzed by MALDI-TOF MS. Bacillomycin D was not found inside the cells (data not shown), indicating that YczE is involved not in the antibiotic's export but rather in its synthesis, in a yet-unidentified manner. Finally, it can be ruled out that the posttranscriptional effects of DegU and YczE on bacillomycin D synthesis are mediated through the same pathway, since DegU did not activate the yczE promoter (see Fig. S3B in the supplemental material).
FIG. 6.
Production of bacillomycin D by FZB42 derivatives as indicated by growth inhibition of the Saccharomyces cerevisiae indicator strain used in the agar diffusion assay. (A) FZB42, CH4 (yczE), and TF1 (degU). (B) Top, CH41 (PbmyD::Pspac), CH42 (PbmyD::Pspac yczE), and CH43 (PbmyD::Pspac degU) without IPTG; bottom, CH41 (PbmyD::Pspac), CH42 (PbmyD::Pspac yczE), and CH43 (PbmyD::Pspac degU) after induction by IPTG.
Posttranscriptional control exerted by YczE is absolutely essential for bacillomycin D synthesis.
In order to examine whether DegU and/or YczE is required for the posttranscriptional expression of bacillomycin D, we replaced the natural PbmyD promoter with the IPTG-inducible Pspac promoter in FZB42 and the respective mutant strains. In the absence of IPTG, no bacillomycin D production was recorded. However, in the presence of IPTG, CH41, containing the IPTG-inducible promoter, expressed large amounts of bacillomycin D as indicated by its fungicidal action against baker's yeast. In contrast, IPTG did not turn on bacillomycin D production in strain CH42, bearing the same promoter but deficient in yczE. In strain CH43, harboring the degU mutation, production of bacillomycin D was strongly reduced but not completely silenced (Fig. 6B).
DISCUSSION
We have demonstrated here that the bmy operon, governing nonribosomal synthesis of the cyclic lipopeptide bacillomycin D, the main component of antifungal compounds produced by FZB42, is under direct transcriptional control of DegU, in contrast to the related cyclic lipopeptide surfactin, which is part of the ComA regulon (31, 36). Expression of bmy is dependent on a σA-dependent promoter, Pbmy, which is activated by interaction with DegU during early stationary phase. A similarly organized promoter has been reported to control the expression of iturin A in B. subtilis RB14 (44), though the reported transcriptional start, which is situated 1 bp downstream of our main transcriptional start, differs slightly from the one identified here. Although a direct interaction between DegU and the iturin promoter has not been investigated, it is very likely that expression of the giant gene clusters governing synthesis of iturin-like compounds in bacilli (12, 18, 44) are controlled by the same transcriptional regulator.
DegU binds to and activates Pbmy.
DegU is a two-component system response regulator that controls many cellular processes, including exoprotease production, competence development, motility, and osmotic response, and triggers post-exponential-phase responses under growth-limiting conditions (9, 10, 22, 39). Here we have pinpointed a key function of DegU in transcription regulation of a nonribosomally synthesized antibiotic. A series of in vivo and in vitro data indicated that DegU directly activates the expression of Pbmy. The results from the EMSA and the DNase I footprinting experiments coincided and indicated that DegU occupied two distinct DNA binding sites at the bmy promoter. The first site, site I, is located close to the transcriptional start, between bp −123 and −99, whereas the second one, site II, is situated further upstream, between bp −201 and −172. Binding of DegU to site II is essential for the optimal activation of the promoter (Fig. 3A). We propose that the binding of DegU to site I triggers a sharp DNA bend directly downstream of it (seen as hypersensitivity in the footprinting analysis), thus enabling the site II-bound DegU to activate the promoter. This is a rather common transcriptional activation mechanism (4).
The protection that DegU offers to the DNA at its two binding sites is relatively weak, but strong hypersensitive sites can be observed adjacent to the two DNA binding sites, implying that the binding of DegU to its sites rearranges the local DNA architecture, probably by inducing strong DNA bending, constraint, or even unwinding, which makes the DNA more accessible to DNase I attack. Similarly, DegU was proposed to activate the comK promoter by altering the shape of the approximately four DNA helices that separate the tandem ComK boxes and thereby facilitating the binding of ComK to them (17).
There are several reasons why the binding of DegU to the DNA only weakly protects the latter against DNase I attack. First, in both our experiments and the previous study (17), unphosphorylated DegU was used for the footprinting analysis. Although in many studies response regulators are used in their unphosphorylated state in order to demonstrate DNA binding, the use of the phosphorylated form often results in more distinct/extended regions being protected against DNase I cleavage (8, 36). Therefore, footprinting analysis was also performed with DegU after incubation with “cold” acetyl phosphate, and results very similar to those with the unphosphorylated DegU were obtained. Even though incubation of a response regulator with acetyl phosphate should result in its phosphorylation, no direct proof that transphosphorylation actually took place can be provided. Another reason for the weak protection patterns of DegU is the nature of its binding sites. A/T-rich DNA regions, such as the DNA binding sites of DegU, are more curved and therefore less accessible to DNase I, even when the DNA is naked without any protein bound to it. Thus, the A/T-rich DNA binding sites appear to be “protected,” even in the absence of their binding partner.
Despite the fact that studies directly monitoring the binding of DegU to DNA promoter regions in vitro are limited (17, 32, 37), two possible motifs have been proposed as putative DegU recognition sites. In vitro data from the aprE and comK promoters indicated that DegU recognizes an A/T-rich motif (37), whereas in vivo studies with the wapA promoter and an alignment of DegU-regulated promoters indicated AGAA-N11-TTCAG as the recognition site for DegU (10). Although none of these studies provides conclusive evidence and they contradict each other, degenerate forms of the latter motif could be identified in the DegU protected regions within sites I and II at the bmy promoter region, whereas the A/T-rich motifs proposed as putative DegU binding sites were part of the hypersensitive sites that were generated at the bmy promoter region upon addition of DegU in the DNase I footprints. In any case, further experimental evidence, involving extensive site-directed mutagenesis, will be required to identify the consensus sequence recognized by DegU in Pbmy and/or other promoters.
It is generally assumed that DegU has two modes of action: phosphorylated DegU (DegU-P) directly activates degradative enzyme production and represses motility, whereas unphosphorylated DegU directly stimulates competence (9) through binding to the comK promoter (17). The assumption that only unphosphorylated DegU is required for competence has been based on the observation that either hyperphosphorylation of DegU or complete inactivation of the degSU operon decreases competence, whereas inactivation of degS alone leaves competence unaffected. Moreover, a DegU mutant with an impaired phosphorylation site has no effect on competence (9). However, there are alternative explanations of why neither the hyperactive form of DegU nor the complete absence of DegU allows competence, whereas the modest activity of the unphosphorylated DegU is sufficient to activate competence. DegU has opposing effects on different members of the DNA uptake gene cascade. On one hand, it coactivates the comK promoter (17), but on the other hand, it represses the srf operon (24) and therefore also inhibits the expression of comS. Reduced ComS levels result in an enhanced MecA/ClpCP-mediated degradation of ComK (47). Thus, it may well be that the final action of DegU on comK expression is only positive, when the levels of DegU and/or its DNA binding affinity are relatively low (the unphosphorylated DegU form has probably a weaker binding affinity to its DNA targets). In contrast, the negative effect on comS expression prevails when the cellular amounts or the activity of DegU increase. This scenario is supported by recent microarray results (33), in which the DegU regulon was defined by comparing a degS mutant with its isogenic strain bearing a DegU-containing plasmid. comK was not part of the induced genes, whereas all the phosphorylated DegU-dependent genes were. This means that the phosphorylation state of DegU alone does not define the targets of DegU. In our case, DegU activates the expression of the bmy operon during stationary-phase growth. Therefore, it seems plausible that DegU-P is more suitable for optimal promoter binding and activation. However more direct evidence is required to verify this suggestion.
DegQ positively affects bmy expression via DegU.
DegQ is a small pleiotropic regulatory protein, which consists of 46 amino acids and controls the expression of degradative enzymes, intracellular proteases, and several other secreted enzymes (1, 29). Recently it was also shown to enhance the production of the peptide antibiotics plipastatin and iturin A (45, 46). Here we demonstrate that DegQ transcriptionally activates the biosynthesis of a nonribosomally synthesized antibiotic and therefore is important for the high production of bacillomycin D in its host strain. However, the effects of DegQ are only indirect and are mediated via DegU for a number of reasons: (i) DegQ shares no homology to typical transcriptional regulators, i.e., DNA binding proteins; (ii) DegQ overexpression cannot complement the loss of DegU in terms of bacillomycin D synthesis; and (iii) DegQ affects bmy expression only when both DegU recognition sites are kept intact in the bmyD promoter region (Fig. 2D). Our results corroborate the earlier observation that DegQ controls the expression of sacB (encoding a levansucrase) only in the presence of DegU (29). We suggest that DegQ modulates the activity of DegU, via an unknown mechanism. Interestingly, DegQ shows homology to a small region of the eukaryotic A kinase anchor proteins (11). Therefore, a possible role could be that it facilitates the transphosphorylation to DegU.
ComA probably affects bmy expression via DegQ/DegU.
While DegU was shown to directly bind to the bmy promoter region, it is likely that ComA controls bmy expression indirectly via DegQ, which serves as an auxiliary factor to DegU. The absence of putative ComA binding boxes within the bmyD upstream region supports this assumption. However, the two ComA boxes found in the degQ gene in B. subtilis are conserved in FZB42, and therefore ComA likely promotes the expression of degQ in this organism, as has been shown in B. subtilis (29). Unfortunately, it was impossible to verify the proposed indirect role of ComA, as we were unable to obtain a comA-deficient mutant of FZB42 in which degQ is expressed from an IPTG-inducible promoter.
DegU and YczE posttranscriptionally control bacillomycin D production.
Deletion of both degU and yczE specifically abolished the production of bacillomycin D without influencing the production of other peptide antibiotics produced by FZB42. While DegU has an additional effect on Pbmy activity, YczE exerts its effect only in a posttranscriptional manner. Neither is involved in the export of the lipopeptide into the external milieu, and they exert their effects through independent pathways. This conclusion is supported by the results obtained with strains in which the IPTG-inducible Pspac promoter was inserted in order to replace the natural Pbmy promoter. Efficient synthesis of bacillomycin D occurred only in the wild-type background, while in the presence of the degU mutation, the Pspac-dependent synthesis was strongly reduced. In the presence of the yczE mutation, the bacillomycin D synthesis was completely abolished, suggesting that yczE is essential for an unknown posttranscriptional event during synthesis.
A recent report (43) describes the existence of a membrane-bound, organelle-like protein complex responsible for synthesis of the polyketide bacillaene in B. subtilis, a process resembling nonribosomal synthesis of lipopeptides. It can be speculated that the giant nonribosomal peptide synthetase involved in bacillomycin D synthesis in FZB42 assumes a similar superstructure, and the integral membrane protein YczE is a candidate for anchoring bacillomycin D synthetase at the membrane.
Supplementary Material
Acknowledgments
We are indebted to Christiane Müller for technical assistance. We are also grateful to Tzu-Fang Huang for kindly providing strain TF1.
This work was financially supported by the German Ministry of Education and Research as a part of the competence network Genome Research on Bacteria (GenoMik/GenoMik-Plus).
Footnotes
Published ahead of print on 7 September 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amory, A., F. Kunst, E. Aubert, A. Klier, and G. Rapoport. 1987. Characterization of the sacQ genes from Bacillus licheniformis and Bacillus subtilis. J. Bacteriol. 169:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniewski, C., B. Savelli, and P. Stragier. 1990. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J. Bacteriol. 172:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besson, F., F. Peypoux, G. Michel, and L. Delcambe. 1978. Mode of action of iturin A, an antibiotic isolated from Bacillus subtilis, on Micrococcus luteus. Biochem. Biophys. Res. Commun. 81:297-304. [DOI] [PubMed] [Google Scholar]
- 4.Browning, D., and S. J. Busby. 2004. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 2:57-65. [DOI] [PubMed] [Google Scholar]
- 5.Chen, X. H., A. Koumoutsi, R. Scholz, A. Eisenreich, K. Schneider, I. Schneider, B. Morgenstern, B. Voss, W. R. Hess, O. Reva, H. Junge, B. Voigt, B. R. Jungblut, J. Vater, R. Süssmuth, H. Liesegang, A. Strittmatter, G. Gottschalk, and R. Borriss. 2007. Comparative analysis of the complete genome sequence of the plant growth promoting Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. doi: 10.1038/nbt1325. [DOI] [PubMed]
- 6.Chen, X. H., J. Vater, J. Piel, P. Franke, R. Scholz, K. Schneider, A. Koumoutsi, G. Hitzeroth, N. Grammel, A. W. Strittmatter, G. Gottschalk, R. D. Süssmuth, and R. Borriss. 2006. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB42. J. Bacteriol. 188:4024-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutting, S. M., and P. B. Van der Horn. 1990. Molecular biological methods for Bacillus. Wiley, Chichester, United Kingdom.
- 8.Dahl, J. L., B. Y. Wei, and R. J. Kadner. 1997. Protein phosphorylation affects binding of the Escherichia coli transcription activator UhpA to the uhpT promoter. J. Biol. Chem. 272:1910-1919. [DOI] [PubMed] [Google Scholar]
- 9.Dahl, M. K., T. Msadek, F. Kunst, and G. Rapoport. 1992. The phosphorylation state of the DegU response regulator acts as a molecular switch allowing either degradative enzyme synthesis or expression of genetic competence in Bacillus subtilis. J. Biol. Chem. 267:14509-14514. [PubMed] [Google Scholar]
- 10.Dartois, V., M. Debarbouille, F. Kunst, and G. Rapoport. 1998. Characterization of a novel member of the DegS-DegU regulon affected by salt stress in Bacillus subtilis. J. Bacteriol. 180:1855-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dransfield, D. T., J. L. Yeh, A. J. Bradford, and J. R. Goldenring. 1997. Identification and characterization of a novel A-kinase-anchoring protein (AKAP120) from rabbit gastric parietal cells. Biochem. J. 322:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duitman, E. H., L. W. Hamoen, M. Rembold, G. Venema, H. Seitz, W. Saenger, F. Bernhard, R. Reinhardt, M. Schmidt, C. Ullrich, T. Stein, F. Leenders, and J. Vater. 1999. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc. Natl. Acad. Sci. USA 96:13294-13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duitman, E. H., D. Wyczawski, L. G. Boven, G. Venema, O. P. Kuipers, and L. W. Hamoen. 2007. Novel methods for genetic transformation of natural Bacillus subtilis isolates used to study the regulation of the mycosubtilin and surfactin synthetases. Appl. Environ Microbiol. 73:3490-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finking, R., and M. A. Marahiel. 2004. Biosynthesis of nonribosomal peptides. Annu. Rev. Microbiol. 58:453-488. [DOI] [PubMed] [Google Scholar]
- 15.Gonzy-Treboul, G., C. Karmazyn-Campelli, and P. Stragier. 1992. Developmental regulation of transcription of the Bacillus subtilis ftsAZ operon. J. Mol. Biol. 224:967-979. [DOI] [PubMed] [Google Scholar]
- 16.Guillen, N., Y. Weinrauch, and D. A. Dubnau. 1989. Cloning and characterization of the regulatory Bacillus subtilis competence genes comA and comB. J. Bacteriol. 171:5354-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamoen, L. W., A. F. Van Werkhoven, G. Venema, and D. Dubnau. 2000. The pleiotropic response regulator DegU functions as a priming protein in competence development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 97:9246-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofemeister, J., B. Conrad, B. Adler, B. Hofemeister, J. Feesche, N. Kucheryava, G. Steinborn, P. Franke, N. Grammel, A. Zwintscher, F. Leenders, G. Hitzeroth, and J. Vater. 2004. Genetic analysis of the biosynthesis of non-ribosomal peptide- and polyketide-like antibiotics, iron uptake and biofilm formation by Bacillus subtilis A1/3. Mol. Genet. Genomics 272:363-378. [DOI] [PubMed] [Google Scholar]
- 19.Idris, E. E., D. J. Iglesias, M. Talon, and R. Borriss. 2007. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 19:619-626. [DOI] [PubMed] [Google Scholar]
- 20.Koumoutsi, A., X. H. Chen, A. Henne, H. Liesegang, G. Hitzeroth, P. Franke, J. Vater, and R. Borriss. 2004. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 186:1084-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunst, F., M. Pascal, J. Lepesant-Kejzlarova, J. A. Lepesant, A. Billault, and R. Dedonder. 1974. Pleiotropic mutations affecting sporulation conditions and the syntheses of extracellular enzymes in Bacillus subtilis 168. Biochimie 56:1481-1489. [DOI] [PubMed] [Google Scholar]
- 22.Kunst, F., and G. Rapoport. 1995. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J. Bacteriol. 177:2403-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landy, M. W., G. H., Roseman, S. B., and L. G. Colio. 1948. Bacillomycin, an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc. Soc. Exp. Biol. Med. 67:539-541. [DOI] [PubMed] [Google Scholar]
- 24.Mader, U., H. Antelmann, T. Buder, M. K. Dahl, M. Hecker, and G. Homuth. 2002. Bacillus subtilis functional genomics: genome-wide analysis of the DegS-DegU regulon by transcriptomics and proteomics. Mol. Genet. Genomics 268:455-467. [DOI] [PubMed] [Google Scholar]
- 25.Maget-Dana, R., and F. Peypoux. 1994. Iturins, a special class of pore-forming lipopeptides: biological and physicochemical properties. Toxicology 87:151-174. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 27.Mootz, H. D., R. Finking, and M. A. Marahiel. 2001. 4′-Phosphopantetheine transfer in primary and secondary metabolism of Bacillus subtilis. J. Biol. Chem. 276:37289-37298. [DOI] [PubMed] [Google Scholar]
- 28.Msadek, T., F. Kunst, D. Henner, A. Klier, G. Rapoport, and R. Dedonder. 1990. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J. Bacteriol. 172:824-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Msadek, T., F. Kunst, A. Klier, and G. Rapoport. 1991. DegS-DegU and ComP-ComA modulator-effector pairs control expression of the Bacillus subtilis pleiotropic regulatory gene degQ. J. Bacteriol. 173:2366-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano, M. M., N. Corbell, J. Besson, and P. Zuber. 1992. Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol. Gen. Genet. 232:313-321. [DOI] [PubMed] [Google Scholar]
- 31.Nakano, M. M., L. A. Xia, and P. Zuber. 1991. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J. Bacteriol. 173:5487-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogura, M., K. Shimane, K. Asai, N. Ogasawara, and T. Tanaka. 2003. Binding of response regulator DegU to the aprE promoter is inhibited by RapG, which is counteracted by extracellular PhrG in Bacillus subtilis. Mol. Microbiol. 49:1685-1697. [DOI] [PubMed] [Google Scholar]
- 33.Ogura, M., H. Yamaguchi, K. Yoshida, Y. Fujita, and T. Tanaka. 2001. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res. 29:3804-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peypoux, F., F. Besson, G. Michel, and L. Delcambe. 1981. Structure of bacillomycin D, a new antibiotic of the iturin group. Eur. J. Biochem. 118:323-327. [DOI] [PubMed] [Google Scholar]
- 35.Peypoux, F., J. M. Bonmatin, and J. Wallach. 1999. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 51:553-563. [DOI] [PubMed] [Google Scholar]
- 36.Roggiani, M., and D. Dubnau. 1993. ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA. J. Bacteriol. 175:3182-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimane, K., and M. Ogura. 2004. Mutational analysis of the helix-turn-helix region of Bacillus subtilis response regulator DegU, and identification of cis-acting sequences for DegU in the aprE and comK promoters. J. Biochem. (Tokyo). 136:387-397. [DOI] [PubMed] [Google Scholar]
- 38.Spizizen, J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA 44:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steil, L., T. Hoffmann, I. Budde, U. Volker, and E. Bremer. 2003. Genome-wide transcriptional profiling analysis of adaptation of Bacillus subtilis to high salinity. J. Bacteriol. 185:6358-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein, T. 2005. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol. 56:845-857. [DOI] [PubMed] [Google Scholar]
- 41.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79-83. [DOI] [PubMed] [Google Scholar]
- 42.Stragier, P., C. Bonamy, and C. Karmazyn-Campelli. 1988. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52:697-704. [DOI] [PubMed] [Google Scholar]
- 43.Straight, P. D., J. M. Willey, and R. Kolter. 2006. Interactions between Streptomyces coelicolor and Bacillus subtilis: role of surfactants in raising aerial structures. J. Bacteriol. 188:4918-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuge, K., T. Akiyama, and M. Shoda. 2001. Cloning, sequencing, and characterization of the iturin A operon. J. Bacteriol. 183:6265-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuge, K., T. Ano, M. Hirai, Y. Nakamura, and M. Shoda. 1999. The genes degQ, pps, and lpa-8 (sfp) are responsible for conversion of Bacillus subtilis 168 to plipastatin production. Antimicrob. Agents Chemother. 43:2183-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuge, K., S. Inoue, T. Ano, M. Itaya, and M. Shoda. 2005. Horizontal transfer of iturin A operon, itu, to Bacillus subtilis 168 and conversion into an iturin A producer. Antimicrob Agents Chemother. 49:4641-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turgay, K., L. W. Hamoen, G. Venema, and D. Dubnau. 1997. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev. 11:119-128. [DOI] [PubMed] [Google Scholar]
- 48.Typas, A., and R. Hengge. 2006. Role of the spacer between the −35 and −10 regions in σS promoter selectivity in Escherichia coli. Mol. Microbiol. 59:1037-1051. [DOI] [PubMed] [Google Scholar]
- 49.Voskuil, M. I., and G. H. Chambliss. 1998. The −16 region of Bacillus subtilis and other gram-positive bacterial promoters. Nucleic Acids Res. 26:3584-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, M., E. Ferrari, E. Chen, and D. J. Henner. 1986. Identification of the pleiotropic sacQ gene of Bacillus subtilis. J. Bacteriol. 166:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.