Abstract
Analyses of the distribution of virulence factors among different Escherichia coli pathotypes, including Shiga toxin-producing E. coli (STEC), may provide some insight into the mechanisms by which different E. coli strains cause disease and the evolution of distinct E. coli types. The aim of this study was to examine the DNA sequence of the gene for enterohemolysin, a plasmid-encoded toxin that readily causes the hemolysis of washed sheep erythrocytes, and to assess the distribution of enterohemolysin subtypes among E. coli isolates from various human and animal sources. The 2,997-bp ehxA gene was amplified from 227 (63.8%) of 356 stx- and/or eae-positive E. coli strains isolated from cattle and sheep and from 24 (96.0%) of 25 STEC strains isolated from humans with diarrheal disease. By using PCR and restriction fragment length polymorphism (RFLP) analysis of ehxA, six distinct PCR-RFLP types (A to F) were observed, with strains of subtypes A and C constituting 91.6% of all the ehxA-positive strains. Subtype A was associated mainly with ovine strains with stx only (P < 0.001), and subtype C was associated with bovine eae-positive strains (P < 0.001). Eleven ehxA alleles were fully sequenced, and the phylogenetic analysis indicated the presence of three closely related (>95.0%) ehxA sequence groups, one including eae-positive strains (subtypes B, C, E, and F) and the other two including mainly eae-negative STEC strains (subtypes A and D). In addition to being widespread among STEC strains, stx-negative, eae-positive strains (atypical enteropathogenic E. coli strains) isolated from cattle and sheep have similar ehxA subtypes and hemolytic activities.
Diarrheagenic Escherichia coli pathotypes, such as Shiga toxin-producing E. coli (STEC) and enteropathogenic E. coli (EPEC), are conveniently subdivided according to the expression of pathogenic determinants that are implicated in animal and human disease (26). The acquisition of such virulence factors often reflects the extensive horizontal transfer of genetic material, such as the insertion of mobile elements, and can provide some insight into the evolution of separate E. coli pathotypes (8). However, the convenient subdivision of E. coli strains into specific groups often fails to address fundamental intrapathotype variation and interpathotype similarities. For example, all STEC strains exhibit a marked cytotoxic effect on human vascular endothelial cells mediated by the Shiga toxins encoded by stx1 and/or stx2 (27). Some STEC strains also express a number of proteins, including intimin (encoded by the eae allele), that coordinate the formation of attaching and effacing (A/E) lesions on gastrointestinal epithelial cells (19), but other STEC strains are eae negative and do not produce such proteins. Similarly, all EPEC strains are eae positive and typically possess a cluster of plasmid-borne genes (bfp) that encode bundle-forming pili that stabilize microcolony formation (26). However, atypical EPEC strains, commonly isolated from ruminants, are bfp negative (26, 41). Therefore, the structural analysis of virulence determinants within and between different E. coli diarrheagenic pathotypes should provide valuable clues on the evolutionary relationship of these pathotypes and on possible mechanisms by which pathogenesis occurs. One such virulence factor is the plasmid-encoded enterohemolysin of STEC that readily causes the hemolysis of washed sheep erythrocytes (4, 5, 34, 39) by serotypes including O157:H7 (5, 32) and O111:H− (33). Indeed, serum samples from patients with hemolytic-uremic syndrome (HUS) react specifically to the hemolysin from O157:H7 strains (32).
The main genetic determinants for the production of the O157:H7 STEC enterohemolysin (4) are associated with pO157 (34). This large plasmid of approximately 94 kb contains the ehx locus, which has the gene order ehxCABD and is >60% homologous to the alpha-hemolysin gene of E. coli (35). Like alpha-hemolysin, enterohemolysin is a pore-forming RTX (repeats in toxin) cytolysin that is active on sheep erythrocytes and certain bovine lymphoma cell lines (2, 32, 34, 35, 44). Overnight incubation on washed sheep blood agar is required for enterohemolysin expression, in contrast to the rapid (4-h) hemolysis associated with alpha-hemolysin (4). Enterohemolysin is also likely to be synthesized as an inactive protoxin requiring the acylation activity of EhxC protein and secretion, mediated by EhxB and EhxD, by a specific membrane translocator system (38, 44).
The precise role of enterohemolysin in STEC disease remains to be fully established, but enterohemolysin from a STEC O128:H12 isolate has been observed to induce increased levels of the proinflammatory cytokine interleukin-1β from human monocytes during in vitro studies (39). Other studies using bacterial strains from which pO157 has been removed have failed to provide definitive proof of the importance of the large plasmid or enterohemolysin in STEC pathogenesis (18, 43); however, ehxA is found in many STEC serotypes, such as O157:H7, O26:H11, O103:H2, O111:H−, O113:H21, O5:H−, and O84:H2/H−, that are commonly associated with diarrheal disease and HUS, and thus, ehxA has been commonly used as a possible marker for STEC (4, 13, 24, 31, 32, 33, 45, 46). Several ehxA-positive serotypes, including O157:H− (16) and O111:H− (33), do not exhibit the enterohemolytic phenotype on washed sheep blood agar, indicating that the precise conditions for the regulation and optimum expression of enterohemolysin may remain to be determined.
More recently, stx-negative E. coli strains that possess the A/E-lesion determinant have been increasingly recognized as having the same enterohemolytic phenotype on washed sheep erythrocytes as STEC (1, 10, 21). The link between atypical EPEC strains that are bfp negative and human diarrheal disease has yet to be readily established (29, 37, 41, 42). However, some stx-negative strains of STEC-associated serotypes O157:H7 and O157:H−, commonly associated with human clinical disease (diarrhea and HUS), have also occasionally been isolated in clinical cases (36). Furthermore, atypical EPEC strains (serotypes O26:H11, O69:H32, O76:H−, O84:H38, O115:H−, O123:H11, O123:H−, O145:H−, O149:H−, O149:H7, and O168:H8) lacking bundle-forming pili have been isolated from cattle and sheep on a number of occasions (1, 10, 21), but no information is available on the relationship between the ehxA gene from these atypical EPEC strains and that for the enterohemolysin more commonly associated with STEC. Therefore, the aim of this work was to identify and characterize the genetic and phenotypic diversity of the enterohemolysin from stx- and/or eae-positive E. coli strains from various sources (animal and human) to better understand the possible evolutionary relationship among bacteria from different E. coli diarrheagenic pathotypes. In addition, this study describes a rapid technique for the typing of ehxA genes from E. coli and the identification of three new ehxA sequences that are similar to those from STEC O157:H7.
MATERIALS AND METHODS
Bacterial strains and culture conditions for enterohemolysin expression.
Most of the bacterial strains used in this study have been described elsewhere (3, 11, 12, 13). Of the strains examined for ehxA, 215 were positive for stx only (99 for stx1 only, 33 for stx1 and stx2, and 83 for stx2 only) and 141 were eae positive (115 were positive for eae only, 25 were stx1 and eae positive, and 1 was stx2 and eae positive). These strains were isolated from rectoanal mucosal swabs taken from healthy cattle and sheep on the lower North Island, New Zealand, over a period of 3 months. The E. coli strains (n = 25; 1 positive for stx1 only, 2 stx1 and stx2 positive, 6 positive for stx2 only, 9 stx1 and eae positive, 5 stx2 and eae positive, and 2 eae positive) from human patients with diarrheal disease were obtained from the Enteric Reference Laboratory, ESR Ltd. Luria-Bertani broth or agar was used routinely for the growth of bacteria. For the detection of enterohemolytic activity, strains were inoculated from a well-spaced single colony onto washed sheep blood agar plates and the plates were incubated at 37°C for 18 h, followed by 6 h at room temperature. Defibrinated sheep blood was washed three times in phosphate-buffered saline (pH 7.4) at 950 × g and added (5%, vol/vol) to Luria-Bertani agar cooled to 50°C (9).
PCR-RFLP subtyping.
All bovine and ovine bacterial strains that were stx and eae positive (n = 38), stx positive only (n = 115), or eae positive only (n = 115) were chosen for further study to detect the presence of ehxA by PCR amplification of the complete 2,997-bp ehxA gene with oligonucleotide primers (Invitrogen, Auckland, New Zealand) ehxARFLP F (5′ ATGACAGTAAATAAAATAAAGAAC 3′) and ehxARFLP R (5′ TCAGACAGTTGTCGTTAAAGTTG 3′), corresponding to positions 1 to 24 (ehxARFLP F) and 2975 to 2997 (ehxARFLP R), and the Px2 system (Thermo Hybaid, Ashford, United Kingdom). Amplification was performed with a DNA template prepared from heat-treated bacterial cells as described previously (11). The PCR mixture included the DNA template and 2.5 pM (each) primers, and the volume was made up to 20 μl by using the Taq polymerase-deoxynucleoside triphosphate supermix (Invitrogen, Auckland, New Zealand). For PCR amplification of the ehxA gene, an initial denaturing cycle of 95°C for 5 min was followed by 30 cycles of 95°C for 45 s (denaturing), 52°C for 45 s (annealing), and 72°C for 2 min, with a final extension step at 72°C for 5 min. Initially, 5 μl of each PCR mixture was used to detect the presence of the 2,997-bp ehxA amplicon by agarose gel electrophoresis (Innovative Sciences Ltd., Dunedin, New Zealand). Approximately 0.5 μl of 10× gel loading buffer (Invitrogen, Auckland, New Zealand) was added to the sample before it was electrophoresed across a 2.5% (wt/vol) agarose (Boehringer Mannheim, Germany) gel in Tris-acetate-EDTA buffer. Each gel was electrophoresed at 180 V (approximately 15 V cm−1) for 55 min. Approximately 8 μl of a 10,000× SYBR safe DNA stain (Molecular Probes, Auckland, New Zealand) solution was added to each 100-ml molten agarose (2.5%, wt/vol)-Tris-acetate-EDTA buffer gel mix immediately prior to pouring in order to visualize DNA on a transilluminator. For those strains that were ehxA positive, approximately 1.1 μl of 10× restriction enzyme buffer and 5 U of TaqI restriction endonuclease (Invitrogen, Auckland, New Zealand) were added to 9 μl of each PCR mixture and mixed gently. Digestion mixtures were incubated for 90 min at 65°C and then subjected to DNA electrophoresis and visualization as described previously. Restriction fragment length polymorphism (RFLP) electrophoretic gel images were stored as TIFF files by using a Gel Logic 200 imaging system (Kodak; Biolab, Auckland, New Zealand) to compare the images with those generated in silico. Virtual gels of GenBank ehxA gene sequences AF043471 (O8:H19), AY258503 (O113:H21), and AB032930 (O128:H12), corresponding to subtype A; X79839 (O157:H7), corresponding to subtype B; and X94129 (O111:H−), corresponding to subtype C, were constructed using Vector NTI Advance (version 9.1.0; Invitrogen Corporation). The TaqI sites from each ehxA sequence were identified, and the apparent electrophoretic mobility of the TaqI fragments was assessed using the Vector NTI Advance program.
Colony blot hybridization.
Bacteria were streaked out onto gridded Hybond N+ nylon membranes (GE Healthcare Life Sciences, Auckland, New Zealand) immobilized on MacConkey agar plates and grown for 16 h at 37°C. The membrane was removed from the agar plate, and bacteria were lysed using sodium dodecyl sulfate (10%, wt/vol). Bacterial DNA was denatured, and the membrane was neutralized according to the manufacturer's instructions. Excess bacterial cell debris was removed by washing in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and DNA was fixed to the membrane by baking at 80°C for 2 h. A 534-bp fragment of ehxA was amplified using hlyAF and hlyAR primers, corresponding to positions 70 to 90 and 581 to 602 of the ehxA sequence, as described previously (11) and labeled using the AlkPhos direct labeling and detection system according to the instructions of the manufacturer (GE Healthcare Life Sciences). Hybridization reactions were performed overnight at 50°C. Membranes were washed at 50°C, and ehxA-positive bacteria were visualized using CDP-Star (GE Healthcare Life Sciences). E. coli O157:H7 NCTC12900 and E. coli DH5α were used as positive and negative controls, respectively.
DNA sequencing.
The complete ehxA gene sequence was obtained from 11 ehxA-positive strains (2 strains each of ehxA subtypes A, B, C, D, and F and 1 strain of ehxA subtype E) displaying a wide variety of enterohemolytic characteristics. Initially, the ehxA gene was amplified using the ehxARFLP F and ehxARFLP R primers as described previously. The PCR product was purified using the QIAGEN PCR purification kit and sequenced using the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Auckland, New Zealand). Initial sequencing was performed with the ehxARFLP F and ehxARFLP R primers and then proceeded with internally designed oligonucleotides for the sequencing of the whole ehxA gene fragment. Capillary separation of sequencing reaction mixtures was performed on an ABI 3730 machine (Applied Biosystems) at the Allan Wilson Centre Genome Service Facility (Massey University, New Zealand).
Serological analysis.
The evaluation of O (O1 to O181)- and H (H1 to H56, excluding H13, H22, and H50)-antigens from selected strains was carried out according to standard World Health Organization methods using antisera raised for all known O and H groups (Statens Serum Institute, Copenhagen, Denmark). O-antigens were identified using overnight broth cultures steamed for 1 h. H-antigens were identified from strains after repeated passage through semisolid agar medium followed by treatment with 0.05% (vol/vol) formalin. Strains that did not agglutinate any of the specified O or H antisera were designated O nontypeable (ONT) and H nontypeable (HNT), respectively, and strains that were considered nonmotile were designated H−.
Phylogenetic analysis.
DNA sequences were edited with Contig Express (Vector NTI Advance, version 9.1.0; Invitrogen Corporation). Phylogenetic trees were compiled with MEGA version 3.1 (22; http://www.megasoftware.net/) using the neighbor-joining method of Saitou and Nei (30). ClustalW included in the DNASTAR (University of Wisconsin) software was used to calculate genetic distances (40).
Statistical analysis.
Comparisons of the distribution of ehxA PCR-RFLP subtypes with that of (i) E. coli pathotypes and (ii) strains from cattle or sheep was performed using χ2 analysis (GenStat 7.0; VSN International, Hemel Hempstead, United Kingdom) where a P value of <0.05 was considered significant.
Nucleotide sequence accession numbers.
The complete ehxA sequences of strains O90:H8 AGR047 (subtype C), O131:H25 AGR053 (subtype C), O153:H− AGR119 (subtype F), O98:H− AGR151 (subtype A), O101:H− AGR158 (subtype E), O121:H19 AGR270 (subtype B), O91:H− AGR340 (subtype A), O5:H− AGR374 (subtype F), ONT:H− AGR670 (subtype D), ONT:H− AGR674 (subtype D), and O157:H7 ER03/4238 (subtype B) were submitted to GenBank and have been assigned the accession numbers EF204919 to EF204929, respectively.
RESULTS
Identification of novel ehxA subtypes by PCR-RFLP analysis.
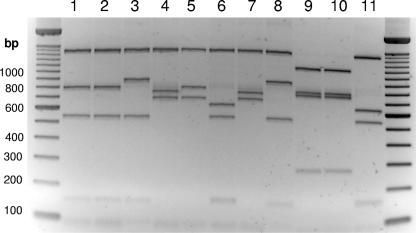
E. coli strains that were stx and/or eae positive were examined by PCR to detect the presence of the full-length 2,997-bp ehxA gene. Overall, 227 (63.8%) of the stx- or eae-positive strains from animals were ehxA positive, of which 134 (59.0%) were sheep strains and 93 (41.0%) were cattle strains (Table 1). Twenty-four (96.0%) of the 25 human E. coli isolates examined were ehxA positive. Of the 134 ehxA-positive sheep E. coli strains that were stx or eae positive, 103 (76.9%) were stx positive only, 4 (3.0%) were stx and eae positive, and 27 (20.1%) were eae positive only. Of the 93 ehxA-positive cattle E. coli strains that were stx or eae positive, 3 (3.2%) were stx positive only, 21 (22.6%) were stx and eae positive, and 69 (74.2%) were eae positive only. After an initial PCR screening for the presence of ehxA, the remainder of the ehxA PCR product was cut with TaqI and electrophoresed on a 2.5% agarose gel to determine the ehxA PCR-RFLP type (Fig. 1). Six distinct ehxA subtypes could be differentiated after digestion with TaqI and agarose gel electrophoresis (Fig. 1). ehxA subtypes A and E had the most similar PCR-RFLP profiles but could be readily distinguished when broad-toothed gel combs were used to give high-definition electrophoresis profiles. The predicted lengths of TaqI restriction fragments from GenBank ehxA sequences AF043471 (O8:H19), AY258503 (O113:H21), AB032930 (O128:H12), X79839 (O157:H7), and X94129 (O111:H−) corresponded to the lengths of fragments of subtype A, B, or C. Subtypes D, E, and F appeared to be novel.
TABLE 1.
Distribution of ehxA-positive STEC and EPEC strains and associated ehxA PCR-RFLP subtypes
| PCR-RFLP subtype | No. (%) of strains | No. (%) of strains from:
|
||
|---|---|---|---|---|
| Cattle | Sheep | Humans | ||
| A | 115 (45.8) | 4 | 103 | 8 |
| B | 5 (2.0) | 1 | 0 | 4 |
| C | 115 (45.8) | 74 | 29 | 12 |
| D | 3 (1.2) | 3 | 0 | 0 |
| E | 2 (0.8) | 2 | 0 | 0 |
| F | 11 (4.4) | 9 | 2 | 0 |
| ehxA positive | 251 (65.9) | 93 (66.4) | 134 (62.0) | 24 (96.0) |
| ehxA negative | 130 (34.1) | 47 (33.6) | 82 (38.0) | 1 (4.0) |
FIG. 1.
RFLP profiles of ehxA PCR amplicons after TaqI digestion. PCR products were subjected to agarose (2.5%, wt/vol) gel electrophoresis for the analysis of exhA PCR-RFLP profiles. Lanes: 1, O90:H8 AGR047 (subtype C); 2, O131:H25 AGR053 (subtype C); 3, O153:H− AGR119 (subtype F); 4, O98:H− AGR151 (subtype A); 5, O101:H− AGR158 (subtype E); 6, O121:H19 AGR270 (subtype B); 7, O91:H− AGR340 (subtype A); 8, O5:H− AGR374 (subtype F); 9, ONT:H− AGR670 (subtype D); 10, ONT:H− AGR674 (subtype D); and 11, O157:H7 ER03/4238 (subtype B).
Four further E. coli strains (one positive for stx2 only and three positive for eae only) were identified as ehxA positive by colony blot analysis using a 534-bp ehxA fragment, but the full-length ehxA gene could not be amplified using the PCR primers or the conditions outlined above, indicating that there may be other ehxA subtypes or that the ehxA gene in these strains was not complete.
ehxA PCR-RFLP subtyping of E. coli strains from human patients with diarrheal disease and ruminants.
In this study, two PCR-RFLP types (A and C) represented the majority (230 of 251; 91.6%) of the strains that were identified as ehxA positive (Table 1). The remaining PCR-RFLP types (B, D, E, and F) were noted less frequently. Only 4 (8.3%) of 48 stx-positive, eae-negative strains from cattle were ehxA positive, compared to 103 (61.7%) of 167 stx-positive, eae-negative sheep strains. Of the eae-positive strains, 81.3% of cattle strains (74 of 91) and 59.2% of sheep strains (29 of 49) were ehxA positive. Of the strains positive for stx only, 51.8% (117 of 226) were ehxA positive, of which 114 (99.0%) were ehxA subtype A (P < 0.001) (Table 2). All the stx- and eae-positive STEC strains (n = 38) were ehxA positive and were subtype C, except for four O157:H7 isolates that were subtype B and three O5:H− isolates that were subtype F. Most (96 of 217; 82.1%) eae-positive, stx-negative atypical EPEC strains were ehxA positive, and 84 (87.5%) of 96 had ehxA subtype C (P < 0.001) (Table 2). However, four additional subtypes (A, B, E, and F) were less frequently associated with the eae-positive, stx-negative strains. Further studies may indicate whether these data represent a general trend or whether E. coli populations isolated from geographically distinct areas or from different animal cohorts have different ratios of ehxA subtypes.
TABLE 2.
Virulence factors associated with ehxA PCR-RFLP subtypes of E. coli strains
| PCR-RFLP subtype | No. (%) of strains with virulence determinant(s):
|
||
|---|---|---|---|
| stx only | stx and eae | eae only | |
| A | 114 | 0 | 1 |
| B | 0 | 4 | 1 |
| C | 0 | 31 | 84 |
| D | 3 | 0 | 0 |
| E | 0 | 0 | 2 |
| F | 0 | 3 | 8 |
| ehxA positive | 117 (51.8) | 38 (100) | 96 (82.1) |
| ehxA negative | 109 (48.2) | 0 (0) | 21 (17.9) |
Hemolytic activity on sheep blood agar plates.
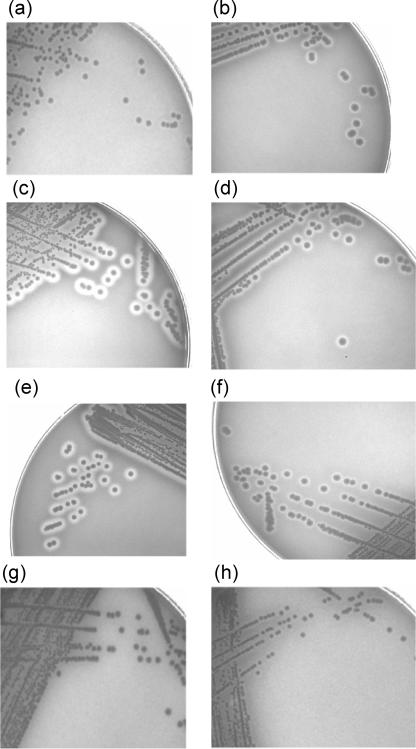
Enterohemolytic activity is exhibited as a narrow zone or a very narrow zone of hemolysis on washed sheep red blood cells (31). In the present study, hemolytic activity on washed sheep blood agar characteristically required overnight incubation but varied by strain, from barely discernible zones of hemolysis more suggestive of enterohemolytic activity as previously described (4, 31, 32, 34) (Fig. 2a) to large (≥3-mm) zones of visible clearing of the agar surrounding individual bacterial colonies (Fig. 2c). The degree of hemolysis varied between and within ehxA subtypes, but under the incubation conditions and with the solid growth media described above, the eae-positive, stx-negative subtype F strains generally displayed the most extensive clearing of the washed sheep blood agar, similar to alpha-hemolysis (Fig. 2c). The hemolytic activity associated with the ehxA subtype F O5:H− STEC strains, however, was more typically characteristic of enterohemolytic activity, with small zones of turbidity (Fig. 2h). Whether this variable hemolytic activity is attributable to altered enterohemolysin expression or secretion is unknown. However, a variation in the hemolytic activities of ehxA-positive STEC strains has been noted previously (9, 39). Generally, cattle strains were more likely than sheep strains to be hemolytic (Table 3). Under the incubation conditions tested, strains of the less frequently identified ehxA PCR-RFLP subtypes B, E, and F were hemolytic on the blood agar plates. Most bacterial strains (93.0%) that were ehxA subtype C were hemolytic. However, approximately 40% of the subtype A and all (n = 3) of the subtype D strains did not display the hemolytic phenotype (Table 3).
FIG. 2.
Enterohemolytic phenotypes and hemolytic scores of ruminant E. coli strains after growth (18 h at 37°C and 6 h at room temperature) on washed sheep blood agar plates. (a) O157:H7 ER03/4238 (subtype B); (b) O131:H25 AGR053 (subtype C); (c) O153:H− AGR119 (subtype F); (d) O98:H− AGR151 (subtype A); (e) O101:H− AGR158 (subtype E); (f) O121:H19 AGR270 (subtype B); (g) ONT:H− AGR674 (subtype D); (h) O5:H− AGR374 (subtype F).
TABLE 3.
Hemolytic phenotypes of ehxA-positive E. coli strains after growth (18 h at 37°C and 6 h at room temperature) on washed sheep blood agar
| PCR-RFLP subtype | No. of indicated strains from:
|
|||||
|---|---|---|---|---|---|---|
| Cattle
|
Sheep
|
Humans
|
||||
| Hemolytic | Nonhemolytic | Hemolytic | Nonhemolytic | Hemolytic | Nonhemolytic | |
| A | 4 | 0 | 58 | 46 | 8 | 0 |
| B | 1 | 0 | 0 | 0 | 4 | 0 |
| C | 66 | 7 | 29 | 1 | 12 | 0 |
| D | 0 | 3 | 0 | 0 | 0 | 0 |
| E | 2 | 0 | 0 | 0 | 0 | 0 |
| F | 9 | 0 | 2 | 0 | 0 | 0 |
Serotyping of ehxA-positive and -negative strains.
Of the 132 strains for which serotyping was attempted, including the 38 eae-positive STEC strains, 40 were O-antigen nontypeable (Table 4). The most common O serogroup was O84 (n = 16), of which 11 strains were serotype O84:H− and 5 were O84:H2. Some serotypes corresponded to different ehxA subtypes, with single O153:H− strains being of subtype C and F and O91:H− strains being of subtype A or C or being ehxA negative. Both eae-positive STEC strains and stx-negative, eae-positive strains of serotypes O145:H− and O26:H11 had identical ehxA subtypes (Table 4). Whether the stx prophage had been lost during storage is unknown, but all strains were subcultured from the original −85°C freezer stocks to eliminate the likelihood of the loss of stx during successive subcultures.
TABLE 4.
Serotypes of STEC and eae-positive E. coli strainsa
| PCR-RFLP subtype | Serotype(s) with virulence determinant(s):
|
||
|---|---|---|---|
| stx only | stx and eae | eae only | |
| A | O75:H8, O91:H21, O91:H−, O113:H21, O128:H−, O128:H2, O130:H11, ONT:H7, ONT:H8, ONT:H10, ONT:H−, OR:H2, OR:H− | O98:H− | |
| B | O157:H7 | O121:H19 | |
| C | O26:H11, O26:H−, O84:H2, O84:H−, O84:H−, O145:H−, ONT:H11, ONT:H−, ONT:HNT, OR:H−, OR:H11, OR:H21 | O26:H−, O26:H11, O70:H11, O76:H−, O90:H8, O91:H−, O103:H25, O103:HR, O108:H25, O115:H−, O129:H−, O131:H25, O136:H−, O145:H46, O145:H−, O150:H−, O153:H−, O172:H−, O177:H11, O180:H−, OR:H11, ONT:H?, ONT:H8, ONT:H25, ONT:H− | |
| D | ONT:H− | ||
| E | O101:H− | ||
| F | O5:H− | O103:H−, O153:H− | |
| None | O9:H51, O65:H−, O69:H6, O91:H−, O91:H−, O149:H8, O150:H8, O174:H8, ONT:H6, ONT:H8, ONT:H10, ONT:H14 | O37:H−, O49:H10, O109:H−, O158:H11, ONT:H10, ONT:H−, ONT:H− | |
Serotypes listed in bold correspond to strains obtained from human patients with diarrheal disease and identified in this study. Underlined designations represent serotypes of strains from which the full-length ehxA PCR product was not obtained but which hybridized with the 534-bp ehxA probe during colony blotting.
Comparative sequence analysis and phylogenetic profiling.
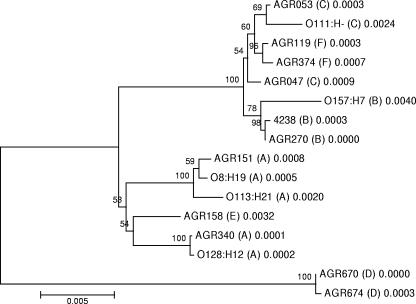
The ehxA gene from strains that possessed representative enterohemolysin PCR-RFLP subtypes was fully sequenced and aligned along with five other enterohemolysin gene sequences available from GenBank. Virtual gel profiles generated from in silico TaqI digestion of ehxA sequence subtypes from GenBank and ehxA sequence subtypes from this work matched exactly with TaqI profiles generated using the PCR-RFLP method. A phylogenetic tree was generated using the neighbor-joining method with MEGA (version 3.1) (Fig. 3). Based on the ehxA sequence analysis and alignment, subtypes B, C, and F were closely related and were associated with eae-positive strains exclusively. Subtypes A and E formed a distinct group; all strains with these subtypes were stx positive and eae negative, except two subtype E strains that were stx negative and eae positive. The ehxA sequences from the two subtype D strains (stx positive and eae negative) were the most distantly related and were well separated from those of the other strains representative of the other five ehxA PCR-RFLP subtypes (Fig. 3). These data were reflected in the percentages of sequence similarity calculated using a sequence identity matrix for the 16 ehxA genes (Table 5). The ehxA sequences of AGR670 and AGR674 were identical (100.0%), and there was 95.6 to 96.6% identity to sequences associated with non-subtype D ehxA types. All other ehxA (n = 14) sequences had ≥98.1% sequence similarity. Two ehxA subtype A sequences, those of AGR340 (O91:H−) and O128:H12, were identical (100.0%). An analysis of the amino acid sequences of the protoxins of AGR670 and AGR674 indicated the deletion of a codon encoding one of four concurrent glycine residues in the 11th RTX toxin repeat associated with enterohemolysin. All other enterohemolysin sequences had the predicted 13 tandem arrays of the 9-amino-acid repeat consensus sequence GGXGXDX[L/I/V/W/Y/F]X (where X is any amino acid) associated with RTX proteins and spanning amino acid residues 706 to 832 at the C terminus (17, 23, 32). The lysine residues at positions 550 and 675 required for the activation of the protoxin through fatty acylation of the enterohemolysin were also present in all deduced amino acid sequences (38), as were histidine 841 (14) and aspartate 845 (15), required for pH- and Ca2+-dependent activity, respectively.
FIG. 3.
Phylogenetic positions of E. coli ehxA sequences from this study and the following ehxA sequences available in GenBank, based on neighbor joining: AF043471 (O8:H19), AY258503 (O113:H21), and AB032930 (O128:H12), corresponding to subtype A; X79839 (O157:H7), corresponding to subtype B; and X94129 (O111:H−), corresponding to subtype C. Bootstrap values (expressed as percentages of 1,000 replications) of ≥50% are shown at branch points. The number after the ehxA PCR-RFLP subtype corresponds to the branch length and indicates the genetic distance between the two ehxA sequences that the branch connects. Bar, 0.5% sequence dissimilarity.
TABLE 5.
Percentages of identity of enterohemolysin gene sequence pairs as determined using ClustalW alignment
| Sequence from strain | % Identity to sequence from strain:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4238 | AGR047 | AGR053 | AGR119 | AGR151 | AGR158 | AGR270 | AGR340 | AGR374 | AGR670 | AGR674 | O111 | O113 | O128 | O157 | O8 | |
| 4238 | 100.0 | 99.7 | 99.6 | 99.7 | 98.4 | 98.6 | 100.0 | 98.5 | 99.6 | 96.1 | 96.1 | 99.4 | 98.3 | 98.5 | 99.5 | 98.4 |
| AGR047 | 100.0 | 99.8 | 99.8 | 98.4 | 98.7 | 99.8 | 98.6 | 99.8 | 96.1 | 96.1 | 99.5 | 98.3 | 98.5 | 99.3 | 98.4 | |
| AGR053 | 100.0 | 99.8 | 98.3 | 98.6 | 99.7 | 98.5 | 99.8 | 96.0 | 96.0 | 99.7 | 98.2 | 98.4 | 99.2 | 98.3 | ||
| AGR119 | 100.0 | 98.4 | 98.6 | 99.7 | 98.5 | 99.9 | 96.1 | 96.0 | 99.6 | 98.3 | 98.5 | 99.3 | 98.4 | |||
| AGR151 | 100.0 | 99.0 | 98.4 | 99.1 | 98.3 | 96.5 | 96.5 | 98.1 | 99.6 | 99.0 | 98.0 | 99.9 | ||||
| AGR158 | 100.0 | 98.6 | 99.3 | 98.6 | 96.6 | 96.5 | 98.4 | 98.9 | 99.3 | 98.2 | 99.0 | |||||
| AGR270 | 100.0 | 98.5 | 99.7 | 96.1 | 96.1 | 99.4 | 98.3 | 98.5 | 99.6 | 98.4 | ||||||
| AGR340 | 100.0 | 98.5 | 96.5 | 96.5 | 98.3 | 99.0 | 100.0 | 98.1 | 99.1 | |||||||
| AGR374 | 100.0 | 96.0 | 96.0 | 99.5 | 98.2 | 98.4 | 99.2 | 98.3 | ||||||||
| AGR670 | 100.0 | 100.0 | 95.8 | 96.5 | 96.5 | 95.7 | 96.5 | |||||||||
| AGR674 | 100.0 | 95.8 | 96.4 | 96.5 | 95.6 | 96.5 | ||||||||||
| O111 | 100.0 | 98.1 | 98.3 | 99.3 | 98.2 | |||||||||||
| O113 | 100.0 | 99.0 | 97.9 | 99.8 | ||||||||||||
| O128 | 100.0 | 98.1 | 99.1 | |||||||||||||
| O157 | 100.0 | 98.0 | ||||||||||||||
| O8 | 100.0 | |||||||||||||||
DISCUSSION
As yet, the role of enterohemolysin as an E. coli virulence factor has not been fully elucidated. It is likely that the enterohemolysin is expressed during human infection and subsequent disease, as patients suffering from O157-associated HUS produce serum antibodies specific to the enterohemolysin from STEC O157 in almost all cases (32). Any possible role for enterohemolysin in bacterial colonization and pathogenesis in ruminants remains to be established, and although receptors for stx1 on bovine intestinal epithelia have been noted previously (20), STEC strains do not normally cause disease in cattle. To our knowledge, E. coli strains that are ehxA and bfp positive have not been recognized. Therefore, by virtue of being ehxA positive, eae positive, and stx negative, atypical EPEC strains from cattle and sheep that lack the bundle-forming pilus may have a closer evolutionary relationship with STEC strains than with other EPEC strains, despite the absence of stx genes (1, 10, 21). However, the expression of enterohemolysin by these strains or STEC in the animal host has not been established. Atypical EPEC strains that lack the bfp locus are becoming more apparent, especially in ruminants (41). However, additional studies are required to identify the role of enterohemolysin in pathogenesis and to determine whether the formation of A/E lesions without concomitant bundle-forming pilus expression (as in typical EPEC strains) or the expression of stx (as in STEC strains) is sufficient for diarrheal disease to occur.
With in vitro culture on washed sheep blood agar, a variety of enterohemolysin activity levels were noted in this study (Fig. 2). It has also been suggested previously that the variation in levels of enterohemolysin secretion and, therefore, of visible hemolysis may be a characteristic of the double or single methionine residue in the N-terminal region of EhxB (39). However, this possibility has not been substantiated, and additional work is required to establish a correlation between the number of methionine residues and enterohemolytic activity. Previous studies have noted the discrepancy between the presence of the ehxA gene and the enterohemolytic phenotype. None of nine STEC O157:H− strains from Finland were hemolytic on enterohemolysin agar (16), and 2 of 22 STEC O111:H− strains displayed the nonhemolytic phenotype despite being ehxA positive (33). The enterohemolysins of the two PCR-RFLP subtype D strains that were sequenced had a deletion in tandem repeat 11 that may have been a cause of the nonhemolytic phenotype. However, for other apparently nonhemolytic strains, different culture conditions may be required for the effective expression of enterohemolysin.
Recent work indicates that genes found on pO157 regulate some chromosomal genes involved in colonization by STEC O157 strains and the persistence of these strains in cattle (25); however, there was no discernible activity associated with enterohemolysin. Analyses of the pO157 plasmid and other large plasmids from STEC strains suggest that many virulence factors are on potentially mobile elements and that their acquisition and evolution may be in part a reflection of the association of specific STEC serotypes with pathogenicity (8). The genetic diversity of ehxA and enterohemolysin expression as determined in the present study may give some insight into the potential virulence of E. coli strains isolated from ruminants.
This is the first study to use a simple and rapid method, such as PCR-RFLP analysis, for the comprehensive subtyping of ehxA genes and the comparison of exhA genes from atypical EPEC strains with the ehxA alleles from STEC strains in order to assess the genetic relatedness of the corresponding enterohemolysins. The ehxA gene was more commonly associated with those strains that were eae positive (86.2%) than with those that were stx positive only (51.8%). Previously, ehxA has been used as a marker for identifying specific eae- and ehxA-positive STEC serotypes such as O26, O103, O111, O145, and O157, which are more commonly associated with outbreaks of serious human clinical disease, including bloody diarrhea and HUS, than other serotypes (3, 5, 13, 31, 32, 33, 45, 46). Indeed, all the eae-positive STEC strains examined in this study (n = 38) were also ehxA positive. STEC strains that are eae negative are only rarely isolated as a cause of diarrheal disease (45). However, STEC O113:H21 strains, which are often eae negative and exhibit ehxA subtype A, are a well-recognized serotype associated with severe diarrhea and HUS (3, 28) and provide an example of a particularly virulent serotype.
The association of ehxA with eae-positive, stx-negative ruminant E. coli strains has only recently been noted (1, 10, 21). The bacterial strains in this study were isolated from apparently healthy animals, and therefore, the role of eae- and ehxA-positive strains in ruminant diarrheal disease remains equivocal. Only bacterial strains that were stx and/or eae positive were assessed for the presence of ehxA in this study. Other studies have found that ehxA may commonly be associated with E. coli strains lacking the stx and eae virulence factors (6, 21). Strains positive for ehxA only (stx negative and eae negative) were isolated from 6 (3.1%) of 191 bovine fecal samples derived from animals with gastrointestinal infections in Australia (21). A total of 338 strains that were isolated from effluent from municipal wastewater treatment were also found to be ehxA positive. However, none were stx positive and only two were eae positive (6). These data indicate that the ehx locus may be commonly associated with environmental E. coli strains that lack well-recognized virulence factors such as stx and eae, in addition to strains from cattle and sheep, and that enterohemolytic functionality may not necessarily be directly involved with virulence per se but may have a role in survival and persistence outside of the gastrointestinal tract under conditions in which trace elements such as iron are required for maintenance.
By using a single restriction endonuclease, six distinct ehxA PCR-RFLP subtypes could be distinguished in this study. The only previous study investigating the sequence diversity and evolution of the ehxA gene separated STEC strains from diverse geographical areas into two major groups that corresponded to strains that were ehxA and eae positive and strains that were ehxA positive and eae negative by PCR analysis (7). Although eae-positive, stx-negative strains were not included in the study by Boerlin et al. (7), STEC strains were divided into two groups comprising eae-positive and eae-negative STEC strains upon analysis of the various ehxA alleles identified. The same division of STEC strains according to ehxA sequence similarity was also found in this present study, but all the eae-positive, stx-negative strains were grouped with the eae-positive STEC strains. This study has identified an additional subtype (D) not noted in previous work that has a distinct PCR-RFLP profile type and a DNA sequence that represents a new ehxA group associated with stx-positive, eae-negative strains from cattle. In addition, the eae-positive PCR-RFLP subtype E strains clustered with the eae-negative PCR-RFLP subtype A strains, indicating that there may be some intermediate ehxA subtypes that do not fit the previous ehxA grouping model. A further example of a strain that did not conform to the previous ehxA grouping scheme was a single O98:H− (AGR151) strain that was eae positive but had an ehxA PCR-RFLP profile that corresponded to the subtype A profile more usually associated with the eae-negative, stx-positive strains. The ehxA gene from this strain was fully sequenced and, upon phylogenetic analysis, grouped with the cluster comprising mostly eae-negative, stx-positive strains (Fig. 3). For the most part, RFLP analysis of the complete ehxA sequence is able to distinguish STEC strains that are positive for stx only from E. coli strains that are positive for eae only or eae-positive STEC strains. In addition to the analysis of ehxA, further sequence analysis of the large plasmid of STEC has confirmed the clear division of this pathotype into two groups (7), but the analysis of the same sequences of the large plasmids of stx-negative, eae-positive strains is required to determine whether these strains too are more closely related to eae-positive STEC. Furthermore, the ehxA sequence analysis of the stx-negative, eae-negative strains isolated from wastewater (6) is warranted to assess the relationship of these strains to those stx-positive and/or eae-positive strains examined in this work.
The predominance of subtypes A and C and the apparent scarcity of subtypes D and E requires confirmation from further ehxA subtyping studies. Indeed, two of the three subtype D strains were isolated from the same dairy cow, and the remaining strain was isolated from the same cohort of dairy cattle on the same day, indicating that this ehxA subtype may be rare. The two subtype E strains were isolated from cattle from two separate sites. Subtype F, although uncommon, may be fairly widespread in cattle and sheep as it was isolated from animals on seven separate sampling occasions and from all four separate locations where sampling took place.
No O157 strains were identified among this collection of bacteria isolated from cattle and sheep (11); however, STEC O157:H7 and O157:H− strains have been identified previously using this TaqI PCR-RFLP method as having a specific subtype found exclusively in STEC serogroup O157 strains (7), and this subtype corresponds to subtype B from the present study. Therefore, the ehxA PCR-RFLP subtypes of four New Zealand O157:H7 human strains were included in this study to give a characteristic profile for this serotype (Fig. 1). The ehxA gene of one of the New Zealand O157:H7 strains (ER03/4238) was also sequenced to establish the identity of its sequence to others available in the GenBank database. Of note, a single strain (O121:H19) gave a TaqI ehxA PCR-RFLP profile that was indistinguishable from that of the ehxA gene of O157. When sequenced, the ehxA gene from this stx-negative, eae-positive subtype B strain grouped closely with the sequence from the New Zealand O157:H7 ER03/4238 strain. These data indicate that the use of ehxA subtype A, for the identification of eae-negative STEC strains that are less frequently associated with disease than other STEC strains, and the use of subtype B, for the identification of the serotypes O157:H7 and O157:H− that are commonly associated with large outbreaks of diarrheal disease and HUS, cannot be exhaustively relied upon for distinguishing eae-negative from eae-positive strains.
It should be highlighted that the use of descriptive terms for diarrheagenic E. coli pathotypes, such as EPEC and STEC, must be viewed with caution as there have been several instances in which analyses of virulence factors, such as ehxA, shared by STEC and stx-negative E. coli strains have indicated that there may be some shared evolutionary relationships. In contrast, within EPEC and STEC groups, there are other virulence factors, such as bfp, eae, and ehxA, that clearly separate bacteria of each pathotype into different groups.
In summary, this exhaustive study has shown a strong link not only between the presence of the ehxA and eae alleles in STEC strains from cattle and sheep but also between ehxA and eae-positive atypical EPEC strains isolated from cattle and sheep. There is no apparent difference in ehxA genes from ruminant E. coli strains and those isolated from humans that may indicate specific roles for enterohemolysin in the separate hosts. Whether the reduced frequency of ehxA in stx-positive, eae-negative STEC strains may be in part a reflection of the apparently reduced virulence of these strains and their association with hemorrhagic colitis and HUS in humans is unknown, but several important roles of the enterohemolysin in pathogenesis have been identified only in humans. However, these roles and an immune response to enterohemolysin from atypical EPEC strains and eae-negative STEC strains such as those of O113:H21 in the context of human infection remain to be established. Generally, the ehxA gene is highly conserved (>95%) among a large number of atypical EPEC and STEC strains, but as demonstrated by the data from this study, certain distinguishing features of different ehxA subtypes are readily established by PCR-RFLP methods that give some possible insight into the pathogenicity and associated virulence factors of these subtypes. It is likely that the horizontal transfer of the ehx locus to E. coli may have occurred at least three times, (i) to eae-positive E. coli strains (including atypical EPEC and eae-positive STEC strains), (ii) to eae-negative STEC strains, and (iii) to ehxA subtype D eae-negative STEC strains, but selection pressures ensure that the locus remains highly conserved. Recent data also indicate that ehxA is present in stx-negative, eae-negative E. coli strains (6, 21), but the ehxA subtypes of these strains remain to be established. Further studies are also required to establish the role of the enterohemolysin in the animal host and to determine the mechanisms by which enterohemolysin expression may be regulated.
Acknowledgments
This work was supported by AgResearch Repositioning funds.
We thank John Koolaard for excellent statistical assistance.
Footnotes
Published ahead of print on 24 August 2007.
REFERENCES
- 1.Aidar-Ugrinovich, L., J. Blanco, M. Blanco, J. E. Blanco, L. Leomil, G. Dahbi, A. Mora, D. L. Onuma, W. D. Silveira, and A. F. Pestana de Castro. 2007. Serotypes, virulence genes, and intimin types of Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) isolated from calves in São Paulo, Brazil. Int. J. Food Microbiol. 115:297-306. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, M. E., and R. A. Welch. 1996. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 64:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, J., A. L. Cookson, C. Pope, and C. Nichol. 2006. New Zealand strains of Escherichia coli serogroup O113, abstr. P13.1.03, p. 117. Abstr. 6th Int. Symp. Shiga Toxin (Verocytotoxin)-Producing E. coli Infect., Melbourne, Australia.
- 4.Beutin, L., J. Prada, S. Zimmermann, R. Stephan, I. Ørskov, and F. Ørskov. 1988. Enterohemolysin, a new type of hemolysin produced by some strains of enteropathogenic E. coli (EPEC). Zentbl. Bakteriol. Mikrobiol. Hyg. A 267:576-588. [DOI] [PubMed] [Google Scholar]
- 5.Beutin, L., M. A. Montenegro, I. Ørskov, F. Ørskov, J. Prada, S. Zimmermann, and R. Stephan. 1989. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J. Clin. Microbiol. 27:2559-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boczek, L. A., C. H. Johnson, E. W. Rice, and B. K. Kinkle. 2006. The widespread occurrence of the enterohaemolysin gene ehlyA among environmental strains of Escherichia coli. FEMS Microbiol. Lett. 254:281-284. [DOI] [PubMed] [Google Scholar]
- 7.Boerlin, P., S. Chen, J. K. Colbourne, R. Johnson, S. de Grandis, and C. Gyles. 1998. Evolution of the enterohemorrhagic Escherichia coli hemolysin plasmid and the locus for enterocyte effacement in Shiga toxin-producing E. coli. Infect. Immun. 66:2553-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunder, B. W., H. Schmidt, M. Frosch, and H. Karch. 1999. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 145:1005-1014. [DOI] [PubMed] [Google Scholar]
- 9.Chart, H., C. Jenkins, H. R. Smith, D. Hedges, and B. Rowe. 1998. Haemolysin production by strains of verocytotoxin-producing Escherichia coli. Microbiology 144:103-107. [DOI] [PubMed] [Google Scholar]
- 10.Cookson, A. L., C. M. Hayes, G. R. Pearson. J. M. Roe, A. D. Wales, and M. J. Woodward. 2002. Isolation from a sheep of an attaching and effacing Escherichia coli O115:H− with a novel combination of virulence factors. J. Med. Microbiol. 51:1041-1049. [DOI] [PubMed] [Google Scholar]
- 11.Cookson, A. L., S. C. S. Taylor, and G. T. Attwood. 2006. The prevalence of Shiga toxin-producing Escherichia coli in cattle and sheep in the lower North Island, New Zealand. N. Z. Vet. J. 54:28-33. [DOI] [PubMed] [Google Scholar]
- 12.Cookson, A. L., S. C. S. Taylor, J. Bennett, F. Thomson-Carter, and G. T. Attwood. 2006. Serotypes and analysis of distribution of Shiga toxin-producing Escherichia coli from cattle and sheep in the lower North Island, New Zealand. N. Z. Vet. J. 54:78-84. [DOI] [PubMed] [Google Scholar]
- 13.Cookson, A. L., D. Croucher, C. Pope, J. Bennett, F. Thomson-Carter, and G. T. Attwood. 2006. Isolation, characterization, and epidemiological assessment of Shiga toxin-producing Escherichia coli O84 isolates from New Zealand. J. Clin. Microbiol. 44:1863-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortajarena, A. L., F. M. Goñi, and H. Ostolaza. 2002. His-859 is an essential residue for the activity and pH dependence of Escherichia coli RTX toxin α-haemolysin. J. Biol. Chem. 277:23223-23229. [DOI] [PubMed] [Google Scholar]
- 15.Cortajarena, A. L., F. M. Goñi, and H. Ostolaza. 2003. Asp-863 is a key residue for calcium-dependent activity of Escherichia coli RTX toxin α-haemolysin. FEBS Lett. 546:271-275. [DOI] [PubMed] [Google Scholar]
- 16.Eklund, M., M. Bielaszewska, U. M. Nakari, H. Karch, and A. Siitonen. 2006. Molecular and phenotypic profiling of sorbitol-fermenting Escherichia coli O157:H− human isolates from Finland. Clin. Microbiol. Infect. 12:634-641. [DOI] [PubMed] [Google Scholar]
- 17.Felmlee, T., and R. A. Welch. 1988. Alternations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc. Natl. Acad. Sci. USA 85:5269-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fratamico, P. M., S. Bhaduri, and R. L. Buchanan. 1993. Studies on Escherichia coli serotype O157:H7 strains containing a 60-MDa plasmid and on 60-MDa plasmid-cured derivatives. J. Med. Microbiol. 39:371-381. [DOI] [PubMed] [Google Scholar]
- 19.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 73:2573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoey, D. E., C. Currie, R. W. Else, A. Nutikka, C. A. Lingwood, D. L. Gally, and D. G. E. Smith. 2002. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J. Med. Microbiol. 51:143-149. [DOI] [PubMed] [Google Scholar]
- 21.Hornitzky, M. A., K. Mercieca, K. A. Bettelheim, and S. P. Djordjevic. 2005. Bovine feces from animals with gastrointestinal infections are a source of serologically diverse atypical enteropathogenic Escherichia coli and Shiga toxin-producing E. coli strains that commonly possess intimin. Appl. Environ. Microbiol. 71:3405-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 23.Lally, E. T., R. B. Hill, I. R. Kieba, and J. Korostoff. 1999. The interaction between RTX toxins and target cells. Trends Microbiol. 7:356-361. [DOI] [PubMed] [Google Scholar]
- 24.Lehmacher, A., H. Meier, S. Aleksic, and J. Bockemühl. 1998. Detection of hemolysin variants of Shiga toxin-producing Escherichia coli by PCR and culture on vancomycin-cefixime-cefsulodin blood agar. Appl. Environ. Microbiol. 64:2449-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim, J. Y., H. Sheng, K. S. Seo, Y. H. Park, and C. J. Hovde. 2007. Characterization of an Escherichia coli O157:H7 plasmid O157 deletion mutant and its survival and persistence in cattle. Appl. Environ. Microbiol. 73:2037-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obrig, T. G., C. B. Louise, C. A. Lingwood, B. Boyd, L. Barley-Maloney, and T. O. Daniel. 1993. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268:15484-15488. [PubMed] [Google Scholar]
- 28.Paton, A. W., M. C. Woodrow, R. M. Doyle, J. A. Lanser, and J. C. Paton. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosa, A. C. P., A. T. Mariano, A. M. S. Pereira, A. Tibana, T. A. Gomes, and J. R. Andrade. 1998. Enteropathogenicity markers in Escherichia coli isolated from infants with acute diarrhoea and healthy controls in Rio de Janeiro. J. Med. Microbiol. 47:781-790. [DOI] [PubMed] [Google Scholar]
- 30.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 31.Sandhu, K. S., R. C. Clarke, and C. L. Gyles. 1997. Hemolysin phenotypes and genotype of eaeA-postive and eaeA-negative bovine verotoxigenic Escherichia coli. In P. S. Paul, D. H. Francis, and D. A. Benfield (ed.), Mechanisms in the pathogenesis of enteric disease. Plenum Press, New York, NY. [DOI] [PubMed]
- 32.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt, H., and H. Karch. 1996. Enterohemolytic phenotypes and genotypes of Shiga toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic-uremic syndrome. J. Clin. Microbiol. 34:2364-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt, H., C. Kernbach, and H. Karch. 1996. Analysis of the EHEC hly operon and its location in the physical map of the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:907-914. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt, H., E. Maier, H. Karch, and R. Benz. 1996. Pore-forming properties of the plasmid-encoded hemolysin of enterohaemorrhagic Escherichia coli O157:H7. Eur. J. Biochem. 241:594-601. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt, H., J. Scheef, H. I. Huppertz, M. Frosch, and H. Karch. 1999. Escherichia coli O157:H7 and O157:H− strains that do not produce Shiga toxin: phenotypic and genetic characterization of isolates associated with diarrhea and hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scotland, S. M., H. R. Smith, B. Said, G. A. Willshaw, T. Cheasty, and B. Rowe. 1991. Identification of enteropathogenic Escherichia coli isolated in Britain as enteroaggregative or as members of a subclass of attaching-and-effacing E. coli not hybridising with the EPEC adherence-factor probe. J. Med. Microbiol. 35:278-283. [DOI] [PubMed] [Google Scholar]
- 38.Stanley, P., C. Hyland, V. Koronakis, and C. Hughes. 1999. An ordered reaction mechanism for bacterial toxin acylation by the specialized acyltransferase HlyC: formation of a ternary complex with acylACP and protoxin substrates. Mol. Microbiol. 34:887-901. [DOI] [PubMed] [Google Scholar]
- 39.Taneike, I., H.-M. Zhang, N. Wakisaka-Saito, and T. Yamamoto. 2002. Enterohemolysin operon of Shiga toxin-producing Escherichia coli: a virulence function of inflammatory cytokine production from human monocytes. FEBS Lett. 524:219-224. [DOI] [PubMed] [Google Scholar]
- 40.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trabulsi, L. R., R. Keller, and T. A. Tardelli Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vieira, M. A., J. R. C. Andrade, L. R. Trabulsi, A. C. Rosa, A. M. Dias, S. R. Ramos, G. Frankel, and T. A. Gomes. 2001. Phenotypic and genotypic characteristics of Escherichia coli strains of non-enteropathogenic E. coli (EPEC) serogroups that carry EAE and lack the EPEC adherence factor and Shiga toxin DNA probe sequences. J. Infect. Dis. 183:762-772. [DOI] [PubMed] [Google Scholar]
- 43.Wadolkowski, E. A., J. A. Burris, and A. D. O'Brien. 1990. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 58:2438-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welch, R. A., M. E. Bauer, A. D. Kent, J. A. Leeds, M. Moayeri, L. B. Regassda, and D. L. Swenson. 1995. Battling against host phagocytes: the wherefore of the RTX family of toxins? Infect. Agents Dis. 4:254-272. [PubMed] [Google Scholar]
- 45.Willshaw, G. A., S. Scotland, H. R. Smith, and B. Rowe. 1992. Properties of verocytotoxin-producing Escherichia coli of human origin of O serogroups other than O157. J. Infect. Dis. 166:797-802. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, W. L., M. Bielaszewska, A. Liesegang, H. Tschäpe, H. Schmidt, M. Bitzan, and H. Karch. 2000. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26 strains. J. Clin. Microbiol. 38:2134-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]