Abstract
Degradation of bis(2-chloroethyl) ether (BCEE) was observed to occur in two bacterial strains. Strain ENV481, a Xanthobacter sp. strain, was isolated by enrichment culturing of samples from a Superfund site located in the northeastern United States. The strain was able to grow on BCEE or 2-chloroethylethyl ether as the sole source of carbon and energy. BCEE degradation in strain ENV481 was facilitated by sequential dehalogenation reactions resulting in the formation of 2-(2-chloroethoxy)ethanol and diethylene glycol (DEG), respectively. 2-Hydroxyethoxyacetic acid was detected as a product of DEG catabolism by the strain. Degradation of BCEE by strain ENV481 was independent of oxygen, and the strain was not able to grow on a mixture of benzene, ethylbenzene, toluene, and xylenes, other prevalent contaminants at the site. Another bacterial isolate, Pseudonocardia sp. strain ENV478 (S. Vainberg et al., Appl. Environ. Microbiol. 72:5218-5224, 2006), degraded BCEE after growth on tetrahydrofuran or propane but was not able to grow on BCEE as a sole carbon source. BCEE degradation by strain ENV478 appeared to be facilitated by a monooxygenase-mediated O-dealkylation mechanism, and it resulted in the accumulation of 2-chloroacetic acid that was not readily degraded by the strain.
Ether-containing aliphatic compounds have been used widely in an array of industrial and agricultural applications, and in many cases they have become important environmental pollutants. Among the ether-containing compounds that have received recurring attention is bis(2-chloroethyl) ether (BCEE). BCEE has been used commercially as a solvent for fats and greases, a cleaning fluid for textiles, a constituent of paints and varnishes, and an insecticide. Most recently, it has been used as a chemical intermediate in the production of a commercial fungicide, and it may be present in trace amounts in the final product (3). It also has been identified in leachate and groundwater at several former industrial landfills, and it has been speculated that BCEE is generated as a chlorination by-product in waste streams containing ethylene or propylene (29). It has been classified as a probable human carcinogen by the U.S. EPA, with a 10−6 cancer risk at a water concentration of 0.03 μg/liter (22); consequently, U.S. EPA region III has established a risk-based concentration for BCEE of only 9.6 × 10−3 μg/liter in tap water (23). These relatively low regulatory concentrations have created renewed concern about the potential sources, transport, and fate of this compound in the environment.
Concern over the release of BCEE to the environment is exacerbated by its mobility and persistence. It has an aqueous solubility of approximately 17,000 mg/liter, an organic carbon partitioning coefficient (log Koc) value of 1.2, and a Henry's law constant of 1.8 × 10−5 atm m3/mol (24). It also is chemically stable and not readily degraded by abiotic mechanisms. These properties limit the natural attenuation (e.g., sorption, volatilization, and biodegradation) of BCEE in groundwater and facilitate its migration, and they limit the effectiveness of conventional groundwater remediation technologies, such as carbon adsorption and air stripping (5).
Biological degradation is a potential treatment alternative for remediating ether-contaminated environments (19, 20, 26, 27). Mechanisms for biological ether scission have been reviewed previously in detail (28). The most common scission mechanisms for short-chain alkyl ethers is O dealkylation, whereby a carbon adjacent to the ether bond is hydroxylated, forming a hemiacetal. Because hemiacetals are unstable in aqueous solutions, they disproportionate to an alcohol and an aldehyde. In some cases, however, enzymatic reactions may facilitate transformation of the hemiacetal (17). An O-dealkylation mechanism has been suggested for BCEE degradation by a Rhodococcus strain (9) and Rhodococcus strain DTB (12). In the case of chlorinated ethers like BCEE, however, additional biological reactions may be required prior to ether scission. For example, Janssen and colleagues (7) implicated a dehalogenase reaction in the degradation of BCEE by bacterial strain GJ70. Although a complete degradation pathway for BCEE was not proposed, the results suggested that dehalogenation preceded ether scission in this strain. The dechlorinated product [presumably 2-(2-chloroethoxy)ethanol (2CEE)] could then (i) undergo ether scission; (ii) be oxidized further to the carboxylic acid ether [e.g., 2-(2-chloroethoxy)acetic acid] prior to scission, as occurs with 2-chlorovinylether in Ancylobacter strains (27); or (iii) be dehalogenated again to diethylene glycol (DEG), which ultimately undergoes further oxidation or scission.
In this report we describe the isolation of a new BCEE-degrading bacterium, Xanthobacter sp. strain ENV481, and we perform a detailed analysis of BCEE degradation products for this strain. We also evaluate BCEE degradation by tetrahydrofuran (THF)-degrading Pseudonocardia sp. strain ENV478 (25). Our results demonstrate that BCEE can be used by strain ENV481 as a sole source of carbon and energy and that the strain performs sequential dehalogenations of BCEE, leading to the production of DEG, which is then oxidized to 2-hydroxyethoxyacetic acid (2HEAA) and utilized by the strain. Conversely, strain ENV748 appears to oxidize BCEE directly to a hemiacetal that disproportionates to 2-chloroethanol and, presumably, 2-chloroacetaldehyde. The results demonstrate the importance of multiple enzymatic processes, including dehalogenation and oxidative ether scission, in the degradation of BCEE, and they suggest that biological treatment may be a viable remedial alternative for treating BCEE-contaminated environments.
MATERIALS AND METHODS
Chemicals.
BCEE was 99% grade and purchased from Aldrich Chemical Co. (Milwaukee, WI). 2HEAA was synthesized as previously described (27). R2A medium was from BBL, Inc. (Cockeysville, MD). Unless otherwise stated, all other chemicals were of at least reagent grade and purchased from Aldrich Chemical Co. (Milwaukee, WI), Mallinckrodt Specialty Chemical Co. (Paris, KY), J. T. Baker, Inc. (Phillipsburg, NJ), or Sigma Chemical Co. (St. Louis, MO).
Isolation and characterization of a BCEE-degrading bacterium.
A microcosm study was performed with aquifer soil and groundwater collected from a Superfund landfill site located in southern New Jersey. Approximately 5 ml of microcosm slurry was removed from one of the microcosms and added to 50 ml of basal salts medium (BSM) (4). Four consecutive 1:10 dilutions were made from this medium with the addition of 25 mg/liter (0.17 mM) BCEE. After depletion of BCEE, the primary enrichment cultures were subcultured twice in BSM with 100 mg/liter (0.70 mM) of BCEE and then plated onto R2A agar plates. Individual colonies were selected and screened for their ability to grow on BCEE, and a single pure culture was selected for further study and designated strain ENV481. Strain ENV481 was characterized by sequencing 1,412 bp of its 16S rRNA gene. Genomic DNA was isolated from bacterial cells by using an UltraClean microbial DNA isolation kit (Mo Bio Laboratories, Inc., CA) according to the directions of the manufacturer. The DNA encoding the 16S rRNA genes was first PCR amplified with primers 27f and 1522r, and the purified PCR product was used directly in DNA sequencing reactions with primers 27f, 357f, 704f, 926f, 1242f, 342r, 685r, 907r, 1392r, and 1522r (10). The complete sequence was assembled and edited with the Lasergene program (DNAStar, Madison, WI). The assembled sequence was used to query the GenBank database by using the BLAST algorithm (1) to identify sequences most closely related to that of strain ENV481.
Bacterial growth.
Strain ENV481 was grown in 250-ml flasks with 100 ml of sterile BSM. Flasks were closed with silicone stoppers, and 50 ml of oxygen was added to each flask through a sterile 0.2-μm nylon filter (Nalgene, Rochester, NY). Flasks were incubated with shaking (250 rpm) at 28 ± 1°C. Growth substrates, except 2-chloroacetaldehyde, were added to flasks at an initial concentration of 100 to 200 mg/liter and then periodically thereafter as growth was observed and substrates were utilized. 2-Chloroacetaldehyde was added at a concentration of 25 mg/liter. Cell growth was monitored by measuring the optical density at 550 nm (OD550) of the culture in a 1-cm cuvette (Spectronic Instruments, Rochester, NY). Strain ENV478 was grown on THF or propane as previously described (25). Growth rates and doubling times were measured by calculating the bacterial concentration (dry weight) during the exponential growth phase. The yield was determined by measuring the difference between initial and final bacterial concentrations (dry weight) and the amount of substrate utilized. The dry weight of bacterial suspensions was determined gravimetrically after drying washed cells at 105°C.
Determination of the degradation kinetics and products.
Strain ENV481 was grown on BSM amended with BCEE, 2CEE, or DEG as described above. After growth, the cells were concentrated by centrifugation (10,000 × g for 10 min) and washed three times in chloride-free BSM. Equivalent amounts of nitrogen in the medium were supplied by adding (NH4)2SO4 instead of NH4Cl. The final OD550 of bacterial suspensions were 0.65 (growth on BCEE), 1.65 (growth on 2CEE), and 0.66 (growth on DEG). The washed culture (20 ml) was added to 160-ml serum vials, and the vials were closed with Teflon-lined septa and aluminum crimp seals. BCEE, 2CEE, or DEG was added to a final concentration of 100 mg/liter. After defined periods of time, samples of the culture liquor from each set of vials were collected, centrifuged to remove cells (10,000 × g for 10 min), filtered through 0.25 μM nylon filter units, and analyzed for BCEE, chloride, and possible products of BCEE degradation (see below). All experiments were performed in triplicate.
Anaerobic degradation of BCEE by strain ENV481.
Strain ENV481 was grown in 200 ml of LB medium supplemented with 70 μl of DEG in a 0.5-liter flask. The cells were harvested via centrifugation, rinsed three times with chloride-free BSM, and suspended to an OD550 of 1. The washed culture was placed in a 150-ml serum vial and sealed with Teflon-lined butyl rubber septa. The vial was then purged with oxygen-free nitrogen (flow of 1 liter per minute) for 30 min, using a long syringe needle as the inlet and a short needle as the outlet, so that the incoming nitrogen would bubble up through the suspension. The purged vial was transferred to an anaerobic chamber for all subsequent handling. A portion (5 ml) of the culture was collected and analyzed for oxygen content by use of a CHEMets 0- to 1-ppm dissolved oxygen kit (Chemetrics, Inc., Calverton, VA), revealing that the dissolved oxygen content was <0.1 mg/liter. Subsamples (40 ml) of the culture were transferred in triplicate to sterile 150-ml vials that were subsequently sealed using Teflon-lined septa. BCEE was then added to the samples to a final concentration of 250 μM, and the samples were incubated without shaking for 20 h at room temperature (∼25°C). Samples were withdrawn through the septa for gas chromatography analysis and ion-exchange chromatography as described below. The first samples were taken after 10 min of incubation.
Determining the products of BCEE degradation by strain ENV478.
Pseudonocardia sp. strain ENV478 (25) was grown initially in 500-ml flasks in 200 ml BSM containing 0.2% (wt/vol) yeast extract. After 2 days of growth, the cultures were fed THF (100 mg/liter) or propane (1:5 [vol/vol] in the headspace). After several additions of THF or propane, the cultures were concentrated by centrifugation, and the cell pellets were washed and suspended in BSM to 0.5 to 0.7 g (dry weight)/liter. Cell suspension (200 ml) was added to 500-ml bottles fitted with Teflon-lined caps and lure lock fittings, and BCEE was added to each vial to a final concentration of 100 mg/liter (0.70 mM). The samples were incubated with shaking at 28°C, and subsamples were removed periodically, filtered through 0.25-μm filters, and analyzed for BCEE and potential degradation products.
Analytical methods.
Whenever possible, analytical methods performed during this project followed U.S. EPA method SW-846, available online at http://www.epa.gov/epaoswer/hazwaste/test/index.htm. (The specific test methods used are identified herein by their U.S. EPA method numbers.) Samples for chemical analysis were filtered through a 0.25-μm syringe-mounted nylon filter to remove bacterial cells, and 1 μl of sample was injected directly onto the gas chromatograph by using an autosampler as described below. The recovery efficiency of this method for each compound analyzed was not measured, but each experiment was performed with replicate samples containing killed cells and/or cell-free medium to account for any analytical deficiencies. BCEE and its breakdown products 2CEE, DEG, and ethylene glycol were analyzed with a Varian 3800 gas chromatograph equipped with a flame ionization detector essentially as described by U.S. EPA method 8015B. A Restek Stabilwax column (diameter of 0.53 mm, length of 30 m, and film thickness of 1.00 μm; Bellefonte, PA) was set at 50°C for 2 min and was then ramped to 200°C at 8°C/min and held for 4.25 min for a total run time of 25 min. A 1.0-μl splitless injection onto the column was made using a Varian CP-8400 autosampler. The injector temperature was set to 200°C and the flame ionization detector to 250°C. Gas flow rates were set as follows: column flow, 5.0 ml/min; makeup gas, 25 ml/min; air, 200 ml/min; and, hydrogen, 30 ml/min. Helium was used for both the column and the makeup gas. Results were compared to a five-point standard curve prepared with authentic compounds. In addition, a colorimetric assay that used Purpald reagent (Sigma-Aldrich, St. Louis, MO) was used for detecting aldehydes in bacterium-free medium (13). Analysis of volatile fatty acids, 2-chloroacetic acid, 2HEAA, and chloride was performed with an ion chromatograph (Dionex Co., Sunnyvale, CA) using U.S. EPA method 9056A.
RESULTS
Isolation and characterization of strain ENV481.
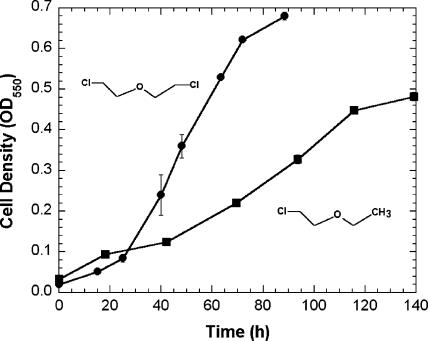
Enrichment culturing of microcosm samples with BCEE as a sole source of carbon and energy resulted in the isolation of a single bacterial culture capable of growth on BCEE. The gram-negative isolate formed yellow-green colonies on R2A medium, and the sequenced 1,412 bases of the 16S rRNA gene (GenBank accession no. EF592179) had 100% identity with that of Xanthobacter flavus strain JW/KR-1, which was isolated from the rhizosphere of rice plants (14). The strain was designated Xanthobacter sp. strain ENV481. Strain ENV481 grew on BCEE and 2-chloroethyl ethyl ether (Fig. 1), 2-propanol, ethanol, l-alanine, sodium lactate, R2A medium, and yeast extract. The strain did not grow on methyl tert-butyl ether (MTBE), diethyl ether, THF, 1,4-dioxane, 1,1,1-trichloroethane, 1,1-dichloroethane, 1,2-dichloroethane (1,2-DCA), glucose, sucrose, l-histidine, dl-phenylalanine, or a mixture of benzene, toluene, ethyl benzene, and xylenes (BTEX).
FIG. 1.
Growth of ENV481 on BCEE (•) or 2-chloroethyl ethyl ether (▪). Symbols represent means (n = 3), and error bars represent 1 standard error.
Biodegradation of BCEE by strain ENV481.
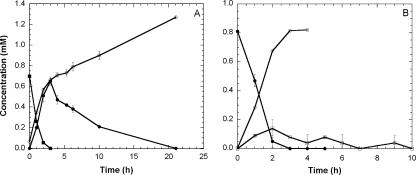
BCEE-grown strain ENV481 degraded BCEE at an initial rate of approximately 2.4 ± 0.06 mM h−1 g (dry weight)−1 and was able to degrade BCEE at initial concentrations of at least 173 mg/liter (1.2 mM) without apparent inhibition. BCEE degradation resulted in the formation of stoichiometric amounts of 2CEE and chloride ions (Fig. 2A). Likewise, lactate-grown strain ENV481 degraded BCEE at an initial rate of 2.3 ± 0.2 mM h−1 g (dry weight)−1 without an apparent lag period, suggesting constitutive expression of the degradative genes involved in the initial reaction. The addition of relatively high concentrations of BTEX (100 mg/liter) neither inhibited nor enhanced BCEE degradation by the strain, and BTEX was not degraded in BCEE-grown cultures. Strain ENV481 degraded 2CEE at the rate of 0.83 ± 0.03 mM h−1 g (dry weight)−1 and produced stoichiometric amounts of chloride ions. Stoichiometric amounts of DEG did not accumulate during degradation of 2CEE, presumably because DEG was degraded at a greater rate (1.1 ± 0.06 mM h−1 g [dry weight]−1) than the former compound. Ethylene glycol was not detected as a product of DEG degradation, but a transient accumulation of 2HEAA was observed.
FIG. 2.
Biodegradation of BCEE (A) and 2CEE (B) by strain ENV481. Symbols represent BCEE (▪), 2CEE (•), DEG (□), and chloride (○). (A) The assays contained 0.18 g (dry weight)/liter (OD550 of 0.65) of strain ENV481. (B) The assays contained 0.46 g (dry weight)/liter (OD550 of 1.65) of strain ENV481. Symbols represent means (n = 3), and error bars represent 1 standard error.
To further evaluate the BCEE degradation pathway in strain ENV481, the culture was incubated with a number of potential degradation products. The strain grew well on 2CEE and DEG (Table 1), which are possible prescission degradation products (see below). It also grew on 2-chloroethanol and 2-chloroacetic acid (Table 1), which are potential postscission degradation products (see below), and it was able to degrade 2-chloroacetaldehyde. Growth on 2-chloroacetaldehyde was not confirmed because of the apparent toxicity of this compound at the concentrations typically used for growth studies. The strain also grew on 2HEAA, which is a potential prescission degradation product and a product known to accumulate during monooxygenase-mediated degradation of 1,4-dioxane (25). The strain did not grow in liquid cultures on 100 or 200 mg/liter diglycolic acid. No potential chlorinated ether scission products, e.g., chloroethanol and chloroacetaldehyde, were detected in the culture liquor of cells fed either BCEE or 2CEE.
TABLE 1.
Strain ENV481 growth and biodegradation kinetics
| Growth substrate | Growth rate constant (h−1) | Doubling time (h) | Yield (g [dry wt] liter−1 g substrate−1) | q (mmol/g [dry wt]/h)a | K (mmol/liter)a |
|---|---|---|---|---|---|
| BCEE | 0.040 | 16 | 0.18 | 2.2 ± 0.4 | 0.14 ± 0.09 |
| 2CEE | 0.040 | 17 | 0.20 | 0.38 ± 0.1 | 0.31 ± 0.3 |
| 2-Chloroethyl ethyl ether | 0.019 | 36 | 0.24 | ND | ND |
| DEG | 0.045 | 16 | 0.29 | ND | ND |
| 2HEAA | 0.017 | 41 | 0.23 | ND | ND |
| 2-Chloroacetic acid | 0.012 | 60 | 0.08 | ND | ND |
| 2-Chloroethanol | 0.027 | 25 | 0.25 | ND | ND |
| Ethylene glycol | 0.061 | 11 | 0.26 | ND | ND |
q and K are defined in Results. ± values represent 95% confidence intervals. ND, not determined.
Dechlorination of BCEE to 2CEE and chloride, followed by dechlorination of 2CEE, was modeled using Monod kinetics (15) as follows:
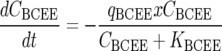 |
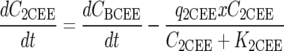 |
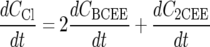 |
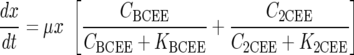 |
where Ci is the aqueous concentration of compound i (where i is BCEE, 2CEE, or chloride) (mmol/liter), t is time (h), Ki is the concentration of substrate i at which half the maximum utilization rate of compound i is achieved (i.e., half velocity coefficient of compound i) (mmol/liter), qi is the maximum utilization rate of compound i (mmol/g [dry weight]/h), x is the ENV481 cell concentration (g [dry weight]/liter), and μ is the ENV481 maximum growth rate constant (h−1).
The kinetic model assumes that oxygen is present in excess and that there is no competitive inhibition between BCEE and 2CEE; the latter assumption was verified in parallel experiments where BCEE degradation was not impacted by the addition of equimolar concentrations of 2CEE. The ENV481 maximum growth rate constant (μ) was determined independently in the batch bacterial growth experiments, and initial values of x were measured.
The four equations shown above were simultaneously regressed to the microcosm experimental data in which sequential BCEE and 2CEE degradation and strain ENV481 concentration were measured. Regression to the experimental data was performed using the Microsoft Excel Solver function and a nonlinear least-squares analysis similar to that described by Smith et al. (18).
Regressed values of q and K for BCEE and 2CEE are listed in Table 1. Regression results show that the experimental results are reasonably described by the model, with an r2 value of 0.92. Regression results also show that the maximum utilization rate for BCEE is approximately 10 times that of 2CEE, indicating that BCEE was more readily biodegradable than 2CEE for the conditions of this study.
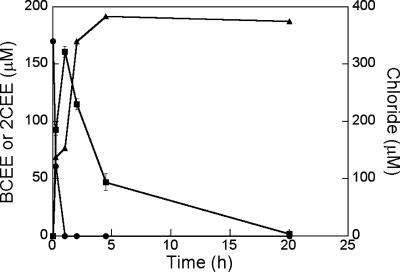
To evaluate the possible role of a monooxygenase enzyme or the lack of monooxygenase activity in BCEE degradation by ENV481, the strain was incubated anaerobically with BCEE. The culture degraded BCEE under anaerobic conditions (Fig. 3), but the degradation rate (0.4 mM h−1 g [dry weight]−1) was slower than the aerobic degradation rate. BCEE degradation resulted in the sequential production of stoichiometric amounts of 2CEE and chloride ions (109% yield), even in the absence of oxygen. These results indicate that an oxygenase enzyme was not responsible for the initial transformations of BCEE.
FIG. 3.
Biodegradation of BCEE by strain ENV481 under anaerobic conditions. Symbols represent BCEE (•), 2CEE (▪), and chloride (▴). Values represent means (n = 3), and error bars represent 1 standard error of the mean. The first sample was collected after 10 min of incubation.
BCEE degradation by Pseudonocardia sp. strain ENV478.
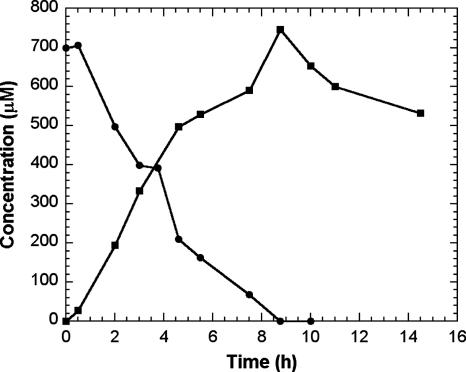
Pseudonocardia sp. strain ENV478 was shown previously to degrade BCEE after growth on THF or propane (25), but it did not grow on BCEE as a sole carbon source. Analysis of culture medium of THF- or propane-grown cells incubated with BCEE revealed the presence of 2-chloroethanol and 2-chloroacetic acid (Fig. 4). 2-Chloroacetaldehyde was not detected in the culture medium, possibly because it was rapidly oxidized to 2-chloroacetic acid and/or because of the higher analytical detection limit for this catabolite. The strain also degraded BCEE after growth on lactate but at a slower rate than after growth on THF (data not shown).
FIG. 4.
Biodegradation of BCEE by strain ENV478. Symbols represent BCEE (•) and 2-chloroacetic acid (▪). Assays contained 1 g (dry weight)/liter ENV478. Values represent means (n = 3), and error bars representing 1 standard error are smaller than the sizes of the symbols.
DISCUSSION
BCEE-contaminated groundwater was identified in and around two large Superfund landfill sites in the northeastern United States. In microcosm studies with samples from one of these sites, we observed significant decreases in BCEE concentrations in aerobic microcosms, but only after most of the other contaminants in the samples, especially BTEX, had been biodegraded (unpublished results). Enrichment culturing of the aerobic microcosm samples with BCEE as a sole carbon source led to the isolation of strain ENV481.
Strain ENV481 grew readily on BCEE as a sole carbon and energy source, and it also grew on several potential BCEE degradation products, including 2CEE, DEG, 2HEAA, 2-chloroethanol, 2-chloroacetaldehyde, and 2-chloroacetic acid (Table 1). Phylogenetic analysis of the isolate, based on 16S rRNA gene sequencing, suggested that it was most closely related to members of the genus Xanthobacter. Some Xanthobacter strains have been shown to grow on short-chain chlorinated aliphatic compounds and to dehalogenate some chlorinated ethers, including BCEE (8). The most studied of these strains is Xanthobacter autotrophicus strain GJ10, which was isolated in Europe more than 20 years ago (8). Strain GJ10 produces at least two broad-substrate hydrolytic dehalogenase enzymes, and it also is able to grow aerobically on 1,2-DCA (7). Strain ENV481 does not grow on 1,2-DCA, but it can grow on or degrade the 1,2-DCA degradation products 2-chloroethanol, 2-chloroacetaldehyde, and 2-chloroacetic acid.
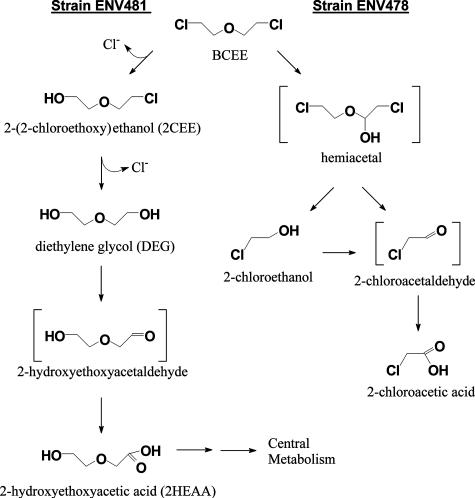
Based on our analyses, the most likely pathway for BCEE degradation in strain ENV481 is sequential dehalogenation of the parent compound leading to the formation of DEG (Fig. 5). Decreases in BCEE concentrations in strain ENV481 cultures were accompanied by the sequential formation of 2CEE and DEG and the concomitant production of chloride ions at a ratio of ∼2 mol of chloride per mol of BCEE degraded (Fig. 2A and B). No chlorinated ether scission products (e.g., 2-chloroethanol, 2-chloroacetaldehyde, or 2-chloroacetate) were detected in BCEE-fed strain ENV481 cultures, and the strain was able to grow on either 2CEE or DEG as a sole source of carbon and energy (Table 1). Thus, our results suggest that strain ENV481 dehalogenates BCEE completely to DEG prior to ether scission.
FIG. 5.
Proposed BCEE biodegradation pathways in strains ENV481 and ENV78. Compounds shown in brackets were not analyzed in this study but are expected based on the other products detected.
Dehalogenation of BCEE has been reported to occur in an Acinetobacter strain (7), but a complete pathway for BCEE degradation was not provided. Similarly, Ancylobacter aquaticus strain AD25 was able to dehalogenate monochlorinated 2-chlorovinylether (27), and Pseudomonas sp. strain AD1 performed sequential dehalogenation reactions with 1,3-dichloro-2-propanol via the action of a single haloalkane dehalogenase (27). Although the dehalogenase responsible for BCEE degradation in strain ENV481 has not yet been identified, it is possible that a single dehalogenase performs both dehalogenation reactions. This is supported in part by the near stoichiometric production of 2CEE from BCEE before further catabolism of 2CEE (Fig. 2A). Several haloalkane dehalogenases studied to date have been shown to have broad substrate ranges (7, 16) and to dehalogenate chlorinated ethers (7, 27) and alcohols (11). The dehalogenation of chlorinated alcohol ethers by these enzymes, however, may be a novel reaction. The involvement of multiple dehalogenases in the same strain also cannot be ruled out completely (7, 9).
The mechanism for DEG catabolism in strain ENV481 has not yet been elucidated fully, but it did result in the production of 2HEAA that was catabolized further by the strain. This suggests that one of the β carbons of DEG is oxidized completely to a carboxylic acid prior to ether scission. Glycolate and glyoxylate were identified as apparent products of 2HEAA catabolism, and the strain was able to grow on either glyoxylate or glycolate as a sole carbon source (data not shown).
Results of our study also demonstrated that strain ENV481 can degrade BCEE in the absence of molecular oxygen (Fig. 3), suggesting that the strain does not employ a typical O-dealkylation mechanism during which monooxygenation of an α-carbon is followed by hemiacetal decomposition (6, 12, 17, 20, 25, 28). This mechanism was proposed for BCEE degradation in Rhodococcus sp. strain DEE5151 (11) and Rhodococcus sp. strain DBT (12), but it was not demonstrated fully. With BCEE, O dealkylation would lead to the production of 2-chloroethanol and 2-chloroethaldehyde. Although strain ENV481 was able to grow on or degrade both of these potential catabolites, these products were not detected in cultures fed BCEE. Likewise, the strain was not able to degrade nonchlorinated ethers, including MTBE, diethyl ether, THF, and 1,4-dioxane, which are reported to be degraded by broad-substrate monooxygenases via O-dealkylation mechanisms (2, 6, 20, 21, 25). Monooxygenation-mediated O dealkylation of BCEE, however, does appear to be the degradation pathway used by THF- or propane-grown Pseudonocardia sp. strain ENV478, as demonstrated by the production of 2-chloroethanol and 2-chloroacetic acid from BCEE by this strain (Fig. 4). Although strain ENV478 does have low-level constitutive monooxygenase activity (25), it was not able to grow on BCEE as a sole carbon and energy source.
Based on our analysis of BCEE degradation products generated by strains ENV481 and ENV478 and growth of strain ENV481 on potential BCEE catabolites, we propose the biodegradation pathways presented in Fig. 5. Strain ENV481 utilizes sequential dehalogenation to produce DEG and oxidizes DEG to 2HEAA prior to ether scission, and strain ENV478 oxidizes BCEE directly and cleaves it via an O-dealkalation mechanism. The isolation of strain ENV481 from a BCEE-contaminated landfill site suggests that dehalogenation may play an important role in controlling the fate of BCEE in the environment. Furthermore, the inability of strain ENV481 to grow on BTEX, which comprises the bulk of the contamination at the site from which the strain was isolated, suggests that BCEE may support the growth and survival of the strain ENV481 in situ. BCEE also is present in other East Coast landfills, and BCEE degradation was observed to occur in microcosms constructed from one of these sites (John T. Wilson, personal communication). Experiments to determine the presence of strain ENV481-like organisms in other BCEE-contaminated landfills are planned to better evaluate the role of these bacteria in affecting the fate of BCEE in contaminated environments.
Acknowledgments
We thank Anthony Soto, Randi Rothmel, and Paul Hedman for providing chemical analyses for this project.
This work was supported in part by a grant (no. CU-1422) from the Strategic Environmental Research and Development Program to R.J.S.
Footnotes
Published ahead of print on 14 September 2007.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Scaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernhardt, D., and H. Dickmann. 1991. Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental Rhodococcus strain. Appl. Microbiol. Biotechnol. 36:120-123. [DOI] [PubMed] [Google Scholar]
- 3.Canada Environmental Protection Act. 1993. Priority substances list assessment report: bis(2-chloroethyl) ether. Publication no. 40-215/9E. Environment Canada, Ottawa, Ontario, Canada.
- 4.Hareland, W. A., R. L. Crawford, P. J. Chapman, and S. Dagley. 1975. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J. Bacteriol. 121:272-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang, F. Y. C., K. Y. Li, and C. C. Liu. 1999. Treatability studies of groundwater contaminated with bis(2-chloroethyl) ether. Environ. Prog. 18:55-59. [Google Scholar]
- 6.Hur, H.-G., L. M. Newman, L. P. Wackett, and M. J. Sadowsky. 1997. Toluene 2-monooxygenase-dependent growth of Burkholderia cepacia G4-PR1 on diethyl ether. Appl. Environ. Microbiol. 63:1606-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen, D. B., J. Gerritse, J. Brackman, C. Kalk, D. Jager, and B. Witholt. 1988. Purification and characterization of a bacterial dehalogenase with activity toward halogenated alkanes, alcohols and ethers. Eur. J. Biochem. 171:67-72. [DOI] [PubMed] [Google Scholar]
- 8.Janssen, D. B., A. Scheper, L. Dijkhuizen, and B. Witholt. 1985. Degradation of halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10. Appl. Environ. Microbiol. 49:673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jesenska, A., M. Pavlova, M. Strouhal, R. Chaloupkova, I. Tesinska, M. Monincova, Z. Prokop, M. Bartos, I. Pavlik, I. Rychlik, P. Mobius, Y. Nagata, and J. Damborsky. 2005. Cloning, biochemical properties, and distribution of mycobacterial haloalkane dehalogenases. Appl. Environ. Microbiol. 71:6736-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, J. L. 1994. Similarity analysis of rRNAs, p. 683-700. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. ASM Press, Washington, DC.
- 11.Kim, Y.-H., and K.-H. Engesser. 2004. Degradation of alkyl ethers, aralkyl ethers, and dibenzyl ether by Rhodococcus sp. strain DEE5151, isolated from diethyl ether-containing enrichment cultures. Appl. Environ. Microbiol. 70:4398-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Horn, M., L.-A. Garde, R. Tressl, L. Adrian, and H. Gorisch. 2003. Biodegradation of bis(1-chloro-2-propyl) ether via initial ether scission and subsequent dehalogenation by Rhodococcus sp. strain DTB. Arch. Microbiol. 179:234-241. [DOI] [PubMed] [Google Scholar]
- 13.Quesenberry, M. S., and Y. C. Lee. 1996. A rapid formaldehyde assay using Purpald reagent: application under periodation conditions. Anal. Biochem. 234:50-55. [DOI] [PubMed] [Google Scholar]
- 14.Rainey, F. A., and J. Wiegel. 1996. 16S ribosomal DNA sequence analysis confirms the close relationship between the genera Xanthobacter, Azorhizobium, and Aquabacter and reveals a lack of phylogenetic coherence among Xanthobacter species. Int. J. Syst. Bacteriol. 46:607-610. [Google Scholar]
- 15.Rittmann, B. E., and P. L. McCarty. 2001. Environmental biotechnology: principals and applications. McGraw-Hill, New York, NY.
- 16.Sato, Y., M. Monincova, R. Chaloupkova, Z. Prokop, Y. Ohtsubo, K. M. Tsuda, J. Damborsky, and Y. Nagata. 2005. Two rhizobial strains, Mesorhizobium loti MAFF303099 and Bradyrhizobium japonicum USDA110, encode haloalkane dehalogenases with novel structures and substrate specificities. Appl. Environ. Microbiol. 71:4372-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith, C. A., K. T. O'Reilly, and M. R. Hyman. 2003. Characterization of the initial reactions during the cometabolic oxidation of methyl tert-butyl ether by propane-grown Mycobacterium vaccae JOB5. Appl. Environ. Microbiol. 69:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, L. H., P. L. McCarty, and P. K. Kitanidis. 1998. Spreadsheet method for evaluation of biochemical reaction rate coefficients and their uncertainties by weighted nonlinear least-squares analysis of the integrated Monod parameters. Appl. Environ. Microbiol. 64:2044-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffan, R. J., Y. Farhan, C. W. Condee, and S. Drew. 2003. Bioremediation at a New Jersey site using propane-oxidizing bacteria, p. 506-516. In E. E. Moyer and P. T. Kostecki (ed.), MTBE remediation handbook. Amherst Scientific Publishers, Amherst, MA.
- 20.Steffan, R. J., K. McClay, S. Vainberg, C. W. Condee, and D. Zhang. 1997. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane-oxidizing bacteria. Appl. Environ. Microbiol. 63:4216-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiemer, B., J. R. Andreesen, and T. Schrader. 2003. Cloning and characterization of a gene cluster involved in tetrahydrofuran degradation in Pseudonocardia sp. strain K1. Arch. Microbiol. 179:266-277. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Environmental Protection Agency. 1987. Health effects assessment for bis(2-chloroethyl)ether. Publication no. EPA/600/8-88/023. U.S. Environmental Protection Agency, Washington, DC.
- 23.U.S. Environmental Protection Agency. 2007. Human health risk assessment. http://www.epa.gov/reg3hwmd/risk/human/index.htm.
- 24.U.S. Environmental Protection Agency. 1996. Soil screening guidance: user's guide, 2nd ed. Office of Solid Waste and Emergency Response publication no. 9355.4-23. U.S. Environmental Protection Agency, Washington, DC.
- 25.Vainberg, S., K. McClay, H. Masuda, D. Root, C. W. Condee, G. J. Zylstra, and R. J. Steffan. 2006. Biodegradation of ether pollutants by Pseudonocardia sp. strain ENV478. Appl. Environ. Microbiol. 72:5218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vainberg, S., A. P. Togna, P. M. Sutton, and R. J. Steffan. 2002. Treatment of MTBE-contaminated water in fluidized bed bioreactor (FBR). J. Environ. Eng. 128:842-851. [Google Scholar]
- 27.van den Wijngaard, A., J. Prins, A. J. A. C. Smal, and D. B. Janssen. 1993. Degradation of 2-chloroethylvinylether by Ancylobacter aquaticus AD25 and AD27. Appl. Environ. Microbiol. 59:2777-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White, G. F., N. J. Russell, and E. C. Tidswell. 1996. Bacterial scission of ether bonds. Microbiol. Rev. 60:216-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. 1998. Selected chloroalkyl ethers, environmental health criteria, no. 201. ISBN 9789241572019. WHO Press, Geneva, Switzerland.