Abstract
ATP, by activating purinergic 2 (P2) receptors on group III and IV afferents, is thought to evoke the metabolic component of the exercise pressor reflex. Previously we have shown that injection of PPADS, a P2 receptor antagonist, into the arterial supply of skeletal muscle of decerebrated cats attenuated the responses of group III and IV afferents to static contraction while the muscles were freely perfused. We have now tested the hypothesis that injection of PPADS (10 mg kg−1) attenuated the responses of group III (n = 13) and group IV afferents (n = 9) to post-contraction circulatory occlusion. In the present study, we found that PPADS attenuated the group III afferent responses to static contraction during circulatory occlusion (P < 0.05). Likewise, PPADS abolished the group IV afferent responses to static contraction during occlusion (P = 0.001). During a 1 minute period of post-contraction circulatory occlusion, four of the 13 group III afferents and eight of the nine group IV afferents maintained their increased discharge. A Fischer's exact probability test revealed that more group IV afferents than group III afferents were stimulated by post-contraction circulatory occlusion (P < 0.02). In addition, the nine group IV afferents increased their mean discharge rate over baseline levels during the post-contraction circulatory occlusion period, whereas the 13 group III afferents did not (P < 0.05). PPADS abolished this post-contraction increase in discharge by the group IV afferents (P < 0.05). Our findings suggest that P2 receptors on group IV afferents play a role in evoking the metabolic component of the exercise pressor reflex.
The exercise pressor reflex is evoked by thin fibre afferents responding to both mechanical and metabolic stimuli arising in contracting muscles. The metabolic component of the reflex is widely believed to signal the spinal cord and brainstem that the blood/oxygen supply to exercising muscle is not adequate to meet its metabolic demand (Mitchell et al. 1983). Nevertheless, the substance evoking the metabolic component of the exercise pressor reflex has not been identified with any certainty.
Alam & Smirk (1937) were the first to demonstrate experimentally the metabolic nature of the stimulus evoking the exercise pressor reflex. These investigators showed that the pressor response to dynamic exercise was maintained by post exercise circulatory occlusion of the working muscles. They suggested that circulatory occlusion trapped metabolites in the working muscles, and that these trapped metabolites stimulated muscle afferents to increase arterial pressure by a reflex mechanism. Since that time, many investigators, using either static or dynamic exercise, have replicated Alam and Smirk's finding (Rowell et al. 1976; Bonde-Petersen et al. 1978; Freund et al. 1979; Victor et al. 1988; Mitchell et al. 1989; Sinoway et al. 1989; Ettinger et al. 1991). Consequently, postexercise circulatory occlusion has become the standard method used to investigate the metabolic component of the exercise pressor reflex, an effect which has also been called the ‘muscle metaboreflex’(Rowell & Sheriff, 1988; Rowell & O'Leary, 1990).
The sensory arm of the exercise pressor reflex arc is composed of group III and IV muscle afferents (Coote & Pérez-González, 1970; McCloskey & Mitchell, 1972). Collectively, these sensory nerves have been called thin fibre muscle afferents. This term distinguishes thin fibre afferents from muscle spindles and Golgi tendon organs (i.e. group I and II), both of which are thickly myelinated and conduct impulses more rapidly than do their thin fibre counterparts. Group III afferents are believed to be primarily responsive to mechanical stimuli, whereas group IV afferents are believed to be primarily responsive to metabolic stimuli (Kaufman et al. 1983, 1984; Hayes et al. 2006). Recently, evidence has been accumulating that suggests that adenosine triphosphate (ATP), a P2 receptor agonist, evokes the metabolic component of the exercise pressor reflex (Hanna et al. 2002; Li & Sinoway, 2002; Hanna & Kaufman, 2003; Li et al. 2003; Hanna & Kaufman, 2004; Kindig et al. 2006). This evidence prompted us to test the hypothesis that pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS), a P2 receptor antagonist, attenuated the responses of thin fibre muscle afferents to postcontraction circulatory occlusion.
Methods
General
The Institutional Care and Use Committee of the University of California, Davis approved all procedures in this report. Cats (n = 40; weight range: 2.5–4.0 kg) were anaesthetized with halothane (3–4%) and oxygen. The trachea was cannulated and the lungs were mechanically ventilated (Harvard Apparatus, Holliston, MA, USA) with 3% halothane in oxygen until after the decerebration and surgery were completed. Catheters were placed in the right common carotid artery and the right jugular vein. Arterial blood pressure was measured by connecting the carotid catheter to a pressure transducer (model P23XL, Statham, Hato Rey, PR, USA). Heart rate was calculated beat-to-beat from the arterial pressure pulse (Gould Biotach).
The cat was placed in a Kopf stereotaxic and spinal unit and given dexamethasone (4 mg; i.v.). A midcollicular decerebration was performed and all neural tissue rostral to the section was removed. Haemostasis was achieved and the cranial vault filled with agar (37°C). A laminectomy was performed to expose the L6–S1 dorsal roots. The left triceps surae muscles were isolated and the calcaneal bone was severed. The left leg was fixed in place with a clamp and knee brace so that the angle between the upper and lower leg was approximately 115 deg. The free end of the left calcaneal tendon was attached to a force transducer (model FT-10C, Grass Instruments) in order to measure the tension developed by the left triceps surae muscles. All visible branches of the left sciatic nerve except for those innervating the triceps surae muscles were cut. The left femoral and obturator nerves were also cut. At the conclusion of the experiment the cat was humanely killed with an overdose of pentobarbital (Cardinal) followed by an injection of saturated KCl solution.
Recording impulse activity from group III and IV afferents
Afferent impulses were recorded from thin filaments dissected from either L7 or S1 dorsal roots. We located the receptive fields of group III and IV afferents by probing the left triceps surae muscles with both noxious and non-noxious stimuli. Noxious probing consisted of vigorously pinching the muscles with the fingers; likewise non-noxious probing consisted of either gently stroking the triceps surae with a blunt rod or gently squeezing the muscles with the fingers. The afferent signals were passed through a high impedance probe (HIP 511, Grass Instruments), amplified and filtered (100–3000 Hz; P511, Grass Instruments). Action potentials were displayed on a computer monitor (Spike2; Cambridge Electronics Design, Cambridge, UK) and on a storage oscilloscope (HP 54603B).
The conduction velocity of an afferent was calculated by dividing the conduction distance between the recording electrode on the dorsal root and the stimulating electrode on the tibial nerve by the conduction time, which was measured on the storage oscilloscope. Group III fibres had conduction velocities between 2.5 and 30 m s−1. Group IV fibres had conduction velocities of less than 2.5 m s−1 (Kaufman & Forster, 1996). Afferents having a conduction velocity of greater than 30 m s−1 were discarded.
Protocols
The left triceps surae muscles were contracted statically for 60 s while they were freely perfused as well as while their circulation was occluded. Contraction was induced by electrical stimulation of the tibial nerve (15 Hz; 25 μs; 1.5–2 × motor threshold). We have shown previously that this method of contracting the triceps surae muscles does not electrically stimulate the axons of group III and IV afferents (Hanna & Kaufman, 2003). In addition, static contraction for 60 s was also evoked during circulatory occlusion of the triceps surae muscles. We occluded the circulation of the muscles by tightening snares placed around the left iliac artery and the left common iliac vein for 3 min prior to contraction. The occlusion was maintained during the contraction as well as for 1 min after the contraction ended. The order of the two types of contraction was varied randomly. If a group III or IV afferent increased its discharge over baseline during static contraction while the circulation to the triceps surae muscles was occluded it was included in the study, and its response to static contraction determined after P2 receptor blockade with PPADS.
P2 receptor blockade with PPADS
Before injecting PPADS, we tightened the same snares as those that were used to occlude the circulation of the triceps surae muscles. We injected PPADS (10 mg kg−1) into the popliteal artery, trapping it within the circulation of the lower leg. We released the snares after 15 min and allowed the leg to be freely perfused for 15 min before initiating contraction. We have shown previously that popliteal arterial injections of this dose of PPADS blocked both the reflex pressor responses and the thin fibre muscle afferent responses to popliteal arterial injection of α,β-methylene-ATP (Hanna et al. 2002; Hanna & Kaufman, 2004). In addition, we have shown that this dose of PPADS attenuated the responses of group III and IV afferents to static contraction while the triceps surae muscles were freely perfused (Kindig et al. 2006).
Data analysis
Baseline impulse activity was counted for 60 s prior to a manoeuvre (i.e. contraction while the muscles were freely perfused or their circulation was occluded), during the manoeuvre, and for 60 s after the manoeuvre ended. Activity is expressed as impulses per second. The criterion for a response to static contraction was defined as an increase of at least 12 impulses in 60 s. The tension time index (Perez-Gonzalez, 1981) was calculated by integrating the area between the tension trace during static contraction and its baseline level (Spike2). Peak developed tension was calculated by subtracting the resting tension from the maximum tension. All values are expressed as the mean ± standard error of the mean. Two by two way repeated measures ANOVA followed by Tukey's post hoc test or a Fisher's Exact Probability Test were used to determine statistical significance. The criterion for statistical significance was set at P < 0.05.
Results
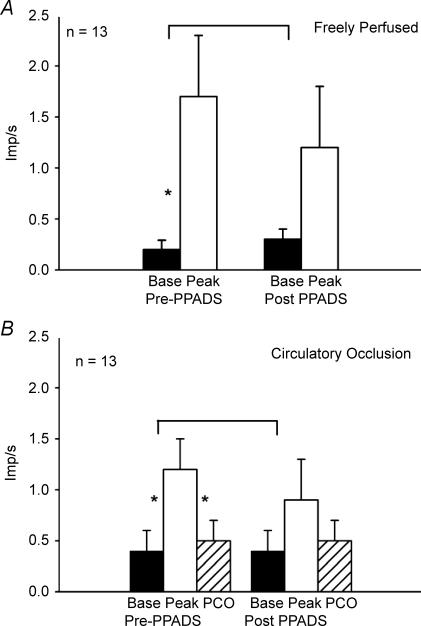
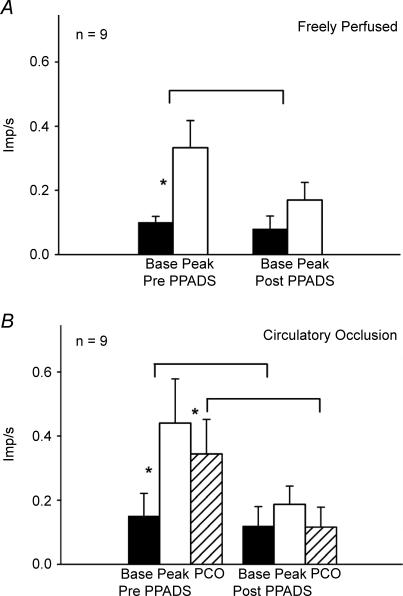
We recorded the impulse activity of 23 group III afferents and 17 group IV afferents with endings in the triceps surae muscles. During circulatory occlusion, 13 of the 23 group III afferents, and nine of the 17 group IV afferents increased their discharge rate over baseline levels when the triceps surae muscles were statically contracted for 1 min. The responses of 12 of these 13 group III afferents and eight of these nine group IV afferents to static contraction while the triceps surae muscles were freely perfused have been reported previously by Kindig et al. (2006) (Figs 1A and 2A); their data have been included in this report in order to compare the responses of these afferents to contraction while the muscles were freely perfused with the responses to contraction while the circulation to the muscles was occluded. Each of the 13 group III afferents, but none of the nine group IV afferents, responded to non-noxious probing of their receptive fields in the triceps surae muscles. Noxious pinch of the triceps surae muscles was required to discharge each of the nine group IV afferents when probing for their receptive fields.
Figure 1. Summary of responses of 13 group III afferents to static contraction while the triceps surae muscles were freely perfused (A) and while their circulation was occluded (B).
A, discharge rates of group III afferents responding to static contraction while the circulation to the muscles was freely perfused, both before (Pre) and after (Post) PPADS was injected into the popliteal artery (10 mg kg−1). B, discharge rates of group III afferents responding to static contraction while the circulation to the muscles was occluded, both before (Pre) and after (Post) PPADS was injected into the popliteal artery (10 mg kg−1). Filled bars represent baseline means (base) and open bars represent means during contraction (peak). Hatched bars represent the mean discharge rate during a 1 min period of post-contraction circulatory occlusion (PCO). Vertical brackets represent standard errors. Asterisks represent a significant difference (P < 0.05) between baseline and either contraction or PCO. Horizontal brackets represent a significant difference (P < 0.05) between the increase in discharge due to contraction before and after PPADS.
Figure 2. Summary of responses of nine group IV afferents to static contraction while the triceps surae muscles were freely perfused (A) and while their circulation was occluded (B).
A, discharge rates of group IV afferents responding to static contraction while the circulation to the muscles was freely perfused, both before (Pre) and after (Post) PPADS was injected into the popliteal artery (10 mg kg−1). B, discharge rates of group IV afferents responding to static contraction while the circulation to the muscles was occluded, both before (Pre) and after (Post) PPADS was injected into the popliteal artery (10 mg kg−1). Filled bars represent baseline means (base) and open bars represent means during contraction (peak). Hatched bars represent the mean discharge rate during a 1 min period of post-contraction circulatory occlusion (PCO). Vertical brackets represent standard errors. Asterisks represent a significant difference (P < 0.05) between baseline and either contraction or PCO. Horizontal brackets represent a significant difference (P < 0.05) between the increase in discharge due to contraction before and after PPADS, and a significant difference (P < 0.05) in discharge due to PCO before and after PPADS.
Four of the 13 group III afferents and eight of the nine group IV afferents maintained their increase in discharge during the 1 min period of post-contraction circulatory occlusion (Figs 1B and 2B). A Fisher's Exact Probability Test revealed that more group IV afferents responded to post-contraction circulatory occlusion than did group III afferents (P < 0.02). Moreover, the group IV afferents (n = 9) increased their mean discharge rate over baseline levels during post-contraction circulatory occlusion, whereas the group III afferents (n = 13) did not (P < 0.05). In addition, the group III afferents appeared to generate less of a response to static contraction while the circulation to the triceps surae muscles was occluded than they did to static contraction while the muscles were freely perfused (Fig. 1). Nevertheless, this trend was not statistically significant (P = 0.089). In contrast, the group IV afferents appeared to generate about the same response to static contraction while the circulation to the muscles was occluded as that while the muscles were perfused (Fig. 2).
PPADS, injected into the popliteal artery of the limb undergoing static contraction, prevented the responses of the group IV afferents to post-contraction circulatory occlusion (Figs 2B and 3). PPADS also attenuated the responses of these unmyelinated afferents to static contraction both while the triceps surae muscles were freely perfused and while their circulation was occluded (Figs 2A and B and 3). Likewise PPADS attenuated the responses of the group III afferents to static contraction both while the triceps surae muscles were freely perfused and while their circulation was occluded (Fig. 1). The tension time indices and peak developed tensions before PPADS was injected into the popliteal artery did not differ significantly from those after PPADS was injected for either the group III or group IV afferents when the circulation to the triceps surae muscles was either freely perfused or was occluded (Table 1, P > 0.05).
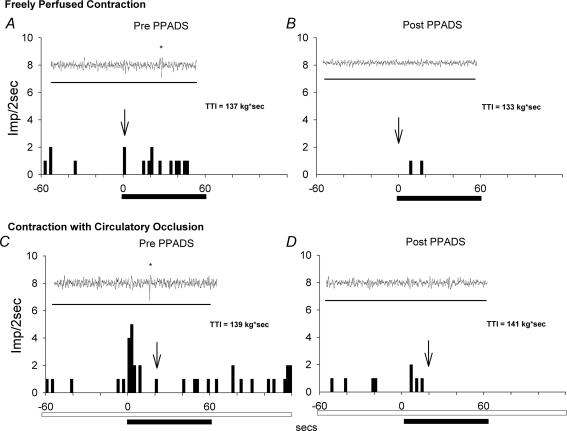
Figure 3. Responses of a single group IV afferent (conduction velocity = 1.7 m s−1) to static contraction while while the triceps surae muscles were freely perfused (A and B) and while their circulation was occluded (C and D) both before (Pre PPADS; A and C) and after (Post PPADS; B and D) PPADS, which was injected into the popliteal artery (10 mg kg−1).
Static contraction started at time zero and lasted for 60 s (filled horizontal bar). Circulatory occlusion was started 3 min prior to contraction and maintained during and 1 min after contraction (open horizontal bar). Only the 60 s immediately prior to contraction is shown. Insets show the recording of the action potential, as denoted by the arrow. Horizontal bar in inset represents 280 ms. Abbreviation: TTI, tension time index. Stars identify action potentials discharged by group IV afferent.
Table 1.
Tension time indices (TTI) and peak developed tensions (Dev Ten) for static contraction while the triceps surae muscles were freely perfused and while their circulation was occluded
| Before PPADS | After PPADS | |||
|---|---|---|---|---|
| TTI (kg) (s) | Peak Dev Ten (kg) | TTI (kg) (s) | Peak Dev Ten (kg) | |
| Group III afferents freely perfused (n = 12) | 226 ± 26 | 4.5 ± 0.5 | 215 ± 17 | 4.5 ± 0.3 |
| Group IV afferents freely perfused (n = 9) | 155 ± 28 | 3.1 ± 0.5 | 145 ± 12 | 2.9 ± 0.3 |
| Group III afferents circulatory occlusion (n = 12) | 212 ± 23 | 4.3 ± 0.4 | 193 ± 21 | 4.1 ± 0.4 |
| Group IV afferents circulatory occlusion (n = 9) | 165 ± 24 | 3.2 ± 0.4 | 148 ± 25 | 2.8 ± 0.4 |
Note that there were no pair wise comparisons of TTIs or Peak Dev Ten within the Group III category that were significantly different from each other (P > 0.05). Likewise, there were no pair wise comparisons of TTIs or Peak Dev Ten within the Group IV category that were significantly different from each other. Also note that the TTI and peak developed tension were not available for one Group III afferent.
As a control, we examined the responses of three group IV afferents to two successive static contractions while the circulation to the triceps surae muscles was occluded. The interval between the two contractions was similar to that described above, but PPADS was not injected into the popliteal artery. We found that the first static contraction stimulated each of the three afferents, increasing activity from 0.02 ± 0.01 to 0.3 ± 0.02 impulses s−1 (n = 3; P < 0.05). Likewise, we found that the second static contraction stimulated each of the three group IV afferents, increasing activity from 0.03 ± 0.03 to 0.3 ± 0.08 impulses s−1 (n = 3; P < 0.05).
In addition, we measured the pressor and cardioaccelerator responses to popliteal arterial injections of lactic acid (0.2–1 ml, 24 mmol; n = 4), and capsaicin (1–2 μg; n = 4) before and after PPADS (Table 2). PPADS had no effect on the pressor responses to either lactic acid (P > 0.05) or capsaicin (P > 0.05). In addition, PPADS had no effect on the cardioaccelerator response to lactic acid (P > 0.05), and in fact increased this response to capsaicin (P < 0.05).
Table 2.
Pressor responses to injection of lactic acid and capsaicin into the popliteal artery
| Before PPADS | After PPADS | |||
|---|---|---|---|---|
| Baseline (mmHg) | Peak (mmHg) | Baseline (mmHg) | Peak (mmHg) | |
| Lactic acid 24 mmol, 0.2–1 ml | 117 ± 9 | 153 ± 12* | 131 ± 8 | 181 ± 8* |
| Capsaicin 1–2 μg | 131 ± 9 | 175 ± 13* | 128 ± 16 | 168 ± 23* |
PPADS did not attenuate the pressor responses to either lactic acid (n = 4) or capsaicin (n = 4)
Table 3.
Cardioaccelerator responses to injection of lactic acid and capsaicin into the popliteal artery
| Before PPADS | After PPADS | |||
|---|---|---|---|---|
| Baseline (bpm) | Peak (bpm) | Baseline (bpm) | Peak (bpm) | |
| Lactic acid 24 mmol, 0.2–1 ml | 195 ± 19 | 202 ± 15 | 198 ± 27 | 211 ± 19 |
| Capsaicin 1–2 μg | 200 ± 16 | 218 ± 10 | 207 ± 21 | 229 ± 17† |
Note that after PPADS the cardioaccelerator response to capsaicin (n = 4) was significantly larger than the response before PPADS (P < 0.05; see cross). PPADS had no effect on the cardioaccelerator responses to lactic acid (n = 4). Asterisks (*) signify that the peak responses to lactic acid and capsaicin were significantly different from baseline (P < 0.05)
Discussion
We have shown that Purinergic 2 (P2) receptor blockade with PPADS prevented the responses of group IV afferents to post-contraction circulatory occlusion. We have also shown that PPADS attenuated the responses of both group III and IV afferents to static contraction while the triceps surae muscles were freely perfused and while their circulation was occluded. In our experiments, PPADS was injected into the popliteal artery, trapped there for 15 min, and was allowed subsequently to circulate systemically. This method of administration makes it likely that the site of action for PPADS was the endings of group III and IV afferents innervating the triceps surae muscles. The site of action for PPADS could not have been in the dorsal horn of the spinal cord, for example, because the dorsal roots were sectioned.
If P2 receptors on thin fibre muscle afferents are responsible for signalling a mismatch between blood supply and demand in exercising muscle, then one should expect them to respond to exogenous administration of P2 receptor agonists. Moreover, one might expect that this stimulation would be prevented by prior administration of PPADS. Both expectations have been, in fact, confirmed experimentally. Specifically, group IV muscle afferents in cats have been shown to be stimulated by α,β-methylene-ATP, an effect prevented by PPADS (Hanna & Kaufman, 2004). Moreover, the only group III afferents stimulated by α,β-methylene-ATP were those conducting impulses at less than 5 m s−1 (Hanna & Kaufman, 2004). Likewise, group IV muscle afferents in rats have been shown to be stimulated by ATP (Reinöhl et al. 2003). The finding that P2 receptor agonists stimulate slowly conducting group III and group IV afferents supports the hypothesis that these afferents respond to metabolic events in exercising muscles.
In the present and prior studies, circulatory occlusion decreased the responses of group III afferents to static contraction, regardless of whether the contraction was maintained (Kaufman et al. 1984) or intermittent (Mense & Stahnke, 1983). Group III afferents are mechanosensitive as evidenced both by their responsiveness to gentle non-noxious probing of their receptive fields and by their responsiveness to tendon stretch (Kaufman et al. 1983). In addition, group III afferents usually respond in a graded manner to the level of tension developed by the statically contracting muscles (Kaufman et al. 1983; Kaufman & Rybicki, 1987). Consequently, the decrease in response to contraction by group III afferents during circulatory occlusion in our experiments might have been caused partly by the decrease in tension development by the triceps surae muscles.
One important issue for our studies was that the responses of the group III and IV afferents to static contraction were repeatable. If this was not the case, then our finding that PPADS attenuated the responses of the afferents to static contraction may have been caused by deterioration of the preparation. We found, however, that the responses of three group IV afferents to contraction while the circulation was occluded were repeatable. In addition, we have previously reported that group III and IV afferents displayed repeatable responses to static contraction while the triceps surae muscles were freely perfused (Rotto et al. 1990a,b). A second important issue was that the antagonistic action of PPADS was specific for P2 receptors. We have in part addressed this issue by showing that PPADS had no effect on the pressor and cardioaccelerator responses to popliteal arterial injections to either capsaicin, a TPRV1 receptor agonist, or lactic acid, an ASIC receptor agonist.
Although group III afferents respond less to static contraction during circulatory occlusion than they do to static contraction while the muscles were freely perfused (Mense & Stahnke, 1983; Kaufman et al. 1984), the opposite seems to be the case during dynamic exercise induced by electrical stimulation of the mesencephalic locomotor region (Adreani & Kaufman, 1998; Hayes et al. 2006). We can only speculate as to the cause of this discrepancy. It might be attributable to the sensitizing action of ischaemic metabolites on group III mechanosensitive afferents when they respond to an oscillating stimulus such as a dynamically exercising muscle. During circulatory occlusion, the triceps surae muscles did not show any signs of fatigue (i.e. decreases in tension development) while they were dynamically exercising (Adreani & Kaufman, 1998; Hayes et al. 2006) whereas, these muscles did show decrements in tension development while they were statically contracting (Kaufman et al. 1984; present study). Consequently, any sensitizing effect by ischaemic metabolites on the discharge of group III mechanoreceptors might be countered by the decrease in tension developed by a fatiguing muscle with a reduced blood supply.
During the 1 min contraction period, group IV afferents responded similarly when the triceps surae muscles were freely perfused as when their circulation was occluded. This finding may appear at first glance to contrast with that reported previously by our laboratory (Kaufman et al. 1984). Two factors may explain this discrepancy. The first is that in the present study, the contracting triceps surae muscles developed less peak tension than did those in the previous study (Kaufman et al. 1984). As a consequence, the metabolism of the muscles may have been less in the present study than in the previous one. Second, in the present study we reported group IV afferent discharge as an average calculated over the entire period of contraction (i.e. 60 s), whereas in the previous study (Kaufman et al. 1984), we reported group IV discharge as the peak rate calculated over a 5 s period. We believe that the present method of reporting afferent discharge is a more complete representation of afferent response to contraction than is the former method.
In the past, attempts have been made to attenuate the exercise pressor reflex by injecting receptor antagonists into the arterial supply of contracting skeletal muscle. Specifically, antagonists to bradykinin 2 receptors (Pan et al. 1993) and purinergic 2 receptors (Hanna & Kaufman, 2003) have been found to block much of the reflex pressor response to static contraction while the muscles were freely perfused. Likewise, preventing prostaglandin synthesis with cyclooxygenase blockers has also been shown to prevent this reflex pressor response to contraction (Stebbins et al. 1988). However, neither the study using a bradykinin 2 receptor antagonist nor the study using cyclooxygenase antagonists tested the effect of these agents on the pressor response to post-contraction circulatory occlusion. In contrast, the study using the P2 receptor antagonist (Hanna & Kaufman, 2003) performed this test, finding that P2 receptor blockade prevented the reflex pressor response to post-contraction circulatory occlusion. Our present finding that PPADS prevented the responses of group IV afferents to post-contraction circulatory occlusion is consistent both with the findings of the reflex study (Hanna & Kaufman, 2003) and with previous findings that these C-fibres are metabosensitive (Mense & Stahnke, 1983; Kaufman et al. 1984).
The hypothesis that the metabolite signalling a mismatch between blood/oxygen supply and demand in exercising muscle also relaxes vascular smooth muscle to increase blood flow has always been appealing. Such a substance would dilate the vascular beds of exercising muscles while simultaneously evoking the exercise pressor reflex to constrict the vascular beds of non-exercising tissues. However, stimulation of P2X receptors with α,β-methylene-ATP constricts hind limb vascular smooth muscle in cats (Bivalacqua et al. 2002), rats (Kluess et al. 2005) and dogs (Buckwalter et al. 2003). Thus, if ATP functions to evoke the muscle metaboreflex, the hypothesis that a single substance causes reflex vasoconstriction and metabolic vasodilatation is not supported.
Although PPADS in our experiments abolished the responses of the group IV afferents to post-contraction circulatory occlusion, we hesitate to conclude that adenosine triphosphate, the naturally occurring agonist for P2 receptors, is the sole substance responsible for signalling a mismatch between blood/oxygen supply and demand in the working muscles. The effect of either a bradykinin 2 receptor antagonist or a prostaglandin synthesis antagonist on the pressor response to circulatory occlusion immediately following static contraction remains to be determined. Perhaps, several substances, such as bradykinin, prostaglandins and ATP, need to be present near the endings of group IV afferents for them to respond to post-contraction circulatory occlusion. Removal of one substance by receptor blockade or by preventing its synthesis might prevent the cascade of events needed to stimulate group IV afferents during ischaemia. As such, ATP may play an important role in signalling the central nervous system that during exercise blood/oxygen flow to contracting muscles is not adequate to meet metabolic demand.
Acknowledgments
This work was supported by NIH grant HL 30710. We thank Ms. Yao Dong for her surgical assistance.
References
- Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol. 1998;84:1827–1833. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Shah MK, De Witt BJ, Inscho EW, Kadowitz PJ. Comparative responses to α,β-methylene-ATP in cat pulmonary, mesenteric, and hindquarter vascular beds. J Appl Physiol. 2002;93:1287–1295. doi: 10.1152/japplphysiol.00262.2002. [DOI] [PubMed] [Google Scholar]
- Bonde-Petersen F, Rowell LB, Murray RG, Blomqvist GG, White R, Karlsson E, Campbell W, Mitchell JH. Role of cardiac output in the pressor responses to graded muscle ischemia in man. J Appl Physiol. 1978;45:574–580. doi: 10.1152/jappl.1978.45.4.574. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Hamann JJ, Clifford PS. Vasoconstriction in active skeletal muscles: a potential role for P2X purinergic receptors? J Appl Physiol. 2003;95:953–959. doi: 10.1152/japplphysiol.00173.2003. [DOI] [PubMed] [Google Scholar]
- Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol. 1970;208:261–278. doi: 10.1113/jphysiol.1970.sp009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger S, Gray K, Whisler S, Sinoway L. Dichloroacetate reduces sympathetic nerve responses to static exercise. Am J Physiol Heart Circ Physiol. 1991;261:H1653–H1658. doi: 10.1152/ajpheart.1991.261.5.H1653. [DOI] [PubMed] [Google Scholar]
- Freund PR, Rowell LB, Murphy TM, Hobbs SF, Butler SH. Blockade of pressor response to muscle ischemia by sensory nerve block in man. Am J Physiol Heart Circ Physiol. 1979;236:H433–H439. doi: 10.1152/ajpheart.1979.237.4.H433. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Hayes SG, Kaufman MP. α,β-Methylene ATP elicits a reflex pressor response arising from muscle in decerebrate cats. J Appl Physiol. 2002;93:834–841. doi: 10.1152/japplphysiol.00237.2002. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol. 2003;94:1437–1445. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol. 2004;96:1166–1169. doi: 10.1152/japplphysiol.01020.2003. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol. 2006;290:H2239–H2246. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems, Control of Respiratory and Cardiovascular Systems. part II. New York: Oxford University Press; 1996. pp. 381–447. [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:160–165. [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol. 1984;57:644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol. 2006;290:H1214–H1219. doi: 10.1152/ajpheart.01051.2005. [DOI] [PubMed] [Google Scholar]
- Kluess HA, Buckwalter JB, Hamann JJ, Clifford PS. Acidosis attenuates P2X purinergic vasoconstriction in skeletal muscle arteries. Am J Physiol Heart Circ Physiol. 2005;288:H129–H132. doi: 10.1152/ajpheart.00574.2004. [DOI] [PubMed] [Google Scholar]
- Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol. 2003;95:577–583. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol. 2002;283:H2636–H2643. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Stahnke M. Responses in muscle afferent fibers of slow conduction velocity to contractions and ischemia in the cat. J Physiol. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anesthesia and cardiovascular responses to static exercise in man. J Physiol. 1989;417:13–24. doi: 10.1113/jphysiol.1989.sp017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H-L, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol. 1993;75:2061–2068. doi: 10.1152/jappl.1993.75.5.2061. [DOI] [PubMed] [Google Scholar]
- Perez-Gonzalez JF. Factors determining the blood pressure responses to isometric exercise. Circ Res. 1981;48:I76–I86. [PubMed] [Google Scholar]
- Reinöhl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett. 2003;338:25–28. doi: 10.1016/s0304-3940(02)01360-5. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates the responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol. 1990a;259:H745–H750. doi: 10.1152/ajpheart.1990.259.3.H745. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol. 1990b;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Hermansen L, Blackmon JR. Human cardiovascular and respiratory responses to graded muscle ischemia. J Appl Physiol. 1976;41:693–701. doi: 10.1152/jappl.1976.41.5.693. [DOI] [PubMed] [Google Scholar]
- Rowell L, O'Leary D. Reflex control of the circulation during exercise: Chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Sheriff DD. Are muscle ‘chemoreflexes’ functionally important? News Physiol Sci. 1988;3:240–253. [Google Scholar]
- Sinoway L, Prophet S, Gorman I, Mosher TJ, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol. 1989;66:429–436. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res. 1988;59:645–654. doi: 10.1161/01.res.59.6.645. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest. 1988;82:1301–1305. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]