Abstract
Anabaena is a filamentous, N2-fixing, and morphologically diverse genus of cyanobacteria found in freshwater and brackish water environments worldwide. It contributes to the formation of toxic blooms in freshwater bodies through the production of a range of hepatotoxins or neurotoxins. In the Baltic Sea, Anabaena spp. form late summer blooms, together with Nodularia spumigena and Aphanizomenon flos-aquae. It has been long suspected that Baltic Sea Anabaena may produce microcystins. The presence of microcystins has been reported for the coastal regions of the Baltic proper, and a recent report also indicated the presence of the toxin in the open Gulf of Finland. However, at present there is no direct evidence linking Baltic Sea Anabaena spp. to microcystin production. Here we report on the isolation of microcystin-producing strains of the genus Anabaena in the open Gulf of Finland. The dominant microcystin variants produced by these strains included the highly toxic MCYST-LR as well as [d-Asp3]MCYST-LR, [d-Asp3]MCYST-HtyR, MCYST-HtyR, [d-Asp3,Dha7]MCYST-HtyR, and [Dha7]MCYST-HtyR variants. Toxic strains were isolated from the coastal Gulf of Finland as well as from the easternmost open-sea sampling station, where there were lower salinities than at other stations. This result suggests that lower salinity may favor microcystin-producing Anabaena strains. Furthermore, we sequenced 16S rRNA genes and found evidence for pronounced genetic heterogeneity of the microcystin-producing Anabaena strains. Future studies should take into account the potential presence of microcystin-producing Anabaena sp. in the Gulf of Finland.
The Baltic Sea is one of the largest brackish water basins in the world. Its salinity is much lower than that of marine waters, due to high freshwater input and limited exchange with the North Sea via the narrow Danish sounds. Anabaena spp. are present in the coastal waters of the Baltic Sea (31, 40), in the open Baltic proper (45), and in the Gulf of Finland (17, 22), where its presence is restricted to the summer months (22). The annual late-summer blooms of cyanobacteria consist also of Nodularia spumigena, along with Aphanizomenon flos-aquae (45). Together, these make up one of the largest cyanobacterial blooms in the world, covering areas of more than 100,000 km2 (16). The expanse and intensity of these blooms have increased in recent decades (8), and consequently, the blooms have attracted a great deal of public attention.
Recently, microcystin-LR was detected in low concentrations at the entrance to the Gulf of Finland (17). The toxin producer could not be identified, but it was suspected to be Anabaena (17). However, to date, no clear evidence has been presented that links microcystin production in the Baltic Sea directly to the genus Anabaena. It is currently believed that the toxicity of cyanobacterial blooms in the Baltic Sea is attributed exclusively to the genus Nodularia spumigena, which produces the hepatotoxic cyclic pentapeptide nodularin (40). In freshwater environments, Aphanizomenon flos-aquae produces neurotoxins (43) but, for the Baltic Sea, it has been reported to be nontoxic (37, 40).
Microcystin-producing Anabaena strains have been identified in freshwater environments in Canada, Denmark, Egypt, Finland, France, and Norway (39). Microcystins are specific inhibitors of eukaryotic serine/threonine protein phosphatases 1 and 2A (30) and have been linked to liver cancer in humans (20). Although the binding mechanisms of microcystins to protein phosphatases are documented in detail (11), the ecological function of these toxins is largely unknown. Microcystins are small cyclic peptides comprised of seven amino acids (cyclo-d-Ala1-X2-d-MeAsp3-Z4-Adda5-d-Glu6-Mdha7). Structural variation has been encountered in all seven positions, but the most variable amino acids are found at the X and Z positions. The X and Z positions contain l-amino acids, the most common of which are leucine and arginine, respectively, but other amino acids are also found (5). Position seven, not normally referred to as a variable residue, usually contains N-methyldehydroalanine, but seven other amino acids have been reported at this position (5). Position five is occupied by 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid (Adda), an unusual beta-amino acid found only in microcystins and nodularins, and is believed to play a critical role in the toxicity of these compounds (38). Over 70 different microcystin variants have been described previously, while the number of new microcystins reported continues to grow (4). Microcystin variants differ in toxicity with acute 50% lethal dose values ranging from 50 to 1,200 μg kg−1 (mouse, intraperitoneally), although nontoxic variants also exist (39).
We isolated a range of planktonic Anabaena strains from the Gulf of Finland and screened the cultures for microcystins by using liquid chromatography-mass spectrometry (LC-MS). We identified a number of Anabaena strains producing microcystins. The results presented here unequivocally identify Anabaena as a microcystin producer in the Baltic Sea. Furthermore, our results demonstrate unexpected genetic heterogeneity of Baltic Sea Anabaena strains.
MATERIALS AND METHODS
Sampling and identification of Anabaena strains.
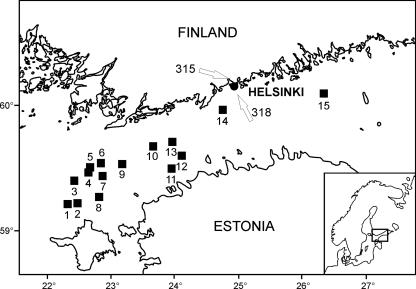
Our study included 15 sampling stations in the Gulf of Finland (Fig. 1). Samples were taken onboard R/V Aranda between 14 and 29 July 2004. Altogether, we isolated 49 Anabaena strains from the open-sea sampling stations (Table 1). In addition, we analyzed two microcystin-producing Anabaena strains, which had previously been isolated from the coastal waters of Helsinki City, Finland (Fig. 1) (14). Anabaena strain 315 was isolated in August 1997 from a plankton sample taken at Seurasaari in Helsinki. Anabaena strain 318 was isolated in June 1998 from the coastal waters of Helsinki City (indicated by the arrows in Fig. 1). Open sea plankton samples were obtained with a 10-μm plankton net from surface water in one single lift at one station at depths of between 0 and 10 m. Phytoplankton net samples, 50 and 100 μl in volume, were plated on Z8 growth medium with salt added (NaCl, 8.75 g liter−1) but free of nitrogen (26). Strains were subsequently purified by plating filaments individually under the microscope to monotypic cultures. Each Anabaena strain was identified by microscopy according to the criteria of Tikkanen (49). After purification, the strains were grown in liquid medium at room temperature (22 to 24°C) under continuous illumination at 8 μmol m−2 s−1.
FIG. 1.
Locations of sampling stations in the Gulf of Finland. Open-sea Anabaena strains were isolated from stations 1 to 15 in 2004. Anabaena strains 315 and 318 were isolated from the coastal waters of Helsinki (the locations are indicated with white arrows). (Modified from reference 19a.)
TABLE 1.
Number of Anabaena strains isolated from each sampling station and data for environmental parametersa
| Station | Lat. | Long. | No. of strains isolated | Salinity (PSU) | Temp (oC) | Oxygen | PO4 | PTOT | SiO4 | NO3+NO2 | NH4 | NTOT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 59.1259 | 22.1894 | 1 (0) | 6.66 | 15.86 | 6.35 | 0.02 | 0.44 | 8.20 | 0.02 | 0.22 | 20.80 |
| 1b | 59.1259 | 22.1896 | 3 (0) | 6.61 | 16.34 | 6.54 | ND | 0.42 | 8.75 | 0.01 | 0.08 | 20.45 |
| 2 | 59.1330 | 22.2851 | 2 (0) | NM | NM | NM | NM | NM | NM | NM | NM | NM |
| 3 | 59.2400 | 22.2551 | 2 (0) | 6.24 | 15.52 | 6.58 | 0.10 | 0.70 | 6.90 | 0.03 | 0.13 | 23.30 |
| 4 | 59.2802 | 22.3858 | 2 (0) | 6.23 | 15.10 | 6.60 | 0.15 | 0.72 | 6.90 | ND | 0.07 | 25.25 |
| 5 | 59.3020 | 22.4031 | 2 (0) | 6.25 | 15.53 | 6.58 | 0.11 | 0.60 | 6.85 | 0.01 | 0.10 | 22.20 |
| 6 | 59.3218 | 22.5014 | 3 (0) | 6.23 | 14.99 | 6.66 | 0.17 | 0.62 | 7.00 | 0.02 | 0.18 | 22.25 |
| 7 | 59.2551 | 22.5206 | 3 (0) | 6.40 | 16.44 | 6.18 | 0.02 | 0.59 | 7.70 | 0.01 | 0.08 | 23.10 |
| 8 | 59.1701 | 22.4700 | 1 (0) | 6.67 | 16.52 | 6.65 | ND | 0.48 | 7.85 | ND | 0.09 | 21.85 |
| 9 | 59.3250 | 23.1000 | 2 (0) | 6.30 | 15.08 | 6.58 | 0.18 | 0.75 | 7.60 | ND | 0.17 | 24.00 |
| 10 | 59.3882 | 23.3903 | 3 (0) | 6.12 | 17.07 | 6.74 | ND | 0.67 | 6.70 | ND | 0.15 | 23.90 |
| 11 | 59.2901 | 23.5702 | 5 (0) | 6.26 | 17.33 | 6.67 | ND | 0.68 | 7.45 | 0.01 | 0.13 | 24.55 |
| 12 | 59.3515 | 24.0700 | 7 (0) | 5.96 | 16.78 | 7.02 | ND | 0.69 | 6.35 | ND | 0.15 | 25.90 |
| 13 | 59.4181 | 23.5798 | 1 (0) | 5.93 | 16.61 | 6.84 | ND | 0.68 | 6.35 | ND | 0.15 | 24.60 |
| 14 | 59.5701 | 24.4500 | 5 (0) | 5.64 | 17.03 | 6.70 | ND | 0.72 | 4.85 | ND | 0.18 | 30.85 |
| 15 | 60.0400 | 26.2087 | 7 (5) | 5.03 | 15.80 | 6.81 | 0.02 | 0.62 | 2.80 | ND | 0.15 | 25.05 |
The data shown are mean values measured from the values of depths 3 and 7 m, with the numbers of toxic strains shown in parenthesis. Oxygen, PO4, PTOT, SiO4, NO3+NO2, NH4, and NTOT are expressed as milligrams per liter. Station 1 was sampled twice and is indicated as 1a and 1b. Lat., latitude; Long., longitude; PSU, practical salinity units; PTOT, total phosphorus; NTOT, total nitrogen; NM, not measured; ND, not detected (concentration was under the detection limit, which was 0.05 μM for PO4-phosphorus, 0.1 μM for NO3 + NO2-nitrogen, and 0.06 μM for NO3-nitrogen).
Extraction of microcystins from Anabaena strains and from water sample.
Cells for microcystin determination were collected by centrifuging 40-ml cultures at 7,000 × g for 7 min at 4°C. The extraction of weighed, freeze-dried cells was performed with 1 ml of 75% (vol/vol) aqueous methanol (Merck, Darmstadt, Germany), supplemented with glass beads (0.5 millimeter; Scientific Industries, New York). The mixture was homogenized with a FastPrep cell disrupter (FP120; Bio101 Savant, Thermo Electron Corporation, Mitford, MA) three times at speed 5 for 20 s, and the mixture was subsequently centrifuged at 10,000 × g for 5 min. The supernatant was used for microcystin analysis in LC-MS.
Water samples were collected by a rosette sampler from depths of 0, 3, and 7 m (1 liter in volume) to make a direct toxin analysis. Water samples were filtered through a 1.0-μm filter (47-mm polycarbonate filters; Osmonics, Minnetonka, MN) and subsequently frozen at 20°C. Water sample handling was performed as sample handling for the Anabaena strain was, but the water sample was additionally bath sonicated (Sonorex Super 10P; Bandelin Electronic, Berlin, Germany) for 15 min and mixing and sonication were repeated three times. Prior to microcystin analysis, the supernatant was concentrated by vacuum centrifuging (Heto vacuum centrifuge; Heto-Holten A/S, Allerød, Denmark) and reconstituted to 50 μl.
Microcystin analysis.
The analysis of microcystins was carried out using a high-performance liquid chromatograph, combined with a diode array detector (Agilent 1100; Agilent Technologies, Santa Clara, CA) and a mass spectrophotometer (Agilent XCT Plus ion trap). Microcystins from the methanolic extracts were separated with a Zorbax Eclipse XDB-C8 column (4.6 by 150 mm; particle diameter, 5 μm; Agilent Technologies, Santa Clara, CA). For liquid chromatography, the mobile phase consisted of a gradient of 0.1% aqueous (water for high-pressure liquid chromatography, CHROMASOLV Plus; Sigma-Aldrich, Steinheim, Germany) formic acid (50% solution, Fluka; Sigma-Aldrich, Steinheim, Germany) (solvent A) and 0.1% formic acid in acetonitrile (Sigma-Aldrich, Steinheim, Germany) (solvent B). The linear gradient was as follows: 20% solvent A at 0 min, 80% solvent A at 50 min, and 100% solvent A at 65 min. A flow rate of 0.6 ml min−1 was used with the column temperature set to 40°C. Electrospray ionization was performed in positive ion mode. Nebulizer gas (N2) pressure was 50 lb/in2, and drying gas flow and temperature were 10 liters min−1 and 350°C, respectively. The capillary voltage was set at 3,270 V, with a capillary exit offset value of 317.4 V, a skimmer 1 potential of 41.5 V, and a trap drive value of 82.8. Spectra were recorded as averages of four using ultra scan mode and a scan range from 50 to 1,200 m/z. MS2 spectra were recorded in an auto-MS mode by using the following parameters: 5 to 10 precursor ions from 800 to 1,200 m/z, an isolation width of 4.0 m/z, and a fragmentation amplitude value of 0.50 V.
Microcystin analysis of filtered water sample was carried out as described earlier, with the following exceptions: the separation column was a Luna C18(2) column (150 by 2.0 mm; particle diameter, 5 μm; Phenomenex, Torrance, CA), the solvent A concentration was 60% at 60 min, the gradient flow rate was 0.15 ml min−1, the nebulizer gas (N2) pressure was 30 lb/in2, and the drying flow was 8 liters min−1.
Anabaena strains 90 (9, 42) and 66A (32, 42) as well as Microcystis viridis strain NIES102 (18) were used as reference strains. The identification of the microcystins was based on characteristic MH+ values corresponding with the range of published microcystins and the loss of neutral fragment 134 in the ion source (an event not so clearly observed from the MS2 spectrum). In addition, the occurrence of high intensity ion m/z 599 [(MeAsp)-Arg-Adda-(Glu) with H+] and less intense ion m/z 375 or 361 [Adda-134-Glu-(M)dha with H+; not present in all MS2 spectra] in the MS2 spectrum as well as comparison of LC-MS properties of microcystins produced by reference strains were used for identification. Microcystins have a characteristic UV spectrum with a maximum absorbance of 238 nm, and in the case of major variants, the UV spectrum attested also to the presence of microcystins. Peak areas from the chromatographic MH+ ion signals of the methanolic extracts and the MCYST-LR standard (a gift from Zbigniew Grzonka, Faculty of Chemistry, University of Gdańsk, Poland) were used for microcystin quantitation.
Collecting environmental data from the sampling stations.
Water samples for the determination of phosphate, total phosphorus, silicate, nitrate, nitrite, ammonium, and total nitrogen concentrations were obtained with a rosette sampler, and measurements were carried out at the time of sampling by using an autoanalyzer and following the guidelines of the Baltic Sea Monitoring Programme (13). A conductivity-temperature-depth probe provided continuous measurements of temperature, oxygen, and salinity. However, conductivity-temperature-depth sampling was not conducted at station 2 (Table 1).
Statistical analysis.
Since all microcystin-producing Anabaena strains were detected at one sampling station, station 15 (see Results), we used principal component analysis (PCA) to relate the presence of the microcystin-producing Anabaena strains to environmental conditions. PCA analysis was performed with Canoco for Windows, version 4.52 (48), and the PCA plot was constructed in CanoDraw 4.5.
16S rRNA gene PCR amplification and sequencing.
DNA from the strains was extracted using a modified cetyltrimethylammonium bromide (CTAB)-based extraction method (19). The quality and quantity of extracted DNA were determined by UV spectrophotometric measurements. The 16S rRNA gene was amplified from Anabaena strains with the primer pair pA (6) and B23S (27) as previously described (10). The 16S rRNA gene was sequenced using the internal sequencing primers 16S544R, 16S1092R, and 16S979F (35). The sizes of the PCR amplification products were checked in agarose gels, and PCR products were purified using Montage PCR centrifugal filter devices (Millipore, Billerica, MA). Sequencing was performed in the DNA sequencing laboratory of the Institute of Biotechnology, University of Helsinki, Helsinki, Finland.
Phylogenetic analysis of 16S rRNA gene sequences.
Phylogenetic analysis was performed for microcystin-producing Anabaena strains in order to resolve their relationship to Anabaena strains originating from freshwater environments. Representative 16S rRNA gene sequences from toxic and nontoxic Anabaena spp. from a range of environments were aligned using ClustalW program, version 1.4, as implemented in the BioEdit sequence alignment editor (version 7.0.1). The alignment was refined manually as ambiguous sites were excluded. Altogether, 1,354 bp of the 16S rRNA gene was used for phylogenetic analyses. Trees were constructed using neighbor-joining (NJ), maximum parsimony (MP), and maximum likelihood (ML) algorithms in the PAUP, version 10b, program (47). The GTR+I+G evolutionary model of substitution was used for NJ and ML using parameters (base frequencies, rate matrix of substitution types, and shape of gamma distribution) estimated from the data. In MP and ML analyses, we used heuristic searches, tree bisection, and reconnection branch rearrangement with a rearrangement limit of 1,000. To assess the reliability of the tree constructions, 1,000 bootstrap resamplings were performed for each tree with the same heuristic search. Nodularia sp. strain PCC73104/1 (accession number AJ133184), Nodularia sp. strain PCC7804 (AJ133181), Cyanospira rippkae (AY038036), Anabaenopsis sp. strain PCC9215 (AY038033), and Anabaena cylindrica (AF091150) were used as outgroups in all analyses. Uncorrected P distances of 16S rRNA gene sequences were calculated in PAUP, version 10b.
Nucleotide sequence accession numbers.
The nucleotide sequences of 16S rRNA genes have been deposited in the GenBank database under the accession numbers EF547190 to EF547196.
RESULTS
Microcystin analysis of isolated Anabaena strains.
We isolated 49 Anabaena strains from the open Gulf of Finland (Table 1) and demonstrated that five of these strains were microcystin producers. The toxic strains BIR246 (microcystin content, 2.1 μg mg−1 dry weight), BIR250A (3.9 μg mg−1), BIR257 (1.9 μg mg−1), BIR258 (1.3 μg mg−1). and BIR260 (2.1 μg mg−1) were all isolated from the easternmost sampling station, station 15 (Fig. 1). In addition, we analyzed two toxic strains, 315 and 318, which were isolated previously from the coastal waters of Helsinki. Microcystin content in strains 315 and 318 was 1.8 and 1.3 μg mg−1 dry weight, respectively.
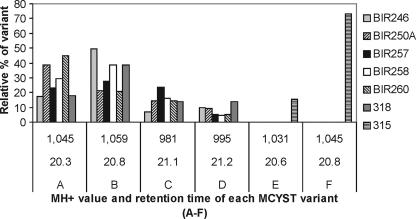
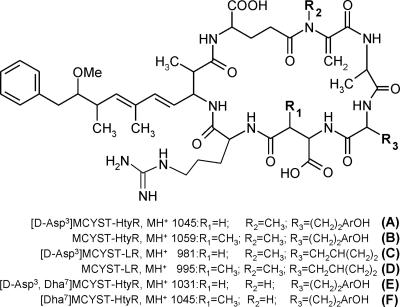
Strains BIR246, BIR250A, BIR257, BIR258, and BIR260 as well as strain 318 produced the same set of microcystin variants (Fig. 2). Four dominant variants were detected, and they represented 80 to 90% of the total microcystin content (Fig. 2). The MS2 spectrum of variant A strongly indicated that the structure for A was [d-Asp3]MCYST-HtyR (Fig. 2 and 3). We identified variant B as MCYST-HtyR (Fig. 2 and 3), which has been documented from Anabaena flos-aquae NRC 525-17 (12) and is the only variant having an m/z value of 1,059. Variants C and D were classified as [d-Asp3]MCYST-LR and MCYST-LR, respectively (Fig. 2 and 3), because their fragmentation of the MH+ ions and retention times were in agreement with the same microcystins produced by reference strain Anabaena 90 (9, 42). In addition to these four main microcystin variants, 23 other minor variants were described for these six Anabaena strains (data not shown).
FIG. 2.
Dominant microcystin variants produced by seven Anabaena strains. The y axis represents the relative proportion (%) of each microcystin variant out of all variants of each strain produced. Strains BIR246, BIR250A, BIR257, BIR258, BIR260, and 318 produced the same dominant variants, whereas the microcystin pattern produced by strain 315 was different. Variant A, [d-Asp3]MCYST-HtyR; variant B, MCYST-HtyR, variant C, [d-Asp3]MCYST-LR; variant D, MCYST-LR; variant E, [d-Asp3, Dha7]MCYST-HtyR; and variant F, [Dha7]MCYST-HtyR.
FIG. 3.
Microcystin structures of the main variants produced by seven Anabaena strains.
Strain 315 showed an unusual pattern of microcystin variants compared to those of the other toxic strains (Fig. 2). Almost 90% of the total microcystin content in strain 315 could be assigned to two variants, E and F (in Fig. 2 and 3). The structure of variant E was [d-Asp3,Dha7]MCYST-HtyR, proven by the coelution and congruent MS2 spectrum with microcystins from the reference strain Anabaena 66A (32). For variant F, the classified structure was [Dha7]MCYST-HtyR based on the coelution with the corresponding compound of the reference strain Anabaena 66A (32) and their high similarity in MS2 spectra. Altogether, strain 315 produced 10 microcystin variants, from which seven were not found in other isolated Anabaena strains (data not shown). From all the microcystin-producing strains, many unpublished microcystin variants were detected. However, these variants were produced in amounts insufficient for structural classification.
Toxin analysis of the filtered water sample.
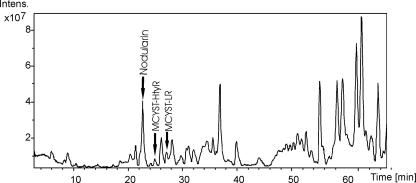
Nodularin and two variants of microcystins were detected by an LC-MS analysis of a filtered water sample from sampling station 15 (Fig. 4). The detected microcystin variants were MCYST-HtyR (m/z, 1,059) and MCYST-LR (m/z, 995). The total microcystin concentration in the water sample was 0.02 μg liter−1.
FIG. 4.
LC-MS chromatogram of the filtered water sample. Peaks representing nodularin, MCYST-HtyR, and MCYST-LR are indicated with black arrows. The y axis represents the intensity of each peak, and the x axis represents the retention time (in minutes).
Hydrographical data of the study area and statistical analysis.
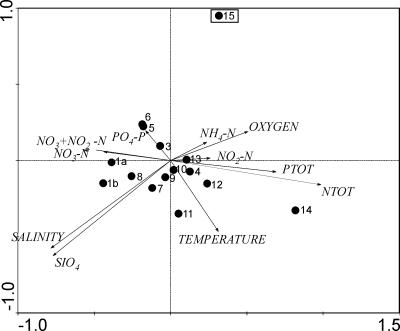
The environmental parameters from the sampling stations are listed in Table 1 (the mean values were calculated from values at depths of 3 and 7 m). All microcystin-producing Anabaena strains isolated from the open-sea sampling stations (strains BIR246, BIR250A, BIR257, BIR258, and BIR260) were found exclusively at the easternmost station, sampling station 15 (Fig. 1; Table 1). PCA analysis demonstrated that station 15 had lower salinity and lower silicate concentration than other stations did (Fig. 5). The first and second principal components accounted for 94.4% of the total variance.
FIG. 5.
PCA plot based on the environmental parameters measured from sampling stations (values listed in Table 1). Numbers correlate to the station numbering in Fig. 1 as well as in Table 1 (station 1 was sampled twice [indicated in the PCA plot as 1a and 1b]). Low-salinity and low-silicate concentrations were the variables to explain the divergence of station 15 (station indicated with a square) from the other stations. All open-sea microcystin-producing Anabaena strains were isolated from the station 15. The first and second principal components accounted for 94.4% of the total variance.
16S rRNA gene phylogeny of the Baltic Sea microcystin-producing Anabaena strains.
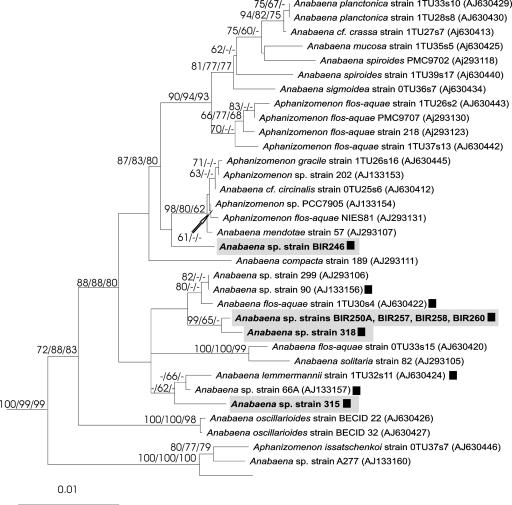
Microcystin-producing Anabaena strains were genetically heterogeneous since they were divided into four branches in the 16S rRNA gene tree (Fig. 6). Since all tree-constructing methods resulted in similar topologies, only the NJ tree is shown. The 16S rRNA gene sequences of four microcystin-producing Anabaena strains, BIR250A, BIR257, BIR258, and BIR260, were identical. These four Baltic Sea strains grouped with toxic Anabaena strain 318, isolated from the coastal waters of Helsinki (Fig. 1). Anabaena strain BIR246 was genetically distinct from freshwater hepatotoxic Anabaena strains as well as from the other Baltic Sea Anabaena strains (Table 2; Fig. 6).
FIG. 6.
Neighbor-joining tree based on 16S rRNA gene sequences (1,354 bp). Four branches, including the microcystin-producing Anabaena strain isolated from the Gulf of Finland, are boxed. Numbers at the nodes indicate bootstrap values of more than 50% for NJ, MP, and ML analysis. The taxa Nodularia sp. strain PCC73104/1 (accession number AJ133184), Nodularia sp. strain PCC7804 (AJ133181), Cyanospira rippkae (AY038036), Anabaenopsis sp. strain PCC9215 (AY038033), and Anabaena cylindrica (AF091150) were used as outgroups in all analyses. ▪, hepatotoxic strains studied by LC-MS.
TABLE 2.
Uncorrected P similarities of 16S rRNA gene sequences (1,354 bp) of the Baltic Anabaena strains
| Strain | BIR246 | BIR250A | BIR257 | BIR258 | BIR260 | 315 |
|---|---|---|---|---|---|---|
| BIR246 | ||||||
| BIR250A | 98.0 | |||||
| BIR257 | 98.0 | 100 | ||||
| BIR258 | 98.0 | 100 | 100 | |||
| BIR260 | 98.0 | 100 | 100 | 100 | ||
| 315 | 98.1 | 99.0 | 99.0 | 99.0 | 99.0 | |
| 318 | 97.9 | 99.7 | 99.7 | 99.7 | 99.7 | 98.9 |
DISCUSSION
Our results demonstrated that hepatotoxic microcystins, together with hepatotoxic nodularin, may occur in the open Gulf of Finland during the late summer cyanobacterial blooms, and we unequivocally proved that microcystins were produced by Anabaena sp. Anabaena is a common component of the cyanobacterial blooms in the Baltic proper as well as in the Gulf of Finland (e.g., 17, 45). Microcystins have been detected in water samples from the coastal southern Baltic Sea (28, 31) as well as at the entrance to the Gulf of Finland (17). However, these studies could not demonstrate conclusively which cyanobacteria were responsible for microcystin production. Anabaena isolates obtained previously from the Baltic Sea plankton habitats did not produce microcystins (K. Rantasärkkä, unpublished data), whereas benthic Anabaena strains were reported to be hepatotoxic or cytotoxic but not microcystin producing (14, 46).
All seven toxic Anabaena strains produced two to four dominant microcystin variants. It is common for Anabaena to produce a number of main toxin variants simultaneously (41). The 50% lethal dose (LD50) toxicity values for [d-Asp3]MCYST-HtyR (variant A) (Fig. 2 and 3) and [d-Asp3]MCYST-LR (variant C) are 160 to 300 μg kg−1 (39). For MCYST-HtyR (variant B), the LD50 value ranges between 80 and 100 μg kg−1, and for MCYST-LR (variant D), the LD50 value is 50 μg kg−1 (39). Also, variants [d-Asp3,Dha7]MCYST-HtyR (variant E) and [Dha7]MCYST-HtyR (variant F) have proved to be toxic (39). Variants [d-Asp3]MCYST-LR and MCYST-LR are prevalent in Anabaena strains isolated from Finnish freshwater habitats (42), and MCYST-LR was detected also at the entrance to the Gulf of Finland (17) as well as at the coastal waters of the southern Baltic proper (28).
Two microcystin variants, MCYST-LR and MCYST-HtyR, together with the nodularin, were detected in the filtered water sample from station 15. The concentration of MCYST-LR was about twice as much as MCYST-HtyR, whereas MCYST-HtyR was produced in greater amounts than MCYST-LR was in most of the isolated strains. Anabaena strains also produced demethylated microcystin variants, which were not detected in the filtered water sample. Temperature might be one factor contributing to this difference because a rise in temperature has been reported to increase the demethylated microcystin variants produced in freshwater Anabaena strains (36). At the time of sampling, the temperature of the surface water column at station 15 was 15.8°C, whereas isolated strains were grown at 22 to 24°C in the laboratory.
All of the microcystin-producing Anabaena strains were isolated from the easternmost sampling station (station 15). Thus, it is possible that some environmental factor(s) at sampling station 15 favored microcystin-producing strains. It is generally accepted that cyanobacteria produce toxins in conditions that are high in the nutrients that usually also favor the growth of these organisms (39). The concentration of PO4 phosphorus, which is considered to promote microcystin production in freshwater Anabaena strains (36), was nevertheless lower at station 15 than at stations at the entrance to the Gulf of Finland (Table 1). The PCA demonstrated that low concentrations of silicate and salinity distinguished station 15 from other stations. However, cyanobacteria are not known to require silicate and the connection between the occurrence of microcystin-producing Anabaena and low silicate concentrations remains speculative. Based on the PCA analysis, salinity appears to be the best explanation for the confined presence of microcystin-producing Anabaena strains to the eastern part of the Gulf of Finland. Furthermore, the coastal waters of Helsinki City, where two toxic Anabaena strains (315 and 318) were isolated, had salinities lower than 5.7‰ in 1997 and 1998 (according to monitoring data of the Helsinki City Environment Centre). The Gulf of Finland has a strong salinity gradient from the western and more saline part to the less-saline eastern part, with salinity being affected by freshwater inflow (21). Salinity has been identified as a possible controlling factor for diazotrophic cyanobacterial blooms, e.g., for distribution (26) and genetic diversity (23) of Aphanizomenon in the Baltic Sea. Thus, we might speculate that low-salinity waters favor toxin-producing Anabaena. However, future studies are needed to assess the effect of different salinities on the fitness of the Baltic Sea Anabaena strains and their microcystin production.
Microcystin-producing Baltic Sea Anabaena strains were genetically heterogeneous, since studied strains dispersed to three different branches in the complex 16S rRNA gene tree. However, since the 16S rRNA gene similarities of the Baltic microcystin-producing Anabaena strains were at least 97.9% (Table 2), they all belonged to the same species according to the “species” description based on 16S rRNA gene similarity of >97.5% (44). Conversely, in the Baltic Sea plankton, hepatotoxic Nodularia spumigena (3, 24) as well as nontoxic Aphanizomenon flos-aquae (2, 23) populations have shown to be genetically homogeneous. Thus, the Anabaena population appears to be genetically more diverse than Aphanizomenon or Nodularia populations in the Baltic Sea.
In previous studies, microcystin-producing freshwater Anabaena strains have been genetically heterogeneous but have grouped together (including also a few nontoxic Anabaena strains) when distinguished by 16S rRNA genes (29, 34), internal transcribed spacer sequence 1-S (ITS1-S) (the spacer region of the ribosomal operon) (11), rpoB (RNA polymerase beta-subunit) (34), and rbcLX (RubisCO) (34) genes. Although the phylogeny of the Anabaena strains from freshwater environments has been studied intensively, the paucity of strains has impeded studies of the phylogeny of the brackish Anabaena. Based on our results, microcystin-producing Baltic Sea Anabaena strains are genetically more diverse than are freshwater hepatotoxic Anabaena strains in the 16S rRNA tree. It has been suggested that toxic and nontoxic cyanobacteria might be separated using proxies, such as 16S rRNA, to indirectly determine toxin production. For example, to identify Australian neurotoxic Anabaena circinalis strains, specific clustering of A. circinalis in rpoC1 (7) and 16S rRNA (1) gene trees has been used for detection. Similarly, hepatotoxic and nontoxic Nodularia strains have been distinguished by 16S rRNA genes as well as by hetR, ITS1-S, and PC-IGS loci (24, 25). Even recently, Janse et al. (15) suggested that the toxic Microcystis strains could be separated from the nontoxic ones by using rRNA ITS sequences (15). However, the unsuitability of molecular taxonomy to separate toxic and nontoxic strains has been stated previously especially in the cases of Microcystis (29, 33) and Planktothrix (29) and our results proved that unsuitability in case of the Anabaena strains.
To relate the toxin production unequivocally to a certain genus or species requires strain isolation or single-filament or aggregate picking. Altogether, only a few Anabaena strains have been isolated from the Baltic Sea (K. Rantasärkkä, unpublished data) and Anabaena has not been included in toxin screenings (e.g., see references 37 and 45), probably due to its role (considered minor) as a component of cyanobacterial blooms in the Baltic Sea. However, in the Gulf of Finland, Anabaena species may exist even as dominating components in phytoplankton, together with Aphanizomenon (17). Our results demonstrate that the assumption that Anabaena strains are nontoxic in the Baltic Sea is incorrect, and future toxin screenings should take into account the presence of microcystin variants produced by Anabaena, especially in the low-salinity coastal waters, where the recreational value is the greatest. Further work will be required to determine the distribution of microcystin-producing Anabaena in the Baltic Sea.
Acknowledgments
This work was supported by the Baltic Sea Research Programme (BIREME, 202441) and grants from the Academy of Finland to K.S. (grant no. 53305 and 214457) and to D.P.F. (grant no. 212943).
We thank Kerttu Koskenniemi and Kaisa Rantasärkkä for assistance on the R/V Aranda in collecting the strains. Kerttu Koskenniemi is also acknowledged for kindly preparing the map. We are indebted to Lyudmila Saari and Eeva Eronen, who purified and maintained the cultures. The critical comments of Hermanni Kaartokallio, Harri Kuosa, and Christina Lyra improved the final manuscript. The crew of R/V Aranda (vessel of the Finnish Institute of Marine Research) is greatly appreciated for their kind assistance.
Footnotes
Published ahead of print on 31 August 2007.
REFERENCES
- 1.Baker, J. A., B. Entsch, B. A. Neilan, and D. B. McKay. 2002. Monitoring changing toxigenicity of a cyanobacterial bloom by molecular methods. Appl. Environ. Microbiol. 68:6070-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker, G. L. A., A. Konopka, B. A. Handley, and P. K. Hayes. 2000. Genetic variation in Aphanizomenon (cyanobacteria) colonies from the Baltic Sea and North America. J. Phycol. 36:947-950. [Google Scholar]
- 3.Barker, G. L. A., P. K. Hayes, S. L. O'Mahony, P. Vacharapiyasophon, and A. E. Walsby. 1999. A molecular and phenotypic analysis of Nodularia (cyanobacteria) from the Baltic Sea. J. Phycol. 35:931-937. [Google Scholar]
- 4.Codd, G. A., J. Lindsay, F. M. Young, L. F. Morrison, and J. S. Metcalf. 2005. Harmful cyanobacteria: from mass mortalities to management measures, p. 1-23. In J. Huisman, H. C. P. Matthijs, and P. M. Visser (ed.), Harmful cyanobacteria. Springer, Dordrecht, The Netherlands.
- 5.Diehnelt, C. W., S. M. Peterman, and W. L. Budde. 2005. Liquid chromatography-tandem mass spectrometry and accurate m/z measurements of cyclic peptide cyanobacteria toxins. Trends Anal. Chem. 24:622-634. [Google Scholar]
- 6.Edwards, U., T. Rogall, H. Blöcker, M. Emde, and E. C. Böttger. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fergusson, K. M., and C. P. Saint. 2000. Molecular phylogeny of Anabaena circinalis and its identification in environmental samples by PCR. Appl. Environ. Microbiol. 66:4145-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finni, T., K. Kononen, R. Olsonen, and K. Wallström. 2001. The history of cyanobacterial blooms in the Baltic Sea. Ambio 30:172-178. [PubMed] [Google Scholar]
- 9.Fujii, K., K. Sivonen, T. Nakano, and K. Harada. 2002. Structural elucidation of cyanobacterial peptides encoded by peptide synthetase gene in Anabaena species. Tetrahedron 58:6863-6871. [Google Scholar]
- 10.Gkelis, S., P. Rajaniemi, E. Vardaka, M. Moustaka-Gouni, T. Lanaras, and K. Sivonen. 2005. Limnothrix redekei (Van Goor) Meffert (cyanobacteria) strains from Lake Kastoria, Greece form a separate phylogenetic group. Microb. Ecol. 49:176-182. [DOI] [PubMed] [Google Scholar]
- 11.Gulledge, B. M., J. B. Aggen, H.-B. Huang, A. C. Nairn, and A. R. Chamberlin. 2002. The microcystins and nodularins: cyclic polypeptide inhibitors of PP1 and PP2A. Curr. Med. Chem. 9:1991-2003. [DOI] [PubMed] [Google Scholar]
- 12.Harada, K.-I., K. Ogawa, Y. Kimura, H. Murata, M. Suzuki, P. M. Thorn, W. R. Evans, and W. W. Carmichael. 1991. Microcystins from Anabaena flos-aquae NRC 525-17. Chem. Res. Toxicol. 4:535-540. [DOI] [PubMed] [Google Scholar]
- 13.HELCOM. 1984. Guidelines for the Baltic Monitoring Programme for the second stage (no. 12). Helsinki Commission, Helsinki, Finland.
- 14.Herfindal, L., L. Oftedal, F. Selheim, M. Wahlsten, K. Sivonen, and S. O. Døskeland. 2005. A high proportion of Baltic Sea benthic cyanobacterial isolates contain apoptogens able to induce rapid death of isolated rat hepatocytes. Toxicon 46:252-260. [DOI] [PubMed] [Google Scholar]
- 15.Janse, I., W. E. A. Kardinaal, M. Meima, J. Fastner, P. M. Visser, and G. Zwart. 2004. Toxic and nontoxic Microcystis colonies in natural populations can be differentiated on the basis of rRNA gene internal transcribed spacer diversity. Appl. Environ. Microbiol. 70:3979-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahru, M. 1997. Using satellites to monitor large-scale environmental change in the Baltic Sea, p. 43-61. In M. Kahru and C. W. Brown (ed.), Monitoring algal blooms: new techniques for detecting large-scale environmental change. Springer-Verlag, Berlin, Germany.
- 17.Karlsson, K. M., H. Kankaanpää, M. Huttunen, and J. Meriluoto. 2005. First observation of microcystin-LR in pelagic cyanobacterial blooms in the northern Baltic Sea. Harmful Algae 4:163-166. [Google Scholar]
- 18.Kaya, K., and M. M. Watanabe. 1990. Microcystin composition of an axenic clonal strain of Microcystis viridis and Microcystis viridis-containing waterblooms in Japanese freshwaters. J. Appl. Phycol. 2:173-178. [Google Scholar]
- 19.Kolmonen, E., K. Sivonen, J. Rapala, and K. Haukka. 2004. Diversity of cyanobacterial and heterotrophic bacteria in cyanobacterial blooms in Lake Joutikas, Finland. Aquat. Microb. Ecol. 36:201-211. [Google Scholar]
- 19a.Koskenniemi, K. C. Lyra, P. Rajaniemi-Wacklin, J. Jokela, and K. Sivonen. 2007. Quantitative real-time PCR detection of toxic Nodularia cyanobacteria in the Baltic Sea. Appl. Environ. Microbiol. 73:2173-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuiper-Goodman, T., I. Falconer, and J. Fitzgerald. 1999. Human health aspects, p. 113-153. In I. Chorus and J. Baltram (ed.), Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management. E & FN Spon, London, United Kingdom.
- 21.Kullenberg, G. 1981. Physical oceanography, p. 135-181. In A. Voipio (ed.), The Baltic Sea. Elsevier Scientific Publishing Company, Amsterdam, The Netherlands.
- 22.Laamanen, M., and H. Kuosa. 2005. Annual variability of biomass and heterocysts of the N2-fixing cyanobacterium Aphanizomenon flos-aquae in the Baltic Sea with reference to Anabaena spp. and Nodularia spumigena. Boreal Env. Res. 10:19-30. [Google Scholar]
- 23.Laamanen, M., L. Forsström, and K. Sivonen. 2002. Diversity of Aphanizomenon flos-aquae (cyanobacterium) populations along a Baltic Sea salinity gradient. Appl. Environ. Microbiol. 68:5296-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laamanen, M. J., M. F. Gugger, J. M. Lehtimäki, K. Haukka, and K. Sivonen. 2001. Diversity of toxic and nontoxic Nodularia isolates (cyanobacteria) and filaments from the Baltic Sea. Appl. Environ. Microbiol. 67:4638-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehtimäki, J., C. Lyra, S. Suomalainen, P. Sundman, L. Rouhiainen, L. Paulin, M. Salkinoja-Salonen, and K. Sivonen. 2000. Characterization of Nodularia strains, cyanobacteria from brackish water, by genotypic and phenotypic methods. Int. J. Syst. Evol. Microbiol. 50:1043-1053. [DOI] [PubMed] [Google Scholar]
- 26.Lehtimäki, J., P. Moisander, K. Sivonen, and K. Kononen. 1997. Growth, nitrogen fixation, and nodularin production by two Baltic Sea cyanobacteria. Appl. Environ. Microbiol. 63:1647-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepère, C., A. Wilmotte, and B. Meyer. 2000. Molecular diversity of Microcystis strains (Cyanophyceae, Chroococcales) based on 16S rRNA sequences. Syst. Geogr. Plant 70:275-283. [Google Scholar]
- 28.Luckas, B., J. Dahlmann, K. Erler, G. Gerdts, N. Wasmund, C. Hummert, and P. D. Hansen. 2005. Overview of key phytoplankton toxins and their recent occurrence in the North and Baltic Seas. Environ. Toxicol. 20:1-17. [DOI] [PubMed] [Google Scholar]
- 29.Lyra, C., S. Suomalainen, M. Gugger, C. Vezie, P. Sundman, L. Paulin, and K. Sivonen. 2001. Molecular characterization of planktic cyanobacteria of Anabaena, Aphanizomenon, Microcystis, and Planktothrix genera. Int. J. Syst. Evol. Microbiol. 51:513-526. [DOI] [PubMed] [Google Scholar]
- 30.MacKintosh, C., K. A. Beattie, S. Klumpp, P. Cohen, and G. A. Codd. 1990. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 264:187-192. [DOI] [PubMed] [Google Scholar]
- 31.Mazur, H., and M. Pliński. 2003. Nodularia spumigena blooms and the occurrence of hepatotoxin in the Gulf of Gdańsk. Oceanologia 45:305-316. [Google Scholar]
- 32.Namikoshi, M., K. Sivonen, W. R. Evans, W. W. Carmichael, L. Rouhiainen, R. Luukkainen, and K. L. Rinehart. 1992. Structures of three new homotyrosine-containing microcystins and a new homophenylalanine variant from Anabaena sp. strain 66. Chem. Res. Toxicol. 5:661-666. [DOI] [PubMed] [Google Scholar]
- 33.Otsuka, S., S. Suda, R. Li, M. Watanabe, H. Oyaizu, S. Matsumoto, and M. M. Watanabe. 1999. Phylogenetic relationships between toxic and non-toxic strains of the genus Microcystis based on 16S and 23S internal transcribed spacer sequence. FEMS Microb. Lett. 172:15-21. [DOI] [PubMed] [Google Scholar]
- 34.Rajaniemi, P., P. Hrouzek, K. Kaštovská, R. Willame, A. Rantala, L. Hoffmann, J. Komárek, and K. Sivonen. 2005. Phylogenetic and morphological evaluation of the genera Anabaena, Aphanizomenon, Trichormus and Nostoc (Nostocales, Cyanobacteria). Int. J. Syst. Evol. Microbiol. 55:11-26. [DOI] [PubMed] [Google Scholar]
- 35.Rajaniemi-Wacklin, P., A. Rantala, M. A. Mugnai, S. Turicchia, S. Ventura, J. Komárková, L. Lepistö, and K. Sivonen. 2005. Correspondence between phylogeny and morphology of Snowella spp. and Woronichinia naegeliana, cyanobacteria commonly occurring in lakes. J. Phycol. 42:226-232. [Google Scholar]
- 36.Rapala, J., K. Sivonen, C. Lyra, and S. I. Niemelä. 1997. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Repka, S., M. Meyerhöfer, K. von Bröckel, and K. Sivonen. 2004. Associations of cyanobacterial toxin, nodularin, with environmental factors and zooplankton in the Baltic Sea. Microb. Ecol. 47:350-358. [DOI] [PubMed] [Google Scholar]
- 38.Rinehart, K. L., M. Namikoshi, and B. W. Choi. 1994. Structure and biosynthesis of toxins from blue-green algae (cyanobacteria). J. Appl. Phycol. 6:159-176. [Google Scholar]
- 39.Sivonen, K., and G. Jones. 1999. Cyanobacterial toxins, p. 41-111. In I. Chorus and J. Baltram (ed.), Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. E & FN Spon, London, United Kingdom.
- 40.Sivonen, K., K. Kononen, W. W. Carmichael, A. M. Dahlem, K. L. Rinehart, J. Kiviranta, and S. I. Niemelä. 1989. Occurrence of the hepatotoxic cyanobacterium Nodularia spumigena in the Baltic Sea and structure of the toxin. Appl. Environ. Microbiol. 55:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivonen, K., M. Namikoshi, R. Luukkainen, M. Färdig, L. Rouhiainen, W. R. Evans, W. W. Carmichael, K. L. Rinehart, and S. I. Niemelä. 1995. Variation of cyanobacterial hepatotoxins in Finland, p. 163-169. In M. Munawar and M. Luotola (ed.), The contaminants in the Nordic ecosystem: dynamics, processes and fate. Ecovision World Monograph Series. SPB Academic Publishing, Amsterdam, The Netherlands.
- 42.Sivonen, K., M. Namikoshi, W. R. Evans, W. W. Carmichael, F. Sun, L. Rouhiainen, R. Luukkainen, and K. L. Rinehart. 1992. Isolation and characterization of a variety of microcystins from seven strains of the cyanobacterial genus Anabaena. Appl. Environ. Microbiol. 58:2495-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivonen, K., S. I. Niemelä, R. M. Niemi, L. Lepistö, T. H. Luoma, and L. A. Räsänen. 1990. Toxic cyanobacteria (blue-green algae) in Finnish fresh and coastal waters. Hydrobiologia 190:267-275. [Google Scholar]
- 44.Stackebrandt, E., and B. M. Goebel. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 45.Stal, L. J., P. Albertano, B. Bergman, K. von Bröckel, J. R. Gallon, P. K. Hayes, K. Sivonen, and A. E. Walsby. 2003. BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea—responses to a changing environment. Continental Shelf Res. 23:1695-1714. [Google Scholar]
- 46.Surakka, A., L. M. Sihvonen, J. M. Lehtimäki, M. Wahlsten, P. Vuorela, and K. Sivonen. 2005. Benthic cyanobacteria from the Baltic Sea contain cytotoxic Anabaena, Nodularia, and Nostoc strains and an apoptosis-inducing Phormidium strain. Environ. Toxicol. 20:285-292. [DOI] [PubMed] [Google Scholar]
- 47.Swofford, D. L. 2003. PAUP version 4b10: phylogenetic analysis using parsimony (and other methods). Sinauer Associates, Sunderland, MA.
- 48.ter Braak, C. J. F., and P. Šmilauer. 2002. Canoco reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca, NY.
- 49.Tikkanen, T. 1986. Kasviplanktonopas. Suomen luonnonsuojeluliiton tuki Oy. Forssan kirjapaino, Forssa, Finland.