Abstract
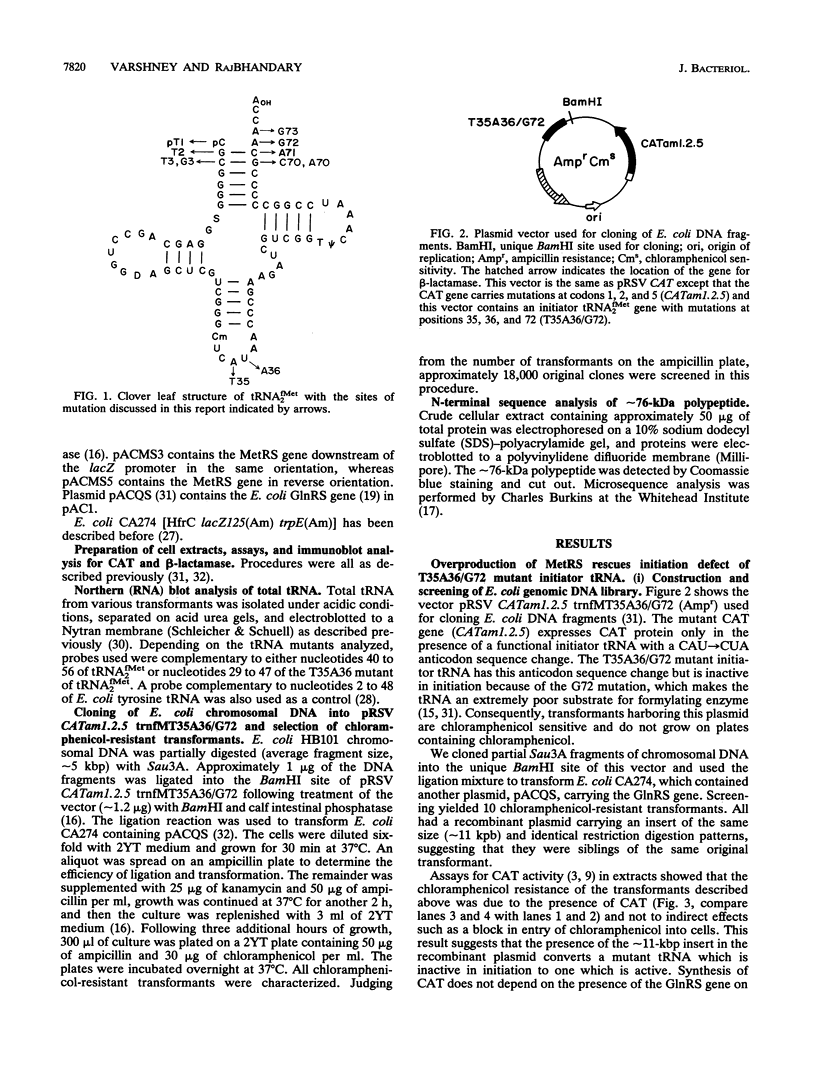
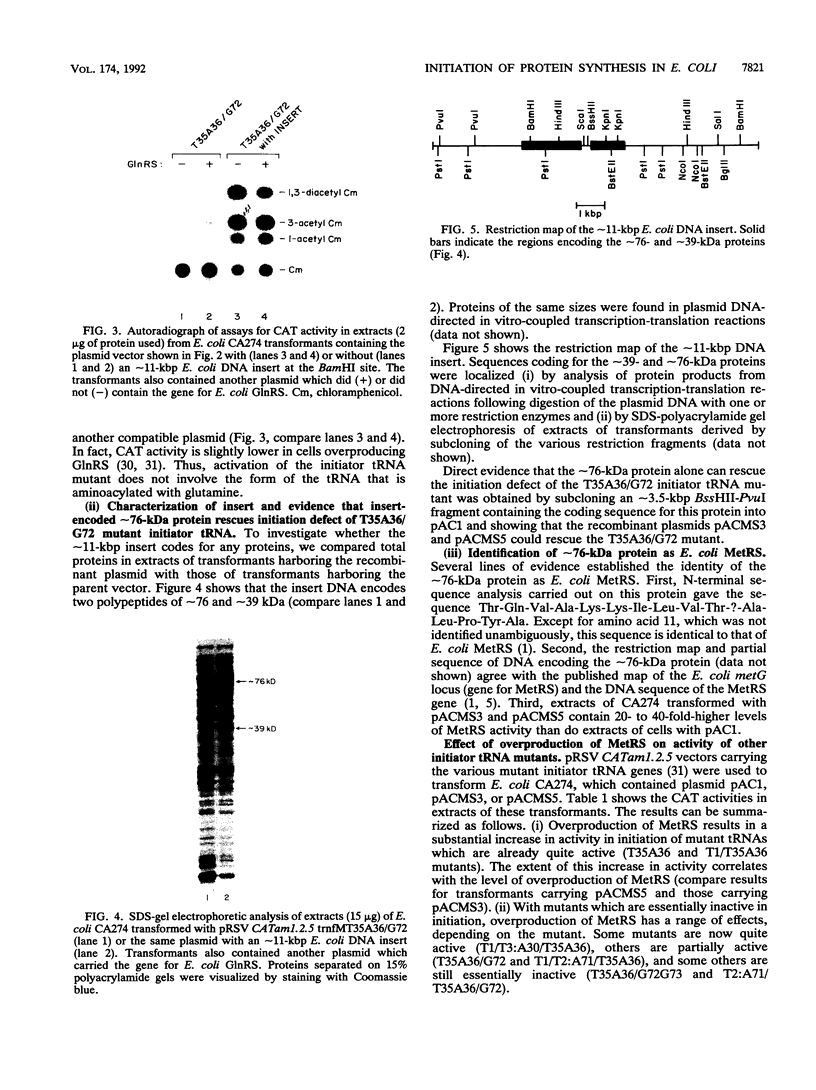
We showed recently that a mutant of Escherichia coli initiator tRNA with a CAU-->CUA anticodon sequence change can initiate protein synthesis from UAG by using formylglutamine instead of formylmethionine. We further showed that coupling of the anticodon sequence change to mutations in the acceptor stem that reduced Vmax/Km(app) in formylation of the tRNAs in vitro significantly reduced their activity in initiation in vivo. In this work, we have screened an E. coli genomic DNA library in a multicopy vector carrying one of the mutant tRNA genes and have found that the gene for E. coli methionyl-tRNA synthetase (MetRS) rescues, partially, the initiation defect of the mutant tRNA. For other mutant tRNAs, we have examined the effect of overproduction of MetRS on their activities in initiation and their aminoacylation and formylation in vivo. Some but not all of the tRNA mutants can be rescued. Those that cannot be rescued are extremely poor substrates for MetRS or the formylating enzyme. Overproduction of MetRS also significantly increases the initiation activity of a tRNA mutant which can otherwise be aminoacylated with glutamine and fully formylated in vivo. We interpret these results as follows. (i) Mutant initiator tRNAs that are poor substrates for MetRS are aminoacylated in part with methionine when MetRS is overproduced. (ii) Mutant tRNAs aminoacylated with methionine are better substrates for the formylating enzyme in vivo than mutant tRNAs aminoacylated with glutamine. (iii) Mutant tRNAs carrying formylmethionine are significantly more active in initiation than those carrying formylglutamine. Consequently, a subset of mutant tRNAs which are defective in formylation and therefore inactive in initiation when they are aminoacylated with glutamine become partially active when MetRS is overproduced.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D. G., Ebel J. P., Jakes R., Bruton C. J. Methionyl-tRNA synthetase from Escherichia coli. Primary structure of the active crystallised tryptic fragment. Eur J Biochem. 1982 Oct;127(3):449–457. [PubMed] [Google Scholar]
- Baumstark B. R., Spremulli L. L., RajBhandary U. L., Brown G. M. Initiation of protein synthesis without formylation in a mutant of Escherichia coli that grows in the absence of tetrahydrofolate. J Bacteriol. 1977 Jan;129(1):457–471. doi: 10.1128/jb.129.1.457-471.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone J. P., Sedivy J. M., Sharp P. A., RajBhandary U. L. Introduction of UAG, UAA, and UGA nonsense mutations at a specific site in the Escherichia coli chloramphenicol acetyltransferase gene: use in measurement of amber, ochre, and opal suppression in mammalian cells. Mol Cell Biol. 1986 Sep;6(9):3059–3067. doi: 10.1128/mcb.6.9.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan A. M., Feng L., Donahue T. F. tRNAi(met) functions in directing the scanning ribosome to the start site of translation. Science. 1988 Oct 7;242(4875):93–97. doi: 10.1126/science.3051379. [DOI] [PubMed] [Google Scholar]
- Dardel F., Fayat G., Blanquet S. Molecular cloning and primary structure of the Escherichia coli methionyl-tRNA synthetase gene. J Bacteriol. 1984 Dec;160(3):1115–1122. doi: 10.1128/jb.160.3.1115-1122.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delk A. S., Rabinowitz J. C. Partial nucleotide sequence of a prokaryote initiator tRNA that functions in its non-formylated form. Nature. 1974 Nov 8;252(5479):106–109. doi: 10.1038/252106a0. [DOI] [PubMed] [Google Scholar]
- Giegé R., Ebel J. P., Clark B. F. Formylation of mischarged E. coli tRNA Met f . FEBS Lett. 1973 Mar 15;30(3):291–295. doi: 10.1016/0014-5793(73)80672-6. [DOI] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualerzi C. O., Pon C. L. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990 Jun 26;29(25):5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Kan Y. W. In vivo aminoacylation of human and Xenopus suppressor tRNAs constructed by site-specific mutagenesis. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2185–2188. doi: 10.1073/pnas.84.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y. M., Schimmel P. Modeling with in vitro kinetic parameters for the elaboration of transfer RNA identity in vivo. Biochemistry. 1989 Jun 13;28(12):4942–4947. doi: 10.1021/bi00438a005. [DOI] [PubMed] [Google Scholar]
- Jahn M., Rogers M. J., Söll D. Anticodon and acceptor stem nucleotides in tRNA(Gln) are major recognition elements for E. coli glutaminyl-tRNA synthetase. Nature. 1991 Jul 18;352(6332):258–260. doi: 10.1038/352258a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. P., Seong B. L., RajBhandary U. L. Structural and sequence elements important for recognition of Escherichia coli formylmethionine tRNA by methionyl-tRNA transformylase are clustered in the acceptor stem. J Biol Chem. 1991 Sep 25;266(27):18012–18017. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Perona J. J., Swanson R., Steitz T. A., Söll D. Overproduction and purification of Escherichia coli tRNA(2Gln) and its use in crystallization of the glutaminyl-tRNA synthetase-tRNA(Gln) complex. J Mol Biol. 1988 Jul 5;202(1):121–126. doi: 10.1016/0022-2836(88)90524-4. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Rabinowitz J. C. Initiation of protein synthesis by folate-sufficient and folate-deficient Streptococcus faecalis R. Biochemical and biophysical properties of methionine transfer ribonucleic acid. J Biol Chem. 1974 Feb 25;249(4):1198–1206. [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon switching changes the identity of methionine and valine transfer RNAs. Science. 1988 Nov 4;242(4879):765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. In vitro conversion of a methionine to a glutamine-acceptor tRNA. Biochemistry. 1985 Dec 3;24(25):7309–7314. doi: 10.1021/bi00346a043. [DOI] [PubMed] [Google Scholar]
- Seong B. L., Lee C. P., RajBhandary U. L. Suppression of amber codons in vivo as evidence that mutants derived from Escherichia coli initiator tRNA can act at the step of elongation in protein synthesis. J Biol Chem. 1989 Apr 15;264(11):6504–6508. [PubMed] [Google Scholar]
- Seong B. L., RajBhandary U. L. Mutants of Escherichia coli formylmethionine tRNA: a single base change enables initiator tRNA to act as an elongator in vitro. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8859–8863. doi: 10.1073/pnas.84.24.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K. W., Rabinowitz J. C. Isolation and characterization of the Saccharomyces cerevisiae MIS1 gene encoding mitochondrial C1-tetrahydrofolate synthase. J Biol Chem. 1988 Jun 5;263(16):7717–7725. [PubMed] [Google Scholar]
- Smith J. D., Celis J. E. Mutant tyrosine transfer RNA that can be charged with glutamine. Nat New Biol. 1973 May 16;243(124):66–71. [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Hoben P., Sumner-Smith M., Uemura H., Watson L., Söll D. Accuracy of in vivo aminoacylation requires proper balance of tRNA and aminoacyl-tRNA synthetase. Science. 1988 Dec 16;242(4885):1548–1551. doi: 10.1126/science.3144042. [DOI] [PubMed] [Google Scholar]
- Varshney U., Lee C. P., RajBhandary U. L. Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J Biol Chem. 1991 Dec 25;266(36):24712–24718. [PubMed] [Google Scholar]
- Varshney U., Lee C. P., Seong B. L., RajBhandary U. L. Mutants of initiator tRNA that function both as initiators and elongators. J Biol Chem. 1991 Sep 25;266(27):18018–18024. [PubMed] [Google Scholar]
- Varshney U., RajBhandary U. L. Initiation of protein synthesis from a termination codon. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1586–1590. doi: 10.1073/pnas.87.4.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T., Gross M., Sigler P. B. Isoleucyl initiator tRNA does not initiate eucaryotic protein synthesis. J Biol Chem. 1984 Apr 25;259(8):4706–4709. [PubMed] [Google Scholar]
- Yarus M. Intrinsic precision of aminoacyl-tRNA synthesis enhanced through parallel systems of ligands. Nat New Biol. 1972 Sep 27;239(91):106–108. doi: 10.1038/newbio239106a0. [DOI] [PubMed] [Google Scholar]








