Abstract
Mycobacterium avium is a potential pathogen occurring in drinking water systems. It is a slowly growing bacterium producing a thick cell wall containing mycolic acids, and it is known to resist chlorine better than many other microbes. Several studies have shown that pathogenic bacteria survive better in biofilms than in water. By using Propella biofilm reactors, we studied how factors generally influencing the growth of biofilms (flow rate, phosphorus concentration, and temperature) influence the survival of M. avium in drinking water biofilms. The growth of biofilms was followed by culture and DAPI (4′,6′-diamidino-2-phenylindole) staining, and concentrations of M. avium were determined by culture and fluorescence in situ hybridization methods. The spiked M. avium survived in biofilms for the 4-week study period without a dramatic decline in concentration. The addition of phosphorus (10 μg/liter) increased the number of heterotrophic bacteria in biofilms but decreased the culturability of M. avium. The reason for this result is probably that phosphorus increased competition with other microbes. An increase in flow velocity had no effect on the survival of M. avium, although it increased the growth of biofilms. A higher temperature (20°C versus 7°C) increased both the number of heterotrophic bacteria and the survival of M. avium in biofilms. In conclusion, the results show that in terms of affecting the survival of slowly growing M. avium in biofilms, temperature is a more important factor than the availability of nutrients like phosphorus.
Mycobacterium avium is a pathogen infecting both humans and animals. In humans it may cause pulmonary, soft tissue, and, especially in children, lymph node infections (11, 17). Recent findings suggest that it may also cause hypersensitivity pneumonitis-like disease (32, 33). The risk of healthy individuals for getting M. avium infections is regarded as low. In contrast, the risk is increased with immunodeficiency, especially in AIDS patients, where infections are disseminated and often fatal. M. avium is an ubiquitous organism found in drinking water systems worldwide (2, 6, 12, 43, 58), and it has been detected in biofilms (12). Different warm water systems, such as hot tubs, circulating bath systems, and hospital water systems, have been recognized as potential sources of diseases caused by M. avium (32, 33, 51, 57). There are few reports on the association between M. avium and drinking water (2). Transmission from environmental sources is thought to occur via respiratory and gastrointestinal tracts (55). The risk associated with this emerging pathogen in water systems has been recognized by authorities: the U.S. Environmental Protection Agency has included M. avium in the contaminate candidate list for possible regulation in drinking water (54).
All mycobacterial cells, including M. avium, have some unique characteristics which probably enhance their persistence in biofilms. Mycobacterial cell walls have a high lipid content that makes them hydrophobic and allows them to adhere to surfaces easily. Their tolerance to disinfectant chemicals, like chlorine and ozone, is several orders of magnitude higher than that of Escherichia coli (23, 52). Mycobacteria are also capable of replicating in free-living amoebae (4), which may be important in oligotrophic water environments. Biofilm formation of mycobacteria has been linked to the synthesis of glycopeptidolipid, and several genes involved in the synthesis have been identified recently (59).
Several environmental factors influence the survival and growth of microbes in drinking water systems (42). The effects of pipe material (39, 47), disinfection (50, 52), and assimilable organic carbon (AOC) (12, 39, 53) have been studied for mycobacteria. The effects of several other factors known to affect the survival of heterotrophic bacteria in general remain unexplored. In boreal regions like Finland and Japan, drinking waters have a high organic matter content and phosphorus is the limiting nutrient for microbial growth (29, 36, 46). An increase in water flow velocity may enhance microbial growth by enhancing mass transfer of the nutrients into biofilms (30, 41). The effect of flow velocity is not straightforward, and conflicting results exist (8, 44). If flow velocity is too high, biofilms are detached (5). Temperature is a well-known factor influencing microbial growth in drinking water systems either directly by increasing growth or indirectly by, e.g., decreasing the effect of chlorine (42). In boreal regions, temperatures in water distribution systems may vary from as low as 3 to almost 20°C (53, 60). M. avium is a thermotolerant species capable of multiplying at 42°C. Whether temperatures in boreal drinking water systems are high enough for the multiplication of M. avium in biofilms is not known.
The aim of this study was to find out, how flow velocity, phosphorus, and temperature affect the survival of M. avium in experimentally produced biofilms. Because, like all bacteria, only a small minority of mycobacteria are culturable (24, 34, 35), we applied microscopic methods based on fluorescence in situ hybridization (FISH) of specific rRNA regions developed recently (24) in parallel with culture to detect both culturable and nonculturable M. avium.
MATERIALS AND METHODS
Experimental setting.
Biofilms were grown in Propella (Xenard; Mechanique de Precision, Seichamps, France) biofilm-monitoring reactors (40). The reactors included 20 polyvinyl chloride (PVC) coupons, with the area of a coupon being 2.01 cm2. The reactors were placed in a containment cabinet with a separate ventilation system, and we disinfected waste waters with chlorine before draining them into a sewer. In each experiment, two reactors (A and B) were used in parallel and their flow velocities, concentrations of phosphorus, and temperatures were adjusted as described in Table 1. In the basic run, water flow through the Propella reactor was maintained at about 185 ml/h and the flow rate was about 0.24 m/s, corresponding to a Reynolds number of over 15,000 (turbulent flow). The retention time of water in the reactor was 12.4 h. In the water flow experiment, the flow velocity of reactor A was adjusted to 0.1 m/s, also a turbulent flow rate. In the phosphorus experiment, the phosphorus concentration in reactor B was increased by adding Na2HPO4 with a separate flow so that the increase in Propella was about 10 μg PO4-P/liter. In temperature experiments, the temperatures were adjusted to 7 and 20°C, with a temperature element incorporated into the Propella devices. A cooling or warming effect was achieved by circulating cold or warm water, respectively, through the temperature element. Before spiking, the reactors were run for 4 weeks with drinking water from a municipal distribution supply using bank-filtrated lake water as raw water. The characteristics of the drinking water are described in Table 1.
TABLE 1.
Propella and inlet water quality during the spiking experiments
| Test | Mean ± SD (no. of observations)a
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Propella water
|
Inlet water
|
||||||||||||||
| Flow velocity (m/s)
|
Total phosphorus (μg/liter)
|
Temp (°C)
|
pH | EC (μS/cm) | Chlorine (mg/liter) | TOC (mg/liter) | AOC (μg acetate eq. C/liter) | Total phosphorus (μg/liter) | MAP (μg PO4-P/liter) | HPC bacteria (CFU/ml) | Total bacteria (cells/ml) | ||||
| A | B | A | B | A | B | ||||||||||
| Flow velocity | 0.10 ± 0.00 (12) | 0.24 ± 0.00 (12) | ND | ND | 15.6 ± 2.0 (7) | 16.2 ± 1.4 (5) | 8.0 ± 0.1 (5) | 201 ± 23 (5) | 0.17 ± 0.01 (4) | 2.8 ± 0.2 (5) | 71 ± 15 (5) | 3.0 ± 1.8 (5) | 0.20 ± 0.13 (5) | 5.1 × 103 ± 9.0 × 102 (5) | 1.5 × 105 ± 3.0 × 104 (5) |
| Phosphorus | 0.24 ± 0.00 (16) | 0.24 ± 0.00 (13) | 4.2 ± 0.9 (5) | 13.8 ± 3.2 (5) | 14.8 ± 0.8 (16) | 15.0 ± 0.9 (15) | 7.9 ± 0.2 (5) | 189 ± 8 (5) | 0.15 ± 0.06 (5) | 2.8 ± 0.2 (5) | 106 ± 79 (5) | 3.9 ± 0.6 (5) | 0.18 ± 0.09 (5) | 4.3 × 102 ± 4.3 × 102 (5) | 7.1 × 104 ± 2.6 × 104 (5) |
| Temp | 0.25 ± 0.00 (9) | 0.25 ± 0.00 (9) | ND | ND | 7.0 ± 0.5 (14) | 20.0 ± 0.5 (14) | 7.8 ± 0.2 (5) | 194 ± 17 (5) | 0.17 ± 0.08 (5) | 3.5 ± 0.4 (5) | 55 ± 20 (5) | 7.8 ± 2.3 (5) | 0.26 ± 0.16 (5) | 1.6 × 103 ± 1.4 × 103 (5) | 1.8 × 105 ± 1.5 × 104 (5) |
EC, electrical conductivity; ND, not determined.
Spiking.
M. avium strain E89 isolated from brook water was grown in Middlebrook 7H9 broth with oleic acid-albumin-dextrose-catalase enrichment (Difco) at 35°C for 10 days. The cells were centrifuged (15 min, 1,650 × g, Sorvall GLC-3), washed with 0.9% NaCl, suspended in autoclaved municipal drinking water, and spiked into Propella reactors. The concentrations of mycobacteria in the reactors at the time of the spiking were 104 CFU/ml.
Biofilm analyses.
Biofilm samples were taken six times during the 4-week experiments. The samples were taken a day after spiking, on the day 4, and thereafter once a week. At each sampling, three PVC coupons were removed and biofilms were detached from the coupons by 2 min of sonication with a probe (MSE Soniprep 150 ultrasonic disintegrator, no. 41371-250, probe no. 38121-1154, 9.5-mm diameter, 20 kHz; MSE, Loughborough, England) in 25 ml of deionized water filtered through a 0.1-μm membrane filter. Control samples of two coupons were taken before the spiking to ensure the absence of inherent M. avium in the system. For the cultures of M. avium, 13-ml and 1-ml portions of the biofilm suspension as well as 10-fold serial dilutions made in sterile deionized water were decontaminated with 0.005% cetylpyridinium chloride for 30 min (48). After decontamination, the samples were filtered on sterile cellulose nitrate filters (50-mm diameter, 0.2-μm pore size; Sartorius, Göttingen, Germany) which were then rinsed with sterile deionized water. The filters were placed on Mycobacteria 7H11 agar with oleic acid-albumin-dextrose-catalase enrichment (Difco), sealed with Parafilm, packed in plastic bags, and incubated at 35°C for 4 weeks. FISH analyses of M. avium were performed from the detached biofilm suspension. One milliliter of the suspension was filtered on 0.2-μm Anodisc filters (Whatman, Maidstone, England). Bacteria on filters were fixed for 15 min with 80% ethanol. After air drying, 50 μl of hybridization buffer containing 200 nM MAV148 peptide nucleic acid probe (24) was added to the filters. Bacteria were hybridized at 59°C for 90 min, and subsequently, the filters were immersed in 59°C washing buffer for 30 min (24). Hybridized bacteria were detected with an Olympus BX-51 TF epifluorescence microscope using an UPlanFI 100×/1.30 oil objective (Olympus Co. Ltd., Tokyo, Japan). The microscope was equipped with a fluorescence mirror unit (U-41001) with an excitation filter (460 to 500 nm), dichroic mirror (505 nm), and emission filter (510 to 560 nm). Bacteria were counted using an ocular grid. Heterotrophic plate counts (HPCs) were analyzed using the spread plate method on R2A agar (Difco) by incubation at 22°C for 7 days (1). The total number of bacteria was analyzed by filtering 5 ml to 7 ml of the biofilm suspension on a Nuclepore membrane (0.2-μm pore size; Whatman, Maidstone, England) and staining with 4′, 6-diamidino-2-phenylindole (DAPI) stain (final concentration, 5 μg/ml) for 15 min. All biofilm results were calculated per surface area (cm2).
Water analyses.
Water samples were taken from the inlet and effluent of Propella reactors simultaneously with biofilm samples. For the cultures of M. avium, volumes up to 500 ml and 10-fold serial dilutions made in sterile deionized water were decontaminated and incubated in a manner similar to that used for biofilm samples. FISH analyses for M. avium were performed from a 3-ml sample of effluent water. Water samples were filtered on 0.2-μm Anodisc filters (Whatman, Maidstone, England), and hybridization was performed as described above for the biofilms. HPCs and the total number of bacteria were analyzed as described above for the biofilms. The concentration of AOC was determined by a modification (37) of the method of van der Kooij et al. (56), and the concentration of microbially available phosphorus was determined by bioassay (27). Total organic carbon (TOC) was analyzed by a high-temperature combustion method with a Shimadzu 5000 TOC analyzer (Shimadzu, Kyoto, Japan) (13). Total phosphorus concentration was determined using the ascorbic acid method (14), and residual free chlorine was determined with a Palintest Micro 1000 chlorometer (Palintest Ltd., Tyne and Wear, United Kingdom). Electric conductivity (EC) was measured with a WTW pH/Cond 340i meter according to the manufacturer's instructions (Weilheim, Germany).
Statistical analyses.
The median concentrations of HPCs, total bacteria, culturable M. avium, and M. avium analyzed by FISH were calculated from the results obtained from the three parallel Propella coupons detached at each sampling. The effects of the flow rate, phosphorus, and temperature on the microbiological variable measured were analyzed by nonparametric, two-tailed, Wilcoxon-signed rank test (SPSS 13.0 for Windows; SPSS, Inc., Chicago, IL).
RESULTS
Flow velocity.
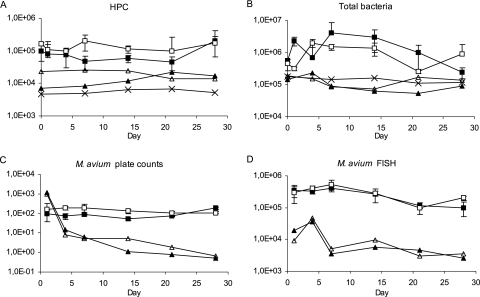
The HPCs of biofilms were, on average, 1.5 times higher with the flow velocity of 0.24 m/s than those with the flow velocity of 0.1 m/s (P < 0.05) (Fig. 1A). The effect of flow rate on HPC was not significant in reactor effluent water. The effect was also insignificant for the total counts of bacteria in both biofilms and effluent waters (Fig. 1B). M. avium spiked into the model system persisted in the biofilms and effluent water for the entire study period. M. avium plate counts from biofilms were two to three times higher at the higher flow velocity during the first 2 weeks than those grown at the lower flow rate; however, this difference was not seen after the second week (Fig. 1C). There was no statistical difference in the culturability of M. avium, i.e., the ratio of FISH to plate counts, between the two flow velocities in either biofilms or waters. Plate and FISH counts of M. avium in effluent waters and FISH counts in biofilms decreased during the experiment but were at similar levels with both flow rates (Fig. 1C and D).
FIG. 1.
HPC (A), number of total bacteria (B), M. avium plate count (C), and M. avium analyzed by FISH (D) in the effluent waters and biofilms with different flow velocities. Symbols for biofilms: ▪, 0.1 m/s, CFU, or cells/cm2; □, 0.24 m/s, CFU, or cells/cm2. Symbols for water: ▴, 0.1 m/s, CFU, or cells/ml; ▵, 0.24 m/s, CFU, or cells/ml; ×, inlet water, CFU, or cells/ml. Error bars indicate standard deviations.
Phosphorus.
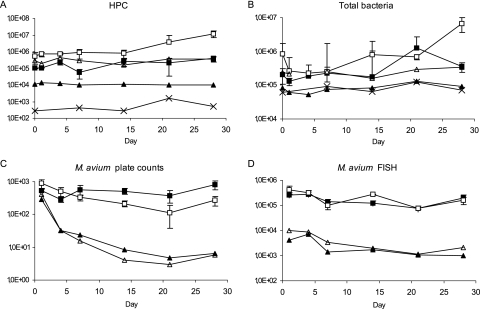
The addition of phosphorus had a dramatic effect on the HPCs, which on average were 7 and 30 times higher in biofilm and effluent water samples, respectively, when phosphorus concentration was 13.8 μg/liter than when the concentration was 4.2 μg/liter (P < 0.05) (Fig. 2A). A similar effect of phosphorus concentration was seen in total counts of bacteria in effluent waters (P < 0.05) (Fig. 2B). The plate and FISH counts of M. avium decreased in biofilms during the first 3 weeks but started to increase after that (Fig. 2C and D). After the first week, the plate counts of M. avium in biofilms were 2.7 times higher, on average, with the lower phosphorus concentration but the FISH counts of M. avium were similar with both concentrations (Fig. 2C and D). In the effluent waters, the plate counts of M. avium were similar or higher with the lower concentration but the FISH counts were, on average, 1.7 times lower with the lower concentration (P < 0.05) (Fig. 2C and D). These results led to a decreased ratio of FISH to plate counts, i.e., a higher culturability of M. avium, in effluent waters with the lower concentration of phosphorus. The ratios of FISH to plate counts were 173 and 317, with concentrations of 4.2 and 13.8 μg/liter, respectively (P < 0.05). The ratios were similar in biofilms, 247 and 615, with the phosphorus concentrations of 4.2 and 13.8 μg/liter, respectively, but the difference did not reach statistical significance.
FIG. 2.
HPC (A), number of total bacteria (B), M. avium plate count (C), and M. avium analyzed by FISH (D) in the effluent waters and biofilms with different phosphorus concentrations. Symbols for biofilms: ▪, 4.2 μg/liter, CFU, or cells/cm2; □, 13.8 μg/liter, CFU, or cells/cm2. Symbols for water: ▴, 4.2 μg/liter, CFU, or cells/ml; ▵, 13.8 μg/liter, CFU, or cells/ml; ×, inlet water, CFU, or cells/ml. Error bars indicate standard deviations.
Temperature.
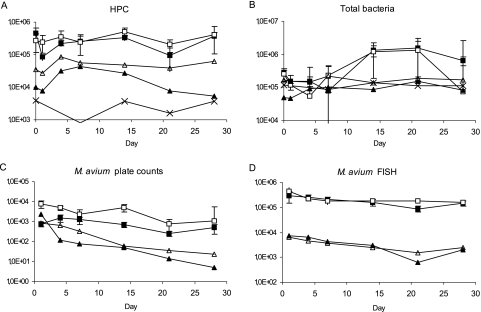
The increase in temperature increased the HPCs and total bacterial counts in effluent waters. The results were on average four and two times higher at 20°C than at 7°C, respectively (P < 0.05) (Fig. 3A and B). Also, the plate counts of M. avium were 2 to 10 times higher in biofilms at 20°C than those at 7°C (P < 0.05) (Fig. 3C). After a few days, the same trend was also detected in plate counts of M. avium in effluent waters. In contrast, FISH counts of M. avium were similar throughout the study for both biofilms and effluent waters (Fig. 3C and D). These results led to a decreased ratio of FISH to plate counts in biofilms, i.e., higher culturability, at a higher temperature. The ratio was 265 at 7°C and 67 at 20°C (P < 0.05).
FIG. 3.
HPC (A), number of total bacteria (B), M. avium plate count (C), and M. avium analyzed by FISH (D) in the effluent waters and biofilms with different temperatures. Symbols for biofilms: ▪, 7°C, CFU, or cells/cm2; □, 20°C, CFU, or cells/cm2. Symbols for water: ▴, 7°C, CFU, or cells/ml; ▵, 20°C, CFU, or cells/ml; ×, inlet water, CFU, or cells/ml. Error bars indicate standard deviations.
DISCUSSION
The data showed that M. avium spiked into Propella reactor adhered directly to existing biofilms and persisted in PVC coupons for the entire 4-week study period, confirming our preliminary results on the adherence of M. avium to similar drinking water biofilms (25). The concentrations of M. avium remained rather constant from the first sampling. This indicates that the adherence from water phase to biofilms happened during the first day. This result agrees with those of Dailloux and coworkers (7), who showed that the adherence of Mycobacterium xenopi, another slow-growing mycobacterium, took place in hours. In all experiments, M. avium concentrations in biofilms showed a slight increase after a 3-week period. However, due to the short study period, it was impossible to estimate whether this was caused by growth or normal fluctuation between the attached and free-living cells.
The survival levels of culturable and FISH-analyzed M. avium in biofilms were similar, with flow velocities of 0.1 m/s and 0.24 m/s. Other heterotrophic bacteria present in the biofilm grew better with a higher flow velocity, as reported in earlier studies (30, 41). The increased bacterial growth with higher velocity has been attributed to a more rapid mass transfer of nutrients to biofilm microbes (41). Since M. avium is slow growing, perhaps rapid availability of nutrients is not as necessary for its growth as it is for more rapidly growing organisms. Both flows in our experiment were turbulent, and apparently 0.1 m/s was sufficient to transfer nutrients to this slow-growing organism and maintain its survival.
When phosphorus was added to the model system, the HPCs in biofilms and water and total number of bacteria in water were increased. Finnish drinking waters have a high content of organic matter (20), originating from large areas of peatlands and forests. Phosphorus, instead of carbon, has been shown to be the nutrient limiting the microbial growth in both drinking waters (26, 28, 36) and biofilms (26, 29). We added 10 μg/liter phosphorus, but the addition of phosphorus amounts as low as 1 μg/liter has been enough to increase HPCs and microbial biomass in phosphorus-limited drinking water and biofilms, as reported earlier (26). The effect of the addition of phosphorus on M. avium was different. The plate counts of M. avium in biofilms or water were similar or higher after the first week, with the phosphorus concentration at 4.2 μg/liter. In contrast, the numbers of M. avium analyzed by FISH in effluent waters were slightly higher, with the phosphorus concentration at 13.8 μg/liter. The culturability of M. avium was thus higher, i.e., the ratio of FISH to plate counts is lower in biofilms at a lower phosphorus concentration. These results show that M. avium does not benefit from the addition of phosphorus as other heterotrophic bacteria do, and phosphorus does not appear to be a limiting nutrient for its growth. Perhaps the need for phosphorus (e.g., for nucleic acid synthesis) by slowly growing mycobacteria is lower than that of faster-growing bacteria. The higher concentration of phosphorus may also increase competition with other microbes in biofilms so that the slower-growing M. avium does not survive as well. M. avium appears to survive better in biofilm with a lower phosphorus concentration and less competition. Leys and coworkers (31) have suggested that the ability of mycobacteria to degrade recalcitrant polycyclic aromatic hydrocarbons in low-nutrient soils makes them competitive with faster-growing organisms. Norton and coworkers (39) observed the inhibitory effect of competition on M. avium. They found out that the elimination of other microbes from copper pipes resulted in growth and almost pure culture of M. avium in biofilm, while the percentage of M. avium was much lower before elimination. It has been shown in previous field studies (12, 53) and in nondisinfected pilot systems (39) that the increase in AOC increases the growth of M. avium and other slowly growing mycobacteria. More studies on AOC, microbially available phosphorus, and other nutrients in controlled circumstances, however, are needed to fully understand their role in the survival of M. avium in drinking water systems. Our results for phosphorus indicate that controlling M. avium in drinking water systems is not possible by reducing the phosphorus concentration. The opposite is true for other heterotrophic bacteria, which can be controlled by removing phosphorus through coagulation and filtration processes (38, 28).
Of the variables tested, water temperature had the clearest effect on the survival of M. avium in biofilms. Compared to a temperature of 7°C, a temperature of 20°C increased the survival of culturable M. avium and culturability. The effect of temperature on other heterotrophic bacteria was similar, but it was seen more clearly in effluent waters than in biofilms. These results agree with those of earlier studies showing that the growth of heterotrophic bacteria increases in drinking water at temperatures above about 15°C (3, 22, 45). An increase in temperature also enhances the bacterial numbers and biomass in biofilms (8, 16, 29). Earlier results for the effect of temperature on mycobacteria are similar. In natural water or water filtrate, Mycobacterium intracellulare, a close relative of M. avium, grows at temperatures between 15.5°C (15) and 20°C (18). Several studies have shown that more mycobacteria can be found from environmental sources during warmer months of the year (9, 19), and a seasonal pattern has also been detected for drinking waters (21). Though a temperature of 20°C increased the survival of M. avium, M. avium growth could not be detected during the 4-week study period. M. avium is a thermotolerant species able to multiply at 42°C and resisting temperatures up to 53°C (39). It has been isolated more frequently from hot than from cold water systems of buildings (10). These results indicate that a temperature higher than 20°C and/or a longer incubation period is needed for growth of M. avium in these kinds of water distribution systems. The better survival of M. avium at 20°C than at 7°C, however, suggests that keeping the drinking water temperature as low as possible is also important for controlling M. avium in the systems.
In conclusion, this study showed that M. avium persisted in drinking water biofilms for weeks without a decline in concentrations. Factors found to influence the survival of M. avium included temperature, nutrient conditions, and competition with other microbes. The availability of phosphorus as such was not essential for survival. The results here were obtained on PVC material. Since the pipe material may have interactions with system variables (49), it is necessary to also know the effect of the system variables on other surfaces.
Acknowledgments
This work has been realized as part of the research project SAFER: Surveillance and Control of Microbiological Stability in Drinking Water Distribution Networks, which is supported by the European Union within the Fifth Framework Programme, “Energy, Environment and Sustainable Development Program,” no. EVK1-2002-00108.
We are solely responsible for this work, the work does not represent the opinion of the European Community, and the European Community is not responsible for any use that might be made of data appearing herein.
We thank Marjo Tiittanen and Tiina-Maria Heiskanen for excellent laboratory assistance.
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.American Public Health Association, American Water Works Association, and Water Environment Federation. 2005. Standard methods for the analysis of water and wastewater, 21st ed. American Public Health Association, Washington, DC.
- 2.Aronson, T., A., Holtzman, N. Glover, M. Boian, S. Froman, O. G. W. Berlin, H. Hill, and G. Stelma, Jr. 1999. Comparison of large restriction fragments of Mycobacterium avium isolates recovered from AIDS and non-AIDS patients with those of isolates from potable water. J. Clin. Microbiol. 37:1008-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batté, M., C. Féliers, P. Servais, V. Gauthier, J.-C. Joret, and J.-C. Block. 2006. Coliforms and other microbiological indicators occurrence in water and biofilm in full-scale distribution systems. Water Sci. Technol. 54:41-48. [DOI] [PubMed] [Google Scholar]
- 4.Cirillo, J. D., S. Falkow, L. C. Tompkins, and L. E. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloete, T. E., D. Westaard, and S. J. van Vuuren. 2003. Dynamic response of biofilm to pipe surface and fluid velocity. Water Sci. Technol. 47:57-59. [PubMed] [Google Scholar]
- 6.Covert, T. C., M. R. Rodgers, A. L. Reyes, and G. N. Stelma, Jr. 1999. Occurrence of nontuberculous mycobacteria in environmental samples. Appl. Environ. Microbiol. 65:2492-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dailloux, M., M. Albert, C. Laurain, S. Andolfatto, A. Lozniewski, P. Hartemann, and L. Mathieu. 2003. Mycobacterium xenopi and drinking water biofilms. Appl. Environ. Microbiol. 69:6946-6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donlan, R. M., and W. O. Pipes. 1988. Selected drinking water characteristics and attached microbial population density. J. Am. Water Works Assoc. 11:70-76. [Google Scholar]
- 9.Donoghue, H. D., E. Overend, and J. L. Stanford. 1997. A longitudinal study of environmental mycobacteria on a farm in south-west England. J. Appl. Microbiol. 82:57-67. [DOI] [PubMed] [Google Scholar]
- 10.du Moulin, G. C., K. D. Stottmeier, P. A. Pelletier, A. Y. Tsang, and J. Hedley-Whyte. 1988. Concentration of Mycobacterium avium by hospital hot water systems. JAMA 260:1599-1601. [DOI] [PubMed] [Google Scholar]
- 11.Falkinham, J. O., III. 1996. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 9:177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falkinham, J. O., III, C. D. Norton, and M. W. LeChevallier. 2001. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution system. Appl. Environ. Microbiol. 67:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finnish Standards Association SFS. 1997. Water analysis: guidelines for the determination of total organic carbon (TOC) and dissolved organic carbon (DOC). SFS-EN 1484. Finnish Standards Association SFS, Helsinki, Finland.
- 14.Finnish Standards Association SFS. 1997. Water quality. Determination of phosphorus. Ammonium molybdate spectrometric method. SFS-EN 1189. Finnish Standards Association SFS. Helsinki, Finland.
- 15.George, K. L., B. C. Parker, H. Gruft, and J. O. Falkinham III. 1980. Epidemiology of infection by nontuberculous mycobacteria. II. Growth and survival in natural waters. Am. Rev. Respir. Dis. 122:89-94. [DOI] [PubMed] [Google Scholar]
- 16.Hallam, N. B., J. R. West, C. F. Forster, and J. Simms. 2001. The potential for biofilm growth in water distribution systems. Water Res. 35:4063-4071. [DOI] [PubMed] [Google Scholar]
- 17.Inderlied, C. B., C. A. Kemper, and L. E. M. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazda, J. 1973. Die Bedeutung von Wasser für die Verbreitung von potentiell pathogenen Mykobakterien. I. Möglichkeiten für eine Vermehrung von Mykobakterien. Zentbl. Bakteriol. Parisitenkd. Infektrakh. Hyg. Abt. 1 Orig. B 158:161-169. [PubMed] [Google Scholar]
- 19.Kirschner, R. A., Jr., B. C. Parker, and J. O. Falkinham III. 1992. Epidemiology of infection by nontuberculous mycobacteria. Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. Am. Rev. Respir. Dis. 145:271-275. [DOI] [PubMed] [Google Scholar]
- 20.Kortelainen, P. 1993. Content of total organic carbon in Finnish lakes and its relationship to catchment characteristics. Can. J. Fish. Aquat. Sci. 50:1477-1483. [Google Scholar]
- 21.Kubalek, I., and S. Komenda. 1995. Seasonal variations in the occurrence of environmental mycobacteria in potable water. APMIS 103:327-330. [DOI] [PubMed] [Google Scholar]
- 22.LeChevallier, M. W., N. J. Welch, and D. B. Smith. 1996. Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 62:2201-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Dantec, C., J.-P. Duguet, A. Montiel, N. Dumoutier, S. Dubrou, and V. Vincent. 2002. Chlorine disinfection of atypical mycobacteria isolated from a water distribution system. Appl. Environ. Microbiol. 68:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehtola, M. J., E. Torvinen, I. T. Miettinen, and C. W. Keevil. 2006. Fluorescence in situ hybridization (FISH) using peptide nucleic acid probes for rapid detection of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. paratuberculosis in potable water biofilms. Appl. Environ. Microbiol. 72:848-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehtola, M. J., E. Torvinen, J. Kusnetsov, T. Pitkänen, L. Maunula, C.-H. von Bonsdorff, P. J. Martikainen, S. A. Wilks, C. W. Keevil, and I. T. Miettinen. 2007. Survival of Mycobacterium avium, Legionella pneumophila, Escherichia coli, and caliciviruses in drinking water biofilms grown under high shear turbulent flow. Appl. Environ. Microbiol. 73:2854-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehtola, M. J., I. T. Miettinen, and P. J. Martikainen. 2002. Biofilm formation in drinking water affected by low concentrations of phosphorus. Can. J. Microbiol. 48:494-499. [DOI] [PubMed] [Google Scholar]
- 27.Lehtola, M. J., I. T. Miettinen, T. Vartiainen, and P. J. Martikainen. 1999. A new sensitive bioassay for determination of microbially available phosphorus in water. Appl. Environ. Microbiol. 65:2032-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehtola, M. J., I. T. Miettinen, T. Vartiainen, and P. J. Martikainen. 2002. Changes in content of microbially available phosphorus, assimilable organic carbon and microbial growth potential during drinking water treatment processes. Water Res. 36:3681-3690. [DOI] [PubMed] [Google Scholar]
- 29.Lehtola, M. J., J. Talis, I. M. Miettinen, T. Vartiainen, and P. J. Martikainen. 2004. Formation of biofilms in drinking water distribution networks, a case study in two cities in Finland and Latvia. J. Ind. Microbiol. Biotechnol. 31:489-494. [DOI] [PubMed] [Google Scholar]
- 30.Lehtola, M. J., M. Laxander, I. T. Miettinen, A. Hirvonen, T. Vartiainen, and P. J. Martikainen. 2006. The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Res. 40:2151-2160. [DOI] [PubMed] [Google Scholar]
- 31.Leys, N. M., L. Bastiaens, W. Verstraete, and D. Springael. 2005. Influence of the carbon/nitrogen/phosphorus ratio on polycyclic aromatic hydrocarbon degradation by Mycobacterium and Sphingomonas in soil. Appl. Microbiol. Technol. 66:726-736. [DOI] [PubMed] [Google Scholar]
- 32.Mangione, E. J., G. Huitt, D. Lenaway, J. Beebe, A. Bailey, M. Figoski, M. P. Rau, K. D. Albrecht, and M. A. Yakrus. 2001. Nontuberculous mycobacterial disease following hot tub exposure. Emerg. Infect. Dis. 7:1039-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marras, T. K., R. J. Wallace, L. L. Koth, M. S. Stulbarg, C. T. Cowl, and C. L. Daley. 2005. Hypersensitivity pneumonitis reaction to Mycobacterium avium in household water. Chest 127:664-671. [DOI] [PubMed] [Google Scholar]
- 34.Martin, E. C., B. C. Parker, and J. O. Falkinham III. 1987. Epidemiology of infection by nontuberculous mycobacteria. VII. Absence of of mycobacteria in Southeastern groundwaters. Am. Rev. Respir. Dis. 136:344-348. [DOI] [PubMed] [Google Scholar]
- 35.McCoy, W. F., and B. H. Olson. 1986. Relationship among turbidity, particle counts and bacteriological quality within water distribution lines. Water Res. 20:1023-1029. [Google Scholar]
- 36.Miettinen, I. T., T. Vartiainen, and P. J. Martikainen. 1997. Phosphorus and bacterial growth in drinking water. Appl. Environ. Microbiol. 63:3242-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miettinen, I. T., T. Vartiainen, and P. J. Martikainen. 1999. Determination of assimilable organic carbon in humus-rich drinking waters. Water Res. 33:2277-2282. [Google Scholar]
- 38.Nishijima, W., E. Shoto, and M. Okada. 1997. Improvement of biodegradation of organic substance by addition of phosphorus in biological activated carbon. Water Sci. Technol. 36:251-257. [Google Scholar]
- 39.Norton, C. D., M. W. LeChevallier, and J. O. Falkinham III. 2004. Survival of Mycobacterium avium in a model distribution system. Water Res. 38:1457-1466. [DOI] [PubMed] [Google Scholar]
- 40.Parent, A., S. Fass, M. L. Dincher, D. Reasoner, D. Gatel, and J.-C. Block. 1996. Control of coliform growth in drinking water distribution systems. Water Environ. J. 10:442-445. [Google Scholar]
- 41.Percival, S. L., J. S. Knapp, D. S. Wales, and R. G. J. Edyvean. 1999. The effect of turbulent flow and surface roughness on biofilm formation in drinking water. J. Ind. Microbiol. Biotechnol. 22:152-159. [Google Scholar]
- 42.Percival, S. L., J. T. Walker, and P. R. Hunter. 2000. Microbiological aspects of biofilms and drinking water. CRC Press, Boca Raton, FL.
- 43.Peters, M., C. Müller, S. Rüsch-Gerdes, C. Seidel, U. Göbel, H. D. Pohle, and B. Ruf. 1995. Isolation of atypical mycobacteria from tap water in hospitals and homes: is this a possible source of disseminated MAC infection in AIDS patients? J. Infect. 31:39-44. [DOI] [PubMed] [Google Scholar]
- 44.Peyton, B. M. 1996. Effects of shear stress and substrate loading rate on Pseudomonas aeruginosa biofilm thickness and density. Water Res. 30:29-36. [Google Scholar]
- 45.Reasoner, D. J., J. C. Blannon, E. E. Geldreich, and J. Barnick. 1989. Nonphotosynthetic pigmented bacteria in a potable water treatment and distribution system. Appl. Environ. Microbiol. 55:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sathasivan, A., S. Ohgaki, K. Yamamoto, and N. Kamiko. 1997. Role of inorganic phosphorus in controlling regrowth in water distribution system. Water Sci. Technol. 35:37-44. [Google Scholar]
- 47.Schulze-Röbbecke, R., and R. Fischeder. 1992. Occurrence of mycobacteria in biofilm samples. Tuber. Lung Dis. 73:141-144. [DOI] [PubMed] [Google Scholar]
- 48.Schulze-Röbbecke, R., A. Weber, and R. Fischeder. 1991. Comparison of decontamination methods for the isolation of mycobacteria from drinking water samples. J. Microbiol. Methods 14:177-183. [Google Scholar]
- 49.Simões, L. C., N. Azevedo, A. Pachec, C. W. Keevil, and M. J. Vieira. 2006. Drinking water biofilm assessment of total and culturable bacteria under different operating conditions. Biofouling 22:91-99. [DOI] [PubMed] [Google Scholar]
- 50.Steed, K. A., and J. O. Falkinham III. 2006. Effect of growth in biofilms on chlorine susceptibility of Mycobacterium avium and Mycobacterium intracellulare. Appl. Environ. Microbiol. 72:4007-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugita, Y., N. Ishii, M. Katsuno, R. Yamada, and H. Nakajima. 2000. Familiar cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br. J. Dermatol. 142:789-793. [DOI] [PubMed] [Google Scholar]
- 52.Taylor, R. H., J. O. Falkinham III, C. D. Norton, and M. W. LeChevallier. 2000. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 66:1702-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torvinen, E., S. Suomalainen, M. J. Lehtola, I. T. Miettinen, O. Zacheus, L. Paulin, M.-L. Katila, and P. J. Martikainen. 2004. Mycobacteria in water and loose deposits of drinking water distribution systems in Finland. Appl. Environ. Microbiol. 70:1973-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.U.S. Environmental Protection Agency. 2005. Drinking water contaminant candidate list 2; final notice. Fed. Register 70:9071-9077. [Google Scholar]
- 55.Vaerewijck, M. J. M., G. Huys, J. C. Palomino, J. Swings, and F. Portaels. 2005. Mycobacteria in drinking water distribution systems: ecology and significance for human health. FEMS Microbiol. Rev. 29:911-934. [DOI] [PubMed] [Google Scholar]
- 56.van der Kooij, D., W. A. M. Hijnen, and A. Visser. 1982. Determining the concentration of easily assimilable organic carbon in drinking water. J. Am. Water Works Assoc. 74:540-545. [Google Scholar]
- 57.von Reyn, C. F., J. N. Maslow, T. W. Barber, J. O. Falkinham III, and R. D. Arbeit. 1994. Persistent colonization of potable water as a source of Mycobacterium avium infection in AIDS. Lancet 343:1137-1141. [DOI] [PubMed] [Google Scholar]
- 58.von Reyn, C. F., R. D. Waddell, T. Eaton, R. D. Arbeit, J. N. Maslow, T. W. Barber, R. J. Brindle, C. F. Gilks, J. Lumio, J. Lähdevirta, A. Ranki, D. Dawson, and J. O. Falkinham III. 1993. Isolation of Mycobacterium avium complex from water in the United States, Finland, Zaire, and Kenya. J. Clin. Microbiol. 31:3227-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamazaki, Y., L. Danelishvili, M. Wu, M. MacNab, and L. E. Bermudez. 2006. Mycobacterium avium genes associated with the ability to form biofilm. Appl. Environ. Microbiol. 72:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zacheus, O., and P. J. Martikainen. 1997. Physicochemical quality of drinking and hot waters in Finnish buildings originated from groundwater or surface water plants. Sci. Total Environ. 204:1-10. [DOI] [PubMed] [Google Scholar]