Abstract
In the nervous system, zinc can influence synaptic responses and at extreme concentrations contributes to epileptic and ischaemic neuronal injury. Zinc can originate from synaptic vesicles, the extracellular space and from intracellular stores. In this study, we aimed to determine which of these zinc pools is responsible for the increased hippocampal excitability observed in zinc-depleted animals or following zinc chelation. Also, we investigated the source of intracellularly accumulating zinc in vulnerable neurons. Our data show that membrane-permeable and membrane-impermeable zinc chelators had little or no effect on seizure activity in the CA3 region. Furthermore, extracellular zinc chelation could not prevent the accumulation of lethal concentrations of zinc in dying neurons following epileptic seizures. At the electron microscopic level, zinc staining significantly increased at the presynaptic membrane of mossy fibre terminals in kainic acid-treated animals. These data indicate that intracellular but not extracellular zinc chelators could influence neuronal excitability and seizure-induced zinc accumulation observed in the cytosol of vulnerable neurons.
In the mammalian brain, zinc is found sequestered into synaptic vesicles, in the extracellular space or tightly bound to intracellular proteins. It has been suggested that zinc has a modulatory role in synaptic transmission and it has been implicated in cell death in ischaemia, epilepsy and traumatic brain injury. Synaptically released zinc has been shown to interact with receptors that determine neuronal excitability and increased levels of free intracellular zinc are observed in dying neurons.
Extracellularly applied zinc can modulate excitatory and inhibitory synaptic responses (Forsythe et al. 1988; Mayer & Vyklicky, 1989; Draguhn et al. 1990; Chen et al. 1997; Paoletti et al. 1997; Vogt et al. 2000; Lin et al. 2001; Molnar & Nadler, 2001). In addition to its effect on synaptic transmission, zinc has been found at elevated concentrations in vulnerable neurons; cells that show signs of distress and eventually die after seizures. A major increase in intracellular zinc concentration eventually leads to apoptosis or necrosis (Kim et al. 1999; Jiang et al. 2001). As increased intracellular free zinc can induce neuronal death, it has been suggested to play a crucial role in selective neurodegeneration produced by neurotoxic agents and ischaemia (Koh et al. 1996; Weiss & Sensi, 2000). However, experiments carried out on zinc-depleted (zinc is completely depleted from the tissue using chelators) or zinc-deficient (animals are kept on a zinc free diet, zinc levels are decreased but there is still a significant amount in the tissue) animals showed that this may not be the case. These animals had a lower threshold for seizures which are longer and stronger, and the number of degenerating cells was increased (Cole et al. 2000; Lee et al. 2000a; Dominguez et al. 2003; Takeda et al. 2003). While under pathological conditions intracellularly accumulated zinc can be neurotoxic, it can also play a role in neuroprotection. It has been demonstrated that the effects of zinc on neuronal cell death are concentration-dependent and cell-type specific (Cote et al. 2005).
Mossy fibres of the dentate granule cells contain an unusually high concentration of zinc in their synaptic terminals (Maske, 1955). Several studies have shown that synaptic activity increases extracellular zinc concentration in the micromolar range (Assaf & Chung, 1984; Howell et al. 1984; Li et al. 2001a,b), but a recent study by Kay (2003) casts doubt on this observation. Using fluorometric measurements, he observed very little (nanomolar levels) release from synaptic terminals during increased activity. Nonetheless, synaptically released zinc has been convincingly shown to modulate synaptic NMDA responses (Vogt et al. 2000; Molnar & Nadler, 2001). These seemingly contradicting results could be explained by the relatively high affinity of NMDA receptors for zinc (Yamada et al. 2002). In addition to fluorimetric techniques, zinc can also be detected with Timm-staining (Timm, 1958). Recent developments in this method (Danscher, 1982, 1996), including the introduction of the sodium tungstate (Seress & Gallyas, 2000), now permit visualization of zinc in presynaptic terminals, synaptic vesicles and synapses.
The origin of zinc that influences excitability and causes cell death is unknown. One possibility is that zinc can translocate from zinc-rich presynaptic terminals during increased synaptic transmission (Frederickson et al. 1989; Li et al. 2001b). Another possibility is that the accumulating zinc has an extracellular origin other than synaptic vesicles. A third possibility is that zinc is released from intracellular stores (Berendji et al. 1997; Aizenman et al. 2000; Bossy-Wetzel et al. 2004). Here, we aimed to determine the origin of zinc that modifies network excitability and that accumulates in the soma of degenerating neurons following epileptic seizures. We also investigated how the distribution of zinc at the synapse is modified by seizure activity.
Methods
Animals
Male Sprague-Dawley rats (>35 days old) were used for all extracellular recording experiments. For the experiments with sodium diethlydithiocarbamate trihydrate (DEDTC), animals received a single intraperitoneal (i.p.) injection (0.2 g kg−1) 30 min before being killed. For the experiments using a zinc-free diet, male Sprague-Dawley rats (40 to 50 days old) were maintained on freely accessible zinc-free pellets (LabDiet, USA) for at least 80 days. Male Sprague-Dawley rats (14 to 21 days old) were used in all patch-clamp experiments. The protocols were approved by the Animal Protection Committee of Université Laval.
Hippocampal slice preparation
Extracellular recording experiments
Rats were anaesthetized by isoflurane volatile inhalation and decapitated. The brains were quickly removed and transverse slices were prepared in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 130, NaHCO3 25, KCl 3.5, NaH2PO4 1.25, MgCl2 5, CaCl2 1 and glucose 10; saturated with 95% O2–5% CO2, pH 7.4. Sections (400 μm) were cut on a VT1000S microtome (Leica Microsystems, Canada) then transferred to a holding chamber at room temperature (20°C) containing the same bubbled ACSF for at least 1 h before recording.
Patch-clamp experiments
Slices (300 μm) were prepared as described above, except for the cutting medium which was an ice-cold solution containing (mm): sucrose 248, NaHCO3 26, KCl 1, MgCl2 9, CaCl2 1 and glucose 10.
Electrophysiology
Extracellular recordings
Field potentials from CA3 pyramidal neurons were recorded using a patch-clamp electrode (1–3 MΩ) filled with ASCF and coupled to a MultiClamp 700A amplifier (Molecular Devices Corporation, USA), filtered at 10 kHz, operating in the current-clamp mode.
Whole-cell patch-clamp recordings
Slices were placed in a submerged chamber and imaged at 40 × with an Olympus BX51W1 upright microscope. The slices were perfused (1–2 ml min−1) with ACSF (see above). Whole-cell current-clamp recordings were made with glass electrodes (4–6.5 MΩ) filled with a solution containing (mm): potassium gluconate 140, NaCl 4, MgATP 4, NaGTP 0.3, Hepes 10 and phosphocreatinine 0.2. Recordings were performed with a Multiclamp 700A amplifier (Molecular Devices Corporation) from visually identified CA3 pyramidal cells. Uncompensated series resistance and input resistance (Ri) were monitored by the delivery of −10 mV voltage steps throughout the experiment, and recordings were discontinued following changes of >15%.
Intraventricular infusion of CaEDTA, ZnEDTA, CuEDTA, tricine and ethylenediamine-N,N′-diacetic-N,N′-di-β-propionic acid
Chelators were continuously delivered (8 μl h−1) by Alzet mini-osmotic pumps (model 2001D, DURECT Corporation, USA) into the lateral ventricle. Pumps were filled with 0.9% NaCl, 30 mm tricine, 250 μm ethylenediamine - N,N′ - diacetic - N,N′ - di-β-propionic acid (EDPA) or 10 mm CaEDTA, ZnEDTA or CuEDTA dissolved in 0.9% NaCl. In all cases, 1 mg cresyl violet was added to verify the injection site. The rats were anaesthetized with 2% isoflurane mixed with oxygen. The ears and scalp were locally anaesthetized by subcutaneous injections of xylocaine (13 mg kg−1) and the head was secured on a stereotaxic apparatus (Narishige Instrument Company, USA). Pumps were implanted according to the manufacturer's instructions. Residual liquid in the pumps was recorded at the end of each experiment. We wanted to determine the exact concentration of CaEDTA in the hippocampus after several hours of perfusion using the implanted minipumps. Therefore, we implanted minipumps delivering CuEDTA and measured the amount of CuEDTA present in the hippocampus after several hours using a chemical reaction with (NH4)2S. At the end of the reaction, we measured the weight of precipitated CuS. Using a calibration curve we determined that after 5 h continuous delivery of 10 mm CuEDTA into the lateral ventricle the concentration of CuEDTA was 4.6 ± 1.3 mm (n = 3) in the contralateral hippocampus. Because CuEDTA, CaEDTA and ZnEDTA have similar molecular weight and charge, we assumed that these chemicals were also present in the contralateral hippocampus at a concentration of approximately 5 mm.
Intrahippocampal injection of ZnCl2
A 1.5 μl solution of 1 mm ZnCl2 and 50 μm sodium pyrithione dissolved in PBS was injected into the left hippocampus with a Hamilton syringe attached to the stereotaxic apparatus. The injection was controlled by Ultra MicroPump II syringe pump (World Precision Instruments, USA) and the needle remained static within the ventricle for 10 min. The general surgical procedures were carried out as described above.
Seizure induction
Animals were injected i.p. with 10 mg kg−1 kainic acid dissolved in 0.9% NaCl 90 min after recovery from anaesthesia. Seizures were stopped with an i.p. injection of 75 mg kg−1 sodium phenytoin. The behaviour of animals was monitored for several hours after kainic acid injection. Only the data from rats that reached status epilepticus (78%) were considered.
Tissue preparation
Rats were deeply anaesthetized with ketamine-xylazine (85 and 13 mg kg−1, respectively, i.p.) and decapitated 10 h after the kainic acid injection. Brains were removed, quickly frozen in dry ice and isopentane, then stored at −80°C. Coronal sections (30 μm) of the contralateral hemisphere were cut using a cryostat and mounted on gelatin-coated glass slides. (N-(6-methoxy-8-quiolyl) paratoluenesulphonamide (TSQ)-staining to label zinc was performed (Vogt et al. 2000), followed by terminal deoxynuclesotidyl transferase biotin-dUTP nick end labelling (TUNEL)-staining to visualize dying neurons (Roche Diagnostics, Germany) according to the manufacturer's instructions. TSQ-stained sections were examined under fluorescence microscope (excitation, 360–370 nm; dichromatic beamsplitter, 400 nm, barrier filter, 420 nm) and photographed before TUNEL-staining. Filter settings for TUNEL-staining were as follows: excitation, 545–550 nm, dichromatic beamsplitter, 600 nm, barrier filter, 610 nm.
Electron microscopy
Animals used for the electron microscopy were deeply anaesthetized (ketamine, 50 mg kg−1) and transcardially perfused after the first or second stage 5 seizure (Racine, 1972) first with a buffered sodium sulphide solution (12 g Na2S.9H2O and 12 g NaH2PO4.H2O in 1000 ml of distilled water, pH 7.4; 0.05 m) for 1 min, then with a buffered 3% glutaraldehyde solution in 0.12 m PBS (pH 7.4) for 20 min, and finally with the sodium sulphide solution again for 15 min. Brains were removed from the skull, postfixed in the buffered 3% glutaraldehyde solution for 2 h and sectioned with a Vibratome 1000 at 50 μm. Free-floating sections were washed with Tris buffer (pH 7.4) for 5 min periods in order to eliminate adsorbed phosphate ions, which would react with silver ions causing an unwanted precipitation. Thereafter, sections were placed in the physical developer containing sodium tungstate as protective colloid, hydroquinone as reducing agent, sodium acetate and acetic acid to adjust the pH and silver nitrate to visualise zinc (for further details see Seress & Gallyas, 2000). The process of development was stopped by placing the sections into 1% sodium thiosulphate for 1 min. Next, the sections were washed with Tris buffer for 5 min, then osmificated with 1% OsO4 for 1 h, dehydrated, and flat-embedded in Durcupan according to routine electron microscopic procedure. After microscopic examination, the area of interest was cut, re-embedded and thin sectioned. Thin sections were stained with uranyl acetate and lead citrate. A JEOL JEM-1200EX II electron microscope was used for electron microscopy analysis. Throughout the animal experiments, Principles of Laboratory Animal Care (NIH publication no. 86–23, revised 1985) and the regulations of the Hungarian Law for the Protection of Animals were observed.
Quantification of zinc staining
Black particles indicating zinc were counted manually in standard-sized boxes on the micrographs. For the spatial distribution, terminals were divided into four compartments and the vesicles counted. All data are expressed as density for reliable comparison.
Quantification of epileptiform discharges
Bursts evoked by the increase of extracellular potassium were quantified using the coastline bursting index (CBI) which calculates the total length of the line representing the burst waveform (Korn et al. 1987). In order to calculate the CBI, the bursts were digitized (10 Hz) and cursors were placed before and after the burst waveform, the total length of the line between the cursors was calculated and a segment of the same length measured from a burst-free zone of the recording was subtracted.
Drugs
CaEDTA, CuEDTA, DEDTC, diethylenetriamin-epentaacetic acid (DTPA), EDPA, N,N,N′,N′-tetrakis2-pyridylmethylethyenediamine (TPEN) and tricine (Sigma-Aldrich, Canada), kainic acid (Ocean Produce, Canada) and ZnEDTA (Fluka, Switzerland) were used in the experiments. These reagents were prepared as stock solutions and stored as recommended. The chelators were diluted in properly oxygenated solutions at pH 7.4.
Statistical analysis
Data are expressed as means ± s.e.m. The statistical significance of differences was assessed with Student's t test unpaired or one-sample t test. In experiments involving multiple comparisons, data were subjected to ANOVA and Scheffe's post hoc test using Origin 7.0 software. The level of significance was set at P < 0.05.
Results
The effect of zinc chelation on the excitability of the CA3 area
Our first aim was to determine whether decreased levels of zinc alter the susceptibility of the hippocampus to electrographic seizures, and if so, which zinc pools are involved. Therefore, we compared the effect of intra- and extracellular zinc chelation and zinc-free diet on the excitability of the CA3 region using a high [K+]o model of epilepsy for status epilepticus (McBain, 1995). Extracellular field potentials were recorded from the stratum pyramidale of the CA3 region and [K+]o was elevated gradually in 1.5 mm steps from 3.5 to 11 mm (Fig. 1). We compared the intensity of the interictal bursts at each level of [K+]o in the following conditions: (a) control; (b) in the presence of the membrane-permeable zinc chelator DEDTC (Lees et al. 1998); (c) animals kept on a zinc-free diet for at least 80 days; and (d) in the presence of CaEDTA, a membrane-impermeable zinc chelator in the extracellular solution. Spontaneous interictal bursts were not observed in any of these conditions until the [K+]o was elevated to 8 mm. To compare the burst intensity, we measured three parameters: CBI, interburst interval and burst duration (Fig. 1A). Subtle changes in synaptic strength were shown to effectively modify interburst interval and burst duration (Staley et al. 1998; Bains et al. 1999; Yee et al. 2003). Therefore, we monitored these parameters to detect possible changes in network excitability that synaptically released zinc might cause. At 8 mm [K+]o no significant difference was observed among the groups we tested (Fig. 1B and C). However at 9.5 and 11 mm [K+]o the DEDTC-treated (7.91 ± 1.74 and 6.01 ± 1.61, respectively; P < 0.01) and zinc-free diet groups (6.32 ± 2.39 and 3.17 ± 0.40, respectively; P < 0.05) showed significantly higher CBI than the control group (2.65 ± 0.42 and 1.62 ± 0.39, respectively) (Fig. 1B and C). In contrast, CBI measurements in the CaEDTA group were not significantly different from the control group at any [K+]o (Fig. 1B and C). Interburst interval (Fig. 1D) and burst duration (Fig. 1E) were not significantly different under any of the above conditions.
Figure 1. Chelation of both intra- and extracellular zinc, but not extracellular zinc alone, increases the excitability in the CA3 region.
Interictal bursts in the CA3 area are induced by gradual elevation of [K+]o. A, burst activity was quantified using the burst duration, the interburst interval and the coastline bursting index (CBI). B, traces are representative of the mean CBI value for each condition. C–E, values are given as the mean ± s.e.m. (Control, n = 10; DEDTC, n = 8; zinc-free diet, n = 6; CaEDTA, n = 6). The chelating methods to decrease zinc concentration not only in the extracellular space but also in intracellular pools significantly increased the CBI at 9.5 and 11 mm [K+]o, whereas all types of zinc depletion had no effect on the burst interval or burst duration.
Certain parameters chosen here to assess the burst intensity could differ from one slice to another. Furthermore the molecule used to chelate extracellular zinc, CaEDTA, is known to possess slow binding kinetics. To confirm our results, we repeated the experiments with two alternative membrane-impermeable zinc chelators as well as two membrane-permeable chelators in in vitro slices (Fig. 2). Interictal burst activity was induced by perfusing the slices with 9.5 mm [K+]o. Once bursting was stable, we applied the chosen chelator onto the slice. Changes in the parameters measured were expressed as percentage of the control value. Both membrane-impermeable zinc chelators, EDPA (100 μm) and tricine (1 mm) combined with DTPA (10 μm), as well as membrane-permeable TPEN (1 μm) caused no significant change in burst amplitude, burst duration, interburst interval or CBI. Conversely, perfusion of the slices with the potent zinc chelator DEDTC (200 μm) significantly increased (P < 0.05) both burst amplitude and CBI, while it had no effect on burst duration and interburst period.
Figure 2. Effect of membrane-permeable and membrane-impermeable zinc chelators on CA3 bursting.
A–C, illustrates the variation of the four parameters studied with membrane-impermeable chelator (A) and membrane-permeable chelators (B and C). Each dot represents the average of three bursts for every minute of recording. D–G, graphs indicate that the interburst interval, the burst duration, the amplitude and the CBI do not show significant change (P > 0.05) for the membrane-impermeable chelators EDPA (n = 7) and tricine–DTPA (n = 7) or for the membrane-permeable chelator TPEN (n = 8). Intra- and extracellular zinc chelation with DEDTC (n = 5) increases the CBI and the amplitude of CA3 spontaneous burst.
These data indicate that seizure susceptibility of the CA3 area is not modified by extracellular zinc chelation. In contrast, zinc chelation or depletion techniques that attenuate both extra- and intracellular zinc levels could lead to more intense interictal bursts.
The effect of membrane-permeable zinc chelation on cellular membrane properties
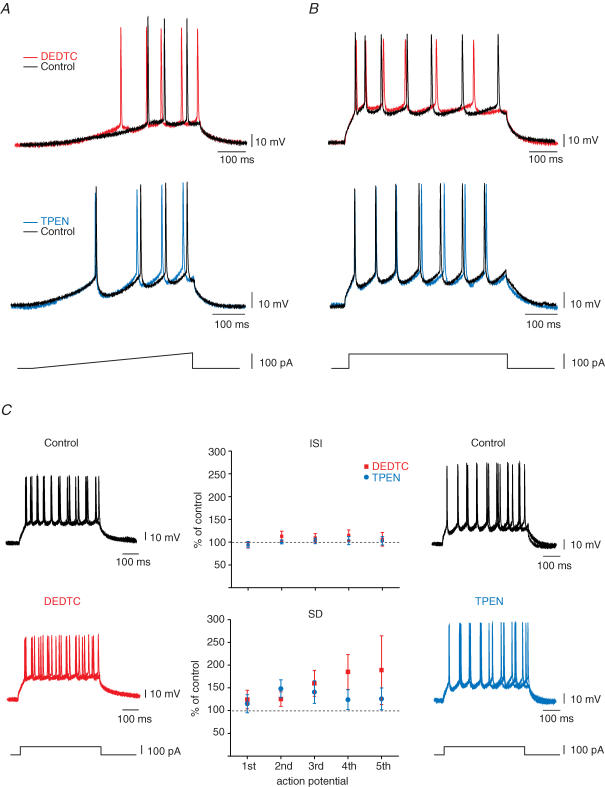
Next, we sought to determine how the membrane-permeable zinc chelator DEDTC influences CBI and burst amplitude. We tested whether this chelator modifies the intrinsic membrane properties of CA3 pyramidal cells. We also compared the effects of DEDTC and TPEN on these parameters because they had different effects on CA3 bursting. We analysed the effect of a 15 min application of DEDTC (200 μm) or TPEN (1 μm) on the firing threshold and the action potential timing in whole-cell current-clamp recordings (Fig. 3). Firing threshold was measured using a 0–100 pA ramp; the precision of action potential generation was measured as a variability of interspike intervals. DEDTC treatment significantly lowered the firing threshold of CA3 pyramidal neurons by 3.2 ± 0.9 mV (n = 11; P < 0.01) whereas the decrease observed with TPEN was not significant (1.1 ± 0.5 mV; n = 7). On the other hand, neither treatment altered the interspike interval generated by 100 pA step-depolarization. Whereas DEDTC tended to increase the heterogeneity of spike timing (140.4 ± 22.1%; P = 0.07), the effect was less pronounced for TPEN (122.4 ± 18.3%).
Figure 3. Effect of DEDTC on intrinsic cellular properties.
CA3 pyramidal cells were recorded in whole-cell current-clamp at membrane potential below the level of spontaneous firing. A and B, superimposed traces represent response to ramp-(A) and step-depolarization (B) in control (black) and after application of 200 μm DEDTC (red) or 1 μm TPEN (light blue). C, graphs indicate that intra- and extracellular chelation with DEDTC or TPEN has no effect on the interspike interval, but tends to change the homogeneity of spike timing in individual cells.
Treatment with both membrane-permeable chelators showed a small but non-significant decrease in spike amplitude (DEDTC, −6.35 ± 1.7 mV, n = 11; TPEN, −4.20 ± 2.2 mV, n = 7), and this change is not related to the depolarization or hyperpolarization caused by the treatment. Moreover, neither the resting potential (control, −55.5 ± 2.6 mV; DEDTC, −55.2 ± 4.3 mV; and control, −48.9 ± 2.5 mV; TPEN, −47.3 ± 1.8 mV), nor the input resistance (control, 279.7 ± 61.8 MΩ; DEDTC, 273.7 ± 51 MΩ; and control, 276 ± 23.8 MΩ TPEN, 266.1 ± 30.2 MΩ) changed significantly after application of chelator.
The origin of free intracellular zinc in dying cells
In the previous experiments, we examined the effect of zinc chelation on the excitability of the CA3 area. With extracellular zinc chelation we assessed whether zinc regulates the activity of the CA3 population by modulating ion channels and neurotransmitter receptors. In addition to its regulatory effect on ion channels and neurotransmitter receptors, zinc is also known to permeate certain receptors during increased activity and trigger cell death (Sensi et al. 1999b, 2000). Here, we investigated whether intracellular free zinc originates from intracellular and/or extracellular sources. It has been reported that CaEDTA reduces lethal zinc accumulation, suggesting that zinc may originate from presynaptic terminals (Koh et al. 1996; Calderone et al. 2004). However at high concentrations, CaEDTA may also affect intracellular zinc levels (Frederickson et al. 2002). In order to quantify the extent to which CaEDTA can influence intracellular stores, we injected 300 mm CaEDTA into the lateral ventricle and visualized mossy fibre zinc content with TSQ-staining. Mossy fibre TSQ-staining was reduced by 56.8% (ΔF/F: control, 0.51 ± 0.12; CaEDTA, 0.22 ± 0.08; n = 4 for both; Fig. 4A).
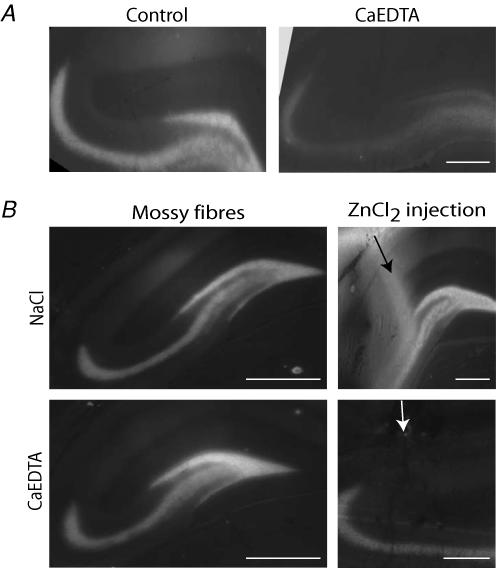
Figure 4. New method to reliably chelate extracellular zinc in ‘in vivo’ experiments.
A, TSQ staining in the hippocampus in a control animal and in a rat injected with CaEDTA. CaEDTA (300 mm) injected into the lateral ventricle resulted in the chelation of the zinc content of mossy fibres, indicating that at this very high concentration CaEDTA does not only act as an extracellular chelator but also influences the intracellular zinc pools. Scale bar, 200 μm. B, in four animals, after the implantation of the pumps, we injected 1 mm ZnCl2 plus 50 μm sodium pyrithione into the hippocampus. In control animals (0.9% NaCl in the pump) this resulted in a strongly diffused zinc labelling in the CA1 and the dentate gyrus. However, this labelling was blocked when the pumps delivered CaEDTA, while mossy fibre staining remained unaffected indicating that this method could be used to differentiate between extra- and intracellular zinc pools. Scale bars, B (first column), 400 μm and A, B (second column), 100 μm.
These data show that intraventricular injection of high concentrations of CaEDTA does not permit reliable differentiation between extracellular and intracellular zinc pools. Therefore, we searched for a technique with which we could deliver chelators at low concentrations. We implanted mini-osmotic pumps that continuously delivered 10 mm CaEDTA into the right ventricle, which resulted in approximately 5 mm in the contralateral hippocampus (see Methods). In the first set of experiments, 2 h after pump implantation we injected ZnCl2 directly into the left hippocampus to demonstrate that this delivery method can successfully chelate zinc in the extracellular space (Fig. 4B). Our data show that whereas diffuse TSQ-labelling was observed in control animals after ZnCl2 injection, this could be prevented with the administration of CaEDTA via the pumps. We also show that CaEDTA used at such a low concentration does not alter mossy fibre staining, indicating that intracellular zinc levels are not modified with this protocol (Fig. 4B).
In the second set of experiments, 2 h after the implantation of the pump the animals received an i.p. injection of kainic acid and were killed 10 h later (a timepoint based on our previous work which showed the highest concentration of zinc in dying neurons (Cote et al. 2005)). Control animals were implanted with pumps delivering 10 mm ZnEDTA. We used TSQ-staining to visualize intracellularly accumulated zinc and TUNEL-staining to determine whether cells were dying (Fig. 5). We found that in the CaEDTA-treated animals (n = 5) the number of TSQ-labelled cells per section was 5.4 ± 1.6 in the CA3 region, 2.3 ± 1.1 in the CA1 and 2.8 ± 0.8 in the hilus. A total of 87.7% of these cells were also TUNEL positive. Of the TUNEL-positive cells, 87.5% were TSQ positive. This indicates that the vast majority of zinc-accumulating neurons will eventually die and most of the dying cells accumulate zinc. In the ZnEDTA-treated control animals (n = 5), 6.7 ± 1.6 TSQ-positive cells were counted per section in the CA3, 2.8 ± 0.8 in the CA1 and 2.5 ± 0.8 in the hilus. A total of 83.7% of the TSQ-labelled cells were TUNEL positive and 79.8% of the TUNEL-positive cells were TSQ-labelled. Data collected from the two groups were not statistically different. We also compared the spatial distribution and TSQ intensity of the labelled cells in these two groups. We found that the intensity of TSQ-staining in animals treated with CaEDTA was somewhat lower (ΔF/F: CaEDTA, 0.32 ± 0.08; ZnEDTA, 0.42 ± 0.08; P < 0.05). However, this did not influence the number of dying cells detected after seizures. These experiments were also performed using two other membrane-impermeable zinc chelators, tricine (n = 4) and EDPA (n = 5). The number of zinc-accumulating and dying cells were not significantly different from ZnEDTA-injected control animals (Fig. 5). These data suggest that the intracellularly accumulating zinc that triggers cell death in vulnerable neurons originates from intracellular rather than extracellular sites.
Figure 5. Extracellular zinc chelation does not block intracellular zinc accumulation after seizures.
Animals were implanted with Alzet mini-osmotic pumps delivering extracellular zinc chelators at a constant rate (8 μl h−1) into the lateral ventricle. ZnEDTA was used as a control. Animals were injected with kainic acid 2 h after the implantation of the pumps, and killed 10 h later. A, cryostat sections were stained with TSQ to visualize zinc accumulation and with TUNEL to assess cell death. Pictures are representative examples of TSQ-stained sections showing the CA3 area of the hippocampus. Arrows indicate zinc-accumulating neurons. B, bar graphs show the normalized number of TSQ- and TUNEL-positive cells. The number of zinc-accumulating neurons and dying cells after seizures were not statistically different in the presence of extracellular zinc chelators. ZnEDTA, n = 6; CaEDTA, n = 6; tricine, n = 6; EDPA, n = 2. Scale bars, 100 μm.
Altered zinc levels after seizures at the pre- and postsynaptic sites
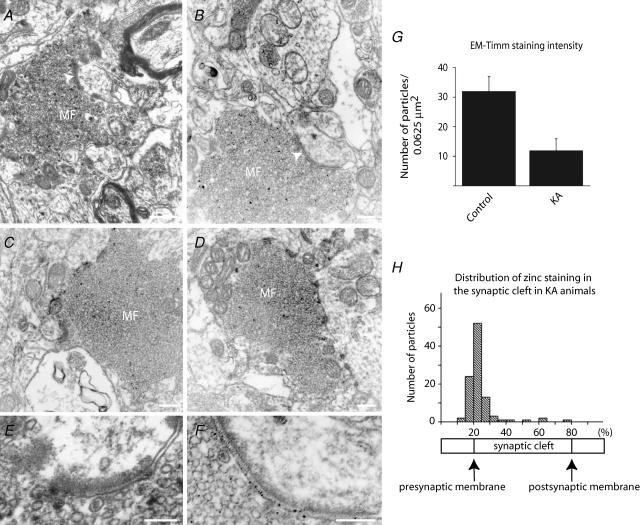
We showed that zinc release from intracellular stores leads to increased zinc concentration in the cytosol of sensitive neurons. This process is relatively slow and becomes apparent only several hours after the first seizures. In contrast, in presynaptic mossy fibre terminals zinc concentration is rapidly decreased following seizures. This dramatic reduction in vesicular zinc staining has been detected using fluorescent zinc probes (Frederickson et al. 1988; Kay & Tóth, 2006). It was assumed that the zinc content in mossy fibres decreases during seizures because zinc is released and translocates into the postsynaptic cell. We pursued the possibility that zinc translocates from mossy fibre terminals to postsynaptic cells by visualizing the zinc content in pre- and postsynaptic elements in control and epileptic animals. We used a highly sensitive sodium tungstate Timm method to detect vesicular zinc at the electron microscopic level. This method produces round silver grains that are small enough to precisely locate zinc both inside synaptic terminals and in the synaptic cleft (Seress & Gallyas, 2000; Seress et al. 2001). We investigated 25 terminals from control and 25 terminals from kainic acid-treated animals. KA-injected animals were killed immediately after the first seizures. We quantified the Timm-staining intensity by counting the silver grains located inside the mossy fibre terminals. Our data indicate that the intensity of zinc staining was significantly decreased by 64.3% (P < 0.05) in kainic acid-treated animals (n = 3) when compared to samples collected from non-treated rats (n = 3) (Fig. 6A–G). In order to determine the distribution of zinc staining inside the synaptic cleft, we expressed the location of silver grains relative to the pre- and postsynaptic membranes (see Methods). Whereas in control animals zinc staining was observed only very rarely inside the synaptic cleft, in kainic acid-treated animals silver grains were apparent in most synaptic contacts established by large mossy fibre terminals. Our data demonstrate that zinc staining was predominantly concentrated on the presynaptic membrane in kainic acid-treated animals. A total of 89.6% of the silver grains were in close proximity with the presynaptic surface and only a small portion of the silver grains were located close to or on the postsynaptic membrane.
Figure 6. Vesicular zinc is diminished in the mossy fibres after seizures.
A–F, electron micrographs of mossy fibres from control (A and E) and kainic acid-treated (B–D and F) animals. Animals were killed after the first major seizure. Zinc is visualized on these sections with a modified Timm staining (Seress & Gallyas, 2000). Black, granular precipitates represent chelatable zinc. White arrows indicate synapses. Zinc is not observed in the synaptic cleft in control animals (E) but is clearly visible after the first seizure (F). G, data collected from 25 terminals; granular precipitates were counted manually in a standard-sized square. H, In epileptic animals, zinc was found predominantly at the presynaptic surface and only very rarely on the postsynaptic site. Data were normalized from 50 synapses, synaptic cleft represents 20–80% of the standardized line, 0–20% represents the cytosol of the presynaptic, 80–100% the postsynaptic cell. Data were collected manually. Scale bars: A–D, 200 nm; E and F, 100 nm.
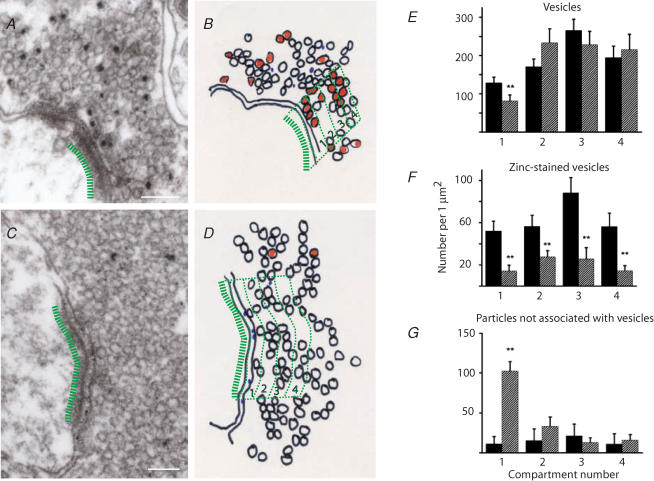
Small particles indicating the presence of zinc were found predominantly (92.4%) over synaptic vesicles in control animals. Next, we investigated whether the distribution of zinc-positive and zinc-negative synaptic vesicles in the mossy fibre terminals is influenced by seizures. We identified four compartments per synapse, each 75 nm wide starting from the active zone (Fig. 7). We calculated the total vesicle density and the density of zinc-positive vesicles in each compartment. We also measured the density of zinc labelling that was not associated with synaptic vesicles in both control and kainic acid-treated animals. The first compartment is composed mostly of docked vesicles. The density of synaptic vesicles was lowest in this compartment and it was significantly decreased in kainic acid-treated animals when compared to controls (control, 128.4 ± 15.1 vesicles μm−2; kainic acid, 81 ± 14.3 vesicles μm−2; P < 0.05). In contrast, synaptic vesicle density did not change significantly in the other three compartments after seizures (Fig. 7E). The density of zinc-positive vesicles decreased in all four compartments in kainic acid-treated animals, and the relative proportion of zinc-positive vesicles was not significantly different in any of the compartments (1st, 39.8%; 2nd, 32.9%; 3rd, 34.3%; 4th, 28.7%; Fig. 7F). These data show that zinc-positive vesicles are evenly distributed within synaptic terminals, and zinc staining is uniformly decreased in each compartment after seizures. We also investigated the distribution of particles that were not associated with synaptic vesicles. In control animals, the relative contribution of these particles was very small (7.6% of the number of particles associated with vesicles). Conversely, in kainic acid-treated animals there was a substantial increase in the number of these particles in the first compartment; this increase is the result of zinc staining observed on the presynaptic membrane surface (Fig. 7G).
Figure 7. The distribution of zinc within the terminals.
Spatial distribution of synaptic vesicles in control (A and B) and in kainic acid-treated (C and D) animals within mossy fibre terminals as a function of distance from the synapse. Electron micrographs of terminals (A and C) were divided into four compartments as shown in B and D. Compartment 1 is closest to the active zone. The number of vesicles were calculated in each compartment and expressed as vesicle density. E, vesicle density of all vesicles in control (filled bars) and kainic acid-treated animals (hatched bars); a significant difference was only observed in compartment 1 (P < 0.05). F, vesicle density of zinc-positive vesicles; vesicle density was significantly lower in each compartment in kainic acid-treated animals (P < 0.05), G, density of particles that are not associated with vesicles. The robust increase in the first compartment is due to the appearance of particles on the presynaptic membrane in kainic acid-treated animals as shown on Fig. 6F. Scale bars, 100 nm.
Discussion
In this study we have demonstrated that (a) decreasing extracellular zinc concentration does not modify the excitability of the CA3 network; however, diminished intracellular zinc content can increase the interictal burst intensity, most probably via a decrease in neuronal firing threshold. (b) Following seizures, intracellularly accumulating zinc in vulnerable neurons is not released from presynaptic terminals but rather from intracellular stores. (c) Epileptic seizures decrease mossy fibre zinc content, and extracellular zinc staining is largely associated with the presynaptic, not the postsynaptic, membrane surface.
Extracellular zinc chelation had no effect on the excitability of the CA3 area in our experiments. Membrane-impermeable zinc chelators may have been ineffective under our experimental conditions because either zinc was not released, or because the sum of its effects on various receptors and different types of cells is close to zero (Timofeeva & Nadler, 2006). Alternatively, our zinc-chelating methods were not fast or strong enough to chelate synaptically released zinc. However, although multiple research groups have proposed that zinc is released from the mossy fibres (Assaf & Chung, 1984; Howell et al. 1984; Vogt et al. 2000; Li et al. 2001a,b; Qian & Noebels, 2005), recent data suggest that alternative possibilities exist (Kay, 2003; Kay & Tóth, 2006). Because our data show that extracellular zinc chelators do not change significantly the excitability of the CA3 area, this suggests that the primary role of zinc is not the direct control of activity of the CA3 neuronal population.
CaEDTA has been shown to successfully chelate extracellular zinc (Vogt et al. 2000; Molnar & Nadler, 2001; Ruiz et al. 2004); however, it has been suggested that its binding kinetics are too slow to successfully chelate synaptically released zinc, because calcium ions must unbind before zinc ions can bind to EDTA (Vogt et al. 2000). Due to this potential problem with CaEDTA, we also tested other extracellular zinc chelators: tricine and EDPA. Neither of these chelators need to unbind to another ion before binding to zinc, and EDPA chelates zinc faster than CaEDTA (Kay, 2003). However, none of the three currently available membrane-impermeable zinc chelators tested in this study affected either network excitability or seizure-induced intracellular zinc accumulation.
In this study, we have demonstrated that zinc chelation with DEDTC enhances hippocampal excitability, whereas chelation with TPEN and selective chelation of extracellular zinc did not increase the intensity of interictal bursts. Our data indicate that in the presence of the membrane-permeable chelator DEDTC, the action potential threshold is significantly decreased which could increase the number of cells contributing to a burst, and therefore increase the CBI. TPEN is a more specific zinc chelator than DEDTC which could explain the differences observed with the two membrane-permeable zinc chelators. Changes in burst amplitude, CBI and spike timing caused by DEDTC might result from non-specific chelation of other heavy metal ions such as copper, which plays a crucial role in neuronal excitability (Mathie et al. 2006). Alternatively, DEDTC and TPEN vary in their ability to remove zinc from proteins. Zinc is a cofactor for many enzymes and a structural element of several non-enzymatic proteins (Vallee & Falchuk, 1993); functional disruption of one or more of these proteins potentially could lead to changes in neuronal excitability.
While seizure-induced cell death was decreased with zinc-free diet (Cote et al. 2005), the present study provides evidence that the use of extracellular zinc chelators do not decrease the number of degenerating neurons in epileptic animals. A zinc-free diet reduces zinc concentration in both the extra- and intracellular space. In contrast, CaEDTA, EDPA and tricine only chelate extracellular zinc. Therefore, we conclude that the lethal concentration of free zinc in the cytosol of vulnerable neurons originates from intracellular stores. Opposite conclusions have also been reached (Koh et al. 1996; Lee et al. 2000b) from studies showing that a single intraventricular injection of high CaEDTA concentration (100–300 mm) reduces neuronal death after seizures and ischaemia. However, CaEDTA at such a high concentration might have led to osmotic imbalance in the brain tissue which, in turn, could deplete intracellular zinc content even though this chelator is nominally membrane impermeant. The osmotic shock from such a high concentration of CaEDTA may simply permeabilize the cells. This possibility is further supported by the observation that this concentration of CaEDTA can chelate vesicular zinc from the mossy fibres (Frederickson et al. 2002). Even though the concentration of CaEDTA that we used is much lower, there was a small but significant (21%) decrease in the concentration of intracellularly accumulated zinc compared to the concentration in the ZnEDTA-treated group. However, this decrease was not large enough to have an impact on the fate of the vulnerable cells. The CaEDTA concentration we used (5 mm in the tissue) is higher than the concentration that is generally applied to efficiently chelate extracellular zinc in in vitro slice experiments (1–2.5 mm) (Vogt et al. 2000). Therefore, we are confident that extracellular zinc was sufficiently chelated.
Zinc uptake in degenerating neurons via calcium-permeable AMPA receptors has been convincingly demonstrated in cell cultures (Sensi et al. 1999a). In these experiments, zinc was applied extracellularly in the micromolar range, but in vivo zinc release from the mossy fibres may never reach this level (Kay, 2003). This is further supported by the observation that following seizures, zinc accumulation and neuronal cell death are not altered in vesicular zinc transporter (ZnT3) knockout mice (Lee et al. 2000a). Our data suggest that even though calcium-permeable AMPA receptors are zinc permeable and permit accumulation of lethal concentrations of exogenously applied zinc in vitro, endogenously released zinc may not reach comparable levels in the extracellular space in vivo. Nonetheless, these receptors could play an important role in neuronal vulnerability as they are permeable to calcium which can lead to zinc release from intracellular pools (Bossy-Wetzel et al. 2004). Intracellular zinc release from mitochondria (Sensi et al. 2003) and metallothioneins (Maret, 1994; Maret & Vallee, 1998) have been demonstrated, therefore both could act as zinc reservoirs responsible for accumulating cytosolic free zinc.
Immediately after seizures, zinc concentration in the mossy fibres is dramatically decreased. Previously it has been suggested that this decrease is the result of massive zinc release during seizures, which leads to zinc ‘translocation’ into the postsynaptic cells (Frederickson et al. 1989; Suh et al. 2001). If zinc is released at high concentration and translocates to postsynaptic cells, we would expect to see an increased zinc concentration in the synaptic cleft and on the postsynaptic membrane surface. However, our data show that even though vesicular zinc concentration is decreased, zinc concentration is not increased inside the synaptic cleft or on the postsynaptic membrane, but it is restricted to the presynaptic membrane. Because we perfused the animals rapidly after the first seizure, we presume that the zinc distribution accurately reflects the state of increased synaptic activity. Such an asymmetrical trans-synaptic distribution might arise in several ways: either (a) zinc is released and a very rapid uptake system removes it immediately from the synaptic cleft or (b) zinc is tightly bound to postsynaptic membrane receptors or other proteins, and it is not accessible with our staining method, and (c) zinc is not released but ‘externalized’ during increased synaptic activity (Kay, 2003, 2006; Kay & Tóth, 2006). Such a rapid uptake system must work several times more efficiently than the glutamate uptake system because glutamate can reach not only the postsynaptic site but also neighbouring synapses (Asztely et al. 1997; Vogt & Nicoll, 1999). While we cannot rule out the possibility that such an uptake system exists, the previously proposed externalization model (Kay, 2003) offers an alternative explanation for our results. If zinc is loosely bound to vesicular proteins and does not diffuse into the extracellular space, staining on the pre- but not postsynaptic membrane surface would be expected upon increased synaptic activity.
In previous work, zinc staining in control animals was observed in the synaptic cleft (Seress & Gallyas, 2000). In that study, presynaptic terminals innervating parvalbumin-postive interneurons were investigated, whereas our study focused on large mossy terminals. Interneurons are innervated by small mossy fibre filopodias, while pyramidal cells receive their inputs from the main mossy terminal (Acsady et al. 1998). Differences in zinc staining between small filopodias and the large mossy fibre terminals have also been documented using the autometallographic zinc sulphide-staining method (Danscher, 1996). Therefore, it is possible that the different zinc staining observed in the synapses facing interneurons and pyramidal cells indicates a target cell-specific mechanism that provides this terminal with the ability to differentially regulate the rate of vesicle fusion at these distinct synapse types (Lawrence et al. 2004).
After seizures, the number of zinc-positive vesicles in mossy terminals decreased by 68.4%, whereas the total number of vesicles was unchanged. If this reduction results from a preferential exocytosis of zinc-positive vesicles during increased activity, we would expect a 23.2% decrease in the total number of vesicles because zinc-positive staining was observed in 33.9% of the vesicles. The fact that the number of vesicles is not changed after seizures and the number of zinc-stained vesicles is uniformly decreased in the terminal, suggests that zinc staining is diminishing not due to increased release but rather due to a mechanism that eliminates free, histologically detectable intravesicular zinc. This mechanism is not known; one possibility is that seizures alter the pH in the synaptic vesicles leading to an altered binding between the ion and intravesicular proteins and macromolecules.
Our data indicate that after seizures zinc content is decreased in mossy fibre terminals and is augmented in vulnerable interneurons. It is tempting to conclude that the reduction in zinc levels in the presynaptic terminals is the cause of the increase in zinc levels in postsynaptic cells. However, our study provides evidence that the link between decreased zinc concetration in mossy fibre terminals and increased zinc level in postsynaptic cells is more complex. Simple translocation of zinc is unlikely because (a) the time course of the two events is very different. Decreases in mossy fibre zinc content can be observed immediately after seizures, whereas postsynaptic increase is only detectable 10 h later. (b) Extracellular zinc chelation does not have measurable effects on excitability. (c) Extracellular chelators do not prevent somatic zinc accumulation in vulnerable cells. (d) In addition, zinc can only be detected on the pre- but not on the postsynaptic membrane surfaces at mossy fibre synapses innervating CA3 pyramidal cells. While further experiments are required to unveil the mechanism for the decrease in zinc staining in mossy fibres after seizures, our data indicate that intracellularly accumulated zinc in vulnerable neurons is released from intracellular stores. These two consequences of seizure activity are not directly related to each other.
Acknowledgments
This work was supported by Canadian Institute of Health Research (CIHR) (Fellowship and Operating grant to K.T.), and N.L. was supported by Centre de Recherche sur le Cerveau, le Comportement et la Neuropsychiatrie (CRCN) and Natural Sciences and Engineering Research Council of Canada (NSERC) scholarships. We would like to thank Drs Richard Miles and Alan Kay for their comments on the manuscript.
References
- Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999;2:720–726. doi: 10.1038/11184. [DOI] [PubMed] [Google Scholar]
- Berendji D, Kolb-Bachofen V, Meyer KL, Grapenthin O, Weber H, Wahn V, Kroncke KD. Nitric oxide mediates intracytoplasmic and intranuclear zinc release. FEBS Lett. 1997;405:37–41. doi: 10.1016/s0014-5793(97)00150-6. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E, Gotz T, Han J, Ellisman MH, Perkins GA, Lipton SA. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- Calderone A, Jover T, Mashiko T, Noh KM, Tanaka H, Bennett MV, Zukin RS. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004;24:9903–9913. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol. 1997;51:1015–1023. doi: 10.1124/mol.51.6.1015. [DOI] [PubMed] [Google Scholar]
- Cole TB, Robbins CA, Wenzel HJ, Schwartzkroin PA, Palmiter RD. Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res. 2000;39:153–169. doi: 10.1016/s0920-1211(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Cote A, Chiasson M, Peralta MR, 3rd, Lafortune K, Pellegrini L, Tóth K. Cell type-specific action of seizure-induced intracellular zinc accumulation in the rat hippocampus. J Physiol. 2005;566:821–837. doi: 10.1113/jphysiol.2005.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danscher G. Exogenous selenium in the brain. A histochemical technique for light and electron microscopical localization of catalytic selenium bonds. Histochemistry. 1982;76:281–293. doi: 10.1007/BF00543951. [DOI] [PubMed] [Google Scholar]
- Danscher G. The autometallographic zinc-sulphide method. A new approach involving in vivo creation of nanometer-sized zinc sulphide crystal lattices in zinc-enriched synaptic and secretory vesicles. Histochem J. 1996;28:361–373. doi: 10.1007/BF02331399. [DOI] [PubMed] [Google Scholar]
- Dominguez MI, Blasco-Ibanez JM, Crespo C, Marques-Mari AI, Martinez-Guijarro FJ. Zinc chelation during non-lesioning overexcitation results in neuronal death in the mouse hippocampus. Neuroscience. 2003;116:791–806. doi: 10.1016/s0306-4522(02)00731-5. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Westbrook GL, Mayer ML. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988;8:3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ, Hernandez MD, Goik SA, Morton JD, McGinty JF. Loss of zinc staining from hippocampal mossy fibers during kainic acid induced seizures: a histofluorescence study. Brain Res. 1988;446:383–386. doi: 10.1016/0006-8993(88)90899-2. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Hernandez MD, McGinty JF. Translocation of zinc may contribute to seizure-induced death of neurons. Brain Res. 1989;480:317–321. doi: 10.1016/0006-8993(89)90199-6. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Koh JY, Cha YK, Thompson RB, LaBuda CJ, Balaji RV, Cuajungco MP. Depletion of intracellular zinc from neurons by use of an extracellular chelator in vivo and in vitro. J Histochem Cytochem. 2002;50:1659–1662. doi: 10.1177/002215540205001210. [DOI] [PubMed] [Google Scholar]
- Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308:736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn2+ induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- Kay AR. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn. J Neurosci. 2003;23:6847–6855. doi: 10.1523/JNEUROSCI.23-17-06847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. Imaging synaptic zinc: promises and perils. Trends Neurosci. 2006;29:200–206. doi: 10.1016/j.tins.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Kay AR, Tóth K. Influence of location of a fluorescent zinc probe in brain slices on its response to synaptic activation. J Neurophysiol. 2006;95:1949–1956. doi: 10.1152/jn.00959.2005. [DOI] [PubMed] [Google Scholar]
- Kim EY, Koh JY, Kim YH, Sohn S, Joe E, Gwag BJ. Zn2+ entry produces oxidative neuronal necrosis in cortical cell cultures. Eur J Neurosci. 1999;11:327–334. doi: 10.1046/j.1460-9568.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Korn SJ, Giacchino JL, Chamberlin NL, Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987;57:325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, Grinspan ZM, McBain CJ. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J Physiol. 2004;554:175–193. doi: 10.1113/jphysiol.2003.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Cole TB, Palmiter RD, Koh JY. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: evidence against synaptic vesicle origin. J Neurosci. 2000a;20:RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Park J, Kim YH, Kim DH, Kim CG, Koh JY. Induction by synaptic zinc of heat shock protein-70 in hippocampus after kainate seizures. Exp Neurol. 2000b;161:433–441. doi: 10.1006/exnr.1999.7297. [DOI] [PubMed] [Google Scholar]
- Lees GJ, Cuajungco MP, Leong W. Effect of metal chelating agents on the direct and seizure-related neuronal death induced by zinc and kainic acid. Brain Res. 1998;799:108–117. doi: 10.1016/s0006-8993(98)00483-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Hough CJ, Frederickson CJ, Sarvey JM. Induction of mossy fiber→Ca3 long-term potentiation requires translocation of synaptically released Zn2+ J Neurosci. 2001a;21:8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hough CJ, Suh SW, Sarvey JM, Frederickson CJ. Rapid translocation of Zn2+ from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J Neurophysiol. 2001b;86:2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- Lin DD, Cohen AS, Coulter DA. Zinc-induced augmentation of excitatory synaptic currents and glutamate receptor responses in hippocampal CA3 neurons. J Neurophysiol. 2001;85:1185–1196. doi: 10.1152/jn.2001.85.3.1185. [DOI] [PubMed] [Google Scholar]
- McBain CJ. Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol. 1995;73:2853–2863. doi: 10.1152/jn.1995.73.2.2853. [DOI] [PubMed] [Google Scholar]
- Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci U S A. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W, Vallee BL. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc Natl Acad Sci U S A. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maske H. Uber den topochemischen nachwels von zinkim ammonshorn verschiedener saugetiere. Naturwissenschaften. 1955;42:424. [Google Scholar]
- Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther. 2006;111:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L., Jr The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J Physiol. 1989;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar P, Nadler JV. Synaptically-released zinc inhibits N-methyl-D-aspartate receptor activation at recurrent mossy fiber synapses. Brain Res. 2001;910:205–207. doi: 10.1016/s0006-8993(01)02720-2. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM. Endogenous zinc inhibits GABAA receptors in a hippocampal pathway. J Neurophysiol. 2004;91:1091–1096. doi: 10.1152/jn.00755.2003. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003;100:6157–6162. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999a;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Weiss JH. Glutamate triggers preferential Zn2+ flux through Ca2+ permeable AMPA channels and consequent ROS production. Neuroreport. 1999b;10:1723–1727. doi: 10.1097/00001756-199906030-00018. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Weiss JH. AMPA/kainate receptor-triggered Zn2+ entry into cortical neurons induces mitochondrial Zn2+ uptake and persistent mitochondrial dysfunction. Eur J Neurosci. 2000;12:3813–3818. doi: 10.1046/j.1460-9568.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- Seress L, Abraham H, Paleszter M, Gallyas F. Granule cells are the main source of excitatory input to a subpopulation of GABAergic hippocampal neurons as revealed by electron microscopic double staining for zinc histochemistry and parvalbumin immunocytochemistry. Exp Brain Res. 2001;136:456–462. doi: 10.1007/s002210000601. [DOI] [PubMed] [Google Scholar]
- Seress L, Gallyas F. The use of a sodium tungstate developer markedly improves the electron microscopic localization of zinc by the Timm method. J Neurosci Methods. 2000;100:33–39. doi: 10.1016/s0165-0270(00)00227-2. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nat Neurosci. 1998;1:201–209. doi: 10.1038/651. [DOI] [PubMed] [Google Scholar]
- Suh SW, Thompson RB, Frederickson CJ. Loss of vesicular zinc and appearance of perikaryal zinc after seizures induced by pilocarpine. Neuroreport. 2001;12:1523–1525. doi: 10.1097/00001756-200105250-00044. [DOI] [PubMed] [Google Scholar]
- Takeda A, Hirate M, Tamano H, Nisibaba D, Oku N. Susceptibility to kainate-induced seizures under dietary zinc deficiency. J Neurochem. 2003;85:1575–1580. doi: 10.1046/j.1471-4159.2003.01803.x. [DOI] [PubMed] [Google Scholar]
- Timm F. Histochemistry of zinc. Dtsch Z Gesamte Gerichtl Med. 1958;47:428–431. [PubMed] [Google Scholar]
- Timofeeva O, Nadler JV. Facilitation of granule cell epileptiform activity by mossy fiber-released zinc in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2006;1078:227–234. doi: 10.1016/j.brainres.2006.01.051. [DOI] [PubMed] [Google Scholar]
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Vogt KE, Nicoll RA. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci U S A. 1999;96:1118–1122. doi: 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JH, Sensi SL. Ca2+-Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci. 2000;23:365–371. doi: 10.1016/s0166-2236(00)01610-6. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Iwamoto T, Watanabe Y, Sobue K, Inui M. PSD-95 eliminates Src-induced potentiation of NR1/NR2A-subtype NMDA receptor channels and reduces high-affinity zinc inhibition. J Neurochem. 2002;81:758–764. doi: 10.1046/j.1471-4159.2002.00886.x. [DOI] [PubMed] [Google Scholar]
- Yee AS, Longacher JM, Staley KJ. Convulsant and anticonvulsant effects on spontaneous CA3 population bursts. J Neurophysiol. 2003;89:427–441. doi: 10.1152/jn.00594.2002. [DOI] [PubMed] [Google Scholar]