Abstract
In spite of its clinical and nutritional importance, l-alanyl-l-glutamine (Ala-Gln) has not been widely used due to the absence of an efficient manufacturing method. Here, we present a novel method for the fermentative production of Ala-Gln using an Escherichia coli strain expressing l-amino acid α-ligase (Lal), which catalyzes the formation of dipeptides by combining two amino acids in an ATP-dependent manner. Two metabolic manipulations were necessary for the production of Ala-Gln: reduction of dipeptide-degrading activity by combinatorial disruption of the dpp and pep genes and enhancement of the supply of substrate amino acids by deregulation of glutamine biosynthesis and overexpression of heterologous l-alanine dehydrogenase (Ald). Since expression of Lal was found to hamper cell growth, it was controlled using a stationary-phase-specific promoter. The final strain constructed was designated JKYPQ3 (pepA pepB pepD pepN dpp glnE glnB putA) containing pPE167 (lal and ald expressed under the control of the uspA promoter) or pPE177 (lal and ald expressed under the control of the rpoH promoter). Either strain produced more than 100 mM Ala-Gln extracellularly, in fed-batch cultivation on glucose-ammonium salt medium, without added alanine and glutamine. Because of the characteristics of Lal, no longer peptides (such as tripeptides) or dipeptides containing d-amino acids were formed.
Glutamine (Gln) is the most abundant free-form amino acid in human cells. Gln is not an essential amino acid for humans, but it is considered to be a conditionally essential amino acid because of its central role in nitrogen metabolism (7, 40). In spite of such nutritional importance, Gln has hardly been used as a component of parenteral nutrition due to its low solubility and instability in solution. One approach to overcome the physicochemical limitations of Gln is to supply the amino acid as a dipeptide by conjugation with other amino acids. Alanyl-glutamine (Ala-Gln) is the most suitable Gln-containing dipeptide. Since there have been a number of reports on the safety and effectiveness of this dipeptide (2, 7), the clinical and nutritional importance of Ala-Gln has already been established.
Several methods for producing Ala-Gln have been described; chemical or enzymatic condensation of protected alanine (Ala) and Gln (3, 22, 24, 43) and a chemical synthetic process via d-2-chloropropionyl-glutamine (32). These methods, however, have not been satisfactory in regard to cost-effectiveness and quality. Most methods need protection steps and sometimes deprotection steps, lowering production efficiency (8). The protecting reactions also trigger racemization of the substrate amino acid(s), which results in the formation of an undesired by-product, d-alanyl-glutamine. These chemical and enzymatic methods have also been suggested to form other by-products, such as different dipeptides (for example, alanyl-glutamic acid) or longer oligopeptides (for example, tripeptides) (32, 43).
Recently, we identified a new enzyme named l-amino acid α-ligase (Lal) in Bacillus subtilis (38). This enzyme catalyzes dipeptide synthesis from unprotected amino acids in an ATP-dependent manner. The substrate specificity was so wide that it could synthesize 44 different dipeptides, including Ala-Gln and alanyl-alanine (Ala-Ala), but not glutaminyl-alanine and alanyl-glutamic acid. Lal never formed tripeptides or longer peptides and did not react with d-amino acids. These characteristics prompted us to explore the possibility of producing Ala-Gln using an Escherichia coli strain expressing Lal directly from glucose and ammonia, identical to amino acid fermentation.
However, the dipeptide-degrading activity of the cells, which is apparently necessary for protein turnover and homeostasis (20), had to be taken into consideration; E. coli possesses more than 40 peptide bond-breaking activities (20). Four peptidases, encoded by pepN, pepA, pepB, and pepD, have been reported to degrade a broad spectrum of dipeptides (16, 20). Unfortunately, it is unclear to what extent these peptidases contribute to the total dipeptide degradation in the cell, although disruption of the peptidases made the strain unable to grow on certain dipeptides (21). Another consideration when using E. coli expressing Lal is the low affinity of Lal for Gln. The Km of Lal for Gln has been reported to be 107 mM (38), whereas the intracellular concentration of Gln in E. coli under normal conditions has been reported to be around 1.2 mM (29), implying that the enzyme works poorly in vivo.
In the present report, we show how to overcome the above-mentioned complications and demonstrate a novel fermentative process for the production of Ala-Gln.
MATERIALS AND METHODS
Microorganisms, plasmids, and media.
The E. coli strains and plasmids used in this study are listed in Table 1. Strain NM522 was used for plasmid construction. For test tube cultivation, 0.8 ml of an overnight culture in LB medium (10 g/liter Bacto tryptone, 5 g/liter yeast extract, and 5 g/liter NaCl) was transferred into a test tube with 8 ml of TT medium [10 g/liter glucose, 16 g/liter K2HPO4, 14 g/liter KH2PO4, 2 g/liter (NH4)2SO4, 5 g/liter Casamino acid, 1 g/liter citric acid, 2 g/liter MgSO4·7H2O, 50 mg FeSO4·7H2O, 10 mg/liter MnSO4·7H2O, and 10 mg/liter thiamine HCl] and vigorously shaken (220 rpm) for 24 h at 30°C. To maintain the plasmid(s), 100 mg/liter of kanamycin and/or 25 mg/liter of chloramphenicol was added and 0.2 g/liter of proline (Pro) was provided for the proline auxotroph strain. For the amino-acid feeding experiment, 2.5 g/liter each of Ala and Gln was added initially. For cultivation in a jar fermentor, 100 ml of LB medium with kanamycin cultured in a 1-liter Erlenmeyer flask at 30°C for 16 h was transferred into a 5-liter jar fermentor (Mitsuwa Rikagaku Kogyo, Japan) containing 1.9 liters of J medium (10 g/liter glucose, 6 g/liter Na2HPO4, 3 g/liter KH2PO4, 5 g/liter NaCl, 1 g/liter NH4Cl, 5 g/liter yeast extract, 10 mg/liter MnSO4·7H2O, 2 g/liter MgSO4·7H2O, 200 mg/liter FeSO4·7H2O, and 10 mg/liter thiamine HCl) with kanamycin and Pro (0.3 g/liter) and cultivated at 30°C with aeration (2 liter/min) and agitation (600 rpm). When the initial glucose was depleted, continuous feeding of a glucose solution (600 g/liter) was begun. One liter of the glucose solution was supplied at a controlled rate to keep the glucose concentration of the culture medium at 30 ± 10 g/liter. During cultivation, the pH was kept at 7.0 with 28% (vol/vol) NH4OH. At each time point indicated (see Fig. 4), a sample (5 ml) was taken and a portion (1 ml) was used to measure the optical density at 660 nm. The rest of the sample was centrifuged immediately to get a supernatant, which was used for the assay of glucose, amino acids, and dipeptides concentrations. The resting-cell reaction for measuring the Ala-Gln degradation rate was carried out as follows. A test tube with LB medium from the overnight culture was centrifuged, and the cells were washed twice with ice-cold 50 mM KH2PO4 (pH 7.2). The cells were suspended in the same buffer at an optical density of 4 (660 nm). Two milliliters of cell suspension was mixed with 0.1 ml of Ala-Gln solution (100 g/liter); part of the sample (0.1 ml) was taken as time point zero, and the rest was incubated at 30°C with vigorous shaking. Samples (0.1 ml) were taken every 3 h and centrifuged. The Ala-Gln concentrations of the supernatants were assayed as described in “Analytical procedures” below. As the degradation rates were almost constant during the first 9 h, the average value during this period was taken as the degradation rate.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype/phenotype or genea | Source or reference |
|---|---|---|
| Strains | ||
| NM522 | Δ(lac-proAB) hsdΔ5 supE thi [F′ lacIqlacZΔM15 proBA] | Stratagene |
| JM101 | supE thi-1 Δ(lac-proAB) [F′ traD36 lacIqlacZΔM15 proAB] | Stratagene |
| JKYQ1 | JM101 glnB glnE | This work |
| JKYP1 | JM101 pepA | This work |
| JKYP2 | JM101 pepB | This work |
| JKYP3 | JM101 pepD pro* | This work |
| JKYP4 | JM101 pepN | This work |
| JKYP5 | JM101 dpp | This work |
| JKYP6 | JM101 pepD pepN pro* | This work |
| JKYP7 | JM101 pepD pepN pepB pro* | This work |
| JKYP8 | JM101 pepD dpp pro* | This work |
| JKYP9 | JM101 pepD pepN dpp pro* | This work |
| JKYP10 | JM101 pepD pepN pepA dpp pro* | This work |
| JKYP11 | JM101 pepD pepN pepB dpp pro* | This work |
| JKYPQ1 | JKYP9 glnB glnE | This work |
| JKYPQ2 | JKYPQ1 putA pepA | This work |
| JKYPQ3 | JKYPQ2 pepB | This work |
| Plasmids | ||
| pQE-60 | Col E1-based vector; ampicillin resistance | QIAGEN |
| pQE60ywfE | lal; ampicillin resistance | 38 |
| pTK31 | pBR322-based vector; trp promoter; kanamycin resistance | This work |
| pPE56 | pTK31 lal under trp promoter; kanamycin resistance | This work |
| pPE86 | pTK31 lal and ald under trp promoter; kanamycin resistance | This work |
| pPE167 | trp promoter region of pPE86 was replaced by uspA promoter | This work |
| pPE177 | trp promoter region of pPE86 was replaced by rpoH promoter | This work |
Whenever the pepD gene was disrupted, the strain became a proline auxotroph (indicated by pro*), probably because of the close positions of the pepD and proAB genes.
FIG. 4.
Time course of dipeptide fermentation. JKYPQ2/pPE86 (a), JKYPQ3/pPE167 (b), or JKYPQ3/pPE177 (c) was cultivated with J medium in a fed-batch manner. Cell growth (×) and the amounts of Ala (⋄), Gln (□), Ala-Gln (•), and Ala-Ala (▴) that accumulated in the cultivation medium were measured. Each strain was cultivated at least three times, and representative results are shown.
DNA manipulation.
DNA manipulations were basically performed according to the method of Sambrook et al. (31). The primers used in this study are listed in Table 2. The pTK31 plasmid, a derivative of pTrS30 (23) containing the replication origin of pBR322, a kanamycin resistance gene from pUC4K (Pharmacia Biotech), and the tryptophan promoter from E. coli K-12 was used as an expression vector (see the supplemental material). The lal gene was amplified from pQE60ywfE (38) by PCR to be flanked by an XhoI site and a BamHI site. The trp promoter region was amplified from pTrS30 by PCR to be flanked by an EcoRI site and an XhoI site. The 1.4-kb lal gene PCR product was digested with NcoI and BamHI, and the 0.4-kb trp promoter PCR product was digested with EcoRI and XhoI and inserted between the EcoRI and BamHI sites of pTrS30. The resulting plasmid, designed to express lal under the control of the trp promoter, was named pPE56. The l-alanine dehydrogenase (ald) gene was amplified from the B. subtilis (ATCC 15245) genome by PCR to introduce BamHI sites at both termini. The 1.2-kb ald gene PCR product was digested with BamHI and inserted between the BamHI sites of pPE56 in the same direction as lal to yield pPE86. The ribosome-binding site (RBS) of pQE-60 (QIAGEN, Germany) was amplified by PCR to be flanked by a ClaI site and a SphI site. The amplified 0.3-kb RBS region was digested with ClaI and SphI and inserted between the ClaI and SphI sites of pTK31 to yield pTKQE31. The uspA promoter region was amplified from the E. coli W3110 genome by PCR to be flanked by an EcoRI site and a ClaI site. The rpoH promoter region was amplified from the E. coli W3110 genome by PCR to be flanked by an EcoRI site and a ClaI site. The 0.4-kb PCR product of the uspA promoter region was digested with EcoRI and ClaI and inserted between the EcoRI and ClaI sites of pTKQE31 to yield pUATKQE31. The 0.4-kb rpoH promoter region amplified by PCR was similarly inserted into pTKQE31 to yield pRHTKQE31. The uspA promoter and RBS region were cut from pUAKQE31 with EcoRI and NcoI and inserted between the EcoRI and NcoI sites of pPE86. In the resulting plasmid, pPE167, the lal and ald genes were downstream of the uspA promoter-RBS region. A similar construct, but with the rpoH promoter and the RBS region of pRHTKQE31 instead of the uspA-RBS region, was named pPE177.
TABLE 2.
Primers
| Gene or region | Template | Stranda | Primer (5′-3′) |
|---|---|---|---|
| lal | pQE60ywfE | S | TACACTCGAGATTAAAGAGGAGAAATTAA |
| A | TTAGGATCCTCATACTGGCAGCACATACTT | ||
| trp promoter | pTrS30 | S | CAAGAATTCTCATGTTTGACAGCT |
| A | TAACTCGAGATTCCCTTTTTACGTGAAC | ||
| ald | B. subtilis genome | S | AAAGGATCCCATATACAGGAGGAGACAGAT |
| A | TATGGATCCTTAAGCACCCGCCACAGATGA | ||
| RBS of pQE-60 | pQE-60 | S | TACAATCGATATTAAAGAGGAGAAATTAA |
| A | TACAGCATGCAACTGACTGAAATGCCTCAA | ||
| ushA promoter | E. coli genome | S | GATGAATTCATACGAGCGGCTCTATAGATAGTGTAG |
| A | CATAAGCTTATCGATACTCCTTCCATAAAGTTGTCGATGACT | ||
| rpoH promoter | E.coli genome | S | TCAGAATTCAGTTTGATATCAATGGCTTAT |
| A | CTCAATCGATATCTTCTGGCGCTTCAGTGG | ||
| pepD | pKD13 | S | CTAACCCTGTGACCTGCAATACTGTTTTGCGGGTGAGTGTAGGCTGGAGCTGCTTC |
| A | GAAACTGCCGGAAGGCGATTAAACGCCATCCGGCAGATTCCGGGGATCCGTCGACC | ||
| pepN | pKD13 | S | TTACGCAACAGGAATAGACTGAACACCAGACTCTATGTGTAGGCTGGAGCTGCTTC |
| A | AGAAAACAGGGGTAAATTCCCCGAATGGCGGCGCTAATTCCGGGGATCCGTCGACC | ||
| dpp | pKD13 | S | GCATCCCCACCTCATAACGTTGACCCGACCGGGCAAGTGTAGGCTGGAGCTGCTTC |
| A | CTGTACGGCATTTTGCTATGCTTGTCGCCACTGTTGATTCCGGGGATCCGTCGACC | ||
| pepA | pKD3 | S | ATGGAGTTTAGTGTAAAAAGCGGTAGCCCGGAGAAAGTGTAGGCTGGAGCTGCTTC |
| A | TTACTCTTCGCCGTTAAACCCAGCGCGGTTTAACAGCATATGAATATCCTCCTTAG | ||
| pepB | pKD3 | S | ATGACAGAAGCGATGAAGATTACCCTCTCTACCCAAGTGTAGGCTGGAGCTGCTTC |
| A | TTACGCCGTTAACAGATTAGCTATCGTGCGCACACCCATATGAATATCCTCCTTAG | ||
| glnB | pKD3 | S | CTGGACGATGTCCGCGAAGCACTGGCCGAAGTCGGTGTGTAGGCTGGAGCTGCTTC |
| A | TGCCGCGTCGTCCTCTTCACCGGTACGGATGCGAATCATATGAATATCCTCCTTAG | ||
| glnE | pKD3 | S | GTTGAGCGGCTGCCAGAGCCTTTAGCCGAGGAATCAGTGTAGGCTGGAGCTGCTTC |
| A | CTGCCAGCTTGCCCGCACCAGTTCACGCTCTGCGGTCATATGAATATCCTCCTTAG | ||
| putA | pKD3 | S | CATCATGGATATTTCACGATAACGTTAAGTTGCACCGTGTAGGCTGGAGCTGCTTC |
| A | AGATGCCGGAGGAGGTTGTAACATCCTCCGGCTACCCATATGAATATCCTCCTTAG |
S, sense; A, antisense.
Gene disruptions were performed based on the phage lambda Red recombinase system (5). pKD46, pKD3, pKD13, and pCP20 were obtained from the E. coli Genetic Stock Center. The primer sets used for disruption are listed in Table 2. The 1.0-kb FLP recombination target-flanked kanamycin or chloramphenicol resistance gene was introduced into the strain carrying the Red helper plasmid (pKD46) by electroporation. Following PCR to check for correct integration, the kanamycin or chloramphenicol resistance gene was eliminated by introducing a FLP recombinase expression plasmid (pCP20).
Analytical procedures.
Bacterial growth was expressed as the optical density at 660 nm after dilution with distilled water. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of proteins was carried out according to the method of Laemmli (15). The glucose concentration was measured using Detaminar GL-E (Kyowa Medex, Japan). Dipeptides and amino acids were derivatized using 9-fluorenylmethoxy carbonyl chloroformate and measured by high-performance liquid chromatography as described previously (38), except that the gradient program was slightly modified as follows: 0 to 2 min, solvent A-solvent B at 75:25; 2 to 26 min, a linear increase in solvent B to A-B at 55:45; 26 to 28 min, a linear increase to A-B at 5:95; 28 to 33 min, held at A-B at 5:95; 33 to 35 min, a linear decrease in solvent B to A-B at 75:25. Intracellular amino acids or dipeptides were extracted with ice-cold ethanol as previously described (29). For determination of the dry weight of cells, 5-ml of culture was filtered through a 0.45-μm-pore-size filter (diameter, 47 mm), the filter was washed with 10 ml of water and dried at 105°C to a constant weight, and the mean bacterial dry weight was calculated to be 0.33 mg ml−1 A660 unit−1 for the TT medium culture. The intracellular volume of the cell was calculated based on 1.6 μl mg dry cell weight−1 (29). Data are expressed as the mean values of at least three independent experiments, except for one figure that shows representative data (see Fig. 4).
RESULTS
Lal expression in a strain with the wild-type background.
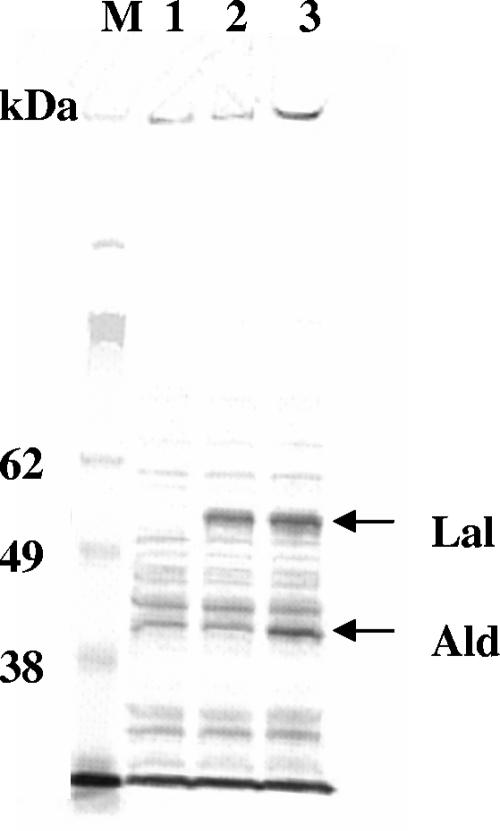
We first examined whether the expression of Lal in a wild-type E. coli strain evoked Ala-Gln dipeptide accumulation. An E. coli strain expressing Lal under the control of the trp promoter was constructed (JM101/pPE56). Expression of the enzyme was confirmed by SDS-PAGE analysis of cell extracts (Fig. 1). When the strain was cultivated in TT medium in a test tube, no dipeptide (either Ala-Gln or other dipeptides) was detected in the cultivation fluid. Inside the cells, only Ala-Ala was detected (Table 3). Since the JM101 host strain alone never accumulated dipeptides (Table 3), the occurrence of Ala-Ala in the JM101/pPE56 cells suggested the expression of active Lal in the strain. This view was supported by the fact that JM101 expressing His-tagged Lal also accumulated Ala-Ala intracellularly, and furthermore, by the fact that His-tagged Lal purified from cells using a nickel column confirmed activity (data not shown).
FIG. 1.
Confirmation of Lal and Ald expression by SDS-PAGE analysis. Shown are crude cell extracts of JM101/pTK31 (lane 1), JM101/pPE56 (lane 2), and JM101/pPE86 (lane 3). Lane M, marker. The arrows show the expected band positions of Lal (52.3 kDa) and Ald (39.7 kDa).
TABLE 3.
Impact of enhancement of amino acid biosynthesis on the accumulation of amino acids and dipeptides
| Strain (genotype)a | Intracellular concnb (mM)
|
Extracellular concnb (mM)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Ala | Gln | Ala-Ala | Ala-Gln | Ala | Gln | Ala-Ala | Ala-Gln | |
| JM101 (wt) | 6.7 ± 1.4 | 0.4 ± 0.2 | 0.0 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 |
| JM101/pPE56 (wt/lal) | 8.2 ± 4.3 | 0.6 ± 0.5 | 1.7 ± 1.1 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 |
| JKYQ1 (ΔglnEB) | 5.8 ± 4.7 | 5.3 ± 3.1 | 0.0 | 0.0 | 0.9 | 2.4 | 0.0 | 0.0 |
| JM101/pPE86 (wt/lal, ald) | 34.6 ± 15.5 | 0.3 ± 0.3 | 5.5 ± 4.0 | 0.0 | 16.1 | 0.0 | 0.0 | 0.0 |
| JKYQ1/pPE56 (ΔglnEB/lal) | 10.9 ± 4.9 | 52.0 ± 26.6 | 0.4 ± 0.2 | 0.1 ± 0.2 | 0.1 | 15.4 | 0.0 | 0.0 |
| JKYQ1/pPE86 (ΔglnEB/lal, ald) | 49.0 ± 16.5 | 10.6 ± 8.7 | 1.4 ± 0.5 | 0.6 ± 0.3 | 12.7 | 2.5 | 0.0 | 0.1 |
The relevant genotypes are expressed as host genotype/overexpressed gene from plasmid. wt, wild type.
Values are means ± standard deviations. As the extracellular concentrations were within 10% of the mean values, standard deviations of the extracellular concentrations are omitted.
The observation that Ala-Gln was not detected inside or outside the cells, despite the presence of an active Lal, prompted us to investigate what obstructed the formation of Ala-Gln.
Influence of peptide-degrading activities.
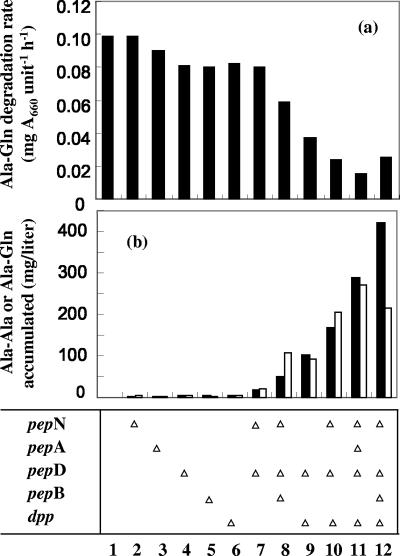
To examine whether inactivation of one or a few dipeptidases and aminopeptidases encoded by the pepD, pepN, pepA, and pepB genes exerted a positive effect on accumulation of the Ala-Gln dipeptide, single, double, or triple disruptants of pepD, pepN, pepA, and pepB were constructed. Each strain was incubated with Ala-Gln, and the extracellular concentration of Ala-Gln was measured periodically to assess the Ala-Gln degradation rate.
As shown in Fig. 2a, single disruption of each gene slightly reduced the rate of degradation of Ala-Gln, except for the pepN disruptant (JKYP4, bar 2), which showed degrading activity identical to that of the parental strain (JM101, bar 1). The degradation rate of the ΔpepD ΔpepN strains was almost the same as that of the ΔpepD strain (JKYP3, bar 3), supporting the hypothesis that PepN hardly contributes to Ala-Gln degradation. On the other hand, a combination of ΔpepD, ΔpepN, and ΔpepB (JKYP7, bar 8) reduced the degradation rate. It should be noted that the growth rate of the double or triple disruptant (JKYP6 or JKYP7) was slightly lower than that of the wild-type strain, JM101 (data not shown).
FIG. 2.
Influence of disruption of the dpp or pep gene on the Ala-Gln degradation rate (a) and extracellular dipeptide accumulation (b). (a) Each strain was incubated with Ala-Gln, and the rate of Ala-Gln degradation was measured. (b) The same strain containing pPE56 was cultivated in a test tube with TT medium supplemented with Ala and Gln for 24 h. The amounts of Ala-Ala (empty bars) and Ala-Gln (filled bars) that accumulated in the medium (extracellularly) were assayed. The relevant genotype of the strain shown in panel a, which was used as the host strain in panel b, is indicated at the bottom. The open triangles show the genes disrupted. The strains employed were JM101 (column 1), JKYP4 (column 2), JKYP1 (column 3), JKYP3 (column 4), JKYP2 (column 5), JKYP5 (column 6), JKYP6 (column 7), JKYP7 (column 8), JKYP8 (column 9), JKYP9 (column 10), JKYP10 (column 11), and JKYP11 (column 12). While each value is the mean of at least three experiments, standard deviations are not shown, since each measurement was within 10% of the mean value.
It has been demonstrated that E. coli imports dipeptides via a transport system coded for by the dpp operon (1). The influence of inactivation of this system was examined (Fig. 2a). Disruption of dpp alone (JKYP5, bar 6) showed an effect similar to disruption of pepD. However, the combination of Δdpp and ΔpepD exerted a synergistic effect rather than an additive effect (JKYP8, bar 9). The degradation rate was further reduced and was six times lower than the wild-type level when pepA disruption was introduced into the ΔpepD ΔpepN Δdpp strain (JKYP10, bar 11).
Since a reduction in degradation activity toward externally added Ala-Gln was confirmed, the effect of the above-described genetic manipulation on internally synthesized Ala-Gln was investigated. The Lal-expressing plasmid pPE56 was introduced into each mutant strain and cultivated in a test tube in the presence of Ala and Gln. After 24 h of cultivation, the amounts of dipeptides accumulated extracellularly were assayed (Fig. 2b). Although negligible amounts were detected in strains with the wild-type background, when each single mutation was conferred, small but significant amounts of dipeptides (Ala-Gln and Ala-Ala) appeared. More extensive disruption of peptidases was associated with greater accumulation of dipeptides. Simultaneous disruption of the dpp and pep genes boosted this accumulation. These results almost correlated with those of the experiments in which Ala-Gln was added to E. coli strains (Fig. 2a). The differences were the effect of ΔpepN and the degree of synergy between the disruptions of the dpp and pep genes. While the reason for these differences is not clear at present, the reduction in dipeptide-degrading activity by combinatorial inactivation of the dpp and pep genes was effective at increasing the production of Ala-Gln.
Effectiveness of enhancement of the amino acid supply.
One possibility for Lal to efficiently synthesize dipeptides in vivo is to elevate the intracellular concentration of the substrate amino acid(s) to levels high enough for Lal activity. This can be achieved by increasing the metabolic flux toward substrate amino acids. In E. coli, Gln biosynthesis is known to be negatively regulated by GlnE and GlnB (18, 28); therefore, we performed an experiment in which regulation of Gln biosynthesis was attenuated by constructing a strain from JM101 by disrupting the glnE and glnB genes. The glnE-glnB-deficient strain, JKYQ1, was transformed with pPE56, a Lal expression plasmid, and cultivated in the absence of Ala and Gln. As shown in Table 3, the glnE-glnB deficiency resulted in an increase in intracellular Gln and excretion of the amino acid, confirming that the genetic manipulation deregulated Gln biosynthesis. On this background, expression of Lal resulted in the intracellular appearance of Ala-Gln, although the amount of the dipepide detected in cells was small and variable (Table 3).
Whether simultaneous elevation of Ala and Gln levels boosted Ala-Gln production was further assessed. To enhance Ala synthesis, l-alanine dehydrogenase from B. subtilis (34) was used. A plasmid coexpressing the gene (ald) coding for l-alanine dehydrogenase and Lal was constructed (pPE86). Expression of Ald and Lal was confirmed by SDS-PAGE analysis (Fig. 1). The intracellular concentrations of Ala and Ala-Ala were increased by the additional expression of Ald (compare JM101/pPE56 and JM101/pPE86 or JKYQ1/pPE56 and JKYQ1/pPR86 in Table 3), confirming the contribution of Ald to Ala synthesis. Corresponding to the elevation in intracellular concentrations of Ala and Gln, the amount of Ala-Gln that accumulated in cells increased severalfold (Table 3). It should be noted that Ala-Gln was detected even outside JKYQ1/pPE86 cells. These results suggest that a simultaneous increase in the intracellular levels of both substrate amino acids made it possible for cells with Lal activity to synthesize the dipeptide faster than the rate of degradation of the dipeptide.
Construction of an Ala-Gln-producing strain.
As described above, the reduction in dipeptide-degrading activities caused by a combinatorial disruption of the dpp and pep genes and elevation of intracellular concentrations of the substrate amino acids was found to have a large impact on Ala-Gln production. Thus, these two strategies were integrated by constructing a strain with higher productivity of Ala-Gln.
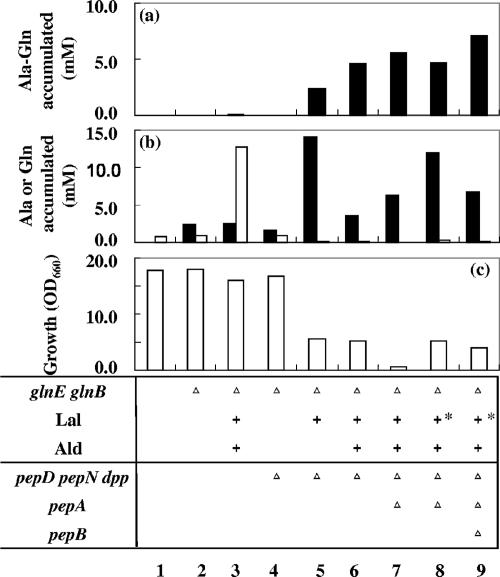
The starting strain was the pepD-, pepN-, and dpp-deficient mutant JKYP9. This strain did not accumulate Gln in the cultivation medium, but as in strain JM101, disruption of glnE and glnB (JKYPQ1) conferred Gln overproductivity (Fig. 3b, bar 4). On this genetic background (pepD pepN dpp glnE glnB), expression of Lal alone (JKYPQ1/pPE56) triggered extracellular accumulation of a significant amount of Ala-Gln (Fig. 3a, bar 5). Coexpression of Ald and Lal almost doubled the Ala-Gln titer (JKYPQ1/pPE86, bar 6). The fact that the amount of accumulated dipeptide in JKYPQ1/pPE86 was 50-fold higher than in JKYQ1/pPE86 clearly indicates the importance of limiting dipeptide-degrading activity.
FIG. 3.
Influences of metabolic manipulations on the production of Ala-Gln, amino acids, and cell growth. Each strain was cultivated in a test tube with TT medium for 24 h. The amounts of Ala-Gln (a), Ala (b, empty bars), and Gln (b, filled bars) that accumulated in the medium (extracellularly) and of cell growth (c) were measured. Ala-Ala was not detected in the medium. The relevant genotype of the strain is indicated at the bottom. +, overexpression of Lal or Ald; +*, Lal expression under the control of the uspA promoter; ▵, disruption of the gene(s). The strains employed were JM101 (column 1), JKYQ1 (column 2), JKYQ1/pPE86 (column 3), JKYPQ1 (column 4), JKYPQ1/pPE56 (column 5), JKYPQ1/pPE86 (column 6), JKYPQ2/pPE86 (column 7), JKYPQ2/pPE167 (column 8), and JKYPQ3/pPE167 (column 9). While each value is the mean of at least three experiments, standard deviations are not shown, since each measurement was within 10% of the mean value.
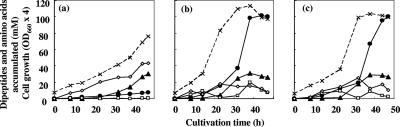
The pepA gene was further deleted from JKYPQ1. The putA gene was also disrupted to reduce the amount of proline, which was required by the pepD-disrupted strain in our experiments. By the additional disruption of pepA (JKYPQ2/pPE86, bar 7) the Ala-Gln titer was raised, but cell growth was greatly impaired (Fig. 3c). The growth rate remained low even when the strain was cultivated in a jar fermentor under sufficient aeration and pH-controlled conditions (Fig. 4a). The Ala-Gln titer was 7.4 mM after 47 h of cultivation.
Since the expression of Lal apparently exerted a negative effect on cell growth (Fig. 3c), separating the production period from the growth period could improve both growth and productivity. For this purpose, Lal was expressed under the control of the rpoH or uspA promoter, since expression of these genes has been reported to be elevated in the stationary phase (9, 25). This modification of Lal expression yielded a small increase in growth, an increase in the amount of Gln produced, and a slight decrease in the Ala-Gln titer (Fig. 3, column 8), suggesting an alleviated growth-inhibitory effect of Lal.
Thus, we constructed a host strain containing all of the useful genotypes described above by introducing a pepB disruption into the KYPQ2 strain. The resultant strain was designated KYPQ3 (pepA pepB pepD pepN dpp glnE glnB putA). To confirm phase transition during fermentation, the final strains (JKYPQ3/pPE167 and JKYPQ3/pPE177) were cultured in a jar fermentor in a fed-batch manner (Fig. 4b and c). In both cases, the growth rates were higher than that of JKYPQ2/pPE86, and when cells entered the stationary phase as a result of consumption of proline, which was required, Ala-Gln rapidly accumulated in the medium. After 47 h of cultivation, the extracellular concentrations of Ala-Gln and Ala-Ala reached 100.3 mM (24.7 g/liter) and 26.2 mM (4.2 g/liter), respectively, in JKYPQ3/pPE167 and 100.1 mM (24.6 g/liter) and 26.8 mM (4.3 g/liter), respectively, in JKYPQ3/pPE177. Molar yields of Ala-Gln against consumed glucose were 11.5% and 11.6% for JKYPQ3/pPE167 and JKYPQ3/pPE177, respectively. No other dipeptides, including d-amino-acid-containing dipeptides or longer oligopeptides, were detected in the cultivation medium (Fig. 5). These results clearly demonstrate that separating the production phase from the growth phase is effective at increasing the production of Ala-Gln.
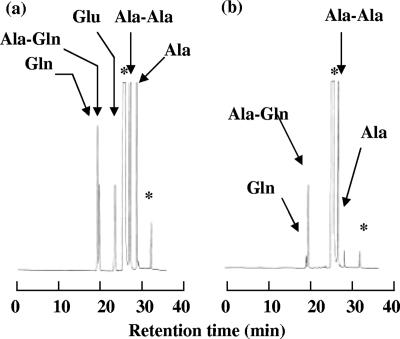
FIG. 5.
High-performance liquid chromatography analysis of the fermentation medium. (a) Authentic samples of Ala, Gln, glutamic acid (Glu), Ala-Gln, and Ala-Ala. (b) The supernatant from 47-h cultivation of JKYQ3/pPE167. The peaks indicated by asterisks were derived from 9-fluorenylmethoxy carbonyl reagent.
DISCUSSION
In this report, we demonstrate that the dipeptide Ala-Gln can be produced through a fermentative process with Lal in a genetically engineered E. coli strain. This fermentative process never forms longer oligopeptides or dipeptides containing d-amino acid(s) and does not need any protection or deprotection steps or addition of substrate amino acids. The only dipeptide by-product, Ala-Ala, can be easily separated from Ala-Gln by chromatography or crystallization (unpublished observation). Therefore, Ala-Gln fermentation seems to be very advantageous in regard to quality and cost-effectiveness compared with other Ala-Gln-manufacturing methods.
There have been many reports on dipeptides as microbial metabolites (11, 12, 14, 17, 27, 30, 39), but all of them except one (14) concern dipeptides containing an unusual amino acid(s), such as bacilycin (30) or alahopcin (11, 12). In contrast to the number of reports on the occurrence of dipeptides in nature, their biosynthesis has rarely been studied. Only bacilycin synthesis in B. subtilis has been studied to some extent; it relies on the bac operon, which involves bacD (a synonym for lal) (36), and expression of the bac operon is regulated by guanosine 3′ diphosphate 5′ diphosphate (13). It was also reported that Salmonella enterica serovar Typhimurium lacking peptidases N, A, B, and D accumulated a heterogeneous mixture of small peptides (42).
While Lal activity is indispensable for Ala-Gln fermentation, two metabolic manipulations (reduction of dipeptide degradation and elevation of intracellular levels of the substrate amino acids) were shown to be essential. Since it is impossible to inactivate all dipeptide-degrading activities, the combination of the two metabolic manipulations is apparently necessary for Ala-Gln production. It is worth noting that the necessity for increasing the amino acid supply had an unexpected benefit, since it prevented the formation of other dipeptides despite the wide substrate specificity of Lal.
Peptidases had a cumulative effect on the Ala-Gln breakdown (Fig. 3), as expected from their overlapping substrate specificities. Despite a long history of peptidase research, the contribution of each peptidase to the overall peptide-degrading activity of the cell is still largely unknown. It was reported that PepA, -B, -D, and -N have cysteinylglycine-hydrolyzing activity in E. coli (37). PepA, -B, -D, and -N have been shown to participate in protein breakdown in S. enterica serovar Typhimurium (41). On the other hand, Chandu and Nandi reported that PepN was exclusively responsible for the breakdown of several chromogenic peptidase substrates (4), implying that its contribution to hydrolysis would differ for each peptide.
Ala-Gln and Ala-Ala accumulated inside JKYQ/pPE86 cells, whereas only Ala-Gln was detected extracellularly (Table 3). This unbalanced distribution could be attributed to preferences in the dipeptide uptake system. DppA, the periplasmic substrate binding protein of the Dpp system, is known to prefer Ala-Ala over Ala-Gln (35). Other peptide uptake systems, Opp (6) and/or Tpp (26), might also be involved. In addition to these possibilities, completely unknown processes that release dipeptides into extracellular spaces could also be involved.
One unexpected finding of the current study was the growth-inhibitory effect of Lal. This effect seemed to be more severe when Lal expression was combined with pep disruption. It was previously reported that a pepA, -B, -D, and -N-deficient mutant grew more slowly than the wild-type strain (10). Certain small peptides, such as leucine-containing peptides, have been reported to inhibit E. coli growth (19). Taken together, these data indicate that dipeptides accumulating in cells due to peptidase deficiency may inhibit cell growth. However, at present, we cannot specify the dipeptides that cause this growth inhibition, since exogenously added Ala-Gln or Ala-Ala had no effect on growth (unpublished observation) and we could not find any other peptide accumulating in the cell.
Considering the wide substrate specificity of Lal and a related enzyme, d-alanine-d-alanine ligase (33), other dipeptides could be produced in a manner similar to that presented here. To explore these possibilities, more precise studies of the intracellular degradation and synthesis rates and the transport systems of each dipeptide are needed.
Supplementary Material
Acknowledgments
We thank Aya Kubota-Hada, Reiko Kohda, and Mayumi Fukano for their technical assistance. We also thank Akio Ozaki and Yoshiyuki Yonetani for helpful discussions.
Footnotes
Published ahead of print on 24 August 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abouhamad, W. N., and M. D. Manson. 1994. The dipeptide permease of Escherichia coli closely resembles other bacterial transport systems and shows growth-phase-dependent expression. Mol. Microbiol. 14:1077-1092. [DOI] [PubMed] [Google Scholar]
- 2.Abumard, N. N., E. L. Morse, H. Lochs, P. E. Williams, and S. A. Adibi. 1989. Possible sources of glutamine for parenteral nutrition: impact on glutamine metabolism. Am. J. Physiol. 257:E228-E234. [DOI] [PubMed] [Google Scholar]
- 3.Akabori, S., S. Sakakibara, and Y. Shimonishi. 1961. Protection of amide-nitrogen for peptide synthesis. A novel synthesis of peptide containing C-terminal glutamine. Bull. Chem. Soc. Jpn. 34:739. [Google Scholar]
- 4.Chandu, D., and D. Nandi. 2003. PepN is the major aminopeptidase in Escherichia coli: insights on substrate specificity and role during sodium-salicylate-induced stress. Microbiology 149:3437-3447. [DOI] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felice, M. D., J. Guardiola, A. Lamberti, and M. Iaccarino. 1973. Escherichia coli K-12 mutants altered in the transport systems for oligo- and dipeptides. J. Bacteriol. 116:751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furst, P., K. Pogan, and P. Stehle. 1997. Glutamine dipeptide in clinical nutrition. Nutrition 13:731-737. [DOI] [PubMed] [Google Scholar]
- 8.Gill, I., R. Lopez-Fandino, X. Jorba, and E. N. Vulfson. 1996. Biologically active peptides and enzymatic approaches to their production. Enzyme Microb. Technol. 18:162-183. [DOI] [PubMed] [Google Scholar]
- 9.Hengge-Aronis, R. 1996. Regulation of gene expression during entry into stationary phase, p. 1497-1512. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, DC.
- 10.Hermsdorf, C. L., S. Simmonds, and A. Sauders. 1979. Soluble di- and aminopeptidases in Escherichia coli K-12. Int. J. Peptide Protein Res. 13:146-151. [DOI] [PubMed] [Google Scholar]
- 11.Higashide, E., S. Horii, H. Ono, N. Mizokami, T. Yamazaki, M. Shibata, and M. Yoneda. 1985. Alahopcin, a new dipeptide antibiotic produced by Streptomyces albulus subsp. ochragerus subsp. nov. J. Antibiot. 38:285-295. [DOI] [PubMed] [Google Scholar]
- 12.Horii, S., H. Fukase, E. Higashide, and M. Yoneda. 1985. Structure of alahopcin (nourseimycin), a new dipeptide antibiotic. J. Antibiot. 38:302-311. [DOI] [PubMed] [Google Scholar]
- 13.Inaoka, T., K. Takahashi, M. Ohnishi-Kameyama, M. Yoshida, and K. Ochi. 2003. Guanine nucleotides guanosine 5′-diphosphate 3′-diphosphate and GTP co-operatively regulate the production of an antibiotic bacilysin in Bacillus subtilis. J. Biol. Chem. 278:2169-2176. [DOI] [PubMed] [Google Scholar]
- 14.Kodaira, Y. 1961. Toxic substances to insects, produced by Aspergillus ochraceus and Oopsra destructor. Agric. Biol. Chem. 25:261-262. [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Lazdunski, A. M. 1989. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. FEMS Microbiol. Rev. 63:265-276. [DOI] [PubMed] [Google Scholar]
- 17.Lee, M. D., A. A. Fantini, N. A. Kuck, M. Greenstein, R. T. Testa, and B. Borders. 1987. New antitumor antibiotic, LL-D05139β. Fermentation, isolation, structure determination and biological activities. J. Antibiot. 40:1657-1663. [DOI] [PubMed] [Google Scholar]
- 18.Maheswaran, M., and K. Forchhammer. 2003. Carbon-source-dependent nitrogen regulation in Escherichia coli is mediated through glutamine-dependent GlnB signalling. Microbiology 149:2163-2172. [DOI] [PubMed] [Google Scholar]
- 19.Miller, C. G. 1975. Peptidases and proteases of Escherichia coli and Salmonella typhimurium. Annu. Rev. Microbiol. 29:485-504. [DOI] [PubMed] [Google Scholar]
- 20.Miller, C. G. 1996. Protein degradation and proteolytic modification, p. 938-954. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, DC.
- 21.Miller, C. G., and G. Schwartz. 1978. Peptidase-deficient mutants of Escherichia coli. J. Bacteriol. 135:603-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monter, B., B. Herzog, P. Stehle, and P. Furst. 1991. Kinetically controlled synthesis of dipeptides using ficin as biocatalyst. Biotechnol. Appl. Biochem. 14:183-191. [PubMed] [Google Scholar]
- 23.Nishi, T. 1988. Studies on the maximization of foreign gene expression in Escherichia coli. Ph.D. thesis. Tokyo University, Tokyo, Japan.
- 24.Nozaki, H., I. Kira, S. Suzuki, and K. Yokozeki. May 2006. Dipeptide production method, l-amino acid amide hydrolase used therin, and production method of l-amino acid amide hydrolase. U.S. patent 7,037,673.
- 25.Nystrom, T., and F. C. Neidhardt. 1994. Expression and role of the universal stress protein, UspA, of Escherichia coli during growth arrest. Mol. Microbiol. 11:537-544. [DOI] [PubMed] [Google Scholar]
- 26.Payne, J. W., B. M. Grail, S. Gupta, J. E. Ladbury, N. J. Marshall, R. O'Brien, and G. M. Payne. 2000. Structure basis for recognition of dipeptides by peptide transporters. Arch. Biochem. Biophys. 384:9-23. [DOI] [PubMed] [Google Scholar]
- 27.Poetsch, M., and H. Zahner. 1985. Metabolic products from microorganisms. 230. Amiclenomycin-peptides, new antimetabolites of biotin. Taxonomy, fermentation and biological properties. J. Antibiot. 38:312-319. [DOI] [PubMed] [Google Scholar]
- 28.Reitzer, L. J. 1996. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagines, l-alanine, and d-alanine, p. 391-407. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, DC.
- 29.Roe, A. J., D. McLaggan, L. Davidson, C. O'Byrne, and I. R. Booth. 1998. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J. Bacteriol. 180:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers, H. J., G. G. F. Newton, and E. P. Abraham. 1965. Production and purification of bacilysin. Biochem. J. 97:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Sano, T., T. Sugaya, K. Inoue, S. Mizutaki, Y. Ono, and M. Kasai. 2000. Process research and development of l-alanyl-l-glutamine, a component of parenteral nutrition. Org. Process Res. Dev. 4:147-152. [Google Scholar]
- 33.Sato, M., K. Kirimura, and K. Kino. 2005. d-Amino acid dipeptide production utilizing d-alanine-d-alanine ligase with novel substrate specificity. J. Biosci. Bioeng. 99:623-628. [DOI] [PubMed] [Google Scholar]
- 34.Siranosian, K. J., K. Ireton, and A. D. Grossman. 1993. Alanine dehydrogenase (ald) is required for normal sporulation in Bacillus subtilis. J. Bacteriol. 175:6789-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith, M. W., D. R. Tyreman, G. M. Payne, N. J. Marshall, and J. W. Payne. 1999. Substrate specificity of the periplasmic dipeptide-binding protein from Escherichia coli: experimental basis for the design of peptide prodrugs. Microbiology 145:2891-2901. [DOI] [PubMed] [Google Scholar]
- 36.Steinborn, G., M. Hajirezaei, and J. Hofemeister. 2005. bac genes for recombinant bacilysin and anticapsin production in Bacillus host strains. Arch. Microbiol. 183:71-79. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki, H., S. Kamatani. E. Kim, and H. Kumagai. 2001. Aminopeptidases A, B, and N and dipeptidase D are the four cysteinylglycinases of Escherichia coli K-12. J. Bacteriol. 183:1489-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabata, K., H. Ikeda, and S. Hashimoto. 2005. ywfE in Bacillus subtilis codes for a novel enzyme, l-amino acid ligase. J. Bacteriol. 187:5195-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umezawa, H., T. Aoyagi, H. Suda, M. Hamada, and T. Takeuchi. 1976. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J. Antibiot. 29:97-99. [DOI] [PubMed] [Google Scholar]
- 40.Wernerman, J. 2004. Suggestion for present and future use of parenteral glutamine. Clin. Nutr. Suppl. 1:37-42. [Google Scholar]
- 41.Yen, C., L. Green, and C. G. Miller. 1980. Degradation of intracellular protein in Salmonella typhimurium peptidase mutants. J. Mol. Biol. 143:21-33. [DOI] [PubMed] [Google Scholar]
- 42.Yen, C., L. Green, and C. G. Miller. 1980. Peptide accumulation during growth of peptidase deficient mutants. J. Mol. Biol. 143:35-48. [DOI] [PubMed] [Google Scholar]
- 43.Yokozeki, K., and S. Hara. 2005. A novel and efficient enzymatic method for the production of peptides from unprotected starting materials. J. Biotechnol. 115:211-220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.