Abstract
In Lactococcus lactis, the interactions between oxidative defense, metal metabolism, and respiratory metabolism are not fully understood. To provide an insight into these processes, we isolated and characterized mutants of L. lactis resistant to the oxidizing agent tellurite (TeO32−), which generates superoxide radicals intracellularly. A collection of tellurite-resistant mutants was obtained using random transposon mutagenesis of L. lactis. These contained insertions in genes encoding a proton-coupled Mn2+/Fe2+ transport homolog (mntH), the high-affinity phosphate transport system (pstABCDEF), a putative osmoprotectant uptake system (choQ), and a homolog of the oxidative defense regulator spx (trmA). The tellurite-resistant mutants all had better survival than the wild type following aerated growth. The mntH mutant was found to be impaired in Fe2+ uptake, suggesting that MntH is a Fe2+ transporter in L. lactis. This mutant is capable of carrying out respiration but does not generate as high a final pH and does not exhibit the long lag phase in the presence of hemin and oxygen that is characteristic of wild-type L. lactis. This study suggests that tellurite-resistant mutants also have increased resistance to oxidative stress and that intracellular Fe2+ can heighten tellurite and oxygen toxicity.
Lactococcus lactis interacts with oxygen in a complex fashion. When grown with aeration in conventional growth media, it produces damaging reactive oxygen species (ROS), such as H2O2, OH·, and O2−. It can defend itself against these compounds with superoxide dismutase and peroxidases. However, the peroxidase activity is low, resulting in H2O2 accumulation. In the presence of iron, this is converted to the OH· radical via the Fenton reaction. The OH· radical is very reactive and damages a wide variety of biomolecules. While L. lactis is not an obligate anaerobe, a clear manifestation of its oxygen sensitivity is the very poor survival of aerated cells in stationary phase (2, 20). This picture is complicated by the ability of L. lactis to carry out respiration when supplied with exogenous hemin (4). Respiring cultures grow to a high optical density, acidify the medium less, and have a greatly extended survival time in stationary phase in comparison with nonrespiring aerated cultures (4). The basis for the survival in stationary phase has been examined by Rezaïki et al. (20), who found that respiring cells scavenge oxygen from the medium and so prevent the formation of ROS.
There has been significant but not extensive research into the molecular details of L. lactis oxidative stress and oxidative defense. Superoxide dismutase- and thioredoxin reductase-deficient mutants have been shown to have defective growth under aerobic conditions (22, 29). In addition, insertional mutagenesis studies aimed at identifying genes involved in thermal resistance and acid resistance revealed that mutants deficient in high-affinity phosphate transport, purine metabolism, and the stringent response are resistant to multiple stressors, including oxidative stress (3, 6, 19). These studies also identified several genes of unknown function that impact on oxidative defense. To date, the direct identification of L. lactis insertion mutants affected in oxidative defense has not been reported.
Potassium tellurite (K2TeO3) is used as a selective and differential agent in a number of different bacteriological media for the isolation of food-borne pathogens. In general, gram-positive bacteria are much more tellurite resistant than gram-negative bacteria. The mechanism of tellurite toxicity is not fully understood, although there is now strong evidence that it imposes oxidative stress through the generation of ROS and in particular O2− during intracellular reduction to tellurium (18, 26). It is likely that its oxidizing action has some similarities to paraquat, which also generates intracellular O2−. The aim of this study was to identify genes involved in the oxidative defense mechanisms of L. lactis. We hypothesized that L. lactis tellurite-resistant insertion mutants would also be resistant to oxidative stress. Twenty tellurite-resistant strains were isolated from a pool of random insertion mutants. The tellurite-resistant mutants were found to be resistant to oxidative stress and contained insertions in genes encoding a metal ion transporter, a phosphate uptake system, a compatible solute uptake system, and a redox regulator homolog. Moreover, it was found that the metal ion transporter mutant is defective in Fe2+ uptake and has altered growth properties under respiration-conducive conditions.
MATERIALS AND METHODS
Bacterial strains, chemicals, and enzymes.
L. lactis subsp. cremoris MG1363 was grown using M17 supplemented with 0.5% glucose (GM17) or 1% glucose (2× GM17) (Oxoid, Basingstoke, United Kingdom) and was incubated at 30°C or 37°C as required. L. lactis containing pGh9::ISS1 (13) was grown in the presence of 2 μg/ml erythromycin. Escherichia coli JM109 (Promega, Madison, WI) and E. coli XL1-Blue (Stratagene) were used in cloning experiments and were grown using either Luria-Bertani medium (Oxoid, Basingstoke, United Kingdom) or brain heart infusion medium (Oxoid, Basingstoke, United Kingdom) containing 300 μg/ml erythromycin at 30°C or 37°C as required. Cysteine (Sigma-Aldrich, St. Louis, MO) and potassium tellurite (K2TeO3) in a 3.5% solution (Oxoid) or as a powder (Sigma-Aldrich) were added to growth media as required. Restriction enzymes, DNA ligase, and Expand DNA polymerase were obtained from Roche (Indianapolis, IN).
Construction of a transposon library using pGh9::ISS1 and isolation of tellurite-resistant mutants.
A random L. lactis transposon library was prepared essentially as described by Maguin et al. (13). The library (containing ∼16,000 mutants) was scraped from the surface of agar plates, mixed, and stored at −80°C in 40% glycerol. An aliquot of this library was grown to log phase and spread onto GM17 agar containing 2 μg/ml erythromycin and 0.1 mM or 0.05 mM tellurite. In the middle of the plate was placed a 6-mm-diameter disk to which 10 μl cysteine (1 M) was added. Plates were incubated at 36.5°C for 2 days. Out of ∼3 × 105 mutants plated, there were 106 colonies on the plate containing 0.1 mM tellurite (resistant mutant frequency was ∼3.5 × 10−4 per mutant) and ∼900 colonies on the plate containing 0.05 mM tellurite (resistant mutant frequency was ∼3 × 10−3 per mutant). Twenty tellurite-resistant mutants were purified by streaking onto GM17 agar containing 2 μg/ml erythromycin and 0.1 mM tellurite and incubated at 36.5°C and were the subject of further study.
Characterization of tellurite-resistant mutants.
Chromosomal DNA was obtained from tellurite-resistant mutants by standard methods (9) and the ISS1 flanking sequences were cloned in E. coli as described previously, except that cultures were grown at the permissive temperature (13). Sequencing of the ISS1 flanking regions was performed using primer ISS1-seq1 (5′-CACGATAGCTTAGATTGTAACG-3′) (for EcoRI loopouts) or ISS1-seq2 (5′-GAACCGAAGAAATGGAACGCTC-3′) (for HindIII loopouts). The ISS1 insertion site for 12 out of 20 mutants was determined by plasmid rescue. Inverse PCR was performed on each of the mutants (following EcoRI digestion and religation) using primers ErmUS (5′-AACGAGCTCATACACCAATCAGTGCAA-3′) and ISS1-seq1. The band sizes obtained by inverse PCR for the eight uncharacterized mutants were the same as those for bands obtained from the characterized mntH or pstA insertion mutants. PCR was performed on undigested chromosomal DNA of the eight uncharacterized mutants by use of primers specific for the mntH (MntH-DS-Xho [5′-AGCCTCGAGTGCTTTTCGCGCTCGCTC-3′]) or pstA (PstA-check [5′-AATTGGGTCAAGGGCTGATG-3′]) genes and ISS1-seq1. These PCR products were sequenced to identify the exact ISS1 insertion site in these mutants. The sequences of the flanking genomic regions were compared to the L. lactis subsp. cremoris MG1363 genome (31) and to other genes in databases by use of BLAST programs (1). To isolate stable ISS1 mutants, the integrated plasmid was excised as described previously (13). Tellurite-resistant mutants were confirmed by growing them to late log phase, diluting the culture 1 in 100 in fresh GM17, and streaking a loopful onto GM17 agar containing 0.4 mM tellurite and 0.5 mM cysteine or GM17 agar containing 0.4 mM tellurite. Wild-type (wt) L. lactis MG1363 was also included, and the plates were incubated overnight at 37°C.
Survival after aerated growth.
Overnight nonaerated cultures of L. lactis MG1363 and the mutants grown at 30°C were diluted 1 in 1,000 in 2× GM17 and incubated at 30°C under aerated conditions. The medium was less than 1/10 of the culture tube volume, and the cultures were shaken at 230 rpm. Dilutions were carried out after 9 and 24 h of incubation, and 10-μl spots were plated onto GM17 agar to determine viable cell numbers.
Iron uptake analysis.
Measurement of Fe2+ uptake was performed using the colorimetric ferrozine assay (24), which can measure the bacterially mediated depletion of iron from solutions (11). Cultures of L. lactis MG1363 and the mntH mutant were harvested at late log growth phase, washed once, resuspended in 50 mM NaCl to similar densities (wt optical density at 600 nm [OD600] = 5.45; mntH mutant OD600 = 5.6), and kept on ice for ∼30 min. Cells (0.5 ml) were mixed with 0.5 ml NaCl (50 mM) and were warmed by placing in a water bath set at 30°C for 10 min. The assay was started by the addition of 10 μl of 10 mM FeSO4·6H2O, which was freshly prepared to minimize oxidation. To measure the effect of Mn2+ on iron uptake, 10 μl of 100 mM MnSO4·H2O (i.e., at a concentration 10 times higher than that of iron) was added 1 min prior to the addition of iron. Samples (150 μl) were taken from the assay after 30 min, and the cells were removed by centrifugation for 1 min at 18,000 × g. A portion (100 μl) of supernatant was assayed for Fe2+ concentration using the ferrozine assay as previously described (30). Control reaction mixtures containing no cells showed that the level of Fe2+ remained constant for the duration of the experiment and was not affected by the presence of other metal ions (Mn2+, Zn2+, or Mg2+).
Respiration growth studies.
Overnight nonaerated cultures of L. lactis MG1363 and the mntH mutant grown at 30°C were diluted 1 in 1,000 in 2× GM17 and incubated at 30°C under either aerated or respiration-conducive conditions. Cultures were grown for 20 h with shaking at 180 rpm in Erlenmeyer flasks filled to less than 1/10 flask volume either in the absence (aeration) or in the presence (respiration) of autoclaved hemin (final concentration, 10 μg/ml; Sigma) or filtered hemin precursor protoporphyrin IX (PPIX) (final concentration, 10 μg/ml; Sigma). At this point, the OD600 values of the cultures and the pH levels of the spent supernatants were measured.
Hemin-induced toxicity assay.
Overnight nonaerated cultures of L. lactis MG1363 and the mntH mutant grown at 30°C were diluted 1 in 1,000 in 2× GM17 with or without autoclaved hemin (10 μg/ml final) and incubated at 30°C under aerated conditions. Cultures were shaken at 230 rpm in culture tubes filled to less than 1/10 total tube volume, and the OD600 was followed during the incubation.
RESULTS
Isolation and characterization of L. lactis mutants with greater resistance to tellurite.
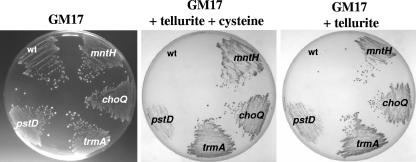
Random insertional mutagenesis was performed on L. lactis by use of pGh9::ISS1, and tellurite-resistant mutants were selected on plates containing tellurite (0.1 or 0.05 mM) with a filter disk containing cysteine placed in the center (see Materials and Methods). Previous work has shown that cysteine significantly heightens the toxicity of tellurite to Staphylococcus aureus, and this was also found to be the case with L. lactis (reference 27 and unpublished data). Twenty mutants were isolated, and the genes which contained the integrated pGh9::ISS1 plasmids were identified by sequence comparison with genes from the L. lactis subsp. lactis MG1363 genome (31) and other bacteria (Table 1). It was found that 12 mutants had insertions in the same position in a gene encoding a protein similar to the proton-dependent transporter MntH. MntH is a member of the Cβ class of the natural resistance-associated macrophage protein (Nramp) family (21), members of which have been shown to mediate the uptake of Mn2+ and or Fe2+, the latter with much lower affinity (12, 15). Five mutants had independent insertions in the pstA and pstD genes, which encode components of the Pst high-affinity phosphate transporter. One pstA mutant contained another pGh9::ISS1 located elsewhere in the chromosome, which was not identified. Two mutants had the same insertion in a gene encoding the ATPase component of a putative proline/glycine-betaine/carnitine transport system (ChoQ), which most probably functions in osmoprotection. Finally, one mutant had an insertion just upstream of the trmA gene, which was previously identified as being involved in temperature resistance in L. lactis MG1363 (3). One of each class of mutant (mntH, pstD, choQ, and trmA) was selected and stabilized by excision of the transposed plasmid (see Materials and Methods). Each of these stable mutants was confirmed as being more resistant than the wt both to a combination of tellurite and to cysteine or tellurite alone (Fig. 1).
TABLE 1.
Genes affected in the tellurite-resistant mutants and their putative functions
| Genea | No. of mutants | Insertion site(s)b | Homologous proteins | Putative function | Reference |
|---|---|---|---|---|---|
| llmg_1490 | 12 | 1184 | MntH | Mn2+/Fe2+ transport (Nramp) | 21 |
| llmg_1896 | 3 | 11,c 58, 87 | PstA | Phosphate transport (ATPase) | 19 |
| llmg_1899 | 2 | 101, 507 | PstD | Phosphate transport (permease) | 19 |
| llmg_1700 | 2 | 268 | ChoQ | Proline/glycine-betaine/carnitine/choline transport (ATPase) | 5 |
| llmg_0640 | 1 | −43d | TrmA (Spx) | Temperature resistance | 3 |
Gene is from the L. lactis subsp. cremoris MG1363 genome (31).
Insertion site(s) of pGh9::ISS1 is in nucleotides from the start of the gene.
Two insertions of pGh9::ISS1 were integrated into the chromosome of this mutant.
The insertion of pGh9::ISS1 was 43 bp upstream from the gene.
FIG. 1.
Growth of tellurite-resistant mutants but not wt L. lactis MG1363 on tellurite-containing agar. Log-phase-grown cells were diluted (10−2) and streaked onto GM17 agar, GM17 agar containing 0.4 mM tellurite plus 0.5 mM cysteine, or GM17 agar containing 0.4 mM tellurite. Plates were incubated overnight at 37°C. The pstD mutant grew slower on tellurite containing agars and produced pinpoint colonies after 24 h.
Tellurite-resistant mutants have improved survival following aerated growth.
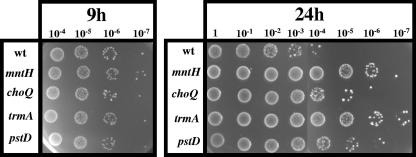
Following fermentative growth under aerated conditions, L. lactis cells have severe protein and DNA damage, high spontaneous mutation frequencies, and poor survival due to oxidative stress (20). We investigated whether the tellurite-resistant mutants had improved oxidative stress resistance by measuring their survival following aerated growth. After 9 h of growth (early stationary phase), cell viability was the same for the mutants as for the wt (Fig. 2). However, 24 h after growth, the mutants showed survivals approximately 1,000-fold (mntH and trmA) and >10-fold (pstD and choQ) greater than that of the wt. This suggests that the mutants are more resistant to the toxic oxidative effects resulting from aerated growth.
FIG. 2.
Survival of wt L. lactis MG1363 and tellurite-resistant mutants following growth under aerated conditions. Overnight nonaerated cultures were diluted 1 in 1,000 in 2× GM17 and incubated at 30°C with shaking. After 9 and 24 h, viable cell numbers were determined by diluting the cultures and spotting 10 μl onto GM17 agar. Plates were incubated overnight at 30°C. This experiment was performed twice, with similar results observed each time.
mntH insertion mutagenesis impairs Fe2+ uptake.
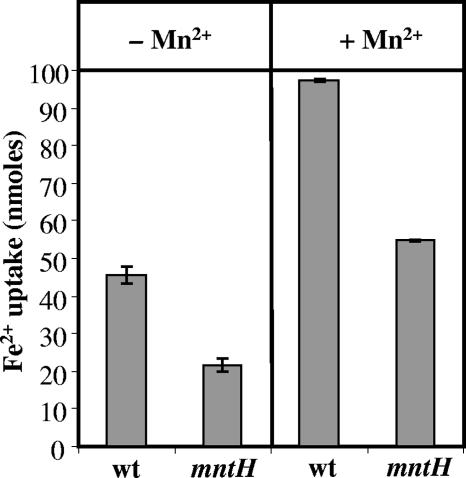
It has been shown that free iron is a major contributor to mortality following the aerated growth of L. lactis due to its participation in the Fenton reaction (20). We hypothesized that the mntH mutant is more resistant to tellurite and oxidative stress because it is unable to transport iron efficiently and consequently has lower intracellular iron levels. Measurement of iron uptake by the wt and the mntH mutant was performed using the ferrozine assay to monitor iron depletion from a solution after 30 min. The removal of iron by the mntH mutant was significantly (>2-fold) lower than that by the wt (Fig. 3, left). This confirms that MntH is a major iron transporter in L. lactis. Residual iron transport activity in the mntH mutant suggests that L. lactis also contains other iron transporters. As manganese has been reported to be the major metal ion transported by MntH proteins in gram-negative bacteria (12, 15), we tested the effect of manganese addition on iron uptake by wt and mntH mutant cells. Manganese actually increased iron uptake by both strains (Fig. 3). Other metal ions, Zn2+ or Mg2+, had little or no effect (iron uptake was not affected by Zn2+ and was slightly inhibited by Mg2+; data not shown), suggesting that this effect is not a general metal ion phenomenon. The addition of a lower concentration of Mn2+ (the same concentration as Fe2+) also stimulated Fe2+ uptake (data not shown). This suggests that manganese is not a competitive inhibitor of iron uptake but instead stimulates iron transport at least partially independently of MntH.
FIG. 3.
Uptake of iron by L. lactis MG1363 (wt) and the mntH mutant either in the absence (left) or in the presence (right) of manganese. The total amount of Fe2+ added to the cells was 100 nmol (final concentration, 99 μM). Mn2+ was added at a 10-fold-higher concentration (990 μM) shortly before the addition of Fe2+. Fe2+ remaining in the supernatant was measured after 30 min. Triplicate iron uptake assays were performed.
The mntH mutant affects respiration and is more resistant to hemin-induced oxidative stress.
L. lactis undergoes respiration metabolism in the presence of oxygen and exogenous hemin or the hemin precursor PPIX (4). Since iron in the form of hemin is required for functional cytochrome activity and respiration, we investigated whether MntH-mediated iron transport affects respiration in L. lactis. Growth characteristics of the wt and the mntH mutant under static, aerated, and respiration (aerated plus hemin or PPIX) growth conditions were examined. No significant differences in final biomass and pH between the wt and the mntH mutant under static and aerated conditions were observed (Table 2). Under respiration conditions with exogenous hemin or PPIX, the final biomasses were similar between the two strains, but the pH for the mntH mutant was consistently lower than that for the wt (Table 2). This suggests that MntH-mediated iron transport has an effect upon respiration in L. lactis.
TABLE 2.
L. lactis MG1363 (wt) and mntH mutant growth yields and pH values under static, aerated, and respiration-conducive conditions
| Growth condition | OD600 (wt/mntH mutant) | pH (wt/mntH mutant) |
|---|---|---|
| Static | 1.8/1.9 | 4.4/4.4 |
| Aeration | 2.0/1.9 | 4.4/4.4 |
| Respiration (hemin) | 2.9/2.7 | 5.3/4.8 |
| Respiration (PPIX) | 2.9/2.8 | 5.3/4.9 |
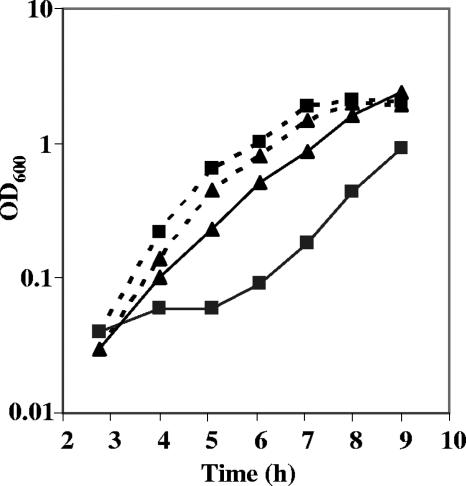
It has been reported that the addition of hemin to stationary-phase-exiting cultures of L. lactis results in an increase in the lag phase due to oxidative stress caused by precocious hemin uptake (7). It is most probable that the toxic effect of hemin is due to the iron component. It was hypothesized that the mntH mutant contains lower intracellular iron levels and would be more resistant to the toxic effects of hemin. Overnight cultures of the wt and the mntH mutant were diluted into growth media with or without added hemin and incubated with aeration. While the addition of hemin resulted in a significant lag phase for the wt, it had a negligible effect on the mntH mutant (Fig. 4). This suggests that iron in hemin in combination with iron transported by MntH causes significant oxidative stress. This result may also explain the lower final pH observed for the mntH mutant culture when grown under respiration conditions.
FIG. 4.
Effect of hemin on the growth of wt L. lactis MG1363 and the mntH mutant under aerobic conditions. Overnight nonaerated cultures were diluted 1 in 1,000 in 2× GM17 (broken lines) or 2× GM17 containing hemin (solid lines) and incubated at 30°C with shaking. The optical densities of the wt (squares) and the mntH mutant (triangles) were monitored for 9 h. This experiment was performed three times, with similar results observed each time.
DISCUSSION
In this study, we set out to use tellurite resistance as a means of identifying L. lactis genes involved in redox functions and oxidative stress. It has previously been reported (2, 20) that a key manifestation of oxidative stress is L. lactis is death during stationary phase in nonrespiring aerated cultures. All tellurite-resistant insertion mutants analyzed displayed increased survival under these conditions. This demonstrates that tellurite resistance is indeed a valuable selectable phenotype for revealing genes whose products impose oxidative stress. This is consistent with the well-accepted model that tellurite toxicity is due to the production of ROS (18, 25, 26).
Of the genes identified, mntH is arguably of the most interest. MntH belongs to the very widespread Nramp family of high-affinity proton-linked divalent cation transporters. This family is found in virtually all cellular life. In bacteria, many genomes contain several genes that encode diverged Nramp members. Fully characterized Nramp examples from bacteria have been found to be most active in transporting Mn2+ and Fe2+, the latter with much lower affinity (12, 15). This has resulted in a convention of naming Nramp members found in bacteria “MntH” (H+-dependent manganese transporter). The nramp gene family has been subjected to extensive duplication and divergence, and there is also evidence for horizontal gene transfer (21). The bacterial mntH genes have been divided into three major classes (A, B, and C), and class C has been divided into Cα, Cβ, and Cγ. The L. lactis MG1363 genome contains two putative mntH genes (31). One of these encodes a class B MntH (MntA; llmg_2171) that is most closely related to MntH sequences from Thermoanaerobacter and Clostridium, so the gene is likely to have entered the L. lactis genome by horizontal transfer. The other mntH gene is the one identified in this study. It encodes a class Cβ MntH. Its closest relatives are all found in lactic acid bacteria, in particular, Enterococcus, Oenococcus, and Lactobacillus brevis. Other class Cβ sequences are found in a wide variety of other lactic acid bacteria. While the complexity of this family makes it difficult to trace horizontal gene transfer events and reliably determine which mntH sequences are orthologs and which are paralogs, it is clear that the MntH class Cβ transporters are widely distributed in the lactic acid bacteria and have been there for a long time.
The class Cβ MntH transporters encoded by lactic acid bacteria have not been studied extensively. Hayashi et al. (10) found that the L. brevis mntH gene is a marker for hop resistance in beer-brewing fermentations, and it was suggested that Mn2+ and Mg2+ uptake is used to defend against the ionophores found in hop bitter compounds. Groot et al. (8) hypothesized that the MntH proteins potentially encoded by the Lactobacillus plantarum genome contribute to the accumulation of high intracellular manganese ion compounds. However, inactivating the genes alone or in combination had no measurable effect, so the roles of these genes in L. plantarum remain obscure. In the case of L. lactis, an attempt has been made to express the class Cβ MntH in E. coli, but it was apparently not functional (21). We have obtained strong evidence that the L. lactis class Cβ MntH is responsible for a large proportion of iron uptake under the experimental conditions used. The tellurite resistance of the mntH mutant is consistent with the well-known ability of iron to generate ROS, in particular the hydroxyl radical, which is especially damaging to biomolecules. This has been shown to be the case for L. lactis by Rezaïki et al. (20), who demonstrated a strong correlation between free iron availability in the growth medium and oxidative damage in aerated stationary-phase cultures. The metal ion specificity of MntH in L. lactis is not yet known. It was found here that manganese, but not other metal ions, stimulated an increase in iron uptake in L. lactis in an at least partially MntH-independent manner. This suggests that manganese does not competitively inhibit iron transport by MntH, unlike what has been reported for other MntH proteins, but does not rule out Mn2+ as a substrate for MntH (12, 15).
It was of interest to determine the effect of mntH inactivation on respiratory metabolism. Our hypothesis was that the lesion would have little effect in cultures with exogenous hemin but a significant effect on cultures with exogenous PPIX (hemin precursor). The rationale was that iron-deficient cells would be defective in the ability to synthesize hemin from PPIX. This, however, proved not be the case. The mntH mutant is clearly capable of respiratory metabolism in the presence of either hemin or PPIX, although in both instances, a key marker for respiratory metabolism was less marked in the mutant than in the parent. Specifically, the stationary-phase pH was lower. It may be that the defective iron uptake is reducing the amount of cytochrome in the PPIX-supplemented culture, with observed respiratory metabolism accounted for by mntH-independent iron uptake. However, why there is a similar reduction of respiratory activity in the hemin-supplemented culture remains obscure. A partial answer may be inferred from our observation that hemin-induced aerated culture lag phase is eliminated in the mntH mutant. This suggests that the amount of hemin entering the cell is less in the mntH mutant. However, it is also possible that oxidative stress is additive with respect to cytoplasmic free iron and hemin and that the mntH mutant cells were at a lower oxidative stress level than the wt before the hemin was added and so were hemin resistant. This remains to be resolved.
It was concluded that the L. lactis class Cβ MntH transporter is required for maximal iron uptake and that its inactivation depletes the cells of iron sufficiently to protect against oxidative stress but not sufficiently to prevent respiratory metabolism taking place in the presence of exogenous PPIX. This may be of significance to the dairy industry, as inactivation of mntH results in cells that in conventional growth media can gain the great majority of benefits of respiration with respect to biomass production but have additional resistance to oxidative stress imposed by (i) the presence of exogenous hemin in the aerated lag phase or (ii) aeration during growth in the absence of hemin.
Another gene of interest identified in the course of this study is trmA. This has previously been identified in L. lactis as a site of insertion mutations that relieve temperature sensitivity in a recA or clpP background and also has been shown to increase temperature and puromycin resistance when disrupted in a wt background (3, 6). There are eight genes in the trmA family encoded by the L. lactis MG1363 genome (31), and they have homology to the oxidative stress regulator Spx from Bacillus subtilis (16). Recent work has revealed novel roles of L. lactis TrmA homologs SpxB and TrmA in regulating peptidoglycan acetylation and lysozyme resistance (28).
The bases for the tellurite resistance of the choQ and pst mutants remain obscure. The ChoQ homolog from Listeria monocytogenes (OpuCA) is regulated by the stress-induced sigma-B system and plays a role in osmoregulation and virulence (5, 23). Insertions in genes encoding the high-affinity phosphate uptake system (PstABCDEF) have been identified in a number of searches for stress-resistant insertion mutants (3, 19). It may be that phosphate depletion induces a number of stress responses that cause cross-resistance to a range of stressors, including oxidative stress, and this has already been shown when examining H2O2 resistance (3, 19). It is feasible that the choQ mutant has a similar basis.
In conclusion, selection for tellurite resistance is an effective means of obtaining insertions in genes involved in oxidative stress or defense. This study has lead to the identification of a novel iron transporter and to the demonstration that several previously discovered genes contribute to oxidative stress or impede oxidative defense.
Acknowledgments
We thank Raquel Lo, Terry Walsh, Willa Huston, and the Bug Masters for helpful discussions on various aspects of this work.
This research was supported by grants from ARC (grant no. DP0665546) and NHMRC (grant no. 290526).
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duwat, P., S. D. Ehrlich, and A. Gruss. 1995. The recA gene of Lactococcus lactis: characterization and involvement in oxidative and thermal stress. Mol. Microbiol. 17:1121-1131. [DOI] [PubMed] [Google Scholar]
- 3.Duwat, P., S. D. Ehrlich, and A. Gruss. 1999. Effects of metabolic flux on stress response pathways in Lactococcus lactis. Mol. Microbiol. 31:845-858. [DOI] [PubMed] [Google Scholar]
- 4.Duwat, P., S. Sourice, B. Cesselin, G. Lamberet, K. Vido, P. Gaudu, Y. Le Loir, F. Violet, P. Loubiere, and A. Gruss. 2001. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J. Bacteriol. 183:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette l-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frees, D., P. Varmanen, and H. Ingmer. 2001. Inactivation of a gene that is highly conserved in Gram-positive bacteria stimulates degradation of non-native proteins and concomitantly increases stress tolerance in Lactococcus lactis. Mol. Microbiol. 41:93-103. [DOI] [PubMed] [Google Scholar]
- 7.Gaudu, P., G. Lamberet, S. Poncet, and A. Gruss. 2003. CcpA regulation of aerobic and respiration growth in Lactococcus lactis. Mol. Microbiol. 50:183-192. [DOI] [PubMed] [Google Scholar]
- 8.Groot, M. N., E. Klaassens, W. M. de Vos, J. Delcour, P. Hols, and M. Kleerebezem. 2005. Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology 151:1229-1238. [DOI] [PubMed] [Google Scholar]
- 9.Harwood, C. R., and S. M. Cutting. 1990. Molecular biological methods for Bacillus. John Wiley and Sons, Ltd., Chichester, England.
- 10.Hayashi, N., M. Ito, S. Horiike, and H. Taguchi. 2001. Molecular cloning of a putative divalent-cation transporter gene as a new genetic marker for the identification of Lactobacillus brevis strains capable of growing in beer. Appl. Microbiol. Biotechnol. 55:596-603. [DOI] [PubMed] [Google Scholar]
- 11.Huston, W. M., M. P. Jennings, and A. G. McEwan. 2002. The multicopper oxidase of Pseudomonas aeruginosa is a ferroxidase with a central role in iron acquisition. Mol. Microbiol. 45:1741-1750. [DOI] [PubMed] [Google Scholar]
- 12.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 13.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Makui, H., E. Roig, S. T. Cole, J. D. Helmann, P. Gros, and M. F. M. Cellier. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 35:1065-1078. [DOI] [PubMed] [Google Scholar]
- 16.Nakano, S., E. Kuster-Schock, A. D. Grossman, and P. Zuber. 2003. Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 100:13603-13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Perez, J. M., I. L. Calderon, F. A. Arenas, D. E. Fuentes, G. A. Pradenas, E. L. Fuentes, J. M. Sandoval, M. E. Castro, A. O. Elias, and C. C. Vasquez. 2007. Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS ONE 2:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rallu, F., A. Gruss, S. D. Ehrlich, and E. Maguin. 2000. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35:517-528. [DOI] [PubMed] [Google Scholar]
- 20.Rezaïki, L., B. Cesselin, Y. Yamamoto, K. Vido, E. van West, P. Gaudu, and A. Gruss. 2004. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol. Microbiol. 53:1331-1342. [DOI] [PubMed] [Google Scholar]
- 21.Richer, E., P. Courville, I. Bergevin, and M. F. M. Cellier. 2003. Horizontal gene transfer of “prototype” Nramp in bacteria. J. Mol. Evol. 57:363-376. [DOI] [PubMed] [Google Scholar]
- 22.Sanders, J. W., K. J. Leenhouts, A. J. Haandrikman, G. Venema, and J. Kok. 1995. Stress response in Lactococcus lactis: cloning, expression analysis, and mutation of the lactococcal superoxide dismutase gene. J. Bacteriol. 177:5254-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleator, R. D., J. Wouters, C. G. Gahan, T. Abee, and C. Hill. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 67:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stookey, L. L. 1970. Ferrozine: a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 25.Taylor, D. E. 1999. Bacterial tellurite resistance. Trends Microbiol. 7:111-115. [DOI] [PubMed] [Google Scholar]
- 26.Tremaroli, V., F. Stefano, and D. Zannoni. 2007. Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress of cells of Pseudomonas pseudoalcaligenes KF707. Arch. Microbiol. 187:127-135. [DOI] [PubMed] [Google Scholar]
- 27.Turner, M. S., R. Lo, and P. M. Giffard. 2007. Inhibition of Staphylococcus aureus growth on tellurite-containing media by Lactobacillus reuteri is dependent upon CyuC and thiol production. Appl. Environ. Microbiol. 73:1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veiga, P., C. Bulbarela-Sampieri, S. Furlan, A. Maisons, M. P. Chapot-Chartier, M. Erkelenz, P. Mervelet, P. Noirot, D. Frees, O. Kuipers, J. Kok, A. Gruss, G. Buist, and S. Kulakauskas. 7 May 2007. SpxB regulates O-acetylation dependent resistance of Lactococcus lactis peptidoglycan to hydrolysis. J. Biol. Chem. [Epub ahead of print.] [DOI] [PubMed]
- 29.Vido, K., H. Diemer, A. van Dorsselaer, E. Leize, V. Juillard, A. Gruss, and P. Gaudu. 2005. Roles of thioredoxin reductase during the aerobic life of Lactococcus lactis. J. Bacteriol. 187:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viollier, E., P. W. Inglett, K. Hunter, A. N. Roychoudhury, and P. van Cappellen. 2000. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 15:785-790. [Google Scholar]
- 31.Wegmann, U., M. O'Connell-Motherway, A. Zomer, G. Buist, C. Shearman, C. Canchaya, M. Ventura, A. Goesmann, M. J. Gasson, O. P. Kuipers, D. van Sinderen, and J. Kok. 2007. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 189:3256-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]