Abstract
Previous studies have shown that alcohol (EtOH) intoxication impairs lung immunity by affecting cytokines pivotal to the inflammatory process. The objective of this study was to test the hypothesis that acute alcohol intoxication impairs lung innate immunity by down-regulating the expression of pro-inflammatory mediators while simultaneously up-regulating anti-inflammatory mediators. EtOH was administered to the mice 0.5 h prior to an intra-tracheal injection of E. coli lipopolysaccharide (LPS). The animals were killed either 4 or 24 h after LPS to recover plasma, lungs and bronchoalveolar lavage fluid. Lung inflammatory cytokines TNF-α, IL-1β, IL-6, MIF, IL-10, TGF-β and receptors for TNF-α, IL-1β, IL-6 and TGF-β as well as gp130 and corticosterone levels were evaluated at mRNA and protein level. While the mRNA expression and the soluble TNF-Rp55 levels were significantly up-regulated by EtOH, LPS-induced TNF-α activity, TNF-Rp55 mRNA expression and soluble TNF-Rp55 levels were significantly suppressed. The LPS-induced expression of IL-1β, IL-6, MIF, gp130, and receptors IL-1RI, IL-1RII and IL-6Rα were also significantly impaired by EtOH. EtOH increased significantly basal IL-10 activity at 3 h, which continued to remain elevated even at 24 h. The EtOH effect on IL-10 activity persisted even in LPS-challenged mice. EtOH and LPS augmented lung corticosterone levels independently of each other. EtOH suppressed up-regulation of TGF-β1 mRNA expression by LPS and blocked completely LPS-induced TGF-β1 secretion. In conclusion, the data suggest that the suppression of acute lung inflammation by EtOH intoxication is largely due to impairment by EtOH of pro-inflammatory cytokine signaling at the levels of cytokine expression and secretion as well as receptor expression and soluble receptor activity. The augmentation by EtOH of anti-inflammatory mediators' secretion most likely shifts the cytokine balance in the anti-inflammatory direction.
Keywords: Lung, pro- and anti-inflammatory cytokines, cytokine receptors, acute alcohol intoxication, inflammatory response, corticosterone
Introduction
Exposure of the lungs to an acute immune challenge evokes a local innate immune response. The response is characterized by an up-regulation in multiple inflammatory mediators' (e.g., cytokines, chemokines and adhesion molecules) expression, many of which display redundant biological functions (Szarka et al., 1997; Starcher and Williams, 1989). Activation of the innate immune system also induces the translocation and activation of transcription factors (e.g., NF-κB and AP-1), which regulate the expression of the inflammatory genes [Vanden Berghe et al., 2006; Bozinoyski et al., 2002]. The gender and the nutritional status of the host are some other factors that can influence the innate immune response (Imahara et al., 2005; Kishino and Moriguchi, 1992; Watzl et al., 1993).
E. coli lipopolysaccharide (LPS) administration into rodent lungs induces the expression of inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), macrophage inflammatory proteins (MIPs) and the intracellular adhesion molecule (ICAM-1) to signal directional migration of polymorphonuclear leukocytes (PMNs) to the insult site (Mizgerd, 2002; Nelson and Summer, 1998). The macrophage inhibitory factor (MIF) is another pivotal cytokine that is up-regulated in response to E. coli LPS (Calandra, 2003). By acting as a physiologic counter-regulator of glucocorticoid action, it can regulate the magnitude of the inflammatory response (Calandra et al., 1995; Donnelly et al., 1997). While the recruited PMNs aid the resident alveolar macrophages (AMs) in responding efficiently to the immune challenge (Cox et al., 1995; Ishii et al., 1998), the local endogenous anti-inflammatory mediators (e.g. IL-10, IL-13, lipoxins and glucocorticoids) help in containing and resolving the inflammation (Chapman et al., 2006; Fan et al., 2001; Serhan, 2004; Scannell and Maderna, 2006).
The susceptibility of excessive EtOH drinkers to pulmonary infections is ascribed primarily to the adverse effects of EtOH on the lung immunity (Bomalaski and Phair, 1982; Cook, 1998; Jerrells et al., 1994; MacGregor and Louria, 1997; Nelson and Summer, 1998; Szabo, 1999). Ethanol, by affecting the expression of the inflammatory mediators, can alter the normal course of an inflammatory response (Mandrekar et al., 2006; Zhang et al., 2002). Acute and chronic EtOH consumption affects the ability of the lungs not only to generate and secrete some of the mediators evoked during local inflammation (D'Souza et al., 1996; Nelson et al., 1998; Standiford and Danforth, 1997; Zhang et al., 1999; Zhang et al., 2002), but also to express their receptors (D'Souza et al., 1994). Cofactors such as nutritional deficiencies, aging, smoking and use of other recreational drugs can augment the adverse effects of EtOH (Waltz et al., 1993; Husain et al., 2001).
Previously published animal studies suggest that the extent of EtOH-induced immune changes appear to depend on the amount of EtOH consumed, the duration for which it is consumed, the route of administration, and the strain and gender of animal species used (D'Souza et al., 1989; Sarphie et al., 1997; Spitzer, 1999; Thurman et al., 2001). In addition, the effects of acute and chronic EtOH intake on the immune system are mostly different from each other; the acute intake usually down-regulates the inflammatory response while the chronic intake augments it (Thakur et al., 2006; Valles et al., 2003). In vivo and in vitro studies performed mostly in rats using a single point in time for evaluation show that acute EtOH exposure can impair the production of pro-inflammatory cytokines TNF-α, MIP-2, CINC and the recruitment of PMNs in response to an immune challenge (Boe et al., 2003; D'Souza et al., 1989; Nelson et al., 1989; Zhang et al., 2002). However, the precise mechanisms by which acute EtOH intoxication impairs lung innate immunity are still not completely clear.
To further understand the mechanisms by which acute ETOH intoxication impairs lung innate immunity, we evaluated the effects of acute EtOH intoxication on several pro- and anti-inflammatory mediators and their receptors during the early and late phases of E. coli LPS-induced lung inflammation. Two points in time, 3 and 24 h post LPS instillation, were selected for evaluation. These were based on a time course study we performed to determine the time required for initiation and resolution of LPS-induced lung inflammation. Our central hypothesis is that acute EtOH intoxication impairs lung innate immunity against E.coli LPS by down-regulating the expression of multiple inflammatory mediators and their receptors while simultaneously up-regulating anti-inflammatory mediators, and these effects may, at least, be partially mediated by EtOH-induced glucocorticoid release.
Materials and Methods
Mice and ethanol administration
Eight-week old, specific pathogen-free male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in sterile filter-top cages in the Veterans Administration Medical Center (VAMC) animal facility with a 12 h light-dark cycle and constant temperature and humidity. Animal housing and all experimental procedures were performed in compliance with the guidelines approved by the IACUC of the VAMC (Lexington, Kentucky). The mice had ad libitum access to standard Purina chow and water and were used for the experiments one week after their arrival at the animal facility. On the day of the experiment, the mice were weighed and divided into two groups (EtOH and Control). Mice in the EtOH group received an intra-peritoneal (IP) injection of 4.4 g EtOH·kg−1 body weight, 0.5 h before an intra-tracheal (IT) injection of LPS or saline. Mice in the Control group received an IP injection of 400 μL saline, 0.5 h before IT saline or LPS injection.
E.coli LPS inoculation
Lipopolysaccharide (E. coli 026:B6), obtained from Difco Laboratories (Detroit, MI), was used as the local immune challenge. The stock LPS (1mg·ml−1) was prepared freshly in LPS-free saline and administered IT into the lungs at a concentration of 1 mg·kg−1 body weight under avertin (tribromoethyl alcohol + amyl alcohol) anesthesia. After recovery from anesthesia, the mice were returned to their cages and allowed free access to food and water until the time of euthanasia. No mortality was observed with either the dose of EtOH or LPS used over the experimental period. Three or 24 h after the IT injection, the mice were anesthetized with Na pentobarbital (NembutalRR, 60 mg·kg−1 body weight) and blood was collected from the inferior vena cava. The mice were then exsanguinated by severing the inferior vena cava; the lungs perfused with saline and snap frozen immediately in liquid nitrogen for RNA and protein extraction. In another set of experiments, the bronchoalveolar lavage (BAL) was performed with a fixed volume of EDTA containing PBS, and the BAL fluid recovered was assayed for secreted cytokines, soluble cytokine receptors and corticosterone (CS), the predominant glucocorticoid produced in mice.
Quantitation of mRNA expression for cytokines and cytokine receptors
The total lung RNA was extracted using TriZOL reagent obtained from Life Technologies (Gaithersburg, MD) according to the manufacturer's instructions. The mRNA expression for cytokines and cytokine receptors was evaluated using RiboQuant multiprobe assay system and instructions from BD Pharmingen (San Diego, CA). The gels were scanned using PhosphoImager (Molecular Dynamics, Sunnyvale, CA), and the images obtained quantified using ImageQuant program (Molecular Dynamics, Sunnyvale, CA). The data obtained were normalized to housekeeping gene (GAPDH) and the integrated optical density (IOD) expressed as ratios.
Quantitation of cytokine and cytokine receptor proteins using Western Blot Analysis
The lung protein extraction and gel electrophoresis were performed according to the instructions from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The extracted proteins were run on 4 - 20% tris-HCl gels (Biorad Laboratories, Hercules, CA). For a given run, all lanes were loaded with an identical amount of protein. The separated proteins were transferred to Trans-blot transfer membranes (Bio-Rad Laboratories Hercules, CA), and immunoblotted according to instructions from Santa Cruz Biotechnology (Santa Cruz, CA), the manufacturer of the antibodies. The immunocomplexes formed were detected by ECL Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ), and the intensity of the light produced by the enhanced chemiluminescent reaction was detected by exposure of the membrane to Kodak blue light-sensitive film. The bands obtained were quantified using Scion Image Beta 4.0.2 program (Scion Corporation, Frederick, MD). The integrated optical density is presented as arbitrary units (AU) after deducting the background.
Quantitation of cytokines and cytokine receptors in BAL fluid
The cell-free supernatant from the first 1 ml of BAL fluid collected was used for measuring secreted cytokines and soluble cytokine receptors using ELISA kits (R&D Systems, Inc., Minneapolis, MN and Biosource International, Carmarillo, CA). The TGF-β kit used measured the total (latent and active) TGF-β secretion. All samples were assayed in duplicates, in accordance with the manufacturers' instructions and the activity expressed as ml−1 of BAL fluid.
Statistical Analysis
The data were analyzed using one-way ANOVA followed by Newman-Keuls post test when comparing the four groups within each time point, and by Student t-test when comparing identical treatment groups between the two time points. Data are presented as mean ± SEM, with significance set at P ≤ 0.05.
Results
The blood EtOH concentration at the 3 h time point was 261 ± 15 mg/dl in EtOH/Sal group and 235 ± 13 mg/dl in EtOH/LPS. No EtOH was detected in the blood at the 24 h time point.
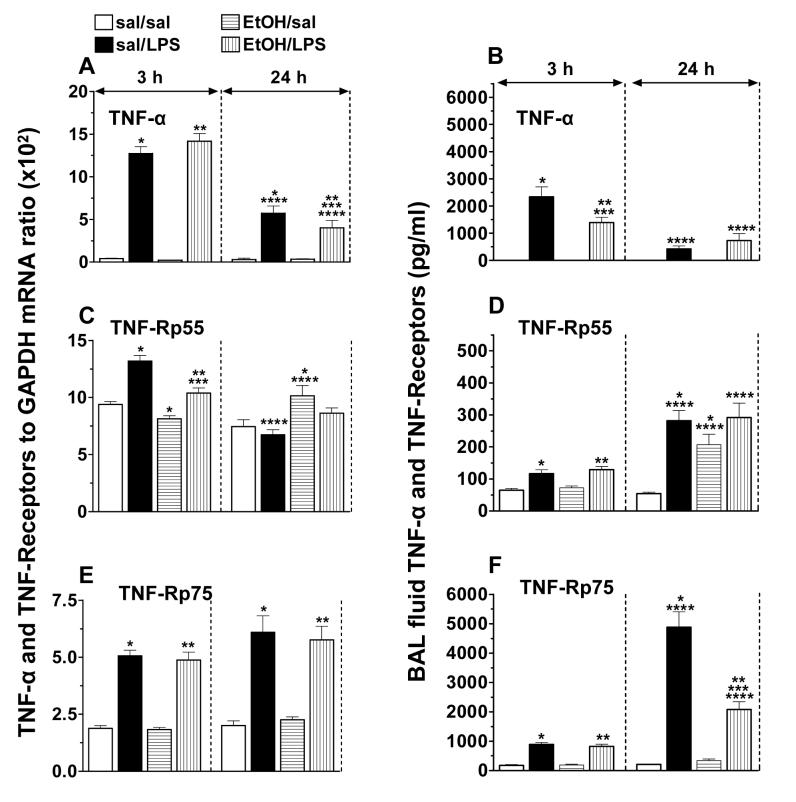
Lung TNF-α, TNF-Rp55 and TNF-Rp75 mRNA levels, and BAL fluid TNF-α and soluble TNF receptors
To understand further the mechanisms by which acute EtOH intoxication impairs TNF-α mediated lung innate immunity, the expression of lung TNF-α and of its receptors was evaluated during LPS-induced lung inflammation under conditions of acute EtOH intoxication.
Although EtOH did not affect the constitutive or LPS-induced peak TNF-α mRNA expression, it accelerated significantly (80% vs. 50%) the down-regulation of LPS-induced TNFα mRNA levels expression at 24 h (Fig. 1A). Consistent with published data [Nelson et al., 1989; Kolls et al., 1995], LPS-induced peak TNF-α secretion was blunted (40%) by acute EtOH intoxication. Low levels of LPS-induced TNF- α were still detectable at 24 h (Fig. 1B), but did not differ between saline and EtOH-treated mice.
Fig. 1.
Mouse lung TNF-α (A), TNF-Rp55 (C) and TNF-Rp75 (E) mRNA levels and BAL fluid TNF-α (B), TNF-Rp55 (D) and TNF-Rp75 (F) activities following alcohol and lipopolysaccharide treatment. Plotted are means ± SEM for 6 - 8 mice/group. Abbreviations: saline (sal); alcohol (EtOH); lipopolysaccharide (LPS). *) p < 0.05 vs. sal/sal; **) p < 0.05 vs. EtOH/sal; ***) p < 0.05 vs. sal/LPS; ****) p < 0.05 vs. similarly treated 3 h group.
Ethanol induced a small (13%) decrease in basal TNF-Rp55 mRNA levels at 3 h and suppressed (21%) the LPS-induced up-regulation. At 24 h, EtOH induced a modest (36%) up-regulation in the basal TNF-Rp55 mRNA levels (Fig. 1C). The basal levels of soluble TNFRp55 were not affected by EtOH at 3 h, but at 24 h, EtOH increased the levels 3.8-fold and masked the LPS effect. LPS increased soluble TNFRp55 activity 1.8-fold at 3 h and 5-fold at 24 h (Fig. 1D).
LPS up-regulated TNF-Rp75 mRNA levels (2.7-fold) that remained elevated even at 24 h. Neither basal nor LPS-induced TNF-Rp75 mRNA levels were affected by EtOH (Fig. 1E). LPS induced significant shedding of TNF-Rp75 as early as 3 h. However, a massive (23-fold) shedding of TNF-Rp75 was observed 24 h post LPS challenge and this was markedly (74%) suppressed by EtOH (Fig. 1F).
The data confirm previous findings that the effects of acute EtOH intoxication on lung TNF-α in the early phase of LPS-induced lung inflammation are post-transcriptional [Kolls et al., 1995]. The data suggest further that acute EtOH intoxication modulates TNF-α signaling not only by impairing cytokine production, but also by affecting the expression and shedding of its cell-surface receptors.
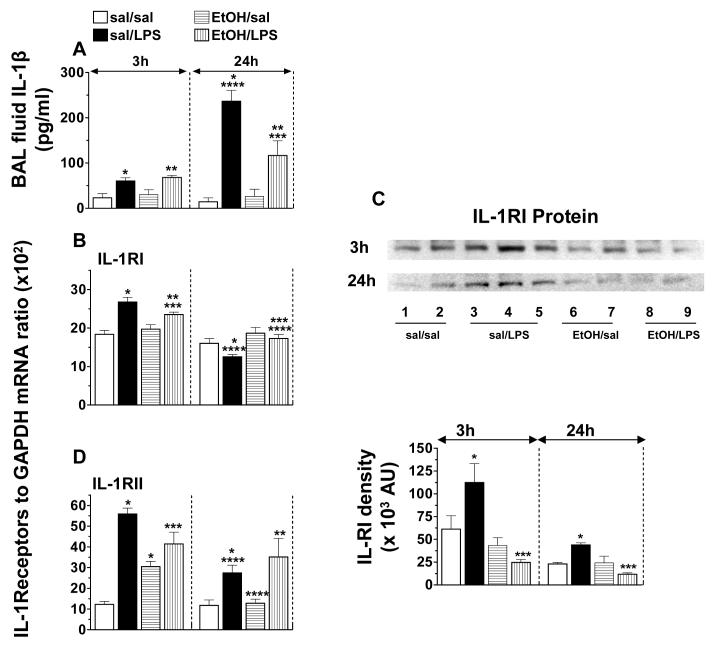
BAL fluid IL-1β activity and lung IL-1RI and IL-1RII mRNA and protein levels
The overlapping patterns of gene expression and common inflammatory responses of IL-1β and TNF-α prompted us to investigate if the effects of acute EtOH intoxication on IL-1β signaling are similar to those on TNF-α.
A small significant increase in BAL fluid IL-1β activity was observed in response to LPS at 3 h; however, the increase was 10-fold higher at 24 h. The peak LPS-induced IL-1β secretion was suppressed (50%) by acute EtOH intoxication (Fig. 2A).
Fig. 2.
Mouse BAL fluid IL-1β (A) activity, lung Il-1RI (B) and IL-1RII (D) mRNA levels, and IL-1RI (C) protein expression. Plotted are means ± SEM for 6 - 8 mice/group. Abbreviations: saline (sal); alcohol (EtOH); lipopolysaccharide (LPS); arbitary units (AU). *) p < 0.05 vs. sal/sal; **) p < 0.05 vs. EtOH/sal; ***) p < 0.05 vs. sal/LPS; ****) p < 0.05 vs. similarly treated 3 h group.
LPS-induced an up-regulation (45%) in IL-1RI mRNA levels in the early phase of the inflammatory response. Although the basal IL-1RI mRNA levels were unaffected by EtOH, the LPS effect on the receptor was significantly impaired (Fig.2B). Western blot analysis of whole lung proteins show that, consistent with the mRNA data, LPS induced an up-regulation in IL-RI protein expression, and this up-regulation was suppressed by EtOH (Fig.2C).
A 3-fold increase in IL-RII mRNA levels was observed 3 h post LPS challenge. EtOH increased IL-RII mRNA levels and suppressed significantly the LPS effect. At 24 h, LPS-induced IL-1RII mRNA levels showed a trend to decline to baseline (Fig.2D).
The data suggest that acute EtOH intoxication may impair IL-1β signaling not only by affecting its production, but also by affecting IL-1RI expression. Considering the overlapping properties of TNF-α and IL-1β, up-regulation by LPS of IL-1RII (decoy) may be one possible mechanism by which IL-1β cell signaling is regulated. EtOH, by interfering with the up-regulation of the decoy receptor, may affect IL-1β signaling.
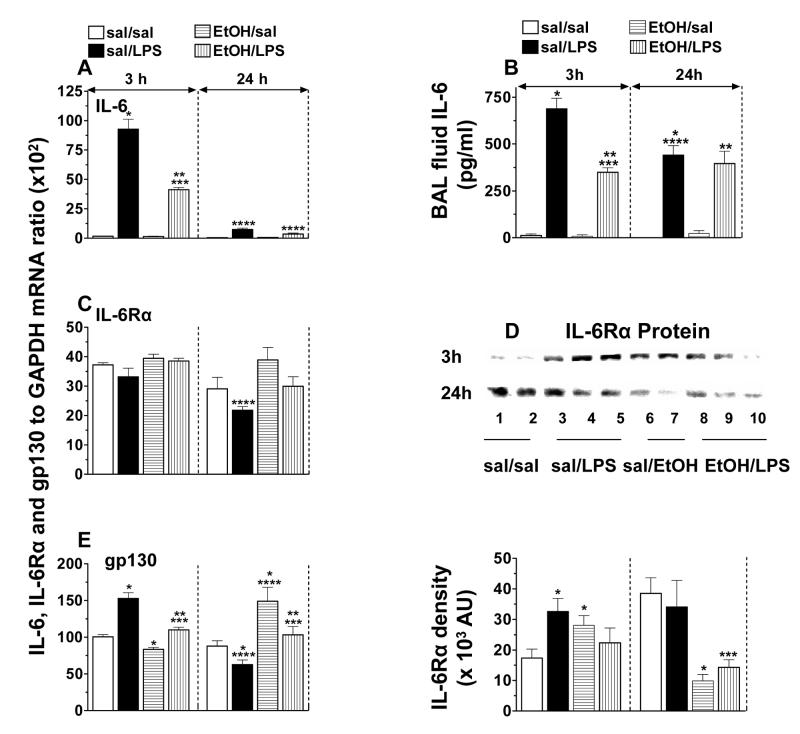
Lung IL-6, IL-6Rα and gp130 mRNA and protein levels, and BAL fluid IL-6
The production of IL-6 at the site of inflammation suggests a major role for it in inflammatory and immune responses. Therefore, we have evaluated the effect of acute EtOH intoxication on lung IL-6, IL-6Rα, and gp130, the intracellular protein involved in IL-6 signaling.
A massive increase in lung IL-6 mRNA levels and protein secretion was observed in the early phase of LPS-induced lung inflammation. While the LPS-induced IL-6 mRNA expression declined close to baseline by 24 h, the protein secretion still remained significantly elevated. Although the basal IL-6 (mRNA and protein) levels were unaffected by EtOH, it blunted (50%) significantly the LPS-induced mRNA levels and protein secretion (Figs. 3A-3B).
Fig. 3.
Mouse lung Il-6 (A), IL-6Rα (C) and gp130 (E) mRNA levels, BAL fluid IL-6 (B) activity, and IL-6Rα (D) protein expression. Plotted are means ± SEM for 6 - 8 mice/group. Abbreviations: saline (sal); alcohol (EtOH); lipopolysaccharide (LPS); arbitrary units (AU). *) p < 0.05 vs. sal/sal; **) p < 0.05 vs. EtOH/sal; ***) p < 0.05 vs. sal/LPS; ****) p < 0.05 vs. similarly treated 3 h group.
The basal IL-6Rα mRNA expression was unaffected by LPS in the early phase of inflammation, but in the later phase a modest (34 %) down-regulation was observed. Acute EtOH intoxication, either alone or in association with LPS, did not have any significant effect on IL-6Rα mRNA levels (Fig. 3C). LPS and EtOH, each, showed a trend to increase lung IL-6Rα protein expression at 3 h, however, at 24 h, EtOH, alone as well as in association with LPS down-regulated the protein expression (Fig. 3D).
LPS up-regulated (53%) gp130 mRNA levels at 3 h in the control mice, and the levels declined below baseline by 24 h. EtOH alone up-regulated (69%) basal gp130 mRNA levels at 24 h and suppressed the LPS effect (Fig. 3E).
The data suggest that acute EtOH intoxication may impair LPS-induced IL-6 signaling by suppressing IL-6 mRNA expression and protein secretion as well as receptor IL-6Rα and gp130 expression.
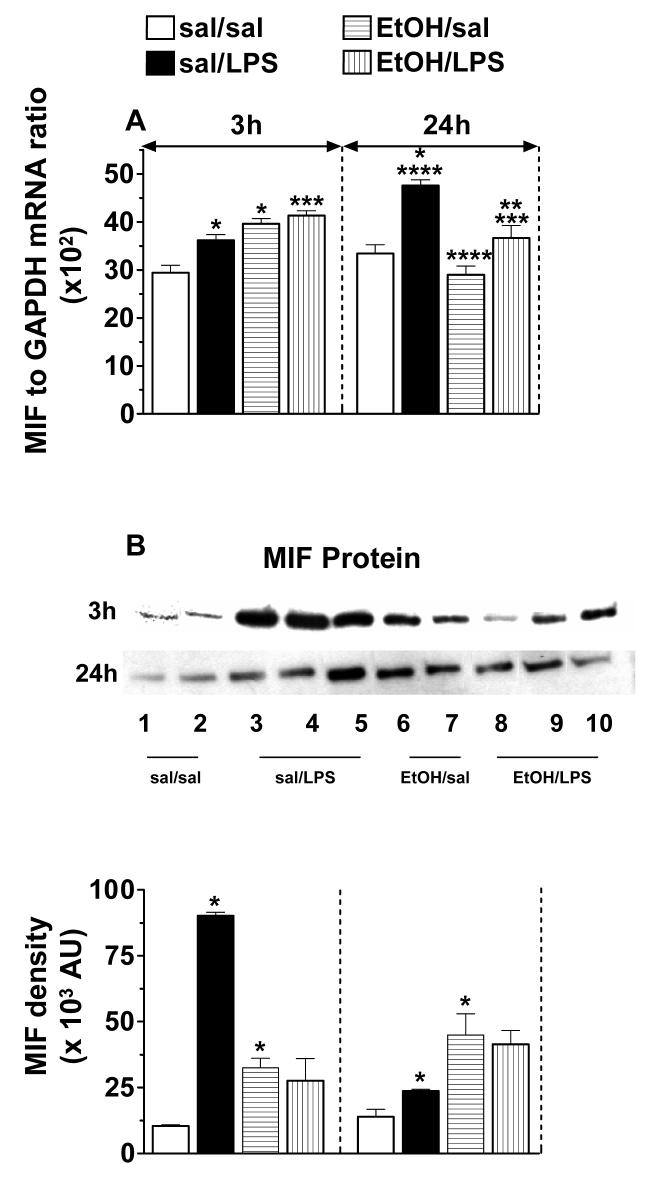
Lung MIF mRNA and protein levels
The MIF is both a cytokine and endocrine factor, and recently published data suggest a regulatory role for MIF in the expression and functions of TNF-α and IL-1 receptors [Calandra, 2003; Calandra et al., 1995)]. Considering its widespread distribution and its crucial role in innate immunity, we investigated the effect of EtOH on MIF expression.
In the control mice, LPS increased MIF mRNA levels significantly (23% and 43%) at 3 and at 24 h respectively. Ethanol, up-regulated MIF mRNA levels, masked the LPS effect at 3 h and suppressed it partially (23%) at 24 h (Fig. 4A). Consistent with the mRNA expression, the protein expression was also up-regulated by LPS at 3 h in control mice and remained slightly above basal levels at 24h. EtOH showed a trend to up-regulate MIF protein expression and suppressed the LPS effect (Fig. 4B).
Fig. 4.
Mouse lung MIF (A) mRNA levels and MIF (B) protein expression. Plotted are means ± SEM for 6 - 8 mice/group. Abbreviations: saline (sal); alcohol (EtOH); lipopolysaccharide (LPS); arbitary units (AU). *) p < 0.05 vs. sal/sal; **) p < 0.05 vs. EtOH/sal; ***) p < 0.05 vs. sal/LPS; ****) p < 0.05 vs. similarly treated 3 h group.
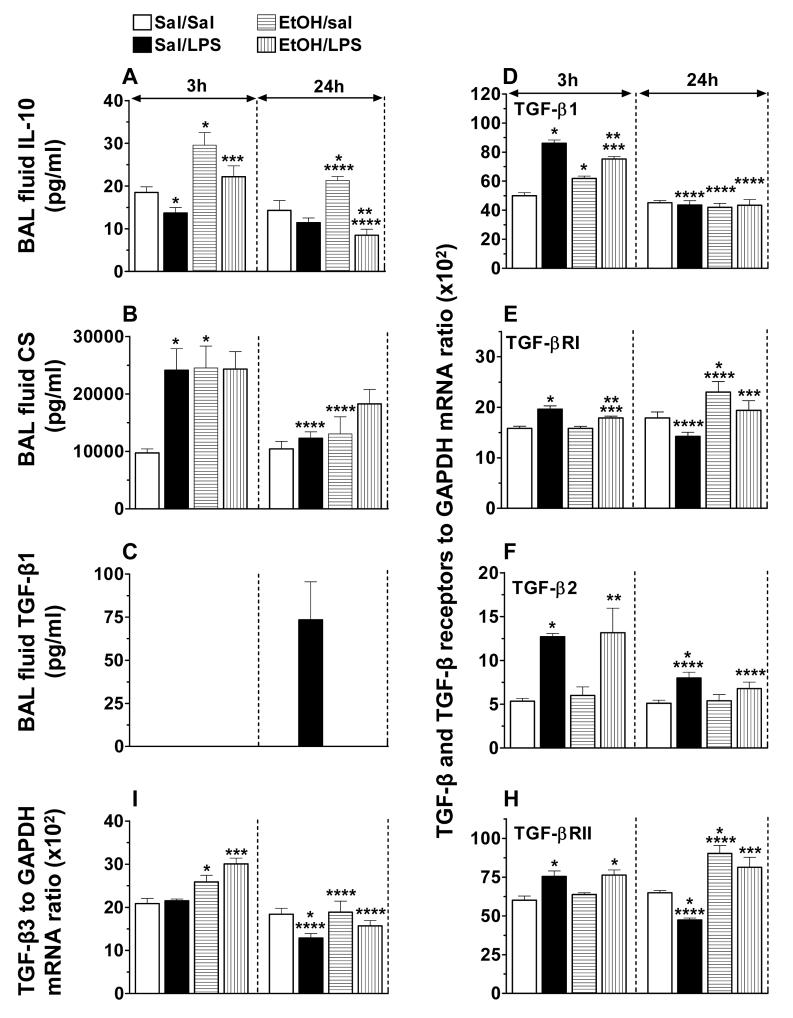
BAL fluid IL-10, CS and TGF-β, and TGF-β and TGF-β Receptors mRNA levels
To determine whether EtOH-induced suppression of LPS-induced pro-inflammatory mediators signaling is associated with an enhanced production of anti-inflammatory mediators, we investigated IL-10, TGF-β, and CS activity in the BAL fluid.
The basal IL-10 activity in BAL fluid (minimum detectable levels 4.0 pg/ml) was significantly (26%) down-regulated by LPS. EtOH alone and in association with LPS induced a 42-62% increase in the IL-10 activity at 3 h, and the activity remained significantly elevated even at 24 h in EtOH-treated mice not challenged with LPS. This EtOH-induced increase in IL-10 activity was suppressed by LPS at 24h (Fig. 5A).
Fig. 5.
Mouse BAL fluid IL-10 (A), corticosterone (B) and TGF-β1 (C) activities, and lung TGF-β1 (D), TGF-βR1 (E), TGF-β2 (F), TGF-βRII (H) and TGF-β3 (I) mRNA levels. *) p < 0.05 vs. sal/sal; **) p < 0.05 vs. EtOH/sal; ***) p < 0.05 vs. sal/LPS; ****) p < 0.05 vs. similarly treated 3 h group.
Corticosterone activity was detectable in BAL fluid recovered from saline treated control mice. EtOH and LPS each increased further (2.5-fold) the CS levels at 3 h and the effects of the two were not additive. The CS levels declined to baseline by 24 h (Fig. 5B). CS activity was also detectable in the plasma, but the levels were not significantly different between groups (data not shown).
LPS administration elevated mRNA levels for TGF-β1 (1.7-fold) and TGF-βRI (1.24-fold) in the early phase of inflammation. At this point in time, EtOH also increased the mRNA levels for TGF-β1 (1.25-fold), but not for TGF-βRI. When the immune insult was superimposed on EtOH, a further increase in TGF-β1 mRNA, and a small but significant increase in TGF-βRI mRNA levels was observed. The effect of EtOH and LPS on TGF-β1 and TGF-βRI mRNA levels was not additive (Fig. 5D&E). At 24 h, however, EtOH induced a significant up-regulation (25%) in TGF-βR1 mRNA expression and masked the LPS effect (Fig. 5E).
LPS induced a 2.4-fold increase in TGF-β2 and a 1.25-fold in TGF-βRII mRNA levels in control mice at the early time point, and the levels declined close to baseline by 24h. EtOH had no significant effect on the basal or LPS-induced TGF-β2 mRNA levels (Fig. 5F). At 24 h, EtOH induced a 1.4-fold increase in TGF-βRII mRNA and masked the LPS effect (Fig. 5H). TGF-β1 was detectable only in BAL fluid recovered from control mice 24 h post LPS instillation. This LPS-induced secretion of TGF-β1 was blocked completely by EtOH (Fig. 5C). LPS had no significant effect on TGF- β3 mRNA expression in the early phase of the inflammatory response, but in the later phase, it down-regulated the expression below basal levels. TGF - β3 mRNA expression was up-regulated by EtOH independent of LPS (Fig. 5I). The BAL fluid was also assayed for TGF-β2 and TGF-β3 isoforms, but these were not in the detectable range of the assay used for any of the treatment groups investigated.
Discussion
Results from our study confirm previous findings that acute EtOH intoxication impairs LPS-induced lung TNF-α production and that the effects of EtOH are post-transcriptional (Kolls et al., 1995; Nelson et al., 1989). The study further demonstrates that EtOH impairs LPS-induced inflammatory response by impairing signaling by other pro-inflammatory cytokines such as IL-1β, IL-6 and MIF at their production and receptor sites while simultaneously augmenting the generation of anti-inflammatory mediators CS and IL-10. In addition, acute EtOH intoxication impairs LPS-induced TGF-β signaling by blocking TGF-β1 secretion and modulating the expression of its receptors.
The immune stimulus recognition by pattern-recognition Toll-like receptors (TLRs) has received a lot of attention in recent years (Akashi-Takamura and Miyake., 2006). TLR4, in particular, has been implicated in Gram-negative LPS signaling, innate immunity and inflammation (Miyake, 2004). Published in vivo and in vitro studies suggest that EtOH suppresses cytokine responses through TLR signaling (Dai and Pruett, 2006; Oak et al., 2006]. In rodent lung, acute EtOH intoxication has also been reported to dampen LPS-induced lung inflammation by impairing TNF-α, MIP-2 and CINC secretion [Boe et al, 2003; D'Souza et al., 1989; Nelson et al., 1989; Zhang et al., 2002].
The up-regulation in TNF-α secretion, its receptors' expression and unaltered levels of the soluble forms of the two TNF receptors observed in our study, in the early phase of LPS-induced acute inflammation, are consistent with enhanced TNF-α signaling (Grell, 1995-1996). The decline in LPS-induced TNF-α secretion and TNF-Rp55 expression, in association with increased levels of the soluble forms of the two TNF receptors, in the later phase, is suggestive of down-regulation in TNF-α signaling and, possibly, the onset of inflammation resolution. The increase in the levels of the soluble TNF receptors may represent an important physiological response targeted at neutralizing any unbound TNF-α. Interestingly, in the later phase, TNFRp75 mRNA expression was also significantly elevated in response to LPS suggesting that the function of this receptor may extend beyond mediating TNF-α signaling. An earlier study suggests a dominant role for TNF-Rp75 in suppressing TNF-induced inflammation (Peschon et al., 1998).
Ethanol suppressed LPS-induced TNF-α secretion without affecting the mRNA expression supporting previous findings that the effect of EtOH on TNF-α is post-transcriptional (Kolls et al., 1995). EtOH also suppressed LPS-induced TNF-Rp55 expression at transcriptional level in the early phase of inflammation. At 24 h, EtOH alone produced a significant increase in both the basal mRNA expression and the soluble form of TNF-Rp55; this is the point in time at which the LPS-induced mRNA expression returned to baseline. At this point in time, EtOH also suppressed markedly the LPS-induced increase in the soluble TNF-Rp75 levels. Our data on TNF-α and its receptors suggest that acute EtOH intoxication may delay TNF-α mediated amplification of the inflammatory response. Since TNF-α receptors play an important role in TNF-α signaling and apoptosis, these findings may have important implications for individuals who might encounter a local immune challenge in the first 24 h post heavy drinking.
The IL-1β signal is transduced by the binding of IL-1β to its cell-surface receptor IL-1R1 (Boraschi and Tagliabue, 2006; Dinarello, 2002). IL-1β is also reported to have greater affinity for IL-1RII, and both the membrane-bound and soluble forms of this receptor are thought to function as natural inhibitors of IL-1β signaling (Boraschi and Tagliabue, 2006). The increase in IL-1β secretion, IL-1R1 and IL-1RII expression in response to LPS was suppressed significantly by EtOH. The much greater increase, observed in our study, in IL-1RII compared to IL-1RI in response to LPS support the notion that IL-1RII receptor may have an important role in containing IL-1β signaling (Bourke et al., 2003). EtOH, by affecting both IL-1β secretion and the expression of its receptors, may impair the normal course of IL-1β signaling.
Acute EtOH intoxication suppressed LPS-induced IL-6 mRNA expression and IL-6 secretion while simultaneously affecting IL-6Rα protein expression. These findings suggest that EtOH impairs IL-6 signaling at cytokine generation and receptor expression levels, which may have important implications, considering that this cytokine plays an important role in inflammation and activation of hypothalamic-pituitary- adrenal (HPA) axis (Hadid et al., 1999; Kamimura et al., 2003; Gabay, 2006).
A possible role in counter-regulating the anti-inflammatory effects of glucocorticoids has been ascribed to MIF, an important component of the neuron-endocrine system (Aeberli et al., 2006; Calandra and Roger, 2003). In addition, MIF is reported to regulate innate immunity by inducing the expression of TLR4, TNF-α and IL-β, and amplify its own production in response to endotoxin (Aeberli et al., 2006; Toh et al., 2006; Roger et al., 2003). In our study, LPS-induced an up-regulation in lung MIF mRNA levels in the early phase of inflammation and the levels continued to rise even in the late phase. The increase in mRNA levels was accompanied by a dramatic increase in the protein expression. Acute EtOH intoxication up-regulated MIF mRNA and protein levels and masked the LPS effect. The up-regulation in MIF expression following acute EtOH intoxication may be glucocorticoid-mediated, considering that EtOH up-regulated lung CS levels.
The presence of low levels of IL-10 and CS in BAL fluid of saline-instilled mice, observed in our study, is consistent with other reports of the normal lung environment being immunosuppressive (Fernandez et al., 2004). Considering the constant exposure of the lungs to low levels of inhaled particles, low levels of anti-inflammatory mediators may be a requisite for the maintenance of lung immune homeostasis. While LPS had no effect on lung IL-10 secretion, EtOH alone increased it, and EtOH and LPS each elevated lung CS levels. The HPA axis and glucocorticoid responses are required to maintain homeostasis within the body between the brain and the immune system (Gaillard, 2001; Gottesfeld et al., 2002). Stressors such EtOH intoxication and inflammatory stimuli can activate the HPA axis to release hormones that stimulate the synthesis and release of endogenous glucocorticoids (Beishuizen and Thijs, 2003). In our study, we found very high levels of CS in the lungs of all intoxicated mice. Since the activation of HPA and the release of CS by acute EtOH intoxication are rapid (Rivier, 1993), we can only speculate that the balance of lung pro- and anti-inflammatory mediators most likely shifts to a more immunosuppressive state even before the lung encounters the immune challenge. Further studies are needed to determine if the dampening of the LPS-induced lung inflammation by EtOH observed in our study is glucocorticoid-mediated.
The role of TGF-β in inflammation is controversial (Bone et al., 1996; Garcia-Lazaro et al., 2005; Turner et al., 1991). On one hand, TGF-β is shown to inhibit in vitro response of macrophages to LPS (Bogdan and Nathan, 1993; Imai et al., 2000) and, on the other hand, to promote inflammation and lung tissue injury (Pittet et al., 2001). In the present study, the mRNA levels of TGF-β1, TGF-β2, TGF-βRI and TGF-βRII were all significantly elevated in the early phase of LPS-induced inflammation. Interestingly, TGF-β1 was the only isoform that was secreted in the late phase of LPS-induced inflammation, and EtOH suppressed partially the mRNA expression of TGF-β1, TGF-βRI, and completely blocked the cytokine secretion. We speculate that TGF-β may play a role in down-regulating LPS-induced early response cytokines, as TGF-β activity was detectable in the lavage at a point in time when the early response cytokines declined close to baseline. Further studies are needed to determine if the effects of EtOH on TGF-β signaling are direct or mediated.
In conclusion, we can say that acute EtOH intoxication alters the normal course of LPS-induced lung inflammation by down-regulating the production of pro-inflammatory mediators TNF-α, IL-1β, IL-6 and simultaneously up-regulating that of anti-inflammatory mediators such as IL-10 and corticosterone, and modulating TGF-β signaling. Furthermore, the EtOH-induced changes in both the expression of membrane associated and soluble cytokine receptors suggest that EtOH may affect signaling by these molecules at the receptor sites. Another important finding from the study is that EtOH intoxication creates an immunosuppressive state in the lung by up-regulating local IL-10 and CS levels. This, most probably, is an early event mediated by the activation of the HPA axis by EtOH. Considering the degree to which the innate immune response is suppressed by EtOH is largely dependent on blood EtOH levels, and based on present findings, it is tempting to speculate that EtOH at high concentration may also impair expression of TLR receptors and/or enhance their soluble forms, thereby impairing recognition and clearance of the stimuli and possibly sensitizing the lung to subsequent immune stimuli.
Acknowledgments
The authors are grateful to Dr. Ion V. Deaciuc for reviewing the manuscript and to Duree Kainath, Casie Woosley and Kasey Cox for their technical assistance. The study was supported by NIAAA RO1AA013168 (NBD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aeberli D, Leech M, Morand EF. Macrophage migration inhibitory factor and glucocorticoid sensitivity. Rheumatology. 2006;45(8):937–943. doi: 10.1093/rheumatology/kel142. [DOI] [PubMed] [Google Scholar]
- Akashi-Takamura S, Miyake K. Toll-like receptors (TLRs) and immune disorders. J. Infect. Chemother. 2006;12(5):233–240. doi: 10.1007/s10156-006-0477-4. [DOI] [PubMed] [Google Scholar]
- Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J. Endotoxin. Res. 2003;9(1):3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- Boe DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense to Streptococcus pneumoniae. Alcohol. Clin. Exp. Res. 2003;27(11):1838–1845. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann. N.Y. Acad. Sci. 1993;23(685):713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- Bone RC. Sir Isaac Newton, Sepsis, SIRS and CARS. Crit. Care Med. 1996;24(7):1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Bomalaski JS, Phair JP. Alcohol, immunosuppression, and the lung. Arch. Intern. Med. 1982;142:2073–2074. [PubMed] [Google Scholar]
- Boraschi D, Tagliabue A. The interleukin-1 receptor family. Vitam. Horm. 2006;74:229–254. doi: 10.1016/S0083-6729(06)74009-2. [DOI] [PubMed] [Google Scholar]
- Bourke E, Cassetti A, Villa A, Fadlon E, Colotta F, Mantovani A. IL-1 beta scavenging by type II IL-1 decoy receptor in human neutrophils. J. Immunol. 2003;170(12):5999–6005. doi: 10.4049/jimmunol.170.12.5999. [DOI] [PubMed] [Google Scholar]
- Bozinovski S, Jones JE, Vlahos R, Hamilton JA, Anderson GP. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NF kappa B and AP-1 in vivo. J. Biol. Chem. 2002;277(45):42808–42814. doi: 10.1074/jbc.M207840200. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Metz CN, Speigel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T. Macrophage migration inhibitory factor and host innate immune responses to microbes. Scand. J. Infect. Dis. 2003;35(9):573–576. doi: 10.1080/00365540310016277. [DOI] [PubMed] [Google Scholar]
- Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immune. 2003;3(10):791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KE, Coutinho A, Gray M, Gilmour JS, Seville JS, Sack JR. Local amplification of glucocorticoids by 11 beta-hydroxysteroid dehydrogenase type 1 and its role in the inflammatory response. Ann. N.Y. Acad. Sci. 2006;1088:265–273. doi: 10.1196/annals.1366.030. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol. Clin. Exp. Res. 1998;22(9):1927–1942. [PubMed] [Google Scholar]
- Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am. J. Respir. Cell Mol. Biol. 1995;12(2):232–237. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- Dai Q, Pruett SB. Ethanol suppresses LPS-induced Toll-like receptor 4 clustering, reorganization of the actin cytoskeleton, and associated TNF-alpha production. Alcohol. Clin. Exp. Res. 2006;30(8):1436–1444. doi: 10.1111/j.1530-0277.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- D'Souza NB, Bagby GJ, Nelson S, Lang CH, Spitzer JJ. Acute alcohol infusion suppresses endotoxin-induced serum tumor necrosis factor. Alcohol. Clin. Exp. Res. 1989;13:295–298. doi: 10.1111/j.1530-0277.1989.tb00329.x. [DOI] [PubMed] [Google Scholar]
- D'Souza NB, Mandujano JF, Nelson S, Summer WR, Shellito JE. CD4+T lymphocyte depletion attenuates lipopolysaccharide-induced tumor necrosis factor secretion by alveolar macrophages in the mouse. Lymph. Cytokine Res. 1994;13(6):359–366. [PubMed] [Google Scholar]
- D'Souza NB, Nelson S, Summer WR, Deaciuc IV. Expression of tumor necrosis factor-alpha and interleukin-6 cell-surface receptors on the alveolar macrophage in alcohol-treated rats. Alcohol. Clin. Exp. Res. 1994;18(6):1430–1435. doi: 10.1111/j.1530-0277.1994.tb01446.x. [DOI] [PubMed] [Google Scholar]
- D'Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol modulates alveolar macrophage tumor necrosis factor-alpha, superoxide anion, and nitric oxide secretion in the rat. Alcohol. Clin. Exp. Res. 1996;20(1):156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002;5(27):S1–13. [PubMed] [Google Scholar]
- Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WAW, Metz CN, Bruce LJ, Bucala R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat. Med. 1997;3(3):320–323. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281(5):L1037–L1050. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Jose P, Avdiushko MG, Kaplan AM, Cohen DA. Inhibition of Il-10 receptor function in alveolar macrophages by Toll-like receptor agonists. J. Immunol. 2004;172(4):2613–2620. doi: 10.4049/jimmunol.172.4.2613. [DOI] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8(Suppl 2):S3–S8. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard RC. Interaction between the hypothalamic-pituitary-adrenal axis and the immunological system. Ann. Endocrinol. 2001;62(2):155–163. [PubMed] [Google Scholar]
- Garcia-Lazaro JF, Thieringer F, Luth S, Czochra P, Meyer E, Renteria IB, Galle PR, Lohse AW, Herkel J, Kanzler S. Hepatic over-expression of TGF-beta1 promote LPS-induced inflammatory cytokine secretion by liver cells and endotoxemic shock. Immunol. Lett. 101:217–222. doi: 10.1016/j.imlet.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z, Moore AN, Dash PK. Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J. Neurotrauma. 2002;19(3):317–326. doi: 10.1089/089771502753594882. [DOI] [PubMed] [Google Scholar]
- Grell M. Tumor necrosis (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J. Inflamm. 19951996;47(12):8–17. [PubMed] [Google Scholar]
- Hadid R, Spinedi E, Chautard T, Giacomini M, Gaillard RC. Role of several mediators of inflammation on mouse hypothalamic-pituitary-adrenal axis response during acute endotoxemia. Neuroimmunomodulation. 1999;6(5):336–343. doi: 10.1159/000026393. [DOI] [PubMed] [Google Scholar]
- Husain K, Scott BR, Reddy SK, Somani SM. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol. 2001;25(2):89–97. doi: 10.1016/s0741-8329(01)00176-8. [DOI] [PubMed] [Google Scholar]
- Imai K, Takeshita A, Hanazawa S. Transforming growth factor-beta inhibits lipopolysaccharide-stimulated expression of inflammatory cytokines in mouse macrophages through downregulation of activation protein 1 and CD14 receptor expression. Infect. Immun. 2000;68(5):2418–2423. doi: 10.1128/iai.68.5.2418-2423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imahara SD, Jelacic S, Junker CE, O'Keefe GE. The influence of gender on human innate immunity. Surgery. 2005;138(2):275–282. doi: 10.1016/j.surg.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Hashimoto K, Nomura A, Sakamoto T, Uchida Y, Ohtsuka M, Hasegawa S, Sagai M. Elimination of neutrophils by apoptosis during the resolution of acute pulmonary inflammation in rats. Lung. 1998;176(2):89–98. doi: 10.1007/pl00007597. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Slukvin I, Sibley D, Fuseler J. Increased susceptibility of experimental animals to infectious organisms as a consequence of ethanol consumption. Alcohol Alcohol. 1994;2(Suppl 2):425–430. [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. Il-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Kishino Y, Moriguchi S. Nutritional factors and cellular immune responses. Nutr. Health. 1992;8(23):133–141. doi: 10.1177/026010609200800308. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Xie J, Lei D, Greenberg S, Summer WR, Nelson S. Differential effects of in vivo ethanol on LPS-induced TNF and nitric oxide production in the lung. Am. J. Physiol. 1995;268:L991–L998. doi: 10.1152/ajplung.1995.268.6.L991. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Louria DB. Alcohol and infection. Curr. Clin. Top. Infect. Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol. Clin. Exp. Res. 2006;30(1):135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Miyake K. Endotoxin recognition molecules, Toll-like receptor 4-MD-2. Semin. Immunol. 2004;16(1):11–6. doi: 10.1016/j.smim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: Essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J. Immuno. 2001;166:4042–4048. doi: 10.4049/jimmunol.166.6.4042. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J. Infect. Dis. 1989;60:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Nelson S, Summer WR. Lower respiratory tract infections. Innate immunity, cytokines, and pulmonary host defense. Infect. Dis. Clin. N. America. 1998;12(3):555–567. doi: 10.1016/s0891-5520(05)70198-7. [DOI] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J. Immunol. 2006;176(12):7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 1998;160(2):943–952. [PubMed] [Google Scholar]
- Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J. Clin. Invest. 2001;107(12):1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Acute interactions between cytokines and alcohol on ACTH and corticosterone secretion in the rat. Alcohol. Clin. Exp. Res. 1993;17(5):946–950. doi: 10.1111/j.1530-0277.1993.tb05646.x. [DOI] [PubMed] [Google Scholar]
- Roger T, Froidevaux C, Martin C, Calandra T. Macrophage migration inhibitory factor (MIF) regulates host responses to endotoxin through modulation of Toll-like receptor 4 (TLR4) J. Endotoxin Res. 2003;9(2):119–123. doi: 10.1179/096805103125001513. [DOI] [PubMed] [Google Scholar]
- Sarphie TG, D'Souza NB, Van Thiel DH, Hill D, McClain CJ, Deaciuc IV. Dose- and time-dependent effects of ethanol on functional and structural aspects of the liver sinusoid in the mouse. Alcohol. Clin. Exp. Res. 1997;21(6):1128–1136. [PubMed] [Google Scholar]
- Scannell M, Maderna P. Lipoxins and annexin-1: resolution of inflammation and regulation of phagocytosis of apoptotic cells. Sci. World J. 2006;6:1555–1573. doi: 10.1100/tsw.2006.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Hitches Cell Biol. 2004;122(4):305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- Spitzer JA. Gender differences in some host defense mechanisms. Lupus. 1999;8(5):380–383. doi: 10.1177/096120339900800510. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Danforth JM. Ethanol feeding inhibits proinflammatory cytokine expression from murine alveolar macrophages ex vivo. Alcohol. Clin. Exp. Res. 1997;21(7):1212–1217. [PubMed] [Google Scholar]
- Starcher B, Williams I. A method for intratracheal instillation of endotoxin into the lungs of mice. Lab. Anim. 1989;23:234–240. doi: 10.1258/002367789780810536. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J. Immunol. Methods. 1997;(202):49–57. doi: 10.1016/s0022-1759(96)00236-0. [DOI] [PubMed] [Google Scholar]
- Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am. J. Physiol. Gastrintest. Liver Physiol. 2006;290(5):G998–1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman RG, Gaebele E, Yin M, Uesugi T, Arteel GE. Strain differences in the development of alcohol-induced liver fibrosis in the mouse. Hepatology. 2001;34(4):464A. Pt. 2 of 2. [Google Scholar]
- Toh ML, Aeberli D, Lacey D, Yang Y, Santos LL, Clarkson M, Sharma L, Clyne C, Morand EF. Regulation of IL-1 and TNF receptor expression and function by endogenous macrophage migration inhibitory factor. J. Immunol. 2006;177(7):4818–4825. doi: 10.4049/jimmunol.177.7.4818. [DOI] [PubMed] [Google Scholar]
- Turner M, Chantry D, Katskis P, Berger A, Brennan FM, Feldmann M. Induction of the interleukin 1 receptor antagonist protein by transforming growth factor-beta. Eur. J. Immunol. 1991;21(7):1635–1639. doi: 10.1002/eji.1830210708. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Azorin I, Guasch R, Pascual M, Gomez-Lechon MJ, Renau-Piqueras J, Guerri C. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol. Clin. Exp. Res. 2003;27(12):1979–1986. doi: 10.1097/01.ALC.0000099261.87880.21. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Ndlovu MN, Hoya-Arias R, Dijsselbloem N, Gerlo S, Haegeman G. Keeping up NF-κB appearances: Epigenetic control of immunity or inflammation-triggered epigenetics. Biochem. Pharmacol. 2006;72(9):1114–1131. doi: 10.1016/j.bcp.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Waltz B, Lopez M, Shahbazian M, Chen G, Colombo LL, Huang D, Way D, Watson RR. Diet and ethanol modulate immune responses in young C57BL/6 mice. Alcohol. Clin. Exp. Res. 1993;17(3):623–630. doi: 10.1111/j.1530-0277.1993.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Boe DM, Zhong Q, Schwarzenberger P, Kolls JK, Nelson S, Summer WR. Acute alcohol intoxication suppresses the CXC chemokine response during endotoxemia. Alcohol. Clin. Exp. Res. 2002;26(1):65–73. [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Stoltz DA, Summer WR, Nelson S. Granulocyte Colony-Stimulating Factor modulates the pulmonary host response to endotoxin in the absence and presence of acute ethanol intoxication. J. Infect. Dis. 1999;179:1441–1448. doi: 10.1086/314763. [DOI] [PubMed] [Google Scholar]
- Zhang P, Nelson S, summer WR, Spitzer JA. Acute ethanol intoxication suppresses the pulmonary inflammatory response in rats challenged with intrapulmonary endotoxin. Alcohol. Clin. Exp. Res. 1997;21(5):773–778. [PubMed] [Google Scholar]