Abstract
The bacterial candidate phylum Termite Group I (TG-1) presently consists mostly of “Endomicrobia,” which are endosymbionts of flagellate protists occurring exclusively in the hindguts of termites and wood-feeding cockroaches. Here, we show that public databases contain many, mostly undocumented 16S rRNA gene sequences from other habitats that are affiliated with the TG-1 phylum but are only distantly related to “Endomicrobia.” Phylogenetic analysis of the expanded data set revealed several diverse and deeply branching lineages comprising clones from many different habitats. In addition, we designed specific primers to explore the diversity and environmental distribution of bacteria in the TG-1 phylum.
Termite Group I (TG-1) represents a deep branch in the tree of bacterial 16S rRNA gene sequences (18) and has been recognized as a candidate phylum (10). TG-1 comprises a large number of the bacteria in the hindguts of Reticulitermes species (6, 25), where they occur as intracellular symbionts of flagellate protists (22). These symbionts, for which the name “Endomicrobia” has been proposed, form a monophyletic lineage occurring exclusively in the hindguts of termites and wood-feeding cockroaches (11, 22).
However, a few sequences only distantly related to the “Endomicrobia” but clearly affiliated with the TG-1 phylum have been reported to also occur in habitats other than termite guts (3, 19, 20, 24). Moreover, Nakajima et al. (17) obtained two sequences from the gut of Reticulitermes speratus that fall outside the “Endomicrobia” lineage. At present, public databases contain a growing number of sequences from various habitats that are phylogenetically unassigned but seem to be affiliated with the TG-1 phylum.
In this study, we screened public databases for hitherto unrecognized TG-1 sequences and conducted a comprehensive phylogenetic analysis of the expanded data set. In addition, we designed specific PCR primers to investigate the diversity and environmental distribution of major lineages of TG-1 bacteria in soils, sediments, and intestinal tracts.
Data mining.
Sequences affiliated with the TG-1 phylum were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/) using various characteristic oligonucleotide signatures deduced from the originally available 16S rRNA gene sequences, and they were added to the database of the ARB program suite (15). By continuously adapting the signatures to the growing data set (data not shown), we obtained approximately 50 previously unassigned phylotypes that fell into the radiation of the TG-1 phylum. Most of these phylotypes were from large-scale diversity studies of various environments, including soils, sediments, and intestinal tracts. Sequences were aligned with the ARB Fast Aligner tool. The alignment was manually corrected, and highly variable regions and ambiguous positions were excluded from the analysis. Rigorous chimera checking with Bellerophon (9) and fractional treeing (14) identified only one sequence as a putative chimera (accession no. DQ830579), which was removed from the data set. All shorter sequences (500 to 1,300 bp) were added to the core tree using the parsimony tool implemented in ARB.
Primer design and PCR.
Phylogenetic analysis of the nearly full-length sequences (>1,300 bp) fully supported the conclusion that the TG-1 phylum forms a separate line of descent in the bacterial tree, consisting of several diverse and deeply branching lineages (Fig. 1). Designing a single phylum-specific primer that excluded all representatives of other phyla proved to be impossible. A primer set for specific amplification of “Endomicrobia” (primer set 1) (Table 1) was designed in a previous study (22). Based on the expanded data set, we designed three additional primer sets (primer sets 2 to 4) that covered most of the other lineages in the TG-1 phylum. When applicable, information from shorter sequences was included to improve primer design.
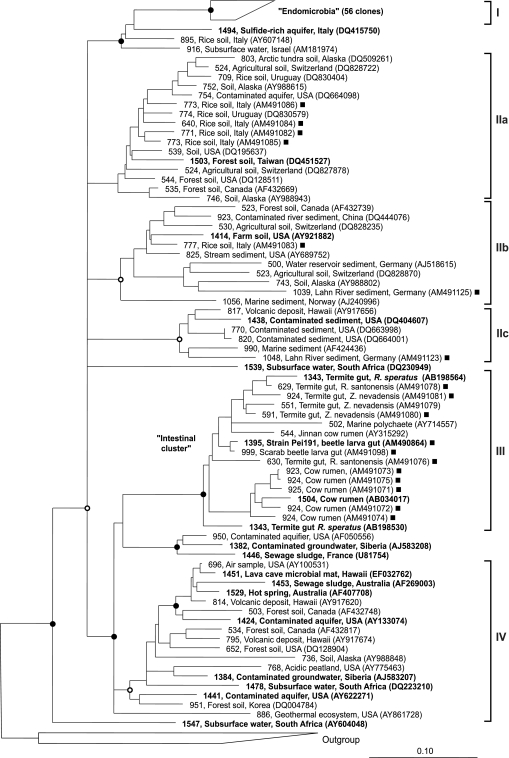
FIG. 1.
Phylogenetic tree of all sequences in the TG-1 phylum obtained in this and previous studies. A core tree was constructed with 1,262 unambiguously aligned sequence positions of all nearly full-length sequences retrieved from GenBank (in bold type), using maximum-likelihood analysis (fastDNAml). Short sequences (>500 bp), positionally filtered by base frequency (50%), were added without changing the global tree topology using the ARB parsimony tool. The scale bar is only approximate because the procedure distorts branch length. Representatives of 10 other phyla were used as the outgroup. Clones obtained in this study are indicated by filled squares. The topology of the core tree and individual clusters was tested separately by neighbor-joining and parsimony analysis (DNAPARS) with bootstrapping (seqboot; 1,000 bootstraps). Only nodes supported by high bootstrap values are marked (filled circles, >95%; open circles, >75%); nodes not supported by all analyses are shown as multifurcations. Original sequence definitions in the GenBank database were replaced with a consistent nomenclature including sequence length (in base pairs), habitat, geographic origin, and accession number. Roman numerals indicate the lineages referred to in the text and in Table 1.
TABLE 1.
Primer sets used for amplification of major lineages in the TG-1 phylum and annealing temperatures and MgCl2 concentrations used in the PCR assaya
| Primer set | Target group(s)b | Primer | E. coli position | Sequence (5′ to 3′) | Annealing temp (°C) | MgCl2 concn (mM) |
|---|---|---|---|---|---|---|
| 1 | I | TG1-209fc | 210-237 | AATGCGTTTTGAGATGGTCCTG | 54.0 | 2 |
| TG1-1325rc | 1325-1343 | GATTCCTACTTCATGTGG | ||||
| 2 | II | EluD540f | 539-562 | AGGTGGCAAGCGTTACTCGGAAT | 63.9 | 1.5 |
| EluD1300r | 1286-1306 | TCTGAACTGGGGCCGGCTTTT | ||||
| 3 | III | EluB22f | 22-40 | GCTCAGAGTTAACGCTGGC | 61.7 | 1.5 |
| EluB998r | 981-998 | GTCGTTCGAGCCCAGGTAA | ||||
| 4 | IV | EluC104f | 104-126 | GGCAGACGAGTGAGTARCACGTA | 57.0 | 2 |
| EluC1173r | 1153-1173 | ACGTTATCCGCGGCAGTCTCC | ||||
| T | II-IV | 27fd | 8-27 | AGAGTTTGATCCTGGCTCAG | 55.0 | 2 |
| Elu1058r | 1042-1058 | CCATGCAGCACCTCGGC |
The primers were used for PCR-based screening of various habitats for the presence of TG-1 bacteria. DNA was extracted by bead beating (16), and humic substances were removed by passing aqueous extracts over an Autoseq G-50 column (Amersham Bioscience). For PCR amplification a standard protocol optimized for the primer pairs was used (Table 1). The products of two identical reactions were combined, cleaned with a MinElute PCR purification kit (QIAGEN), and cloned with an pGEM-T Easy vector kit (Promega). Positive clones were amplified with M13 vector primers and checked for inserts on a 1% agarose gel. Clones with inserts that were the correct lengths were sorted by restriction fragment length polymorphism analysis as previously described (21). Inserts were sequenced on both strands.
Phylogenetic analysis.
Phylogenetic analysis of the resulting clone libraries documented that all primer pairs were highly specific and amplified only 16S rRNA genes of bacteria in the TG-1 phylum.
Primer set 1 gave a PCR product only with the termite hindgut samples (Table 2). This corroborates the specificity of this primer set for its target group, the “Endomicrobia” (lineage I in Fig. 1), which seem to be restricted to termites and wood-feeding cockroaches harboring gut flagellates (1, 22). “Endomicrobia” sequences from termite guts were not further investigated since they are the subject of a separate study (11).
TABLE 2.
Detection of TG-1 bacteria in various habitats using the new designed primer sets for specific amplification of major lineages in the phyluma
| Habitat or strain | Detection of TG-1 bacteria withb,c:
|
|||
|---|---|---|---|---|
| Primer set 1 | Primer set 2 | Primer set 3 | Primer set 4 | |
| Lahn River sedimentd | − | − | − | − |
| Marburg forest soild | − | − | − | − |
| Italian rice soile | − | 6/0 (14) | − | − |
| Cow rumenf | − | − | 5/0 (16) | − |
| Reticulitermes santonensis gutg | + | 3/3 (16) | 5/3 (17) | − |
| Zootermopsis nevadensis gutg | + | 3/3 (19) | 5/2 (12) | − |
| Pachnoda ephippiata gutg | − | − | ± | − |
| Strain Pei191h | − | − | + | − |
See Table 1.
+, PCR product of the expected size; −, no PCR product; ±, results with different preparations varied. If clone libraries were analyzed, the number of TG-1 phylotypes/number of “Endomicrobia” phylotypes among the total clones tested (in parentheses) is indicated.
Each reaction mixture (25 μl) contained reaction buffer (Invitrogen), MgCl2 (see Table 1), deoxynucleoside triphosphates (200 μM each), primers (0.3 μM each), dimethyl sulfoxide (1 μl), DNA extract (200 to 300 ng), and Taq DNA polymerase (1.25 U; Invitrogen). The thermal cycling consisted of an initial denaturation step of 5 min at 96°C, followed by 30 cycles consisting of 30 s at 94°C, 30 s at the annealing temperature (Table 1), and 45 s at 72°C.
Topsoil under Fagus sylvatica and anoxic sediment of the Lahn River were freshly collected in Marburg, Germany.
Dried rice soil (Oryza sativa) from wetland rice fields of the Italian Rice Research Institute in Vercelli (Italy) was regenerated for 3 days as described by Frenzel et al. (5).
Rumen contents of a freshly slaughtered Holstein cow.
Isolated from the hindgut of Pachnoda ephippiata.
Primer set 2 matched the sequences of three deeply branching lineages (lineages IIa, IIb, and IIc) comprising clones from soil or sediment samples. An amplification product of the expected size was obtained only with Italian rice soil. The resulting clone library contained several phylotypes falling into lineage IIa and a single phylotype falling into lineage IIb (Fig. 1). Generally, the large number of sequences from soils and sediments retrieved from public databases suggests that bacteria in lineage II are widely distributed in these habitats.
Primer set 3 was designed to target the sequences in lineage III, which consists of sequences from intestinal habitats and also includes strain Pei191, the first isolate from the TG-1 phylum obtained from the gut of a beetle larva (O. Geissinger and A. Brune, unpublished data). Several new phylotypes were also obtained from the hindguts of Reticulitermes santonensis and Zootermopsis nevadensis (Table 2). All were distantly related to the clones previously retrieved from an R. speratus gut wall sample (17), underlining the finding that termite guts harbor a second lineage of TG-1 bacteria besides the “Endomicrobia.” Cow rumen amplification products yielded a diverse but monophyletic group of sequences clustering with a single TG-1 sequence (accession no. AB034017) in a bacterial clone library from this habitat and erroneously assigned to the Proteobacteria (23).
Primer set 2 also yielded PCR products with the termite gut samples, but cloning analysis revealed that the products consisted exclusively of “Endomicrobia” sequences, which indicated that this primer set insufficiently discriminates against this group. Also, the termite clone libraries obtained with primer set 3 contained sequences belonging to the “Endomicrobia” (Table 2). Although both primer sets had two or more mismatches with all nontarget sequences within the TG-1 phylum, they apparently lack differentiating power for the corresponding subgroups if too many “Endomicrobia” are present in a sample, a problem encountered only with lower termites.
None of the DNA samples used in this study gave a PCR product with primer set 4, designed to detect sequences from lineage IV, which comprises clones from many different habitats (Fig. 1). Lahn River sediment yielded PCR products only with primer set T (Table 1), which was designed to detect most TG-1 sequences other than those of lineage I. This primer set yielded PCR products for all habitats tested but turned out to be nonspecific. Sequence analysis revealed that only about 10% of the clones in each clone library fell into the TG-1 phylum (lineage III in the Pachnoda ephippiata gut; lineages IIb and IIc in Lahn River sediment). Other clones were mostly representatives of the Bacteroidetes and the Acidobacteria, which was in agreement with the lack of discrimination of this primer set with a few representatives of these phyla (data not shown).
Abundance in the environment.
TG-1 bacteria are highly abundant only in bacterial clone libraries from the hindguts of lower termites (6, 7, 8, 18, 25). Fluorescence in situ hybridization corroborated the conclusion that “Endomicrobia” constitute a significant portion of the gut microbiota (11, 22). In contrast, bacterial clone libraries from other habitats generally contain only small numbers of sequences from members of the TG-1 phylum, if they contain any at all. In rumen fluid clone libraries (150 clones) and farm soil clone libraries (1,700 clones), only a single clone each fell into the TG-1 phylum (23, 24). Clone libraries of P. ephippiata larva gut homogenates (113 clones) (4) did not contain any clones affiliated with the TG-1 phylum, although a representative of the “intestinal cluster” was obtained from this species with primer set T (this study). Possible explanations for these phenomena may lie in either a low relative abundance of such bacteria in the respective communities or a mismatch in the “universal” Bacteria primers used in these studies.
Conclusion.
The results of this study document that bacteria affiliated with the TG-1 phylum form a separate line of descent, as proposed previously on the basis of a much smaller data set (10, 18). This phylum consists of numerous diverse and deeply branching lineages comprising bacteria from a wide range of chemically and geographically distinct habitats, including soils, sediments, and intestinal tracts. Although TG-1 bacteria seem to be numerically abundant only in the hindguts of lower termites (lineage I; “Endomicrobia”), the great diversity and wide environmental distribution of other lineages suggest a hitherto unrecognized role in the environment.
Nucleotide sequence accession numbers.
Sequences determined in this study have been deposited in the EMBL database under accession numbers AM491071 to AM491086, AM491098, AM491123, and AM491125 (http://www.ebi.ac.uk/).
Acknowledgments
This study was funded by the Max Planck Society. D.P.R.H. received a fellowship from the International Max Planck Research School for Environmental, Cellular, and Molecular Microbiology.
Footnotes
Published ahead of print on 17 August 2007.
REFERENCES
- 1.Brune, A., and U. Stingl. 2005. Prokaryotic symbionts of termite gut flagellates: phylogenetic and metabolic implications of a tripartite symbiosis, p. 39-60. In J. Overmann (ed.), Molecular basis of symbiosis. Springer, Berlin, Germany. [DOI] [PubMed]
- 2.DeSantis, T. Z., P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu, and G. L. Andersen. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dojka, M. A., P. Hugenholtz, S. K. Haack, and N. R. Pace. 1998. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl. Environ. Microbiol. 64:3869-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egert, M., B. Wagner, T. Lemke, A. Brune, and M. W. Friedrich. 2003. Microbial community structure in midgut and hindgut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 69:6659-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frenzel, P., F. Rothfuss, and R. Conrad. 1992. Oxygen profiles and methane turnover in a flooded rice microcosm. Biol. Fertil. Soils 14:84-89. [Google Scholar]
- 6.Hongoh, Y., M. Ohkuma, and T. Kudo. 2003. Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera, Rhinotermitidae). FEMS Microbiol. Ecol. 44:231-242. [DOI] [PubMed] [Google Scholar]
- 7.Hongoh, Y., H. Yuzawa, M. Ohkuma, and T. Kudo. 2003. Evaluation of primers and PCR conditions for the analysis of 16S rRNA genes from a natural environment. FEMS Microbiol. Lett. 221:299-304. [DOI] [PubMed] [Google Scholar]
- 8.Hongoh, Y., P. Deevong, T. Inoue, S. Moriya, S. Trakulnaleamsai, M. Ohkuma, C. Vongkaluang, N. Noparatnaraporn, and T. Kudo. 2005. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 71:6590-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 10.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda-Ohtsubo, W., M. Desai, U. Stingl, and A. Brune. Phylogenetic diversity of “Endomicrobia” and their specific affiliation with termite gut flagellates. Microbiology, in press. [DOI] [PubMed]
- 12.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, NY.
- 13.Lemke, T., U. Stingl, M. Egert, M. W. Friedrich, and A. Brune. 2003. Physicochemical conditions and microbial activities in the highly alkaline gut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 69:6650-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig, W., S. H. Bauer, M. Bauer, I. Held, G. Kirchhof, R. Schulze, I. Huber, S. Spring, A. Hartmann, and K. H. Schleifer. 1997. Detection and in situ identification of representatives of a widely distributed new bacterial phylum. FEMS Microbiol. Lett. 153:181-190. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, et al. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lueders, T., and M. W. Friedrich. 2003. Evaluation of PCR amplification bias by terminal restriction fragment length polymorphism analysis of small-subunit rRNA and mcrA genes by using defined template mixtures of methanogenic pure cultures and soil DNA extracts. Appl. Environ. Microbiol. 69:320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima, H., Y. Hongoh, R. Usamib, T. Kudo, and M. Ohkuma. 2005. Spatial distribution of bacterial phylotypes in the gut of the termite Reticulitermes speratus and the bacterial community colonizing the gut epithelium. FEMS Microbiol. Ecol. 54:247-255. [DOI] [PubMed] [Google Scholar]
- 18.Ohkuma, M., and T. Kudo. 1996. Phylogenetic diversity of the intestinal bacterial community in the termite Reticulitermes speratus. Appl. Environ. Microbiol. 62:461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reardon, C. L., D. E. Cummings, L. M. Petzke, B. L. Kinsall, D. B. Watson, B. M. Peyton, and G. G. Geesey. 2004. Composition and diversity of microbial communities recovered from surrogate minerals incubated in an acidic uranium-contaminated aquifer. Appl. Environ. Microbiol. 70:6037-6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spear, J. R., J. J. Walker, T. M. McCollom, and N. R. Pace. 2005. Hydrogen and bioenergetics in the Yellowstone geothermal ecosystem. Proc. Natl. Acad. Sci. USA 102:2555-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stingl, U., and A. Brune. 2003. Phylogenetic diversity and whole-cell hybridization of oxymonad flagellates from the hindgut of the wood-feeding lower termite Reticulitermes flavipes. Protist 154:147-155. [DOI] [PubMed] [Google Scholar]
- 22.Stingl, U., R. Radek, H. Yang, and A. Brune. 2005. “Endomicrobia”: cytoplasmic symbionts of termite gut protozoa form a separate phylum of prokaryotes. Appl. Environ. Microbiol. 71:1473-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajima, K., S. Arai, K. Ogata, T. Nagamine, H. Matsui, M. Nakamura, R. I. Aminov, and Y. Benno. 2000. Rumen bacterial community transition during adaptation to high-grain diet. Anaerobe 6:273-284. [Google Scholar]
- 24.Tringe, S. G., C. von Mering, A. Kobayashi, A. A. Salamov, K. Chen, H. W. Chang, M. Podar, J. M. Short, E. J. Mathur, J. C. Detter, P. Bork, P. Hugenholtz, and E. M. Rubin. 2005. Comparative metagenomics of microbial communities. Science 308:554-557. [DOI] [PubMed] [Google Scholar]
- 25.Yang, H., D. Schmitt-Wagner, U. Stingl, and A. Brune. 2005. Niche heterogeneity determines bacterial community structure in the termite gut (Reticulitermes santonensis). Environ. Microbiol. 7:916-932. [DOI] [PubMed] [Google Scholar]