Abstract
Cells containing reporters which are specifically induced via selected promoters are used in pharmaceutical drug discovery and in environmental biology. They are used in screening for novel drug candidates and in the detection of bioactive compounds in environmental samples. In this study, we generated and validated a set of five Bacillus subtilis promoters fused to the firefly luciferase reporter gene suitable for cell-based screening, enabling the as yet most-comprehensive high-throughput diagnosis of antibiotic interference in the major biosynthetic pathways of bacteria: the biosynthesis of DNA by the yorB promoter, of RNA by the yvgS promoter, of proteins by the yheI promoter, of the cell wall by the ypuA promoter, and of fatty acids by the fabHB promoter. The reporter cells mainly represent novel antibiotic biosensors compatible with high-throughput screening. We validated the strains by developing screens with a set of 14,000 pure natural products, representing a source of highly diverse chemical entities, many of them with antibiotic activity (6% with anti-Bacillus subtilis activity of ≤25 μg/ml]). Our screening approach is exemplified by the discovery of classical and novel DNA synthesis and translation inhibitors. For instance, we show that the mechanistically underexplored antibiotic ferrimycin A1 selectively inhibits protein biosynthesis.
The differentiated regulatory response of the soil bacterium Bacillus subtilis to different types of stress has led to the choice of this bacterium as a preferred model organism for studying the mechanism of action (MOA) of antibiotics. Based on so-called reference compendia of antibiotic-triggered mRNA expression profiles, promoter regions have been identified which are selectively and strongly induced by antibiotic killing of bacteria via similar MOAs (13, 20). Because of genetic engineering of strains which harbor such promoters fused to reporter genes, cellular biosensors are now available that can signal the presence of many different types of antibiotics. Currently, cellular biosensors based on various microbial species containing reporters which are specifically induced via selected promoters are widely used in pharmaceutical drug discovery and in environmental biology (1, 4, 12, 18, 28, 29, 33, 35). Nevertheless, only in the case of B. subtilis have genomewide, systematic approaches for the identification of appropriate antibiotic biomarkers based on mRNA expression profiling been reported so far (11, 19). We previously exemplified the approach with the identification and high-throughput screening application of FapR regulator-dependent promoters selectively and strongly responding to inhibitors of fatty acid biosynthesis (11). In addition, Hutter et al. (19) reported five high-throughput screening (HTS)-compatible strains carrying promoter-reporter fusions for a limited spectrum of antibiotic mechanisms: inhibition of fatty acid biosynthesis (fabHB promoter), inhibition of the chromosomal topology-changing activities of DNA gyrase and topoisomerase IV by quinolones (promoters of dinB, yneA, and yorB), and inhibition of cell wall biosynthesis by the lipid II antagonist vancomycin (ytrA promoter). However, the previously reported B. subtilis promoter-reporter fusions still lack the diagnosis of important antibiotic mechanisms addressing a broader spectrum of essential steps in DNA synthesis and replication or cell wall and protein biosyntheses. In this study, we generated and validated a set of five antibiotic biosensors enabling the as yet most-comprehensive HTS-compatible diagnosis of antibiotic interference in the five major biosynthetic pathways of bacteria: biosynthesis of DNA, RNA, proteins, cell wall, and fatty acids.
While the previously described biomarker-containing strains have been validated only on the basis of a limited number of a few tens of antibiotics, in this study we tested our cellular biosensors on a large set of diverse chemical entities in order to comprehensively estimate the profile of detectable antibiotics. We tested the strains on our unique library of approximately 14,000 pure natural products. Natural products are an unsurpassed source of evolved chemical diversity and therefore represent a rich starting point for screening programs aimed at generating pharmacologically active small molecule leads. In the past, natural products have been a very successful source of new drugs (7, 30). Our compilation of highly diverse structures includes numerous reference antibiotics with known MOAs, as well as antibiotics which are mechanistically not characterized yet. Moreover, the library represents a source of numerous antibiotics representing novel structural entities. Here we exemplify our validation approach by reporting the screening results obtained with promoters indicative of antibiotics targeting DNA replication and protein synthesis.
MATERIALS AND METHODS
Biomarker construction and host strain generation.
Standard cloning techniques were applied using Escherichia coli XL1Blue (Stratagene, La Jolla, CA). Firefly luciferase was amplified from pBestluc (Stratagene) (for primers, see Table 1) and cloned into the shuttle vector pHT304 (resistance markers, ampicillin in E. coli and macrolide-lincomycin-streptogramin B in B. subtilis [2]) via PstI and HindIII. The upstream regions of the B. subtilis genes yheI, yorB, ypuA (each approximately 500 bp long), and yvgS (approximately 250 bp long) were amplified (for primers, see Table 1) and cloned in front of the luciferase genes using KpnI and PstI. The resulting constructs carrying the promoter-reporter fusions were transformed into B. subtilis 1S34, a non-spore-forming derivative of strain 168 (31). Construction of the fabHB promoter-reporter construct and assay development with this promoter have been described previously (11).
TABLE 1.
Primers used in this work
| Oligonucleotide name | Sequencea |
|---|---|
| lucFF-1 | 5′-ATATCTGCAGTAAGGAGGAAAAAATGGAAGACGCCAAAAACATAAAG-3′ |
| lucFF-2 | 5′-ATATAAAGCTTTTACAATTTGGACTTTCCGCCCTTC-3′ |
| yheI-1 | 5′-TATAGAGCTCGGTACCTTCTTACTATTTTCACTTCCGTCAAAC-3′ |
| yheI-2 | 5′-TATACTGCAGGTCGACTCATCAGCCGCCTTCTATTTTTTCCTT-3′ |
| yorB-1 | 5′-TATAGAGCTCGGTACCCGGGATATATTGGGATAAAGATTCAGA-3′ |
| yorB-2 | 5′-TATACTGCAGGTCGACTTTTGAAATTTTTGGTACTACTAAATTA-3′ |
| ypuA-1 | 5′-TATAGAGCTCGGTACCCCGCCTCATGTGTATGCGG-3′ |
| ypuA-2 | 5′-TATACTGCAGCAATTTCACAAGCAGCTGGAT-3′ |
| yvgS-1 | 5′-TATAGAGCTCGGTACCATGTTTAATTGGAAGCTGCCAAACCGT-3′ |
| yvgS-2 | 5′-TATACTGCAGGTCGACGAAAATAGTTGACAAACATAGATGA-3′ |
Restriction sites used for cloning of PCR products are underlined.
Assay development, screening, and second-line test.
Four cellular biomarker induction assays (using the yheI, yorB, yvgS, and ypuA promoter-reporter fusion constructs) were developed for high-throughput screening in 384-well microtiter plates. Each biomarker-carrying B. subtilis strain was grown in LB medium (yorB, yvgS, and ypuA strains) or Belitzky minimal medium (34) (yheI strain) with 5 μg/ml erythromycin to an optical density at 600 nm of 0.9 at 37°C, diluted to an optical density at 600 nm of 0.1 (yheI strain), 0.01 (yorB strain), or 0.02 (yvgS and ypuA strains), and stored overnight at 4°C. The next day, 40 μl of the cell cultures were incubated at 37°C with 0.5 μl of each screening compound (dissolved in dimethyl sulfoxide [DMSO]) for 4 h (yheI strain), 3 h (yorB strain), 1.5 h (yvgS strain), or 1 h (ypuA strain), respectively. The screening compounds were tested at three different concentrations: 25.0 μg/ml, 6.25 μg/ml, and 1.56 μg/ml. Luminescence was measured immediately after addition of 25 μl 0.1 M citrate buffer (pH 5) with 2 mM luciferin using a charge-coupled-device camera-based luminescence detector (“Lumibox”; Bayer Technology Services GmbH, Leverkusen, Germany) which captures 384 images simultaneously. The signal induction was not influenced by DMSO concentrations up to 2%. Each microtiter plate contained wells as negative controls (no compound addition) and positive controls (addition of reference compounds causing maximal signal induction).
The following agents were used as reference compounds: ciprofloxacin (Bayer) (for yorB, marker for inhibition of DNA synthesis), rifampin (Sigma-Aldrich) (for yvgS; RNA synthesis), linezolid (Pfizer) (for yheI; protein biosynthesis), and vancomycin (Sigma-Aldrich) (for ypuA; cell wall biosynthesis). Additional reference compounds for test validation were the following (Table 1): DNA ligase inhibitor compound no. 3 (6), moiramide B, moxifloxacin, polymerase IIIC inhibitor compound no. 1 (24), and streptovaricin (Bayer); ramoplanin (Biosearch Italia, Geranzano, Italy); trovafloxacin (Pfizer); and actinomycin D, actinonin, azaserine, azithromycin, cefoxitin, cerulenin, chloramphenicol, doxycycline, erythromycin, ethidium bromide, fusidic acid, gentamicin, kanamycin, mersacidin, methicillin, monensin, nalidixic acid, netropsin, N-ethyl maleimide, nisin, novobiocin, mitomycin C, oxacillin, polymyxin B, puromycin, triclosan, trimethoprim, and tunicamycin (Sigma-Aldrich). The Bayer collection of pure natural products consisted of approximately 14,000 individual substances which were each characterized by nuclear magnetic resonance spectroscopy. The compounds were dissolved in DMSO.
In screening of the pure natural products, we set strict cutoff values for hit identification which were at least five standard deviations above the noninduced signal of the negative control (250% for yorB, 200% for yvgS, and 200% for yheI). In the case of the ypuA assay, the cutoff value of 170% was only 3.5 standard deviations above the noninduced signal due to the limited induction value of 180% obtained with the reference compound vancomycin. Screening with all biosensors revealed acceptable assay quality (see Results and Discussion; see also Table 3). The initial hits were retested for hit confirmation. Retests were performed at additional compound concentrations (12.5 μg/ml, 3.13 μg/ml, and concentrations below 1.56 μg/ml).
TABLE 3.
Screening of 14,000 natural products with antibiotic biomarker strains
| Biomarker | Reference antibiotic | MIC of reference antibiotic [μM (μg/ml)] | Maximal signal induction by reference antibiotic (%) | SD (%) | Z′ factor | Detection cutoff (%) | Minimal signal-inducing concn of reference antibiotic at detection cutoff (μM) | No. (%) of confirmed hits |
|---|---|---|---|---|---|---|---|---|
| yheI | Linezolid | 3.13 (0.93) | 980 | 13 | 0.84 | 200 | 0.1 | 26 (0.2) |
| yorB | Ciprofloxacin | 0.71 (0.25) | 1,380 | 9 | 0.87 | 250 | 0.06 | 12 (0.1) |
Second-line MOA studies by incorporation of radiolabeled metabolic precursors were performed as described previously (14) using Staphylococcus aureus 133 (DSMZ no. 11823; DSMZ, Braunschweig, Germany).
RESULTS AND DISCUSSION
Assay development and small-scale test validation.
The development of the cellular antibiotic biosensors was elaborated in three key phases. First, after selection of the appropriate candidate promoter regions represented by the upstream sequences of the genes yheI, yorB, yvgS, ypuA, and fabHB from B. subtilis, we fused each of these sequences with the firefly luciferase gene in order to generate cellular biosensors enabling diagnosis of interference with the major bacterial biosynthetic pathways. Second, we developed assays with a 384-well microtiter plate format and tested several parameters for creating optimal assay protocols for each biosensor strain (preculture handling and storage, cell inoculum, growth medium, incubation time, and solvent [DMSO] sensitivity; see Materials and Methods). Third, a panel of 39 reference antibiotics was used to confirm the MOA-specific induction of the biomarkers (Table 2). The fabHB promoter-reporter fusion was selectively induced by fatty acid biosynthesis inhibitors, a finding confirming previously reported results (11, 19). The yvgS promoter activity was not only indicative of rifampin activity, as demonstrated previously (19). It also responded to streptovaricin, another transcription inhibitor. This observation might indicate that yvgS promoter induction is not only rifampin specific but also indicative of agents generally interfering with transcription. The ypuA promoter-reporter fusion represented a novel biomarker which indicated a broad spectrum of cell wall biosynthesis inhibitors and cell envelope stressing agents: among others, the lipid II antagonist vancomycin, the cell membrane perturbing agent polymyxin B, and the β-lactams. The yorB marker has recently been described as being strongly induced by quinolones (DNA gyrase/topoisomerase IV inhibitors) (19). We found that many more compounds induced the reporter: the DNA-alkylating agent mitomycin C, the folate biosynthesis inhibitor trimethoprim, and the recently reported novel inhibitors of the DNA polymerase IIIC (anilinouracils) (24) and the bacterial NAD+-dependent DNA ligase (pyridochromanones) (6) as strong inducers (>500%). In addition, the DNA gyrase inhibitor novobiocin was detected, even though the signal was only raised to 250%. Finally, the novel yheI marker was significantly induced by the protein biosynthesis inhibitors chloramphenicol, doxycycline, fusidic acid, and linezolid (Fig. 1).
TABLE 2.
Antibiotic biomarker validation based on a selection of reference agentsa
| Target area | Antibiotic | Induction of promoter:
|
||||
|---|---|---|---|---|---|---|
| yorB | yvgS | yheI | ypuA | fabHB | ||
| DNA synthesis | Moxifloxacin | + | − | − | − | − |
| Ciprofloxacin | + | − | − | − | − | |
| Trovafloxacin | + | − | − | − | − | |
| Nalidixic acid | + | − | − | − | − | |
| Novobiocin | + | − | − | − | − | |
| Pol IIIC inhibitorb | + | − | − | − | − | |
| Trimethoprim | + | − | − | − | − | |
| DNA ligase inhibitorc | + | − | − | − | − | |
| Azaserine | + | − | − | − | − | |
| Mitomycin C | + | − | − | − | − | |
| RNA synthesis | Streptovaricin | − | + | − | − | − |
| Rifampin | − | + | − | − | − | |
| Protein synthesis | Linezolid | − | − | + | − | − |
| Doxycycline | − | − | + | − | − | |
| Fusidic acid | − | − | + | − | − | |
| Chloramphenicol | − | − | + | − | − | |
| Erythromycin | − | − | −d | − | − | |
| Azithromycin | − | − | −d | − | − | |
| Gentamicin | − | − | − | − | − | |
| Kanamycin | − | − | − | − | − | |
| Puromycin | − | − | − | − | − | |
| Actinonin | − | − | − | − | − | |
| Cell wall | Cefoxitin | − | − | − | + | − |
| synthesis-cell | Oxacillin | − | − | − | + | − |
| envelope stress | Methicillin | − | − | − | + | − |
| Nisin | − | − | − | + | − | |
| Mersacidin | − | − | − | + | − | |
| Ramoplanin | − | − | − | + | − | |
| Vancomycin | − | − | − | + | − | |
| Tunicamycin | − | − | − | + | − | |
| Polymyxin B | − | − | − | + | − | |
| Fatty acid synthesis | Moiramide B | − | − | − | − | −e |
| Cerulenin | − | − | − | − | + | |
| Triclosan | − | − | − | − | + | |
| Diverse MOAs | Netropsin | − | − | − | − | − |
| Ethidium bromide | − | − | − | − | − | |
| Actinomycin D | − | − | − | − | − | |
| Monensin | − | − | − | − | − | |
| N-ethyl maleimide | − | − | − | − | − | |
+/−, reporter signal induced by at least/less than 250% (yorB), 200% (yvgS and yheI), 170% (ypuA), or 200% (fabHB) in comparison to the noninduced signal.
DNA polymerase IIIC inhibitor compound no. 1 (anilinouracil) (24).
DNA ligase inhibitor compound no. 3 (pyridochromanone) (6).
The macrolides also induce the yheI promoter as found in expression profiling experiments (11, 13). The biosensor does not respond to these antibiotics due to plasmid-encoded macrolide-lincomycin-streptogramin B resistance.
Moiramide B targets the first enzyme in fatty acid synthesis, the acetyl-coenzyme A carboxylase, which is regulated differently from the other fatty acid/phospholipid biosynthesis enzymes (13).
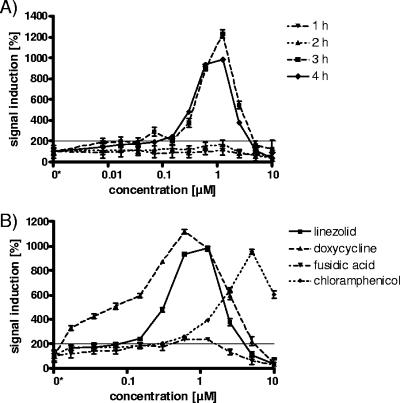
FIG. 1.
Typical time course of yheI biomarker induction by linezolid between 1 and 4 h and concentration-induction patterns with different translation inhibitors. (A) Linezolid induces the biosensor cells at the earliest after a 3-h exposure time, leading to a maximum signal of 1,300%. (B) The concentration-induction pattern reveals that linezolid, doxycycline, and lead to an approximately 10-fold signal induction, while fusidic acid doubles the signal after the optimal assay incubation time of 4 h. Using 200% signal induction as a significance cutoff, low concentrations of antibiotics (0.02 μM doxycycline, 0.1 μM linezolid, 0.6 μM fusidic acid, and 0.3 μM chloramphenicol) can be detected. These concentrations are 1 to 2 orders of magnitude below the corresponding MICs (MICs, 3.13 μM for linezolid, fusidic acid, and doxycycline; 12.4 μM for chloramphenicol). The fourfold-increased MIC of chloramphenicol is reflected by the maximal signal-inducing concentration of 5 μM in comparison to the other reference antibiotics, which reach the induction maximum around 1 μM. The growth-inhibitory effects of the antibiotics at and above their MICs lead to reduced numbers of viable cells and therefore to a reduction of the signal intensities.
Large-scale test validation and drug screening using a library of pure natural products.
While we recently described the HTS compatibility of the fabHB promoter reporter strain (11), we aimed to deliver a highly validated proof of concept for each of the remaining assay systems. Therefore, we screened the antibiotic biomarker strains with our library of 14,000 diverse pure natural compounds. In general, our screening results confirmed the appropriateness of each reporter strain for the discovery of pathway-specific inhibitors on a high-throughput scale. For instance, the yvgS biomarker strain, which could be maximally induced by a factor of 4.1 in the presence of the reference antibiotic rifampin, exhibited an acceptable standard deviation of 13% and a screening window coefficient Z′ of 0.8. The Z′ coefficient reflects the signal difference between a positive and a negative control sample together with the signal variance associated with the measurements. Values of at least 0.5 indicate suitability for high-throughput applications. While the yvgS reporter strain described by Hutter et al. (19) was not suitable for HTS, the strain constructed in this study turned out to be HTS compatible. One reason might be the difference in the yvgS upstream region length cloned in front of the reporters (a 245-bp fragment in this study, in contrast to a 180-bp region cloned by Hutter et al.). Although the ypuA biomarker strain was only maximally induced by a factor of 1.8 by the reference antibiotic vancomycin, the HTS revealed acceptable statistical data (standard deviation, 17%; Z′ coefficient, 0.5). However, we want to exemplify our validation approach in more detail here by reporting the screening results obtained with the two remaining promoters, yorB and yheI (Table 3).
First, the yorB promoter-reporter fusion, which was previously described as a marker for a limited spectrum of antibiotics (mainly quinolones [19]), turned out to possess a largely broadened diagnostic potential, as already indicated in testing of our limited set of reference antibiotics (see above). The yorB biosensor screening revealed 12 confirmed hits (hit rate, 0.1%). Ten of them have been described as interfering with DNA replication by covalent DNA binding and strand breaking or by inhibition of gyrase and topoisomerase IV (Table 4). Two hits were novel and are currently under further evaluation (data not shown). The yorB gene is probably part of the lexA regulon, which comprises a group of genes regulating the B. subtilis SOS response triggered by DNA damages (3, 25). The yorB promoter-reporter fusion delivered the highest signal-to-noise ratio among promoters of the SOS regulon in B. subtilis. Since the gene yorB is part of an SPβc2 prophage sequence, it might be strongly repressed under nonstressed conditions by a phage-specific regulator, leading to very high induction by DNA damaging events. It is consistent that quinolones lead to induction of the yorB promoter. This compound class inhibits the activity of DNA gyrase and topoisomerase IV, which includes cleavage and rejoining of both DNA strands. The ternary enzyme-DNA-quinolone complexes lead to the stopping of DNA synthesis and to rapid fragmentation of chromosomal DNA (8, 9). Similarly, direct “attacks” on DNA by covalently binding agents which cause strand breaks are also expected to induce the SOS response genes, including yorB. We included in our set of reference compounds the anticancer drug mitomycin C, which cross-links DNA by alkylation (16) and indeed leads to strong yorB promoter induction. The natural product library screening with the yorB marker revealed mainly such types of DNA-targeting antibiotics as hits (Table 4). Many of them are applied in anticancer chemotherapy (e.g., bleomycin) (17). The hit novobiocin was the exception, since this antibiotic inhibits DNA gyrase activity by blocking the ATP binding site (26). Remarkably, no noncovalently binding agents, such as the intercalator ethidium bromide and minor-groove binder netropsin, induce the yorB promoter. Altogether, we consider the yorB marker indicative of a much broader range of DNA synthesis-blocking agents than previously reported. Further evaluation of the remaining two hits having unknown MOAs will reveal whether they might even act via mechanisms similar to those of synthetic reference antibiotics, such as quinolones, anilinouracils, pyridochromanones, or trimethoprim.
TABLE 4.
yorB biomarker-inducing compounds identified in screening of 14,000 natural products
| Compound | MOA | Signal (%) at 12.5 μMa |
|---|---|---|
| Bleomycin | DNA strand breaking | 452 |
| Alternariol monomethyletherb | DNA strand breaking | 252 |
| Desmethylether-sibiromycin | Covalent binding to DNA | 693 |
| Myxin | DNA strand breaking | 326 |
| Porfiromycin (methylmitomycin C) | DNA cross-linking | 400 |
| Anthramycin | Covalent binding to DNA | 1,110 |
| Streptonigrin | DNA strand breaking | 677 |
| Phleomycin | DNA strand breaking | 398 |
| Novobiocin | Inhibition of DNA gyrase and topoisomerase IV (targeting ATP-binding subunits) | 250 |
Luminescence data measured during hit confirmation (compound concentration of 12.5 μM or, in the case of novobiocin, 6.25 μM). Values are percentages of the noninduced biomarker signal. The cutoff for hit consideration was 250%.
Another hit also represented an alternariol derivative.
Second, the yheI promoter-reporter fusion represents a new tool for the discovery of a broad spectrum of translation inhibitors. Only limited information about the expression of yheI in B. subtilis was available previously (27). Recent studies have revealed that transcription of yheI and its probable operon neighbor yheH is induced by SpoIIID and sigma factor E, two transcription factors involved in asymmetric division during sporulation of B. subtilis (10). Fukushima et al. (15) reported the first data indicating that YheH/YheI might play roles in a signaling pathway during sporulation initiation. However, a concrete function has not yet been assigned to YheI and YheH. These gene products exhibit significant homologies to multidrug efflux ABC transporters or multidrug resistance proteins (see http://www.genome.jp/kegg/brite.html). The homology findings suggest that the yheIH operon might be inducible in B. subtilis by a limited spectrum of antibiotic chemotypes. Our screening of the library of pure natural products revealed that among the 26 confirmed hits (hit rate, 0.2%), a surprisingly large diversity of chemical structures induced the yheI promoter (Table 5; Fig. 2). Eighty percent (21) of the yheI marker-inducing hits were already known translation inhibitors. They mainly target the 50S ribosomal subunit and block the peptidyl transferase activity. Thus, the high selectivity of the yheI promoter induction for translation inhibitors suggests that the induction is coupled to translation arrest. This regulatory coupling needs to be further elucidated. Remarkably, protein biosynthesis inhibitors leading to mistranslated peptides (aminoglycosides, such as streptomycin, gentamicin, and kanamycin), to truncated peptides (puromycin), or to nondeformylated peptides (actinonin) did not induce the yheI marker.
TABLE 5.
yheI biomarker-inducing compounds identified in screening of 14,000 natural products, compared to some noninducing protein biosynthesis inhibitors
| Compound | Structural class | Ribosomal subunit specificity | Signal (%) at concna (μg/ml) of:
|
|||
|---|---|---|---|---|---|---|
| 12.5 | 6.25 | 3.13 | 1.56 | |||
| yheI biomarker-inducing compounds | ||||||
| Chloramphenicol | 50S | 140 | 440 | 840 | 570 | |
| Tylosinb | 16-Membered macrolide | 50S | 880 | 490 | 340 | 200 |
| Viridogriseinb | Streptogramin B type | 50S | 180 | 430 | 520 | 110 |
| Pristinamycin 1Cb | Streptogramin B type | 50S | 10 | 20 | 160 | 470 |
| Mikamycin A | = Streptogramin A | 50S | 150 | 350 | 520 | 350 |
| Sparsomycin | 50S | 240 | 180 | 150 | 120 | |
| Thiostrepton | Thiopeptide | 50S | 380 | 190 | 160 | 110 |
| Thiopeptin B | Thiopeptide | 50S | 210 | 130 | 110 | 100 |
| A10255J | Thiopeptide | EF-Tu?c | 20 | 20 | 90 | 200 |
| Althiomycin | 50S | 360 | 270 | 230 | 200 | |
| Cephalosporin P1 | Steroid | EF-G | 10 | 30 | 190 | 290 |
| Helvolic acid | Steroid | EF-G | 250 | 170 | 140 | 120 |
| Bamicetin | Amicetin-type nucleoside | 50S | 370 | 250 | 190 | 150 |
| Amicetin | Amicetin-type nucleoside | 50S | 330 | 190 | 270 | 130 |
| Pleuromutilin | 50S | 200 | 340 | 110 | 230 | |
| Hygromycin A | 50S | 240 | 160 | 170 | 140 | |
| Oxytetracycline | Tetracycline | 30S (50S) | 20 | 100 | 440 | 480 |
| Ferrimycin A1d | Sideromycin | Unknown | 20 | 30 | 440 | 770 |
| Trinactine | Polynactin | 650 | 210 | 190 | 130 | |
| Noninducing protein biosynthesis inhibitors of the natural product library | ||||||
| Actinoninf | 120 | 80 | 85 | 90 | ||
| Streptomycing | Aminoglycoside | 30S | 88 | 89 | 100 | 95 |
| Puromycin | Aminoacyl-adenosine analogue | 50 | 80 | 101 | 110 | |
| Fusidic acidh | Steroid | EF-G | 20 | 20 | 20 | 20 |
| Siomycin Ah | Thiopeptide | 50S | 20 | 20 | 20 | 30 |
Luminescence data measured during hit confirmation at four different compound concentrations between 1.56 and 12.5 μg/ml. Values represent percentages of the noninduced biomarker signal. In a case where a value is above 200% (bold numbers), the biomarker system is considered to be induced. Underlined values (below 50%) indicate antibiotic effects during cell incubation.
Three screening hits represented tylosins, and three additional ones were viridogriseins. Due to a plasmid-encoded resistance determinant in the biosensor cell, yheI marker induction by 14-membered macrolides, such as erythromycin, by lincomycin, or by streptogramin B cannot be expected. Nevertheless, close congeners of streptomycin B do indeed induce the biomarker.
A related compound (GE2270A) binds to EF-Tu.
Ferrimycin consists of a siderophore carrier and a probable bioactive moiety. Only limited data are available concerning its antibiotic MOA. yheI marker induction indicates that ferrimycin might indeed inhibit protein biosynthesis.
Trinactin is known to interfere with biomembranes by acting as an ionophore.
Actinonin inhibits the peptide deformylase, an essential enzyme involved in the posttranslational processing of proteins.
Besides streptomycin, the aminoglycosides gentamicin, kanamycin, and neomycin also do not induce the yheI biomarker. Only spectinomycin, which in contrast to the other aminoglycosides does not cause misreading during translation, induces the biomarker at high concentrations (25 μM).
Fusidic acid and siomycin A are indeed inducing agents below 1.56 μg/ml. At screening concentrations above 1.56 μg/ml, they already inhibit bacterial growth and repress the luciferase signal.
FIG. 2.
Subset of known antibiotics identified in screening of 14,000 pure natural products using the yheI biomarker strain. Structurally diverse chemotypes of antibiotics reported to target the ribosomal function were identified as inducers of the yheI promoter (see Table 5). Some representative structures are presented.
Evaluating mechanistic specificity of hits: ferrimycin A1.
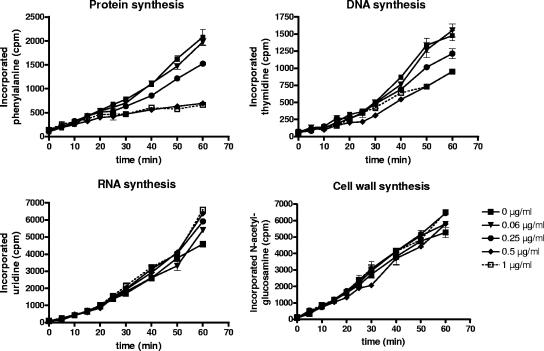
The risk of finding nonspecifically acting antibiotics in a biosensor assay can be well assessed by our approach of screening a chemically diverse library of pure natural products including numerous reference antibiotics. For instance, among 14,000 pure natural products, only 1 compound out of 26 hits was found which induced the yheI marker, although it presumably does not kill cells via translation inhibition. This agent, which is called trinactin, is rather known to be membrane perturbing. However, we did not evaluate its role in interfering with translation. Four hit compounds have not been characterized before with respect to their MOAs. Secondary assays are ongoing to verify their translation-specific MOA (data not shown). For one of these hits, we here demonstrate that the promoter induction assay is predictive for novel translation inhibitors. We found ferrimycin A1 as a hit compound representing an antibiotic which was poorly mechanistically studied (21-23, 32). It was speculated that it might interfere with protein biosynthesis on the basis of preliminary metabolite incorporation assays with a closely related antibiotic A 22,765 (22). However, no clear evidence has been provided that the action of ferrimycin A1 is based on selective inhibition of protein synthesis without interfering with the other major biosynthetic pathways. Therefore, we performed metabolite incorporation studies with the precursors for DNA, RNA, protein, and cell wall biosynthesis in a key pathogen (S. aureus). Our data strongly support the idea that ferrimycin A1 is a selective protein biosynthesis inhibitor (Fig. 3). Ferrimycin A1 consists of a siderophore moiety responsible for transporting the antibiotic into the cell and a second putatively bioactive component (5). The compound probably needs to be cleaved within the cell, like albomycin, before the second component might indeed inhibit translation.
FIG. 3.
MOA studies for ferrimycin A1 by the incorporation of radiolabeled metabolic precursors into whole cells of S. aureus 133. Labeling was performed at different compound concentrations (as indicated).
The antibiotic biosensor screening approach is well suited for finding antibiotic compounds which predominantly act via single cellular processes. Nevertheless, side activities of the identified compounds within the bacterial cell might not be excluded. In addition, detection of multiprocess inhibitory agents might be hampered, since the cellular stress response might be modified in a way not leading to significant induction of the applied promoter-reporter fusions. However, by allowing researchers to identify key cellular processes affected by otherwise-uncharacterized compounds, the reporter strains could be very valuable in enabling researchers to rationally formulate multihurdle natural products or antibiotic combinations against which resistance may be less likely to be developed.
Conclusion.
In this study, we demonstrated that our B. subtilis reporter strains with an optimized set of promoters, showing MOA-specific transcriptional activation patterns, represent an efficient way towards a highly comprehensive high-throughput diagnosis of bioactive compounds interfering with the five major biosynthetic pathways in bacteria. Such assays combine the advantages of the traditional whole-cell screening approaches and the directed, rational strategies of target-based assays. As shown by the yorB and yheI marker screening results, such assay systems delivered reasonable hit rates of 0.1 to 0.2%, whereas 6% of the tested compounds exhibit anti-B. subtilis activity (≤25 μg/ml). Due to the limited concentration window in which compounds might be detected as inducing agents, we run our screens at different compound concentrations (between 1.56 and 25 μg/ml). Nevertheless, some compounds, such as fusidic acid and siomycin A in the case of the yheI biosensor assay, could not be detected due to their very strong antibacterial activities (below 1.56 μg/ml; Table 5).
Our large-scale validation approach, screening a compound library of 14,000 pure natural products, allows comprehensive estimation of the MOA specificities and the HTS compatibilities of our biosensors. This study demonstrates the importance of testing a large diversity of chemical structures for obtaining a representative chemical profile of the cellular biosensor responses. Screening of our pure natural product collection enabled us to predict the MOA of mechanistically underexplored antibiotics, such as ferrimycin A1, and to identify antibacterials which might represent promising lead candidates in antibiotic drug discovery. Moreover, the biosensors might be especially suitable for detecting the MOA of a broad spectrum of antibiotics in environmental samples with high sensitivity.
Acknowledgments
We thank S. Hartke and M. Haas for technical assistance.
Footnotes
Published ahead of print on 24 August 2007.
REFERENCES
- 1.Alksne, L. E., P. Burgio, W. Hu, B. Feld, M. P. Singh, M. Tuckman, P. J. Petersen, P. Labthavikul, M. McGlynn, L. Barbieri, L. McDonald, P. Bradford, R. G. Dushin, D. Rothstein, and S. J. Projan. 2000. Identification and analysis of bacterial protein secretion inhibitors utilizing a SecA-LacZ reporter fusion system. Antimicrob. Agents Chemother. 44:1418-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arantes, O., and D. Lereclus. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115-119. [DOI] [PubMed] [Google Scholar]
- 3.Au, N., E. Kuester-Schoeck, V. Mandava, L. E. Bothwell, S. P. Canny, K. Chachu, S. A. Colavito, S. N. Fuller, E. S. Groban, L. A. Hensley, T. C. O'Brien, A. Shah, J. T. Tierney, L. L. Tomm, T. M. O'Gara, A. I. Goranov, A. D. Grossman, and C. M. Lovett. 2005. Genetic composition of the Bacillus subtilis SOS system. J. Bacteriol. 187:7655-7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi, A. A., and F. Baneyx. 1999. Stress responses as a tool to detect and characterize the mode of action of antibacterial agents. Appl. Environ. Microbiol. 65:5023-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, V. 2005. Bacterial iron transport related to virulence. Contrib. Microbiol. 12:210-233. [DOI] [PubMed] [Google Scholar]
- 6.Brotz-Oesterhelt, H., I. Knezevic, S. Bartel, T. Lampe, U. Warnecke-Eberz, K. Ziegelbauer, D. Habich, and H. Labischinski. 2003. Specific and potent inhibition of NAD+-dependent DNA ligase by pyridochromanones. J. Biol. Chem. 278:39435-39442. [DOI] [PubMed] [Google Scholar]
- 7.Chapman, T. 2004. Drug discovery: the leading edge. Nature 430:109-115. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. R., M. Malik, M. Snyder, and K. Drlica. 1996. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J. Mol. Biol. 258:627-637. [DOI] [PubMed] [Google Scholar]
- 9.Chopra, I., L. Hesse, and A. O'Neill. 2002. Exploiting current understanding of antibiotic action for discovery of new drugs. J. Appl. Microbiol. 92(Suppl.):4S-15S. [PubMed] [Google Scholar]
- 10.Eichenberger, P., M. Fujita, S. T. Jensen, E. M. Conlon, D. Z. Rudner, S. T. Wang, C. Ferguson, K. Haga, T. Sato, J. S. Liu, and R. Losick. 2004. The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol. 2:e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer, H. P., N. A. Brunner, B. Wieland, J. Paquette, L. Macko, K. Ziegelbauer, and C. Freiberg. 2004. Identification of antibiotic stress-inducible promoters: a systematic approach to novel pathway-specific reporter assays for antibacterial drug discovery. Genome Res. 14:90-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freiberg, C., H. Brotz-Oesterhelt, and H. Labischinski. 2004. The impact of transcriptome and proteome analyses on antibiotic drug discovery. Curr. Opin. Microbiol. 7:451-459. [DOI] [PubMed] [Google Scholar]
- 13.Freiberg, C., H. P. Fischer, and N. A. Brunner. 2005. Discovering the mechanism of action of novel antibacterial agents through transcriptional profiling of conditional mutants. Antimicrob. Agents Chemother. 49:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freiberg, C., G. Schiffer, N. Brunner, T. Lampe, J. Pohlmann, M. Brands, D. Haebich, and K. Ziegelbauer. 2004. Identification and characterization of the first class of potent bacterial acetyl-CoA carboxylase inhibitors with antibacterial activity. J. Biol. Chem. 279:26066-26073. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima, S., M. Yoshimura, T. Chibazakura, T. Sato, and H. Yoshikawa. 2006. The putative ABC transporter YheH/YheI is involved in the signalling pathway that activates KinA during sporulation initiation. FEMS Microbiol. Lett. 256:90-97. [DOI] [PubMed] [Google Scholar]
- 16.Gale, E. F., E. Cundliffe, P. E. Reynolds, M. H. Richmond, and M. J. Waring. 1981. The molecular basis of antibiotic action, 2nd ed. John Wiley & Sons, London, United Kingdom.
- 17.Galm, U., M. H. Hager, S. G. Van Lanen, J. Ju, J. S. Thorson, and B. Shen. 2005. Antitumor antibiotics: bleomycin, enediynes, and mitomycin. Chem. Rev. 105:739-758. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, L. H., B. Ferrari, A. H. Sorensen, D. Veal, and S. J. Sorensen. 2001. Detection of oxytetracycline production by Streptomyces rimosus in soil microcosms by combining whole-cell biosensors and flow cytometry. Appl. Environ. Microbiol. 67:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutter, B., C. Fischer, A. Jacobi, C. Schaab, and H. Loferer. 2004. Panel of Bacillus subtilis reporter strains indicative of various modes of action. Antimicrob. Agents Chemother. 48:2588-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutter, B., C. Schaab, S. Albrecht, M. Borgmann, N. A. Brunner, C. Freiberg, K. Ziegelbauer, C. O. Rock, I. Ivanov, and H. Loferer. 2004. Prediction of mechanisms of action of antibacterial compounds by gene expression profiling. Antimicrob. Agents Chemother. 48:2838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knusel, F., and J. Nuesch. 1965. Mechanism of action of sideromycins. Nature 206:674-676. [DOI] [PubMed] [Google Scholar]
- 22.Knusel, F., J. Nuesch, M. Scherrer, and B. Schiess. 1967. The action of siderochromes on the incorporation of low-molecular substances into intact bacterial cells. Pathol. Microbiol. (Basel) 30:900-908. (In German.) [PubMed] [Google Scholar]
- 23.Knusel, F., B. Schiess, and W. Zimmermann. 1969. The influence exerted by sideromycins on poly-U-directed incorporation of phenylalanine in the S-30 fraction of Staphylococcus aureus. Arch. Mikrobiol. 68:99-106. [DOI] [PubMed] [Google Scholar]
- 24.Kuhl, A., N. Svenstrup, C. Ladel, M. Otteneder, A. Binas, G. Schiffer, M. Brands, T. Lampe, K. Ziegelbauer, H. Rubsamen-Waigmann, D. Haebich, and K. Ehlert. 2005. Biological characterization of novel inhibitors of the gram-positive DNA polymerase IIIC enzyme. Antimicrob. Agents Chemother. 49:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarevic, V., A. Dusterhoft, B. Soldo, H. Hilbert, C. Mauel, and D. Karamata. 1999. Nucleotide sequence of the Bacillus subtilis temperate bacteriophage SPbetac2. Microbiology 145:1055-1067. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, R. J., O. M. Singh, C. V. Smith, T. Skarzynski, A. Maxwell, A. J. Wonacott, and D. B. Wigley. 1996. The nature of inhibition of DNA gyrase by the coumarins and the cyclothialidines revealed by X-ray crystallography. EMBO J. 15:1412-1420. [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, J. T., M. B. Connelly, C. Amolo, S. Otani, and D. S. Yaver. 2005. Global transcriptional response of Bacillus subtilis to treatment with subinhibitory concentrations of antibiotics that inhibit protein synthesis. Antimicrob. Agents Chemother. 49:1915-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascher, T., S. L. Zimmer, T. A. Smith, and J. D. Helmann. 2004. Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis. Antimicrob. Agents Chemother. 48:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell, R. J., and M. B. Gu. 2004. Construction and characterization of novel dual stress-responsive bacterial biosensors. Biosens. Bioelectron. 19:977-985. [DOI] [PubMed] [Google Scholar]
- 30.Newman, D. J., G. M. Cragg, and K. M. Snader. 2003. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 66:1022-1037. [DOI] [PubMed] [Google Scholar]
- 31.Piggot, P. J. 1973. Mapping of asporogenous mutations of Bacillus subtilis: a minimum estimate of the number of sporeulation operons. J. Bacteriol. 114:1241-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sackmann, W., P. Reusser, L. Neipp, F. Kradolfer, and F. Gross. 1962. Ferrimycin A, a new iron-containing antibiotic. Antibiot. Chemother. 12:34-45. [PubMed] [Google Scholar]
- 33.Shapiro, E., and F. Baneyx. 2002. Stress-based identification and classification of antibacterial agents: second-generation Escherichia coli reporter strains and optimization of detection. Antimicrob. Agents Chemother. 46:2490-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stulke, J., R. Hanschke, and M. Hecker. 1993. Temporal activation of beta-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J. Gen. Microbiol. 139:2041-2045. [DOI] [PubMed] [Google Scholar]
- 35.Sun, D., S. Cohen, N. Mani, C. Murphy, and D. M. Rothstein. 2002. A pathway-specific cell based screening system to detect bacterial cell wall inhibitors. J. Antibiot. (Tokyo) 55:279-287. [DOI] [PubMed] [Google Scholar]