Abstract
Previous studies demonstrated a direct correlation with loss of kangai-1 (KAI1), a metastasis suppressor, and poor prognosis in human prostate and other cancers. In this study, we have characterized the age-dependent downregulation of KAI1 in the TRAMP model which was reversed when mice were fed a genistein-enriched diet. We demonstrated here that doses of genistein (5 and 10 μM) - which could be achieved by supplement intake- significantly induced the expression of KAI1, both at the mRNA and protein levels (up to 2.5-fold), and decreased the invasiveness of TRAMP-C2 cells by more than 2.0-fold. We have pinpointed KAI1 as the suppressor of invasion, since its targeted knockdown by siRNA restored the invasive potential of genistein-treated TRAMP-C2 cells to control levels. This work provides the first evidence that genistein treatment may counteract KAI1 downregulation, which is observed in many cancer types and therefore, could be used in anti-metastatic therapies.
Keywords: Genistein, KAI1/kangai, CD82, TRAMP, prostate cancer, metastasis, phytoestrogen
Introduction
It is estimated that more than 200,000 new cases of prostate cancer (CaP) will be diagnosed in the United States in 2007, of which more than 10% will die. These estimates make CaP the leading cause of cancer deaths in US men [1]. Although the dysregulation of cell division is at the basis of tumor formation, the most serious threat to the patient’s survival is the malignant cells’ ability to invade surrounding tissues and form distant metastases [2]. It has been reported that 35% of CaP patients develop metastases, in which bone metastasis is the most frequent (~90%) [3]. The metastatic process is complex and involves several steps including invasion, apoptotic evasion as well as angiogenesis [4]. Therefore, natural products or drugs which interfere with these processes are useful agents in the fight against cancer progression.
The reduced incidence of clinically relevant CaP in Asian males [5] suggests that the established chemopreventive action of the phytoestrogen, genistein, a major component of soy and a staple of the Asian diet, is not restricted to its anti-proliferative effects but might also span invasion and metastasis. Previous studies have shown that genistein inhibits the invasion of different tumor cell lines in vitro. These effects were seen in head and neck [6], bladder [7], prostate [8-9], colon [10], hepatocellular [11] and breast [12] cancers among others. On the other hand, genistein has been shown to reduce tumor metastasis in animal models [13-16]. From these studies, several mechanisms by which genistein might exert its anti-metastatic properties have been proposed. Tumor invasion is a crucial part of the metastatic process and involves a number of steps including alterations in cellular adhesion and motility, proteolytic disruption of the basement membrane followed by migration through the extracellular matrix, acquisition of an angiogenic phenotype and the subsequent growth and proliferation of cells at a new site [17]. Mechanistic studies have shown that genistein can hinder several of these steps. Genistein at high doses has been shown to affect 1) cellular detachment by increasing the formation of focal adhesion complexes [18], 2) invasion via downregulation of matrix metalloproteinases (MMPs): MMP-2 [6], 8 [19], 9 [6], and 13 [19], downregulation of urokinase plasminogen activator (uPA) secretion [20], as well as upregulation of tissue inhibitor of matrix metalloproteinases-1 (TIMP1) [12].
Kangai-1/CD82 (KAI1) has been first identified as a prostate cancer metastasis suppressor [21]. Subsequent studies demonstrated a direct correlation with loss of KAI1 expression and poor prognosis in human prostate as well as other cancers [22-24]. KAI1 belongs to the transmembrane 4 superfamily, whose members have been suggested to be involved in regulating metastasis-related cellular processes [25]. A reduced KAI1 expression is associated with altered adhesion to components of the extracellular matrix, reduced cell–cell interactions, and increased cell motility; all of this giving an increased invasive and metastatic potential to these cells.
Since genistein has been shown to reduce the invasive potential of several cell lines as well as metastasis in vivo, and given that the identification of genistein targets that mediate these effects remains crucial; we aimed at determining whether achievable genistein levels would modulate KAI1 expression in a prostate cancer cell line (TRAMP-C2) as well as in the Transgenic Adenocarcinoma Mouse Prostate model (TRAMP) and determine whether KAI1 contributes to the observed anti-invasive effect of genistein.
Materials and Methods
Cell culture and reagents
TRAMP-C2 cell line (gift from Dr. Norman M. Greenberg), was maintained at 37°C with 5% CO2 in phenol red-free IMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Quality Biologicals, Gaithersburg, MD), 2 mM glutamine, 100 units/ml penicillin G sodium and 100 μg/ml streptomycin sulfate (Sigma, St. Louis, MO). Twenty-four hours after seeding (4×105 cells/100 mm plate), genistein (Sigma, St Louis, MO) was added to a final concentration of 5 or 10 μM. Genistein containing media was replenished every day for the experiment duration. Control cells received equal amounts of ethyl alcohol in the media, the solvent of genistein.
Animal handling and tissue preparation
TRAMP (The Jackson laboratory, Bar Harbor, Maine) and FVB mice (Charles River Laboratories, Wilmington, MA) were maintained at the Georgetown University animal facilities. Male and female TRAMP mice were mated with FVB counterparts, and heterozygous male offspring were confirmed by genotyping as described previously [26]. Four-weeks old transgenic males were fed genistein-free purified AIN-76A pellets (Harlan Teklad, Indianapolis, IN) supplemented with 0, 250 and 1000 mg genistein per kilogram diet (n=15/diet group) (Sigma, St. Louis, MO) until 20 weeks of age, for a total sample number of 45 mice. Another group were kept on a regular diet and 10 mice were sacrificed at 5, 9, 18 and 24 weeks of age (n=10/age group) for a total sample number of 40 mice. Animal care and treatments were conducted in accordance with established guidelines and protocols approved by the Georgetown University Animal Care and Use Committee. After completion of genistein treatment (20 weeks) or reaching endpoint ages, mice were sacrificed, blood collected, and various organs (prostate, seminal vesicles, hearts, livers, lungs, kidneys and testes) dissected out, weighed, fixed in 4% paraformaldehyde for 48 hrs, dehydrated and paraffin-embedded. Portions of prostatic lobes (dorsolateral, ventral and anterior) were rapidly frozen on dry ice and stored at -80°C, until processed for mRNA and protein analysis.
SiRNA treatment and plasmid transfection
For siRNA experiments, TRAMP-C2 cells were seeded at a density of 5×105 cells per 6-well plate. After attachment, cells were treated with 0, 5 and 10 μM genistein for 4 days, trypsinized, counted and transfected with control or KAI1 siRNA using Transpass R1 siRNA transfection reagent (New England Biolabs, Ipswich, MA) at a final concentration of 100 nM. Twenty-four hours post-transfection, cells were treated with 0, 5 or 10 μM genistein for 3 more days and proteins were extracted or the invasion assay was performed after re-suspension. For plasmid transfection, TRAMP-C2 cells were transfected with pECFP-C1-Empty Vector or pECFP-C1-CD82 (Addgene plasmid 1818, 28) using GeneJammer transfection reagent (Stratagene, La Jolla, CA) in the presence of complete growth medium for 3 days. Proteins were then extracted or the invasion assay performed.
Reverse transcription
Reverse transcription polymerase chain reaction (RT-PCR). RNA was extracted with TRIzol solution as suggested by the manufacturer (Invitrogen, Carlsbad, CA). KAI1 or GAPDH genes were amplified using the Reverse-It one step kit (Abgene, Rochester, NY). Mouse specific primers were designed by using the Primer Quest program (Integrated DNA Technologies, Coralville, IA). KAI1-Forward 5’- TGAGGATTGGCCTGTGAACACTGA-3, KAI1-Reverse 5’-ATACTGGGAGCCAT T TCGAGCTGT-3’ and GAPDH-Forward 5’-GTGTTCCTACCCCCAATGTG-3′;GAPDH -Reverse: 5′-C TT GCTCAGTGTCCTTGCTG-3’. PCR reactions were initiated at 94°C for 2 minutes, followed by 28 cycles of 94°C for 1 minute, 1-minute annealing temperature (58 °C), 72°C for 1 minute followed by final extension at 72°C for 5 minutes. The yielded PCR products (440 and 349 base pairs, respectively) were separated on 1.5% agarose gels and visualized by ethidium bromide fluorescence using the Fuji LAS-1000 imager (Tokyo, Japan). Images were imported to Photoshop.
Western blot analysis
Protein extracts were prepared from TRAMP-C2 cells treated with or without 5 and 10 μM, siRNA transfected cells or plasmid transfected cells. Alternatively, proteins were extracted from dorsolateral prostates of TRAMP/FVB mice in the age or genistein treatment groups. Membranes were probed with an antibody against KAI1 purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Membranes were stripped and re-probed with GAPDH (1/10000 dilution) antibody purchased from Abcam (Cambridge, MA) to ensure for equal loading. Molecular weight markers (Invitrogen, Carlsbad, CA) were run to confirm the molecular size of the immunoreactive proteins.
Immunofluorescence staining
TRAMP-C2 cells were plated on ECL-coated (Upstate, Charlottesville, VA) Lab–Tek chamber slide. After 7 days of treatment with 0, 5 and 10 μM genistein, cells were fixed in chilled methanol at -20°C for 30 minutes, blocked with 1% BSA at room temperature for 1 hour then probed with KAI1 antibody (Santa Cruz) at a concentration of 1/50 overnight at 4°C followed by incubation with the Alexa Fluor secondary mouse antibody (Molecular probes, Invitrogen, Carlsbad, CA) for 1 hour. Slides were washed, counterstained with propidium iodide, mounted and viewed with a fluorescent Olympus BX 40 fluorescent microscope.
In vitro invasion assay
A quantitative measure of the degree of in vitro invasion of TRAMP-C2 cells across Matrigel was obtained in the Boyden Chamber assay (BD Biosciences) according to the manufacturer protocol. Briefly, after rehydration of the invasion chambers, a 0.5 ml suspension of TRAMP-C2 (40,000 cells) in SFM (treated with or without genistein (5 and 10 μM) for 7 days, or 2) transfected with pECFP-C1-Empty Vector or pECFP-C1-CD82 for 3 days, or 3) treated with genistein (5 and 10 μM) for 4 days then with control siRNA or KAI1 siRNA for 3 days with or without genistein) was placed in the upper compartment of a BD Biocoat Matrigel Invasion Chamber with a 8 μm pore size polycarbonate filter coated with a thin Matrigel layer and incubated for 24 h at 37°C with 10% FBS-supplemented media in the lower compartment. Non-migrating cells were removed with a cotton swab; remaining cells were fixed in methanol and stained with Toliudine Blue. Filters were removed from the chamber and mounted for visualization under the Olympus BX-40 microscope equipped with an Olympus DP-70 camera. The number of cells migrating to the lower side of the filter was determined by counting the invaded cells in 5 random fields from triplicate filters for each treatment. Representative pictures were taken at a magnification of 10X.
Results
The prostate cancer progression in TRAMP mice is associated with an age-dependent decrease in KAI1 levels which are retained by the consumption of genistein
To examine the effect of dietary genistein on KAI1 expression, we started by determining the levels of KAI1 transcripts and protein levels in the dorsolateral prostates (DLPs) of TRAMP/FVB mice of various ages (5, 9, 18 and 24 weeks of age). The histopathological grades of DLPs of TRAMP/FVB mice in the above-mentioned age groups were described previously [28] and represent normal, prostatic intraepithelial neoplasia (PIN), well-differentiated (WD) and poorly differentiated (PD) carcinoma, respectively. We have observed a statistically significant decrease (up to 50%) in KAI1 mRNA and protein levels by 18 weeks of age, followed by more than 90% reduction by 24 weeks of age (Figure 1A). Concomitant with the reduction in the incidence of PD cancer in the DLPs of TRAMP/FVB mice consuming a genistein-rich diet that we previously reported [28], we observed a dose-dependent increase in KAI1 mRNA and protein levels (up to 4.0 and 7.0-fold) in the 250 and 1000 mg/kg diet groups, respectively (Figure 1B).
Figure 1.
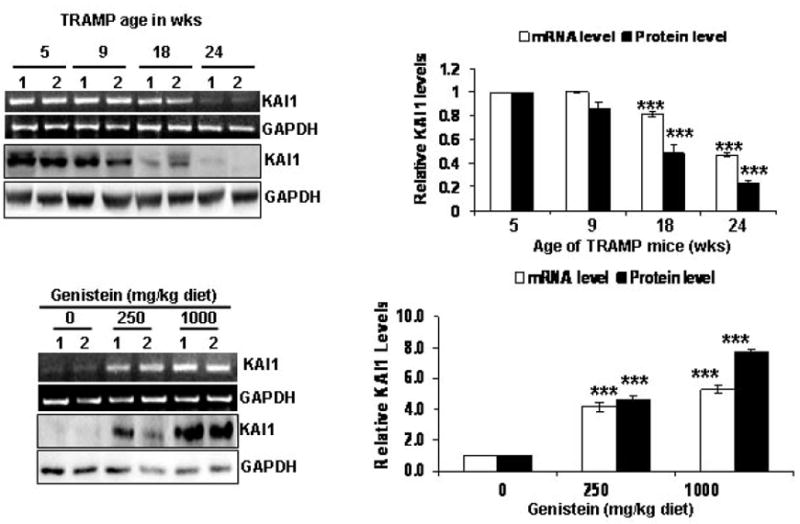
The age-dependent downregulation of KAI1 in the dorsolateral prostates of TRAMP mice is reversed by dietary genistein. The relative mRNA and protein levels of KAI1 in the different age groups were determined by 1) RT-PCR: 500 ng RNA were subjected to 1-step RT-PCR with appropriate KAI1 and GAPDH primers. Photographs in A are representative of 2 samples (designated 1 and 2) from each age group. 2) WB: KAI1 protein levels were determined by running 50 μg proteins from DLP lysates on SDS-PAGE, and immunoblotting with KAI1 antibody. Immunoblots were stripped and probed for GAPDH to ensure equal loading. Values are the mean relative mRNA and protein levels ± SEM from three different blots, normalized to levels in normal DLPs. ***, indicate p<0.001. (B) Effect of genistein consumption on KAI1 levels in TRAMP DLPs was determined by 1) RT-PCR, similarly to A, 500ng of RNA from 2 samples of each genistein treatment group as well as from the control group were subjected to 1-step RT-PCR with The quantitative analyses of PCR products are on the right. Values are the mean relative message levels ± SEM from three different agarose gels. *** indicate p<0.001. 2)WB, similarly to A, with 50 μg of tissue lysates from two samples of each genistein treatment group as well as control group were subjected to SDS-PAGE, The quantitative analyses of immunoblots are on the right. Values are the mean relative protein levels ± SEM from three different immunoblots, normalized to levels in normal DLPs. ***, indicates p<0.001.
KAI1 expression decreases the invasive ability of the TRAMP-derived cell line, TRAMP-C2
We have examined the levels of KAI1 message and protein levels in the TRAMP-derived TRAMP-C2 cell line. KAI1 (mRNA and protein) is expressed at very low levels in TRAMP-C2 cells (Figure 2A). To determine the functional significance of KAI1 expression on TRAMP-C2 in terms of invasion, these cells were transiently transfected with a human KAI1 expression vector. The expression of hKAI1 was demonstrated via RT-PCR with human specific KAI1 primers (Figure 2A), while no effect on endogenous mKAI1 message levels. The successful expression of KAI1 at the protein level was demonstrated by Western blot analysis (Figure 2A, right). The transient expression of hKAI1 in TRAMP-C2 cells resulted in a statistically significant 2.0-fold decrease in the number of invading cells through a matrigel-coated 8 μm pore size polycarbonate filter after 24hrs (Figure 2B). This decrease was significantly different than the slight decrease observed upon transfection with the empty vector control.
Figure 2.
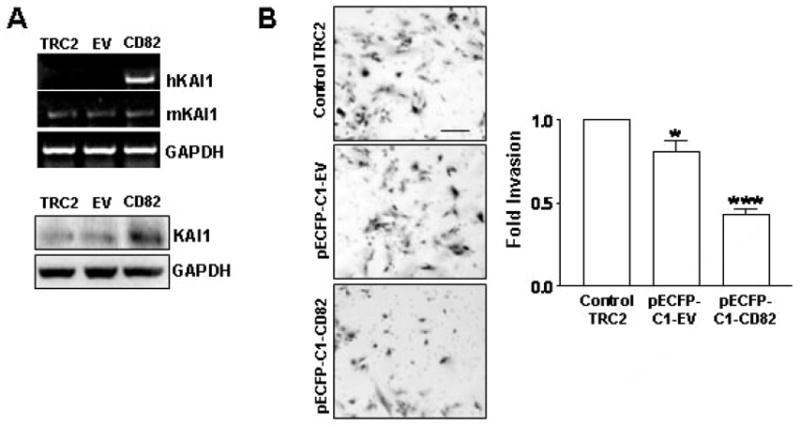
KAI1 expression decreases invasion of TRAMP-C2 cells. TRAMP-C2 cells were left untreated (TRAMP-C2 control), transfected with pECFP-C1-empty vector (EV) or pECFP-C1-CD82 (KAI1) for 3 days. A) 500 ng of RNA from each treatment were subjected to 1 step-RT-PCR with primers for murine KAI1 (mKAI1), human KAI1 (hKAI1) and GAPDH (up). Equal amounts of protein lysates (50 μg) were analyzed by Western blotting using anti-KAI1 antibody or anti-GAPDH antibody to ensure for equal loading (down). B) Forty thousand cells from above treatments were subjected to the BD biosciences Boyden Chamber assay (see Materials and Methods). The invaded cells from 3 different filters for each treatment were counted and results were plotted as fold invasion normalized to number of invaded cells in the TRAMP-C2 control group. * and ***, indicate p<0.05 and p<0.001, respectively. Representative photographs are on the left.
Genistein treatment induces KAI1 expression and reduces the invasive potential of TRAMP-C2 cells in vitro
Having observed a significant dose-dependent increase in KAI1 protein levels in the DLPs of TRAMP/FVB mice fed the genistein-containing diets as compared to their age-matched control diet-fed counterparts, we aimed to determine whether genistein could induce the expression of KAI1 in TRAMP-C2 cells. Western blot analysis revealed that genistein treatment for 7 days resulted in a dose–dependent increase in KAI1 mRNA and protein levels (up to 2.5-fold in 10μM treated cells) (Figure 3A). This increase was also corroborated by increase in KAI1 immunoreactivity in TRAMP-C2 cells by immunofluorescence staining (Figure 3B).
Figure 3.
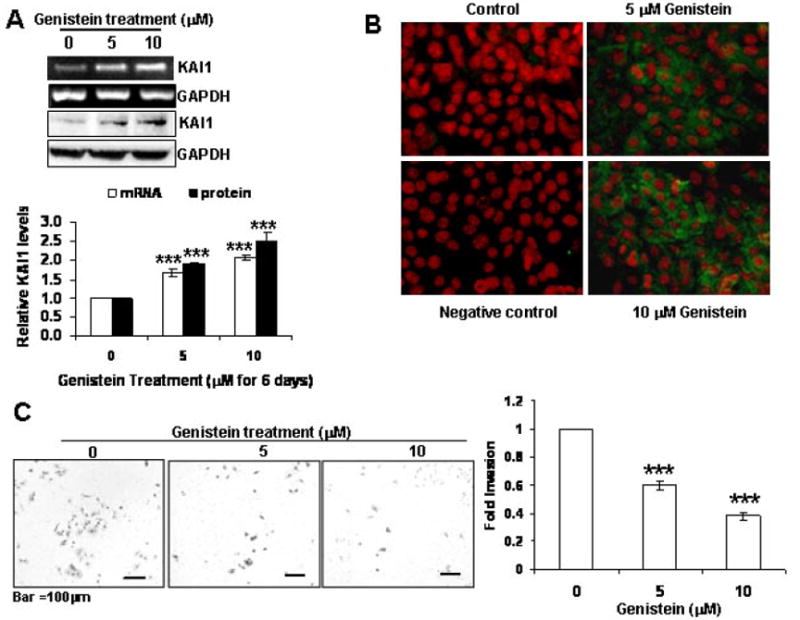
Genistein induces the expression of KAI1 in TRAMP-C2 cells and reduces their invasive potential. TRAMP-C2 cells were treated with genistein (0, 5 and 10 μM) for 7 days. A) KAI1 and GAPDH transcript and protein levels were analyzed by 1 step RT-PCR of 500 ng RNA from each treatment group and 50 μg protein lysates subjected to SDS-PAGE and immunoblotting with anti-KAI1 and anti-GAPDH antibodies to ensure for equal loading. KAI1 mRNA and protein levels were quantified from 3 independent experiments and plotted with normalization to levels in untreated TRAMP-C2 cells (down) ***, indicates p<0.001 B) KAI1 expression was examined by immunocytochemistry with anti-KAI1 antibody. TRAMP-C2 were plated on chamber slides and treated with 0, 5 or 10 μM genistein for 7 days then fixed in methanol, incubated with Anti-KAI1 antibody (or – antibody for negative control slide) and subsequently Alexa Fluor-tagged secondary antibody, counterstained with propidium iodide. Slides were then mounted and examined using a fluorescence microscope. Photographs were taken at the same magnification (20X) and then transported to Photoshop. C) Forty thousand cells from above treatments were subjected to the BD biosciences Boyden Chamber assay. The invaded cells from 3 different filters for each treatment were counted and results were plotted as fold invasion normalized to number of invaded cells in the untreated TRAMP-C2 control group. ***, indicates p<0.001. Representative photographs are on the left.
Previous studies have shown that genistein decreases the invasive potential of prostate cancer cells, albeit at supraphysiological levels (~50 μM). Therefore, we aimed at determining whether the low genistein doses used (5 and 10 μM) that induced the expression of KAI1, would affect TRAMP-C2 invasion. In fact, low doses of genistein treatment reduced the number of invaded cells by more than 60% in the 10 μM genistein group (Figure 3C).
The invasiveness of genistein-treated TRAMP-C2 cells is restored by siRNA targeting KAI1
Having observed an induction of KAI1 levels by genistein concomitant with a decrease in invasion, we wanted to determine whether KAI1 increase plays a role in the observed invasion reduction. TRAMP-C2 cells were treated with 0 or 10 μM genistein (the dose that maximally induced KAI1) and subjected to control scrambled siRNA or KAI1 specific siRNA. The effectiveness of the siRNA has been demonstrated via Western Blot analysis (Figure 4A). These same cells were subjected to the Boyden chamber invasion assay and whereas 10 μM genistein of control siRNA-transfected cells decreased the number of invaded cells by more than 50% (Figure 4B), this same dose failed to decrease the number of invaded cells transfected with KAI1 siRNA (Figure 4B), suggesting that KAI1 expression is in fact responsible for the anti-invasive effects of genistein in our cell system.
Figure 4.
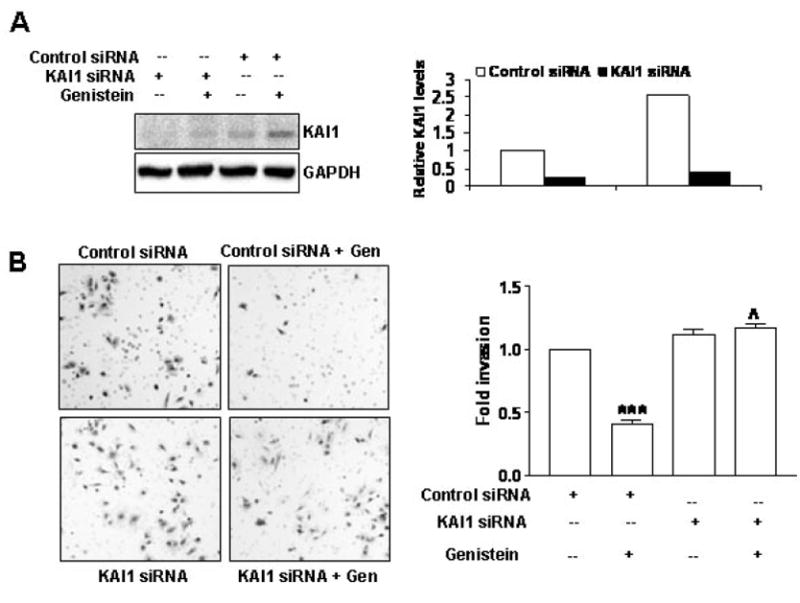
KAI1 siRNA restores the invasive potential of genistein-treated TRAMP-C2 cells. A) KAI1 protein levels in TRAMP-C2 cells transiently transfected with scrambled (control siRNA) or KAI1 siRNA for 72 hrs with or without 10 μM genistein after 4 days of genistein treatment (0 or 10 μM). Quantitative analysis of KAI1 protein levels from duplicate experiments are shown on the right. B: Evaluation of invasion after inhibition of KAI1 and genistein treatment (similarly to A). Forty thousand cells from above treatments were subjected to the BD biosciences Boyden Chamber assay. The invaded cells from 3 different filters for each treatment were counted and results were plotted as fold invasion normalized to number of invaded cells in the untreated control siRNA transfected TRAMP-C2 control group. ***, indicates p<0.001, compared to control siRNA transfected, untreated TRAMP-C2; ˄˄˄, indicates p<0.001 Representative photographs are on the left
Discussion
In this work, we have characterized for the first time the age-dependent decrease in KAI1 levels in the dorsolateral prostates of TRAMP/FVB mice and showed that KAI1 levels were restored in TRAMP/FVB mice consuming a genistein-rich diet, in a prevention regimen. The observed KAI1 retention in the DLPs of TRAMP/FVB mice does not seem to be solely due indirectly to the inhibition of PD cancer in TRAMP/FVB mice. In fact, we have shown that low levels of genistein can induce KAI1 expression in the TRAMP-derived cell line, TRAMP-C2. This induction occurred at both the mRNA and protein levels, suggesting a transcriptional regulation of KAI1 by genistein.
The induction of KAI1 has been previously shown to be dependent on p53 [30]. However, we do not believe this to be the case in this study due to the SV40-transformed nature of the TRAMP model and its derived cell line. Other factors have been demonstrated to induce KAI1 re-expression including nerve growth factor and phorbol esters [31-32]. However, in each of these mentioned cases, it remains to be determined whether the effects on KAI1 expression are directly due to these agents or indirectly as a result of altered cell behavior. The common downregulation of KAI1 in metastatic disease does not involve loss of heterozygosity at the KAI1 locus or mutations within the KAI1 gene [21]. However, the KAI1 promoter is associated with a CpG island; and genistein has been shown to act as a demethylating agent in vitro at a similar dose as the one used in this work (5 μM) [33]; therefore, genistein might induce KAI1 expression via promoter demethylation, which remains to be determined.
The proximal region of the KAI1 promoter contains potential binding sites for several transcription factors including AP1 and Sp1 sites [34]. Phytoestrogens, such as genistein have been shown to regulate promoters containing such sites via estrogen receptor signaling [35]. Furthermore, a comprehensive cDNA microarray examining the effects of a similar dose of genistein (10 μM) revealed the induction of these AP-1 factors [36]. Therefore, KAI1 might be induced via AP-1 regulation by genistein.
We have also shown that the expression of KAI1 by transfection or genistein treatment reduced the invasive potential of TRAMP-C2 cells. The anti-invasive mechanism of KAI1 has not been fully elucidated. In fact, KAI1 lacks intrinsic activity, but mediates its functions via binding to surface receptors such as integrins or receptor tyrosine kinases in a cell specific manner [24]. Several attractive scenarios have been put forward to explain KAI1 anti-invasive effects; including upregulation of TIMP-1 [37] and the inactivation of urokinase receptor proteolytic activities [38]. Although previous studies have shown that several pro-and anti-metastatic factors are modulated by genistein treatment, these were done at supraphysiological levels and therefore might not recapitulate what is happening with the doses used in this study or what could be achieved via genistein consumption. In fact, through this work, we have shown that inhibition of KAI1 expression by siRNA restored the number of invaded cells to control levels in the presence of genistein. This result suggests that KAI1 induction is required for the decrease the invasiveness of TRAMP-C2 cells by genistein. This study suggests that genistein treatment could have beneficial effect in preventing metastatic disease by restoring KAI1 and abolishing the invasive potential of cancer cells.
Acknowledgments
This work was supported by NIH grant R01 DK060875 to Partha P. Banerjee. We thank Dr. Norman Greenberg of Fred Hutchinson Cancer Research Center, Seattle, Washington for his generous gift of TRAMP-C2 cells.
Grant support: This work was supported by NIH grant R01 DK060875 to Partha P. Banerjee.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Gopalkrishnan RV, Kang DC, Fisher PB. Molecular markers and determinants of prostate cancer metastasis. J Cell Physiol. 2001;189:245–256. doi: 10.1002/jcp.10023. [DOI] [PubMed] [Google Scholar]
- 3.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res. 2000;6:1038–1045. [PubMed] [Google Scholar]
- 4.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054–5059. [PubMed] [Google Scholar]
- 5.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49:1857–1860. [PubMed] [Google Scholar]
- 6.Alhasan SA, Aranha O, Sarkar FH. Genistein elicits pleiotropic molecular effects on head and neck cancer cells. Clin Cancer Res. 2001;7:4174–4181. [PubMed] [Google Scholar]
- 7.Theodorescu D, Laderoute KR, Calaoagan JM, Gulding KM. Inhibition of human bladder cancer cell motility by genistein is dependent on epidermal growth factor receptor but not p21ras gene expression. Int J Cancer. 1998;78:775–782. doi: 10.1002/(sici)1097-0215(19981209)78:6<775::aid-ijc16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Sarkar FH. Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genistein. Cancer Lett. 2002;186:157–164. doi: 10.1016/s0304-3835(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Chen S, Xu L, Liu Y, Deb DK, Platanias LC, Bergan RC. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65:3470–3478. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 10.Ogasawara M, Matsunaga T, Suzuki H. Differential Effects of Antioxidants on the In Vitro Invasion, Growth and Lung Metastasis of Murine Colon Cancer Cells. Biol Pharm Bull. 2007;30:200–204. doi: 10.1248/bpb.30.200. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J Gastroenterol. 2005;11:6512–6517. doi: 10.3748/wjg.v11.i41.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–4857. [PubMed] [Google Scholar]
- 13.Schleicher RL, Lamartiniere CA, Zheng M, Zhang M. The inhibitory effect of genistein on the growth and metastasis of a transplantable rat accessory sex gland carcinoma. Cancer Lett. 1999;136:195–201. doi: 10.1016/s0304-3835(98)00322-x. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Yee JA, McGuire MH, Murphy PA, Yan L. Soybean isoflavones reduce experimental metastasis in mice. J Nutr. 1999;129:1075–1078. doi: 10.1093/jn/129.5.1075. [DOI] [PubMed] [Google Scholar]
- 15.Menon LG, Kuttan R, Nair MG, Chang YC, Kuttan G. Effect of isoflavones genistein and daidzein in the inhibition of lung metastasis in mice induced by B16F-10 melanoma cells. Nutr Cancer. 1998;30:74–77. doi: 10.1080/01635589809514644. [DOI] [PubMed] [Google Scholar]
- 16.Iishi H, Tatsuta M, Baba M, Yano H, Sakai N, Akedo H. Genistein attenuates peritoneal metastasis of azoxymethane-induced intestinal adenocarcinomas in Wistar rats. Int J Cancer. 2000;86:416–420. doi: 10.1002/(sici)1097-0215(20000501)86:3<416::aid-ijc17>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Gupta GP, Massagué J. Cancer Metastasis: Building a Framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Kyle E, Lieberman R, Crowell J, Kellof G, Bergan RC. Focal adhesion kinase (FAK) phosphorylation is not required for genistein-induced FAK-beta-1-integrin complex formation. Clin Exp Metastasis. 2000;18:203–212. doi: 10.1023/a:1006729106034. [DOI] [PubMed] [Google Scholar]
- 19.Kim MH, Gutierrez AM, Goldfarb RH. Different mechanisms of soy isoflavones in cell cycle regulation and inhibition of invasion. Anticancer Res. 2002;22:3811–3817. [PubMed] [Google Scholar]
- 20.Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol. 2004;25:1389–1395. [PubMed] [Google Scholar]
- 21.Jackson P, Marreiros A, Russell PJ. KAI1 tetraspanin and metastasis suppressor. Int J Biochem Cell Biol. 2005;37:530–534. doi: 10.1016/j.biocel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Adachi M, Taki T, Ieki Y, Huang CL, Higashiyama M, Miyake M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996;56:1751–1755. [PubMed] [Google Scholar]
- 23.Guo X, Friess H, Graber HU, Kashiwagi M, Zimmermann A, Korc M, Buchler MW. KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996;56:4876–4880. [PubMed] [Google Scholar]
- 24.Sridhar SC, Miranti CK. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and Src kinases. Oncogene. 2006;25:2367–2378. doi: 10.1038/sj.onc.1209269. [DOI] [PubMed] [Google Scholar]
- 25.Domanico SZ, Pelletier AJ, Havran WL, Quaranta V. Integrin alpha 6A beta 1 induces CD81-dependent cell motility without engaging the extracellular matrix migration substrate. Mol Biol Cell. 1997;8:2253–2265. doi: 10.1091/mbc.8.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Ingmundson A, Horner SM, Cicchetti G, Allen PG, Pypaert M, Cunningham JM, Mothes W. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic. 2003;4:785–801. doi: 10.1034/j.1600-0854.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 28.El Touny -LH, Banerjee PP. Akt/GSK3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression towards a poorly differentiated phenotype. Carcinogenesis. 2007 doi: 10.1093/carcin/bgm103. doi: 10.1093. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Bergan RC. Genistein inhibits matrix metalloproteinase type 2 activation and prostate cancer cell invasion by blocking the transforming growth factor beta-mediated activation of mitogen-activated protein kinase-activated protein kinase 2-27-kDa heat shock protein pathway. Mol Pharmacol. 2006;70:869–77. doi: 10.1124/mol.106.023861. [DOI] [PubMed] [Google Scholar]
- 30.Marreiros A, Dudgeon K, Dao V, Grimm MO, Czolij R, Crossley M, Jackson P. KAI1 promoter activity is dependent on p53, junB and AP2: evidence for a possible mechanism underlying loss of KAI1 expression in cancer cells. Oncogene. 2005;24:637–649. doi: 10.1038/sj.onc.1208216. [DOI] [PubMed] [Google Scholar]
- 31.Sigala S, Faraoni I, Botticini D, Paez-Pereda M, Missale C, Bonmassar E, Spano P. Suppression of telomerase, reexpression of KAI1, and abrogation of tumorigenicity by nerve growth factor in prostate cancer cell lines. Clin Cancer Res. 1999;5:1211–1218. [PubMed] [Google Scholar]
- 32.Akita H, Iizuka A, Hashimoto Y, Kohri K, Ikeda K, Nakanishi M. Induction of KAI-1 expression in metastatic cancer cells by phorbol esters. Cancer Lett. 2000;153:79–83. doi: 10.1016/s0304-3835(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 33.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 34.Dong JT, Isaacs WB, Barrett JC, Isaacs JT. Genomic organization of the human KAI1 metastasis-suppressor gene. Genomics. 1997;41:25–32. doi: 10.1006/geno.1997.4618. [DOI] [PubMed] [Google Scholar]
- 35.Schultz JR, Petz LN, Nardulli AM. Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors alpha and beta. J Biol Chem. 2005;280:347–354. doi: 10.1074/jbc.M407879200. [DOI] [PubMed] [Google Scholar]
- 36.Ise R, Han D, Takahashi Y, Terasaka S, Inoue A, Tanji M, Kiyama R. Expression profiling of the estrogen responsive genes in response to phytoestrogens using a customized DNA microarray. FEBS Lett. 2005;579:1732–1740. doi: 10.1016/j.febslet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 37.Jee BK, Park KM, Surendran S, Lee WK, Han CW, Kim YS, Lim Y. KAI1/CD82 suppresses tumor invasion by MMP9 inactivation via TIMP1 up-regulation in the H1299 human lung carcinoma cell line. Biochem Biophys Res Commun. 2006;342:655–661. doi: 10.1016/j.bbrc.2006.01.153. [DOI] [PubMed] [Google Scholar]
- 38.Bass R, Werner F, Odintsova E, Sugiura T, Berditchevski F, Ellis V. Regulation of urokinase receptor proteolytic function by the tetraspanin CD82. J Biol Chem. 2005;280:14811–14818. doi: 10.1074/jbc.M414189200. [DOI] [PubMed] [Google Scholar]


