Abstract
Offspring of rats exposed to valproic acid (VPA) on Gestational Day (GD) 12 have been advocated as a rodent model of autism because they show neuron loss in brainstem nuclei and the cerebellum resembling that seen in human autistic cases [20, 37]. Studies of autistic children have reported alterations in acquisition of classical eyeblink conditioning [40] and in reversal of instrumental discrimination learning [9]. Acquisition of discriminative eyeblink conditioning depends on known brainstem-cerebellar circuitry whereas reversal depends on interactions of this circuitry with the hippocampus and prefrontal cortex. In order to explore behavioral parallels of the VPA rodent model with human autism, the present study exposed pregnant Long-Evans rats to 600 mg/kg VPA on GD12 [cf. 37] and tested their offspring from PND26-31 on discriminative eyeblink conditioning and reversal. VPA rats showed faster eyeblink conditioning, consistent with studies in autistic children [40]. This suggests that previously reported parallels between human autism and the VPA rodent model with respect to injury to brainstem-cerebellar circuitry [37] are accompanied by behavioral parallels when a conditioning task engaging this circuitry is used. VPA rats also showed impaired reversal learning, but this likely reflected “carry-over” of enhanced conditioning during acquisition rather than a reversal learning deficit like that seen in human autism. Further studies of eyeblink conditioning in human autism and in various animal models may help to identify the etiology of this developmental disorder.
Keywords: Eyeblink Conditioning, Gestational Valproate Exposure, Discrimination Reversal, Cerebellum, Hippocampus, Development
The purpose of this article is to use a developmental rodent model of eyeblink conditioning to gather evidence bearing on the hypothesis that early gestational injury to the brainstem plays a role in the etiology of autism [3, 37, 39]. Evidence for this hypothesis arises from a disparate set of findings that show remarkable convergence [39]. The first clues came from reports of an association between autism and early gestational exposure to thalidomide and misoprostol [27]. Knowledge concerning the teratology of thalidomide permits inferences concerning the timing of thalidomide exposure based on the presence of craniofacial defects (ear anomolies). When thalidomide cases involving exposure specifically between 20–24 days post-conception were considered, autism occurred at a staggering rate (~30%, [39]). It was subsequently reported that craniofacial defects, indicative of aberrant development during this critical gestational period are elevated in a large sample of idiopathic autism cases, but not in unaffected siblings or in mental retardation [36]. The rate of autism is unusually high (8–11%) in women prescribed the anticonvulsant drug, valproate, during pregnancy and craniofacial defects are elevated in these autism cases also [28,35]. This teratological evidence is supported by genetic evidence for the early gestational origins of autism. The Hoxa family of genes, which regulate brain development specifically during the critical embryonic period, are associated with enlarged head circumference, a common “autism phenotype” [10] and Hoxa1 homozygous mutations in humans result in brainstem defects, ear anomolies, and autism [52].
This evidence concerning the etiology of autism in human populations motivated a search for an animal model [39]. Rats exposed to valproic acid (VPA) on GD12---the rodent equivalent to the period of increased human vulnerability to autism and craniofacial defects ---show brainstem-cerebellar neuron loss resembling that observed in autism [20, 37]. Rodent studies have also shown that Hoxa1 genes play a role in the effects of this gestational VPA exposure [3, 39].
This converging evidence reveals the fundamental advantage of the embryonic-brainstem-injury hypothesis of autism [39]: the timing of the developmental injury is known. This leads to specific predictions that can be readily tested at neurogenetic, neurobiological, and behavioral levels of analysis in both humans and in animal models. This hypothesis also makes testable predictions concerning the role of specific genotype × environment interactions in the etiology of autism. Most importantly for the purposes of the present article, this hypothesis has the important advantage of offering a specific rodent model of autism for behavioral evaluation, the GD12-VPA-exposed-rat [20, 37].
The eyeblink conditioning paradigm offers a number of advantages as a behavioral test of a rodent model of autism [3, 41, 48]. There is an enormous body of empirical research on the behavioral and neurobiological mechanisms of eyeblink conditioning gathered over the past 60 years in both humans [54] and animals models [55]. Much is known concerning the development of eyeblink conditioning in rodents [47, 50] and humans [21, 18], and about the neural mechanisms underlying this development [13]. There is an expanding literature about how disorders of development affect eyeblink conditioning in both animal models [5, 15, 16, 48] and humans [19, 24, 30, 33]---including human autism [40, 41]. Importantly, the necessary and sufficient neural circuitry underlying eyeblink conditioning is known and is conserved across mammalian species [54, 55]. Simple delay conditioning depends critically on an identified brainstem-cerebellar circuit [e.g., 25, 26, 51] whereas task variants such as trace conditioning and discrimination reversal depend on interactions of hippocampus and/or prefrontal cortex with this circuit [e.g., 4, 8, 23, 29, 53, 54, 55]. Such interactions are also demonstrated by the fact that abnormal activity in the intact hippocampus can slow acquisition of delay conditioning under conditions where extensive hippocampal damage has no effect [43].
These advantages of the eyeblink conditioning paradigm create a rich empirical context in which to interpret behavioral findings from the VPA rodent model of autism. Specifically, it permits us to determine how early brainstem-cerebellar injury alters performance on a behavioral task that is mediated by brainstem-cerebellar circuitry; how this effect compares with other developmental injuries to this circuitry assessed with the same rodent eyeblink conditioning procedure (5, 12, 15, 16, 48]; and how this effect compares with eyeblink conditioning in human autism [2, 40, 41].
The present study used a tone-light EBC discrimination/reversal task in weanling-juvenile rats [5, 32]. Weanling-juvenile rats were chosen as subjects on the basis of evidence that eyeblink conditioning effects in autism are larger in children than in adults [40]. This discrimination learning procedure was chosen because acquisition provides a test of VPA-induced alterations in brainstem-cerebellar function [5] whereas reversal provides both a second test of acquisition as well as an additional extinction test that could inform possible VPA-induced alterations in hippocampal or prefrontal function. More specifically, cerebellar impairment slows acquisition to CS+ during both acquisition and reversal phases [5]. In contrast, hippocampal- or prefrontal-impairment causes perseveration of responding to the new CS− (old CS+) during reversal, without altering acquisition to the new CS+ [4, 6, 53]. This preliminary assessment of forebrain function in the VPA rodent model is potentially relevant to reports of impaired executive function and reversal performance in autism [9, 11, 17, 31].
Eyeblink conditioning in autism is unique among all human neurological disorders [3]. In autism, there is a paradoxical enhancement of eyeblink conditioning accompanied by anomalies in conditioned response (CR) timing [2, 40]. In the many other human disorders examined thus far, eyeblink conditioning is either impaired or unaffected (see review of Arndt et al [3], General Discussion). By the same token, existing rodent studies indicate that disrupting cerebellar development impairs eyeblink conditioning [12, 15, 16, 48, 49]. This leads to opposing predictions concerning the outcome of this study. If the VPA rodent model resembles these other instances of developmental brainstem-cerebellar injury, then conditioning should be impaired. On the other hand, if this model shows an “autism behavioral phenotype,” then eyeblink conditioning should be enhanced.
Method
Subjects
A total of 16 Long Evans rats (10 female, 6 male) derived from 7 litters contributed data to this study. They were the offspring of timed-pregnant females that were shipped to the University of Delaware animal facility on GD 4 or 5 from Harlan Laboratories (Fredrick, MD). Pregnant dams were housed in 45 × 24 × 21 cm plastic cages with standard bedding and continuously supplied with rat chow and water. Illumination was provided on a 12:12-hr light-dark cycle, with lights on at 7:00 a.m. Age of pups was determined by checking for births during the light cycle and designating the date of birth as PND0 (typically GD22). On PND3, litters were culled to 8 pups (usually 4 males and 4 females). On PND21, pups were weaned from their mothers and housed in groups of same-sex littermates in cages the same size that they were reared in, until the start of the experiment. Pups were assigned to Groups VPA (n=9) and Saline (n=7) such that no more than 1 male and 1 female from a given litter were assigned to a given behavioral condition (tone+/light− or light+/tone−) and the treatment groups were counterbalanced as closely as possible for sex and modality of CS+ (see below). Behavioral testing took place from PND26-31 (see below), a period of development that typically precedes the estrous cycle in female rats.
VPA Dosing
Valproic acid (Sigma) was purchased as the sodium salt and was dissolved in 0.9% saline for a concentration of 250 mg/ml, pH 7.3, as verified by enzyme-multiplied immunoassay at the University of Rochester. The dosing procedure was based on that of Rodier et al. [37].
Time-mated females arrived from the supplier on GD4 or 5 and remained undisturbed in individual cages with ad lib food and water in the University of Delaware animal facility until GD 11. They were weighed on this day and again on GD 12 immediately prior to dosing, in order to determine the dosing volume. Females were randomly assigned to receive VPA and saline vehicle but in a manner than matched the two groups as closely as possible pre-dosing (GD11–12) body weight. Weights were also taken daily for three days following dosing (GD13–15) and again a week after dosing (GD19). .
VPA-treated dams (n=4) received a single ip injection of 600 mg/kg NaVP in a volume of 2.4 ml/kg at about 11:00 am on GD 12, while control dams (n=3) were treated with a similar volume of saline vehicle.
Surgery
On PND24, pups were surgically implanted with headstages containing stimulating and recording electrodes (see [46] for full description) under ketamine/xylazine anesthesia (i.p injection of 87 mg/kg ketamine/13 mg/kg xylazine in a 0.6–.75 ml/kg injection volume). Differential EMG electrodes were implanted in the left upper eyelid muscle, and a ground electrode was placed s.c. behind the neck. A bipolar stimulating electrode for delivering the US was implanted subdermally just caudal to the left eye. Electrode connectors were secured to the skull with dental acrylic and via galvanized steel wires implanted onto the skull [46]. Following surgery, subjects were returned to individual cages and monitored during recovery from anesthesia. These cages were supplied with ad lib food and water and housed subjects throughout the experiment (except during test sessions). .
Apparatus
The “freely-moving rat preparation” has been described in great detail elsewhere [5, 47, 48]. Animals were tested in stainless steel wire mesh cages enclosed in larger sound-attenuated chambers (BRS/LVE, Laurel, MD) lined with sound-absorbing foam. Each chamber was fitted with a ventilation fan producing low frequency background noise, a house light (15W), and a speaker which delivered the auditory CS. The auditory CS was a 70 dB, 2.8 kHz tone presented for 380 milliseconds (ms) and the visual CS was activation of the house light (against the dark background) for 380 ms [5, 32]. The US was a 2-mA, 100 ms, periocular shock produced by a constant-current, 60-Hz square wave stimulator (World Precision Instruments, Sarasota, FL). Subjects’ headstages were connected to peripheral equipment via cables which passed through an opening in the chamber to a commutator which allowed subjects to move freely about the chamber during test sessions. A custom-built Eyeblink Conditioning System (available from JSA Designs, Raleigh, NC) controlled stimulus presentations and recorded EMG eyelid activity from individual rats. The system interfaced with 16 conditioning chambers (4 sets of 4-chambers that could be independently programmed), permitting simulataneous testing of up to 16 rats.
Design and Procedures
On PND25 subjects were placed in the apparatus and connected to the recording equipment for a brief (1–2 minute) handling/adaptation procedure during which the experimenter blew a burst of air into the rat’s left eye and noted the quality of the blink-evoked EMG signal on an oscilloscope. This provided an indication of recording quality independent of the eyeblink unconditioned response (UR) elicited by the periocular-shock US during training.
On PND26 subjects began training in a tone-light discrimination as described in detail elsewhere [5, 32). Each session consisted of 50 trials of a 380 ms 15-W light CS, and 50 trials of a 380 ms 70db tone CS, one of which (CS+) preceded and coterminated with a 2-mA, 100 ms periocular-shock US (delay interval = 280 ms) and the other of which (CS−) was presented alone without the shock US. Reinforcement of each CS was counterbalanced across modality, creating two subgroups (light+/tone− and tone+/light−). Trials were presented in a pseudorandom order, with a maximum of three consecutive presentations of the same CS occurring at an average intertrial interval (ITI) of 30 sec (range: 18–42 sec, a range that is very effective and commonly used in both developing and adult animals; Stanton & Freeman, 2000, Woodruff-Pak & Steinmetz, 2000b). Within a block of 10 trials, the CS+ was paired with the US on 4 out of 5 trials (the 5th trial was a CS-alone test trial) and the CS− was presented alone on all 5 trials (one of which was “yoked” to the CS+ for the purpose of analyzing CS-alone test trials). The acquisition phase consisted of 2 sessions per day, beginning 5 hr apart (+/− 30 min), over 2 consecutive days (PND26-27) for a total of 4 sessions. Reversal was run over 4 days (PND28-31) and consisted of the same sessions and trials except that the previously reinforced CS (CS+) was now the CS−, and the previously nonreinforced CS (CS−) was now the CS+.
Dependent Measures
Criteria for CRs and URs have been described in great detail previously (e.g., 42, 46, 22]. EMG signals were sampled in 2.5 ms bins during the 800 ms epoch of each trial type (CS+, CS). The raw EMG signal was rectified and integrated and sampled in 2.5 ms bins during the 800 ms epoch of each trial type (CS+, CS−). . Each trial epoch was divided into: a (1) 280-msec Pre-CS baseline period (2) an 80-msec “Alpha” or Startle period commencing at CS onset; (3) a 200 msec CR period, occurring between the end of the startle period and US onset (EMG activity in this period constituted a CR); and (4) UR period, the time from offset of the US to the end of the trial (140 ms; the recording was interrupted during the 100 ms US presentation in order to avoid stimulus artifact in the UR recording). On CS-alone test trials, the CR sampling periods were as described under “(3)” above, except that they extended to the end of the trial and therefore included the period designated as the “UR period” on paired trials. The threshold for registering an EMG response was set 40 arbitrary units above the average baseline amplitude during the pre-CS period [42]. For each response sampling period, onset and peak response latencies, and peak response amplitudes were measured. The percentage and averages of the amplitude measures were computed separately for the CS+ and CS− across trial blocks and sessions. These averages included trials in which amplitudes registered as zero because no response occurred (termed response “magnitude” by some investigators). CR peak latency measures were taken only from trials on which a CR occurred and only from CS-alone test trials to avoid the artificial “ceiling” in CR peak latency imposed by US onset on paired trials.
Data Analysis
Analysis of variance (ANOVAs) on each dependent measure was performed separately on acquisition vs. reversal phases of training. Initial ANOVA involved the factors of treatment group, modality (tone+/light− vs. light+/tone−), and sex as between-subjects variables and stimulus (CS+ vs. CS−) and sessions (1–4 for acquisition; 1–8 for reversal) as within-subjects variables. There were generally no effects of sex, never any effects that altered conclusions concerning the effect of VPA treatment on conditioning, and so data were subsequently pooled across this factor in ANOVA. Effects of modality were also generally not statistically significant and are only reported when significant interactions of modality with treatment and stimulus were found. Therefore, ANOVA usually involved the factors of Treatment × Stimulus × Sessions, or when appropriate, Treatment × Modality × Stimulus × Sessions.
Results
Growth and Body weight
Prior to dosing on GD12, mean (+/− SE) body weights of Saline- and VPA-treated pregnant females were 268 (+/− 3.5) and 277 (+/− 9.2) g, respectively, and did not differ significantly (t < 1.0). Following dosing, body weights of Saline-treated females on GD13, 14, 15 and 19, respectively, were 281 (+/− 4.9), 291 (+/−6.1), 290 (+/−5.4), and 309.5 (+/−7.5) g; whereas the corresponding values for VPA females were 267 (+/− 9.7), 273.5 (+/− 9.6), 275.8 (+/− 9.3), and 302 (+/− 9.8) g. Although maternal body weights of the two groups never differed significantly across any of these days (all ps > 0.35), weight gain was interrupted for 1–2 days in VPA-treated females and VPA significantly reduced body weight as a percentage of saline-control on GD14 (−2.1%, p < .02) but not on any other day. When litters were culled on PND3, VPA- and SAL-treated offspring weighed 8.78 (+/− 0.357) and 8.17 (+/− 0.451) g, respectively, a nonsignificant difference (p > 0.31; averages are combined across sex because body weights of males and female did not differ significantly.) There was also no treatment effect on litter size, VPA, 7.8 (+/− 1.8) littermates vs. Saline, 11 (+/− 0) littermates (p > 0.14).
Sensory processing of CS and US
Measures of sensory processing and motor performance that are independent of learning were assessed [46]. SR maximum amplitude during acquisition, a measure of CS processing, and UR maximum amplitude during the first 10-trial acquisition block, a measure of US efficacy and motor performance, are shown in Table 1. There were no significant treatment effects in either of these measures (all Fs < 1). The absence of these treatment effects indicates that alterations in conditioning in this study were not secondary to primary sensory or motor effects of VPA exposure.
Table 1.
Mean (+/− SE) UR Maximum Amplitudes (UMA), Startle Maximum Amplitudes (SMA), and CR Onset (CL) and Maximum Latencies (CML) to CS+ during acquisition as a function of treatment group (SAL, Saline treated controls; VPA, 600 mg/kg VPA administered on GD12, see text for full explanation). There were no significant treatment effects in any measure.
| Group | UMA | SMA | CL | CML |
|---|---|---|---|---|
| SAL (n = 7) | 548 ± 83 | 11.5 ± 5.5 | 200 ± 14 | 333 ± 15 |
| VPA (n = 9) | 631 ± 78 | 13.5 ± 4.1 | 202 ± 11 | 313 ± 10 |
Acquisition
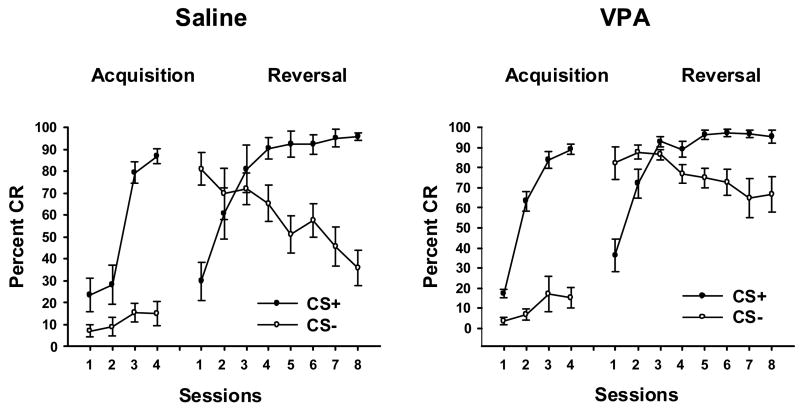
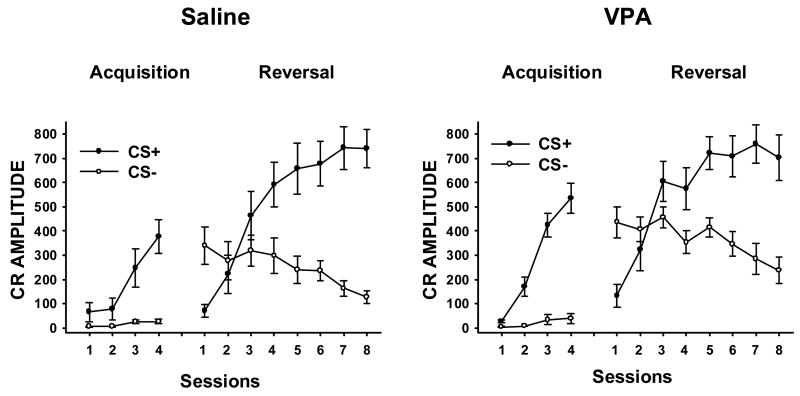
A 2 (Treatment: VPA vs. Saline) × 2 (Stimulus: CS+ vs. CS−) × 4 (sessions) ANOVA was performed on paired CS-US trials from the acquisition phase of the experiment. Separate ANOVAs were performed on the CR percentage (Figure 1) and maximum amplitude measures (Figure 2).
Figure 1.
Mean (± SE) eyeblink CR percentage in PND26-31 rats during acquisition and reversal phases of a tone-light discrimination, as a function of GD12 treatment group (Saline, vehicle control; VPA, 600 mg/kg valproic acid), stimulus (CS+ vs. CS−) and training sessions.
Figure 2.
Mean (± SE) eyeblink CR maximum amplitude (in arbitrary EMG units) in PND26-31 rats during acquisition and reversal phases of a tone-light discrimination, as a function of GD12 treatment group (Saline, vehicle control; VPA, 600 mg/kg valproic acid), stimulus (CS+ vs. CS−) and training sessions.
CR Percentage
VPA treatment on GD12 modestly facilitated acquisition of eyeblink conditioning on PND26-27 (Figure 1, left side of each panel). Percentage CRs to CS+ increased more rapidly in the VPA group whereas CRs to CS− remained low in both treatment groups. This was supported statistically by a significant interaction of Treatment × CS × Sessions [F(3, 42) = 6.72, p < .0009]. Newman-Keuls post-hoc analyses indicated that Group VPA showed increased levels of responding to CS+ relative to Group SAL only in Session 2 (p < .01). While both groups showed increases in %CR across sessions (p < .01), this increase was first significant in Session 2 for Group VPA (p < .01) in contrast to Session 3 for Group Saline (p < .01). There were no significant session- or treatment-related differences in responding to CS− during acquisition. Facilitated eyeblink conditioning in VPA rats is consistent with reports of human autism (see Discussion). That CRs were facilitated to CS+ but not CS− indicates that this is an associative effect and cannot be attributed to nonassociative factors.
CR Maximum Amplitude
Facilitation of eyeblink conditioning by G12 VPA treatment was evident more dramatically in the CR maximum amplitude measure (Figure 2, left of each panel). This was reflected in a statistically significant interaction of Treatment × CS × Sessions [F(3, 42) = 4.44, p < .009] which again reflected a more rapid increase across sessions in responding to CS+ in Group VPA, coupled with a lack of Treatment or Sessions effects in responding to CS−. Newman-Keuls post hoc tests confirmed differences between treatment groups in performance to CS+ over sessions 2–4 (p < .01), while performance to CS− never differed across groups. Performance to CS+ increased across sessions more quickly in Group VPA (Session 2, p < .01) than Group Saline (Session 3, p < .01) and was significantly elevated relative to CS− in both treatment groups during sessions 2–4 (p < .01). As with the CR percentage measure, this result resembles facilitated conditioning in autism and indicates an associative rather than nonassociative basis for the effect (see General Discussion).
CR Latency
CR onset and peak latency were measured on CS-alone test trials in which a CR occurred. CRs to CS− were too infrequent to permit latency analysis and so only CS+ trials were considered. A preliminary 2 × 4 × 2 (Treatment × Sessions × CR-measure) ANOVA on the acquisition data revealed highly significant main effects of Sessions, F(3, 36) = 13.82, p < .0001; and CR-measure, F(1, 12) = 256.94, p < .0001; but no significant main effects or interactions involving the treatment factor (all Fs < 1.37). This occurred because CR onset latency occurred earlier than peak latencies (Table 1) and both measures declined across sessions (from a mean of 221 ms and 356 ms for onset and peak latencies respectively in Session 1, to 171 msec and 273 ms, for these measures respectively, in Session 4. Two rats failed to show CRs to CS+ in Session 1 and so were excluded as cases with missing data in this preliminary ANOVA. Therefore data from these latency measures were averaged across all sessions so that treatment main effects could be reexamined with all animals included in the analysis. Separate ANOVAs on onset and maximum latency confirmed that there were no treatment effects (all Fs < 1, Table 1). The absence of CR latency effects in this study contrasts with the prematurely-timed CRs that have been reported during single-cue conditioning both in autism and in our VPA rodent model (see Discussion).
Reversal
During the reversal phase of training, a 2 (Treatment) × 2 (Stimulus) × 8 (sessions) ANOVA was performed the CR percentage measure. Because modality effects appeared in initial ANOVA, the CR amplitude measure was analyzed with a 2 (Treatment) × 2(Modality) × 2 (Stimulus) × 8 (Sessions) ANOVA (see Data Analysis above).
CR Percentage
For both treatment groups, CRs to the new CS+ (former CS−) increased and CRs to the new CS− (former CS+) declined across reversal sessions (Stimulus × Sessions interaction, F(7,98) = 78.47, p < 0.0001). However, relative to Group Saline, Group VPA again showed facilitated acquisition of CRs to CS+ and showed impaired extinction of CRs to CS− (Figure 2, right of each panel). This resulted in a significant interaction of Treatment × Stimulus × Sessions, F(7, 98) = 2.54, p < .02]. Newman-Keuls post-hoc tests indicated that CRs to CS+ increased across sessions (p < .01) in both groups but Group VPA showed higher percentages than Group Saline in Sessions 2 and 3 (p < .05), i.e., only during the transition between the start of training and asymptotic responding. Both groups also showed a significant decline in CRs to CS− across sessions (p < .01). However, this decline was slower in Group VPA, first becoming significant in Session 7 (p < .01) versus Session 2 in Group Saline (p < .05). This differential extinction of CRs to CS− produced significant treatment group differences on reversal Sessions 2–8 (all ps < .01 except for Session 4, p < .05). These findings suggest that GD12 VPA treatment alters both CR acquisition to CS+ and CR extinction to CS− during reversal of discriminative eyeblink conditioning. However the impairment of extinction to CS− could reflect a “carry over” effect of a stronger association established when this stimulus served as CS+ during acquisition.
CR Maximum Amplitude
During reversal, acquisition of CRs to CS+ was facilitated in Group VPA early in training and CR amplitudes to CS− were elevated in Group VPA across all sessions (Figure 2, right side of each panel). In these respects, the CR amplitude data resemble the CR percentage data. However, unlike the CR percentage data, the rate of decline in CR amplitude across sessions (extinction to CS−) was comparable in the VPA and Saline groups. As a result, the interaction of Treatment × CS was significant [F(1, 12) = 9.00, p < .012] but the Treatment × CS × Sessions interaction was not (F < 1), indicating that the decline in CRs to CS− across sessions occurred at a comparable rate in both the VPA and Saline groups. This contrasts with the differential rate of decline across groups in the %CR measure (Figure 1). Newman-Keuls tests of the Treatment × CS interaction revealed large CR amplitude differences between CS+ versus CS− in both groups (p < .01). There were no group differences in CRs to CS+ but Group VPA responded at a higher level to CS− than Group Saline (p < .01). A marginally significant interaction of Treatment × Modality × Stimulus, F(1, 12) = 3.39, p < .091, suggested a trend for the VPA-induced elevation of CR amplitude to CS− during extinction to be greater when this cue was a light than when CS− was a tone.
In summary, results from both the CR percentage and amplitude measures indicate that GD12 exposure to VPA results in enhanced eyeblink conditioned responding during both the acquisition and reversal phases of this discrimination learning task.
Discussion
The present findings indicate that GD12 exposure to VPA alters both acquisition and reversal of discriminative eyeblink conditioning in PND26-31 rats. In the CR percentage measure, acquisition of CRs to CS+ was facilitated in VPA rats during both the acquisition and reversal phases of the experiment. Facilitation was modest but statistically significant in this CR measure, appearing when CRs were increasing but not at the outset or the end (asymptote) of training. In the CR amplitude measure, CRs to CS+ were much larger in VPA rats even at asymptote during the acquisition phase and there was a statistically non-significant trend toward larger CRs to CS+ in the reversal phase. During acquisition, CRs to CS− remained low and did not differ across treatment groups in both the CR percentage and amplitude measures. This confirms the associative nature of CR acquisition in both treatment groups, and more importantly, indicates that nonassociative factors did not cause facilitation of eyeblink CR performance in the VPA group. If this were the case, CRs would have been elevated to both CS+ and CS− in the VPA group rather than only to CS+. It is also important to note that the absence of group effects in SR and UR measures indicates that differences across treatment groups in CS or US efficacy or in motor performance do not account for facilitated acquisition of CRs shown by VPA rats in this study.
During reversal, extinction of CRs to CS− was impaired in VPA rats, which showed a slower decline in CRs, and a progressively larger elevation in CR percentage over Saline controls, across reversal sessions. However, in the CR amplitude measure, responses to CS− were substantially elevated in VPA rats but declined across sessions at the same rate, relative to Saline-control rats. Given the larger group difference in CR amplitude to CS+ at the end of acquisition, it is most parsimonious to interpret the group difference in extinction of this stimulus (new CS−) during reversal as a “carry-over” effect from acquisition of enhanced CR amplitude to (old) CS+. Such an enhancement could also explain VPA effects in the CR percentage measure because larger CRs to CS− during reversal in VPA rats would tend to cross CR-threshold more frequently and lead to a larger number and slower decline in CR percentage in this group. The role of group differences in acquisition on reversal performance could be addressed in studies that varied the amount of acquisition training and compared VPA and saline groups that were “matched” for acquisition performance. At present, the data from this study provide no conclusive evidence that VPA treatment impairs the rate of extinction of responding to CS− during reversal of discriminative eyeblink conditioning.
The present study reveals a number of parallels between acquisition of eyeblink conditioning in VPA-treated rats and human autism [2, 40]. First, primary sensory processing of CS and US and motor performance (unconditioned eyeblink reflex) are not altered in autism or in the present VPA rodent model. Second, facilitated conditioning appears in both the CR percentage and amplitude measures but the most striking effect is the abnormally large CR amplitude, both in autism [40] and in the VPA rodent model of autism used in the present study. One parallel that is absent in this study is the finding of normal CR timing in VPA rats (Table 1). Studies of eyeblink conditioning in autism have observed prematurely timed CRs [2, 40]. These studies employed single-cue eyeblink conditioning procedures whereas the present study employed discriminative conditioning. We have also observed prematurely timed CRs, together with enhanced CR amplitude, in VPA-treated rats during single-cue eyeblink conditioning [45]. Why CR timing effects are different in single-cue vs. discriminative conditioning is an important question for further study. As noted previously, discriminative conditioning involves a within-subjects nonassociative control. Studies of eyeblink conditioning in autistic individuals have thus far not employed a nonassociative control condition (e.g., involving unpaired presentations of US and CS). The present findings in the VPA rodent model would predict that CR performance of autistic and typically-developing individuals would not differ in such a control group or condition. Associative effects on CR performance point to the involvement of the cerebellum, since this structure is necessary and sufficient for generating eyeblink CRs in all human and animal studies reported to date [54, 55]. In contrast, nonassociative and (sensory, motor, motivational) “performance effects” in eyeblink conditioning generally do not involve the cerebellum. Cerebellar hypoplasia is common in autism [cf. 3] and has been reported in the VPA rodent model [20]. It is paradoxical to observe facilitation of eyeblink conditioning following cerebellar injury. Impaired conditioning has been observed in all studies of cerebellar injury sustained in adulthood and in some cases following developmental injury [54,55]. The developmental rodent model of eyeblink conditioning used in this study has revealed dramatic acquisition impairments in rats showing cerebellar hypoplasia produced by developmental exposure to alcohol [15, 16] or to the antiproliferative agent, methazoxymethanol [12, 48]. Indeed, developmental alcohol exposure dramatically impairs discriminative eyeblink conditioning in rats under the identical training conditions used in this study [5]. Similarly, human eyeblink conditioning is impaired in fetal alcohol syndrome [24] and in prematurely-born, very-low-birth-weight infants [19], developmental conditions associated with cerebellar hypoplasia in human brain imaging studies [1, 44]. So, why is eyeblink conditioning facilitated in autism and in our VPA rodent model but impaired in these other cases? The timing of brain injury is likely to be the most important factor. In all of the other developmental cases just described, the injury occurred either during the third-trimester of human pregnancy, or in the period equivalent to the human third-trimester in rodent studies. Brainstem-cerebellar injury during the first-trimester may be followed by compensatory changes in development of these areas [38], and indeed in the remainder of the brain [39], that are not possible, or are functionally different, when the injury occurs during the third trimester. The early timing of brain injury may be important also in another rodent model---the protein kinase C gamma isoform (PKCg) knockout mouse. This mouse also shows facilitated eyeblink conditioning that is associated with aberrant cerebellar development [7]. Interestingly, there is an association between alterations in genes encoding PKCg and autism [34]. Future studies in which both eyeblink conditioning and anatomical/imaging measures of brainstem-cerebellar injury are examined in the same autistic individuals or the same VPA-exposed rats are needed to confirm and strengthen the apparent association between cerebellar targeting and altered conditioning. Using such an approach in the VPA rodent model, it would be possible to further test specific hypotheses concerning how changes in brain development subsequent to early gestational brainstem injury results in facilitated eyeblink conditioning.
As noted previously, enhanced CR acquisition in VPA rats rendered the reversal findings inconclusive with respect to potential functional targeting by VPA of hippocampal or prefrontal regions. Such targeting would cause slower extinction of CRs to the acquisition CS+ when it became the nonreinforced CS− during reversal [4, 6, 53). Our conservative conclusion is that rate of extinction during eyeblink discrimination reversal is not altered in the G12-VPA-exposed rodent model of autism. The literature on human autism provides mixed behavioral evidence for alterations of executive function in general, and discrimination reversal in particular [9, 11, 17, 31]. The question of whether the VPA rodent model “captures” forebrain targeting in autism has not been studied extensively, although preliminary reports are intriguing [14]. This may be a fruitful area for further interdisciplinary research that integrates brain and behavioral data in autism and in various rodent models of this disorder.
The present findings provide behavioral evidence in support of the hypothesis that the rat exposed to VPA on GD12 is a useful rodent model of autism [3, 37, 39]. This hypothesis is unique in predicting facilitated eyeblink conditioning as an autism-like behavioral phenotype in this rodent model. As noted above, cerebellar hypoplasia in the VPA rodent model [20] together with the outcome of other studies of eyeblink conditioning following impaired cerebellar development [48, 49] would lead to just the opposite prediction---impaired conditioning. Indeed, the prediction in the first study of conditioning in autism was that CRs would be impaired and the investigators were very surprised to find that CRs were enhanced [40]. The likely role of the timing of brain injury in this effect supports the hypothesis that brainstem injury very early in gestation is the cause of at least one form of autism [39]. Eyeblink conditioning has been studied in many human brain disorders, such as temporal lobe amnesia, Alzheimer’s Disease, schizophrenia, mental retardation, and Downs Syndrome, to name only a few, but only in autism has facilitation of conditioning been observed (see review of Arndt et al [3]). This behavioral phenotype and the findings from the VPA rodent model make many other testable predictions concerning the hypothesis of early brainstem injury in autism [3]. For example, eyeblink conditioning may be especially enhanced in autistic individuals with craniofacial features indicative of early gestational injury, or in cases of autism associated with human exposure to VPA [28] or thalidomide [27]. Further studies of eyeblink conditioning in autism and in teratological or genetic rodent models should advance progress in identifying the fundamental causes of this disorder.
Acknowledgments
This research was supported in part by the University of Delaware, and by NIH grant 1-PO1-HD35466. The authors thank Jerome Pagani for technical assistance. Address correspondence to: Mark Stanton, Department of Psychology, University of Delaware, Newark, DE 19716, stanton@psych.udel.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allin M, Matsumoto H, Santhouse AM, Nosarti C, AlAsady MHS, Stewart AL, Rifkin L, Murray RM. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 2001;124:60–66. doi: 10.1093/brain/124.1.60. [DOI] [PubMed] [Google Scholar]
- 2.Arndt TL, Chadman KC, Watson DJ, Tsang V, Peloso ED, Rodier PM, Stanton ME. Devel Psychobiol. 2006. Eyeblink Conditioning in Autism and a Rodent Model, II: Long-Delay Eyeblink Conditioning in Autism. submitted manuscript. [Google Scholar]
- 3.Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23:189–99. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Berger TW, Orr WB. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behav Brain Res. 1983;8:49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- 5.Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal Alcohol Exposure Impairs Acquisition of Eyeblink Conditioned Responses During Discrimination Learning and Reversal in Weanling Rats. Devel Psychobiol. 2006 doi: 10.1002/dev.20178. in press. [DOI] [PubMed] [Google Scholar]
- 6.Chachich M, Powell DA. Both medial prefrontal and amygdala central nucleus lesions abolish heart rate classical conditioning, but only prefrontal lesions impair reversal of eyeblink differential conditioning. Neurosci Lett. 1998;257:151–4. doi: 10.1016/s0304-3940(98)00832-5. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim JJ, Hashimoto K, Thompson RF, Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKC gamma mutant mice. Cell. 1995;83:1233–42. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 8.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: Acquisition and retention. Learn Mem. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 9.Coldren JT, Halloran C. Spatial reversal as a measure of executive functioning in children with autism. J Genet Psychol. 2003;164:29–41. doi: 10.1080/00221320309597501. [DOI] [PubMed] [Google Scholar]
- 10.Conciatori M, Stodgell CJ, Hyman SL, O’Bara M, Militerni R, Bravaccio C, Trillo S, Montecchi F, Schneider C, Melmed R, Elia M, Crawford L, Spence SJ, Muscarella L, Guarnieri V, D’Agruma L, Quattrone A, Zelante L, Rabinowitz D, Pascucci T, Puglisi-Allegra S, Reichelt KL, Rodier PM, Persico AM. Association between the HOXA1 A218G polymorphism and increased head circumference in patients with autism. Biol Psychiatry. 2004;55:413–419. doi: 10.1016/j.biopsych.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- 12.Freeman JH, Jr, Barone S, Jr, Stanton ME. Disruption of cerebellar maturation by an antimitotic agent impairs the ontogeny of eyeblink conditioning in rats. J Neurosci. 1995;15:7301–7314. doi: 10.1523/JNEUROSCI.15-11-07301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman JH, Nicholson DA. Developmental changes in the neural mechanisms of eyeblink conditioning. Behav Cogn Neurosci Rev. 2004;3:3–13. doi: 10.1177/1534582304265865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia S, Mattson BJ, Rinaldi T, Buhl DL, Markram H. Quantification of neocortical neuronal subpopulations in an animal model of autism. Soc Neurosci Abstr. 2005:448.4. [Google Scholar]
- 15.Goodlett CR, Stanton ME, Steinmetz JE. Alcohol-induced damage to the developing brain: Functional approaches using classical eyeblink conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning, Volume 2: Animal models. Amsterdam: Kluwer Academic Publishers; 2000. pp. 135–153. [Google Scholar]
- 16.Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. Cerebellum. 2004;3:178–187. doi: 10.1080/14734220410017338. [DOI] [PubMed] [Google Scholar]
- 17.Griffith EM, Pennington BF, Wehner EA, Rogers SJ. Executive functions in young children with autism. Child Dev. 1999;70:817–32. doi: 10.1111/1467-8624.00059. [DOI] [PubMed] [Google Scholar]
- 18.Herbert JH, Eckerman CO, Stanton ME. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behav Neurosci. 2003;117:1196–1210. doi: 10.1037/0735-7044.117.6.1196. [DOI] [PubMed] [Google Scholar]
- 19.Herbert JH, Eckerman CO, Goldstein RF, Stanton ME. Contrasts in Infant Classical Eyeblink Conditioning as a Function of Premature Birth. Infancy. 2004;5:367–383. [Google Scholar]
- 20.Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol. 2000;22:319–24. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 21.Ivkovich D, Eckerman CO, Krasnegor NA, Stanton ME. Using eyeblink conditioning to assess neurocognitive development. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning, Volume I: Applications in Humans. Amsterdam: Kluwer Academic Publishers; 2000. pp. 119–142. [Google Scholar]
- 22.Ivkovich D, Paczkowski CM, Stanton ME. Ontogeny of trace versus delay eyeblink conditioning in developing rats. Devel Psychobiol. 2000;36:148–160. doi: 10.1002/(sici)1098-2302(200003)36:2<148::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Ivkovich D, Stanton ME. Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiol Learn Mem. 2001;76:426–446. doi: 10.1006/nlme.2001.4027. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson SW, Stanton ME, Hay AM, Burden JJ, Fuller DS, Croxford JA, Molteno CD, Viljoen DL, Jacobson JL. Impaired short delay eyeblink conditioning in children with FAS: Preliminary findings. ACER. 2005;29(Suppl):45A. [Google Scholar]
- 25.Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends in Neuroscience. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 26.McCormick DA, Thompson RF. Cerebellum: Essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 27.Miller MT, Stromland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis. Int J Dev Neurosci. 2005;23:201–219. doi: 10.1016/j.ijdevneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, Dean JCS. A clinical study of 57 children with fetal anticonvulsant syndrome. J Med Genet. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eyeblink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- 30.Ohlrich ES, Ross LE. Acquisition and differential conditioning of the eyelid response in normal and retarded children. J Exp Child Psychol. 1968;6:181–93. doi: 10.1016/0022-0965(68)90083-0. [DOI] [PubMed] [Google Scholar]
- 31.Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, McMahon WM, Minshew N, Munson JA, Pennington BF, Rogers SJ, Spence MA, Tager-Flusberg H, Volkmar FR, Wrathall D. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism network. J Autism Dev Disord. 2004;34:139–50. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- 32.Paczkowski CM, Ivkovich D, Stanton ME. Ontogeny of eyeblink conditioning using a visual conditional stimulus. Devel Psychobiol. 1999;35:253–263. doi: 10.1002/(sici)1098-2302(199912)35:4<253::aid-dev1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Papka M, Simon EW, Woodruff-Pak DS. A one year longitudinal investigation of eyeblink classical conditioning and cognitive and behavioral tests in adults with Down’s Syndrome. Aging Cogn. 1994;1:89–104. [Google Scholar]
- 34.Philippi A, Roschmann E, Tores F, Lindenbaum P, Benajou A, Germain-Leclerc L, Marcaillou C, Fontaine K, Vanpeene M, Roy S, Maillard S, Decaulne V, Saraiva JP, Brooks P, Rousseau F, Hager J. Haplotypes in the gene encoding protein kinase c-beta (PRKCB1) on chromosome 16 are associated with autism. Mol Psychiatry. 2005;10:950–60. doi: 10.1038/sj.mp.4001704. [DOI] [PubMed] [Google Scholar]
- 35.Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47:551–5. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- 36.Rodier PM, Bryson SE, Welch JP. Minor physical anomalies and physical measurements in autism: Data from Nova Scotia. Teratol. 1997;55:319– 325. doi: 10.1002/(SICI)1096-9926(199705)55:5<319::AID-TERA4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 37.Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370:247–61. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Rodier PM, Ingram JL, Tisdale B. Morphology and function in an animal model of autism. Neurotoxicol Teratol. 2001;23:284. [Google Scholar]
- 39.Rodier PM. Converging evidence for brain stem injury in autism. Devel Psychopath. 2002;14:537–557. doi: 10.1017/s0954579402003085. [DOI] [PubMed] [Google Scholar]
- 40.Sears LL, Finn PR, Steinmetz JE. Abnormal classical eyeblink conditioning in autism. J Autism Dev Disord. 1994;24:737–51. doi: 10.1007/BF02172283. [DOI] [PubMed] [Google Scholar]
- 41.Sears LL, Steinmetz JE. Classical eyeblink conditioning in normal and autistic children. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning, Volume I: Applications in Humans. Amsterdam: Kluwer Academic Publishers; 2000. pp. 143–162. [Google Scholar]
- 42.Skelton RW. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behav Neurosci. 1988;102:586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- 43.Solomon PR, Solomon SD, Schaaf EV, Perry HE. Altered activity in the hippocampus is more detrimental to classical conditioning than removing the structure. Science. 1983;220:329–31. doi: 10.1126/science.6836277. [DOI] [PubMed] [Google Scholar]
- 44.Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: Size reduction in Lobules I-V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 45.Stanton ME, Chadman KK, Watson DJ, Rodier PM. Eyeblink conditioning in autism and a rodent model, I: Altered eyeblink conditioning in weanling and adult rats following gestational exposure to valproic acid. Devel Psychobiol. 2006 submitted manuscript. [Google Scholar]
- 46.Stanton ME, Freeman JH, Skelton RW. Eyeblink conditioning in the developing rat. Behav Neurosci. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- 47.Stanton ME, Freeman JH. Developmental studies of eyeblink conditioning in the rat. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning, Volume 2: Animal models. Amsterdam: Kluwer Academic Publishers; 2000. pp. 17–49. [Google Scholar]
- 48.Stanton ME, Freeman JH. Eyeblink conditioning in the developing rat: An animal model of learning in developmental neurotoxicology. Environ Health Perspect. 1994;102:131–139. doi: 10.1289/ehp.94102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exper Res. 1998;22:270–275. [PubMed] [Google Scholar]
- 50.Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- 51.Steinmetz JE. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- 52.Tischfield MA, Bosley TM, Salih MA, Alorainy IA, Sener EC, Nester MJ, Oystreck DT, Chan WM, Andrews C, Erickson RP, Engle EC. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat Genet. 2005;37:1035–1037. doi: 10.1038/ng1636. [DOI] [PubMed] [Google Scholar]
- 53.Weikart CL, Berger TW. Hippocampal lesions disrupt classical conditioning of cross-modality reversal learning of the rabbit nictitating membrane response. Behav Brain Res. 1985;22:85–89. doi: 10.1016/0166-4328(86)90083-5. [DOI] [PubMed] [Google Scholar]
- 54.Woodruff-Pak DS, Steinmetz JE. Eyeblink Classical Conditioning, Volume 1: Applications in Humans. Boston, MA: Kluwer Academic Publishers; 2000a. p. xxx. [Google Scholar]
- 55.Woodruff-Pak DS, Steinmetz JE. Eyeblink Classical Conditioning, Volume 2: Animal Models. Boston, MA: Kluwer Academic Publishers; 2000b. p. xxx. [Google Scholar]