Abstract
The aim of this work was to determine whether reductive acetogenesis can provide an alternative to methanogenesis in the rumen. Gnotobiotic lambs were inoculated with a functional rumen microbiota lacking methanogens and reared to maturity on a fibrous diet. Lambs with a methanogen-free rumen grew well, and the feed intake and ruminal volatile fatty acid concentrations for lambs lacking ruminal methanogens were lower but not markedly dissimilar from those for conventional lambs reared on the same diet. A high population density (107 to 108 cells g−1) of ruminal acetogens slowly developed in methanogen-free lambs. Sulfate- and fumarate-reducing bacteria were present, but their population densities were highly variable. In methanogen-free lambs, the hydrogen capture from fermentation was low (28 to 46%) in comparison with that in lambs containing ruminal methanogens (>90%). Reductive acetogenesis was not a significant part of ruminal fermentation in conventional lambs but contributed 21 to 25% to the fermentation in methanogen-free meroxenic animals. Ruminal H2 utilization was lower in lambs lacking ruminal methanogens, but when a methanogen-free lamb was inoculated with a methanogen, the ruminal H2 utilization was similar to that in conventional lambs. H2 utilization in lambs containing a normal ruminal microflora was age dependent and increased with the animal age. The animal age effect was less marked in lambs lacking ruminal methanogens. Addition of fumarate to rumen contents from methanogen-free lambs increased H2 utilization. These findings provide the first evidence from animal studies that reductive acetogens can sustain a functional rumen and replace methanogens as a sink for H2 in the rumen.
Methane (CH4) eructated from ruminants represents a loss of 8 to 13% of the digestible energy ingested by the animal (71) and contributes to global warming. The amount of methane produced by ruminants varies with the farming system, the nature of the feed, the feeding level, the feed digestibility, and the animal species (6, 67, 71). The annual production of methane by ruminants, estimated to be 80 to 120 × 106 tons or approximately 15% of total anthropogenic methane emissions (16, 63), is the second largest biogenic source of methane after rice paddy fields. Decreasing methane emissions from ruminant livestock is desirable in order to both reduce greenhouse gases in the atmosphere and improve energy capture during digestion.
Nutritionists have been trying for a long time to mitigate rumen methane emissions in order to enhance animal performance but so far have not been successful. The methods most commonly attempted involve utilization of antibiotics and ionophores (58), halogenated methane analogues (20, 37, 59), heavy metals (70), lipid-rich materials such as coconut oil (21, 26, 54, 55, 56), probiotics (58), bacteriocin (47), and numerous chemicals (1, 4). Immunization against methanogens (79), elimination of ciliate protozoans which support methanogen populations (64), and addition of acetogenic bacteria to rumen fluid in in vitro tests (50, 51, 65) have also been tested. Increasingly, the use of antibiotics or toxic chemicals as inhibitors is becoming unsustainable because of negative effects on the environment and residues in meat and milk products. The key steps for developing successful methane abatement strategies are likely to be steps which exploit natural processes in the rumen. One such approach is based upon establishing nonmethanogenic sinks for the hydrogen produced during fermentation (39, 40). In the rumen, CH4 is produced by methanogens using hydrogen to reduce CO2 according to the following equation: 4H2 + CO2 → CH4 + 2H2O. The nonmethanogenic hydrogenotrophic bacteria residing in the rumen include acetogens that reduce CO2 to form acetate (4H2 + 2CO2 → CH3COOH + 2H2O) by the Wood-Lungdahl pathway (reductive acetogenesis), sulfate-reducing bacteria (SRB) that reduce sulfate to hydrogen sulfide (60, 61), and bacteria that use hydrogen to reduce fumarate to succinate (3). Succinate, the product of fumarate reduction, is decarboxylated to propionate, a valuable animal nutrient (78). When animals are fed normal diets, acetogens and fumarate reducers are not competitive with methanogens in terms of H2 scavenging, and methanogenesis appears to be the dominant hydrogenotrophic mechanism in the rumen microbial ecosystem. In microbial ecosystems such as those in the colons of non-methane-producing human subjects (8, 10) or in the guts of xylagophagous termites (11, 12, 75), reductive acetogenesis is the major pathway for removing hydrogen.
During the last decade the ruminal acetogens, a phylogenetically diverse and metabolically versatile group of bacteria, have been the focus of numerous studies (8, 9, 11, 23, 73, 75). Some ruminal acetogen strains have been found to outcompete methanogens for H2 in vitro (14, 40), and efforts have been made to demonstrate effects from reductive acetogenesis in the rumen; however, so far these efforts have not been successful (19, 50). Reductive acetogenesis has been stimulated in vitro after selective inhibition of methanogens (50, 66). Recent investigations, largely limited to in vitro studies, have shown that addition of sodium fumarate can decrease CH4 production (2, 52). This suggests that fumarate reduction and reductive acetogenesis are potential alternatives to methanogenesis in the rumen. Sulfate reduction, which in ecosystems such as marine sediments is more efficient than methanogenesis, is not an option for methane abatement because the hydrogen sulfide end product is toxic to the host animal.
The use of gnotobiotically reared lambs harboring either a simple or complex microflora is an important method for investigating the role of specific microbes in the rumen (36). Such in vivo systems have already been used to study effects of methanogens in the rumen (33). We report here results from a study in which gnotobiotic lambs were inoculated with a complex ruminal microflora lacking methanogens and hydrogenotrophic bacterial populations and H2-utilizing capacities were measured at regular intervals over a period of 18 to 22 months. Similar measurements were obtained for control animals, which included conventional lambs and a methanogen-free lamb subsequently inoculated with a single species of ruminal methanogen. This study was carried out to determine whether nonmethanogenic hydrogenotrophs normally residing in the rumen can replace methanogens to obtain a functioning nonmethanogenic rumen and to determine whether ruminal acetogens offer possibilities for lowering ruminant methane emissions.
MATERIALS AND METHODS
Animals. (i) Preparation and maintenance.
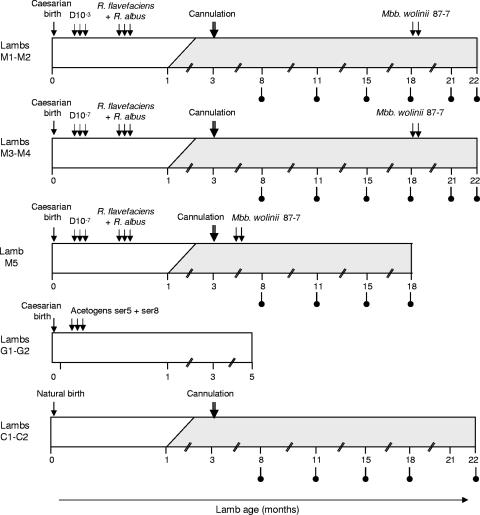
The overall experimental outline is shown in Fig. 1. Seven germfree lambs (lambs M1, M2, M3, M4, M5, G1, and G2) obtained by caesarean section were immediately transferred to sterile isolators equipped for a long-term experiment (27, 29, 30, 33). Lambs M1 and M2 and lambs M3 and M4 were reared in pairs in the same isolator, while lamb M5 was reared alone. Lambs were fed ultrahigh-temperature-sterilized milk from cows until they were 70 days old, and after 6 weeks all animals except lambs G1 and G2 also had access to a ration of dehydrated alfalfa hay in the form of 7-mm pellets sterilized by γ irradiation. After 70 days, the animals were fed twice daily with only pellets. Each lamb was fitted with a permanent rumen plastisol cannula (diameter, 2.5 cm) at 10 weeks. Lambs M1, M2, M3, and M4 were reared in the isolators until the age of 22 months. Lamb M5 was withdrawn from the experiment at 18 months, and lambs G1 and G2 were withdrawn at 5 months. Two conventionally reared lambs (lambs C1 and C2) fed the same diet were used as controls. Feed consumption by each animal was measured throughout the experiment.
FIG. 1.
Experimental outline showing interventions with respect to animal age (in months). Lambs M1, M2, M3, M4, M5, G1, and G2 were reared in sterile isolators, and lambs C1 and C2 were reared conventionally. Most lambs were initially fed sterilized cows' milk (open bars) and then sterile alfalfa hay (shaded bars); the exceptions were lambs G1 and G2, which were fed only milk. The times when rumen samples were removed for H2 utilization tests are indicated by lollipop symbols. Lambs M1 and M2 were inoculated with a 10−3 dilution of the rumen contents (D−3), and lambs M3, M4, and M5 were inoculated with a dilution 10−7 of the rumen contents (D−7). Lamb M5 was inoculated with a pure culture of M. wolinii at the age of 5 months; lambs M1, M2, M3, and M4 received this species at the age of 19 months.
(ii) Inoculation.
During the first week after birth, lambs M1, M2, M3, M4, and M5 were inoculated on three consecutive days with 1 ml of diluted rumen contents removed from an adult sheep fed alfalfa hay. Before dilution, the rumen contents (100 ml) were incubated anaerobically at room temperature for 24 h in the presence of bromoethanesulfonic acid at a final concentration of 0.1 mM to kill methanogens. Lambs M1 and M2 were inoculated with a 10−3 dilution of the rumen contents, and lambs M3, M4, and M5 were inoculated with a 10−7 dilution of the rumen contents. During their second week, each of the five animals was inoculated with 5 ml of a 10-day-old pure culture of each of two hydrogen-producing cellulolytic bacteria, Ruminococcus flavefaciens FD1 and Ruminococcus albus 7, grown on cellulose. These strains originated from the culture collection of the University of Illinois, Urbana-Champaign. At the age of 5 months, lamb M5 was further inoculated with a pure culture of the ruminal methanogen Methanobrevibacter wolinii. M. wolinii 87-7, isolated from a young lamb, was obtained from the INRA culture collection. Lambs M1, M2, M3, and M4 were inoculated with this methanogen at the age of 19 months. Lambs G1 and G2 were inoculated 5 days after birth with a mixture of two acetogen isolates, Ser5 and Ser8, to determine the ability of acetogens to colonize the rumen in the absence of other microbes. Isolates Ser5 and Ser8, purified from the rumen of a 20-h-old lamb, are gram-positive, strictly anaerobic coccobacilli that are able to grow as autotrophs on H2-CO2 and as heterotrophs on various carbohydrates. Their taxonomic position was determined to be within cluster XIV of the clostridia (60, 62).
Microbial inocula were directly introduced into the rumen via a stomach tube. Before fistulation, rumen samples were collected via the stomach tube. After fistulation, rumen samples were collected through the cannula. Immediately after collection, samples were transferred to sterile flasks with stoppers, which were flushed with O2-free CO2 prior to introduction into the isolator.
After bromoethanesulfonic acid treatment, culture enumeration showed that the rumen contents used as a source of inocula contained 5 × 105 reductive acetogens g−1 and 4 × 107 fumarate-reducing bacteria g−1. Thus, lambs M1 and M2 receiving the 10−3 dilutions were expected to receive a complex ruminal community including nonmethanogenic hydrogenotrophs, and lambs M3 and M4 receiving 10−7 dilutions were expected to be free of both acetogens and methanogens. According to the terminology of Ducluzeau and Raibaud (24), lambs M1, M2, M3, M4, and M5, which harbored in their digestive tracts a microflora that was not well defined, were meroxenic, whereas lambs G1 and G2, which were inoculated with a defined bacterial community, were gnotoxenic.
Analyses.
Volatile fatty acid (VFA) and gas concentrations were determined by gas chromatography (DI700 chromatograph; Delsi Instruments) using methods described previously (31, 32, 43). Acetate and succinate concentrations in culture supernatants were determined enzymatically using Boehringer's method (Boehringer-Mannheim, SA, Meylan, France). During identification of bacterial isolates, fermentation products resulting from growth on fumarate were measured by high-performance liquid chromatography (42). Statistical analyses of data were carried out using Student's t test.
Enumeration of microbial communities.
Rumen samples were collected from meroxenic and conventional lambs at regular intervals throughout the experimental period so that methanogens and targeted bacteria could be enumerated. The sampling times for the different groups of lambs are shown in Fig. 1. In general, samples used for enumeration were collected through the cannula just before morning feeding. Reductive acetogens, cellulolytic bacteria, and methanogens were cultured in AC-11 medium (12), Halliwell-Bryant medium (35), and Balch medium (5), respectively. Enumeration in triplicate was carried out as described previously (22, 28, 61). For lambs G1 and G2, acetogens Ser5 and Ser8 were also enumerated in roll tubes containing a medium with only soluble sugars as the growth substrate (49). Fumarate-reducing bacteria were cultured in DSMZ medium 157 (http://www.dsmz.de/microorganisms/html/media/medium000157.html) for Wolinella succinogenes that was modified by removal of sodium formate and inclusion of 20% clarified rumen fluid and 0.1% Trypticase. Cultures under an H2-CO2 (80:20) headspace at 202 kPa were considered fumarate utilization positive when the level of hydrogen decreased and the levels of succinate and propionate increased strongly in comparison to changes in control cultures incubated under N2-CO2. All cell densities were calculated using the most-probable-number method of Clark and Owens (15).
All cultures were incubated for 3 weeks with shaking at 39°C. The establishment of R. flavefaciens and R. albus in 3-month-old lambs was determined by dot blot hybridization with target-specific oligonucleotide probes using methods previously described (7, 74). The presence or absence of protozoans and fungi in the rumens of meroxenic lambs was confirmed by phase-contrast microscopy and by roll tube culture (38), respectively.
Isolation and characterization of dominant ruminal acetogenic strains.
To obtain information on dominant ruminal acetogens in methanogen-free lambs, 50 colonies were picked from agar roll tubes inoculated using the most dilute (10−8) acetogen-positive cultures from most-probable-number enumerations from lambs M1 and M2 at 15 and 18 months of age. Cultures were grown in AC-11 broth under H2-CO2, and the purity of isolates was determined by examination by phase-contrast microscopy. Fifteen representative isolates were incubated to confirm the hydrogenotrophic capabilities, and seven isolates were selected for identification. Isolates 109D, 11A, J2, 22B, B2, 8F, and 69 were cultured on fumarate under an H2-N2 headspace, and the end products were determined. Genomic DNA was extracted from overnight cultures using the bead-beating method of Stahl et al. (74), and 16S rRNA genes were amplified by PCR using the universal eubacterial primers 27f and 1525r of Lane (45). Both strands were sequenced using these primers together with internal primers 357r, 519r, 926f, and 1114f (45) and a Big Dye Terminator kit v3.0 (Roche). Sequences were assembled and edited using Lasergene (DNAstar Inc., Madison, WI) and were aligned with database sequences using the phylogeny software program ARB (53). A dendrogram was generated in ARB using the neighbor-joining method, and similarity matrices were prepared.
VFAs.
Rumen contents for VFA analysis were collected just before morning feeding and 2 h after feed placement for all animals between 9 and 18 months old. For lamb M5 inoculated with M. wolinii 87-7, VFA levels were measured only at the end of the experiment.
Hydrogen utilization in rumen contents.
H2 utilization in rumen contents collected at regular intervals (8, 11, 15, 18, 21, and 22 months) was measured throughout the study (Fig. 1). Rumen contents (15 ml) collected just before morning feeding were placed in screw-cap serum bottles (125 ml) containing the prereduced mineral solution (25 ml) of Bryant and Burkey (13) under O2-free CO2, and H2 (5,400 ± 250 μmol) was injected by syringe. Bottles were incubated with shaking at 39°C for 8, 12, and 24 h. Incubations were carried out in duplicate. Two bottles without rumen contents but with H2 (5,400 ± 250 μmol) and two bottles with rumen contents under CO2 (5,400 ± 200 μmol) were included as controls. At the end of incubations, the total gas remaining in each bottle was measured by displacement using a glass syringe, and the headspace gas composition was determined. The quantity of H2 (Q) was determined from Avagadro's Law (1 mol of gas occupies 22.4 liters under normal conditions of temperature and pressure), and the percentage of hydrogen utilization was calculated as follows: [(Q in controls − Q in tests)/Q in controls] × 100, where Q was expressed in micromoles. Results were expressed as means ± standard deviations of duplicates.
The fate of hydrogen utilized in in vitro incubations was determined from VFA levels measured in 24-h incubations. Hydrogen utilization was distinguished from hydrogen generation from fermentation by measuring hydrogen formation in duplicate incubations of rumen contents under an N2-CO2 (80:20) headspace.
Effect of fumarate on in vitro hydrogen utilization.
To determine the effect of fumarate on hydrogen utilization by the microflora, sodium fumarate was added to in vitro incubations at final concentrations of 18.7 and 37.4 mM.
Calculation of methane production, reducing equivalents, and contribution of reductive acetogenesis to overall fermentation.
The expected methane production in rumen contents from conventional lambs and meroxenic lamb M5 harboring M. wolinii was calculated from the relationship between methane and VFAs established by Van Nevel and Demeyer (76): M = 0.450A − 0.250P + 0.400B, where M is methane, A is acetate, P is propionate, and B is butyrate.
Recoveries of reducing equivalents (2H) in the in vitro fermentations were calculated from the stoichiometry of rumen fermentation (76) as follows, where M is methane, A is acetate, P is propionate, B is butyrate, and V is valerate: percentage of H2 recovery = 2H accepted/2H released × 100, where 2H released = 2A + P + 4B + 3V and 2H accepted = 2P + 2B + 4V + 4M.
The contribution of reductive acetogenesis (x) to overall rumen fermentations was calculated using the method of Faichney et al. (25) as follows: x = (1.8A − 1.1P + 1.6B − 1.3V − 4 M)/5.8A + 11.6B, where M is methane, A is acetate, P is propionate, and B is butyrate.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences (998 to 1,546 nucleotides) of the seven isolates described below have been deposited in the GenBank database under accession numbers EF025905 to EF025911.
RESULTS
Feed consumption.
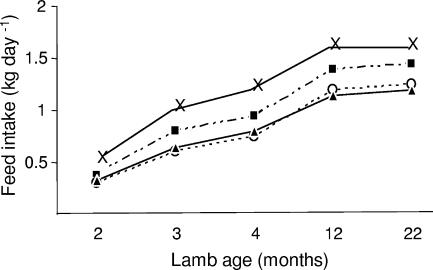
Feed intake for lambs over the experimental period is shown in Fig. 2. For meroxenic lambs, the intake increased gradually during the first year in parallel with that of lambs containing a normal ruminal microflora, After 12 months, the intake stabilized at 1.4 kg day−1 for lambs M1 and M2 and at around 1.2 kg day−1 for lambs M3 to M5, compared with 1.5 kg day−1 for conventional lambs. Introduction of M. wolinii into lambs lacking ruminal methanogens had little effect on feed intake after 18 months (Fig. 2). During the daytime meroxenic lambs, especially before the age of 4 months, consumed feed more irregularly than conventional lambs (data not shown). For all animals, the measured rumen pH was 6.6 to 6.8 before feeding and 5.8 to 6.2 2 h after the start of feeding (data not shown).
FIG. 2.
Consumption of alfalfa pellets by lambs. ▪, meroxenic lambs M1 and M2; ○, meroxenic lambs M3 and M4; ▴, meroxenic lamb M5; ×, conventional lambs C1 and C2.
Establishment of hydrogenotrophic communities in conventional and meroxenic lambs. (i) Conventional lambs C1 and C2.
Hydrogenotrophic communities became established during the first week of life. No hydrogenotrophs were detected 2 days after birth, but all hydrogenotrophs were detected at day 6. Subsequently, methanogens, reductive acetogens (acetogens), SRB, and fumarate-reducing bacteria were found to always be present. After complete weaning (10 weeks) the level of methanogens stabilized at around 108 cells g−1, the level of SRB fluctuated between 105 and 106 cells g−1, and the levels of reductive acetogens and fumarate-reducing bacteria varied from 105 to 107 cells g−1.
(ii) Gnotoxenic lambs G1 and G2.
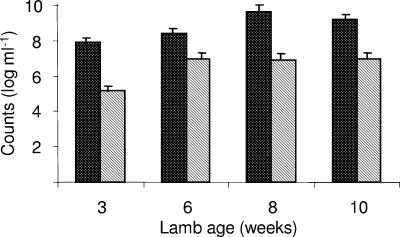
One week after inoculation with acetogens Ser5 and Ser8, the acetogen densities reached 106 to 107CFU g−1 in roll tube cultures (data not shown). The acetogen densities determined from growth on carbohydrate in roll tube cultures increased over the first few weeks and stabilized at 109 to 1010 cells g−1 after 8 weeks (Fig. 3). For the same samples, the acetogen densities were found to be 10- to 100-fold lower when they were determined using growth on H2-CO2 in broth culture (Fig. 3).
FIG. 3.
Population densities of ruminal acetogenic bacteria in gnotoxenic lambs G1 and G2: CFU on complete medium in agar roll tubes under CO2 (dotted bars) and most-probable-number enumeration in broth medium under H2-CO2 (hatched bars). The error bars indicate one standard deviation.
(iii) Meroxenic lambs M1 and M2.
Acetogens were first detected a few weeks after birth, and the population density increased slowly and fluctuated between 5 × 106 and 5 × 108 cells g−1 after the age of 5 months. SRB were not detected before 3 months, and then the densities remained between 5 × 105 and 5 × 107 cells g−1. The first measurement of fumarate-reducing bacteria was carried out when lambs were 4 months old and showed that population densities varied widely (5 × 105 to 2 × 108 cells g−1). When these methanogen-free lambs were inoculated with M. wolinii at the age of 19 months (Fig. 1), this methanogen established itself rapidly and the population density reached 109 cells g−1.
(iv) Meroxenic lambs M3 and M4.
In contrast to expectations, acetogens developed in meroxenic lambs M3 and M4 and were first detected in lambs that were 4.5 months old at a level of 106 cells g−1. In lambs that were more than 6 months old, the acetogen densities fluctuated between 2 × 107 and 5 × 108 cells g−1. SRB appeared at the same time as acetogens, but the population densities varied more widely (105 to 108 cells g−1). The densities of fumarate-reducing bacteria, which were monitored only from 4 months onward, were found to be highly variable (2 × 105 to 5 × 108 cells g−1). M. wolinii inoculated into lambs at 19 months established itself rapidly, and the densities reached were similar to those found in lambs M1 and M2 (108 to 109 cells g−1).
(v) Meroxenic lamb M5.
After inoculation at 5 months (Fig. 1), M. wolinii colonized the rumen of meroxenic lamb M5 quickly, and the population density stabilized at 107 to 108 cells g−1 for the remainder of the experiment. The establishment sequence for acetogenic bacteria, fumarate-reducing bacteria, and SRB in lamb M5 was similar to that in lambs M1 and M2, and the population densities of the four hydrogenotrophs in lamb M5 at the end of the experiment were similar to those in lambs M1, M2, M3, and M4.
Establishment of cellulolytic bacteria.
All meroxenic animals were found to be free of fungi and protozoans. Establishment of R. flavefaciens and R. albus in meroxenic animals was confirmed using rumen samples from 3-month-old animals by dot blot hybridization with targeted oligonucleotide probes. The presence of these species was also detected in conventional lambs. In conventional lambs, the levels of ruminal cellulolytic bacteria remained steady throughout the experiment at around 5 × 108 cells g−1. In all meroxenic animals, the levels of ruminal cellulolytic bacteria fluctuated between 107 and 108 cells g−1.
Ruminal VFA concentrations in meroxenic and conventional lambs.
VFA concentrations (and other measurements for rumen contents described below) were determined mainly using samples collected after lamb behavior, feeding habits, and rumen microflora had stabilized (after 8 months). The ruminal VFA concentrations in 11- to 18-month-old lambs before feeding and 2 h after the start of feeding showed that there was effective fermentation of alfalfa hay in all animals (Table 1). The ruminal VFA concentrations were higher in conventional lambs than in meroxenic lambs, including meroxenic lamb M5 containing a ruminal methanogen. Table 1 shows that the greater the complexity of the rumen community, the higher the VFA concentrations (C1 and C2 > M1 and M2 > M3 and M4). Rumen contents from lambs C1 and C2 with a complete ruminal microflora contained substantially more butyrate. For meroxenic lambs, the ruminal acetate/propionate ratios were markedly lower in samples collected 2 h after the animals received feed. In contrast, the acetate/propionate ratios in the rumens of conventional lambs were little affected by feeding (Table 1). Table 2 shows that after inoculation with M. wolinii the acetate/propionate ratios in lambs M1 and M2 and in lambs M3 and M4 stabilized with respect to postfeeding effects and became similar to those in conventional lambs (Table 1). After establishment of M. wolinii, the levels of methane formation in conventional and meroxenic lambs were 24.5 and 14.1 mmol liter−1 before feeding, respectively, and 39.7 and 33.5 mmol liter−1 2 h after the start of feeding, respectively (data not shown).
TABLE 1.
Ruminal VFA concentrations in conventional lambs C1 and C2 and in meroxenic lambs with (lamb M5) and without (lambs M1 to M4) methanogensa
| Lamb(s) | Time | VFA concn (mmol liter−1)
|
Acetate/ propionate ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Acetate | Propionate | Butyrate | Isobutyrate | Valerate | Isovalerate | |||
| M1 and M2 | T0 | 62.5 ± 10.1 A | 44.2 ± 6.7 (70.7) A,C | 12.5 ± 2.1 (20.0) A | 4.5 ± 0.7 (7.2) A | 0.4 ± 0.02 A | 0.8 ± 0.07 A | 0.3 ± 0.04 A | 3.5 ± 0.5 A |
| T2 | 102 ± 15.3 X | 67.5 ± 7.6 (66.2) X | 23.8 ± 3.1 (23.3) X | 9.0 ± 2.1 (8.8) X | 0.32 ± 0.02 X | 1.0 ± 0.10 X | 0.4 ± 0.10 X | 2.8 ± 0.1 X | |
| M3 and M4 | T0 | 55.7 ± 11.9 A | 40.6 ± 7.31 (72.9) A | 10.2 ± 2.9 (18.3) A,C | 2.8 ± 1.1 (5.0) A | 0.3 ± 0.04 B,C | 0.2 ± 0.02 B | 0.3 ± 0.08 A | 4.0 ± 0.4 A |
| T2 | 81.1 ± 9 Y | 56.0 ± 4.3 (69.0) Y | 20.8 ± 5.3 (25.3) X | 3.5 ± 0.5 (4.3) Y | 0.4 ± 0.08 Y,V | 0.2 ± 0.04 Y | 0.5 ± 0.06 X | 2.7 ± 0.2 X,Y | |
| M5 | T0 | 32.6 ± 2.7 B | 23.6 ± 9.0 (72.4) Y | 6.4 ± 0.5 (19.5) B,C | 1.9 ± 0.3 (5.8) A | 0.3 ± 0.05 B | 0.2 ± 0.03 B | 0.2 ± 0.03 A | 3.7 ± 0.1 A |
| T2 | 85.2 ± 12.1 X,Y | 56.1 ± 6.8 (65.8) Y | 22.2 ± 3.2 (26.0) X | 3.6 ± 0.4 (4.2) Y | 0.4 ± 0.07 V,Z | 0.8 ± 0.06 X | 0.4 ± 0.10 X | 2.5 ± 0.2 Y | |
| C1 And C2 | T0 | 83.1 ± 9.5 C | 53.2 ± 9.0 (64.0) C | 14.8 ± 4.3 (17.8) A | 11.6 ± 3.7 (14.0) B | 0.7 ± 0.05 C | 1.2 ± 0.09 C | 0.9 ± 0.06 B | 3.7 ± 0.3 A |
| T2 | 137.6 ± 15.1 X | 89.7 ± 9.8 (65.1) Z | 25.7 ± 3.1 (18.7) X | 16.1 ± 2.3 (11.7) Z | 1.0 ± 0.02 Y | 1.8 ± 0.4 Z | 1.2 ± 0.2 Y | 3.5 ± 0.2 Z | |
The values are means ± standard deviations of eight samples collected at intervals from lambs that were 11 to 18 months old. Samples were removed just before feeding (T0) and 2 h after the beginning of feeding (T2). The values in parentheses are the percentages of the total VFA. In the same column, concentrations followed by different letters differ significantly (P < 0.05) for the concentrations obtained at T0 (A, B, and C) and at T2 (V, X, Y, and Z).
TABLE 2.
Ruminal VFA concentrations in meroxenic lambs M1 to M4 after ruminal establishment of M. woliniia
| Lambs | Time | VFA concn (mmol liter−1)
|
Acetate/propionate ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Acetate | Propionate | Butyrate | Isobutyrate | Valerate | Isovalerate | |||
| M1 and M2 | T0 | 68.3 ± 6.2 A | 45.5 ± 3.7 (66.6) A | 11.4 ± 2.7 (16.7) A | 9.6 ± 1.7 (14.0) A | 0.5 ± 0.05 A | 0.9 ± 0.06 A | 0.5 ± 0.07 A | 3.6 |
| T2 | 110.5 ± 11.2 X | 75.7 ± 5.5 (68.8) X | 19.7 ± 2.7 (17.9) X | 13.5 ± 2.1 (12.7) X | 0.3 ± 0.02 X | 0.8 ± 0.1 X | 0.4 ± 0.03 X | 3.8 | |
| M3 and M4 | T0 | 52.2 ± 4.2 B | 34.0 ± 3.2 (65.2) B | 8.9 ± 1.2 (17.0) A | 7.5 ± 0.8 (14.3) A | 0.3 ± 0.03 A | 0.8 ± 0.05 A | 0.4 ± 0.03 A | 3.8 |
| T2 | 84.5 ± 6.2 Y | 56.6 ± 4.7 (66.9) Y | 14.8 ± 1.7 (17.5) X | 11.1 (13.1) X | 0.4 ± 0.03 X | 0.8 ± 0.06 X | 0.6 ± 0.04 X | 3.8 | |
The values are means ± standard deviations from two determinations. Samples were removed before feeding (T0) or 2 h after the start of feeding (T2). The values in parentheses are the percentages of the total VFA. In the same column, concentrations followed by different letters differ significantly (P < 0.1) for the concentrations at T0 (A and B) and at T2 (X and Y).
Hydrogen recovery and reductive acetogenesis in rumen samples.
The hydrogen recovery and reductive acetogenesis data for rumen contents calculated from VFA data are shown in Table 3. In rumen contents from meroxenic lamb M5 containing a ruminal methanogen, the H2 recovery was substantially greater than 100% (Table 3). The reasons for this are not known. In rumen contents from meroxenic animals lacking ruminal methanogens, the H2 recovery was less than 50% and was as low as 28%. The average hydrogen recovery was 91% for meroxenic lambs M1 to M4 following inoculation with M. wolinii (data not shown), and the data show the effectiveness of methanogens in capturing hydrogen in the rumen. Reductive acetogenesis made a significant contribution (21 to 25%) to overall ruminal fermentation in meroxenic lambs lacking ruminal methanogens (Table 3) and accounted for around 66% of the hydrogen recovery in these animals.
TABLE 3.
Hydrogen recovery and reductive acetogenesis in rumen contents from lambs lacking (lambs M1 to M4) or containing (lambs M5, C1, and C2) ruminal methanogensa
| Lamb(s) | H2 recovery (%)
|
Reductive acetogenesis (%)
|
||
|---|---|---|---|---|
| T0 | T2 | T0 | T2 | |
| M1 and M2 | 46 | 34 | 24 | 22 |
| M3 and M4 | 28 | 35 | 25 | 21 |
| M5 | 118 | 121 | 0 | 0 |
| C1 and C2 | 107 | 90 | 0 | 0 |
Values were calculated for animals between 11 and 18 months old before feeding (T0) and 2 h after the start of feeding (T2).
Hydrogen utilization in rumen contents from meroxenic and conventional lambs and effect of animal age on ruminal hydrogen consumption.
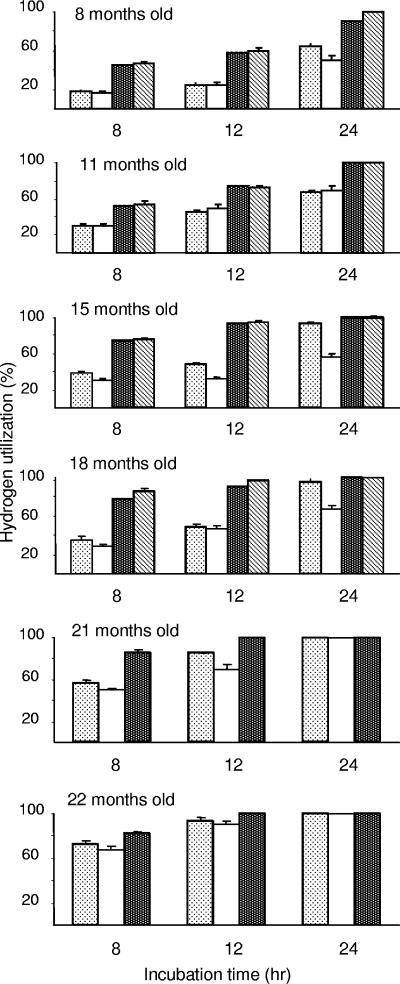
H2 utilization data for incubations under a headspace containing H2 are shown in Fig. 4. In conventional lambs and methanogen-containing lamb M5 at the ages of 8 and 11 months, 45 to 55% of the available H2 was consumed after incubation for 8 h, and 58 to 75% of the available H2 was consumed after 12 h of incubation. After 24 h all available H2 was consumed. When these lambs were 15 and 18 months of age, the H2 consumption increased to about 80% in 8-h incubations and to about 100% in 12-h incubations (Fig. 4). Hydrogen utilization was always much lower in rumen contents from animals lacking ruminal methanogens (Fig. 4). The hydrogen utilization in meroxenic lambs M1 to M4 was lower than that in lambs M5, C1, and C2 containing methanogens (Fig. 4). This was most evident in the 8- and 12-h incubations. Only 20 to 40% of the available H2 was consumed when rumen contents from lambs M1 to M4 at the ages of 8, 11, 15, and 18 months were incubated for 8 h. The results from 24-h incubations of rumen contents from lambs M1 and M2 at 15 and 18 months of age show that H2 utilization was lowest for the less complex microflora from lambs M3 and M4.
FIG. 4.
Hydrogen utilization in rumen contents from lambs M1 to M4 lacking ruminal methanogens and from lamb M5 and conventional lambs C1 and C2 containing ruminal methanogens. Rumen contents from lambs M1 and M2 (open dotted bars), M3 and M4 (open bars), M5 (solid dotted bars), and C1 and C2 (hatched bars) were incubated under an H2-CO2 headspace for 8, 12, or 24 h at 39°C. The error bars indicate one standard deviation.
Following inoculation of meroxenic lambs M1 to M4 with M. wolinii at 19 months, ruminal H2 utilization increased to 50 to 70% in 8-h incubations in the 21- and 22-month-old animals (Fig. 4). When the three groups of meroxenic lambs contained M. wolinii in their rumens, the rumen contents utilized 100% of the available H2 after 24 h of incubation (Fig. 4), and the headspaces contained methane and not H2 (data not shown), showing the effectiveness of the methanogen.
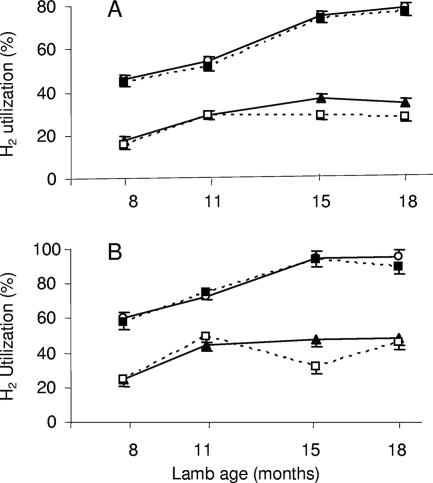
The data in Fig. 4 suggested that there is a relationship between efficiency of ruminal H2 consumption and animal age. To clarify this, the amounts of hydrogen consumed in 8- and 12-h incubations were plotted against animal age for each of the four groups of lambs. The results clearly showed that the H2-consuming capacity of rumen microbial communities in conventional lambs and in meroxenic lamb M5 containing a ruminal methanogen increased with animal age (Fig. 5). The age effect was not evident after 18 months of age. The association between ruminal H2-utilizing capacity and age was less marked for meroxenic lambs lacking methanogens. In this case, the H2-utilizing capacity increased until 11 months of age and then stabilized (Fig. 5).
FIG. 5.
Relationship between animal age and hydrogen utilization in rumen contents from meroxenic and conventional lambs. ▴, lambs M1 and M2; □, lambs M3 and M4; ▪, lamb M5; ○, lambs C1 and C2. Rumen contents were incubated in vitro for 8 h (A) or 12 h (B). The bars indicate one standard deviation.
Fate of hydrogen in incubations of rumen contents.
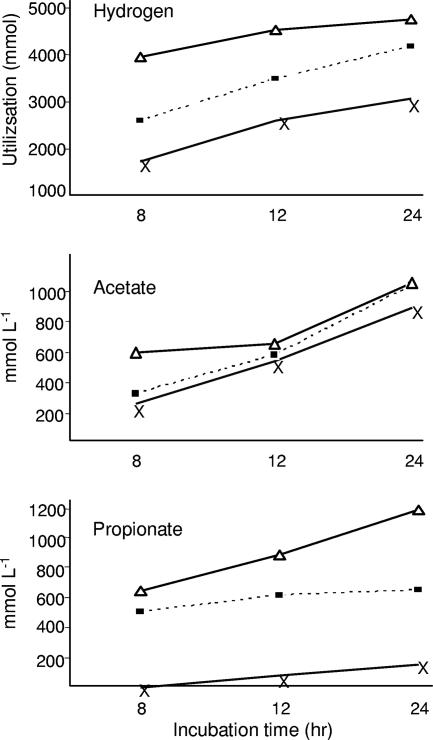
Hydrogen consumption, VFA production, and methane production in rumen contents from the four groups of lambs after 24 h of incubation are shown in Table 4. The VFA levels produced in incubations without H2 in the headspace were low (data not shown). Methane was not detected in incubations of contents from methanogen-free lambs M1 to M4 but was detected in incubations of contents from conventional lambs and from meroxenic lamb M5 containing M. wolinii (Table 4). Table 4 shows that rumen contents from lambs M1 to M4 lacking ruminal methanogens produced high levels of acetate when the contents were incubated under H2-CO2. These acetate levels increased when H2 utilization increased. This finding is in accordance with the hypothesis that reductive acetogenesis is the dominant hydrogenotrophic mechanism. When acetate levels expected from complete hydrogen capture by acetogenesis were calculated from the equation 4H2 + 2CO2 → CH3COOH + 2H2O, the levels were 10 to 20% greater than those measured (Table 4). This suggests that a proportion of ruminal H2 in lambs lacking methanogens was captured by processes other than reductive acetogenesis. Alternative H2 sinks may be associated with the increased propionate and butyrate levels found in incubations (Table 4). In conventional lambs and in meroxenic lambs containing M. wolinii, methanogenesis was the dominant hydrogen sink. In all lambs with ruminal methanogens, the amount of methane measured from incubations exceeded that expected from the stoichiometry of the equation 4H2 +CO2 → CH4 + H2O (Table 4).
TABLE 4.
In vitro hydrogen consumption and production of VFAs and methane in rumen contents from conventional and meroxenic lambs
| Lamb(s) | Age (mo) | H2 utilized (μmol) | Fermentation products (μmol)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Acetate
|
Propionate | Butyrate | Methane
|
|||||
| Expecteda | Measured | Expectedb | Measured | |||||
| M1 and M2 | 11 | 3,340 | 835 | 672 (81)c | 108 | 40 | 0 | 0 |
| 15 | 5,400 | 1,350 | 1,170 (87) | 100 | 40 | 0 | 0 | |
| 18 | 5,405 | 1,351 | 1,210 (90) | 125 | 45 | 0 | 0 | |
| 21 | 5,262 | NDd | ND | ND | ND | 1,315 | 1,547 | |
| M3 and M4 | 11 | 4,062 | 1,015 | 820 (81) | 83 | 27 | 0 | 0 |
| 15 | 3,210 | 802 | 665 (83) | 59 | 17 | 0 | 0 | |
| 18 | 3,920 | 980 | 858 (88) | 67 | 27 | 0 | 0 | |
| 21 | 5,262 | ND | ND | ND | ND | 1,315 | 1,527 | |
| M5 | 8 | 5,560 | ND | ND | ND | ND | 1,390 | 1,450 |
| 11 | 5,504 | ND | ND | ND | ND | 1,376 | 1,547 | |
| 15 | 5,495 | ND | ND | ND | ND | 1,373 | ND | |
| 18 | 5,640 | ND | ND | ND | ND | 1,410 | ND | |
| C1 and C2 | 8 | 5,600 | ND | ND | ND | ND | 1,400 | 1,540 |
| 11 | 5,440 | ND | 215 | 70 | 63 | 1,360 | 1,641 | |
| 15 | 5,560 | ND | 260 | 82 | 78 | 1,389 | 1,646 | |
| 18 | 5,643 | ND | 292 | 78 | 55 | 1,410 | 1,658 | |
| 21 | 5,262 | ND | ND | ND | ND | 1,315 | 1,568 | |
Calculated from the stoichiometry of reductive acetogenesis: 4H2 + 2CO2 → CH3COOH + 2H2O.
Calculated from the stoichiometry of methanogenesis: 4H2 + CO2 → CH4 + 2H2O.
The data in parentheses indicate the amount of measured acetate expressed as a percentage of the amount of acetate from reductive acetogenesis.
ND, not determined.
Effect of fumarate on hydrogen consumption and methane production in vitro.
In Table 5, the results for 8-h incubations show that addition of fumarate significantly increased (7 to 50%) H2 consumption in rumen contents from all lambs containing methanogens (conventional lambs C1 and C2 and meroxenic lamb M5) except those 18 months old. The increased consumption was not fumarate concentration dependent. When fumarate was added to rumen contents from 8-, 11-, and 15-month-old lambs M1 to M4 lacking ruminal methanogens, H2 consumption increased markedly (33 to 76%) in both 8- and 12-h incubations (Table 5). The greatest increases were observed for meroxenic lambs M1 and M2, with the more complex microflora suggesting that the effects of fumarate on ruminal H2 consumption may be community dependent. Effects may also be animal age dependent because fumarate had little effect on H2 consumption in rumen contents from 18-month-old methanogen-free lambs (Table 5). The effects of fumarate on methane production are shown in Table 6. Fumarate at concentrations of 18.7 and 37.4 mM decreased methane production in rumen contents from lamb M5 containing M. wolinii by 10 to 12% and 15 to 24%, respectively. In rumen contents from conventional lambs C1 and C2, the decreases in methane production were smaller and the size of the decreases varied with animal age and incubation period. Methane production in the presence of 18.7 and 37.4 mM fumarate decreased by 3 to 11% and by 7 to 13%, respectively, in 24-h incubations (Table 6).
TABLE 5.
Effect of fumarate on hydrogen utilization in rumen contents from conventional lambs C1 and C2 and meroxenic lambs with (lamb M5) and without (lambs M1 to M4) ruminal methanogens
| Lamb age (mo) | Incubation time (h) | Hydrogen utilized (%) in incubationsa
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lambs M1 and M2
|
Lambs M3 and M4
|
Lamb M5
|
Lambs C1 and C2
|
||||||||||
| No fumarate | 18.7 mM fumarate | 37.4 mM fumarate | No fumarate | 18.7 mM fumarate | 37.4 mM fumarate | No fumarate | 18.7 mM fumarate | 37.4 mM fumarate | No fumarate | 18.7 mM fumarate | 37.4 mM fumarate | ||
| 8 | 8 | 38 ± 2 A | 43 ± 4 A | 60 ± 5 B | 30 ± 2 A | 27 ± 2 A | 40 ± 3 B | 72 ± 2 A | 82 ± 1.5 B | 82.7 ± 2 B | 76 ± 1 A | 80 ± 3 A | 82 ± 4 A |
| 12 | 48 ± 2 A | 59 ± 1 A,B | 69 ± 4 B | 32 ± 2 A | 30 ± 2.5 A | 44 ± 4 A | 92 ± 1.5 A | 96 ± 1.5 A | 96.6 ± 2 A | 95 ± 1 A | 96.7 ± 0.5 A | 97 ± 2 A | |
| 24 | 93 ± 2 A | 95 ± 3 A | 100 A | 57 A | 61 ± 1.5 A | 58 ± 2.5 A | 100 | 100 | 100 | 100 | 100 | 100 | |
| 11 | 8 | 30 ± 2 A | 45 ± 2 B | 53 ± 3 B | 30 ± 2 | NDb | ND | 42 ± 2 A | 51 ± 1 A | 63 ± 3 B | 55 ± 0.3 A | 55.8 ± 1 A | 57 ± 2 A |
| 12 | 45 ± 3 A | 60 ± 5 A,B | 72 ± 4 B | 50 ± 4 A | 72 ± 2 B | 87 ± 3 C | 74 ± 3 A | 75 ± 2 A | 76 ± 10 A | 73 ± 2 A | 77 ± 2.5 A | 81 ± 2 A | |
| 24 | 68 ± 2 A | 78 ± 2 B | 82 ± 3 B | 70 ± 4 A | 77 ± 3 A | 90 ± 2 B | 100 | 100 | 100 | 100 | 100 | 100 | |
| 15 | 8 | 36 ± 2 A | 47 ± 1 A,B | 57 ± 4 B | 29 ± 2 A | 31 ± 1 A | 34 ± 2 A | ND | ND | ND | 70 ± 2 A | 82 ± 2 B | 82 ± 1.5 B |
| 12 | 49 ± 2 A | 61 ± 3 B | 76 ± 3 C | 46 ± 4 A | 43 ± 2 A | 48 ± 1 A | ND | ND | ND | 96 ± 1 A | 97 ± 2 A | 97 ± 1 A | |
| 24 | 95 ± 3 A | 99 ± 1 A | 100 A | 68 ± 3 A | 65 ± 2 A | 70 ± 3 A | ND | ND | ND | 100 | 100 | 100 | |
| 18 | 8 | 18 ± 2 A | 22 ± 3 A | ND | 16 ± 2 A | 18 ± 2 A | ND | 45 ± 4 A | 42 ± 2.5 A | ND | 46 ± 2.5 A | 45 ± 2 | ND |
| 12 | 25 ± 3 A | 27 ± 2 A | ND | 25 ± 2 A | 26 ± 2 A | ND | 58 ± 2 A | 58 ± 3 A | ND | 60 ± 3 A | 59 ± 2.5 A | ND | |
| 24 | 60 ± 3 A | 66 ± 1 A | ND | 50 ± 2 A | 60 ± 2.5 B | ND | 99 ± 1 A | 98 ± 2 A | ND | 100 | 100 | ND | |
The values are means ± standard deviations for two samples. For each group of lambs, values followed by different letters in the same row differ significantly (P < 0.05).
ND, not determined.
TABLE 6.
Effect of fumarate on methane production in rumen contents from conventional lambs C1 and C2 and from meroxenic lamb M5 containing M. wolinii
| Lamb age (mo) | Incubation time (h) | Methane production (μmoles/bottle)a
|
|||||
|---|---|---|---|---|---|---|---|
| Lamb M5
|
Lambs C1 and C2
|
||||||
| No fumarate | 18.7 mM fumarate | 37.4 mM fumarate | No fumarate | 18.7 mM fumarate | 37.4 mM fumarate | ||
| 11 | 8 | 746 | 672 (10) | 633 (15) | 1,041 | 971 (7) | 857 (18) |
| 12 | 1,106 | 884 (11) | 735 (22) | 1,160 | 1,134 (2) | 1,001 (14) | |
| 24 | 1,547 | 1,268 (12) | 1,180 (24) | 1,610 | 1,567 (3) | 1,425 (12) | |
| 15 | 8 | 1,240 | 1,130 (11) | NDb | 1,453 | 1,380 (5) | 1,333 (8) |
| 12 | 1,530 | 1,383 (10) | ND | 1,670 | 1,576 (6) | 1,556 (7) | |
| 24 | 1,860 | 1,675 (10) | ND | 1,940 | 1,801 (7) | 1,798 (7) | |
| 18 | 8 | ND | ND | ND | 1,355 | 1,297 (4) | 1,282 (5) |
| 12 | ND | ND | ND | 1,762 | 1,633 (7) | 1,655 (6) | |
| 24 | ND | ND | ND | 2,006 | 1,788 (11) | 1,750 (13) | |
One sample per animal was used. The values in parentheses are the percent decreases in methane production.
ND, not determined.
Metabolism of fumarate in rumen contents lacking methanogens.
Fermentation end products from fumarate were determined only for rumen contents from methanogen-free lambs. Table 7 shows propionate production from fumarate for lambs M1 and M2. Similar results were obtained for lambs M3 and M4 (data not shown). Figure 6 shows the effects of fumarate on acetate, propionate, and H2 utilization for lamb M1 at 15 months. In all incubations, fumarate was converted predominantly to propionate (Fig. 6 and Table 7), with net production of only small amounts of acetate (Fig. 6) and no measurable butyrate (data not shown). Expected levels of propionate were calculated from the amounts of H2 consumed (Table 4) and the stoichiometry of fumarate reduction to succinate. The expected levels together with the measured levels of propionate are shown in Table 7. In 8- and 12-h incubations, the propionate production was less (43 to 73%) than that expected, whereas in 24-h incubations the production was greater (115 to 178%) than that expected.
TABLE 7.
Propionate production from fumarate added to rumen contents from meroxenic lambs M1 and M2 lacking methanogens
| Lamb (age) | Incubation time (h) | In vitro propionate production (μmoles/bottle)a
|
|||
|---|---|---|---|---|---|
| 18.7 mM fumarate
|
37.4 mM fumarate
|
||||
| Expected | Measured | Expected | Measured | ||
| M1 (15 mo) | 8 | 852 | 500 (59) | 1,316 | 640 (49) |
| 12 | 870 | 612 (70) | 1,566 | 880 (56) | |
| 24 | 574 | 660 (113) | 806 | 1,180 (146) | |
| M1 (18 mo) | 8 | 756 | 544 (72) | 1,180 | 508 (43) |
| 12 | 810 | 568 (70) | 1,460 | 668 (46) | |
| 24 | 538 | 840 (156) | 758 | 1,360 (178) | |
| M2 (15 mo) | 8 | 852 | 488 (57) | 1,316 | 440 (33) |
| 12 | 870 | 630 (72) | 1,560 | 780 (50) | |
| 24 | 574 | 900 (157) | 806 | 960 (119) | |
The values are means of two samples. The values in parentheses are the amounts of measured propionate expressed as a percentage of the expected amount of propionate.
FIG. 6.
Effect of fumarate on hydrogen utilization and production of acetate and propionate in rumen contents from 15-month-old meroxenic lamb M1. The values are the values for one rumen sample at each time point measured in duplicate incubations. The standard deviations were less than 5% of the values and are not shown. The preparations contained no fumarate (×), 18.6 mM fumarate (▪), or 37.2 mM fumarate (▵) and were incubated for 8, 12, or 24 h.
Identification of ruminal reductive acetogens in meroxenic lambs lacking methanogens.
The phenotypic characteristics of reductive acetogens isolated from meroxenic lambs lacking methanogens are shown in Table 8. Of the seven predominant acetogens selected for identification, isolates J2, 8F, and 11A were the most highly active hydrogenotrophs in initial tests. After subculturing, only isolate 8F retained acetogenic capacity when it was grown on H2-CO2. During growth on fumarate under H2-N2, all isolates produced succinate as an end product, but only isolate J2 utilized H2. Isolate J2 also was the most effective at converting fumarate to succinate (approximately 100%), and isolates 69, 11A, and 8F produced only 50, 28, and 9%, respectively, of the succinate expected on an equimolar basis (data not shown). None of the isolates produced propionate from fumarate (Table 8).
TABLE 8.
Identification of predominant ruminal acetogens from meroxenic lambs M1 and M2 lacking methanogens
| Isolate | Morphology | Gram reaction | Growth on fumaratea | Products from fumaratea
|
H2 utilization | 16S rRNA gene identification | |||
|---|---|---|---|---|---|---|---|---|---|
| Succinate | Propionate | Acetate | Formate | ||||||
| B2 | Nonmotile coccus | + | + | + | − | + | + | − | E. gallinarum |
| J2 | Nonmotile short rod | − | + | + | − | + | Tr | + | E. coli |
| 8F | Nonmotile coccobacillus | Variable | + | + | − | + | − | − | C. coccoides |
| 11A | Nonmotile short rod | − | + | + | − | + | − | − | E. coli |
| 22B | Nonmotile coccus | + | + | + | − | + | + | − | E. gallinarum |
| 69 | Nonmotile pointed rod | + | + | + | − | + | + | − | C. symbiosum |
| 109D | Nonmotile curved rod | + | + | + | − | + | − | − | P. australiense |
Cultured under an H2-N2 headspace.
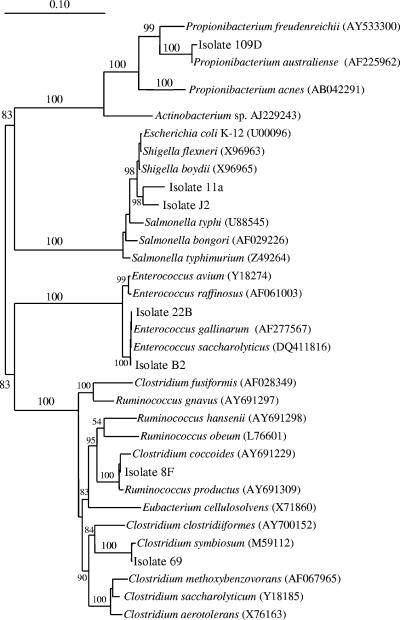
A phylogenetic tree (Fig. 7) showing inferred genetic relationships was generated from 16S rRNA gene sequences, and similarity matrices were prepared. Isolate J2 was found to be most closely related to Escherichia coli (98.1%) and Shigella boydii (98.2%). Isolate B2 was most closely related (99.9%) to Enterococcus gallinarum. Isolate 8F was most closely related (99.8%) to Clostridium coccoides. Isolate 11A was most closely to E. coli (97.7%) and S. boydii (97.7%). Isolate 22B was most closely related (100%) to E. gallinarum. Isolate 69 was most closely (99.6%) related to Clostridium symbiosum, and isolate 109D was most closely related (99.5%) to Propionibacterium australiense.
FIG. 7.
Neighbor-joining tree showing inferred phylogenetic relationships of acetogen isolates from nonmethanogenic lambs. The bootstrap values at the nodes are confidence levels (expressed as percentages) based on 1,000 resamplings. Bar = 10% sequence divergence. The accession numbers of database sequences are given in parentheses.
DISCUSSION
Feed consumption and ruminal fermentation.
Our results show that it is possible to rear lambs to adulthood without methanogenic archaea in their rumens. To the best of our knowledge, this is the first report of nonmethanogenic adult ruminants. Previously, gnotobiotic lambs lacking ruminal methanogens have been reared for short periods (<3 months) to investigate the effect of methanogens on plant cell wall degradation and overall fermentation in vivo (30, 33, 77). The feed intake in meroxenic lambs was lower than that in lambs with a normal ruminal microflora, and the feed intake declined in parallel with decreased microbial complexity in the rumen (Fig. 2). The lower intakes probably arose from the lack of methanogens and/or lack of fungi and protozoans. In sheep fed a fibrous diet, feed intake has been shown to decrease when ruminal fungi are removed (34). The in sacco digestibility of wheat straw decreased in meroxenic lambs when ruminal methanogens were absent (33).
Nevertheless, the ruminal populations in methanogen-free meroxenic lambs were sufficient for growth on a fibrous diet. The levels of ruminal cellulolytic bacteria monitored throughout the study were consistent with those in functional rumens (27, 29, 30). The ruminal VFA levels in the meroxenic lambs were lower than those in the conventional lambs but were in agreement with levels previously observed in meroxenic lambs (31, 32), and results showed that the more complex the ruminal microflora, the higher the VFA levels. The low butyrate levels in meroxenic lambs were probably due to the absence of protozoans (44). The ruminal acetate/propionate ratios in lambs lacking methanogens were lower than those in the conventional lambs, in agreement with results from in vitro incubations of rumen contents in which methanogens were inhibited (17).
Development of hydrogenotroph populations.
One aim of this study was to obtain information on hydrogenotrophy in the rumens of methanogen-free lambs in the presence and absence of ruminal acetogens. In an effort to establish acetogen-free animals, two germfree lambs (lambs M3 and M4) were inoculated with a high dilution (10−7) of methanogen-free rumen contents believed to lack acetogens (the levels of ruminal acetogens in the donor sheep had been estimated to be around 5 × 105 cells g−1). We were surprised to find that after a long delay reductive acetogens were detected in these animals.
(i) Acetogens.
In conventional lambs, a hydrogenotrophic acetogenic community became established rapidly after birth, as observed previously (61, 62). In gnotoxenic lambs G1 and G2, inoculated acetogens established themselves quickly as the sole ruminal occupants, showing that, unlike the cellulolytic bacteria, which require a diverse microbiota for establishment (27, 30, 36), acetogens can colonize and become established independent of other microbes. Acetogens are metabolically very versatile, and heterotrophic growth is favored over autotrophic growth on H2-CO2 (23, 57). In lambs G1 and G2 it is likely that acetogens grew on the mucus and desquamated epithelial cells in the developing rumen (28, 68, 69).
Because acetogens were the sole ruminal inhabitants, rumen contents from lambs G1 and G2 provided an opportunity to test methods of enumeration. Growth on carbohydrates in solid media resulted in acetogen densities that were 10- to 100-fold higher than those after growth on H2-CO2 (Fig. 3). This shows that enumeration on H2-CO2 markedly underestimates true acetogen densities in the rumen. In the present study, such an underestimation may explain why acetogens appeared in lambs M1 to M5 contrary to expectations.
In lambs lacking ruminal methanogens, reductive acetogens became established slowly. The reasons for this are not known, but this observation probably reflected adaptation of populations and slow development during exposure to high levels of hydrogen in vivo. The delay in detection was much longer in lambs M3 and M4 (several months) than in lambs M1 and M2 (6 weeks), suggesting linkage with microbial community complexity or with initial low levels of acetogens. When rumen populations in lambs M1 to M4 stabilized, the acetogen densities measured in methanogen-free lambs were similar to those in conventional ruminants (48).
Characterization of several isolates from meroxenic lambs M1 and M2 revealed that the ruminal acetogens included E. coli and E. gallinarum, bacteria usually not associated with hydrogen consumption in the rumen. No efforts were made to exhaustively isolate acetogens, and it is probable that lambs contained a wider range of acetogens than that shown in Table 8. Two strains of E. coli and a strain of C. coccoides were the most efficient reductive acetogens. Many original isolates lost their reductive acetogenic capacity during subculturing and maintenance. Of the purified bacteria (Table 8), only C. coccoides currently retains its reductive acetogenic capacity. The reasons for this are not known, but unexpected loss of hydrogenotrophic capacity during subculturing has also been observed for isolates of reductive acetogens from mature ruminants (K. Joblin and D. L. Pacheco, unpublished data).
(ii) Methanogens.
The population densities of ruminal methanogens in the conventional lambs were similar to those in ruminants (41). In meroxenic animals, M. wolinii inoculated into “acetogenic” rumens became established rapidly and the population densities reached were high, showing the competitiveness of methanogens in the rumen environment.
(iii) Fumarate-reducing bacteria.
All seven isolates purified on the basis of their initial acetogenic capabilities were found to reduce fumarate to succinate (Table 8), but only one consumed hydrogen during this process. This suggests that although ruminal bacteria such as Selenomonas ruminantium, Fibrobacter succinogenes, E. coli, Veillonella parvulum, and W. succinogenes reduce fumarate using H2 as an electron donor (2, 3), fumarate metabolism in the rumen does not necessarily involve consumption of hydrogen gas.
Hydrogen utilization by hydrogenotrophic communities.
In conventional lambs, acetogens made little contribution to hydrogen utilization (Table 3), indicating that reductive acetogenesis is not significant in the normal rumen. The natural methanogenic community in conventional lambs and a less complex ruminal community in a meroxenic lamb inoculated with a methanogen each consumed hydrogen much more rapidly than communities from meroxenic lambs lacking ruminal methanogens. Ruminal H2 utilization in meroxenic lambs M3 and M4 was lower than that in meroxenic lambs M1 and M2 and was probably related to the lower microbial diversity in the former animals. In our tests, the headspace hydrogen levels were much higher than the hydrogen thresholds for acetogens (40, 73) and so would be expected to favor growth of acetogens. Nevertheless, results for methanogen-free lambs clearly demonstrated that reductive acetogenesis was not as efficient as methanogenesis as a hydrogen sink in the rumen. The competitive advantage of methanogens over acetogens in hydrogen utilization can be explained by higher energy yields, higher substrate affinity, and lower thresholds (23, 57, 73). Nevertheless, some digestive ecosystems, such as those in the human colon (8, 9) and the guts of wood- and grass-feeding termites (11, 12), can be dominated by reductive acetogens rather than methanogens.
In lambs lacking ruminal methanogens, reductive acetogenesis was the major hydrogenotrophic process, although fumarate-reducing bacteria and SRB were present. A major proportion of the reducing equivalents generated in the rumens of methanogen-free meroxenic lambs was not used by reductive acetogens to convert CO2 to acetate. The recovery of hydrogen was low (28 to 46%), and the contribution of reductive acetogenesis to hydrogen capture was small (21 to 25%). These results are similar to findings for young lambs harboring weak methane-producing ruminal communities (25). In the present study, it was not possible to check for hydrogen inside isolators. It is likely that low ruminal hydrogen recovery values for lambs lacking methanogens are largely a consequence of loss of hydrogen gas to the atmosphere. Hydrogen gas has been found to accumulate in lambs treated with chloroform to inhibit methanogens (K. Joblin and G. Naylor, unpublished data). In the human colon, approximately 20% of the hydrogen produced during microbial fermentation is eliminated in breathing (8, 10). Some of the reducing equivalents not accounted for may be in the form of lactate, formate, and ethanol, which were not measured in our experiments. Such reduced compounds can accumulate in the absence of an efficient H2 sink, such as methanogenesis (78).
For both conventional lambs and meroxenic lambs harboring ruminal methanogens, the methane production from rumen contents under H2-CO2 was higher than that expected from stoichiometry (Table 4). The reasons for this are not known, but methane precursors, such as formate or hydrogen, may have been produced during incubation. It has been reported that in mixed cultures hydrogen production by Selenomonas ruminantium, a common rumen bacterium, increased in the presence of methanogens (72).
A novel finding was that the hydrogen consumption capacities of the ruminal microflora increased with animal age (Fig. 4 and 5). The age-related ruminal hydrogen utilization was most marked for conventional lambs and a meroxenic lamb inoculated with a ruminal methanogen. The observed correlation was evident until animals were at least 12 months old. We also found that the effect of fumarate on ruminal H2 utilization was animal age dependent (Table 5). To the best of our knowledge, this is the first report of a relationship between animal age and rumen microbial function in weaned ruminants.
Addition of fumarate to methanogenic rumen contents from conventional lambs and lamb M5 containing M. wolinii reduced methane production by up to 24% (Table 6), and the sizes of the reductions were similar to those found when fumarate was added to a Rusitec simulation of the rumen (52). In general, the size of the decrease correlated with the amount of fumarate converted to propionate (data not shown), indicating that most fumarate was converted via succinate (78). Higher-than-expected quantities of propionate (Table 7) and acetate (Fig. 6) were found in 24-h incubations. These quantities are believed to have arisen from fumarate fermentation to succinate without H2 oxidation. This hypothesis is consistent with the properties of dominant hydrogenotrophic bacteria isolated from the lambs (Table 8).
In conclusion, the present study showed that ruminal methanogens are not essential for effective fermentation in the rumen and that methanogens as a hydrogen sink can be replaced by reductive acetogens. However, reductive acetogenesis was not as efficient as methanogenesis. In the present work, ruminal reductive acetogenesis developed (slowly) after inoculation with incomplete rumen microflora. The inocula may not have included the most efficient reductive acetogens. We believe that inoculation of methanogen-free (or methanogen-suppressed) ruminants with more active reductive acetogens obtained from herbivores or from nonmethanogenic anaerobic ecosystems could increase ruminal hydrogen consumption above that observed in this study. Our results showed that reductive acetogens are unlikely to become established in the rumen unless methanogen-suppressing agents are applied. If safe reliable methods for eliminating or inhibiting ruminal methanogens can be developed and the effects maintained, reductive acetogens have the potential to be an important part of strategies to lower ruminant methane emissions.
Acknowledgments
We thank G. Andant, G. Vert, and C. Demartrin for their skilled technical assistance in rearing the meroxenic lambs and P. Evans for help with constructing the phylogenetic tree.
G.F. and K.J. gratefully acknowledge the French Embassy in Wellington, New Zealand, for support for exchanges between France and New Zealand under the collaborative program “Accord Culturel France/Nouvelle Zélande de 1977.”
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.Anderson, R. C., G. E. Carstens, R. K. Miller, T. R. Callaway, C. L. Schultz, T. S. Edrington, R. B. Harvey, and D. J. Nibet. 2006. Effect of oral nitroethane and 2-nitropropanol administration on methane-producing activity and volatile fatty acid production in the ovine rumen. Bioresour. Technol. 97:2421-2426. [DOI] [PubMed] [Google Scholar]
- 2.Asanuma, N., M. Iwamoto, and T. Hino. 1999. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J. Dairy Sci. 82:780-787. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma, N., and T. Hino. 2000. Activity and properties of fumarate reductase in ruminal bacteria. J. Gen. Appl. Microbiol. 49:119-125. [DOI] [PubMed] [Google Scholar]
- 4.Baker, S. K. 1999. Rumen methanogens, and inhibition of methanogenesis. Aust. J. Agric. Res. 50:1293-1298. [Google Scholar]
- 5.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchemin, K. A., and S. M. McGinn. 2005. Methane emissions from feedlot cattle fed barley or corn diets. J. Anim. Sci. 83:653-661. [DOI] [PubMed] [Google Scholar]
- 7.Bennegadi, N., G. Fonty, L. Millet, T. Gidenne, and D. Licois. 2003. Effects of age and dietary fibre level on caecal microbial communities of conventional and specific pathogen-free rabbits. Microb. Ecol. Health Dis. 15:23-32. [Google Scholar]
- 8.Bernalier, A., M. Lelait, V. Rochet, J.-P. Grivet, G. R. Gibson, and M. Durand. 1996. Acetogenesis from H2 and CO2 by methane- and non-methane-producing human colonic bacterial communities. FEMS Microbiol. Ecol. 19:193-202. [Google Scholar]
- 9.Bernalier, A., V. Rochet, M. Leclerc, J. Doré, and P. Pochart. 1996. Diversity of H2/CO2-utilizing acetogenic bacteria from feces of non-methane-producing humans. Curr. Microbiol. 33:94-97. [DOI] [PubMed] [Google Scholar]
- 10.Bernalier-Donadille, A. 2004. Principales functions de la flore intestinale de l'homme, p. 61-80. In J. C. Rambaud, J.-P. Buts, G. Corthier, and B. Flouré (ed.), Flore microbienne intestinale. Physiologie et pathologies digestives. John Libbey Eurotext, Montrouge, France.
- 11.Breznak, J. A. 1994. Acetogenesis from carbon dioxide in termite guts, p. 303-330. In H. L. Drake (ed.), Acetogenesis. Chapman and Hall, New York, NY.
- 12.Breznak, J. A., and J. M. Switzer. 1986. Acetate synthesis from H2-CO2 by termite gut microbes. Appl. Environ. Microbiol. 52:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryant, M. P., and L. A. Burkey. 1953. Cultural methods and some characteristics of some more numerous group of bacteria in the bovine rumen. J. Dairy Sci. 36:205-217. [Google Scholar]
- 14.Chaucheyras, F., G. Fonty, G. Bertin, and P. Gouet. 1995. In vitro H2 utilization by a ruminal acetogenic bacterium cultivated alone or in association with an archaea methanogen is stimulated by a probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 61:3466-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke, K. R., and N. J. P. Owens. 1983. A simple and versatile micro-computer program for the determination of “most probable number.” J. Microbiol. Methods 1:133-137. [Google Scholar]
- 16.Crutzen, P. 1995. The role of methane in atmospheric chemistry and climate, p. 291-316. In W. Von Engelhardt, S. Leonhard6marek, G. Breves, and G. Gieseke (ed.), Ruminant physiology: digestion, metabolism, growth and reproduction. Ferdinant Enke Verlag, Stuttgart, Germany.
- 17.Czerkawski, J. W., and G. Breckenridge. 1975. New inhibitors of methane production by rumen microorganisms. Experiments with animals and other practical possibilities. Br. J. Nutr. 34:447-457. [DOI] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Demeyer, D., D. Fiedler, and K. G. De Graeve. 1996. Attempted induction of acetogenesis into the rumen fermentation in vitro. Reprod. Nutr. Dev. 36:233-240. [DOI] [PubMed] [Google Scholar]
- 20.Demeyer, D., and V. Fievez. 2000. Ruminants et environnement: la méthanogénèse. Ann. Zootech. 59:95-112. [Google Scholar]
- 21.Dohme, F., A. Machmüller, B. L. Estermann, P. Pfister, A. Wasserfallen, and M. Kreuser. 1999. The role of the rumen ciliate protozoa for methane suppression caused by coconut oil. Lett. Appl. Microbiol. 29:187-192. [Google Scholar]
- 22.Doré, J., B. Morvan, F. Rieu-Lesme, I. Goderel, P. Gouet, and P. Pochart. 1995. Most probable number enumeration of H2-utilizing acetogenic bacteria from the digestive tract of animals and man. FEMS Microbiol. Lett. 130:7-12. [DOI] [PubMed] [Google Scholar]
- 23.Drake, H. L., K. Küsel, and C. Matthies. 2002. Ecological consequences of the phylogenetic and physiological diversities of acetogens. Antonie Leeuwenhoek 81:203-213. [DOI] [PubMed] [Google Scholar]
- 24.Ducluzeau, R., and P. Raibaud. 1979. Ecologie microbienne du tube digestif, p. 1-92. In INRA actualités scientifiques et techniques. Masson, Paris, France.
- 25.Faichney, G. J., N. M. Graham, and D. M. Walker. 1999. Rumen characteristics, methane emissions, and digestion in weaned lambs reared in isolation. Aust. J. Agric. Res. 50:1083-1089. [Google Scholar]
- 26.Fievez, V., F. Dohme, M. Daneels, K. Raes, and D. Demeyer. 2003. Fish oils as potent rumen methane inhibitors and associated effects on rumen fermentation in vitro and in vivo. Anim. Feed Sci. Technol. 104:41-58. [Google Scholar]
- 27.Fonty, G., P. Gouet, J. P. Jouany, and J. Senaud. 1983. Ecological factors determining establishment of cellulolytic bacteria and protozoa in the rumens of meroxenic lambs. J. Gen. Microbiol. 129:213-223. [DOI] [PubMed] [Google Scholar]
- 28.Fonty, G., P. Gouet, J. P. Jouany, and J. Senaud. 1987. Establishment of the microflora and anaerobic fungi in the rumen of lambs. J. Gen. Microbiol. 133:1835-1843. [Google Scholar]
- 29.Fonty, G., P. Gouet, and J. M. Nebout. 1989. Development of the cellulolytic microflora in the rumen of lambs transferred into sterile isolators a few days after birth. Can. J. Microbiol. 35:416-422. [DOI] [PubMed] [Google Scholar]
- 30.Fonty, G., P. Gouet, H. Ratefiarivelo, and J. P. Jouany. 1988. Establishment of Bacteroides succinogenes and measurement of the main digestive parameters in the rumen of gnotoxenic lambs. Can. J. Microbiol. 34:938-946. [DOI] [PubMed] [Google Scholar]
- 31.Fonty, G., J. P. Jouany, M. Chavarot, F. Bonnemoy, and P. Gouet. 1991. Development of the rumen digestive functions in lambs placed in a sterile isolator a few days after birth. Reprod. Nutr. Dev. 31:521-528. [DOI] [PubMed] [Google Scholar]
- 32.Fonty, G., J. P. Jouany, P. Thivend, P. Gouet, and J. Senaud. 1983. A descriptive study of rumen digestion in meroxenic lambs according to the nature and complexity of the microflora. Reprod. Nutr. Dev. 23:857-873. [DOI] [PubMed] [Google Scholar]
- 33.Fonty, G., A. G. Williams, F. Bonnemoy, B. Morvan, S. E. Withers, and P. Gouet. 1997. Effect of Methanobrevibacter sp. MF1 inoculation on glycoside hydrolase and polysaccharide depolymerase activities, wheat straw degradation and volatile fatty acid concentrations in the rumen of gnotobiotically-reared lambs. Anaerobe 3:383-389. [DOI] [PubMed] [Google Scholar]
- 34.Gordon, G. L. R., and M. W. Philips. 1993. Removal of anaerobic fungi from the rumen of sheep by chemical treatment and the effect on feed consumption and in vivo fibre digestion. Lett. Appl. Microbiol. 17:220-223. [Google Scholar]
- 35.Halliwell, G., and M. P. Bryant. 1963. The cellulolytic activity of pure strains of bacteria from the rumen of cattle. J. Gen. Microbiol. 32:441-448. [DOI] [PubMed] [Google Scholar]
- 36.Hobson, P. N., and G. Fonty. 1997. Biological models of the rumen function, p. 661-684. In P. N. Hobson and C. S. Stewart (ed.), The rumen microbial ecosystem, 2nd ed. Blackie Academic and Professional, London, United Kingdom.
- 37.Janssen, P. H., and P. Frenzel. 1997. Inhibition of methanogenesis by methyl fluoride: studies of pure and defined mixed cultures of anaerobic bacteria and archaea. Appl. Environ. Microbiol. 63:4552-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joblin, K. N. 1981. Isolation, enumeration, and maintenance of rumen anaerobic fungi in roll tubes. Appl. Environ. Microbiol. 42:1119-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joblin, K. N. 1996. Options for reducing methane emissions from ruminants in New Zealand and Australia, p. 437-449. In W. J. Bouma, G. I. Pearman, and M. R. Manning (ed.), Greenhouse: coping with climate change. CSIRO Publishing, Collingwood, Australia.
- 40.Joblin, K. N. 1999. Ruminal acetogens and their potential to lower ruminant methane emissions. Aust. J. Agric. Res. 50:1307-1313. [Google Scholar]
- 41.Joblin, K. N. 2005. Methanogenic archaea, p. 47-53. In H. P. S. Makkar and C. S. McSweeney (ed.), Methods in gut microbial ecology for ruminants. Springer, Dordrecht, The Netherlands.
- 42.Joblin, K. N., G. E. Naylor, and A. G. Williams. 1990. The effect of Methanobrevibacter smithii on the xylanolytic activity of anaerobic rumen fungi. Appl. Environ. Microbiol. 56:2287-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jouany, J.-P. 1982. Volatile fatty acid and alcohol determination in digestive contents, silage juices, bacterial cultures and anaerobic fermentor content. Sci. Aliments 2:131-144. [Google Scholar]
- 44.Jouany, J.-P., D. I. Demeyer, and J. Grain. 1988. Effect of defaunating the rumen. Anim. Feed Sci. Technol. 21:229-265. [Google Scholar]
- 45.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-147. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, Chichester, United Kingdom.
- 46.Reference deleted.
- 47.Lee, S. S., J. Hsu, H. C. Mantovani, and J. B. Russell. 2002. The effect of bovicin HC5, a bacteriocin from Streptococcus bovis HC5, on ruminal methane production in vitro. FEMS Microbiol. Lett. 217:51-55. [DOI] [PubMed] [Google Scholar]
- 48.Leedle, J. A. Z., and R. C. Greening. 1988. Postprandial changes in methanogenic and acidogenic bacteria in the rumens of steers fed high- or low-forage diets once daily. Appl. Environ. Microbiol. 64:502-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leedle, J. A. Z., and R. B. Hespell. 1980. Differential carbohydrate media and anaerobic replica plating techniques in delineating carbohydrate-utilizing subgroups in rumen bacterial populations. Appl. Environ. Microbiol. 39:709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Van, T. D., J. A. Robinson, J. Ralph, R. C. Greening, W. J. Smolenski, J. A. Leedle, and D. M. Schaeffer. 1998. Assessment of reductive acetogenesis with indigenous ruminal bacterium populations and Acetitomaculum ruminis. Appl. Environ. Microbiol. 64:3429-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez, S., F. M. McIntosh, R. J. Wallace, and C. J. Newbold. 1999. Effect of adding acetogenic bacteria on methane production by mixed rumen microorganisms. Anim. Feed Sci. 78:1-9. [Google Scholar]
- 52.Lopez, S., C. Valdes, C. J. Newbold, and R. J. Wallace. 1999. Influence of sodium fumarate addition on rumen fermentation in vitro. Br. J. Nutr. 81:59-64. [PubMed] [Google Scholar]
- 53.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, B. Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüβmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K.-H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machemüller, A. 2006. Medium-chain fatty acids and their potential to reduce methanogenesis in domestic ruminants. Agric. Ecosyst. Environ. 112:107-114. [Google Scholar]
- 55.Machmüller, A., and M. Kreuser. 1999. Methane suppression by coconut and associated effects on nutrient and energy balance in sheep. Can. J. Anim. Sci. 79:65-72. [Google Scholar]
- 56.Machmüller, A., D. A. Ossowski, M. Wanner, and M. Kreuser. 1998. Potential of various fatty feeds to reduce methane release from rumen fermentation in vitro (Rusitec). Anim. Feed Sci. Technol. 71:117-130. [Google Scholar]
- 57.Mackie, R. I., and M. P. Bryant. 1994. Acetogenesis and the rumen: syntrophic relationships, p. 331-364. In H. L. Drake (ed.), Acetogenesis. Chapman and Hall, New York, NY.
- 58.McGinn, S. M., K. A. Beauchemin, T. Coates, and D. Colombatto. 2004. Methane emissions from beef cattle: effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J. Anim. Sci. 82:3346-3356. [DOI] [PubMed] [Google Scholar]
- 59.Miller, T. L., and M. J. Wolin. 2001. Inhibition of growth of methane-producing bacteria of the ruminant forestomach by hydroxymethylglutaryl-SCoA reductase inhibitors. J. Dairy Sci. 84:1445-1448. [DOI] [PubMed] [Google Scholar]
- 60.Morvan, B. 1995. Ecologie et physiologie des microorganismes hydrogénotrophes des écosystèmes digestifs—étude particulière de l'écosystème ruminale. Ph.D. thesis. Université de Lyon, Lyon, France.
- 61.Morvan, B., F. Bonnemoy, G. Fonty, and P. Gouet. 1996. Quantitative determination of H2-utilizing acetogenic and sulfate-reducing bacteria and methanogenic archaea from digestive tract of different mammals. Curr. Microbiol. 32:129-133. [DOI] [PubMed] [Google Scholar]
- 62.Morvan, B., J. Doré, F. Rieu-Lesme, L. Foucat, G. Fonty, and P. Gouet. 1994. Establishment of hydrogen-utilizing bacteria in the rumen of the newborn lamb. FEMS Microbiol. Lett. 117:249-256. [DOI] [PubMed] [Google Scholar]
- 63.Moss, A. R., J. P. Jouany, and J. Newbold. 2000. Methane production by ruminants: its contribution to global warming. Ann. Zootech. 49:231-253. [Google Scholar]
- 64.Newbold, C. J., B. Lassalas, and J. P. Jouany. 1995. The importance of methanogens associated with ciliate protozoa in ruminal methane production in vitro. Lett. Appl. Microbiol. 21:230-234. [DOI] [PubMed] [Google Scholar]
- 65.Nollet, L., D. Demeyer, and W. Verstrate. 1997. Effect of 2-bromoethanesulfonic acid and Peptostreptococcus productus 35244 addition on stimulation of reductive acetogenesis in the ruminal ecosystem by selective inhibition of methanogenesis. Appl. Environ. Microbiol. 63:194-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nollet, L., L. Mbanzamihigo, D. Demeyer, and W. Verstrate. 1998. Effect of addition of Peptostreptococcus productus 35244 on reductive acetogenesis in the ruminal ecosystem after inhibition of methanogenesis by cell-free supernatant of Lactobacillus plantarum 80. Anim. Feed Sci. Technol. 41:49-66. [Google Scholar]
- 67.Puchala, R., B. R. Min, A. L. Goetsch, and T. Sahlu. 2005. The effect of condensed tannin-containing forage on methane emission by goats. J. Anim. Sci. 83:182-186. [DOI] [PubMed] [Google Scholar]
- 68.Rieu, F., G. Fonty, B. Gaillard, and P. Gouet. 1990. Electron microscopy study of the bacteria adherent to the rumen wall in young conventional lambs. Can. J. Microbiol. 36:140-144. [DOI] [PubMed] [Google Scholar]
- 69.Rieu, F., G. Fonty, and P. Gouet. 1989. Colony counts and characterization of bacteria adherent to the rumen wall and desquamated epithelial cells in conventional young lambs. Can. J. Microbiol. 35:698-705. [DOI] [PubMed] [Google Scholar]
- 70.Sanchez, J. M., L. Valle, F. Rodriguez, M. A. Morinnigo, and J. J. Borrego. 1996. Inhibition of methanogenesis by several heavy metals using pure cultures. Lett. Appl. Microbiol. 23:439-444. [Google Scholar]
- 71.Sauvant, D., J. P. Jouany, S. Giger-Reverdin, M. Vermorel, and G. Fonty. 1999. Production de CH4 par les ruminants: analyse des processus, quantification et modélisation, spatialisations et bilans, possibilités de réduction des émissions. C. R. Acad. Agric. Fr. 85:70-86. [Google Scholar]
- 72.Scheifinger, C. C., B. Linhan, and M. J. Wolin. 1975. H2 production by Selenomonas ruminantium in the presence and the absence of methanogenic bacteria. Appl. Microbiol. 29:480-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shink, B. 1994. Diversity, ecology and isolation of acetogenic bacteria, p. 197-235. In H. L. Drake (ed.), Acetogenesis. Chapman and Hall, New York, NY.
- 74.Stahl, D. A., B. Flesher, H. R. Mansfield, and L. Montgomery. 1988. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl. Environ. Microbiol. 54:1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tholen, A., and A. Brune. 1999. Localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.). Appl. Environ. Microbiol. 65:4497-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Nevel, C., and D. Demeyer. 1995. Feed additives and other interventions for decreasing methane emissions, p. 329-349. In R. J. Wallace and A. Chesson (ed.), Biotechnology in animal feeds and animal feeding. VCH, Weinheim, Germany.
- 77.Williams, A. G., K. N. Joblin, and G. Fonty. 1994. Interactions between the rumen chytrid fungi and other microorganisms, p. 191-227. In D. O. Mountfort and C. G Orpin (ed.), The anaerobic fungi. Marcel Dekker, New York, NY.
- 78.Wolin, M. J., T. L. Miller, and C. S. Stewart. 1997. Microbe-microbe interactions, p. 467-488. In P. N. Hobson and C. S. Stewart (ed.), The rumen microbial ecosystem. Chapman and Hall, London, United Kingdom.
- 79.Wright, A. D. G., P. Kennedy, C. J. O'Neill, A. F. Toovey, S. Popovski, S. M. Rea, C. L. Pimm, and L. Klein. 2004. Reducing methane emissions in sheep by immunization against rumen methanogens. Vaccine 22:3976-3985. [DOI] [PubMed] [Google Scholar]