Abstract
The component peptides of lacticin 3147 were degraded by α-chymotrypsin in vitro with a resultant loss of antimicrobial activity. Activity was also lost in ileum digesta. Following oral ingestion, neither of the lacticin 3147 peptides was detected in the gastric, jejunum, or ileum digesta of pigs, and no lacticin 3147 activity was found in the feces. These observations suggest that lacticin 3147 ingestion is unlikely to have adverse effects, since it is probably inactivated during intestinal transit.
Lacticin 3147 is a lantibiotic produced by Lactococcus lactis (16, 23). Its antimicrobial activity is mediated by the combined action of its component peptides, LtnA1 and LtnA2 (29). Like other bacteriocins, lacticin 3147 has a number of potential applications for both food and biomedicine (8). In particular, it offers advantages for use in foods, and previous studies have demonstrated its efficacy, both as a food biopreservative and in improving food quality (9, 27). While its broad spectrum offers certain advantages, it may also be a cause for concern, given that it has inhibited all gram-positive bacteria tested to date, including prominent members of the resident gut microbiota such as Lactobacillus, Enterococcus, and Streptococcus spp. (23). Indeed, the direct addition of lacticin 3147 to a human fecal fermentation gut model causes a dramatic reduction in gram-positive populations after only 30 min (22). Consequently, the ingestion of lacticin 3147 or other broad-spectrum bacteriocins in foods has the potential to perturb the commensal gut microbiota. Therefore, as part of a risk assessment for bacteriocins with potential applications in foods, it is important to determine their fate in the gastrointestinal tract (GIT) in vivo. This type of information is also important when considering potential therapeutic applications of orally delivered bacteriocins. This study evaluates the fate of lacticin 3147 in the digestive tract, initially by means of in vitro/ex vivo simulation studies and subsequently in vivo using the pig as a model. A food-grade lacticin 3147 ingredient developed in our laboratory to facilitate food applications was employed (17, 18). This spray-dried lacticin 3147 skim milk powder was manufactured as described previously (22) and reconstituted (10%, wt/vol) in sterile distilled water to prepare the reconstituted lacticin 3147 skim milk (RLSM) used in experiments.
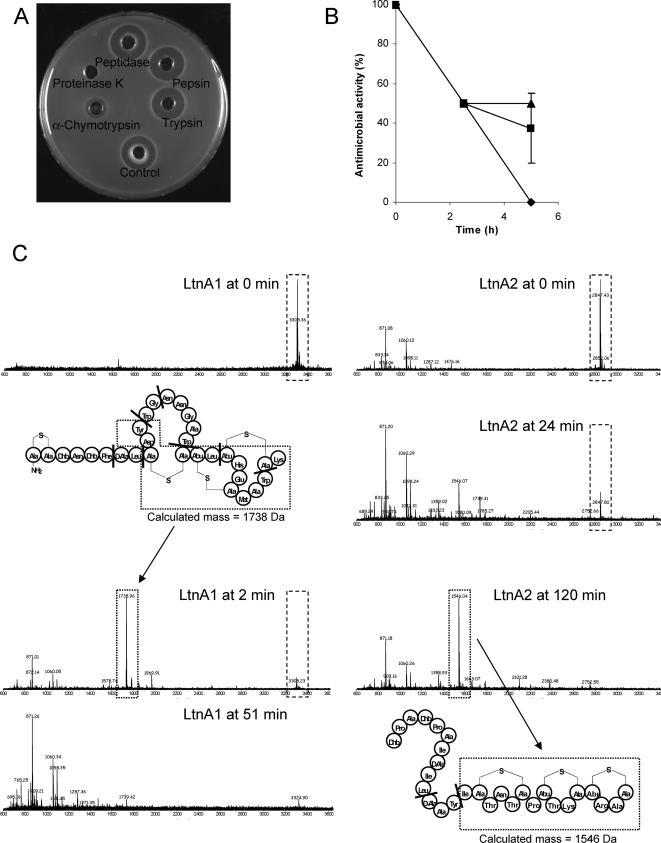
To test lacticin 3147's sensitivity to a range of proteolytic enzymes representative of those found in the mammalian GIT, RLSM was incubated at 37°C for 3 h with 25 mg/ml trypsin, α-chymotrypsin, TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone)-treated α-chymotrypsin, peptidase (all small-intestinal enzymes), or pepsin (stomach enzymes) (all enzymes obtained from Sigma Chemical Co., Poole, Dorset, United Kingdom). Proteinase K, which is known to inactivate lacticin 3147 (23), was used as a positive control and sterile distilled water as a negative control. Lacticin 3147 activity was then assessed using the agar diffusion assay, as described previously (23), using L. lactis HP as the indicator. The only digestive enzyme that affected activity was α-chymotrypsin, but it was not as effective as proteinase K, because although it caused a marked reduction in activity in 3 h, it did not result in complete inactivation (Fig. 1A). In subsequent experiments, RLSM was treated for 5 h with various concentrations of α-chymotrypsin. At the concentration used initially (25 mg/ml, equivalent to 1,350 U/ml), lacticin 3147 activity was reduced by 50% within 2.5 h, with complete inactivation observed after 5 h (Fig. 1B). Lower enzyme concentrations (0.38 and 3.8 U/ml, representing those normally encountered in simulated ileum juice or in porcine ileum digesta, respectively) caused a comparable reduction of 50% within 2.5 h but only slight or no reductions thereafter.
FIG. 1.
(A) Effect of a range of proteolytic digestive enzymes or proteinase K on antimicrobial activity of 10% RLSM following incubation at 37°C for 3 h with 25 mg/ml of each enzyme. The control was incubated with sterile distilled water. (B) Effect of 1,350 U/ml (⧫), 3.8 U/ml (▴), or 0.38 U/ml (▪) α-chymotrypsin on antimicrobial activity of 10% RLSM over time. The activity at 0 h was taken as 100%. Values are the means of the results from duplicate experiments with standard deviations indicated by vertical bars. (C) Digestion of 1 μg/ml of each of the LtnA1 and LtnA2 peptides with 77 μg/ml α-chymotrypsin followed over time by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Peptide digestions were performed in 100 mM Tris HCl-10 mM CaCl2, pH 7.8, at 30°C. The structures of LtnA1 and LtnA2 showing the α-chymotrypsin cleavage sites (indicated by heavy black bars) are also given, with the origin of some of the main peptide fragments generated from the digests indicated.
Each of the lacticin 3147 peptides, LtnA1 and LtnA2 (1 μg/ml separated from purified lacticin 3147 by reverse-phase high-performance liquid chromatography, as described previously [19]), was digested with 77 μg/ml α-chymotrypsin in 100 mM Tris HCl-10 mM CaCl2, pH 7.8, at 30°C. Mass spectrometry was performed on samples taken over a 2-h period, as outlined by Cotter et al. (7). Enzymatic digestion of both peptides was confirmed (Fig. 1C), which is not surprising, as both contain one or more amino acids (tyrosine, phenylalanine, tryptophan, and leucine) at which α-chymotrypsin cleaves (26). The masses of some of the main peptide fragments generated correlated with the calculated masses of the fragments expected based on the α-chymotrypsin cleavage sites (Fig. 1C). However, the LtnA1 peptide was more susceptible to digestion than LtnA2, with α-chymotrypsin breakdown products dominating the spectrum after only 2 min and no LtnA1 detectable after 51 min (Fig. 1C). Breakdown products for LtnA2, on the other hand, did not predominate until 24 min, and complete degradation took up to 120 min. This is most likely due to the fact that LtnA2 possesses only one primary α-chymotrypsin cleavage site, while LtnA1 has five (Fig. 1C). However, even though LtnA2 is more robust to degradation than LtnA1, both peptides are necessary for optimal activity (19, 29).
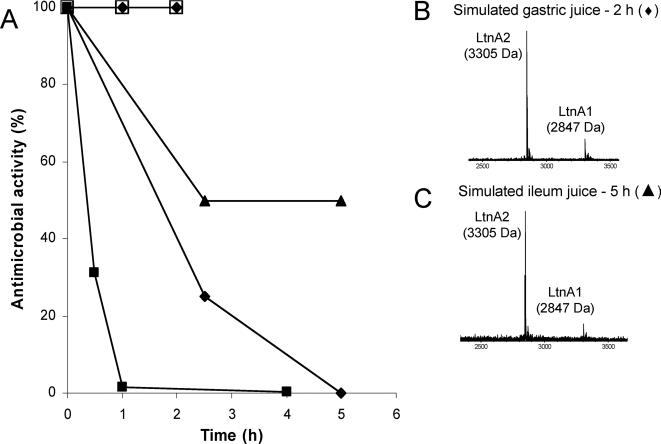
Lacticin 3147 stability and activity were then evaluated under conditions simulating the mammalian GIT. Some investigators have investigated survival or activity of bacteriocin-producing strains but not bacteriocin stability in intestinal simulation studies (2, 21). While Maré et al. (15) confirmed plantaricin production in the ileal section of a gastrointestinal model, the solution used appeared to lack digestive enzymes. In the present study, RLSM was added (1:1) to simulated gastric juice or porcine gastric digesta (pH 2) and to simulated ileum juice or ileum digesta, and each of the mixtures was incubated at 37°C for 2 and 5 h, respectively. Digesta were obtained from 9-week-old pigs at slaughter, and simulated intestinal juices were prepared according to Beumer et al. (3) but with lipase omitted from the ileum juice. Following the addition of RLSM, samples were taken over time (adjusted to pH ∼7 with 1 N NaOH for the gastric assays) and lacticin 3147 activity was determined in activity units (AU)/ml by agar diffusion assays (23), using L. lactis HP and Lactobacillus gasseri ATCC 33233 as indicators for the gastric and ileum assays, respectively. In the same way, purified lacticin 3147, prepared as described by Cotter et al. (7), was added (1:1) to simulated gastric and ileum juices, sampled at intervals, and analyzed by mass spectrometry for the presence of the lacticin 3147 peptides. No loss in lacticin 3147 activity was observed following incubation in either simulated gastric juice or porcine gastric digesta (Fig. 2A), and intact LtnA1 and LtnA2 peptides were detected by mass spectrometry in the simulated juice at the end of the incubation period (Fig. 2B). These data confirmed our initial findings that pepsin does not affect lacticin 3147 activity and suggest that lacticin 3147 would remain intact and active during gastric transit. On the other hand, lacticin 3147 activity was reduced by 50% after 2.5 h in simulated ileum juice but with no further reductions up to 5 h (Fig. 2A), at which time LtnA1 and LtnA2 were still detectable (Fig. 2C). Similarly, ex vivo studies showed that lacticin 3147 activity was reduced in porcine ileal digesta but to a greater extent than under simulated conditions, with only 25% of activity remaining after 2.5 h and complete inactivation occurring after 5 h (Fig. 2A). The inactivation of lacticin 3147 in the ileal environment is most likely due to digestion by α-chymotrypsin, and the differences observed probably reflect the differing concentrations of α-chymotrypsin: 0.38 U/ml was added to the simulated juice while concentrations of ∼2 to 3 U/g have been reported in small-intestinal digesta of pigs postweaning (12, 13). However, similar reductions in lacticin 3147 activity were observed when either 0.38 or 3.8 U/ml of α-chymotrypsin were used in vitro (Fig. 1B), indicating that there are factors other than chymotrypsin concentration that differ between simulated ileum juice and ileum digesta. Overall, our data suggest that once lacticin 3147 reaches the small intestine, its activity will be lost or at least reduced, and this is most likely dependent on α-chymotrypsin concentrations in the gut and gut transit time, the latter being influenced by the food with which lacticin 3147 is ingested.
FIG. 2.
(A) Antimicrobial activity of 10% RLSM during incubation at 37°C in simulated gastric juice (⧫), porcine gastric digesta (□), simulated ileum juice (▴), and porcine ileum digesta (•). Antimicrobial activity from lacticin 3147 skim milk powder mixed (10%) with porcine feces and incubated at 37°C is also shown (▪). Antimicrobial activity at 0 h was taken as 100%. Values are the means of the results from duplicate experiments with the feces/digesta experiments performed with samples obtained from two separate pigs. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry analysis of purified lacticin 3147 added to simulated gastric juice (B) and simulated ileum juice (C) after 2 and 5 h of incubation, respectively, is also shown.
Lacticin 3147 skim milk powder was also mixed (10%, wt/wt) with porcine feces and incubated at 37°C for 4 h, and activity was assessed at intervals by the agar diffusion assay using L. lactis HP and L. lactis HP(pMRC01) as indicators. Within 30 min, only ∼30% of the activity was recovered; after a further 30 min, this level was reduced to 1.5%, and by 4 h, only <0.5% of the initial lacticin 3147 activity remained (Fig. 2A). No inhibition of the lacticin 3147-insensitive strain L. lactis HP(pMRC01) was observed, confirming that the antimicrobial activity was due to the presence of lacticin 3147. Therefore, even if the lacticin 3147 peptides enter the distal GIT, it seems that activity will also be lost in this environment. This may be the result of enzymatic degradation, as α-chymotrypsin transits the small and large intestines and appears in the feces (11), or it may be due to binding of the lacticin 3147 peptides to the surfaces of fecal bacteria.
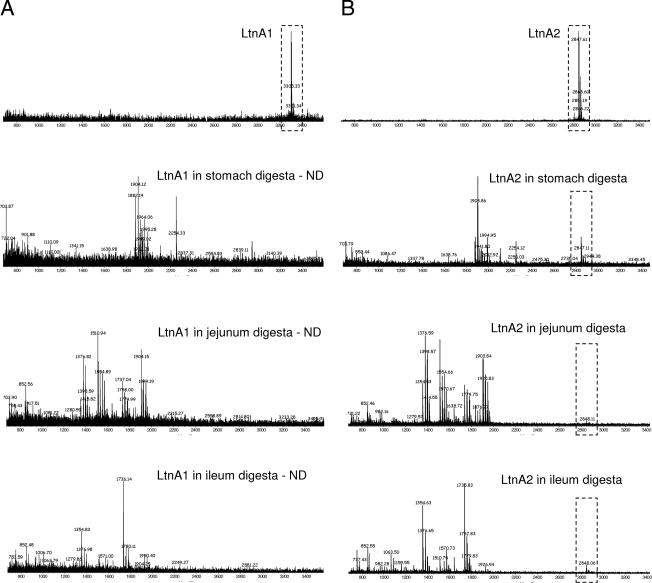
Since the fate of lacticin 3147 in the GIT can be accurately determined only in vivo, feeding studies were performed with pigs (in compliance with EU Council Directives 91/630/EEC[8a] and 98/58/EC[8b]). Pigs provide a suitable model for humans due to similarities in gastrointestinal physiology and anatomy (20). Five weeks postweaning, eight female pigs were blocked by weight, penned individually, and randomly assigned to two groups (n = 4). Having fasted from the previous evening, one group was fed 200 ml of RLSM containing 5,000 AU/ml lacticin 3147 (106 AU of lacticin 3147 ingested), and the other group was fed 200 ml of sterile 10% (wt/vol) reconstituted skim milk as a control. Feeding was staggered by 15 min, and once the RLSM or reconstituted skim milk was consumed (within 5 min), each pig was fed ∼200 g of a commercial starter diet (Easistart; Devenish Nutrition, Belfast, Northern Ireland). Two hours later, all of the pigs were slaughtered (by bleeding after captive bolt stunning) in the same order as they were fed. Previous marker transit studies in pigs showed that the majority of the ingested feed would have transited to the small intestine in this time (4). Stomach, jejunum, and ileum samples were examined for the presence of the lacticin 3147 peptides by mass spectrometry, since results from biological assays were inconclusive due to the lack of specificity of the well diffusion method (data not shown). Neither LtnA1 nor LtnA2 was detected in the digesta of any of the pigs that had ingested lacticin 3147 (data not shown). However, when each of the peptides was spiked into porcine stomach, jejunum, or ileum digesta at concentrations representing those ingested in vivo (37 μg/ml), only LtnA2 was detectable by mass spectrometry (Fig. 3). The fact that LtnA1 was undetectable might be due to the binding of the peptide to the surfaces of intestinal bacteria (it is suggested that LtnA1 binds to lipid II within the bacterial cell wall [29]). On the other hand, even though the mixtures were assayed immediately, LtnA1 may have been degraded by α-chymotrypsin present in the digesta, as it is more susceptible to digestion than LtnA2. Therefore, we can only conclude that LtnA2 was not present in the digesta of pigs fed lacticin 3147; it was absent from the gastric digesta because it had most likely transited the stomach in the 2 h prior to slaughter (4) and from the small intestine probably as a result of α-chymotrypsin degradation. Furthermore, in a longer-term feeding trial where 10 weaned pigs ingested 106 AU of lacticin 3147 daily for 7 days, no lacticin 3147 activity was detected by agar diffusion assay in the feces of any of the pigs at the end of the trial (data not shown). This is not surprising considering the loss of activity observed in porcine feces in earlier ex vivo assays (Fig. 2A).
FIG. 3.
Detection of LtnA1 (A) and LtnA2 (B) by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry when spiked into distilled water (control) or porcine stomach, jejunum, or ileum digesta at 37 μg/ml (concentration representative of the amount ingested by each pig fed lacticin 3147 in subsequent studies). The results are shown for digesta from one of four pigs analyzed. ND, nondetectable.
While we cannot make conclusions as to the presence/absence of the LtnA1 peptide in the intestinal digesta, its effects would most likely be negligible even if it was present, because although it has some antimicrobial activity alone, the combined action of both peptides is required for optimal activity (19, 29). Furthermore, on culturing selected bacterial populations representative of gut microbial species, it was found that lacticin 3147 ingestion did not affect Lactobacillus, coliform, or Enterococcus counts in either the porcine stomach or jejunum (P > 0.05; counts were log10 transformed and analyzed with SAS software [version 9.1] using analyses of variance, with the treatment group as the fixed term) (data not shown). However, this was a preliminary study, and because of the small number of replicates used, the power of the experiment was not sufficient to detect a 1 log difference in these microbial counts (i.e., a difference considered biologically significant). However, based on these findings, we used 10 replicates per treatment in a subsequent experiment, in which pigs ingested 106 AU of lacticin 3147 daily for 7 days. Differences in the fecal counts of Lactobacillus, Enterococcus, coliform, and total anaerobes between lacticin-fed and control pigs were less than the theoretical differences in bacterial population sizes, which could be measured using this approach (i.e., log10 0.58 to 1.09) (data not shown) (P > 0.05; using analyses of covariance on log10-transformed counts, where the fixed term was the treatment and the covariate was the baseline count). However, since only a small proportion of the gut microbiota are cultivable (14), major perturbations of the gut microbiota could theoretically have occurred without being detected using the approaches employed. Nonetheless, a previous study conducted in our laboratory showed that when lacticin 3147 powder is added directly to a fecal fermentation at a concentration similar to that fed to the pigs in the present study, counts of cultivable gram-positive commensal microflora, including Enterococcus and Lactobacillus, were reduced dramatically (22). This suggests that if lacticin 3147 remained intact during intestinal transit, similar reductions would be expected in vivo. Even with the limitations of the approaches used, the fact that no such lacticin 3147-mediated disturbances of these cultivable flora were observed in vivo provides further evidence that lacticin 3147 is degraded during intestinal transit.
Recently, Corr et al. (6) demonstrated that the antilisterial effect of Lactobacillus salivarius UCC118 in mice was mediated by Abp118, a bacteriocin produced by the strain. Others have demonstrated that encapsulated bacteriocins are effective in reducing Campylobacter carriage in poultry and that colicin-producing Escherichia coli reduce E. coli O157 shedding in cattle (5, 24, 25). However, some bacteriocin-producing strains lack efficacy in vivo (2, 10). While the studies outlined above provide indirect evidence that certain bacteriocins can retain bioactivity in the GIT, the investigators did not measure the fate of the bacteriocin peptides in vivo. Bernbom et al. (1) did, however, report the presence of nisin in the small-intestinal contents and feces of human flora-associated rats, but to our knowledge, this is the only report of its type. This is most likely due to difficulties involved in detecting bacteriocin peptides within complex environments. Biological methods used for bacteriocin detection, such as agar diffusion assays, offer the advantage of demonstrating bioactivity but have limitations due to lack of specificity and limited sensitivity. Recently, alternative strategies, such as autoinduction assays (28) and enzyme-linked immunosorbent assays (ELISAs) (1), have been employed for bacteriocin detection in vivo. However, ELISAs do not discriminate between intact and degraded bacteriocins, as the epitope recognized by the antibody will be present on fragments of the molecule as well as the intact peptide. Therefore, ELISAs provide little information as to whether the molecule(s) remains intact in the gut. In fact, Bernbom et al. (1) stated that nisin concentrations estimated in the gut and feces by ELISA were ∼10- to 200-fold higher than the corresponding concentrations estimated by a biological assay and concluded that this was an indication of nisin degradation/inactivation in the GIT. The mass spectrometry approach used in the present study for the detection of the lacticin 3147 peptides has some limitations, in that it is not quantitative and the LtnA1 molecule appears undetectable. Nonetheless, it is capable of detecting intact and therefore biologically active LtnA2, if present in gut samples.
In conclusion, the implication of the findings of this study is that ingestion of antimicrobial peptides such as lacticin 3147 is unlikely to perturb the gut microbiota, because in all probability they will be degraded in the small intestine by α-chymotrypsin with a resultant loss of activity. This is a positive finding from the point of view of consumer safety when considering the addition of lacticin 3147 to foods. On the other hand, it has implications for oral delivery of bacteriocins to the intestine for therapeutic applications. However, strategies such as encapsulation or enteric coating could be used to ensure targeted delivery of active peptide to the desired site. Alternatively, probiotic bacteria that produce bacteriocin in situ could be exploited as a means of delivering functional antimicrobials to the gut. Future work on lacticin 3147 will address this.
Acknowledgments
We thank Paul Cotter for helpful discussions and Michelle Gardiner, Christine Beecher, Jim O'Reilly, and the staff of the Moorepark pig unit for technical assistance.
This work was supported by Science Foundation Ireland through a Centre for Science, Engineering, and Technology award to the Alimentary Pharmabiotic Centre.
Footnotes
Published ahead of print on 31 August 2007.
REFERENCES
- 1.Bernbom, N., T. R. Licht, C.-H. Brogren, B. Jelle, A. H. Johansen, I. Badiola, F. K. Vogensen, and B. Nørrung. 2006. Effects of Lactococcus lactis on composition of intestinal microbiota: role of nisin. Appl. Environ. Microbiol. 72:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernbom, N., T. R. Licht, P. Saadbye, F. K. Vogensen, and B. Nørrung. 2006. Lactobacillus plantarum inhibits growth of Listeria monocytogenes in an in vitro continuous flow gut model, but promotes invasion of L. monocytogenes in the gut of gnotobiotic rats. Int. J. Food Microbiol. 108:10-14. [DOI] [PubMed] [Google Scholar]
- 3.Beumer, R. R., J. de Vries, and F. M. Rombouts. 1992. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 15:153-163. [DOI] [PubMed] [Google Scholar]
- 4.Clemens, E. T., C. E. Stevens, and M. Southworth. 1975. Sites of organic acid production and pattern of digesta movement in the gastrointestinal tract of swine. J. Nutr. 105:759-768. [DOI] [PubMed] [Google Scholar]
- 5.Cole, K., M. B. Farnell, A. M. Donoghue, N. J. Stern, E. A. Svetoch, B. N. Eruslanov, L. I. Volodina, Y. N. Kovalev, V. V. Perelygin, E. V. Mitsevich, I. P. Mitsevich, V. P. Levchuk, V. D. Pokhilenko, V. N. Borzenkov, O. E. Svetoch, T. Y. Kudryavtseva, I. Reyes-Herrera, P. J. Blore, F. Solis de los Santos, and D. J. Donoghue. 2006. Bacteriocins reduce Campylobacter colonization and alter gut morphology in turkey poults. Poult. Sci. 85:1570-1575. [DOI] [PubMed] [Google Scholar]
- 6.Corr, S. C., Y. Li, C. U. Riedel, P. W. O'Toole, C. Hill, and C. G. Gahan. 2007. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 104:7617-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter, P. D., L. H. Deegan, E. M. Lawton, L. A. Draper, P. M. O'Connor, C. Hill, and R. P. Ross. 2006. Complete alanine scanning of the two-component lantibiotic lacticin 3147: generating a blueprint for rational drug design. Mol. Microbiol. 62:735-747. [DOI] [PubMed] [Google Scholar]
- 8.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 8a.Council of the European Communities. 1991. Council Directive of 19 November 1991 laying down minimum standards for the protection of pigs (91/630/EEC). Council of the European Communities, Brussels, Belgium.
- 8b.Council of the European Union. 1998. Council Directive 98/58/EC of 20 July 1998 concerning the protection of animals kept for farming purposes. OJEC L221: 23-27.
- 9.Deegan, L. H., P. D. Cotter, C. Hill, and R. P. Ross. 2006. Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int. Dairy J. 16:1058-1071. [Google Scholar]
- 10.Frana, T. S., S. A. Carlson, D. C. Rauser, B. D. Jones, B. J. Fergen, and R. W. Griffith. 2004. Effects of microcin 24-producing Escherichia coli on shedding and multiple-antimicrobial resistance of Salmonella enterica serotype typhimurium in pigs. Am. J. Vet. Res. 65:1616-1620. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg, D. M. 2000. Proteases in the evaluation of pancreatic function and pancreatic disease. Clin. Chim. Acta. 291:201-221. [DOI] [PubMed] [Google Scholar]
- 12.Hedemann, M. S., and B. B. Jensen. 2004. Variations in enzyme activity in stomach and pancreatic tissue and digesta in piglets around weaning. Arch. Anim. Nutr. 58:47-59. [DOI] [PubMed] [Google Scholar]
- 13.Hedemann, M. S., B. B. Jensen, and H. D. Poulsen. 2006. Influence of dietary zinc and copper on digestive enzyme activity and intestinal morphology in weaned pigs. J. Anim. Sci. 84:3310-3320. [DOI] [PubMed] [Google Scholar]
- 14.Leser, T. D., J. Z. Amenuvor, T. K. Jensen, R. H. Lindecrona, M. Boye, and K. Møller. 2002. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 68:673-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maré, L., G. M. Wolfaardt, and L. M. Dicks. 2006. Adhesion of Lactobacillus plantarum 423 and Lactobacillus salivarius 241 to the intestinal tract of piglets, as recorded with fluorescent in situ hybridization (FISH), and production of plantaricin 423 by cells colonized to the ileum. J. Appl. Microbiol. 100:838-845. [DOI] [PubMed] [Google Scholar]
- 16.Martin, N. I., T. Sprules, M. R. Carpenter, P. D. Cotter, C. Hill, R. P. Ross, and J. C. Vederas. 2004. Structural characterization of lacticin 3147, a two-peptide lantibiotic with synergistic activity. Biochemistry 43:3049-3056. [DOI] [PubMed] [Google Scholar]
- 17.Morgan, S. M., M. Galvin, J. Kelly, R. P. Ross, and C. Hill. 1999. Development of a lacticin 3147-enriched whey powder with inhibitory activity against foodborne pathogens. J. Food Prot. 62:1011-1016. [DOI] [PubMed] [Google Scholar]
- 18.Morgan, S. M., M. Galvin, R. P. Ross, and C. Hill. 2001. Evaluation of a spray-dried lacticin 3147 powder for the control of Listeria monocytogenes and Bacillus cereus in a range of food systems. Lett. Appl. Microbiol. 33:387-391. [DOI] [PubMed] [Google Scholar]
- 19.Morgan, S. M., P. M. O'Connor, P. D. Cotter, R. P. Ross, and C. Hill. 2005. Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrob. Agents Chemother. 49:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moughan, P. J., M. J. Birtles, P. D. Cranwell, W. C. Smith, and M. Pedraza. 1992. The piglet as a model animal for studying aspects of digestion and absorption in milk-fed human infants. World Rev. Nutr. Diet 67:40-113. [DOI] [PubMed] [Google Scholar]
- 21.Portrait, V., S. Gendron-Gaillard, G. Cottenceau, and A. M. Pons. 1999. Inhibition of pathogenic Salmonella enteritidis growth mediated by Escherichia coli microcin J25 producing strains. Can. J. Microbiol. 45:988-994. [DOI] [PubMed] [Google Scholar]
- 22.Rea, M. C., E. Clayton, P. O'Connor, F. Shanahan, B. Kiely, R. P. Ross, and C. Hill. 2007. Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J. Med. Microbiol. 56:940-946. [DOI] [PubMed] [Google Scholar]
- 23.Ryan, M. P., M. C. Rea, C. Hill, and R. P. Ross. 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schamberger, G. P., R. L. Phillips, J. L. Jacobs, and F. Diez-Gonzalez. 2004. Reduction of Escherichia coli O157:H7 populations in cattle by addition of colicin E7-producing E. coli to feed. Appl. Environ. Microbiol. 70:6053-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern, N. J., E. A. Svetoch, B. V. Eruslanov, V. V. Perelygin, E. V. Mitsevich, I. P. Mitsevich, V. D. Pokhilenko, V. P. Levchuk, O. E. Svetoch, and B. S. Seal. 2006. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother. 50:3111-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweeney, P. J., and J. M. Walker. 1993. Proteolytic enzymes for peptide production, p. 277-304. In M. M. Burrell (ed.), Methods in molecular biology, vol. 16. Enzymes of molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 27.Twomey, D., R. P. Ross, M. Ryan, B. Meaney, and C. Hill. 2002. Lantibiotics produced by lactic acid bacteria: structure, function and applications. Antonie Leeuwenhoek 82:165-185. [PubMed] [Google Scholar]
- 28.Wescombe, P. A., M. Upton, K. P. Dierksen, N. L. Ragland, S. Sivabalan, R. E. Wirawan, M. A. Inglis, C. J. Moore, G. V. Walker, C. N. Chilcott, H. F. Jenkinson, and J. R. Tagg. 2006. Production of the lantibiotic salivaricin A and its variants by oral streptococci and use of a specific induction assay to detect their presence in human saliva. Appl. Environ. Microbiol. 72:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiedemann, I., T. Bottiger, R. R. Bonelli, A. Wiese, S. O. Hagge, T. Gutsmann, U. Seydel, L. Deegan, C. Hill, P. Ross, and H. G. Sahl. 2006. The mode of action of the lantibiotic lacticin 3147-a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 61:285-296. [DOI] [PubMed] [Google Scholar]