Abstract
Pfiesteria spp. are mixotrophic armored dinoflagellates populating the Atlantic coastal waters of the United States. They have been a focus of intense research due to their reported association with several fish mortality events. We have now used a clonal culture of Pfiesteria piscicida and several new environmental isolates to describe growth characteristics, feeding, and factors contributing to the encystment and germination of the organism in both laboratory and environmental samples. We also discuss applied methods of detection of the different morphological forms of Pfiesteria in environmental samples. In summary, Pfiesteria, when grown with its algal prey, Rhodomonas sp., presents a typical growth curve with lag, exponential, and stationary phases, followed by encystment. The doubling time in exponential phase is about 12 h. The profiles of proliferation under a standard light cycle and in the dark were similar, although the peak cell densities were markedly lower when cells were grown in the dark. The addition of urea, chicken manure, and soil extracts did not enhance Pfiesteria proliferation, but crude unfiltered spent aquarium water did. Under conditions of food deprivation or cold (4°C), Pfiesteria readily formed harvestable cysts that were further analyzed by PCR and scanning electron microscopy. The germination of Pfiesteria cysts in environmental sediment was enhanced by the presence of live fish: dinospores could be detected 13 to 15 days earlier and reached 5- to 10-times-higher peak cell densities with live fish than with artificial seawater or f/2 medium alone. The addition of ammonia, urea, nitrate, phosphate, or surprisingly, spent fish aquarium water had no effect.
Pfiesteria piscicida is a mixotrophic dinoflagellate that has been the subject of intense research due to its presumed association with massive fish kills and a novel toxic exposure syndrome consisting of characteristic skin lesions, respiratory problems, and short-term memory loss (9, 32, 36, 40, 53). In the Chesapeake Bay region, the increased frequency of fish lesions, mass mortalities in several estuarine and marine fish species, and human health problems reported during the summer of 1997 led to intense media coverage and closures of public waterways to both commercial and recreational use (http://www.dnr.state.md.us/Bay/cblife/algae/dino/pfiesteria/hilltest.html). Because the fish kills and detrimental effects on human health observed in the Chesapeake Bay estuary closely resembled those reported in North Carolina and attributed to P. piscicida blooms (17, 64), it appeared that P. piscicida might be responsible for events in the bay (47).
Although the development of molecular approaches for the environmental detection of P. piscicida and related species (11, 21, 22, 50, 58, 61, 62, 68) has contributed significantly to the accurate assessment of their geographic distributions, the limited information on the environmental factors that may trigger P. piscicida blooms has made them difficult to anticipate. Earlier studies for the development and optimization of toxin bioassays for Pfiesteria spp., however, revealed highly dynamic P. piscicida populations in both the flask (60) and aquarium (23) assay formats, with fluctuating P. piscicida dinospore densities in the water column that were associated with fish deaths. For most dinoflagellates, both proliferation and transitions between life cycle stages may be modulated by physical and environmental conditions, such as nutrient availability, temperature, light exposure, dissolved oxygen content, and salinity (25, 42, 46, 55). However, for mixotrophic species like P. piscicida that employ both heterotrophy and predation, the availability of prey (31, 39, 44, 48, 54) and the effect of grazing by other dinoflagellates, protozoan ciliates, rotifers, and copepods (3, 13, 23, 31, 38, 41) may also be responsible for the fluctuations in cell densities. The life cycle of P. piscicida has been a matter of controversy. The initial description reported a complex cycle consisting of 24 stages, some of which, including several flagellated forms, smooth- and spiny-walled cysts, and amoebae that display either filose or lobose pseudopods, were characterized as toxic (14, 17). More recently, by using rigorous experimental approaches, a simpler life cycle of P. piscicida typical of free-living marine dinoflagellates has been described; it consists of asexual division and sexual reproduction involving dinospores, gametes, zygotes, and cysts (49).
Because it is presumed that dinoflagellate blooms originate from both the massive germination of cysts present in the benthos and the rapid proliferation of dinospores released or already present in the water column, further insight into the effect of environmental factors that may stimulate dinospore proliferation and life stage transitions is of considerable interest, enabling a greater understanding of harmful algal bloom events. In this study, we examined in vitro the potential effect(s) of selected biotic and abiotic factors on the encystment, cyst germination, and proliferation dynamics of P. piscicida dinospores and applied the information gained to study the effect of those factors on the emergence of P. piscicida dinospores from estuarine sediments known to contain P. piscicida cysts (30).
MATERIALS AND METHODS
Cultures.
Clonal P. piscicida isolated from Chicamacomico River, Maryland (1997), was a gift from K. A. Steidinger (Florida Institute of Oceanography, University of South Florida, St. Petersburg) (62). It was maintained in Guillard's f/2 medium (33) with 15-practical-salinity-unit (psu) artificial seawater (ASW [Instant Ocean; Aquarium Systems Inc., Mentor, OH]) at 23°C under a cycle of 14 h of light/10 h of darkness on the prey alga Rhodomonas sp. (CCMP768; Provasoli-Guillard National Center for Culture of Marine Phytoplankton [CCMP], West Boothbay Harbor, ME). Clonal Pfiesteria shumwayae was a gift from R. W. Litaker (NOAA Center for Coastal Fisheries and Habitat Research, Beaufort, NC). Dinoflagellate cultures CCMP429 (Karenia mikimotoi), CCMP448 (Heterocapsa triquetra), CCMP1368 (Prorocentrum lima), CCMP1589 (Prorocentrum micans), CCMP1593 (Akashiwo sanguinea), and CCMP1938 (Gymnodinium catenatum) were obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton.
Experimental fish.
Adult (30- to 60-day-old, 2- to 3-cm-long) and larval (1-day-old, 5- to 8-mm-long) sheepshead minnows (Cyprinodon variegatus) were obtained from Aquatic Biosystems, Inc. (Fort Collins, CO), and gradually acclimated to the salinity used for each experiment at pH 8.0 at 23°C for 1 week. Ten adult fish were maintained in 5 liters of ASW (salinity, 7 psu) in a 6-liter aquarium with a standard air pump (500 ml min−1) and fed TetraMin flakes (approximately one 1-g flake per 20 g of fish every other day; Tetra Sales, Blacksburg, VA) for 10 days, and the aquarium water was then collected and aliquoted. Aliquots were either stored untreated at −20°C, filtered through 0.2- or 0.4-μm-pore-size membranes (Millex PES; Millipore Co., Bedford, MA), or heated at 100°C for 15 min before use as test culture supplements. These aliquots are referred to hereinafter as spent aquarium water (SAW), filtered spent aquarium water (F-SAW), and heat-denatured spent aquarium water (HD-SAW).
Field sediment samples.
Two sediment samples (approximately 2 kg each) were collected from each of 10 drained ponds at the HyRock hybrid striped bass fish farm (Princess Anne, MD) (30; http://www.mdsg.umd.edu/programs/extension/Aquafarmer/Fall97/) during the winter of 2001. The wet sediment samples were stored in separate air-sealed plastic containers in the dark at 4°C until analysis.
Genomic DNA, total RNA extraction, and first-strand cDNA synthesis.
Genomic DNA from dinoflagellates (P. piscicida, K. mikimotoi, H. triquetra, Prorocentrum lima, Prorocentrum micans, A. sanguinea, and G. catenatum) and algal prey (Rhodomonas sp.) was extracted from cultured cells harvested during stationary phase by following the procedure of Saito et al. (62) or by using the FastDNA kit for soil according to the instructions of the manufacturer (Qbiogene, Inc., Irvine, CA). Genomic DNA was stored at −20°C until use. For RNA extraction, 200 μl of RNAlater (QIAGEN, Inc., Valencia, CA) was immediately added to the cells upon harvesting and the suspension was stored at −80°C. RNA was extracted from the cell pellet with the RNeasy mini kit (QIAGEN, Inc.) by following the manufacturer's protocol for yeast. The synthesis of full-length first-strand cDNA was catalyzed by SuperScript II reverse transcriptase (RT) with the SuperScript II kit for first-strand synthesis for RT-PCR (Invitrogen Co., Carlsbad, CA) by using 1 μg of total RNA and oligo(dT)12-16.
Detection and assessment of P. piscicida by PCR-based QC assay.
The quantitative competitive PCR (QC-PCR) assay for P. piscicida reported elsewhere (62) was used for the detection and assessment of the density of P. piscicida cells in this study. After the separation and visualization of QC-PCR products by 1.5% agarose gel electrophoresis, the bands corresponding to the target and the competitor were scanned with a FluorImager 575 (Molecular Dynamics, Sunnyvale, CA) and the intensity was measured with the NIH Image 1.62 software (Research Services Branch, NIMH, NIH, Bethesda, MD).
PCR for P. piscicida and Rhodomonas sp. actin gene.
P. piscicida and Rhodomonas sp. actin gene-specific primers were designed based on the RT-PCR products (≅1 kb) obtained as indicated above (see Fig. S1 in the supplemental material): PpActin-F (forward), 5′-CGGCCGCCCGAAGATGCCCGGCA-3′; PpActin-R (reverse), 5′-TCTCCTTCTCGTTGCTCTCC-3′; RhActin-F (forward), 5′-CCGTGCTGTCTTCCCTTCC-3′; and RhActin-R (reverse), 5′-CGAAGAAGACGAAGCGGCGGTC-3′. These primers amplified products of approximately 600 bp (see Fig. S2 in the supplemental material) with the following protocol: denaturation at 94°C for 4 min; 10 cycles of 94°C for 1 min, 55°C for 40 s, and 72°C for 1 min; 10 cycles of 94°C for 1 min, 53°C for 40 s, and 72°C for 1 min; 10 cycles of 94°C for 1 min, 50°C for 40 s, and 72°C for 1 min; and final extension at 72°C for 10 min.
RT-PCR for P. piscicida and Rhodomonas GAPDH gene.
Rhodomonas sp. (CCMP768) cytosolic and chloroplastic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequences were obtained by PCR amplification of Rhodomonas cDNA by using forward primer dGAPDH-F1 (5′-GTIGGIATEAAYGGITTYGGIMGIATEGG-3′; E is deoxyguanidine-phosphorothioate) and reverse primer dGAPDH-R4 (5′-TAICCIMAYTCRTTRTCRTACCAISWIAC-3′). PCR conditions were denaturation at 94°C for 5 min; 35 cycles of 94°C for 40 s, 45°C for 40 s, and 72°C for 1 min; and extension at 72°C for 10 min. PCR products were cloned into the pGEM-T vector (Promega Co.) and sequenced. Two identical GAPDH clones from eight sequenced clones were very similar to chloroplastic GAPDH genes identified by BLAST searching, and others revealed a cytosolic GAPDH motif sequence and overall homology to cytosolic GAPDH by BLAST searching. By using the Rhodomonas chloroplastic GAPDH gene sequence, specific PCR primers RhGAPDH-F (forward; 5′-GGCTGTCGTTGACTCCTCGTCCC-3′) and RhGAPDH-R (reverse; CCGCAGCGCGCTTCTTCACC) were designed to amplify a 248-bp product from Rhodomonas cDNA (see Fig. S3 in the supplemental material). RT-PCR was performed under the following conditions: denaturation at 94°C for 4 min; 15 cycles of 94°C for 1 min, 65°C for 40 s, and 72°C for 40 s; 20 cycles of 94°C for 1 min, 60°C for 40 s, and 72°C for 40 s; and extension at 72°C for 7 min.
Characterization of in vitro proliferation of P. piscicida.
The in vitro proliferation profiles of P. piscicida were comparatively assessed in different culture formats, including 24- and 6-well plates (BD Biosciences, San Jose, CA), 10- and 20-cm petri dishes (Fisher Scientific, Pittsburgh, PA), and 25-, 250-, and 750-ml vented culture flasks (Corning, Inc., Corning, NY). The culture volumes tested were 1 ml/well in the 24-well plates (n = 48), 5 ml/well in the 6-well plates (n = 12), 20 ml in the 10-cm dishes (n = 3), 50 ml in the 20-cm dishes (n = 3), 20 ml in the 25-ml flasks (n = 3), 200 ml in the 250-ml flasks (n = 3), and 450 ml in the 750-ml flasks (n = 3). Culture flasks were maintained in either an upright or a horizontal position. For all cultures, Guillard's f/2 medium at a salinity of 15 psu was inoculated with 103 P. piscicida cells ml−1 and 1.9 × 105 Rhodomonas cells ml−1 and the cultures were maintained under a cycle of 14 h of light/10 h of darkness at 23°C. Plates were covered with plastic film to prevent evaporation during the experiment. Samples (500 μl; n = 3) were taken daily for the assessment of cell density by counting the P. piscicida dinospores fixed in 4% (wt/vol) formaldehyde in phosphate-buffered saline (PBS; 10 mM phosphate buffer, pH 7.4, containing 150 mM NaCl) in a hemocytometer. Unless indicated otherwise, for all subsequent experiments described below, P. piscicida (103 cells ml−1) was cultured in 175-cm2 vented culture flasks in f/2 medium (salinity, 15 psu; 500 ml) with Rhodomonas sp. (2.2 × 106 cells ml−1) and maintained under the light and temperature regime described above for14 or 28 days, without additional prey inoculation. Culture samples were collected daily, and cell densities were assessed by counting as indicated above. The nuclei of fixed cells were stained by incubation with DAPI (4′,6′-diamidino-2-phenylindole; 100 μg ml−1) in 2.5% (wt/vol) glutaraldehyde-PBS (DAPI/sample ratio, 1:10) for 5 min. Cells were washed three times with PBS, and the number of nuclei per single cell was assessed by fluorescence microscopy (34). The sizes of P. piscicida dinospores in culture were determined from microscopy photographs (at a magnification of ×250; n ≥ 3) of the above-mentioned samples up to day 8. For selected experiments and some particular cases in which the cell counts were very low, the samples were also tested by actin and GAPDH gene-specific PCR.
Effects of light period and exogenous biotic and abiotic factors on in vitro proliferation of P. piscicida.
To examine the potential effect of light regimes on the in vitro proliferation of P. piscicida, cultures (500 ml) seeded with P. piscicida (103 cells ml−1) and Rhodomonas (3 × 104 cells ml−1) in f/2 medium (salinity, 15 psu) in 750-ml culture flasks were maintained at 23°C with either 14 h of light/10 h of darkness (n = 3) or 24 h of darkness (n = 3). Samples (1 ml for cell density assessment and 15 ml for RNA extraction) were collected daily. P. piscicida and Rhodomonas sp. cells were counted in a hemocytometer after fixation with buffered formaldehyde as described above. To examine the potential effects of soil, chicken manure, and fish-derived factors on the in vitro proliferation of P. piscicida, 1 ml of soil or chicken manure extract, SAW, F-SAW, HD-SAW, or fresh f/2 medium was added to the stationary-phase P. piscicida culture (1 ml; 3 × 104 cells ml−1) on 24-well culture plates (BD Biosciences) and the plates were incubated under standard conditions. Culture samples (about 2 ml well−1; n = 3) were collected for cell counting on days 0, 7, 14, and 21. Subsequent experiments were scaled up by adding SAW or ASW (250 ml) to 250-ml culture samples (three each for SAW and ASW) in flasks inoculated with 2.0 × 103 P. piscicida dinospores and 3.8 × 104 Rhodomonas sp. cells ml−1, and the flasks were maintained under the conditions described above. Samples (1 ml) were taken from each flask after gently inverting to mix on days 0.5, 1, 2, 3, 4, 5, 6, and 7, and the cell densities were determined.
In vitro encystment and excystment of P. piscicida.
In preliminary encystment experiments, a P. piscicida stationary-phase culture (average cell density of 2.5 × 104 cells ml−1 in f/2 medium at a salinity of 15 psu) was seeded into 24-well plates (1 ml well−1) and the plates were incubated in the dark at 4°C for various time periods (1, 2, 3, 4, 5, 6, 7, and 8 weeks). The decrease in cell numbers in the culture supernatant was monitored by cell counting (n, 4 replicates) on days 1, 2, 3, 5, 7, 14, and 21. In subsequent experiments, to assess P. piscicida encystment and excystment time course profiles, nine 30-ml samples of stationary-phase culture (3.2 × 104 cells ml−1 with Rhodomonas present at <1% in f/2 medium at a salinity of 15 psu) were maintained in the dark at 4°C without additional algal prey until 100% excystment was observed under the microscope (3 weeks). During the encystment period, six of the nine culture flasks were sampled as follows: (i) to assess the decrease of P. piscicida dinospores in the culture medium on a daily basis, flasks (n = 3) were gently mixed by inversion, a 1-ml sample was obtained, and cells were fixed immediately for counting; (ii) to assess the number of P. piscicida cysts, flasks (n = 3) were carefully drained of culture supernatant, the cysts were removed from the flask surface by harsh shaking with ASW (30 ml; salinity, 15 psu) three times, and the cells were collected by centrifugation (1,000 × g for 15 min at 4°C) and resuspended in ASW (1 ml) for counting (without fixation). No cysts were observed in the removed culture supernatant. After the cysts were enumerated, they were disrupted by using glass beads, the DNA was extracted, and the number of ribosomal DNA (rDNA) copies per cyst was assessed by QC-PCR according to the method of Saito et al. (62). The remaining three flasks with encysted P. piscicida dinospores were incubated at 23°C under a cycle of 14 h of light/10 h of darkness, the excysted P. piscicida dinospores were collected by the removal of culture supernatant medium from flasks by gentle decantation at each time point (0, 3, 9, 12, 15, 18, 21, 24, 36, and 48 h), and the cells were fixed for counting. The culture supernatant removed was immediately replaced by fresh f/2 medium (30 ml; salinity, 15 psu).
SEM analysis of P. piscicida cysts.
The encystment of P. piscicida dinospores was induced as described above in 24-well plates with round microscope glass coverslips (12 mm in diameter; Fisher Scientific) covering the bottoms of the wells. Encystment was monitored to completion under a light microscope, and the coverslips with attached P. piscicida cysts were carefully removed from the culture plates. P. piscicida cysts on coverslips were fixed with 2% glutaraldehyde in Millonig's buffer (pH 7.2) (56), followed by postfixation with 2% osmium tetroxide in Millonig's buffer. Dehydration was performed via sequential transfer through 75 to 100% ethanol (vol/vol). Desiccation was carried out using the standard critical-point drying method with liquid CO2. Fixed and dried cysts were sputter coated with 60% gold and 40% palladium with a thickness of 25 nm, and the coverslips were mounted directly onto stubs for scanning electron microscopy (SEM) analysis (7).
Effect(s) of environmental factor(s) on in vitro encystment and cyst germination of P. piscicida.
To examine the effect of salinity on the in vitro encystment of P. piscicida, stationary-phase cultures (average of 2.5 × 104 cells ml−1 and 1 ml well−1) in f/2 medium at salinities of 1, 3, 5, 7, 15, and 32 psu were seeded into 24-well plates (BD Scientific). The encystment of P. piscicida dinospores was monitored by cell counting (n, 4 replicates) on days 1, 2, 3, 7, 14, and 21. Cyst germination in the encysted cultures was achieved by incubating the plates at 23°C under a standard light cycle (see above) for 7 days, and the dinospore cell numbers in the culture supernatant were assessed (n, 4 replicates) on days 0.5, 1, 2, 3, 4, 5, 6, and 7. To examine the potential effect of desiccation on P. piscicida cysts, 2 days after encystment was complete the culture medium was removed and the plates were maintained in the dark at 4°C for 1, 3, 5, and 7 days. Cyst germination was induced at two different incubation temperatures (13 and 23°C), and the dinospore cell numbers in the culture supernatants were assessed by cell counting, as indicated above. To examine the potential effects of selected factors on the in vitro germination of P. piscicida cysts, encysted cultures (1 ml) were incubated with ammonia, urea, nitrate, and phosphate (final concentrations of 100 nM), SAW (1 ml well−1), and live fish larvae (1 larva well−1). To examine the potential effects of anoxia on in vitro encystment and cyst germination of P. piscicida dinospores, encystment and germination were carried out by the procedures indicated above under anaerobic conditions with the AnaeroPouch system (REMEL, Inc., Lenexa, KS). Encystment and germination profiles were assessed by cell counting, as indicated above, and compared with those of control cultures that were encysted and germinated under the same experimental conditions, as described above.
Optimization of QC-PCR detection of P. piscicida cysts in environmental sediments by spiking-recovery experiments.
In vitro-induced P. piscicida cysts (3 × 103 cells) were spiked into 0.05 g of autoclaved sediment. DNA was extracted from the sediments containing cells with the FastDNA spin kit for soil by following the instructions of the manufacturer (Qbiogene, Inc.) with three additional washes on the DNA-binding matrix before DNA elution. DNA corresponding to 300 P. piscicida cysts was used for QC-PCR (n, 3 replicates) (62).
The mounting medium (1 mg of p-phenylene diamine ml−1 of 90% glycerol in PBS) containing DAPI (100 μg ml−1) was used to visualize nuclei (excitation at 360 nm and emission at 460 nm).
Detection of P. piscicida in environmental sediments.
DNA was extracted from (i) drained pond sediment (0.1 g [wet weight]) suspended in Tris-EDTA (TE) and boiled; (ii) drained pond sediment (0.1 g [wet weight]) subjected to extraction with the FastDNA spin kit for soil (Qbiogene, Inc.) as described above; (iii) the supernatant (15 ml) of a sediment suspension in ASW (10 ml in 50 ml) collected after the sediment was allowed to settle for 10 min at room temperature; (iv) the supernatant (15 ml) of a sediment suspension in f/2 medium (salinity, 7 psu; 10 ml in 50 ml) incubated at 23°C for 7 days under a standard light cycle, and (v) the supernatant (15 ml) of a sediment suspension in ASW (salinity, 7 psu; 10 ml in 50 ml) incubated as for sample iv but in the presence of one adult fish. For all supernatants (samples iii to v), DNA was extracted from the pellet obtained by centrifugation (1,000 × g for 15 min at room temperature) with the kit for soil (Qbiogene, Inc.) as described above. PCR amplification specific for P. piscicida was carried out as described above by following the procedure of Saito et al. (62).
Effect of biotic factors on the cyst germination and dinospore proliferation of P. piscicida and PLDs from environmental sediment.
The sediment slurry (an approximately 10-ml volume of wet sediment in a 50-ml total volume of ASW) was inoculated into flasks containing 450 ml of (i) ASW; (ii) ASW with a live adult fish; (iii) f/2 medium; (iv) ASW with urea (25 mM); (v) ASW with chicken manure extract (0.8 μg of protein ml−1); (vi) ASW with fish extract (0.8 μg of protein ml−1); and (vii) ASW with soil extract. All flasks were maintained under standard culture conditions (23°C under a cycle of 14 h of light/10 h of darkness; salinity, 15 psu) for 30 days. Flask supernatants were sampled (1 ml for counting and 15 ml for DNA extraction) on days 0, 0.5, 1, 2, 3, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 27, and 29. Cell densities of Pfiesteria-like dinoflagellates (PLDs; dinospores morphologically similar to P. piscicida under the microscope that test negative by the P. piscicida-specific PCR assay) were determined by hemocytometer cell counts, and P. piscicida cell numbers were assessed by QC-PCR, as described above.
RESULTS
Characterization of P. piscicida dinospore proliferation in vitro.
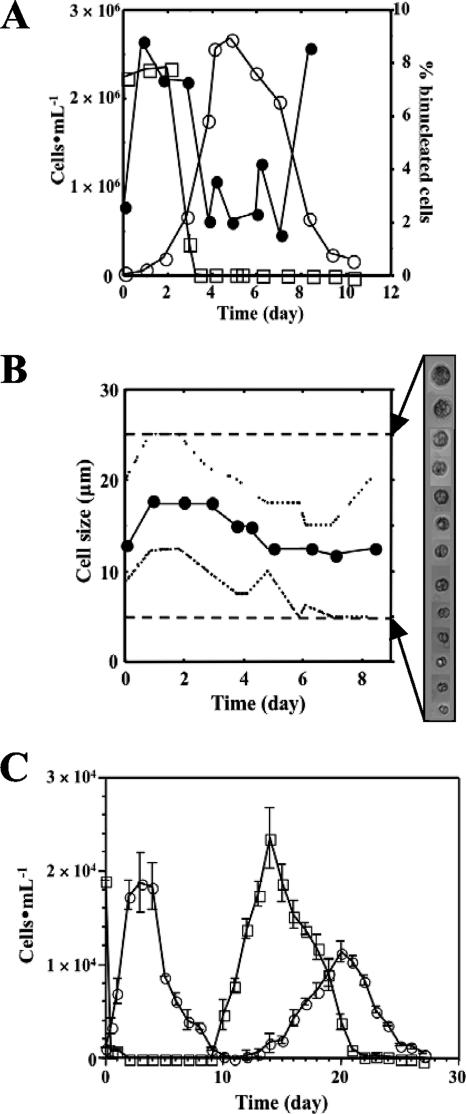
Preliminary studies on the culture of P. piscicida in containers with distinct geometries, including 6- and 24-well plastic plates, petri dishes, and tissue culture flasks, revealed that these containers affected only the length of the lag phase of the growth curve, without any impact on the P. piscicida stationary-phase cell densities (data not shown). It was noteworthy that although the upright-standing 250- and 750-ml flasks yielded considerably longer lag phases (8 to 10 days) than all other formats (3 to 5 days), all cultures reached equivalent cell densities at the stationary phase. The characterization of growth curves for both P. piscicida and its algal prey (Rhodomonas sp.) was carried out using 500-ml horizontal culture flasks (Fig. 1). The growth curves obtained by cell counting over a period of about 2 weeks were typical, consisting of lag, exponential, stationary, and decline phases (Fig. 1A). In these culture flasks, the lag phase was relatively short (up to 48 h) and maximal cell densities were reached at about 5 to 7 days. The doubling time for P. piscicida during the exponential phase of growth under the culture conditions established was approximately 12 h. During this phase, a variety of motile forms, including dinospores, gametes, and/or planozygotes, and multiplying forms (binucleated dinospores, gametes, and/or planozygotes) were regularly observed. As the P. piscicida culture cell density increased, the Rhodomonas cell numbers gradually decreased. When P. piscicida reached the stationary phase, in about 5 to 6 days (cell densities were 1 × 105 to 5 × 105 cells ml−1), the cultures were nearly depleted of Rhodomonas. The decline of P. piscicida cell density after the stationary phase was rapid, and cannibalism among P. piscicida dinospores was often observed. The cell size distribution for P. piscicida in culture was in the range of 5 to 25 μm (Fig. 1B). Larger P. piscicida dinospores were observed 1 to 3 days after the culture was inoculated and coincided with the peak of the algal cell densities. At the end of this period, the level of binucleated cells reached the highest value (Fig. 1A). About 10 to 12 days after the culture inoculation, which typically coincided with the end of the decline phase, the culture was virtually depleted of algal prey and large, round, nonmotile cells, most likely P. piscicida resting cysts, were observed on the bottoms of culture flasks. If the culture was continued beyond the decline phase, a second curve of Rhodomonas proliferation that peaked at approximately day 14 to 15 was observed, followed closely by a similar P. piscicida proliferation curve with a maximal cell density reached at 22 to 24 days (Fig. 1C). The P. piscicida-Rhodomonas proliferation cycle could be continued for a third round, although the peak cell densities of both P. piscicida and Rhodomonas decreased with each cycle, as follows: first proliferation cycle > second proliferation cycle > third proliferation cycle (data not shown).
FIG. 1.
Characterization of P. piscicida on algal prey in vitro. (A) Proliferation profile (○) and percentages of binucleated cells (•) for P. piscicida dinospores maintained on Rhodomonas (□) in f/2 medium at 23°C under a cycle of 14 h of light/10 h of darkness. Cells were counted in a hemocytometer after fixation with 4% (wt/vol) formaldehyde in PBS or staining with DAPI (10 μg ml−1) in PBS containing 2.5% (wt/vol) glutaraldehyde as the final concentration. (B) Size distribution of P. piscicida dinospores in standard culture. Shown are the ranges (broken lines) and averages (circles) of P. piscicida dinospore sizes throughout the 8 days of cultivation on Rhodomonas. Cell sizes were measured on digital microscope images (n, 50 cells per sampling). Dashed lines represent the upper and lower limits of detection. (C) Secondary wave of P. piscicida dinospore proliferation in vitro. Cultures were continuously maintained at 23°C with 14 h of light/10 h of darkness over 28 days without additional prey and fresh medium, and P. piscicida (○) and Rhodomonas (□) cell densities were assessed daily (error bars, standard deviations; n, 3 replicate experiments).
Effects of light period and exogenous biotic and abiotic factors on the in vitro proliferation of P. piscicida.
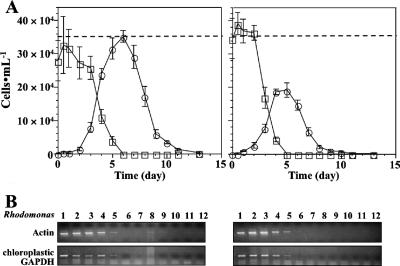
The proliferation profiles of P. piscicida cultured on Rhodomonas at 23°C under a standard light cycle (14 h of light/10 h of darkness) and under 24 h of darkness were compared (Fig. 2). The proliferation profiles of P. piscicida in the dark were similar to those under the standard light cycle, which included the typical lag, exponential, stationary, and decline phases, and the profiles showed similar proliferation and decline rates. However, the P. piscicida peak cell densities reached at the stationary phase were significantly lower in the dark (1.9 × 105 cells ml−1) than under the standard light cycle (3.5 × 105 cells ml−1). In addition, the depletion of Rhodomonas from the culture was slightly faster in the dark, starting at day 3 and reaching the detection limit (<5 × 102 cells ml−1) at day 5 (Fig. 2A). When the cell density of Rhodomonas was below the limit of detection by microscopy, to assess the presence of Rhodomonas in the culture we used species-specific PCR assays for Rhodomonas actin and chloroplastic GAPDH (Fig. 2B) (PCR-based assay detection limits: 10 pg of Rhodomonas genomic DNA for both actin and chloroplastic GAPDH PCR; for the specificity validation, see Fig. S2 in the supplemental material). The results of assays for remaining Rhodomonas cells in the P. piscicida cultures were similar for both cultures, under light and darkness. The signals of both cytosolic and chloroplastic Rhodomonas marker genes detected by RT-PCR gradually decreased in intensity from day 1 to day 6, in parallel with the cell counts, and became undetectable after day 7 (Fig. 2B).
FIG. 2.
Effects of light on proliferation of Pfiesteria spp. on algal prey. (A) Proliferation of P. piscicida and depletion of Rhodomonas with and without light. Cell densities of P. piscicida (○) and Rhodomonas (□) exposed to 14 h of light/10 h of darkness (left panel) and 24 h of darkness (right panel) were determined by counting (error bars, standard deviations; n, 3 replicate experiments). Dashed lines indicate the highest cell density of P. piscicida with light cycle. (B) Detection of Rhodomonas in culture by a specific PCR assay for Rhodomonas actin and chloroplastic GAPDH DNA. Samples were collected every day for the extraction of RNA and the synthesis of cDNA.
The biotic and abiotic supplements (urea, chicken manure, and soil extracts) added to the f/2 medium did not result in the enhancement of either P. piscicida or Rhodomonas proliferation compared to the proliferation in the f/2 medium control (data not shown). The presence of fish-derived factors in SAW, however, resulted in a considerable enhancement of P. piscicida proliferation either in the presence or in the absence of algal prey (Fig. 3). The effects of SAW on P. piscicida dinospores were analyzed in vitro by monitoring the survival of P. piscicida without additional algal prey after stationary phase and its proliferation on algal prey. The P. piscicida dinospores could survive and proliferate without algal prey in the presence of SAW but gradually disappeared from the culture when maintained in ASW or F-SAW (Fig. 3A). Interestingly, in the presence of Rhodomonas, P. piscicida proliferation rates during the exponential phase were higher in the presence of SAW (doubling time, 6 h) than in the presence of ASW or f/2 medium (doubling time, 12 h) (Fig. 3B). Depletion rates of Rhodomonas in ASW and SAW, however, were similar. In the presence of a fish-derived factor(s) in SAW, P. piscicida proliferated faster and the cell density declined rapidly after reaching the peak, with virtually no stationary phase (Fig. 3C). When the SAW was filtered through the 0.2- or 0.4-μm-pore-size membrane (F-SAW) or heated at 100°C (HD-SAW), its effects on P. piscicida proliferation were lost (data not shown).
FIG. 3.
Effect(s) of fish-derived factor(s) on proliferation of P. piscicida dinospores in vitro. (A) Survival of P. piscicida dinospores without prey algae. The stationary-phase P. piscicida culture was maintained without additional prey and fresh medium for an extended 21 days with the supplement of ASW (striped bars), SAW (dotted bars), or F-SAW filtered through a 0.2-μm-pore-size membrane (white bars). Dinospore cell densities were counted on days 0, 7, 14, and 21 after the supplemental inoculation (n, 4 replicates). (B and C) Proliferation of P. piscicida dinospores with and without fish-derived factor(s). SAW or ASW (250 ml) was mixed with the culture (250 ml) of P. piscicida on Rhodomonas (at final densities of 1.0 × 103 P. piscicida cells and 1.9 × 104 Rhodomonas cells ml−1) and the culture was maintained under the standard conditions (error bars, standard deviations; n, 3 replicate experiments). Cell densities of P. piscicida (○ and •) and prey Rhodomonas (□ and▪) were counted. Symbols correspond to fish-derived factor(s) (○ and □) and ASW (• and ▪). Panel B is focused on the growth rates of P. piscicida during the exponential phase from panel C.
In vitro experimentally induced encystment and cyst germination of P. piscicida.
The initial studies of the in vitro encystment of P. piscicida in a small-scale plate format at 4°C under 24 h of darkness revealed 100% encystment within 3 weeks. Longer periods of incubation at 4°C, beyond 3 weeks, reduced the yield of excysted cells (data not shown). Scaling up to 500-ml cultures enabled a more rigorous quantitative assessment of the encystment progression (Fig. 4A). As the cultures were brought to 4°C in the dark, the number of motile P. piscicida dinospores gradually decreased, and after 2 to 3 weeks of incubation, only nonmotile cells attached on the bottom surface of the culture flask were observed (Fig. 4A, inset). The SEM analysis of the in vitro-induced cysts revealed round cells of about 10 to 15 μm in diameter, frequently with attached bacteria on the surface. The P. piscicida cysts could be harvested from the culture flask by harsh shaking with culture medium and counted in a hemocytometer without the need of fixation. Approximately 5 to 10% of the total number of P. piscicida dinospores in the original culture (30 ml; 3.2 × 104 cells ml−1; n = 3) could be recovered as cysts (average total of 6 × 104 cysts) (data not shown). The induction of P. piscicida cyst germination in vitro was achieved by incubating the encysted cultures at 23°C under the standard light cycle. P. piscicida cyst germination profiles (Fig. 4B), as assessed by counting the motile cells in the supernatant culture medium, showed a gradual increase of the cell density, with more than 98% of P. piscicida cells emerging within 24 h, and the maximal density of emerged cells was recorded within 9 to 18 h of incubation (Fig. 4B, inset). The emerged P. piscicida dinospores corresponded to about 10% of the cysts present in the culture. No further cyst germination was observed after 24 h during an additional 10-day incubation period (data not shown). The cysts tested positive for P. piscicida by the species-specific PCR (62). The assessment of rDNA copy numbers in P. piscicida cysts (115 cells; n, 3 replicates) by QC-PCR using a competitor plasmid resulted in ∼100 copies per cell (20 to 40 copies/pg of DNA) (Fig. 5A).
FIG. 4.
Encystment and excystment of P. piscicida cells in vitro. (A) Encystment of P. piscicida in vitro. The stationary-phase P. piscicida dinospore culture was placed at 4°C in darkness for 28 days. Cells in the water column were counted during encystment (n = 3 replicates). The inset is a SEM image of P. piscicida cysts yielded by in vitro culture under these conditions. (B) Excystment of P. piscicida in vitro. A culture of encysted P. piscicida cells was incubated at 23°C under a standard light cycle for monitoring the emergence of dinospores from cysts (error bars, standard deviations; n, 3 replicate experiments). Densities of dinospores emerging between sampling time points are shown in the inset.
FIG. 5.
Assessment of rDNA copy numbers in in vitro-induced P. piscicida cysts. DNA was extracted from P. piscicida cysts collected from cultures of encysted cells without (A) and with (B) the presence of autoclaved sediment. The assessment of rDNA copy numbers was performed by species-specific QC-PCR for P. piscicida (62). rfu, relative fluorescence units.
The potential effects of salinity, temperature, medium supplements, exposure to dryness, and anoxia on in vitro encystment and cyst germination of P. piscicida were evaluated. The numbers of encysting dinospores were higher at lower salinities (complete encystment within 1 week at 1 psu and within 3 weeks at >15 psu), whereas higher salinities resulted in higher numbers of germinating dinospores (8-, 10-, and 13-fold-higher numbers at 7, 15, and 32 psu, respectively, than at 1 to 3 psu). Further, incubation at 23°C resulted in a yield from cyst germination about threefold higher than that from incubation at 13°C at various salinities (3 to 32 psu). In addition, maintaining cysts under dry conditions for 2 days at 4°C negatively affected the yield of germination. The presence of live fish increased cyst germination rates and total numbers of dinospores about fivefold over those of the f/2 medium controls. Culture supplements, such as ammonia, urea, nitrate, and phosphate, which may be released by fish into SAW, had no effect on P. piscicida cyst germination profiles. Similarly, fish extracts or SAW failed to increase cyst germination rates or the total number of released dinospores. The effects of anoxia on P. piscicida encystment or cyst germination were moderate at best. If P. piscicida was encysted at 4°C for 3 weeks under anaerobic conditions, cyst germination in an aerobic environment occurred slightly earlier. Otherwise, no differences in cyst germination rates or total yields of released dinospores under aerobic and anaerobic conditions were observed. Results from experiments aimed at the differential detection of actin and tubulin in cysts and dinospores suggest that tubulin detection may constitute a useful marker for distinguishing cysts from interstitial dinospores in environmental sediments (see Fig. S4 in the supplemental material).
Effect of biotic and abiotic factors on the detection of P. piscicida dinospores and cysts in estuarine sediments.
The performance of the QC-PCR assessment of P. piscicida cysts in environmental sediments was evaluated and optimized by spiking-recovery experiments, in which in vitro-produced P. piscicida cysts were inoculated into autoclaved sediment (300 cysts into a 0.05-g [wet weight] sediment sample) in the DNA extraction vials of the soil kit (Qbiogene, Inc.). DNA was extracted efficiently and reproducibly, as reported elsewhere (62), and the QC-PCR results (n, 3 replicate experiments) revealed approximately 100 copies of rDNA per cyst (Fig. 5B).
The presence of P. piscicida in environmental sediments from 10 drained ponds from a fish farm was detected by the PCR assay optimized as described above (Fig. 6). The application of the P. piscicida-specific PCR assay to DNA extracted directly from 0.1 g (wet weight) of sediment, whether extracted directly from a suspension in TE (Fig. 6A) or with the above-mentioned commercial kit for soil (Fig. 6B), yielded negative results for P. piscicida, as did the DNA extracted from the supernatant of a suspension of 10 ml of wet sediment in 50 ml of ASW allowed to sit for 10 min at room temperature (Fig. 6C). To overcome the potential inhibition of the PCR amplification reaction by factors extracted with the DNA, the samples were diluted up to 1,000-fold, but all DNA samples extracted by the three different procedures outlined above still tested negative (data not shown). When the sediment was suspended in f/2 medium (10 ml of wet sediment in 50 ml of f/2 medium) and the suspensions were incubated at 23°C for 7 days, P. piscicida could be detected in 5 of the 10 sediment sample supernatants (Fig. 6D). If a live fish was present in the suspensions incubated at 23°C, P. piscicida was detected by PCR in all 10 sediment samples within 7 days (Fig. 6E).
FIG. 6.
Detection of P. piscicida in sediments collected from fish farm ponds. DNA was extracted from 0.1 g (wet weight) of sediment boiled with TE (A), 0.1 g (wet weight) of sediment subjected to extraction with the BIO101 FastDNA spin kit for soil (B), the supernatant (15 ml) of a suspension (50 ml) of sediment (10 ml) in ASW (C), the supernatant of a suspension (the same quantities as for panel C) in f/2 medium after incubation at 23°C for 1 week (D), and the supernatant of a suspension (the same quantities as for panel C) in ASW with an adult fish after incubation at 23°C for 1 week (E). Sample numbers correspond to the sediments collected from different fish farming ponds (1 to 10). Positive and negative controls are indicated.
Based on the observation that sediment samples from all 10 ponds examined contained P. piscicida, two of these samples (sediment samples from ponds 1 and 10) were selected to examine in further detail the potential effects of biotic and abiotic factors on the cyst germination and dinospore proliferation of P. piscicida and PLDs in the course of a 29-day incubation in either ASW, ASW with live fish, or f/2 culture medium. Although both sediment samples yielded PLDs in the supernatant (as assessed by light microscopy) (Fig. 7A) and P. piscicida dinospores (as determined by a specific PCR assay) (Fig. 7B) in the presence of fish, critical differences became apparent. The numbers of PLDs that emerged from sediment 1 were relatively low, even in the presence of fish, with a peak cell density at about day 19 (Fig. 7A, left panel). The P. piscicida-specific PCR assay revealed that P. piscicida emerged from sediment 1 during the second week of incubation in the presence of live fish, with a peak cell density between days 11 and 13 (Fig. 7B, left panel). A similar PCR profile was observed for sediment 10 incubated in the presence of fish, but dinospores emerged earlier (first positive sample at day 3, with the highest cell density at day 9) (Fig. 7B, right panel). In contrast to sediment 1, however, sediment 10 yielded P. piscicida-positive supernatants when incubated without fish but did so substantially later, with clear positives appearing at the end of the second week and with maximal PCR signals between the end of the third and fourth weeks, coinciding with the PLD cell density peak (Fig. 7A and B, left panels). The addition of a culture supplement (ammonia, urea, or chicken manure) did not significantly affect the PLD cell densities or the Pfiesteria PCR profiles for the supernatants (data not shown). A QC-PCR analysis of the samples obtained from sediment 10 confirmed much earlier cyst germination by P. piscicida in the presence of fish, with the supernatant cell density reaching the peak at day 9 to 10 and the peak being 5 to 10 times higher than that in ASW or f/2 medium alone (Fig. 7C). Additionally, as an alternative method to distinguish P. piscicida and PLDs, we compared lectin staining patterns on whole cells of several species. The α-mannose-binding lectin from Galanthus nivalis bound to both Pfiesteria spp., while the β-galactosyl-binding lectin from Arachis hypogaea recognized P. shumwayae but not P. piscicida (see Fig. S5 in the supplemental material).
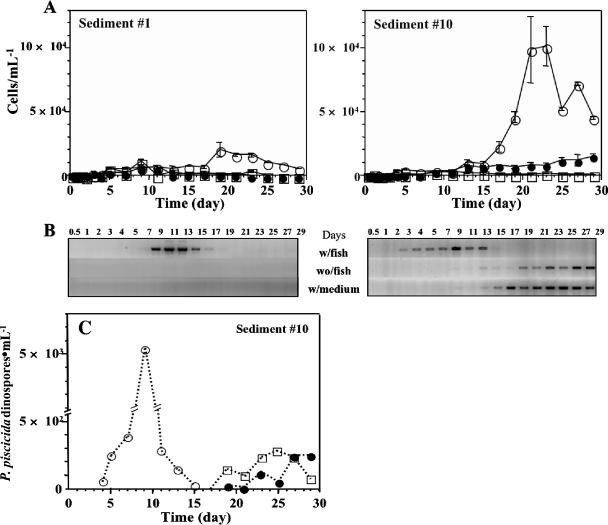
FIG. 7.
Excystment and proliferation of cells of P. piscicida and the Pfiesteria-like complex from environmental sediments. The sediment from two different fish ponds (samples 1 [left panel] and 10 [right panel], corresponding to the samples in Fig. 6) in 50-ml suspensions was incubated in flasks with total volumes of 450 ml under various conditions: (i) with ASW and an adult fish (○; w/fish), (ii) with ASW alone (•; wo/fish), and (iii) with f/2 medium (□; w/medium). Throughout 29 days of incubation at 23°C under a standard light cycle, water samples were collected for counting (1 ml) and PCR detection (15 ml). (A) Densities of emerged P. piscicida-like complex cells in the water column (error bars, standard deviations; n, 3 replicate experiments). (B) Detection of emerged P. piscicida cells by species-specific PCR. (C) QC-PCR assessment of densities of P. piscicida cells emerging from environmental sediment sample 10. The emergence and proliferation of P. piscicida cells in the presence of ASW and an adult fish (○), ASW only (•), and f/2 medium (□) were detected and assessed.
DISCUSSION
The first part of this study was directed at the in vitro characterization of P. piscicida growth curves in the presence of algal prey and the assessment of the potential effects of selected biotic and abiotic factors on the encystment, cyst germination, and dinospore proliferation dynamics of P. piscicida. The culture container geometry affected the length of the lag phase rather than the cell densities reached at the stationary phase, and the duration of the lag phase appeared to relate inversely to the area of the culture medium that was exposed to air, as reported earlier (28). In horizontal 500-ml culture flasks, P. piscicida grown in f/2 medium in the presence of abundant Rhodomonas sp. cells exhibited reproducible growth curves consisting of multiple cycles that revealed a typical predator-prey relationship, with P. piscicida cell densities reaching the highest values as the culture became depleted of Rhodomonas sp. In contrast, the highest numbers of binucleated P. piscicida cells coincided with the presence of abundant Rhodomonas sp. cells, at the beginning of the log phase, suggesting that the high proliferation rates of P. piscicida during this early stage of growth may be conditional, among other factors, on the availability of algal prey. The relatively large proportion of multiplying forms (binucleated dinospores, gametes, planozygotes, and division cysts) during the remainder of the lag phase and the large numbers of putative resting cysts observed at the end of the decline phase when the culture was virtually depleted of algal prey supported this view, which is consistent with the results of studies of other heterotrophic dinoflagellate species (5, 18, 19, 51, 52).
In contrast, several culture medium supplements (ammonia, urea, and extracts of chicken manure, fish, and soil) that have been reported to modulate the proliferation of other dinoflagellate species (24, 26, 66, 67) had no effect on P. piscicida. Previous reports demonstrated that although both Pfiesteria spp. proliferate well on algal prey in nitrogen- and/or phosphate-enriched medium (500 μg of NO3 liter−1 or 500 μg of PO4 liter−1) (15), P. piscicida apparently favors a high-phosphorous environment while P. shumwayae prefers high levels of nitrogen (29). Thus, those supplements used in this study were perhaps not the favorable stimulation factor for Pfiesteria spp. The presence of fish or fish-derived factors, however, significantly enhanced P. piscicida proliferation either in the presence or in the absence of algal prey (Fig. 3), in agreement with earlier reports suggesting that P. piscicida dinospores rapidly emerge from cysts present in sediments and exhibit enhanced proliferation in the presence of fish (16, 20), although the induction mechanisms remain unknown. In this regard, however, our experiments revealed that the fish-derived growth-stimulating factors are particulate and thermolabile, possibly consisting of shed epithelial cells, scale fragments, or mucus. Microscopic observation of the aggressive feeding behavior of P. piscicida (23) and P. shumwayae (65) towards live fish larvae supports this hypothesis.
The profiles of P. piscicida proliferation in the dark were similar to those under the standard light cycle, except for the fact that the peak cell densities reached at the stationary phase were significantly lower in the dark (Fig. 2A). However, if in addition to the regime of darkness and food limitation, the culture was subjected to a low temperature, the dinospores formed cysts that were firmly attached to the bottom surface of the flask. Based on descriptions reported elsewhere (4, 6, 35, 49), these cysts were most likely long-term resting cysts (LTRCs). In contrast to the division cysts that are formed after sufficient feeding or the temporary cysts observed under stressful conditions, resting cysts found at the end of the decline phase of growth in cultures maintained under standard light and temperature regimes with algal prey depletion possibly represent a survival strategy against starvation. Further, the resting cysts may also contribute to the secondary wave of P. piscicida proliferation observed in continuous culture. According to Litaker et al. (49) and Anderson et al. (6), resting cysts can be found in P. piscicida cultures that have been subjected to food limitation for 2 to 3 days. If starvation extends beyond 7 days, resting cysts may transform into LTRCs by the thickening of the cell wall. Resting cysts and LTRCs from various dinoflagellate species, including P. piscicida, may maintain their full viability for several weeks and even months (1, 5, 45, 49). P. piscicida cysts have been reported to survive passage through the digestive apparatuses of grazers (57). In contrast to the results of a previous study that demonstrated in vitro germination when new medium and ample food were provided (49), our experiments showed the germination of in vitro-induced cysts achieved within 24 h following an increase of temperature up to 23°C and exposure to light (Fig. 4B), even in the absence of algal prey or fresh medium. The factors that affect cyst germination have been characterized mainly for autotrophic dinoflagellates. Similar to P. piscicida, several species, such as Gonyaulax spp., Gymnodinium spp., Alexandrium spp., and Scrippsiella sp., show positive effects on germination with increases in water temperature that typically occur during the transition from spring to summer, as well as increases in light intensity (1, 4, 8, 10, 12, 45). In contrast, the yield from cyst germination by P. piscicida was reduced by low salinities or exposure to dry conditions. Interestingly, the presence of live fish resulted in dramatically increased P. piscicida cyst germination rates and total yields compared to those of the controls. Attempts to identify the fish-derived factor(s) responsible for this effect were carried out by adding supplements to the culture medium, such as ammonia, urea, nitrate, and phosphate, that may be introduced by live fish as secretions or excretions, as well as fish extracts or aquarium water in which fish had been maintained. However, these had no effect on P. piscicida cyst germination profiles, as reported previously for Scrippsiella sp. (59). In contrast, Alexandrium catenella germinates faster and with a higher yield in low-nitrate, high-phosphate culture medium (27). Similarly, the possibility that a reduction in the concentration of dissolved oxygen caused by the live fish may act as a cue for cyst germination failed to prove true, an outcome which is comparable to the inhibited excystment of Gonyaulax spp. (4) and Scrippsiella hangoei (45) observed under anaerobiosis. The stimulating effects of live fish on P. piscicida cyst germination may be due to a yet-unidentified fish-derived compound(s) or physical effects, such as turbulence caused by fish movements. Because SAW alone did not show the effects of live fish, however, it is possible that such a fish-derived factor(s) is labile, and this idea will require further analysis.
The second part of this study was focused on evaluating the potential effects of environmental factors on the emergence of P. piscicida dinospores from environmental sediments known to contain P. piscicida cysts. For several species of dinoflagellates, blooms are linked specifically to the germination of resting cysts (2, 5, 37, 43), suggesting that “seed banks” of dinoflagellate cysts may play an important role in bloom initiation (63). Therefore, the specific detection distinguishing viable cysts from toxic dinoflagellate species in environmental sediments to map the location of natural seed banks is critical for anticipating harmful algal blooms. To analyze environmental sediment samples, we tested the efficiency of the quantitative assessment of P. piscicida cysts in sediments by QC-PCR by spiking-recovery experiments, in which in vitro-encysted P. piscicida cells were inoculated into autoclaved sediment. The copy numbers per single cyst were in agreement with the results of direct analyses of in vitro-harvested cysts and dinospores reported elsewhere (62). The application of the widely used molecular detection techniques, however, is problematic for sediment samples, in which cyst density is inherently low. Recently, an RT-PCR-based technique for enumerating P. piscicida viable cysts by targeting specific mRNA transcripts became available (21), but its detection sensitivity is also limited. Results from our study confirmed this observation, since although we validated our PCR detection technique by spiking-recovery assays, we were unable to detect P. piscicida cysts in sediment samples from several drained ponds from a fish farm where P. piscicida blooms had been repeatedly reported (30). It is unlikely that the negative PCR results were due to the presence of DNA polymerase inhibitors because both the DNA extraction and PCR amplification were carried out by following protocols already optimized to prevent false negatives (62). Rather, the densities of P. piscicida cysts or interstitial dinospores were likely below the limit tested in our spiking-recovery optimization experiments. Hence, in this study, we evaluated the induction of cyst germination by incubation in culture medium prior to the PCR-based detection of the emerged dinospores, as an indirect assessment of the numbers of viable cysts and potentially interstitial dinospores present in the sediments. In contrast with the PCR amplification of DNA extracted directly from the sediments, PCR tests of the culture medium supernatants were positive. It is possible that for samples with relatively high cyst and interstitial dinospore densities, the PCR amplification of DNA extracted directly from the sediments may reveal positive results. However, the assay will not discriminate between the two P. piscicida life stages, and results from our preliminary studies (see Fig. S4 in the supplemental material) suggest that this discrimination can be achieved by the comparative detection of tubulin transcripts or protein to determine the populations of flagellated and nonflagellated cells. Although detection methods based on lectins or antibodies are less sensitive than those based on PCR amplification, since they are based on either carbohydrate or protein moieties, they may be useful for the specific detection of particular life stages, species, and strains.
The presence of live fish in the sediment supernatant dramatically increased the proportion of positive P. piscicida PCR results from 50% for sediment samples incubated in f/2 medium to 100% for sediment samples incubated in ASW with live fish. This finding confirmed our initial observation that the presence of live fish had a stimulatory effect on the in vitro cyst germination and dinospore proliferation of P. piscicida and extended it to those cysts present in environmental samples. Longer-term experiments revealed that the presence of live fish dramatically reduced the lag period for P. piscicida detection in the supernatant, with cell densities 5- to 10-fold higher in the cultures with fish than in the controls in which fish were absent. Interestingly, this effect appeared to be specific for P. piscicida since this was not observed for the PLDs.
In summary, this study characterized in vitro, with the use of clonal cultures and with environmental sediments, the potential effects of abiotic and biotic factors on the encystment, cyst germination, and proliferation of P. piscicida dinospores. The presence of live fish significantly induced both faster and greater cyst germination and enhanced the proliferation of dinospores. For dinospores, the growth-enhancing activity is most likely related to the shedding of epithelial cells, scales, mucus, or bacteria from the fish. For the cysts, however, the germination-stimulating effect could not be explained by the results from this study, since none of the potentially secreted or excreted substances individually added to the cultures yielded positive results. Therefore, it is possible that the effects are due to either fish metabolites not yet tested, physical or mechanical changes in the environment caused by the live fish, or the synergistic effect of two or more of these factors. Further experiments to address this question are ongoing.
Supplementary Material
Acknowledgments
We acknowledge Tony Mazzacarro for providing access to HyRock Farm and Timothy Maugel for assistance with the SEM.
This study was supported by grants NIEHS 5-P01-ES09563 and ECOHAB NA860P0192.
Footnotes
Published ahead of print on 17 August 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Anderson, D. M., and B. A. Keafer. 1987. An endogenous annual clock in the toxic marine dinoflagellate Gonyaulax tamarensis. Nature 325:616-617. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. M., and F. M. M. Morel. 1979. Seeding of 2 red tide blooms by the germination of benthic Gonyaulax tamarensis hypnocysts. Estuar. Coast. Mar. Sci. 8:279-293. [Google Scholar]
- 3.Anderson, D. M., and K. Rengefors. 2006. Community assembly and seasonal succession of marine dinoflagellates in a temperate estuary: the importance of life cycle events. Limnol. Oceanogr. 51:860-873. [Google Scholar]
- 4.Anderson, D. M., C. D. Taylor, and E. V. Armbrust. 1987. The effects of darkness and anaerobiosis on dinoflagellate cyst germination. Limnol. Oceanogr. 32:340-351. [Google Scholar]
- 5.Anderson, D. M., and D. Wall. 1978. Potential importance of benthic cysts of Gonyaulax tamarensis and G. excavata in initiating toxic dinoflagellate blooms. J. Phycol. 14:224-234. [Google Scholar]
- 6.Anderson, J. T., D. K. Stoecker, and R. R. Hood. 2003. Formation of two types of cysts by a mixotrophic dinoflagellate, Pfiesteria piscicida. Mar. Ecol. Prog. Ser. 246:95-104. [Google Scholar]
- 7.Anderson, T. F. 1966. Electron microscopy of microorganisms, p. 319-387. In A. W. Pollister (ed.), Physical techniques in biological research, 2nd ed., vol. IIIA. Academic Press, New York, NY. [Google Scholar]
- 8.Band-Schmidt, C. J., C. H. Lechuga-Deveze, D. M. Kulis, and D. M. Anderson. 2003. Culture studies of Alexandrium affine (Dinophyceae), a non-toxic cyst forming dinoflagellate from Bahia Concepcion, Gulf of California. Bot. Mar. 46:44-54. [Google Scholar]
- 9.Bever, C. T., Jr., L. Grattan, and J. G. Morris. 1998. Neurologic symptoms following Pfiesteria exposure: case report and literature review. Md. Med. J. 47:120-123. [PubMed] [Google Scholar]
- 10.Blanco, J. 1990. Cyst germination of two dinoflagellate species from Galicia (NW Spain). Sci. Mar. 54:287-291. [Google Scholar]
- 11.Bowers, H. A., T. Tengs, H. B. Glasgow, Jr., J. M. Burkholder, P. A. Rublee, and D. W. Oldach. 2000. Development of real-time PCR assays for rapid detection of Pfiesteria piscicida and related dinoflagellates. Appl. Environ. Microbiol. 66:4641-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bravo, I., and D. M. Anderson. 1994. The effects of temperature, growth medium and darkness on excystment and growth of the toxic dinoflagellate Gymnodinium catenatum from Northwest Spain. J. Plankton Res. 16:513-525. [Google Scholar]
- 13.Buck, K. R., R. Marin, and F. P. Chavez. 2005. Heterotrophic dinoflagellate fecal pellet production: grazing of large, chain-forming diatoms during upwelling events in Monterey Bay, California. Aquat. Microb. Ecol. 40:293-298. [Google Scholar]
- 14.Burkholder, J. M., H. B. Glasgow, and N. J. Deamer-Melia. 2001. Overview and present status of the toxic Pfiesteria complex (Dinophyceae). Phycologia 40:186-214. [Google Scholar]
- 15.Burkholder, J. M., H. B. Glasgow, N. J. Deamer-Melia, J. Springer, M. W. Parrow, C. Zhang, and P. J. Cancellieri. 2001. Species of the toxic Pfiesteria complex, and the importance of functional type in data interpretation. Environ. Health Perspect. 109:667-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkholder, J. M., and H. B. Glasgow, Jr. 1997. Pfiesteria piscicida and other Pfiesteria-like dinoflagellates: behavior, impacts, and environmental controls. Limnol. Oceanogr. 42:1052-1075. [Google Scholar]
- 17.Burkholder, J. M., H. B. Glasgow, Jr., and C. W. Hobbs. 1995. Fish kills linked to a toxic ambush-predator dinoflagellate: distribution and environmental conditions. Mar. Ecol. Prog. Ser. 124:43-61. [Google Scholar]
- 18.Buskey, E. J. 1995. Growth and bioluminescence of Noctiluca scintillans on varying algal diets. J. Plankton Res. 17:29-40. [Google Scholar]
- 19.Buskey, E. J., C. J. Coulter, and S. L. Brown. 1994. Feeding, growth and bioluminescence of the heterotrophic dinoflagellate Protoperidinium huberi. Mar. Biol. 121:373-380. [Google Scholar]
- 20.Cancellieri, P. J., J. M. Burkholder, N. J. Deamer-Melia, and H. B. Glasgow. 2001. Chemosensory attraction of zoospores of the estuarine dinoflagellates, Pfiesteria piscicida and P. shumwayae, to finfish mucus and excreta. J. Exp. Mar. Biol. Ecol. 264:29-45. [Google Scholar]
- 21.Coyne, K. J., and S. C. Cary. 2005. Molecular approaches to the investigation of viable dinoflagellate cysts in natural sediment from estuarine environments. J. Eukaryot. Microbiol. 52:90-94. [DOI] [PubMed] [Google Scholar]
- 22.Coyne, K. J., D. A. Hutchins, C. E. Hare, and S. C. Cary. 2001. Assessing temporal and spatial variability in Pfiesteria piscicida distributions using molecular techniques. Aquat. Microb. Ecol. 24:275-285. [Google Scholar]
- 23.Drgon, T., K. Saito, P. M. Gillevet, M. Sikaroodi, B. Whitaker, D. N. Krupatkina, F. Argemi, and G. R. Vasta. 2005. Characterization of ichthyocidal activity of Pfiesteria piscicida: dependence on the dinospore cell density. Appl. Environ. Microbiol. 71:519-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyhrman, S. T., and D. M. Anderson. 2003. Urease activity in cultures and field populations of the toxic dinoflagellate Alexandrium. Limnol. Oceanogr. 48:647-655. [Google Scholar]
- 25.Eng-Wilmot, D. L., W. S. Hitchcock, and D. F. Martin. 1977. Effect of temperature on the proliferation of Gymnodinium breve and Gomphosphaeria aponina. Mar. Biol. 41:71-77. [Google Scholar]
- 26.Fan, C., P. M. Glibert, and J. M. Burkholder. 2003. Characterization of the affinity for nitrogen, uptake kinetics, and environmental relationships for Prorocentrum minimum in natural blooms and laboratory cultures. Harmful Algae 2:283-299. [Google Scholar]
- 27.Figueroa, R. I., I. Bravo, and E. Garces. 2005. Effects of nutritional factors and different parental crosses on the encystment and excystment of Alexandrium catenella (Dinophyceae) in culture. Phycologia 44:658-670. [Google Scholar]
- 28.Fogg, G. E. 1965. Algal culture and phytoplankton ecology. The University of Wisconsin Press, Madison, WI.
- 29.Glibert, P. M., J. M. Burkholder, M. W. Parrow, A. J. Lewitus, and D. E. Gustafson. 2006. Direct uptake of nitrogen by Pfiesteria piscicida and Pfiesteria shumwayae, and nitrogen nutritional preferences. Harmful Algae 5:380-394. [Google Scholar]
- 30.Glibert, P. M., and D. E. Terlizzi. 1999. Cooccurrence of elevated urea levels and dinoflagellate blooms in temperate estuarine aquaculture ponds. Appl. Environ. Microbiol. 65:5594-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gransden, S. G., and A. J. Lewitus. 2003. Grazing of two euplotid ciliates on the heterotrophic dinoflagellates Pfiesteria piscicida and Cryptoperidiniopsis sp. Aquat. Microb. Ecol. 33:303-308. [Google Scholar]
- 32.Grattan, L. M., D. Oldach, T. M. Perl, M. H. Lowitt, D. L. Matuszak, C. Dickson, C. Parrott, R. C. Shoemaker, C. L. Kauffman, M. P. Wasserman, J. R. Hebel, P. Charache, and J. G. Morris, Jr. 1998. Learning and memory difficulties after environmental exposure to waterways containing toxin-producing Pfiesteria or Pfiesteria-like dinoflagellates. Lancet 352:532-539. [DOI] [PubMed] [Google Scholar]
- 33.Guillard, R. R. L. 1975. Culture of phytoplankton for feeding marine invertebrates, p. 26-60. In W. L. Smith and M. H. Chanley (ed.), Culture of marine invertebrate animals. Plenum Press, New York, NY.
- 34.Hamada, S., and S. Fujita. 1983. DAPI staining improved for quantitative cytofluorometry. Histochemistry 79:219-226. [DOI] [PubMed] [Google Scholar]
- 35.Hardeland, R. 1994. Induction of cyst formation by low temperature in the dinoflagellate Gonyaulax polyedra Stein: dependence on circadian phase and requirement of light. Experientia 50:60-62. [Google Scholar]
- 36.Haselow, D. T., E. Brown, J. K. Tracy, R. Magnien, L. M. Grattan, J. G. Morris, Jr., and D. W. Oldach. 2001. Gastrointestinal and respiratory tract symptoms following brief environmental exposure to aerosols during a Pfiesteria-related fish kill. J. Toxicol. Environ. Health A 63:553-564. [DOI] [PubMed] [Google Scholar]
- 37.Heaney, S. I., D. V. Chapman, and H. R. Morison. 1983. The role of the cyst stage in the seasonal growth of the dinoflagellate Ceratium hirundinella within a small productive lake. Br. Phycol. J. 18:47-59. [Google Scholar]
- 38.Jeong, H. J., J. S. Kim, J. H. Kim, S. T. Kim, K. A. Seong, T. H. Kim, J. Y. Song, and S. K. Kim. 2005. Feeding and grazing impact of the newly described heterotrophic dinoflagellate Stoeckeria algicida on the harmful alga Heterosigma akashiwo. Mar. Ecol. Prog. Ser. 295:69-78. [Google Scholar]
- 39.Jeong, H. J., J. Y. Park, J. H. Nho, M. O. Park, J. H. Ha, K. A. Seong, C. Jeng, C. N. Seong, K. Y. Lee, and W. H. Yih. 2005. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. Aquat. Microb. Ecol. 41:131-143. [Google Scholar]
- 40.Kane, A. S., D. Oldach, and R. Reimschuessel. 1998. Fish lesions in the Chesapeake Bay: Pfiesteria-like dinoflagellates and other etiologies. Md. Med. J. 47:106-112. [PubMed] [Google Scholar]
- 41.Kim, J. S., and H. J. Jeong. 2004. Feeding by the heterotrophic dinoflagellates Gyrodinium dominans and G. spirale on the red-tide dinoflagellate Prorocentrum minimum. Mar. Ecol. Prog. Ser. 280:85-94. [Google Scholar]
- 42.Kim, Y.-O., and M.-S. Han. 2000. Seasonal relationships between cyst germination and vegetative population of Scrippsiella trochoidea (Dinophyceae). Mar. Ecol. Prog. Ser. 204:111-118. [Google Scholar]
- 43.Kim, Y.-O., M. H. Park, and M. S. Han. 2002. Role of cyst germination in the bloom initiation of Alexandrium tamarense (Dinophyceae) in Masan Bay, Korea. Aquat. Microb. Ecol. 29:279-286. [Google Scholar]
- 44.Koski, M., and C. W. Riser. 2006. Post-bloom feeding of Calanus finmarchicus copepodites: selection for autotrophic versus heterotrophic prey. Mar. Biol. Res. 2:109-119. [Google Scholar]
- 45.Kremp, A., and D. M. Anderson. 2000. Factors regulating germination of resting cysts of the spring bloom dinoflagellate Scrippsiella hangoei from the northern Baltic Sea. J. Plankton Res. 22:1311-1327. [Google Scholar]
- 46.Leong, S. C. Y., M. Nakazawa, and S. Taguchi. 2006. Physiological and optical responses of the harmful dinoflagellate Heterocapsa circularisquama to a range of salinity. Hydrobiologia 559:149-159. [Google Scholar]
- 47.Lewitus, A. J., R. V. Jesien, T. M. Kana, J. M. Burkholder, H. B. Glasgow, and E. May. 1995. Discovery of the “phantom” dinoflagellate in Chesapeake Bay. Estuaries 18:373-378. [Google Scholar]
- 48.Lewitus, A. J., B. M. Willis, K. C. Hayes, J. M. Burkholder, H. B. Glasgow, P. M. Glibert, and M. K. Burke. 1999. Mixotrophy and nitrogen uptake by Pfiesteria piscicida (Dinophyceae). J. Phycol. 35:1430-1437. [Google Scholar]
- 49.Litaker, R. W., M. W. Vandersea, S. R. Kibler, V. J. Madden, E. J. Noga, and P. A. Tester. 2002. Life cycle of the heterotrophic dinoflagellate Pfiesteria piscicida (Dinophyceae). J. Phycol. 38:442-463. [Google Scholar]
- 50.Litaker, R. W., M. W. Vandersea, S. R. Kibler, K. S. Reece, N. A. Stokes, K. A. Steidinger, D. F. Millie, B. J. Bendis, R. J. Pigg, and P. A. Tester. 2003. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. J. Phycol. 39:754-761. [Google Scholar]
- 51.Litaker, R. W., V. E. Warner, C. Rhyne, C. S. Duke, B. E. Kenney, J. Ramus, and P. A. Tester. 2002. Effect of diel and interday variations in light on the cell division pattern and in situ growth rates of the bloom-forming dinoflagellate Heterocapsa triquetra. Mar. Ecol. Prog. Ser. 232:63-74. [Google Scholar]
- 52.Matsuoka, K., H.-J. Cho, and D. M. Jacobson. 2000. Observations of the feeding behavior and growth rates of the heterotrophic dinoflagellate Polykrikos kofoidii (Polykrikaceae, Dinophyceae). Phycologia 39:82-86. [Google Scholar]
- 53.Matuszak, D. L., J. L. Taylor, C. Dickson, and G. C. Benjamin. 1998. Toxic Pfiesteria surveillance for human disease in Maryland. Md. Med. J. 47:144-147. [PubMed] [Google Scholar]
- 54.Menden-Deuer, S., E. J. Lessard, J. Satterberg, and D. Gruenbaum. 2005. Growth rates and starvation survival of three species of the pallium-feeding, thecate dinoflagellate genus Protoperidinium. Aquat. Microb. Ecol. 41:145-152. [Google Scholar]
- 55.Messick, G. A., S. J. Jordan, and W. F. Van Heukelem. 1999. Salinity and temperature effects on Hematodinium sp. in the blue crab Callinectes sapidus. J. Shellfish Res. 18:657-662. [Google Scholar]
- 56.Millonig, G. 1961. Advantage of a phosphate buffer for OsO4 solutions in fixation. J. Appl. Physiol. 32:1637. [Google Scholar]
- 57.Montresor, M., L. Nuzzo, and M. G. Mazzocchi. 2003. Viability of dinoflagellate cysts after the passage through the copepod gut. J. Exp. Mar. Biol. Ecol. 287:209-221. [Google Scholar]
- 58.Oldach, D. W., C. F. Delwiche, K. S. Jakobsen, T. Tengs, E. G. Brown, J. W. Kempton, E. F. Schaefer, H. A. Bowers, H. B. Glasgow, Jr., J. M. Burkholder, K. A. Steidinger, and P. A. Rublee. 2000. Heteroduplex mobility assay-guided sequence discovery: elucidation of the small subunit (18S) rDNA sequences of Pfiesteria piscicida and related dinoflagellates from complex algal culture and environmental sample DNA pools. Proc. Natl. Acad. Sci. USA 97:4303-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olli, K., and D. M. Anderson. 2002. High encystment success of the dinoflagellate Scrippsiella cf. lachrymosa in culture experiments. J. Phycol. 38:145-156. [Google Scholar]
- 60.Quesenberry, M. S., K. Saito, D. N. Krupatkina, J. A. F. Robledo, T. Drgon, W. T. Pecher, N. O'Leary, M. Alavi, T. Miller, R. E. Schneider, R. Belas, J. R. Deeds, A. R. Place, Y. Zohar, and G. R. Vasta. 2002. Bioassay for ichthyocidal activity of Pfiesteria piscicida: characterization of a culture flask assay format. J. Appl. Phycol. 14:241-254. [Google Scholar]
- 61.Rublee, P. A., J. Kempton, E. F. Schaefer, J. M. Burkholder, H. B. Glasgow, and D. Oldach. 1999. PCR and FISH detection extends the range of Pfiesteria piscicida in estuarine waters. Va. J. Sci. 50:325-336. [Google Scholar]
- 62.Saito, K., T. Drgon, J. A. F. Robledo, D. N. Krupatkina, and G. R. Vasta. 2002. Characterization of the rRNA locus of Pfiesteria piscicida and development of standard and quantitative PCR-based detection assays targeted to the nontranscribed spacer. Appl. Environ. Microbiol. 68:5394-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steidinger, K. A. 1975. Implications of dinoflagellate life-cycles on initiation of Gymnodinium breve red tides. Environ. Lett. 9:129-139. [DOI] [PubMed] [Google Scholar]
- 64.Steidinger, K. A., J. M. Burkholder, H. B. Glasgow, Jr., C. W. Hobbs, J. K. Garrett, E. W. Truby, E. J. Noga, and S. A. Smith. 1996. Pfiesteria piscicida gen. et sp. nov. (Pfiesteriaceae fam. nov.), a new toxic dinoflagellate with a complex life cycle and behavior. J. Phycol. 32:157-164. [Google Scholar]
- 65.Vogelbein, W. K., V. J. Lovko, J. D. Shields, K. S. Reece, P. L. Mason, L. W. Haas, and C. C. Walker. 2002. Pfiesteria shumwayae kills fish by micropredation not exotoxin secretion. Nature 418:967-970. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi, M., and S. Itakura. 1999. Nutrition and growth kinetics in nitrogen- or phosphorus-limited cultures of the noxious red tide dinoflagellate Gymnodinium mikimotoi. Fish. Sci. 65:367-373. [Google Scholar]
- 67.Yamaguchi, M., S. Itakura, and T. Uchida. 2001. Nutrition and growth kinetics in nitrogen- or phosphorus-limited cultures of the ‘novel red tide’ dinoflagellate Heterocapsa circularisquama (Dinophyceae). Phycologia 40:313-318. [Google Scholar]
- 68.Zhang, H., and S. Lin. 2002. Detection and quantification of Pfiesteria piscicida by using the mitochondrial cytochrome b gene. Appl. Environ. Microbiol. 68:989-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.