Abstract
A Coxiella-type microbe occurs at 100% frequency in all Amblyomma americanum ticks thus far tested. Using laboratory-reared ticks free of other microbes, we identified the Amblyomma-associated Coxiella microbe in several types of tissue and at various stages of the life cycle of A. americanum by 16S rRNA gene sequencing and diagnostic PCR. We visualized Amblyomma-associated Coxiella through the use of a diagnostic fluorescence in situ hybridization (FISH) assay supplemented with PCR-based detection, nucleic acid fluorescent staining, wide-field epifluorescence and confocal microscopy, and transmission electron microscopy (TEM). Specific fluorescent foci were observed in several tick tissues, including the midgut and the Malpighian tubules, but particularly bright signals were observed in the granular acini of salivary gland clusters and in both small and large oocytes. TEM confirmed intracellular bacterial structures in the same tissues. The presence of Amblyomma-associated Coxiella within oocytes is consistent with the vertical transmission of these endosymbionts. Further, the presence of the Amblyomma-associated Coxiella symbiont in other tissues such as salivary glands could potentially lead to interactions with horizontally acquired pathogens.
Ticks are the most important arthropod vector of disease in the United States and are a major human health concern worldwide (17). Ticks must acquire a blood meal from a vertebrate host at three separate stages of their life cycles in order to metamorphose and lay eggs. A range of viral, bacterial, and protozoan pathogens are acquired by the tick during feeding on one vertebrate host and are subsequently transmitted during the next meal. Blood meals can be separated by a year or longer (17). Pathogens, therefore, must sustain themselves within tick hosts for extended periods and remain transmissible upon feeding. Several pathogens concentrate within the salivary glands and associate with the copious secretions produced from these structures as they are delivered to the host during the blood meal (51). Other pathogens densely colonize Malpighian tubules (large organs that traverse the tick interior responsible for nitrogenous waste elimination) and reproductive tissues. Some disease agents, such as Rickettsia rickettsii, are occasionally vertically transmitted (i.e., transovarially) from female ticks to their progeny (10, 32, 43). Although most pathogens are transmitted horizontally, the maintenance of certain pathogens in tick populations may be significantly impacted by vertical transmission (10). In fact, tissue specificity in relation to the life cycle of the host is obscure for many tick-borne pathogens. We report here the within-tick distribution of a recently discovered Coxiella-type microbe. Our methods involve positive and negative controls at several stages, minimizing the incidence of false positives as well as the false negatives that often plague the characterization of pathogens in low abundance.
The lone star tick, Amblyomma americanum, is a widespread ixodid tick and an emerging disease vector in North America (12). A. americanum aggressively feeds on a variety of bird and mammalian hosts, including humans. It transmits to humans Ehrlichia chafeensis, the causative agents of human monocytotropic ehrlichiosis, and Borrelia lonestari, which may be the cause of southern tick-associated rash illness (15, 59). In addition to these pathogens, A. americanum has also been reported to harbor Francisella tularensis, several species of Rickettsia, and Coxiella burnetii (12, 36).
In addition to disease agents, ticks are known to carry nonpathogenic, symbiotic microbes (9, 13, 22, 50). The presumptive endosymbionts have been described as Rickettsia- and Wolbachia-like microbes on the basis of their ultrastructure (24, 25). Several different ticks have been reported to harbor microbes related to C. burnetii (43). C. burnetii is an obligate intracellular, gram-negative bacterium that often resides in the phagolysosome of infected mammalian cells and is the causative agent of acute Q fever and chronic endocarditis in humans (7, 44, 60, 61). C. burnetii has been detected in many animal species (2). Although more than 40 tick species can be infected with C. burnetii, direct transmission of C. burnetii to humans from arthropods has never been documented (54). In contrast, ticks may play a significant role in the transmission of C. burnetii among wild vertebrates (28, 33). Except for C. burnetii itself, there is no indication that the Coxiella-type microbes harbored by A. americanum cause disease in humans, although their presence could impact the colonization and transmission of other pathogens (31).
As part of a larger study focused on the microbial communities of disease vector ticks, we found a Coxiella-type microbe at 100% frequency in all A. americanum ticks tested (Amblyomma-associated Coxiella) (Clay et al., unpublished data). Contemporaneously, Jasinskas et al. (26) also found this microbe to be pervasively associated with A. americanum using cultivation-independent molecular approaches. Vertical transmission of this Coxiella-type microbe from female ticks to their progeny is supported by the ubiquity of this microbe in A. americanum and its presence in newly hatched, laboratory-reared larvae (Clay et al., unpublished data). In the present study, Coxiella was visualized in several A. americanum tissues and at various stages of the tick life cycle through the use of a diagnostic fluorescence in situ hybridization (FISH) assay supplemented with PCR-based detection, nucleic acid fluorescent staining, both wide-field epifluorescence and confocal microscopy, and transmission electron microscopy (TEM). Our results using FISH for specifically visualizing Amblyomma-associated Coxiella in diverse A. americanum tissues and cells provide compelling evidence to support vertical transmission, reveal a significant concentration of these microbes in tick salivary tissues, and present a valuable point of comparison for more detailed studies of pathogenic C. burnetii in ticks.
MATERIALS AND METHODS
Reagents and DNA manipulation.
DNA manipulations were carried out using standard techniques and enzymes purchased from New England Biolabs (Beverly, MA) and Promega (Madison, WI). DNA sequence was obtained with a Big Dye sequencing kit (Applied Biosystems, Foster City, CA) and analyzed on an ABI3730 automated sequencer operated by the Indiana University Molecular Biology Institute. Computer analysis of DNA sequences, including alignments, was performed with the Vector NTI program from Invitrogen.
Oligonucleotides used and developed in this study are summarized in Table 1. Probe EUB338, which is complementary to a portion of the 16S rRNA gene that is highly conserved in the domain Eubacteria, was used to visualize the entire bacterial population in tick tissues (1). The reverse complement of the PCR primer AAC-1f, designated AAC-1r, was used as a specific FISH probe for Coxiella (Table 1). All oligonucleotides used for FISH were custom synthesized by MWG-Biotech, Inc. (High Point, NC) and conjugated at their 5′ ends with the fluorescent dyes Cy3 and Cy5. In FISH experiments with the EUB338 probe, we developed a negative control probe, non-EUB338, that is the reverse, noncomplementary sequence of EUB338 (Table 1) (57). Likewise, we also developed a negative control probe for the Coxiella-specific probe designated non-AAC-1r, which is the reverse noncomplementary sequence of AAC-1r (Table 1).
TABLE 1.
Oligonucleotide primers used for PCR amplification and FISH analysis
| Primer | Nucleotide sequence | Reference or source |
|---|---|---|
| AAC-1f | 5′-TTTTTAAGGACCTCGCGCTA-3′ | This study |
| AAC-2r | 5′-TTCCCGAAGGCACCAAGTC-3′ | This study |
| AAC-1r | 5′-TAGCGCGAGGTCCTTAAAAA-3′ | This study |
| Non-AAC-1r | 5′-AAAAATTCCTGGAGCGCGAT-3′ | This study |
| EUB338 | 5′-GCTGCCTCCCGTAGGAGT-3′ | 1 |
| Non-EUB338 | 5′-TGAGGATGCCCTCCGTCG-3′ | 1 |
DNA purification and PCRs.
A. americanum and Dermacentor variabilis ticks were obtained from the Oklahoma State University tick facility (Stillwater, OK) or collected locally from sites in southern Indiana. Ticks were processed individually (adult ticks and larvae) and in pools (eggs). To extract genomic DNA, samples were mechanically crushed in liquid N2 with a sterile micropestle in sterile microcentrifuge tubes. DNA was extracted from homogenates with a DNeasy tissue kit (QIAGEN Inc., Valencia, CA) following the manufacturer's protocol. DNA was eluted with 400 μl (two amounts of 200 μl each) of elution buffer provided with the kit.
A standard PCR was performed with 5 μl of the extracted DNA as a template, a 0.8 μM concentration of each primer, 1.75 U of Taq DNA Polymerase (Eppendorf, AG, Hamburg, Germany), a 200 μM concentration of each deoxyribonucleoside triphosphate, 5 μl of 10× PCR buffer, and 5 μl of 25 mM magnesium solution (as supplied) in a total reaction volume of 50 μl. PCR conditions for the AAC-1f/AAC-2r primer pair were as follows: 35 cycles of 1 min at 92°C, 1 min at 55°C, and 2 min at 72°C. The amplification was completed by a final cycle of extension for 20 min at 72°C. PCRs were performed with an MJ Tetrad Thermal Cycler (Bio-Rad Laboratories, Richmond, CA).
Processing of tick samples for microscopy.
Adult and larval A. americanum ticks were processed for cold resin embedding, and adult D. variabilis ticks were processed similarly to serve as a negative control lacking Amblyomma-associated Coxiella. Dissected salivary glands and ovaries from A. americanum and D. variabilis were also processed for paraffin embedding, and smears were prepared from A. americanum eggs.
Each tick was sliced into two roughly equal sections using a sterile razor blade to facilitate penetration of solutions during the process of fixation. One-half of each adult tick was used for DNA extraction followed by PCR analysis, and the other half was fixed with 4% formaldehyde in phosphate-buffered saline (PBS) overnight at 4°C. The embedding procedure, utilizing cold polymerizing resin (Technovit 8100; Kulzer, Germany), was performed according to the manufacturer's instructions. Samples were washed in PBS (four times for 15 min), dehydrated with increasing concentrations of ethanol, and infiltrated with the resin solution (Technovit 8100 base liquid with hardener I) overnight at 4°C. Hardener II was subsequently added, and samples were aligned properly in the conical tip of an embedding capsule (Electron Microscopy Sciences, Hatfield, PA). Several (three to five) larval segments or one-half of an adult tick was polymerized in a single mold. Polymerized capsules were stored at 4°C prior to sectioning. Capsules were sectioned on an ultramicrotome (Porter-Blum MT-2; Sorvall Inc.) using glass knives. Tissue sections (2 μm) were straightened on sterile water, placed on silanized microscope slides (Superfrost/Plus; Fisher Scientific), and dried on a slide warmer at 55°C.
Dissected tissues.
To remove ovaries and salivary glands, ticks were dissected individually on a new slide in a drop of cold PBS using microscissors (VANNAS scissors; Electron Microscopy Sciences). One-half of each pair of salivary glands and one-half of each ovary were frozen in liquid N2 immediately after dissection and kept at −80°C until DNA extraction. The remaining portion of the dissected tissues was used for FISH and Gimenez staining (paraffin embedding) or electron microscopy (epoxy embedding).
For paraffin embedding, immediately after dissection, samples were fixed in 10% neutral buffered formalin. The samples were washed in buffer and dehydrated in a graded ethanol series prior to being embedded in paraffin, sectioned into 5-μm thick sections, and mounted on Superfrost/Plus slides (Fisher Scientific). Prior to hybridization, sections were deparaffinized (three changes of xylene at 5 min each) and rehydrated by immersion for 2 min in a graded dilution series from 100% ethanol to distilled water.
Egg processing.
Freshly laid eggs from a single engorged female were collected over the period of 72 h following egg deposition. The eggs were used for DNA extraction (regular protocol) or for FISH and for analysis using the general DNA stain 4′,6′-diamidino-2-phenylindole (DAPI). After mechanical removal of the chorionic membrane (NaCl-Triton [1:1] bleach solution for 2 to 3 min), eggs were fixed in heptane-paraformaldehyde in PBS for 10 min, devitellinized in methanol, smeared on glass slides, and then air dried. These samples were treated with RNase (2 mg/ml RNase A in PBS at room temperature for 1 h) and stained with the probe conjugated with Cy3, according to the standard FISH protocol, and then stained with DAPI (1 μg/ml in PBS) for 30 min, washed, and mounted (Permount; Fisher Scientific).
FISH, histological staining, and microscopy.
FISH protocols were partially adapted from protocols available from Genedetect.com (http://www.genedetect.com/index.htm). For prehybridization, prewarmed hybridization solution (HS) (Sigma) without the probe was added to slides with mounted sections and incubated at 46°C for 30 min in humid chambers. For hybridization, slides were incubated in a dark humid chamber at 46°C for 3 h in HS with 50 ng/μl of fluorescent probe (probe:HS ratio of 1:10). The volume of solution (10 to 50 μl) added to each sample was adjusted depending upon tissue size. Following hybridization, slides were washed as follows: (i) briefly washed at room temperature in 1× SSC (0.15 M NaCl, 0.015 M sodium citrate), (ii) washed twice for 15 min in 1× SSC at 55°C, (iii) washed twice for 15 min in 0.5× SSC at 55°C, and (iv) washed one final time for 10 min in 0.5× SSC at room temperature. After the washing steps, slides were air dried and mounted in Permount (after cold resin embedding) or glycerol (after paraffin embedding) and stored in darkness at 4°C. All samples were examined by confocal microscopy (Bio-Rad MRC 600) or epifluorescence wide-field microscopy (Nikon E800). Image processing was performed with MetaMorph, version 6.2, software. For samples stained with DAPI, the stain was applied simultaneously with the FISH probe. Additional negative control experiments involved either treatment of slides with RNase (40 μg/ml in RNase buffer, 20 mM Tris-Cl [pH 7.4], 1 mM EDTA) for 1 h at 37°C prior to probe hybridization or experiments performed in the absence of probes. The general histological Gimenez staining protocol was performed as described previously (8).
The microscopy systems used were a Nikon E800 for epifluorescence microscopy and a Bio-Rad MRC 600 confocal head with a Nikon Optiphot microscope, both maintained in the Indiana University Molecular Biology Institute. For all experiments, 60× and 100× objectives were used. Illumination for epifluorescence on the Nikon E800 was from an Osram high-pressure mercury lamp (type 1 × HBO 103 W/2). The filter cubes used for DAPI, fluorescein isothiocyanate (FITC), Cy3, and Cy5 staining were the UV-2E/C, FITC HyQ, tetramethyl rhodamine isocyanate HyQ, and a C-84000 Quad cube, respectively, used in conjunction with a 620- to 660-nm excitation filter installed in a filter wheel (Nikon Instruments, Inc.). Gimenez staining was observed with light from a halogen bright-field lamp. A Hamamatsu Orca ER charge-coupled-device camera was connected to the Nikon E800. The Bio-Rad MRC 600 used a krypton-argon laser with laser lines for fluorochrome excitation at 488 nm and 568 nm. BHS and YHS filter cubes (Bio-Rad Laboratories, Inc.) were used for FITC and Cy3, respectively, in conjunction with the 60× objective. For all confocal microscopy, images were rendered using the COMOS program, version 7.0a. For all epifluorescence and light micrographs, images were processed using Metamorph software, version 6.2.
TEM.
For TEM, dissected salivary glands or ovaries were fixed in 0.1 M cacodylate buffer containing 2.5% glutaraldehyde for 2.5 h at 4°C. The fixed samples were then postfixed in the same buffer containing 1% OsO4 for 1 h at 4°C. Samples were then dehydrated in a graded ethanol dehydration series, embedded in Spurr's low-viscosity resin (Electron Microscopy Sciences), and polymerized at 65°C. Thin sections (ca. 80 nm) from embedded samples were prepared using a Sorvall, Inc., Porter-Blum MT 2 ultramicrotome and were mounted on 200-mesh formvar-coated copper grids; samples were stained with uranyl acetate, lead citrate, and bismuth (4) and observed under a TEM instrument (JEOL 1010; JEOL, Inc., Tokyo, Japan) at 60 and 80 kV. TEM micrographs were processed using Gatan Digital Micrograph software.
RESULTS
Detection of Coxiella-type microbes in laboratory-reared A. americanum ticks.
Small subunit 16S rRNA gene clone libraries generated from 40 single A. americanum ticks, both collected from the field and obtained from the Oklahoma State University rearing facility, revealed the presence of an abundant and ubiquitous microbe similar to, but distinct from C. burnetii (Clay et al., unpublished data). Due to the similarity of these microbes with C. burnetii, we described them as Amblyomma-associated Coxiella bacteria. The Amblyomma-associated Coxiella sequences are identical to those recently reported by Jasinskas et al. (accession no. AY939824) (27; see also Fig. S1 in the supplemental material). Based on 16S rRNA gene sequence alignments with Amblyomma-associated Coxiella isolates, C. burnetii, and other tick-associated microbes, a short insertion region was identified that is unique to the genus Coxiella and matches no other sequences present in the databases (41 nucleotides for the Amblyomma-associated Coxiella sequences located between positions 202 to 224 relative to the E. coli 16S rRNA gene sequence). This insertion is recognizable in both the Amblyomma-associated Coxiella sequence and that of C. burnetii (see Fig. S1 in the supplemental material), but a portion of the primary sequence is divergent between these two microbes, allowing discrimination. A forward-reading primer (AAC-1f) that anneals to this Amblyomma-associated Coxiella-specific region and a second primer (AAC-2r) complementary to a region 835 bp downstream in the 16S rRNA gene sequence (positions 841 to 860 relative to E. coli 16S rRNA gene) were utilized to develop a specific diagnostic PCR assay (Table 1). In this study, the PCR assay was employed to detect and evaluate the Amblyomma-associated Coxiella microbe in A. americanum ticks obtained from the pathogen-free rearing facility at Oklahoma State University.
PCR amplification was performed using the AAC-1f/AAC-2r primer set, and total DNA was isolated from individual adult ticks (12 adults comprised of 3 females and 9 males), individual larvae (a total of 30), and pools of 10 eggs collected from 4 engorged females. These reactions revealed the presence of the Amblyomma-associated Coxiella microbe in all tested stages of tick development (Fig. 1). Results of specific PCR assays of tick extracts and analysis of 16S rRNA gene sequence libraries from these ticks indicated that none of the ticks or eggs sampled were positive for Rickettsia spp., E. chafeensis, B. lonestari, or the Francisella-type symbiont (Dermacentor andersoni symbiont) from the dog tick D. variabilis (data not shown). This suggests that the laboratory-reared A. americanum ticks are effectively free of additional microbes often found in wild populations. Comparison of nucleotide sequences for the 835-bp AAC-1f/AAC-2r 16S rRNA gene amplicons from all ticks indicated 100% identity between Amblyomma-associated Coxiella at these different stages of tick development. No sequence differences between any Amblyomma-associated Coxiella amplicons from any life stage, tissue, or isolate have been observed, suggesting that each tick carries a clonal Amblyomma-associated Coxiella population and that this same microbial lineage is present in all A. americanum ticks. The Amblyomma-associated Coxiella 16S rRNA gene sequence shares 93% identity with that from C. burnetii, indicating that these microbes may qualify as different genera (Table 2). Percent similarities with 16S rRNA gene sequences from other tick-associated, Coxiella-type microbes were also tabulated. The Amblyomma-associated Coxiella microbe from A. americanum ticks is as equally distinct from Coxiella-like symbionts characterized from other tick genera as it is from C. burnetii.
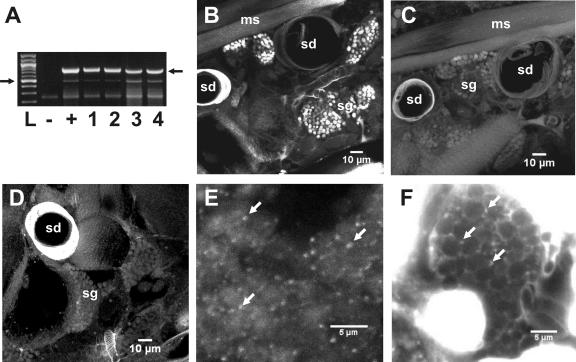
FIG. 1.
Detection of Amblyomma-associated Coxiella symbiont in whole adult tick sections. (A) PCR detection of Amblyomma-associated Coxiella endosymbionts in DNA from A. americanum adults, larvae, and eggs. PCR amplifications used AAC-1f and AAC-2r1 primers. Lanes: −, negative control using an equivalent DNA concentration of an extract from D. variabilis; +, positive control using a plasmid carrying the Amblyomma-associated Coxiella amplicon; 1, single male tick; 2, single female tick; 3, single larva; 4, pool of 10 eggs. Filled arrows indicate the 835-bp Amblyomma-associated Coxiella-specific amplicon and the 600-bp size standard in the ladder. Sodium borohydrate-buffered 1% agarose gel stained with ethidium bromide was used. See Materials and Methods for details. (B) FISH of 2-μm section of the whole adult A. americanum stained with the specific Coxiella AAC-1r-Cy3 probe. (C) Negative control hybridization with non-AAC-1r-Cy3 probe. (D) RNase treatment control hybridized with AAC-1r-Cy3. Salivary gland from the whole tick body section stained with AAC-1r-Cy3 probe (E) and with DAPI (F) is shown at higher magnification. Arrows in panels E and F indicate fluorescent foci. ms, muscles; sg, salivary glands; sd, salivary duct.
TABLE 2.
Identity of the 16S rRNA gene sequences from Coxiella-type bacteriaa
| Organism (accession number) | % Identity
|
|||||
|---|---|---|---|---|---|---|
| Amblyomma- associated Coxiella | C. burnetti | R. sanguineus | O. moubata | H. concinnae | H. longicornis | |
| Amblyomma-associated Coxiella | 100 | 93 | 93 | 93 | 93 | 92 |
| Coxiella burnetii (M21291) | 100 | 94 | 90 | 95 | 93 | |
| Rhipicephalus sanguineus symbiont (D84559) | 100 | 88 | 95 | 93 | ||
| Ornithodoros moubata symbiont (AB001521) | 100 | 94 | 86 | |||
| Hemaphysalis concinnae symbiont (AF521888) | 100 | 92 | ||||
| Hemaphysalis longicornis symbiont (AB001519) | 100 | |||||
Visualization of Amblyomma-associated Coxiella in whole-body sections of unfed A. americanum ticks.
FISH was performed on resin-embedded 2-μm sections from unfed A. americanum adults. Initial experiments were performed with the general bacterial probe EUB338 (Table 1) labeled with FITC. Specific fluorescent foci were observed in several tick tissues, including the midgut and the Malpighian tubules immediately below the cuticle. Particularly bright signals were observed in the granular acini of salivary gland clusters (see Fig. S2 in the supplemental material). As observed by others (23), salivary glands were problematic because they autofluoresce and bind DNA probes nonspecifically. Therefore, great care was taken to perform all microscopy with identical settings and to perform extensive controls on adjacent sections. Hybridization with the FITC-labeled negative control probe non-EUB338 (Table 1) did not generate the fluorescent signals, and RNase treatment of the sections prior to EUB338 addition also abolished the specific labeling (see Fig. S2 in the supplemental material).
To localize the Amblyomma-associated Coxiella microbe, we used the AAC-1r oligonucleotide (Table 1) 5′ end labeled with the Cy3 fluorescent dye. A strong orange-to-yellow signal comprised of punctate fluorescent foci was again observed at several specific sites, including the Malpighian tubules but most notably in the salivary glands within the tick sections (Fig. 1B). This signal was resistant to bleaching. Treatment with RNase prior to probe addition abolished hybridization with the probe, and fluorescence was also absent when sections were hybridized with the non-AAC-1r negative control probe (Table 1) labeled with Cy3 (Fig. 1C and D). At higher resolution, fluorescent foci of approximately 0.5 μm in diameter were observed between secretory granules within the acini, globular multicellular structures connected to salivary ducts that comprise the grape-like morphology of the salivary tissue (Fig. 1E). Several sections revealed fluorescent signal in the posterior portion of the tick, presumptively associated with the midgut, although this was less consistently observed than that in salivary tissue (see Fig. S3 in the supplemental material). Several samples were stained with DAPI, and fluorescent structures of similar dimensions and distribution to those observed in the FISH sections were observed and presumed to be bacteria (Fig. 1F). Simultaneous hybridizations with the FISH probe along with DAPI staining revealed consistent overlap between these signals (data not shown).
To determine whether the Amblyomma-associated Coxiella-specific signals colocalized with the signals observed for the general bacterial probe EUB338, simultaneous hybridization of whole body sections of the adult tick was performed with specific AAC-1r-Cy3 probe (red) and the eubacterial EUB338-FITC probe (green). Overlaid images from sections hybridized with both probes revealed that they labeled the same regions of the tissue, with staining of the same intensity and distribution within the salivary glands (Fig. 2). FISH was also performed on resin-embedded 2-μm sections of whole unfed A. americanum larvae (10 larvae) using the AAC-1r-Cy3 oligonucleotide. Larval tissues are less well defined than those of adult ticks, but some primordial organs could be identified (Fig. 3A). Hybridized sections revealed the presence of fluorescent foci within salivary gland clusters and in the complex epidermal tissue immediately underlying the larval cuticle (Fig. 3B and C). No signals were observed when the non-AAC-1r negative control probe was used (data not shown).
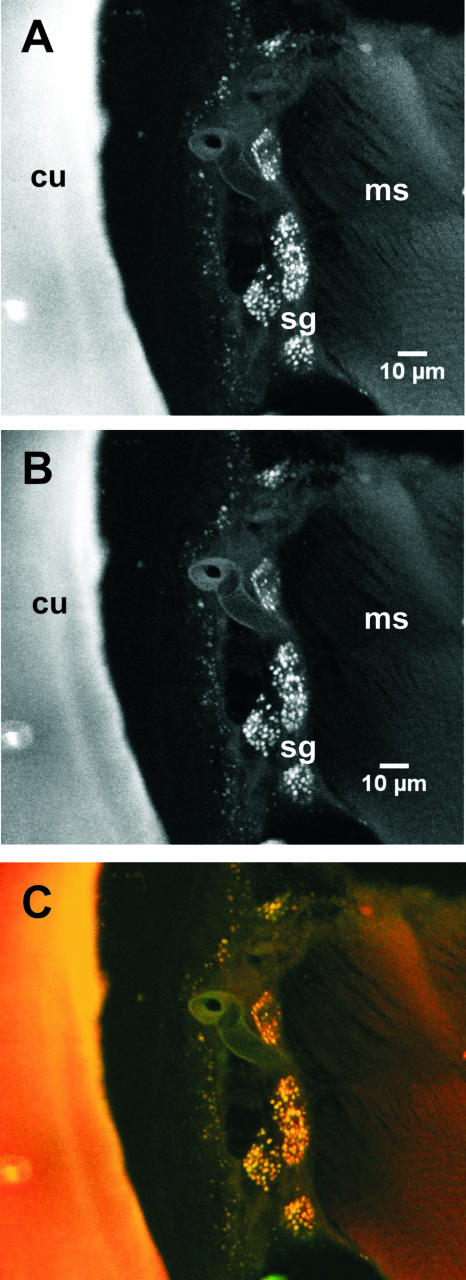
FIG. 2.
Double FISH analysis with Coxiella-specific and eubacterial probes. Confocal microscopy of FISH with double staining of sections from adult female A. americanum. (A) Confocal micrograph of AAC-1r-Cy3-specific staining. (B) Confocal micrograph of Eub338 fluorescein staining. (C) Overlay image of panels A and B. cu, cuticle; ms, muscles; sg, salivary glands.
FIG. 3.
Confocal FISH microscopy of A. americanum larvae. Sections hybridized with AAC-1r-Cy3 probe. (A) A 2-μm section of a whole larva. (B) Higher magnification of the boxed section shown in panel A. (C) Epidermal tissue. Arrows in panels B and C show fluorescent foci. cu, cuticle; ms, muscles; sg, salivary glands; mp, Malpighian tubules.
Detection and localization of Amblyomma-associated Coxiella in dissected salivary tissues.
Unfed adult ticks were carefully dissected, and salivary tissue was harvested (Fig. 4A). DNA was extracted from a portion of these salivary tissues and used as the template for PCR amplification with the AAC-1f/AAC-2r primer set. An abundant Amblyomma-associated Coxiella amplicon was readily obtained from all dissected salivary glands tested (Fig. 4A). Salivary gland tissues excised from male and female adult A. americanum ticks were harvested and embedded in paraffin. Salivary gland tissues were also dissected from a D. variabilis female for use as a Coxiella-free negative control. Sections of the paraffin-embedded tissue (5 μm) were used for FISH experiments using the Amblyomma-associated Coxiella-specific probes. These probes were labeled with Cy5 instead of the Cy3 used in previous experiments because of an improved signal-to-noise ratio. Images were collected under constant conditions of brightness, contrast, and exposure time. Very bright staining was observed in granular acini (Fig. 4B) in both male and female salivary glands of A. americanum ticks. The paraffin-embedded, dissected salivary tissue labeled very similarly to the same tissue visualized in whole ticks, but the resolution was improved due to less background autofluorescence in the Cy5 emission range (Fig. 4C). Numerous Amblyomma-associated Coxiella-specific fluorescent foci of bacterial dimensions were clearly observed between salivary granules. Salivary tissues excised from D. variabilis ticks did not stain significantly above background with the AAC-r1 probe, and the punctate foci were not observed (data not shown). Intensity measurements of these micrographs showed that average fluorescence intensity of A. americanum salivary glands probed with the AAC-r1-Cy5 probe was approximately sixfold above the signal obtained with no probe addition, whereas identically processed and hybridized D. variabilis salivary tissue was indistinguishable from the no-probe control. The common histological protocol of Gimenez staining for examining tissues infected with bacteria confirmed that smears of A. americanum salivary gland tissue are densely colonized with bacteria (Fig. 4D). Despite differences in microscopy and staining techniques, the distribution and size of these presumptive bacteria are very similar to the patterns observed using FISH on salivary tissues and the FISH and DAPI patterns in whole tick sections.
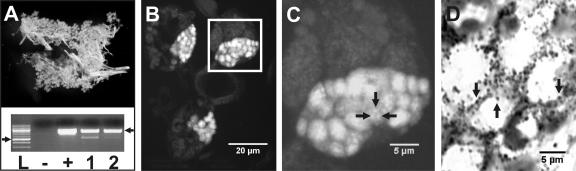
FIG. 4.
Examination of dissected A. americanum salivary tissues. Salivary glands dissected from an adult unfed female A. americanum tick are shown. (A) Dissected salivary glands from a single tick and PCR with specific Amblyomma-associated Coxiella primers (lanes 1 and 2) show Amblyomma-associated Coxiella microbes present in salivary glands from two different ticks (identical conditions and controls as described in the legend of Fig. 1). Filled arrows mark Amblyomma-associated Coxiella amplicon and size standard as described in the legend of Fig. 1. (B) Epifluorescence microscopy of FISH of salivary glands stained with specific AAC-1r-Cy5 probe. (C) Higher magnification of the boxed section shown in panel B. Arrows show fluorescent foci. (D) Gimenez-stained smear preparations of dissected salivary glands observed by bright-field microscopy. Arrows mark presumptive bacteria.
Detection and localization of Amblyomma-associated Coxiella in female reproductive tissues.
The ubiquitous distribution of the Amblyomma-associated Coxiella microbe and its presence in every life stage strongly suggest that this microbe is vertically transmitted. A recent study from a different group also supports this interpretation (26). Vertically transmitted microbes must colonize female reproductive tissue prior to or during fertilization. We therefore examined reproductive tissues of recently engorged female A. americanum ticks. The primordial ovarian tissue in unfed adult female ticks is highly reduced and only matures following a blood meal prior to ovulation. Mature reproductive tissues were dissected from engorged A. americanum females during oviposition (Fig. 5A). To serve as a negative control, mature reproductive tissues were also dissected from D. variabilis females at a similar life stage. During oviposition, oocytes are passed from the ovary into the ovarian lumen and from there are transported into the uterus by peristaltic movement of the ovarian tube and the oviduct (3). It was possible to isolate oocytes at all stages of development from this tissue—from immature oocytes associated with the ovaries (stage I) to mature oocytes localized in the oviduct (stage V) and ready for external deposition (3) (Fig. 5A).
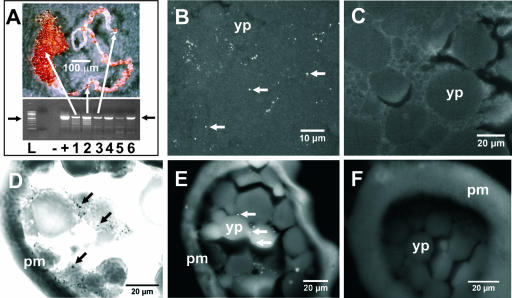
FIG. 5.
Examination of dissected A. americanum reproductive tissues. Reproductive tissues dissected from adult unfed female A. americanum ticks and identically prepared D. variabilis ticks. (A) Ovary dissected from an engorged A. americanum female. PCR of DNA extracted from ovarian tissue (lane 1), oocyte dissected from oviduct (lane 2), and eggs at stage 0 h (lane 3) 20 h (lane 4), 48 h (lane 5), and 72 h (lane 6). Filled arrows mark Amblyomma-associated Coxiella amplicon and size standard as described in the legend of Fig. 1, and white arrows correlate samples to regions of dissected ovarian tissue. (B) Epifluorescence FISH of an A. americanum oocyte at high magnification. (C) Negative control of D. variabilis oocytes stained with AAC-1r-Cy5 probe. (D) Gimenez-stained section from a dissected A. americanum ovary. (E) FISH microscopy of tissue section from a dissected ovary of A. americanum shows oocytes stained with AAC-1r-Cy5 probe. (F) Negative control of tissue section in which no probe was added. Arrows show fluorescent foci and presumptive bacteria. yp, yolk plates; pm, plasma membrane.
Purified DNA samples from ovarian tissue, newly produced oocytes transiting the oviduct, and eggs laid 0 to 72 h following oviposition were examined for the presence of Amblyomma-associated Coxiella using a diagnostic PCR assay. The assays clearly demonstrate the presence of Amblyomma-associated Coxiella throughout these different stages of the life cycle, with a readily obtained Amblyomma-associated Coxiella-specific amplicon (Fig. 5A). No such product was obtained for the D. variabilis tissue. These amplicons and those obtained from salivary tissue and from whole ticks have identical nucleotide sequences.
In parallel, a portion of the dissected ovaries was embedded in paraffin and processed into 5-μm sections. The A. americanum sections were analyzed by FISH with the AAC-1r-Cy5 probe, revealing punctate fluorescent foci distributed throughout the cytoplasm of the oocyte and located in between yolk plates (Fig. 5B and E, yp). These Amblyomma-associated Coxiella-specific particles were observed in both small and large oocytes. Sections stained with the non-AAC-1r-Cy5 negative control probe had no discernible signal, and no fluorescent foci were visible in hybridizations of the AAC-1r-Cy5 probe with sections of D. variabilis ovarian tissue (Fig. 5C and F). Similar particles in both large and small oocytes were localized between yolk plates in Gimenez-stained sections of the dissected A. americanum ovaries (Fig. 5D). Amblyomma-associated Coxiella-specific fluorescent particles were also observed in smears of prefixed eggs collected at 15 h postoviposition (see Fig. S4 in the supplemental material). These structures were consistent among multiple egg smears but were randomly distributed and widely dispersed. Double-staining experiments with FISH and DAPI were in complete agreement with colocalization of both stains to these fluorescent structures (data not shown). The presence of Amblyomma-associated Coxiella within oocytes is consistent with the idea that these microbes are endosymbiotic and vertically transmitted.
Localization of bacterial structures within ovarian and salivary cells by electron microscopy.
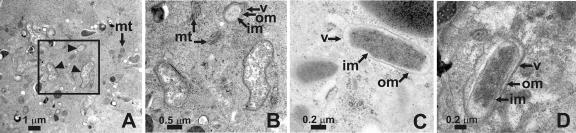
Ovarian and salivary gland tissue from A. americanum was also processed and sectioned for TEM. Intracellular structures, consistent with bacteria in size and structure and distinct from mitochondria and other cellular bodies, were observed in ovarian tissues (Fig. 6A and B). Three membranes were visible surrounding these structures when samples were examined at higher magnification (Fig. 6B and C, v, im, and om). Analysis of salivary gland sections revealed the presence of structures strikingly similar in shape and size within cells (Fig. 6D). In both cases, the presumptive bacteria are clearly intracellular. Given that Amblyomma-associated Coxiella microbes were the only bacteria we routinely detected within laboratory-reared A. americanum ticks and that ovarian and salivary tissues appear to be enriched for Amblyomma-associated Coxiella sequences, it is evident that these structures observed by TEM are in fact Amblyomma-associated Coxiella microbes.
FIG. 6.
TEM analysis of dissected salivary glands and ovaries from A. americanum. (A) TEM image of ovarian tissue from an A. americanum engorged female with endosymbiotic bacteria. Arrowheads indicate bacterium-type structures. (B) Higher magnification of the boxed section shown in panel A. TEM showing detailed structure of endosymbionts in ovary of engorged adult female (C) and in salivary glands of unfed adult female (D). mt, mitochondria; v, cytoplasmic membrane-limited vacuole; om, outer cell membrane; im, inner cell membrane.
DISCUSSION
In this study, we took advantage of a unique sequence motif within the 16S rRNA gene sequence of Coxiella species to develop a PCR assay and a FISH probe specific to the Amblyomma-associated Coxiella symbiont. In a parallel study aimed at determining bacterial diversity within A. americanum, we found the Amblyomma-associated Coxiella microbe at 100% frequency across a large number of A. americanum ticks with broad geographic distribution (Clay et al., unpublished data). During the course of this study, a different group of investigators also characterized the Amblyomma-associated Coxiella microbe (26). They also conclude that Amblyomma-associated Coxiella is a ubiquitous endosymbiont. Based on molecular comparisons, Amblyomma-associated Coxiella is closely related to the Q fever pathogen, C. burnetii, and other Coxiella-type bacteria associated with both ixodid and argasid ticks (5, 29, 30, 35, 43). Using ticks from the Oklahoma State University Laboratory colony and field collections, PCR assays and FISH analyses were complemented with general staining approaches and TEM to localize Amblyomma-associated Coxiella within the tick. Amblyomma-associated Coxiella occurs across different stages of the tick life cycle and abundantly colonizes salivary tissue, Malpighian tubules, the midgut, and ovarian tissues of engorged females.
Early microscopic observations of insect tissues led to the estimate that 15 to 20% of all insects live in symbiotic relationships with bacteria (9). More sophisticated microscopy and molecular methods have largely supported this estimate (20). Many hosts have multiple symbiotic bacteria classified as primary and secondary symbionts (38, 48, 53, 56). Primary symbionts are thought to have a long evolutionary history with the host animal, while secondary symbionts are more recently acquired. Primary symbionts often have small genomes, derived through a process of reductive genome evolution (41), and provide a nutritional benefit to the host (14). The benefit of secondary symbionts to the host is usually not known but may include heat tolerance and protection from pathogens (37, 39). Other arthropods such as spiders (arachnids), mites, and ticks (acarids) are also commonly associated with endosymbiotic bacteria (21, 43). In most cases, the functions of these endosymbionts relative to the host have not been determined. Based on the role of bacterial insect symbionts, a nutritional function is most likely although other functions such as antibiotic production and protection against parasites and pathogens may also be possible (27, 39). The Amblyomma-associated Coxiella appears to be ubiquitous and exhibits a reduction in genome size compared to C. burnetii (26). Both observations are consistent with the idea that Amblyomma-associated Coxiella serves as a primary endosymbiont in A. americanum, perhaps by providing key resources that supplement nutritionally limited blood meals. It is also possible that the Amblyomma-associated Coxiella could negatively impact the host tick through reproductive parasitism, similarly to Wolbachia (58). The ability of A. americanum to survive and/or molt without Amblyomma-associated Coxiella could be tested by using heat or antibiotics to clear the bacteria, similar to the method described in a recent report (62).
Detection of the Amblyomma-associated Coxiella symbiont using FISH.
FISH is a powerful technique for integrating molecular identification of microbes with in situ visualization (40). FISH has been used extensively to examine microbes in diverse environments, including those involved in host associations, but it has only recently been applied to tick-borne bacteria. In one example, Hammer et al. (23) investigated the distribution of Borrelia spp. within laboratory-raised Ixodes ricinus ticks deliberately inoculated with the bacteria through feeding on infected animal hosts. These authors highlighted some of the difficulties in detecting Borrelia within tick tissues, including obtaining appropriate, sectioned material and minimizing background fluorescence. Low numbers of Borrelia cells were visualized clustered near the midgut tissue, with the spirochete cell morphology visible in several images. Although no Borrelia cells were observed in salivary tissues, muscles, or central ganglia, these tissues are often colonized prior to transmission (23). In the present study, we detected Amblyomma-associated Coxiella in A. americanum ticks that had been reared in the laboratory under sterile conditions and were free of other types of bacteria. Two different approaches for tissue and whole-tick embedment were employed, and numerous controls with identical microscopic settings were utilized to validate the fluorescence signals obtained. Cy5-labeled probes provided the best signal-to-noise ratio and were ideal for clear visualization of Amblyomma-associated Coxiella. Examination of the same tissue from dissected D. variabilis, free of Coxiella-type bacteria, also provided a useful control. In contrast to the horizontally acquired Borrelia spp. described by Hammer et al. (23), we found that vertically transmitted Amblyomma-associated Coxiella localized to the salivary tissues, midgut, and Malpighian tubules, as well as ovarian tissues in engorged female ticks. With the appropriate measures and controls, FISH provides a powerful method for tracking tick-borne bacterial infections in situ.
A recent study used quantitative PCR to measure the relative abundance of the Amblyomma-associated Coxiella in three different dissected tissues (26). Amblyomma-associated Coxiella was detected in greatest abundance in midgut tissues, followed by the ovaries, and was present at lowest levels in salivary glands. We also detected Amblyomma-associated Coxiella by FISH in these three tissues (plus PCR detection in salivary glands and ovaries) and Malpighian tubules. However, in contrast, we detected only low numbers of Amblyomma-associated Coxiella microbes in the midgut, with far greater fluorescence signal in salivary glands and ovaries. Great expansion of ovarian tissue occurs in engorged females, perhaps amplifying the resident Amblyomma-associated Coxiella population. In general, clean dissection of tissue such as the midgut can be quite challenging, and direct visualization in whole tick sections using FISH may be less confounded by contamination between tissues than quantitative PCR.
Localization of Amblyomma-associated Coxiella to ovarian tissues and Malpighian tubules.
Vertically transmitted symbiotic bacteria must colonize the reproductive tissue to directly infect progeny. Consistent with vertical transmission, our findings reveal that Amblyomma-associated Coxiella is present in A. americanum ovules within newly developing ovarian tissue following a blood meal. The microbe was present as eggs traversed the ovarian duct, deposited externally, and metamorphosed into larval ticks. Intracellular microbes described as rickettsiae have been reported within the ovaries of several different tick species (18, 19, 24, 46, 52). The Amblyomma-associated Coxiella examined here has been found only in A. americanum ticks, but other related symbionts appear to occupy the same niche in different ticks (Table 2). The mechanisms leading to vertical transmission in ticks and most other arthropods are poorly understood. Primordial ovarian tissue may be colonized by Amblyomma-associated Coxiella during tick development, and subsequently this population may be amplified with the growth of this tissue during ovulation (49). Alternatively, this microbe may colonize this tissue after the final blood meal along with the influx of nutrients during the period of active endocytosis and vitellin formation within developing oocytes (47). In unfed adult females, we could not clearly identify the undeveloped ovaries and therefore cannot answer this question definitively.
Although the reproductive tissues from arthropod hosts of vertically transmitted symbionts must be colonized at some point during host development, it is clear that these same microbes may also reside in somatic tissues of the host (11, 29, 46, 55). The presence of intracellular bacteria morphologically similar to C. burnetii was revealed by TEM within ovarian tissues of Ixodes woodi females and also in Malpighian tubules (29). The same distribution of symbionts closely related to C. burnetii has been reported in association with Ornithodoros moubata, Rhipicephalus sanguineus, and Haemaphysalis longicornis (43). Although these results suggested that the microorganisms are restricted to Malpighian tubules and oocytes, other studies using electron microscopy described rickettsial microorganisms in various organs (ovary, salivary glands, midgut, and Malpighian tubules) of laboratory-reared O. moubata and Argas arboreus (19, 46). Similarly, Amblyomma-associated Coxiella occurs in a variety of tissues of A. americanum including, but not restricted to, the ovaries. Reinhardt et al. (46) reported that the ovaries and Malpighian tubules of O. moubata ticks were infected with two different microorganisms referred to as symbiont A and symbiont B. Our FISH results and PCR assays indicate that Amblyomma-associated Coxiella microbes with identical 16S rRNA sequences are present within both ovaries and salivary glands of A. americanum.
Amblyomma-associated Coxiella in the granular acini of salivary glands.
Similar to several horizontally transmitted pathogens, Amblyomma-associated Coxiella colonizes the salivary tissues. These tissues are comprised of multicellular, secretory bodies (acini, the individual grape-like structures observed in Fig. 1 and 4). Different subtypes of acini can be distinguished by the presence or absence of visible secretory granules (6). For granular acini, each acinus contains 15 to 17 cells of multiple forms that can comprise two types of acini, distinguished by differences in organization and morphology (see reference 49 for a review of salivary gland structure). We found that Amblyomma-associated Coxiella colonizes many of the granular acini. Large acini with numerous granules occur in two classes, type II and type III (see reference 6 for a detailed description of these structures). In our studies we cannot distinguish between type II and type III acini. Within the acinus, a subset of cells is colonized, and the Amblyomma-associated Coxiella bacteria occupy spaces between the secretory granules (Fig. 4B to D).
It is common for tick-borne pathogens localized in the salivary glands, such as Borrelia burgdorferi, to be transmitted into the bloodstream of hosts during feeding. During the blood meal, massive secretions of enzymes, anticoagulants, and numbing agents are mobilized from the salivary glands, promoting the extended feeding periods of ixodid ticks (51). Granular acini and their secretory granules are the source of much of this material. Pathogens associated with secreted material are then introduced into the bloodstream. Conversely, blood-borne pathogens present in infected hosts can colonize the salivary tissues of pathogen-free ticks following ingestion of the blood meal. For horizontally transmitted pathogens such as B. burgdorferi, the acquisition of the infectious agent by ticks and subsequent transmission through the tick salivary tissue are important components of the pathogen life cycle. The abundance of Amblyomma-associated Coxiella salivary tissues raises the possibility that these bacteria could be transmitted to host animals through tick bites. A different intracellular bacterium, Anaplasma marginale, is transmitted to cattle through the salivary glands by feeding ticks (16). There are no reports of horizontal transmission mechanisms for Amblyomma-associated Coxiella, but until now there has been no reason to screen for its presence in vertebrate hosts. Even if they are transmitted to vertebrate hosts, Amblyomma-associated Coxiella microbes may not survive or sufficiently proliferate to allow subsequent transmission to a second tick or damage to the animal host. However, the potential for horizontal transmission of Amblyomma-associated Coxiella, which is closely related to the virulent human pathogen C. burnetii, to human and animal hosts warrants further investigation.
Intracellular bacterial structures revealed by TEM.
Rickettsia-like microorganisms were observed by TEM in early studies examining organs of laboratory-bred O. moubata ticks, including oocytes, Malpighian tubules, and salivary glands (46). More recently, TEM examination of Ixodes woodi ticks from a laboratory colony suggested that female ticks harbored a single endosymbiotic bacterium related to species of Rickettsia and C. burnetii (29). The ultrastructure and intracellular location of these endosymbionts of O. moubata and I. woodi are similar to those we have observed in A. americanum. The intracellular structures we have observed are comparable in shape and size to these endosymbiotic bacteria, residing within vacuoles and surrounded by typical multiple membrane structures for gram-negative bacteria. TEM examination revealed structures within cells of salivary glands and ovarian tissues that are characteristic of intracellular bacteria. The A. americanum ticks we examined were free of any other microbes. These presumptive bacteria are surrounded by three electron-dense layers likely to be membranes, consistent with endosymbiotic bacteria from other arthropods (Fig. 6) (14). The outermost membrane is often host derived, while the one to two internal membranes are analogous to those of free-living bacteria. These structures are clearly distinct from mitochondria and other organelles. At higher resolution, the rod-shaped intracellular bodies observed in salivary tissue and those in the ovaries are very similar (Fig. 6C and D). In mammalian cells, C. burnetii exists in several different morphological forms depending on its stage of growth, and the differently shaped membrane-bound bodies we observe may also reflect such variants (34). In some studies, C. burnetii was deliberately inoculated into ticks, and it can be present in great abundance in specific tissues (45, 52). Although the Amblyomma-associated Coxiella is readily visible at low magnification using FISH, identification of the natural bacteria-type structures in the tick tissue using TEM is more challenging, given their relatively low abundance. These cell-like structures are almost certainly Amblyomma-associated Coxiella microbes as there were no other bacteria detected from the laboratory-reared ticks. It remains a formal possibility, however, that the observed intracellular bodies are not Amblyomma-associated Coxiella but, rather, another microbe present in large numbers in tick tissues that was not detected using other approaches.
Conclusions.
It is intriguing to consider how the Amblyomma-associated Coxiella microbe was acquired by A. americanum. The similarity between this and several other tick endosymbionts to C. burnetii raises the question of whether the symbiont arose from a pathogenic microbe or, conversely, whether the modern pathogen arose from a symbiont. Examination of natural vertebrate host populations may reveal the presence of the Amblyomma-associated Coxiella within a horizontal transmission reservoir. Conversely, inoculation of Amblyomma-associated Coxiella into naïve animal hosts would help determine the pathogenic potential of this microbe. For example, a Francisella symbiont (D. andersoni symbiont) of the western dog tick Dermacentor andersoni caused disease when directly inoculated into guinea pigs, but the pigs remained symptom free when fed upon by the D. andersoni symbiont-infected ticks (42). This could also be true for Amblyomma-associated Coxiella. However, limited genome structure data of Amblyomma-associated Coxiella suggest that it is experiencing reductive genome evolution and thus perhaps has lost the genetic components required for blood-borne infectivity. The prevalence of this microbe in A. americanum also means that colonization of the tick by horizontally acquired pathogens such as E. chafeensis may lead to the interaction of the pathogen with the resident symbiont. These interactions with Amblyomma-associated Coxiella may influence maintenance or transmission of the pathogen, thereby impacting human disease transmission.
Supplementary Material
Acknowledgments
We acknowledge the assistance and input of Sue Childress, Thomas Danhorn, Deanna Oleske, Michael Wade, Curtis Lively, and Robert Pinger during the development of this project.
This research was supported by the Ecology of Infectious Disease program of the National Science Foundation and the National Institutes of Health through grant DEB-03268742.
Footnotes
Published ahead of print on 24 August 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amann, R. I., L. Krumholz, and D. A. Stahl. 1990. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babudieri, B. 1959. Q fever: a zoonosis. Adv. Vet. Sci. 5:81-182. [Google Scholar]
- 3.Balashov, Y. S. 1972. Bloodsucking ticks (Ixodoidea): vectors of disease of man and animals. Misc. Publ. Entomol. Soc. Amer. 8:161-376. [Google Scholar]
- 4.Barrett, J. M., P. M. Heidger, and S. W. Kennedy. 1975. Chelated bismuth as a stain in electron microscopy. J. Histochem. Cytochem. 23:780-783. [DOI] [PubMed] [Google Scholar]
- 5.Bernasconi, M. V., S. Casati, O. Peter, and J. C. Piffaretti. 2002. Rhipicephalus ticks infected with Rickettsia and Coxiella in Southern Switzerland (Canton Ticino). Infect. Genet. Evol. 2:111-120. [DOI] [PubMed] [Google Scholar]
- 6.Binnington, K. C. 1978. Sequential changes in salivary gland structure during attachment and feeding of the cattle tick, Boophilus microplus. Int. J. Parasitol. 8:97-115. [DOI] [PubMed] [Google Scholar]
- 7.Brouqui, P., H. T. Dupont, M. Drancourt, Y. Berland, J. Etienne, C. Leport, F. Goldstein, P. Massip, M. Micoud, A. Bertrand, et al. 1993. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Arch. Intern. Med. 153:642-648. [DOI] [PubMed] [Google Scholar]
- 8.Bruneval, P., J. Choucair, F. Paraf, J. P. Casalta, D. Raoult, F. Scherchen, and J. L. Mainardi. 2001. Detection of fastidious bacteria in cardiac valves in cases of blood culture negative endocarditis. J. Clin. Pathol. 54:238-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY.
- 10.Burgdorfer, W., S. F. Hayes, and A. J. Mavros. 1981. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii, p. 585-594. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, NY.
- 11.Chen, W. J., K. H. Tsai, S. L. Cheng, C. G. Huang, and W. J. Wu. 2005. Using in situ hybridization to detect endosymbiont Wolbachia in dissected tissues of mosquito host. J. Med. Entomol. 42:120-124. [DOI] [PubMed] [Google Scholar]
- 12.Childs, J. E., and C. D. Paddock. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 48:307-337. [DOI] [PubMed] [Google Scholar]
- 13.Clay, K., C. Fuqua, C. Lively, and M. Wade. 2006. Microbial community ecology of tick-borne human pathogens, p. 41-57. In S. Collinge and C. Ray, (ed.), Disease ecology. Oxford University Press, Oxford, United Kingdom.
- 14.Dale, C., and N. A. Moran. 2006. Molecular interactions between bacterial symbionts and their hosts. Cell 126:453-465. [DOI] [PubMed] [Google Scholar]
- 15.Dawson, J. E., J. W. Ewing, W. R. Davidson, J. E. Childs, S. E. Little, and S. M. Standaert. 2005. Human monocytotropic ehrlichiosis, p. 239-257. In J. L. Goodman, D. T. Dennis, and D. E. Sonenshine (ed.), Tick-borne diseases of humans. ASM Press, Washington, DC.
- 16.de la Fuente, J., E. F. Blouin, and K. M. Kocan. 2003. Infection exclusion of the rickettsial pathogen Anaplasma marginale in the tick vector Dermacentor variabilis. Clin. Diagn. Lab. Immunol. 10:182-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennis, D. T., and J. F. Piesman. 2005. Overview of tick-borne infections of humans, p. 3-11. In J. L. Goodman, D. T. Dennis, and D. E. Sonenshine (ed.), Tick-borne diseases of humans. ASM Press, Washington, DC.
- 18.Diehl, P. A., A. Aeschliemann, and F. D. Obenchain. 1982. Tick reproduction: oogenesis and oviposition, p. 272-350. In F. D. Obechain and R. Galun (ed.), Physiology of ticks. Pergamon Press, Oxford, United Kingdom.
- 19.el Shoura, S. M. 1990. Ultrastructure and distribution of intracellular rickettsia-like microorganisms in various organs of the laboratory-reared adult tick Argas (Persicargas) arboreus (Ixodoidea: Argasidae). Exp. Appl. Acarol. 9:137-143. [DOI] [PubMed] [Google Scholar]
- 20.Gil, R., A. Latorre, and A. Moya. 2004. Bacterial endosymbionts of insects: insights from comparative genomics. Environ. Microbiol. 6:1109-1122. [DOI] [PubMed] [Google Scholar]
- 21.Goodacre, S. L., O. Y. Martin, C. F. Thomas, and G. M. Hewitt. 2006. Wolbachia and other endosymbiont infections in spiders. Mol. Ecol. 15:517-527. [DOI] [PubMed] [Google Scholar]
- 22.Grindle, N., J. J. Tyner, K. Clay, and C. Fuqua. 2003. Identification of Arsenophonus-type bacteria from the dog tick Dermacentor variabilis. J. Invertebr. Pathol. 83:264-266. [DOI] [PubMed] [Google Scholar]
- 23.Hammer, B., A. Moter, O. Kahl, G. Alberti, and U. B. Gobel. 2001. Visualization of Borrelia burgdorferi sensu lato by fluorescence in situ hybridization (FISH) on whole-body sections of Ixodes ricinus ticks and gerbil skin biopsies. Microbiology 147:1425-1436. [DOI] [PubMed] [Google Scholar]
- 24.Hayes, S., and W. Burgdorfer. 1989. Interaction between rickettsial endocytobionts and their tick hosts, p. 236-251. In W. Schwemmler (ed.), Insect endocytobiosis: morphology, genetics, evolution. CRC Press, Inc., Boca Raton, FL.
- 25.Hayes, S. F., and W. Burgdorfer. 1981. Ultrastructural comparisons of Wolbachia-like symbiotes of ticks (Acari: Ixodidae), p. 281-333. In W. Burgdorfer and A. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, NY.
- 26.Jasinskas, A., J. Zhong, and A. G. Barbour. 2007. Highly prevalent Coxiella sp. bacterium in the tick vector Amblyomma americanum. Appl. Environ. Microbiol. 73:334-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaltenpoth, M., W. Gottler, G. Herzner, and E. Strohm. 2005. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr. Biol. 15:475-479. [DOI] [PubMed] [Google Scholar]
- 28.Kazar, J. 2005. Coxiella burnetii infection. Ann. N. Y. Acad. Sci. 1063:105-114. [DOI] [PubMed] [Google Scholar]
- 29.Kurtti, T. J., A. T. Palmer, and J. H. Oliver, Jr. 2002. Rickettsiella-like bacteria in Ixodes woodi (Acari: Ixodidae). J. Med. Entomol. 39:534-540. [DOI] [PubMed] [Google Scholar]
- 30.Lee, J. H., H. S. Park, W. J. Jang, S. E. Koh, T. K. Park, S. S. Kang, B. J. Kim, Y. H. Kook, K. H. Park, and S. H. Lee. 2004. Identification of the Coxiella sp. detected from Haemaphysalis longicornis ticks in Korea. Microbiol. Immunol. 48:125-130. [DOI] [PubMed] [Google Scholar]
- 31.Lively, C. M., K. Clay, W. J. Wade, and C. Fuqua. 2005. Competitive coexistence of vertically and horizontally transmitted parasites. Evol. Ecol. Res. 7:1183-1190. [Google Scholar]
- 32.Macaluso, K. R., D. E. Sonenshine, S. M. Ceraul, and A. F. Azad. 2002. Rickettsial infection in Dermacentor variabilis (Acari:Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 39:809-813. [DOI] [PubMed] [Google Scholar]
- 33.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaul, T. F., and J. C. Williams. 1981. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J. Bacteriol. 147:1063-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mediannikov, O., L. Ivanov, M. Nishikawa, R. Saito, Y. N. Sidelnikov, N. I. Zdanovskaya, I. V. Tarasevich, and H. Suzuki. 2003. Molecular evidence of Coxiella-like microorganism harbored by Haemaphysalis concinnae ticks in the Russian Far East. Ann. N. Y. Acad. Sci. 990:226-228. [DOI] [PubMed] [Google Scholar]
- 36.Mixson, T. R., S. R. Campbell, J. S. Gill, H. S. Ginsberg, M. V. Reichard, T. L. Schulze, and G. A. Dasch. 2006. Prevalence of Ehrlichia, borrelia, and rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J. Med. Entomol. 43:1261-1268. [DOI] [PubMed] [Google Scholar]
- 37.Montllor, C. B., A. Maxman, and A. H. Purcell. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27:189-195. [Google Scholar]
- 38.Moran, N. A., and P. Baumann. 2000. Bacterial endosymbionts in animals. Curr. Opin. Microbiol. 3:270-275. [DOI] [PubMed] [Google Scholar]
- 39.Moran, N. A., P. H. Degnan, S. R. Santos, H. E. Dunbar, and H. Ochman. 2005. The players in a mutualistic symbiosis: insects, bacteria, viruses, and virulence genes. Proc. Natl. Acad. Sci. USA 102:16919-16926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moter, A., and U. B. Gobel. 2000. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J. Microbiol. Methods 41:85-112. [DOI] [PubMed] [Google Scholar]
- 41.Nakabachi, A., A. Yamashita, H. Toh, H. Ishikawa, H. E. Dunbar, N. A. Moran, and M. Hattori. 2006. The 160-kilobase genome of the bacterial endosymbiont Carsonella. Science 314:267. [DOI] [PubMed] [Google Scholar]
- 42.Niebylski, M. L., M. G. Peacock, E. R. Fischer, S. F. Porcella, and T. G. Schwan. 1997. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl. Environ. Microbiol. 63:3933-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noda, H., U. G. Munderloh, and T. J. Kurtti. 1997. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 63:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peacock, M. G., R. N. Philip, J. C. Williams, and R. S. Faulkner. 1983. Serological evaluation of Q fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect. Immun. 41:1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Øeháèek, J., and G. Šutáková. 1989. Interaction between Dermacentor reticulatus cells and Coxiella burnetii in vivo. Acta Virol. 33:465-473. [PubMed] [Google Scholar]
- 46.Reinhardt, C., A. Aeschliman, and H. Hecker. 1972. Distribution of Rickettsia-like microorganisms in various organs of an Ornithodorus moubata laboratory strain (Ixodoidea: Argasidae) as revealed by electron microscopy. Z. Parasitenkd. 39:201-209. [DOI] [PubMed] [Google Scholar]
- 47.Rosell, R., and L. B. Coons. 1992. The role of the fat body, midgut and ovary in vitellogenin production and vitellogenesis in the female tick, Dermacentor variabilis. Int. J. Parasitol. 22:341-349. [DOI] [PubMed] [Google Scholar]
- 48.Sandstrom, J. P., J. A. Russell, J. P. White, and N. A. Moran. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217-228. [DOI] [PubMed] [Google Scholar]
- 49.Sauer, J. R., J. L. McSwain, A. S. Bowman, and R. C. Essenberg. 1995. Tick salivary gland physiology. Annu. Rev. Entomol. 40:245-267. [DOI] [PubMed] [Google Scholar]
- 50.Schabereiter-Gurtner, C., W. Lubitz, and S. Rolleke. 2003. Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. J. Microbiol. Methods 52:251-260. [DOI] [PubMed] [Google Scholar]
- 51.Sonenshine, D. E. 2005. The biology of tick vectors of human disease, p. 12-36. In J. L. Goodman, D. T. Dennis, and D. E. Sonenshine (ed.), Tick-borne diseases of humans. ASM Press, Washington, DC.
- 52.Šutáková, G., and J. Øeháèek. 1991. Endocytobionts in Dermacentor reticulatus ticks (Ixodidae): an electron microscope study. Exp. Appl. Acarol. 11:57-72. [DOI] [PubMed] [Google Scholar]
- 53.Thao, M. L., and P. Baumann. 2004. Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr. Microbiol. 48:140-144. [DOI] [PubMed] [Google Scholar]
- 54.Thompson, H. A., D. T. Dennis, and G. A. Dasch. 2005. Q fever, p. 328-342. In J. L. Goodman, D. T. Dennis, and D. E. Sonenshine (ed.), Tick-borne diseases of humans. ASM Press, Washington, DC.
- 55.Tsai, K. H., J. C. Lien, C. G. Huang, W. J. Wu, and W. J. Chen. 2004. Molecular (sub) grouping of endosymbiont Wolbachia infection among mosquitoes of Taiwan. J. Med. Entomol. 41:677-683. [DOI] [PubMed] [Google Scholar]
- 56.Tsuchida, T., R. Koga, H. Shibao, H. Matsumoto, and T. Fukatsu. 2002. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid Acyrthosiphon pisum. Mol. Ecol. 11:2123-2135. [DOI] [PubMed] [Google Scholar]
- 57.Wallner, G., R. Amann, and W. Beisker. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136-143. [DOI] [PubMed] [Google Scholar]
- 58.Werren, J. H. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42:587-609. [DOI] [PubMed] [Google Scholar]
- 59.Wormser, G. P., E. Masters, J. Nowakowski, D. McKenna, D. Holmgren, K. Ma, L. Ihde, L. F. Cavaliere, and R. B. Nadelman. 31 August 2005, posting date. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin. Infect. Dis. 41:958-965. [DOI] [PubMed] [Google Scholar]
- 60.Yuasa, Y., K. Yoshiie, T. Takasaki, H. Yoshida, and H. Oda. 1996. Retrospective survey of chronic Q fever in Japan by using PCR to detect Coxiella burnetii DNA in paraffin-embedded clinical samples. J. Clin. Microbiol. 34:824-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, G. Q., A. Hotta, M. Mizutani, T. Ho, T. Yamaguchi, H. Fukushi, and K. Hirai. 1998. Direct identification of Coxiella burnetii plasmids in human sera by nested PCR. J. Clin. Microbiol. 36:2210-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong, J., A. Jasinskas, and A. G. Barbour. 2007. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS ONE 2:e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.