Abstract
This study aimed to develop a convenient model to investigate the senescence of host defenses and the influence of food and nutrition. A small soil nematode, Caenorhabditis elegans, was grown for 3 days from hatching on a lawn of Escherichia coli OP50 as the normal food source, and subsequently some of the nematodes were fed lactic acid bacteria (LAB). The life spans of worms fed LAB were significantly longer than the life spans of those fed OP50. To investigate the effect of age on host defenses, 3- to 7-day-old worms fed OP50 were transferred onto a lawn of Salmonella enterica serovar Enteritidis for infection. The nematodes died over the course of several days, and the accumulation of salmonella in the intestinal lumen suggested that the worms were infected. The 7-day-old worms showed a higher death rate during the 5 days after infection than nematodes infected at the age of 3 days; no clear difference was observed when the worms were exposed to OP50. We then investigated whether the LAB could exert probiotic effects on the worms' host defenses and improve life span. Seven-day-old nematodes fed LAB from the age of 3 days were more resistant to salmonella than worms fed OP50 until they were infected with salmonella. This study clearly showed that LAB can enhance the host defense of C. elegans and prolong life span. The nematode appears to be an appropriate model for screening useful probiotic strains or dietetic antiaging substances.
Caenorhabditis elegans is a small, free-living soil nematode that feeds on bacteria; it has been extensively used as an experimental system for biological studies because of its simplicity, transparency, ease of cultivation, and suitability for genetic analysis (26). In particular, for aging studies, the worm has the advantage of a short and reproducible life span (7). Recently, since Tan et al. reported infection due to Pseudomonas aeruginosa (31), the organism has also been recognized as an alternative to mammalian models of infection with bacterial pathogens (15, 25). In the field of innate immunity research, C. elegans is becoming one of the most important experimental animals, similar to the fruit fly Drosophila melanogaster (15, 25, 28).
Age at infection is likely one of the most important determinants of morbidity and mortality from disease (22). Because aging is accompanied by functional and metabolic alterations in cells and tissues, senescence of the immune system results in an age-related increase in infection, malignancy, and autoimmunity (9, 23). Elderly humans have increased mortality from many different types of infections (5).
Whether nutritional control can retard the senescence of immune function and decrease mortality from infectious diseases has not yet been established; the difficulty of establishing a model has made this a challenging topic to investigate. Although some studies have shown successful improvement of biomarkers relating to immunological functions (4), few reports have shown a beneficial influence of nutrition on immunity and the resultant outcome of experimental infection (6, 11).
Probiotic bacteria are defined as living microorganisms that exert beneficial effects on human health when ingested in sufficient numbers (24). Lactic acid bacteria (LAB) have been used in various fermented foods since antiquity. Metchnikoff, who first proposed the concept of probiotic bacteria in 1907, hypothesized that lactobacilli were important for human health and longevity (21). LAB are the most commonly used probiotic microorganisms. LAB have been found to have a variety of physiological influences on their hosts, including antimicrobial effects, microbial interference, supplementary effects on nutrition, antitumor effects, reduction of serum cholesterol and lipids, and immunomodulatory effects. However, there have been no reports concerning the influence of LAB on longevity.
We have studied whether food could influence the worm's mean life span and host defenses. In this study, we evaluated whether LAB could contribute to host defenses and prolong the lifetime of C. elegans. Lactobacilli and bifidobacteria were fed to the worms, and their life span and resistance to salmonella were compared with those of worms fed Escherichia coli OP50, an international standard food for C. elegans.
MATERIALS AND METHODS
Nematode.
C. elegans Bristol strain N2 was kindly provided by the Caenorhabditis Genetics Center, University of Minnesota, Minneapolis, MN. The nematodes were maintained and propagated on nematode growth medium (NGM) with standard techniques (29), using E. coli OP50 as the internationally established feed.
Bacterial strains.
Bifidobacterium infantis ATCC 15697, Bifidobacterium longum ATCC 15707, Lactobacillus helveticus NBRC15019, Lactobacillus plantarum NBRC15891, and Lactobacillus rhamnosus NBRC14710 were the LAB used to feed the worms. Lactobacillus spp. were purchased from NITE Biological Resource Center (NBRC), Chiba, Japan. Clostridium perfringens strain 99-279-37 was used as another anaerobic bacterial strain for comparison with LAB. Salmonella enterica serovar Enteritidis strain SE1, originally isolated from a diarrheal specimen, was used as a pathogen. E. coli strain OP50 was kindly provided by Hideyuki Hoshi (Osaka Prefecture University, Japan).
Media.
Culture broth was prepared using GAM broth (Nissui Pharmaceutical Co., Tokyo, Japan) for bifidobacteria and C. perfringens, MRS broth for L. plantarum, and no. 804 broth (see below) for the other lactobacilli. TOS propionate agar (Eiken Chemical Co., Tokyo, Japan), BL agar (Nissui), MRS agar (Oxoid), and medium no. 804 agar were used for B. infantis, B. longum, L. plantarum, and the other two Lactobacillus spp., respectively. Following the instructions of the NBRC, medium no. 804 was prepared by adding 5.0 g Bacto peptone (Becton Dickinson), 5.0 g yeast extract (Oxoid), 5.0 g glucose, 1.0 g MgSO4·7H2O, and 15 g agar (Wako Pure Chemical Industries, Osaka, Japan) to 1 liter of distilled water. C. perfringens was grown using GAM agar (Nissui). Tryptone soya agar (Oxoid) was used to culture E. coli OP50 and S. enterica. The bacterial lawns used for feeding C. elegans were prepared by spreading 25 μl of the bacterial suspension (10 mg/25 μl M9 buffer) on NGM modified to be peptone free (mNGM) in 6.0-cm-diameter plates.
Life span of C. elegans.
The worms were generated from the eggs which were released after adult worms were exposed to a sodium hypochlorite-sodium hydroxide solution as previously described (30). The egg suspension was incubated overnight at 25°C to allow hatching, and the suspension of L1-stage worms was centrifuged at 1,000 rpm for 1 min. The supernatant was removed, and the remaining larvae were transferred onto fresh mNGM plates covered with E. coli OP50 and incubated at 25°C. It is well known that the reproductive system regulates aging in C. elegans (12). To synchronize pubescence, worms were fed on OP50 until maturation. Two days later, life span assays were begun with 3-day-old young-adult worms, which were allocated at 25 each to mNGM plates covered with lawns of each LAB strain. The plates were incubated at 25°C, and the numbers of live and dead worms were scored at least every 24 h. At 25°C, worms produce progeny that develop into adults in 3 days, and it is difficult to identify the original worms. Therefore, the original worms were transferred to fresh plates on a daily basis. A worm was considered dead when it failed to respond to gentle touch with a worm picker. Worms that died as a result of getting stuck to the wall of the plate were eliminated from the analysis.
The mean life span (MLS) was estimated using the formula (36)
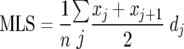 |
where j is the age category (day), dj is the number of worms that died in the age interval (xj, xj+1), and n is the total number of worms. The standard error (SE) of the mean life span estimate was calculated using the equation
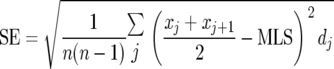 |
Measurement of body size.
The 3-day-old young-adult worms were allocated to mNGM plates covered with lawns of B. infantis or E. coli OP50. The plates were incubated at 25°C, and live worms were examined for their body size measurements every 24 h until they were infected with salmonella. Images of adult nematodes were taken with a VCT-VBIT digital microscope (Shimadzu, Kyoto, Japan) and analyzed using the ImageJ software developed by the National Institutes of Health. In this system, the area of the worm's projection was estimated automatically and used as an index of body size.
Influence of aging on resistance against salmonella infection.
After hatching, the nematodes were grown on E. coli OP50. Each 25 synchronous worms of the desired age were transferred to a plate covered with salmonella, which was incubated at 25°C. The assay plates were prepared in duplicate by spreading 25 μl of bacterial suspensions containing 10 mg of salmonella onto 60-mm-diameter mNGM plates.
Influence of feed on resistance against salmonella infection.
After hatching, the nematodes were grown on E. coli OP50 for 3 days, and then the worms were assigned to a control group that continued to be fed OP50 or to a group that was fed LAB for 4 days. The 7-day-old worms were then transferred onto salmonella lawns. Each group of 25 worms was assigned to one plate and incubated at 25°C. The numbers of live and dead worms were scored at least every 24 h. Survival rates were compared between groups of worms grown on different feeds before the salmonella infection. Each assay was carried out in duplicate.
Bacterial colonization within the C. elegans digestive tract.
The numbers of salmonella cells in the nematodes were determined according to the method of Garsin et al. (8), with some modification. Five worms were picked, and the surface bacteria were removed by washing the worms four times in 4-μl drops of M9 buffer on agar plates. Each nematode was placed in a 0.5-ml microtube containing 20 μl of M9 buffer and was mechanically disrupted using a microtube pestle (Scientific Specialties Inc., Lodi, CA). The volume was adjusted to 500 μl with M9 buffer, and the number of salmonella cells was determined by using mannitol lysine crystal violet brilliant green agar (Nissui).
Statistical analysis.
The rate of nematode survival was calculated by the Kaplan-Meier method, and differences in survival rates were tested for significance by use of the log rank test. Before the statistical analysis of body sizes and bacterial numbers was performed, the values were examined for a normal distribution. If the raw data did not fit a normal distribution, the values that were markedly different in each group were rejected based on the Smirnov-Grubbs test and the group was examined again for a normal distribution. The significance was then examined by Student's or Welch's t test after an F test was performed, unless otherwise stated. If the raw data did not fit a normal distribution, the Mann-Whitney U test was used.
RESULTS
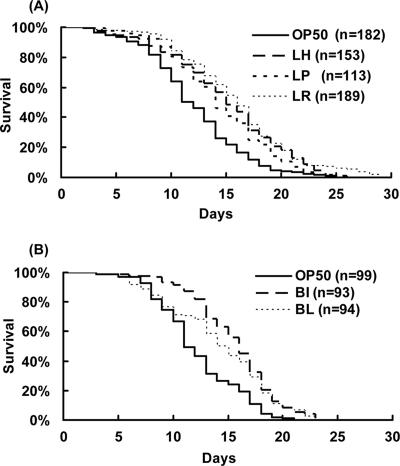
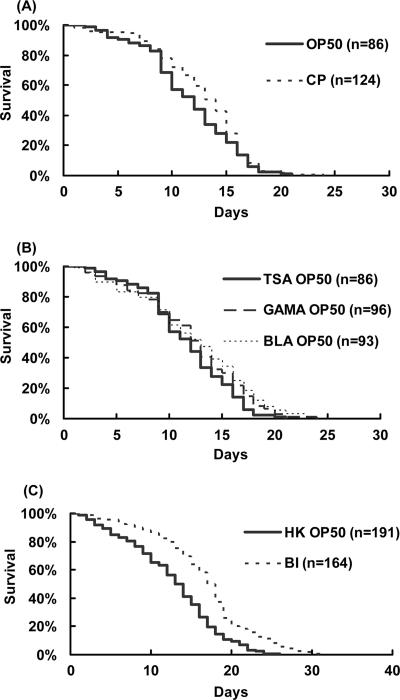
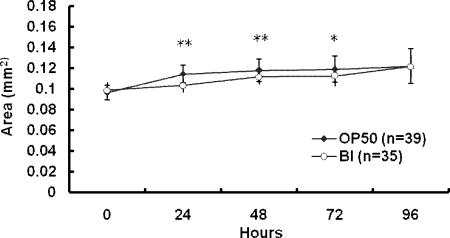
Feeding nematodes bifidobacteria or lactobacilli resulted in increases in the average life spans of the nematodes compared to the life spans of those fed OP50 (Fig. 1A and B). Bifidobacteria and lactobacilli were prepared by anaerobic culture. To examine whether anaerobically cultured bacteria could enhance the longevity of the worms, C. perfringens, an anaerobic pathogen, was also used as feed for the nematodes. However, neither C. perfringens nor OP50, cultured anaerobically on LAB media, increased the life span of the nematodes as much as LAB did (Fig. 2A and B). To examine whether or not the beneficial effects of LAB were brought about by their harmless nature compared to OP50, the survival rate of worms fed on LAB was compared with that of nematodes fed on killed OP50. Heat-killed OP50 did not prolong the worms' longevity as much as LAB did (Fig. 2C). The body sizes of the nematodes fed on B. infantis were a little smaller than those of control worms during the first 3 days after the food source was changed (Fig. 3).
FIG. 1.
Effects of LAB on the life span of C. elegans. Adult worms fed a diet of E. coli strain OP50 for 3 days after hatching were transferred to diets of lactobacilli (A) or bifidobacteria (B). The lactobacilli used were L. helveticus (LH), L. plantarum (LP), and L. rhamnosus (LR). The bifidobacteria used were B. infantis (BI) and B. longum (BL). The life spans of nematodes fed LAB were significantly extended (P < 0.001). The mean life spans (in mean number of days ± standard error) of worms fed L. helveticus, L. plantarum, L. rhamnosus, B. infantis, and B. longum were 14.6 ± 0.43 (25%), 14.2 ± 0.44 (22%), 15.5 ± 0.38 (33%), 15.1 ± 0.40 (29%), and 13.6 ± 0.50 (17%), respectively (the numbers in parentheses are the percent differences in the means relative to the mean for controls fed OP50). The mean life spans of controls for lactobacilli and bifidobacteria were 11.7 ± 0.33 and 11.7 ± 0.38 days, respectively.
FIG. 2.
Comparison of the life span-extending effects of LAB with the effects of other bacteria cultured under anaerobic conditions. (A) Another anaerobic organism, C. perfringens (CP), did not show a life span-extending effect. The mean life span and the percent difference in the mean life span relative to those of controls were 12.1 ± 0.37 days (mean ± standard error) and 8%, respectively. (B) Worms fed OP50 cultured on BL agar (BLA; 11.1 ± 0.54 days; −1% difference) or GAM agar (GAMA; 10.4 ± 0.46 days; −7% difference) did not show any difference from worms fed on organisms cultured on tryptone soya agar (TSA; 11.2 ± 0.45 days). (C) B. infantis (BI; 16.3 ± 0.47 days; 33% difference) prolonged life span over that seen with heat-killed OP50 (HK OP50; 12.27 ± 0.42 days) (P < 0.001).
FIG. 3.
Growth curve of worms fed B. infantis. Images of adult nematodes fed OP50 or LAB were taken with a digital microscope, and the area of the worm's projection was measured and used as an index of body size. The body sizes of the worms fed bifidobacteria were similar to those of controls; however, they were smaller during the first 3 days after their food was changed from OP50 to bifidobacteria, and this effect was statistically significant (*, P < 0.05; **, P < 0.01). All results are presented as means ± standard deviations of the means.
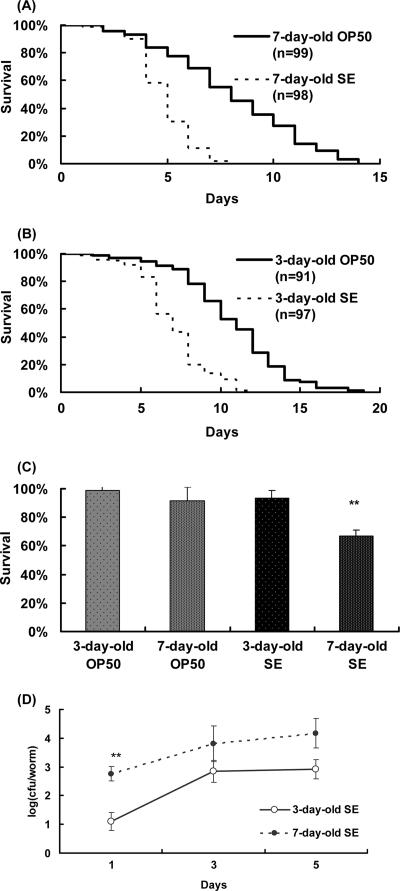
Salmonella killed about 40% of the nematodes within 5 days after the worms were transferred to the lawn of this pathogen at age 7 days (Fig. 4A), while 80% of the worms fed OP50 remained alive after 5 days. The pathogenicity of the salmonella against C. elegans as reported elsewhere (1, 16) was reconfirmed; heat-killed salmonella did not show such pathogenicity, and these worms' life spans were not significantly different from the life spans of those grown on E. coli OP50 (data not shown). The 3-day-old worms were not killed in 5 days when fed either OP50 or salmonella (Fig. 4B). The 3-day-old worms were clearly resistant to salmonella compared to the 7-day-old nematodes (Fig. 4C). The initial number of salmonella cells recovered from those worms in which infection started at age 3 days was smaller than the number recovered from worms infected from age 7 days (Fig. 4D).
FIG. 4.
Survival curve for nematodes infected with Salmonella enterica serovar Enteritidis (SE) at 7 days (A) or 3 days (B) of age. The mean numbers of days of survival of worms after the salmonella infection were 4.4 ± 0.13 (−42%) for 7-day-old worms and 6.6 ± 0.22 (−36%) for 3-day-old worms (the numbers in parentheses are the percent differences in the means relative to the mean for controls fed OP50). The mean survival times of controls fed OP50 were 7.6 ± 0.31 days for 7-day-old worms and 10.3 ± 0.34 days for 3-day-old worms. (C) Survival rate at the fifth day of salmonella infection. The death rate of nematodes infected at age 7 days was greater than that of worms infected at age 3 days (P < 0.01). Survival rates on arbitrary days were determined by using polynomial regression analysis. The values were transformed using trigonometry and examined for a normal distribution. After verifying the homogeneity of variances with the Bartlett test and one-way analysis of variance, multiple comparisons were made using Tukey's test. If the data did not fit a normal distribution, the Mann-Whitney U test was used. (D) The number of salmonella cells recovered from young nematodes on the first day after the infection was significantly lower than the number recovered from worms infected at age 7 days (P < 0.01). All results are presented as means ± standard errors of the means.
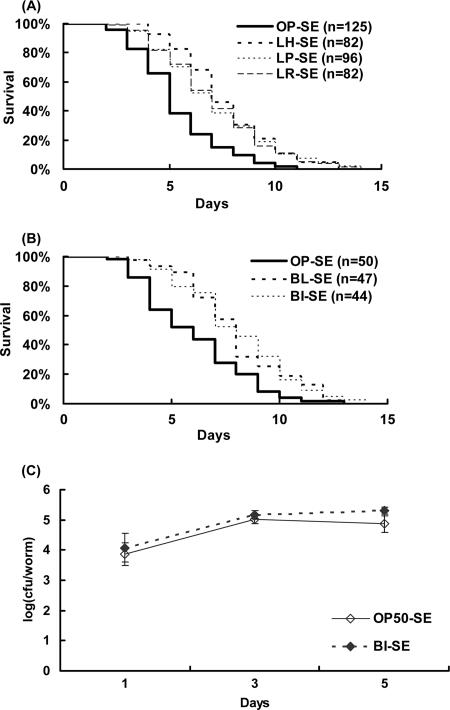
The 7-day-old nematodes fed bifidobacteria or lactobacilli from age 3 days were clearly resistant to subsequent salmonella infection compared with nematodes fed OP50 before the salmonella infection (Fig. 5A and B). However, the number of salmonella cells recovered from worms fed LAB was the same as that recovered from worms grown on OP50 (Fig. 5C).
FIG. 5.
Effects of LAB on resistance of C. elegans to Salmonella enterica serovar Enteritidis (SE). Adult worms fed a diet of E. coli strain OP50 (OP) for 3 days after hatching were transferred to a diet of lactobacilli (A) or bifidobacteria (B). The lactobacilli used were L. helveticus (LH), L. plantarum (LP), or L. rhamnosus (LR). The bifidobacteria used were B. infantis (BI) or B. longum (BL). Four days later, the nematodes were transferred to salmonella plates, and survival curves were drawn. Nematodes fed each type of lactobacilli were significantly more resistant than controls to the pathogen (P < 0.001). The worms fed bifidobacteria were also resistant (P < 0.01). The mean numbers of days of survival of worms fed L. helveticus, L. plantarum, L. rhamnosus, B. infantis, and B. longum before the salmonella infection were 7.1 ± 0.25 (46%), 6.6 ± 0.28 (35%), 6.6 ± 0.27 (35%), 7.2 ± 0.37 (30%), and 7.5 ± 0.35 (35%), respectively (the numbers in parentheses are the percent differences in the means relative to the mean for controls fed OP50). The mean numbers of days of survival of controls for lactobacilli and bifidobacteria were 4.9 ± 0.18 and 5.6 ± 0.35, respectively. (C) Numbers of salmonella cells recovered from nematodes after the infection. All results are presented as means ± standard errors of the means.
DISCUSSION
Nutrition is believed to influence aging and host defense. Recently, resveratrol, a compound contained in red grapes, was discovered to be an effective antiaging substance (3, 33). However, there is no clear experimental evidence showing that specific foods can prolong longevity. The present study has shown that providing LAB as a feed could increase the average life span of C. elegans. It has been reported that even the international food strain OP50 is pathogenic in old nematodes and that worms fed killed OP50 organisms can live longer than those fed live OP50. To examine whether or not the beneficial effects of LAB were brought about by their harmless nature, survival of nematodes fed LAB was compared with that of nematodes fed killed OP50. The beneficial effect was not simply due to the harmless nature of LAB; the survival of nematodes fed heat-killed OP50 was significantly lower than that of nematodes fed LAB. On the basis of the longevity of Bulgarians who eat large quantities of yogurt, Metchnikoff hypothesized that lactobacilli were important for human health and longevity (21). This served as an impetus for research on healthful bacteria, i.e., probiotics. No experimental data about life-prolonging effects of LAB have been published. The present data suggest this possibility, although we should not directly extrapolate the phenomenon to humans, since the present study was performed with nematodes, which are phylogenetically quite distant from mammals.
Similar to the results reported by Laws et al. (18), senescence of the host defense of nematodes was clearly shown by the survival curve during salmonella infection of nematodes in this study. The 3-day-old worms were more resistant to infection than the 7-day-old worms were. However, nematodes fed LAB until age 7 days were more resistant to the pathogen than those fed OP50. LAB were effective not only for longevity but also for host defense. The LAB were digested as feed and were not recovered from the nematodes; it is unlikely that the LAB behaved as protective flora. The mechanism by which the LAB increased the worms' resistance to salmonella remains to be elucidated. Since there was no difference in the numbers of salmonella cells recovered from worms fed LAB and those fed OP50 (Fig. 5C), it is unlikely that the resistance was due to increased expression of antibacterial factors from immunostimulation by LAB. Instead, some type of immunotolerance might be taking place.
Differences in nutritional components between LAB and E. coli OP50 might result in the difference in life span. However, it is not likely that LAB were simply more nutritious or more readily digestible than OP50, because the body sizes of the nematodes were similar except during the first 3 days after the food source was changed from OP50 to LAB (Fig. 3). Calorie restriction is well recognized as a method to extend longevity and slows aging not only in numerous nonmammalian taxa but also in mammals, including mice (34), dogs (14), and, possibly, primates (10). As mentioned above, however, the growth curve did not support the hypothesis that LAB were an extremely low-calorie food compared with OP50, although the first 3 days, when the body sizes of worms fed on LAB were smaller than those of controls, could reflect effective calorie control.
Ubiquinone (or coenzyme Q [CoQ]), an isoprenylated benzoquinone lipid important for electron transport in aerobic respiration, is another possible factor to explain our findings. Since nematodes fed an E. coli mutant that was defective in the ability to synthesize CoQ were reported have an increased life span (17), it is possible that the LAB did not have a full set of enzymes to synthesize CoQ and that this resulted in the extension of life span. However, Ishi et al. observed that the life span of worms could be prolonged by adding CoQ10 into the medium for the worms and discussed the possible role of ubiquinone as an antiaging substance (13).
Unknown components of LAB could contribute to the life-prolonging effects. DAF-16, a forkhead family transcription factor, regulates genes that promote dauer formation in larvae and stress resistance and longevity in adults (19). Recently, Oh et al. found that the c-Jun N-terminal kinase (JNK) family, a subgroup of the mitogen-activated protein kinase superfamily, is a positive regulator of DAF-16 (25a). LAB cell wall components are believed to be primarily responsible for immunostimulation, and differences in cell wall composition may account for the different levels of immunostimulation observed with different probiotics (27). Muramyl dipeptide, a peptidoglycan constituent of LAB, stimulates the production of cytokines by immunocytes. Since JNK is part of a signal transduction cascade that is activated by cytokines including tumor necrosis factor and interleukin-1 in mammals, it is possible that LAB extend the life span and enhance the host defense of nematodes by DAF-16 upregulated via the JNK pathway. The p38 mitogen-activated protein kinase pathway may also play an important role in the immunostimulatory and life-prolonging effects of LAB; this pathway was found to enhance life span via immune effectors distinct from those associated with DAF-16 (32).
C. elegans is a popular animal in molecular research on aging and has been used for some trials in extending the life span with nutrients (2, 13, 38), antioxidant substances (20, 37), and sirtuin activators (35). In those experiments, however, large amounts of chemicals were included in the agar. This study clearly showed that some kinds of food can increase life span and enhance the host defense of C. elegans. Further studies are in progress to clarify which compounds of LAB are essential for this influence, and varieties of worm mutants that have been deprived of signal transduction pathways may become a useful tool for elucidating the mechanisms of the beneficial effects of LAB. The nematode could be an appropriate model to screen useful probiotic strains or dietetic antiaging substances.
Acknowledgments
This study was supported in part by a grant-in-aid from the Graduate School of Human Life Science, Osaka City University.
The nematodes used in this work were kindly provided by T. Stiernagle at the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Footnotes
Published ahead of print on 17 August 2007.
REFERENCES
- 1.Aballay, A., P. Yorgey, and F. M. Ausubel. 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10:1539-1542. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, H., and N. Ishii. 2000. Effects of tocotrienols on life span and protein carbonylation in Caenorhabditis elegans. J. Gerontol. A 55:B280-B285. [DOI] [PubMed] [Google Scholar]
- 3.Baur, J. A., K. J. Pearson, N. L. Price, H. A. Jamieson, C. Lerin, A. Kalra, V. V. Prabhu, J. S. Allard, G. Lopez-Lluch, K. Lewis, P. J. Pistell, S. Poosala, K. G. Becker, O. Boss, D. Gwinn, M. Wang, S. Ramaswamy, K. W. Fishbein, R. G. Spencer, E. G. Lakatta, D. Le Couteur, R. J. Shaw, P. Navas, P. Puigserver, D. K. Ingram, R. de Cabo, and D. A. Sinclair. 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogden, J. D., and D. B. Louria. 2004. Nutrition and immunity in the elderly, p. 79-101. In D. A. Hughes, J. G. Darlington, and A. Bendich (ed.), Diet and human immune function. Humana Press, Totowa, NJ.
- 5.Bradley, S. F., and C. A. Kauffman. 1990. Aging and the response to salmonella infection. Exp. Gerontol. 25:75-80. [DOI] [PubMed] [Google Scholar]
- 6.Effros, R. B., R. L. Walford, R. Weindruch, and C. Mitcheltree. 1991. Influences of dietary restriction on immunity to influenza in aged mice. J. Gerontol. 46:B142-B147. [DOI] [PubMed] [Google Scholar]
- 7.Finch, C. E., and G. Ruvkun. 2001. The genetics of aging. Annu. Rev. Genomics Hum. Genet. 2:435-462. [DOI] [PubMed] [Google Scholar]
- 8.Garsin, D. A., C. D. Sifri, E. Mylonakis, X. Qin, K. V. Singh, B. E. Murray, S. B. Calderwood, and F. M. Ausubel. 2001. A simple model host for identifying gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 98:10892-10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grubeck-Loebenstein, B. 1997. Changes in the aging immune system. Biologicals 25:205-208. [DOI] [PubMed] [Google Scholar]
- 10.Hansen, B. C., N. L. Bodkin, and H. K. Ortmeyer. 1999. Calorie restriction in nonhuman primates: mechanisms of reduced morbidity and mortality. Toxicol. Sci. 52:56-60. [DOI] [PubMed] [Google Scholar]
- 11.Hayek, M. G., S. F. Taylor, B. S. Bender, S. N. Han, M. Meydani, D. E. Smith, S. Eghtesada, and S. N. Meydani. 1997. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J. Infect. Dis. 176:273-276. [DOI] [PubMed] [Google Scholar]
- 12.Hsin, H., and C. Kenyon. 1999. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399:362-366. [DOI] [PubMed] [Google Scholar]
- 13.Ishii, N., N. Senoo-Matsuda, K. Miyake, K. Yasuda, T. Ishii, P. S. Hartman, and S. Furukawa. 2004. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech. Ageing Dev. 125:41-46. [DOI] [PubMed] [Google Scholar]
- 14.Kealy, R. D., D. F. Lawler, J. M. Ballam, S. L. Mantz, D. N. Biery, E. H. Greeley, G. Lust, M. Segre, G. K. Smith, and H. D. Stowe. 2002. Effects of diet restriction on life span and age-related changes in dogs. J. Am. Vet. Med. Assoc. 220:1315-1320. [DOI] [PubMed] [Google Scholar]
- 15.Kurz, C. L., and M. W. Tan. 2004. Regulation of aging and innate immunity in C. elegans. Aging Cell 3:185-193. [DOI] [PubMed] [Google Scholar]
- 16.Labrousse, A., S. Chauvet, C. Couillault, C. L. Kurz, and J. J. Ewbank. 2000. Caenorhabditis elegans is a model host for Salmonella typhimurium. Curr. Biol. 10:1543-1545. [DOI] [PubMed] [Google Scholar]
- 17.Larsen, P. L., and C. F. Clarke. 2002. Extension of life-span in Caenorhabditis elegans by a diet lacking coenzyme Q. Science 295:120-123. [DOI] [PubMed] [Google Scholar]
- 18.Laws, T. R., S. V. Harding, M. P. Smith, T. P. Atkins, and R. W. Titball. 2004. Age influences resistance of Caenorhabditis elegans to killing by pathogenic bacteria. FEMS Microbiol. Lett. 234:281-287. [DOI] [PubMed] [Google Scholar]
- 19.Lee, S. S., S. Kennedy, A. C. Tolonen, and G. Ruvkun. 2003. DAF-16 target genes that control C. elegans life-span and metabolism. Science 300:644-647. [DOI] [PubMed] [Google Scholar]
- 20.Melov, S., J. Ravenscroft, S. Malik, M. S. Gill, D. W. Walker, P. E. Clayton, D. C. Wallace, B. Malfroy, S. R. Doctrow, and G. J. Lithgow. 2000. Extension of life-span with superoxide dismutase/catalase mimetics. Science 289:1567-1569. [DOI] [PubMed] [Google Scholar]
- 21.Metchnikoff, E. 1907. The prolongation of life. Heinemann, London, United Kingdom.
- 22.Miller, E., and N. Gay. 1997. Effect of age on outcome and epidemiology of infectious diseases. Biologicals 25:137-142. [DOI] [PubMed] [Google Scholar]
- 23.Moulias, R., A. Devillechabrolle, B. Lesourd, J. Proust, M. R. Marescot, S. Doumerc, M. Favre Berrone, F. Congy, and A. Wang. 1985. Respective roles of immune and nutritional factors in the priming of the immune response in the elderly. Mech. Ageing Dev. 31:123-137. [DOI] [PubMed] [Google Scholar]
- 24.Naidu, A. S., W. R. Bidlack, and R. A. Clemens. 1999. Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 39:13-126. [DOI] [PubMed] [Google Scholar]
- 25.Nicholas, H. R., and J. Hodgkin. 2004. Responses to infection and possible recognition strategies in the innate immune system of Caenorhabditis elegans. Mol. Immunol. 41:479-493. [DOI] [PubMed] [Google Scholar]
- 25a.Oh, S. W., A. Mukhopadhyay, N. Svrzikapa, F. Jiang, R. J. Davis, and H. A. Tissenbaum. 2005. JNK regulates lifespan in Caenor habditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 102:4494-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riddle, D. L., T. Blumenthal, B. J. Meyer, and J. R. Priess (ed.). 1997. C. elegans II. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 27.Rutherfurd-Markwick, K. J., and H. S. Gill. 2004. Probiotics and immunomodulation, p. 327-344. In D. A. Hughes, L. G. Darlington, and A. Bendich (ed.), Diet and human immune function. Humana Press, Totowa, NJ.
- 28.Schulenburg, H., C. L. Kurz, and J. J. Ewbank. 2004. Evolution of the innate immune system: the worm perspective. Immunol. Rev. 198:36-58. [DOI] [PubMed] [Google Scholar]
- 29.Stiernagle, T. 1999. Maintenance of C. elegans, p. 51-67. In I. A. Hope (ed.), C. elegans: a practical approach. Oxford University Press, New York, NY.
- 30.Sulston, J., and J. Hodgkin. 1988. Methods, p. 587-606. In W. B. Wood (ed.), The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Tan, M. W., S. Mahajan Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troemel, E. R., S. W. Chu, V. Reinke, S. S. Lee, F. M. Ausubel, and D. H. Kim. 2006. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2:1725-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viswanathan, M., S. K. Kim, A. Berdichevsky, and L. Guarente. 2005. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell 9:605-615. [DOI] [PubMed] [Google Scholar]
- 34.Weindruch, R., B. H. Devens, H. V. Raff, and R. L. Walford. 1983. Influence of dietary restriction and aging on natural killer cell activity in mice. J. Immunol. 130:993-996. [PubMed] [Google Scholar]
- 35.Wood, J. G., B. Rogina, S. Lavu, K. Howitz, S. L. Helfand, M. Tatar, and D. Sinclair. 2004. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430:686-689. [DOI] [PubMed] [Google Scholar]
- 36.Wu, D., S. L. Rea, A. I. Yashin, and T. E. Johnson. 2006. Visualizing hidden heterogeneity in isogenic populations of C. elegans. Exp. Gerontol. 41:261-270. [DOI] [PubMed] [Google Scholar]
- 37.Wu, Z., J. V. Smith, V. Paramasivam, P. Butko, I. Khan, J. R. Cypser, and Y. Luo. 2002. Ginkgo biloba extract EGb 761 increases stress resistance and extends life span of Caenorhabditis elegans. Cell. Mol. Biol. 48:725-731. [PubMed] [Google Scholar]
- 38.Zuckerman, B. M., and M. A. Geist. 1983. Effects of vitamin E on the nematode Caenorhabditis elegans. Age 6:1-4. [Google Scholar]