Abstract
Quorum sensing is a regulatory system for controlling gene expression in response to increasing cell density. N-Acylhomoserine lactone (AHL) is produced by gram-negative bacteria, which use it as a quorum-sensing signal molecule. Serratia marcescens is a gram-negative opportunistic pathogen which is responsible for an increasing number of serious nosocomial infections. S. marcescens AS-1 produces N-hexanoyl homoserine lactone (C6-HSL) and N-(3-oxohexanoyl) homoserine lactone and regulates prodigiosin production, swarming motility, and biofilm formation by AHL-mediated quorum sensing. We synthesized a series of N-acyl cyclopentylamides with acyl chain lengths ranging from 4 to 12 and estimated their inhibitory effects on prodigiosin production in AS-1. One of these molecules, N-nonanoyl-cyclopentylamide (C9-CPA), had a strong inhibitory effect on prodigiosin production. C9-CPA also inhibited the swarming motility and biofilm formation of AS-1. A competition assay revealed that C9-CPA was able to inhibit quorum sensing at four times the concentration of exogenous C6-HSL and was more effective than the previously reported halogenated furanone. Our results demonstrated that C9-CPA was an effective quorum-sensing inhibitor for S. marcescens AS-1.
Serratia marcescens is a gram-negative opportunistic human pathogen which is responsible for an increasing number of serious nosocomial infections (8). S. marcescens produces a range of secreted products, including proteases, nucleases, lipases, chitinases, the biosurfactant serrawettin, and hemolysin (8). S. marcescens also produces prodigiosin (2-methyl-3-pentyl-6-methoxyprodigiosin), which is a red pigment and has antibacterial, antifungal, antiprotozoan, and immunosuppressant activities (7, 27). Prodigiosin production is regulated by a range of environmental signals, such as temperature, phosphate limitation, and medium components (7, 27). Nosocomial infections due to some clinical isolates of S. marcescens are also problematic because this pathogen is commonly multidrug resistant (27). Thus, it is necessary to seek a novel treatment technique for Serratia infections without overuse of antibiotics.
Quorum sensing is a regulatory system for controlling gene expression in response to increasing cell density (2, 6). N-Acylhomoserine lactone (AHL) (Fig. 1A) is produced by gram-negative bacteria, which use it as a quorum-sensing signal molecule (2). The LuxI protein family synthesizes AHL, and the LuxR protein family binds AHL and regulates expression of many genes responsible for bioluminescence, production of pigments or antibiotics, etc. (2, 6). In particular, many gram-negative pathogens control the expression of virulence factors, secretion of extracellular protease, pectinase, and rhamnolipid, and biofilm formation via the quorum-sensing system (2).
FIG. 1.
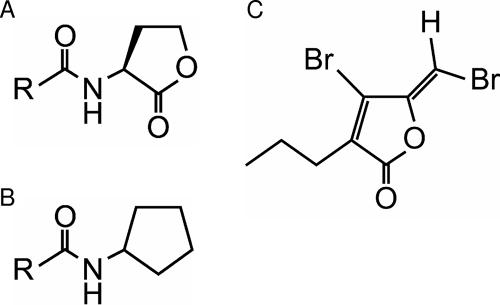
Structures of AHL and quorum-sensing inhibitors used in this study. (A) AHL. (B) Cn-CPA. (C) 4-Bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone.
It has been shown that Serratia strains employ quorum sensing for the regulation of genes encoding extracellular virulence factors (31). Serratia sp. strain ATCC 39006 produces N-butanoyl homoserine lactone (C4-HSL) and N-hexanoyl homoserine lactone (C6-HSL) and regulates production of prodigiosin and carbapenem with AHLs (30). S. liquefaciens (S. marcescens) MG1 produces C4-HSL and C6-HSL and regulates swimming motility and biofilm formation (14, 23). S. marcescens SS-1 produces four AHLs, N-(3-oxohexanoyl) homoserine lactone (3-oxo-C6-HSL), C6-HSL, N-heptanoyl homoserine lactone, and N-octanoyl homoserine lactone, and regulates sliding motility, synthesis of biosurfactant, prodigiosin, and nuclease (10). It was reported previously that signal generation mutants of Serratia strains were deficient in production of exoenzymes and prodigiosin and in biofilm formation (10, 14, 30). Thus, interfering with AHL-mediated quorum sensing could be an effective means of preventing infectious diseases caused by Serratia strains.
It was also reported previously that natural or nonnatural inhibitors were effective for preventing the expression of the genes controlled by the quorum-sensing system. Synthetic compounds modeled on the natural AHLs were evaluated for both their inducing activity and their ability to competitively inhibit the action of AHL in Vibrio fischeri, Burkholderia cenocepacia, and Pseudomonas aeruginosa (1, 3, 13, 22, 24, 28, 29). Halogenated furanones, produced by the red alga Delisea pulchra, inhibit quorum sensing in a number of bacteria (4, 5, 9, 20, 21). In Serratia, it was reported that halogenated furanone affected swarming motility in S. liquefaciens MG1 (4, 21). However, inhibitory effects on other features in Serratia strains, including prodigiosin production and biofilm formation, were not identified.
In a previous study, we found that N-acyl cyclopentylamide (Cn-CPA) (Fig. 1B) inhibited expression of the lasB-lacZ transcriptional fusion gene and biofilm formation without affecting the growth of P. aeruginosa PAO1 (12). In this paper, we demonstrate that Cn-CPA is an effective quorum-sensing inhibitor which interferes with expression of S. marcescens virulence factors regulated by the quorum-sensing system. Additionally, we also compared the inhibitory effects of Cn-CPA and halogenated furanone on quorum sensing.
MATERIALS AND METHODS
Bacterial strains, plasmids, compounds, and growth conditions.
Selected bacterial strains and plasmids used in this study are listed in Table 1. All bacterial strains were cultured in Luria-Bertani (LB) medium (25). S. marcescens AS-1 and AS-1S were grown at 25°C for stable production of prodigiosin. Escherichia coli and Chromobacterium violaceum were grown at 30°C. AHL standards and Cn-CPA were synthesized by a previously described method (11, 12). 4-Bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone was also synthesized by a previously described method (16). Antibiotics were added as required at final concentrations of 100 μg/ml for ampicillin and 50 μg/ml for kanamycin.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Serratia marcescens strains | ||
| AS-1 | Laboratory-maintained strain, natural isolate | This study |
| AS-1S | AS-1 spnI::kan, AHL-negative strain | This study |
| Escherichia coli strains | ||
| XL1-Blue | recA1 endA1 gyrA46 thi hsdR17 supE44 relA1 lac/F′ [proAB+laclq lacZΔM15::Tn10 (Tetr)] | Stratagene |
| S17-1 λpir | thi pro hsdR hsdM+recA RP4 2Tc::Mu-Km::Tn7 | 26 |
| Chromobacterium violaceum CV026 | Mini-Tn5 mutant derived from C. violaceum ATCC 31532, HgrcviI::Tn5 xylE Kanr plus spontaneous Smr, AHL biosensor | 18 |
| Plasmids | ||
| pSTV28 | Cloning vector, Cmr | Takara Bio |
| pUC4K | Cloning vector, Kanr Apr | Pharmacia |
| pASS01 | 3.0-kb Sau3AI fragment from AS-1 genomic DNA in pSTV28 | This study |
| pASS01K | pASS01 containing kan in PstI site | This study |
| pGP704Sac38 | pBR322 derivative with R6K ori, mob RP4, polylinker from M13 tg131 containing sacB; Apr | 19 |
| pGP704SK | pGP704Sac38 containing spnI::kan from pASS01K | This study |
Cloning and disruption of the AHL synthase gene of AS-1.
Chromosomal DNA of AS-1 was extracted to construct a genomic library by the standard protocol (25). DNA was digested partially with Sau3AI, and the fragments were inserted into the BamHI site of cloning vector pSTV28. A genomic library of AS-1 was transformed into E. coli XL1-Blue, and the ability to produce AHL was checked by cross-streaking with a CV026 biosensor. One of the AHL-producing plasmids, pASS01, was digested with various restriction enzymes for construction of a restriction map. Sequencing was performed by using BigDye Terminator version 3.1 and an ABI Prism 3100 genetic analyzer (Applied Biosystems). To disrupt the AHL synthase gene designated spnI, pASS01 was digested partially with PstI and inserted into the 1.3-kb kanamycin cassette (kan) from PstI-digested pUC4K for construction of pASS01K. The spnI::kan region of pASS02K was amplified by PCR using primers 5′-GATCCGAGGCTCAGCAAACA-3′ and 5′-TATTGTCTCCAAACTGGGCG-3′ and inserted into the EcoRV site of pGP704Sac38 for construction of pGP704SK. Disruption of the chromosomal spnI gene in AS-1 was performed by bacterial conjugation (19). Conjugation between E. coli S17-1 λpir with pGP704SK and AS-1 was performed. The chromosomal disruption of spnI was checked by PCR using the same primers, and the mutant was designated AS-1S.
Extraction and bioassays of AHLs.
Bacteria were grown for 15 h, inoculated into 100 ml fresh LB medium (1% inoculum), and incubated for 20 h. Cells were removed by centrifugation, and the supernatant was mixed with an equal volume of acidified ethyl acetate. The ethyl acetate layer was transferred to a new flask, evaporated to dryness, and dissolved in 1 ml of dimethyl sulfoxide. For AHL detection, 0.25 ml of an overnight culture of the CV026 biosensor was mixed with 25 ml of 1.5% LB agar and poured into a petri dish. Eight-millimeter-diameter paper disks (Advantec Inc.) were placed on an agar plate, and 10 μl of culture extract was applied to the disks. The plates were incubated overnight at 28°C, and the area containing a purple pigment was measured to determine the amount of AHLs. AHL samples were also subjected to analytical and preparative thin-layer chromatography (TLC) by using a previously described method (19).
Prodigiosin production assay.
S. marcescens strains were grown for 15 h, inoculated into fresh LB medium (1% inoculum) with or without inhibitors, and incubated for 20 h. Intracellular prodigiosin was extracted from the cells in an acidified ethanol solution (4% 1 M HCl in ethanol) (27). Prodigiosin production was determined by determining the ratio of the absorbance of the extracted prodigiosin solution at 534 nm to the turbidity of the culture suspension as optical density at 600 nm. The effects of inhibitors were evaluated using the relative prodigiosin production (A534/optical density at 600 nm), for which the control value was 100%.
Motility assays.
The medium used for motility assays was LB medium. Swimming assay plates contained 0.3% (wt/vol) agar, and swarming assay plates contained 0.5% (wt/vol) agar (17). Assay plates were inoculated with bacteria from an overnight culture in LB agar (1.5% [wt/vol]) at 25°C with a sterile toothpick. The plates were incubated at 25°C for 20 h.
Biofilm formation assay.
Biofilm formation was determined by a previously described method, with a slight modification (14). The biofilm formation medium used was Difco minimal broth Davis with 0.2% glucose and 0.5% Casamino Acids (8). A full-grown culture of AS-1S was diluted 100-fold in biofilm formation medium, and 200 μl of the dilution was added to each well of a polypropylene microtiter plate (Corning Inc.). After incubation at 25°C for 20 h, 25 μl of a 1% crystal violet solution was added to each well. The plates were incubated at room temperature for 15 min and rinsed with distilled water. The crystal violet was dissolved in 200 μl of 95% ethanol, and biofilm formation was analyzed at 570 nm by using a Spectra Max 250 spectrophotometer (Molecular Devices).
Nucleotide sequence accession numbers.
The nucleotide sequences of the spnI/spnR locus and the 16S rRNA gene from strain AS-1 have been deposited in the DDBJ/EMBL/GenBank databases under accession no. AB234869 and AB270613, respectively.
RESULTS
Effect of the spnI mutation on AHL and prodigiosin production.
S. marcescens AS-1 was isolated from a soil sample as an AHL producer. The nucleotide sequence of the 16S rRNA fragment from AS-1 showed 99.9% identity with that of S. marcescens strain AU736 (accession no. AY043386). First, we attempted to clone the AHL synthase gene from AS-1. To clone the AHL synthase gene, we prepared a genomic library of AS-1. One of the approximately 3,000 plasmids, designated pASS01, showed obvious AHL-producing activity. The nucleotide sequence of a 3.0-kb fragment of pASS01 revealed the presence of two open reading frames, which were the AHL synthase gene (spnI) and the gene homologous to the gene encoding LuxR (spnR). The amino acid sequences of the putative gene products of spnI and spnR showed 75.4 and 94.3% identity to SpnI and SpnR from S. marcescens SS-1, respectively (10). The 159 bp upstream of the translation initiation site of spnR contained a dyad, ACCTGACCGAAAGGTCAGGT, which contains conserved bases of the consensus lux box sequence (5′-RNSTGYAXGATNXTRCASRT-3′) (6). This sequence exhibited 100% sequence identity to the lux box-like sequence present upstream of spnR from SS-1 (10). In addition, the spnI and spnR open reading frames have a 23-bp overlapping region at their 3′ ends. This overlap can be observed in the SS-1 spnI/spnR locus (data not shown).
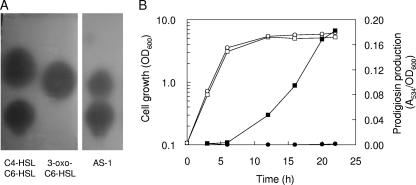
Using a gene replacement method, we constructed the spnI mutant designated AS-1S (spnI::kan). To check whether deletion of the spnI gene affected the biosynthesis of AHLs, the amount of AHLs produced in the culture supernatant was determined. AHLs were extracted with acidified ethyl acetate and fractionated by TLC. A TLC-overlaid CV026 biosensor revealed two AHL spots in the culture supernatant of AS-1, but no spot was observed in the culture supernatant of AS-1S. As determined by comparison with synthetic AHLs, these spots corresponded to 3-oxo-C6-HSL and C6-HSL (Fig. 2A). SpnI from SS-1 produced two AHLs, 3-oxo-C6-HSL and C6-HSL, as major products (10). It was assumed that these two SpnI proteins had similar functions in AHL biosynthesis.
FIG. 2.
Identification and characterization of AHLs produced by S. marcescens AS-1 and AS-1S. (A) TLC analysis of AHLs produced by strain AS-1 and its mutants. AHLs were visualized as pigments produced by strain CV026 and were identified after comparison with the standards C4-HSL, C6-HSL, and 3-oxo-C6-HSL. (B) Production of prodigiosin throughout growth in LB medium in AS-1 (squares) and AS-1S (circles). Samples were taken at various times and analyzed to determine growth (open symbols) and prodigiosin production (filled symbols). OD600, optical density at 600 nm.
Prodigiosin production by AS-1 and AS-1S was determined throughout growth (Fig. 2B). Although cultures of these two strains had similar growth rates, prodigiosin production was increased at early stationary phase in strain AS-1 but not observed at any time in strain AS-1S. Various AHLs which have different acyl chain lengths were added to the cultures of AS-1S, and prodigiosin production was determined. Addition of C6-HSL at a concentration of 5 μM stimulated prodigiosin production in AS-1S, but addition of C4-HSL or N-octanoyl homoserine lactone did not (data not shown). Prodigiosin production by AS-1S was completely restored to wild-type levels by supplying 20 μM C6-HSL (data not shown). It was hypothesized that prodigiosin production was controlled by AHL-mediated quorum sensing and that this molecule could be used as a marker for screening quorum-sensing inhibitors.
Effects of Cn-CPA on prodigiosin production.
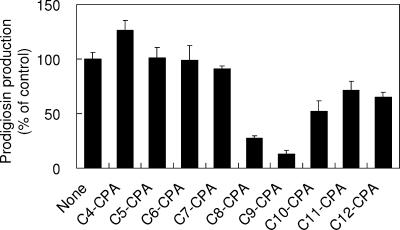
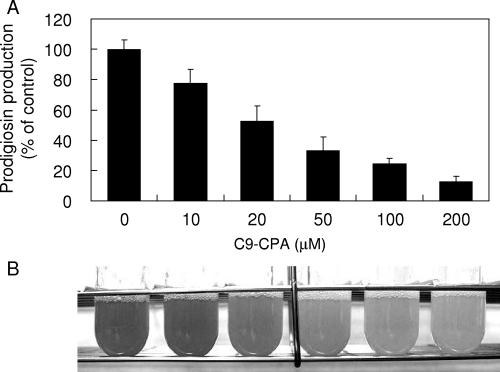
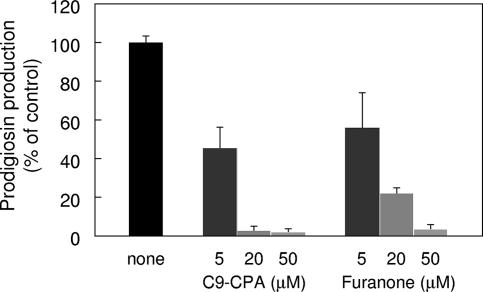
In a previous study, we demonstrated that Cn-CPA had an inhibitory effect on induction of the quorum-sensing-controlled genes in P. aeruginosa PAO1 (12). Therefore, we checked whether Cn-CPA had the ability to interfere with prodigiosin production controlled by quorum sensing in AS-1. A series of Cn-CPAs with acyl chain lengths ranging from 4 to 12 were synthesized and dissolved in dimethyl sulfoxide. Each Cn-CPA was added to an AS-1 culture at a final concentration of 200 μM. After determination of prodigiosin production, two Cn-CPAs, N-octanoyl-cyclopentylamide and N-nonanoyl-cyclopentylamide (C9-CPA), showed high levels of inhibitory effects on prodigiosin production (Fig. 3). The most effective inhibitor was C9-CPA, which inhibited prodigiosin production 87% at a final concentration of 200 μM. Prodigiosin production assays were performed using a range of concentrations of C9-CPA. The results revealed that C9-CPA inhibited prodigiosin production in a dose-dependent manner (Fig. 4). The concentration of C9-CPA required for half-maximal inhibition was approximately 24 μM. The growth of AS-1 was not affected by addition of C9-CPA at any concentration up to 200 μM (data not shown). These results indicate that C9-CPA specifically inhibits the quorum-sensing system.
FIG. 3.
Effects of Cn-CPA on prodigiosin production in S. marcescens AS-1. Intracellular prodigiosin was extracted after 20 h of cultivation. The results were reproduced in three experiments, and the error bars indicate standard deviations.
FIG. 4.
(A) Dose response showing inhibitory effects of C9-CPA on prodigiosin production in S. marcescens AS-1. Intracellular prodigiosin was extracted after 20 h of cultivation. The results were reproduced in three experiments, and the error bars indicate standard deviations. (B) Red-pigmented cultures with various concentrations of C9-CPA.
To investigate the competition between C6-HSL and C9-CPA during prodigiosin production, we used an AHL-negative strain, AS-1S. For the competition assay, C6-HSL was used at a final concentration of 5 μM, which was required for 50% maximal induction of prodigiosin production under our conditions (data not shown). The competition assay was performed using a range of concentrations of C9-CPA and 5 μM C6-HSL. In the presence of exogenous C6-HSL, C9-CPA inhibited prodigiosin production in a dose-dependent manner. When C9-CPA was added at a concentration of 20 μM, which was four times the concentration of C6-HSL, prodigiosin production almost disappeared (Fig. 5). In S. marcescens SS-1, long-chain AHLs (N-decanoyl homoserine lactone and N-dodecanoyl homoserine lactone) are capable of antagonizing sliding motility controlled by quorum sensing (10). However, AHLs with acyl chain lengths of 4 to 10 did not competitively inhibit prodigiosin production induced by 5 μM C6-HSL in AS-1S (data not shown). It was reported previously that halogenated furanone inhibited quorum sensing in S. liquefaciens MG1. Thus, we also compared the inhibitory effects on prodigiosin production of C9-CPA and halogenated furanone. However, although halogenated furanone had an inhibitory effect on prodigiosin production, some prodigiosin was produced at a concentration of 20 μM (Fig. 5). These results indicated that C9-CPA had a greater inhibitory effect on quorum sensing than halogenated furanone.
FIG. 5.
Competition assay with C6-HSL and quorum-sensing inhibitors of prodigiosin production in S. marcescens AS-1. AS-1 was cultivated with 5 μM C6-HSL and various concentrations of C9-CPA or halogenated furanone. Intracellular prodigiosin was extracted after 20 h of cultivation. The results were reproduced in three experiments, and the error bars indicate standard deviations.
Effects of C9-CPA on motility and biofilm formation.
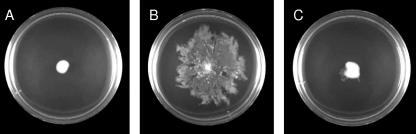
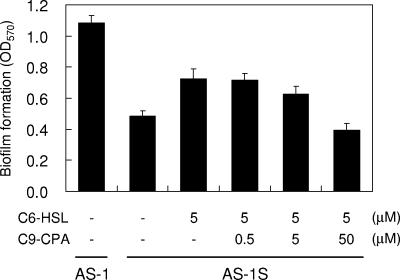
In a previous report, it was shown that in Serratia strains various phenotypes other than prodigiosin production are regulated. For instance, biofilm formation and sloughing in S. liquefaciens MG1 are controlled by quorum sensing (14, 23). Swarming motility in S. liquefaciens MG1 and sliding motility in S. marcescens SS-1 are regulated by AHL-mediated quorum sensing (4, 10). To examine inhibition of these features, workers have investigated whether halogenated furanone affects the swarming motility of S. liquefaciens MG1 (4, 21). Therefore, we checked whether Cn-CPA had the ability to interfere with surface motility on agar plates and biofilm formation in AS-1. Assays were performed on LB medium with 0.3% agar for swimming motility assays or with 0.5% agar for swarming motility assays. AS-1 and AS-1S showed obvious swimming activity with or without AHL or Cn-CPA (data not shown). In contrast, AS-1 showed surface translocation on a swarming assay plate, but AS-1S did not. This defect in swarming motility in AS-1S was restored by adding 5 μM C6-HSL, which was required for 50% maximal induction of prodigiosin production (Fig. 6). These data indicated that quorum sensing in AS-1 controlled swarming motility but did not control swimming motility. A competition assay with C6-HSL was performed at a C9-CPA concentration of 50 μM, and surface translocation of AS-1S disappeared completely (Fig. 6). We also tested biofilm formation on a polypropylene plastic surface. AS-1 and AS-1S produced certain amounts of biomass that adhered to the polypropylene plastic after 20 h. The biofilm formation by AS-1S was reduced to approximately 45% of the parental level. When 5 μM C6-HSL was added to each well, biofilm formation was increased to a level that was approximately 62% of the parental biofilm level (Fig. 7). This behavior implied that biofilm formation by AS-1 was influenced by AHL-mediated quorum sensing. A competition assay with C6-HSL was performed using various concentrations of C9-CPA, and the adherent biomass decreased in a dose-dependent manner (Fig. 7). In particular, the amount of adherent biomass decreased until it was at the control level when C9-CPA was added at a concentration of 50 μM.
FIG. 6.
Analysis of swarming motility of AS-1S on swarming assay plates with 0.5% agar. Plates were incubated at 25°C and monitored after 20 h. (A) AS-1S without any compounds. (B) AS-1S with 5 μM C6-HSL. (C) AS-1S with 5 μM C6-HSL and 50 μM C9-CPA.
FIG. 7.
Quantification of bacteria in biofilms formed on polypropylene plastic by strains AS-1 and AS-1S. Biofilms were allowed to form in a 96-well polypropylene microtiter dish, stained with crystal violet, and estimated by analyzing the absorbance at 570 nm (OD570). The amounts of C6-HSL and C9-CPA used in the reactions are indicated at the bottom. Seven wells of each sample were used for measuring biofilm formation, and the error bars indicate standard deviations.
DISCUSSION
In this work, we demonstrated that C9-CPA was an effective quorum-sensing inhibitor for S. marcescens AS-1. Although several quorum-sensing inhibitors have been evaluated for some bacteria, there have been few reports on quorum-sensing inhibitors for Serratia strains. It was reported previously that halogenated furanone affected swarming motility in S. liquefaciens MG1. Our work provides the first report of nonnatural quorum-sensing inhibitors for S. marcescens. C9-CPA was able to inhibit not only swarming motility but also prodigiosin production and biofilm formation in AS-1. In addition, the inhibitory effect of C9-CPA on quorum sensing in AS-1 was greater than the effect reported for halogenated furanone. We have previously demonstrated that C9-CPA had an inhibitory effect on prodigiosin production in another Serratia strain, S. rubidaea N-1, which is a producer of prodigiosin and a highly salt-tolerant strain (32). It is possible that Cn-CPA inhibits quorum sensing in a broad range of Serratia strains.
The length of the acyl chain of the Cn-CPA most effective for inhibition of quorum sensing in AS-1 was nine. Although the length of the acyl chain of AHLs produced by AS-1 was six (C6-HSL and 3-oxo-C6-HSL), N-hexanoyl-cyclopentylamide did not affect quorum sensing in AS-1. In P. aeruginosa PAO1, which produces two AHLs, C4-HSL and N-(3-oxododecanoyl) homoserine lactone, the most effective quorum-sensing inhibitor was N-decanoyl-cyclopentylamide (12). N-decanoyl-cyclopentylamide had a great inhibitory effect on the las system, which was controlled by N-(3-oxododecanoyl) homoserine lactone. The length of the acyl chain of the most effective Cn-CPA for AS-1 and PAO1 differed considerably from the lengths of the acyl chains of their own AHLs. The interaction between R-protein and the Cn-CPA inhibitor has not been elucidated clearly, so it is thought that investigation of the interaction between purified SpnR and C9-CPA is needed. On the other hand, it was reported that halogenated furanone accelerated LuxR turnover (15). The genes coding for prodigiosin, biosurfactant, and nuclease biosynthesis in S. marcescens SS-1 are negatively regulated by SpnIR (10). In S. marcescens SS-1, AHLs produced via SpnI interact with SpnR, resulting in derepression of these genes (10). The amino acid sequence of SpnR from AS-1 showed high homology with that of SpnR from SS-1 (94% identity). If SpnR from AS-1 works as a negative regulator in quorum sensing as well as SpnR from SS-1, acceleration of SpnR turnover could lead to activation of the genes regulated by quorum sensing. Thus, it was assumed that C9-CPA interferes with the quorum-sensing system by acting as an effective competitor of AHL binding to SpnR.
Many kinds of quorum-sensing inhibitors modeled on the natural AHLs have been designed, synthesized, and evaluated for inhibition of quorum sensing. However, chemosynthesis of these compounds was complicated and required a number of synthetic steps. Cn-CPA can be synthesized easily by using appropriate acyl chlorides and cyclopentylamine through one coupling reaction and several extraction processes (12). Although the relationship between quorum sensing and opportunistic infection by Serratia is not clear, C9-CPA was able to inhibit biofilm formation and motility, which were considered some of the major virulence factors. In the future, it may be possible to use C9-CPA as an antipathogen drug for Serratia infections, as a substitute for current antibiotic drugs.
Acknowledgments
We are grateful to Satoshi Ito of Utsunomiya University and Takenori Ishida of Hiroshima University for technical advice.
This work was supported in part by Grant-in-Aid for Young Scientists (B) 18760588 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.Castang, S., B. Chantegrel, C. Deshayes, R. Dolmazon, P. Gouet, R. Haser, S. Reverchon, W. Nasser, N. Hugouvieux-Cotte-Pattat, and A. Doutheau. 2004. N-Sulfonyl homoserine lactones as antagonists of bacterial quorum sensing. Bioorg. Med. Chem. Lett. 14:5145-5149. [DOI] [PubMed] [Google Scholar]
- 2.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frezza, M., S. Castang, J. Estephane, L. Soulere, C. Deshayes, B. Chantegrel, W. Nasser, Y. Queneau, S. Reverchon, and A. Doutheau. 2006. Synthesis and biological evaluation of homoserine lactone derived ureas as antagonists of bacterial quorum sensing. Bioorg. Med. Chem. 14:4781-4791. [DOI] [PubMed] [Google Scholar]
- 4.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gram, L., R. de Nys, R. Maximilien, M. Givskov, P. Steinberg, and S. Kjelleberg. 1996. Inhibitory effects of furanones from the red alga Delisea pulchra on swarming motility of Proteus mirabilis. Appl. Environ. Microbiol. 62:4284-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg, E. P. 1997. Quorum sensing in gram-negative bacteria. ASM News 63:371-377. [Google Scholar]
- 7.Han, S. B., H. M. Kim, Y. H. Kim, C. W. Lee, E. S. Jang, K. H. Son, S. U. Kim, and Y. K. Kim. 1998. T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. Int. J. Immunopharmacol. 20:1-13. [DOI] [PubMed] [Google Scholar]
- 8.Hejazi, A., and F. R. Falkiner. 1997. Serratia marcescens. J. Med. Microbiol. 46:903-912. [DOI] [PubMed] [Google Scholar]
- 9.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, W. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Hoiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horng, Y. T., S. C. Deng, M. Daykin, P. C. Soo, J. R. Wei, K. T. Luh, S. W. Ho, S. Swift, H. C. Lai, and P. Williams. 2002. The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens. Mol. Microbiol. 45:1655-1671. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda, T., K. Kajiyama, T. Kita, N. Takiguchi, A. Kuroda, J. Kato, and H. Ohtake. 2001. The synthesis of optically pure enantiomers of N-acyl-homoserine lactone autoinducers and their analogues. Chem. Lett. 30:314-315. [Google Scholar]
- 12.Ishida, T., T. Ikeda, N. Takiguchi, A. Kuroda, H. Ohtake, and J. Kato. 2007. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl. Environ. Microbiol. 73:3183-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jog, G. J., J. Igarashi, and H. Suga. 2006. Stereoisomers of P. aeruginosa autoinducer analog to probe the regulator binding site. Chem. Biol. 13:123-128. [DOI] [PubMed] [Google Scholar]
- 14.Labbate, M., S. Y. Queck, K. S. Koh, S. A. Rice, M. Givskov, and S. Kjelleberg. 2004. Quorum sensing-controlled biofilm development in Serratia liquefaciens MG1. J. Bacteriol. 186:692-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manefield, M., T. B. Rasmussen, M. Henzter, J. B. Andersen, P. Steinberg, S. Kjelleberg, and M. Givskov. 2002. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148:1119-1127. [DOI] [PubMed] [Google Scholar]
- 16.Manny, A. J., S. Kjelleberg, N. Kumar, R. de Nys, R. W. Read, and P. Steinberg. 1997. Reinvestigation of the sulfuric acid-catalysed cyclisation of brominated 2-alkyllevulinic acids to 3-alkyl-5-methylene-2(5H)-furanones. Tetrahedron 53:15813-15826. [Google Scholar]
- 17.Matsuyama, T., K. Kaneda, Y. Nakagawa, K. Isa, H. Hara-Hotta, and I. Yano. 1992. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J. Bacteriol. 174:1769-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycrof, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 19.Morohoshi, T., T. Inaba, N. Kato, K. Kanai, and T. Ikeda. 2004. Identification of quorum-sensing signal molecules and the LuxRI homologs in fish pathogen Edwardsiella tarda. J. Biosci. Bioeng. 98:276-281. [DOI] [PubMed] [Google Scholar]
- 20.Rasch, M., C. Buch, B. Austin, W. J. Slierendrecht, K. S. Ekmann, J. L. Larsen, C. Johansen, K. Riedel, L. Eberl, M. Givskov, and L. Gram. 2004. An inhibitor of bacterial quorum sensing reduces mortalities caused by vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum). Syst. Appl. Microbiol. 27:350-359. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen, T. B., M. Manefield, J. B. Andersen, L. Eberl, U. Anthoni, C. Christophersen, P. Steinberg, S. Kjelleberg, and M. Givskov. 2000. How Delisea pulchra furanones affect quorum sensing and swarming motility in Serratia liquefaciens MG1. Microbiology 146:3237-3244. [DOI] [PubMed] [Google Scholar]
- 22.Reverchon, S., B. Chantegrel, C. Deshayes, A. Doutheau, and N. Cotte-Pattat. 2002. New synthetic analogues of N-acyl homoserine lactones as agonists or antagonists of transcriptional regulators involved in bacterial quorum sensing. Bioorg. Med. Chem. Lett. 22:1153-1157. [DOI] [PubMed] [Google Scholar]
- 23.Rice, S. A., K. S. Koh, S. Y. Queck, M. Labbate, K. W. Lam, and S. Kjelleberg. 2005. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J. Bacteriol. 187:3477-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riedel, K., M. Kothe, B. Kramer, W. Saeb, A. Gotschlich, A. Ammendola, and L. Eberl. 2006. Computer-aided design of agents that inhibit the cep quorum-sensing system of Burkholderia cenocepacia. Antimicrob. Agents Chemother. 50:318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 26.Simon, R., J. Quandt, and W. Klipp. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 27.Slater, H., M. Crow, L. Everson, and G. P. Salmond. 2003. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol. Microbiol. 47:303-320. [DOI] [PubMed] [Google Scholar]
- 28.Smith, K. M., Y. Bu, and H. Suga. 2003. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 10:81-89. [DOI] [PubMed] [Google Scholar]
- 29.Smith, K. M., Y. Bu, and H. Suga. 2003. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 10:563-571. [DOI] [PubMed] [Google Scholar]
- 30.Thomson, N. R., M. A. Crow, S. J. McGowan, A. Cox, and G. P. Salmond. 2000. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol. Microbiol. 36:539-556. [DOI] [PubMed] [Google Scholar]
- 31.Wei, J. R., and H. C. Lai. 2006. N-Acylhomoserine lactone-dependent cell-to-cell communication and social behavior in the genus Serratia. Int. J. Med. Microbiol. 296:117-124. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki, G., S. Nishimura, A. Ishida, M. Kanagasabhapathy, X. Zhou, S. Nagata, T. Morohoshi, and T. Ikeda. 2006. Effect of salt stress on pigment production of Serratia rubidaea N-1: a potential indicator strain for screening quorum sensing inhibitors from marine microbes. J. Gen. Appl. Microbiol. 52:113-117. [DOI] [PubMed] [Google Scholar]