Abstract
Exercise is considered to be beneficial for free fatty acid (FFA) metabolism, although reports of the effects of increased physical activity on FFA uptake and oxidation in different tissues in vivo in humans have been inconsistent. To investigate the heredity-independent effects of physical activity and fitness on FFA uptake in skeletal muscle, the myocardium, and liver we used positron emission tomography (PET) in nine healthy young male monozygotic twin pairs discordant for physical activity and fitness. The cotwins with higher physical activity constituting the more active group had a similar body mass index but less body fat and 18 ± 10% higher V˙O2,max (P < 0.001) compared to the less active brothers with lower physical activity. Low-intensity knee-extension exercise increased skeletal muscle FFA and oxygen uptake six to 10 times compared to resting values but no differences were observed between the groups at rest or during exercise. At rest the more active group had lower hepatic FFA uptake compared to the less active group (5.5 ± 4.3 versus 9.0 ± 6.1 μmol (100 ml)−1 min−1, P = 0.04). Hepatic FFA uptake associated significantly with body fat percentage (P = 0.05). Myocardial FFA uptake was similar between the groups. In conclusion, in the absence of the confounding effects of genetic factors, moderately increased physical activity and aerobic fitness decrease body adiposity even in normal-weighted healthy young adult men. Further, increased physical activity together with decreased intra-abdominal adiposity seems to decrease hepatic FFA uptake but has no effects on skeletal muscle or myocardial FFA uptake.
Abnormalities in free fatty acid (FFA) metabolism are evident in the metabolic syndrome (Arner, 2002). Impaired FFA metabolism is associated with reduced lipid oxidation (Turpeinen et al. 1999; Blaak et al. 2000) and increased body adiposity (Miyazaki et al. 2002; Nguyen-Duy et al. 2003; Pietiläinen et al. 2005). Impaired lipid oxidation leads to a higher concentration of circulating FFAs in the blood and a higher FFA supply for tissue uptake. Increased tissue FFA uptake, especially in the skeletal muscle and liver, inhibits glucose metabolism and further creates a predisposition for impaired glucose tolerance (IGT) (Arner, 2002). Physical activity is known to prevent and enhance impaired FFA metabolism (Goodpaster et al. 2001; de Glisezinski et al. 2003). Endurance training has been shown to enhance whole-body lipid oxidation during low- and moderate-intensity exercise, which is due to the increased uptake of FFAs to the cells and enhanced use of intramyocellular lipid storage (Henriksson, 1977; Martin et al. 1993; Klein et al. 1994). The positive effects of increased physical activity on FFA metabolism are well known in metabolic disorders although the results in healthy young adults are inconsistent (Jansson & Kaijser, 1987; Turcotte et al. 1992; Kiens et al. 1993; Bergman et al. 1999).
In healthy young adults, skeletal muscle lipid oxidation during low- and moderate-intensity exercise has been either increased (Jansson & Kaijser, 1987; Turcotte et al. 1992) or decreased (Bergman et al. 1999), while muscle FFA uptake has been similar (Turcotte et al. 1992; Kiens et al. 1993) or enhanced (Henriksson, 1977; Jansson & Kaijser, 1987; Turcotte et al. 1992; Kiens et al. 1993; Bergman et al. 1999) due to endurance training. These divergent results can partly be explained by the different methods and physiological state used.
Free fatty acids are the major oxidative fuel of the heart. Studies investigating the effects of exercise training on myocardial FFA uptake are few and contradictory suggesting either decreased (Heiss et al. 1976) or similar uptake in endurance-trained athletes compared to sedentary subjects (Turpeinen et al. 1996). When measured using positron emission tomography (PET) and insulin stimulation, myocardial glucose uptake was lower (Nuutila et al. 1994) and FFA uptake similar (Takala et al. 1999) in endurance-trained compared to sedentary subjects suggesting a decreased energy demand.
Lifestyle changes are shown to change visceral fat mass and the whole body lipid and glucose metabolism (Slentz et al. 2005; Pietiläinen et al. 2005). Hepatic triglyceride accumulation is linked to insulin resistance and shown to decrease with exercise intervention (Tamura et al. 2005). Effects of lifestyle on hepatic FFA uptake are largely unknown. When studied during hyperinsulinaemia using 14(R,S)-[18F]fluoro-thia heptadecanoic acid (FTHA) and PET, we have recently shown that hepatic FFA uptake is lower in trained as compared to untrained subjects (Iozzo et al. 2004).
The aim of the present study was to investigate the effects of increased physical activity and fitness on muscle, myocardial, and hepatic FFA uptake without the confounding effects of genetic factors. For that purpose apparently healthy non-obese young adult male monozygotic twins discordant for physical activity and fitness were recruited. Twin pairs were divided into the more active and less active group according to physical activity and V˙O2,max. Skeletal muscle FFA and oxygen uptake were measured at rest and during exercise with 18F-labelled FTHA, 15O2 and PET, and compared between the groups. In addition myocardial and hepatic FFA uptake were measured with 18F-labelled FTHA PET in order to study the association in FFA uptake between different organs.
Methods
Subjects
Subjects were selected from among 3065 twin pairs from five consecutive twin-birth cohorts (born 1975–79), as ascertained from the Central Population Register of Finland. Twins were participating in the ongoing FinnTwin16 study where their health habits including numerous questions on physical activity, have been studied by mailed questionnaires three times in adolescence, with a fourth follow-up in young adulthood, which was completed in 2002 (Kaprio et al. 2002). The subjects were initially selected among the monozygotic (MZ) male twins based on the results of this fourth survey. A pair was initially considered eligible for the present study if the healthy brothers had a marked difference in leisure-time physical activity. The criteria for the marked difference were that one brother was inactive and the other exercised at least two to three times per week or that, if both brothers exercised, the more active brother exercised at least twice as much as the less active brother. The process for study subject selection, the inclusion criteria, study subject details, and determination of zygosity have been described in more detail in our previous report from the same larger study (Hannukainen et al. 2005). Based on the inclusion and exclusion criteria a letter of invitation was sent to 26 MZ twin pairs. Subsequently a more detailed telephone interview regarding the current physical activity was performed and as a result 12 consenting twin pairs were selected for the present study. In the first part of the measurements, 2–8 weeks before the PET measurements, physical activity was studied by the questionnaire of Baecke et al. (1982), and a bicycle ergometer test was performed to determine V˙O2,max (Hannukainen et al. 2005). According to the criteria of significant differences in physical activity and at least 9% difference in V˙O2,max, nine twin pairs (age 25.9 ± 1.7 years) were selected for the second part of the study. The cotwins with higher physical activity and V˙O2,max constituting the more active group were compared to the less active group with lower physical activity and V˙O2,max. The more active group had a 18 ± 10% higher relative V˙O2,max (50.9 ± 5.1 versus 43.4 ± 6.7 ml min−1 kg−1, P < 0.001) and a 15 ± 10% (3.8 ± 0.6 versus 3.3 ± 0.5 l min−1, P = 0.002) higher absolute V˙O2,max compared to the less active group. The amount of physical activity of the preceding year was studied by a questionnaire. Physical activity was divided into conditioning exercise (e.g. running, cross country skiing, strength training and intensive ball games) and other physical activity (e.g. light walking, gardening, removal of snow, and field sports). On average the more active group had 4.0 ± 2.9 and the less active group 1.7 ± 1.5 (P = 0.003) conditioning exercise workouts per week and the average time per week spent for those were 229 ± 156 and 98 ± 71 min, respectively (P = 0.013). The weekly frequency of other physical activities was on average 4.3 ± 2.1 in the more active group and 4.4 ± 4.3 in the less active group (P = 0.96) and the average time per week spent for these was 144 ± 110 and 157 ± 162 min, respectively (P = 0.77).
Before starting any measurements, written informed consent was obtained after the purpose, nature and potential risks of the study were carefully explained to the subjects. The Ethical Committee of the Hospital District of South-Western Finland had approved the study protocol, and the study conformed to the Declaration of Helsinki.
Study design
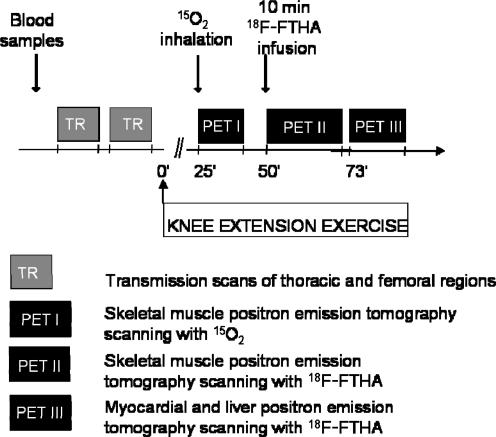
PET studies were performed 2–8 week after the physical activity questionnaire and V˙O2,max measurements. PET studies were performed after at least 12 h fast, and the subjects had avoided strenuous physical exercise for 48 h before the measurements. PET studies started with skeletal muscle oxygen and FFA uptake measurements at rest and during exercise. Thereafter, myocardial and hepatic FFA uptake measurements were performed (Fig. 1).
Figure 1. Study design.
15O2: 15O-labelled oxygen; 18F-FTHA: 18F-labelled 6-thia-hepta-decanoic acid.
Before the PET studies two catheters were inserted. One catheter was inserted into an antecubital vein for saline infusion and injection of a radiotracer 18F-labelled FTHA and another into the opposite radial artery for blood sampling. After the subjects were positioned for the PET camera, a low-intensity one-legged dynamic knee extension exercise (Kalliokoski et al. 2000; Laaksonen et al. 2003) started and lasted until the end of the study. Care was taken to carefully fasten the subjects to the imaging table to avoid any movements in the femoral region during the study. After 25 min of exercise a skeletal muscle oxygen uptake study was started. Skeletal muscle oxygen uptake scanning was measured with the bolus inhalation technique as previously described (Nuutila et al. 2000). Subjects inhaled a mixture of 15O2 (1212 ± 29 MBq, ∼0.43 mSv) and room air as a single bolus which were pumped into a rubber bladder. Dynamic skeletal muscle oxygen uptake scanning started immediately after inhalation and lasted for seven minutes. During the scanning the input function was obtained from arterial blood, which was continuously withdrawn with a pump. After oxygen uptake scanning a 10-min infusion of 18F-labelled FTHA (195 ± 19 MBq ∼2.09 mSv) and a 20 min dynamic PET scanning of the thigh region was started. Thereafte,r a 15 min dynamic scanning of the thoracic region was performed. During 18F-labelled FTHA scans arterial blood samples were drawn for radioactivity, metabolite, and FFA analysis (Mäki et al. 1998) (Fig. 1).
Exercise during PET
Exercise consisted of a right leg dynamic knee-extension exercise (Laaksonen et al. 2003) in which one contraction-cycle lasted for 2 s and a metronome with a sound signal was used to give the proper speed for the exercise. The exercise load (more active 3.4 ± 0.5 kg and less active group 3.2 ± 0.4 kg, P = 0.21) during PET studies was determined by multiplying the quadriceps femoris mass (Saltin, 1985) by 1.5. Exercise load was adjusted to the nearest 0.1 kg. The intention was to strain each gram of muscle by the same load in each subject as all the PET results are expressed for 100 g of muscle. This exercise intensity was selected based on pilot studies so that the subjects could exercise 90 min without exhaustion.
Production of radiotracers 15O2, and 18F-labelled FTHA
Oxygen-15 isotope for radiotracer 15O2 (T1/2 = 2.05 min) was produced with a low energy deuteron accelerator Cyclone 3 (Ion Beam Application, Louvain-la-Neuve, Belgium) as previously described (Crouzel et al. 1993). 15O was produced by the 14N(d,n)15O reaction of natural nitrogen gas (Strijckmans et al. 1985). 18F-labelled FTHA (T1/2 = 109 min) was produced with slight modification of the method previously described (DeGrado et al. 1991; Takala et al. 2002). The purity of the radiotracers was controlled by sterility and pyrogenity tests.
Image acquisition and processing
An ECAT 931/08-12 tomograph (Siemens/CTI Inc., Knoxville, TN, USA) was used for PET scanning. To correct the photon attenuation, transmission scanning of the femoral and thoracic regions was performed before the emission scans. All data were corrected for dead time decay and measured photon attenuation. PET images were reconstructed using the ordered subset expectation maximization with a median root prior algorithm (OSEM-MRP) (Alenius et al. 1998).
Regions of interest (ROIs)
Skeletal muscle ROIs covering the whole quadriceps femoris (QF) muscle group were drawn into four subsequent cross-sectional mid-tight planes in both thighs as previously described (Kalliokoski et al. 2000). The ROIs in oxygen uptake images were copied to images from FFA uptake studies. Myocardial ROIs were drawn on four subsequent mid-heart cross-sectional planes covering the anterior, lateral, and septal walls of the left ventricle. Liver ROI was drawn to the right lobe of the liver (Iozzo et al. 2004).
Calculation of FFA and oxygen uptake
The non-metabolized fraction of 18F-labelled FTHA and plasma and tissue time–activity curves were analysed as previously described (Mäki et al. 1998). In myocardial and liver studies the fractional uptake constant of 18F-labelled FTHA (Ki) was calculated according to the graphical analysis of Patlak & Blasberg (1985). In skeletal muscle due to short scanning time, only the last frame was used to determine the Ki as previously described for [18F]FDG (Stolen et al. 2004). Tissue FFA uptake was calculated by multiplying the Ki with the mean serum FFA concentration during the corresponding PET scan. Muscle oxygen uptake was quantified with non-linear fitting from the 15O2 data as previously described (Nuutila et al. 2000).
Intra-abdominal and subcutaneous fat
Intra-abdominal and subcutaneous fat masses were determined using magnetic resonance imaging (MRI). The MRI studies were performed on a 1.5 T MR imager (Signa Horizon LX, GE Medical Systems, USA) using the body coil. A single T1W FSE image was obtained at the level of the intervertebral disc L2–L3 for analysis of abdominal adipose tissue masses as previously described (Abate et al. 1997). For converting the measured volumes into weight an adipose tissue density of 0.9196 g ml−1 was used.
Biochemical analyses
The subjects underwent a 2 h, 75 g oral glucose tolerance test (OGTT) after at least 12 h fasting. Blood samples were drawn at 0, 10, 20, 30, 60, 90 and 120 min to evaluate the degree of glucose tolerance and the β-cell response to oral glucose load. The plasma insulin response was calculated as an insulin area under the curve (Vauhkonen et al. 1998). A homeostasis model assessment (HOMA) was determined as previously described (Matthews et al. 1985). Plasma glucose was determined with the glucose oxidase method (Analox GM9 Analyser, Analox Instruments Ltd, London, UK). Serum FFA and plasma lactate were both measured by the enzymatic colorimetric methods (ACS-ACOD method, Wako Chemicals USA, Inc., VA, USA for FFA and Roche Diagnostics GmbH, Mannheim, Germany for lactate, respectively). Both methods were analysed with a Roche Modular P800 automatic analyser (Roche Diagnostics GmbH, Mannheim, Germany). Serum insulin was measured using an automated time-resolved immunofluorometric assay (Autodelfia, Wallac, Turku, Finland).
Statistical analyses
Statistical analyses were performed using SAS/STAT statistical analysis program, version 8.2 (SAS Institute Inc., Cary, NC, USA). The normality of the variables was assessed by the Shapiro-Wilk test. Student's t test for paired data was used for normally distributed variables and for hepatic FFA uptake after logarithmic transformation to determine whether there were differences between groups, according to different parameters. The effects of the group and exercise on skeletal muscle FFA uptake and oxygen uptake were assessed using two-way ANOVA and ancova for repeated measurements. Correlations for both groups were calculated separately using the Pearson correlation. Because the subjects were related (MZ twins) we were unable to calculate normal Pearson correlation values for the whole group. Instead, association between continuous parameters in the whole group (i.e. all individuals) was evaluated using a linear mixed model in which twin pair membership was used as a random effect. The resulting β is the slope of the relationship determining how much and in which direction the second parameter changes when the first changes 1 unit. P values of less than 0.05 were considered statistically significant. All results are expressed as means ± standard deviation (s.d.).
Results
Anthropometry and metabolic data
Both groups of twins had asimilar body mass index and similar lean body mass, but the more active twins had a 10% lower whole-body fat percentage. The more active twins had 19% lower abdominal subcutaneous and 20% lower visceral fat mass compared to the less active twins (Table 1). The study groups had similar plasma glucose concentration in OGTT, insulin response and HOMA index values. Further, no differences were observed in serum insulin and FFA concentrations or in plasma glucose and lactate concentrations at baseline or during PET scanning (Table 2).
Table 1.
Anthropometry between more and less active twins
| Less active | More active | Diff.* (95% CI) | P | |
|---|---|---|---|---|
| Weight (kg) | 78.0 ± 10.9 | 75.1 ± 8.1 | −2.8 (−7.4, 1.7) | 0.18 |
| Height (cm) | 176.5 ± 4.8 | 176.3 ± 4.6 | 0.2 (−0.9, 1.4) | 0.64 |
| BMI (kg m−2) | 25.1 ± 3.3 | 24.1 ± 2.5 | −1.0 (−2.2, 0.3) | 0.12 |
| Body fat (%) | 19.6 ± 5.7 | 17.6 ± 5.5 | −2.0 (−4.0, −0.02) | 0.05 |
| Body fat (kg) | 15.8 ± 6.5 | 13.4 ± 5.1 | −2.4 (−4.4, −0.5) | 0.03 |
| Abdominal subcutaneous fat (kg) | 2.4 ± 1.4 | 1.9 ± 1.0 | −0.5 (−0.9, −0.1) | 0.02 |
| Visceral fat (kg) | 1.0 ± 0.4 | 0.8 ± 0.4 | −0.2 (−0.3, − 0.1) | 0.009 |
| LBM (kg) | 62.2 ± 5.6 | 61.7 ± 6.4 | −0.5 (−3.7, 2.7) | 0.75 |
| Waist/hip ratio | 0.87 ± 0.07 | 0.85 ± 0.06 | −0.02 (−0.04, 0.01) | 0.18 |
| Waist (cm) | 85.4 ± 10.7 | 81.8 ± 6.7 | −3.7 (−8.3, 1.0) | 0.11 |
Values are means ±s.d. BMI, body mass index; LBM, lean body mass
Diff., average difference in absolute values between brothers; CI, confidence interval for average difference in absolute values; P, P value for difference between groups (paired t test).
Table 2.
Metabolic data at fasting state, at rest and during 18F-labelled FTHA PET scanning
| Less active | More active | Diff.* (95% CI) | P | |
|---|---|---|---|---|
| Total cholesterol (mmol l−1) | 3.99 ± 0.58 | 3.97 ± 0.58 | −0.02 (−0.28, 0.24) | 0.85 |
| HDL cholesterol (mmol l−1) | 1.19 ± 0.15 | 1.30 ± 0.17 | 0.10 (0.00, 0.20) | 0.04 |
| LDL cholesterol (mmol l−1) | 2.40 ± 0.63 | 2.32 ± 0.56 | −0.08 (−0.38, 0.22) | 0.56 |
| Triglyceride (mmol l−1) | 0.89 ± 0.51 | 0.78 ± 0.18 | −0.11 (−0.43, 0.21) | 0.60 |
| Before PET scanning | ||||
| Insulin (mU l−1) | 7.44 ± 4.98 | 6.56 ± 3.97 | −0.89 (−1.87, 0.09) | 0.07 |
| Glucose (mmol l−1) | 5.78 ± 0.30 | 5.64 ± 0.28 | −0.14 (−0.30, 0.02) | 0.08 |
| FFA (mmol l−1) | 0.35 ± 0.13 | 0.27 ± 0.13 | −0.08 (−0.18, 0.03) | 0.12 |
| Lactate (mmol l−1) | 0.78 ± 0.29 | 0.73 ± 0.31 | −0.04 (−0.24, 0.15) | 0.61 |
| Skeletal muscle PET scanning | ||||
| FFA (mmol l−1) | 0.42 ± 0.25 | 0.32 ± 0.23 | −0.11 (−0.30, 0.09) | 0.30 |
| Lactate (mmol l−1) | 0.58 ± 0.20 | 0.59 ± 0.25 | 0.06 (−0.29, 0.18) | 0.88 |
| Myocardial and hepatic PET scanning | ||||
| FFA (mmol l−1) | 0.44 ± 0.24 | 0.33 ± 0.24 | −0.11 (−0.30, 0.09) | 0.20 |
| Lactate (mmol l−1) | 0.56 ± 0.17 | 0.54 ± 0.14 | − 0.01 (− 0.13, 0.11) | 0.83 |
Values are means ±s.d. FTHA, 6-thia-hepta-decanoic acid; PET, positron emission tomography; CI, confidence interval for average difference in absolute values
Diff., average difference in absolute values between brothers; FFA, serum free fatty acid concentration; P, P value for difference between groups (paired t test).
Skeletal muscle FFA and oxygen uptake
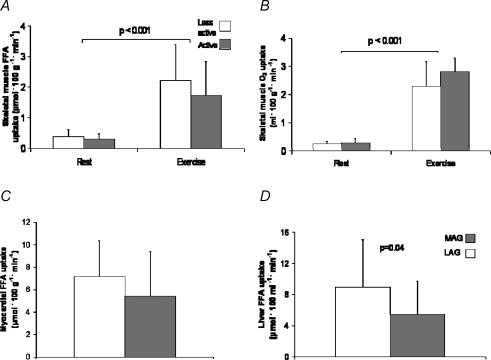
Skeletal muscle oxygen uptake and FFA uptake were similar between the groups at rest (Figs 2A and B). Exercise increased skeletal muscle oxygen uptake 10 times and FFA uptake six times compared to resting values (P < 0.001, both) in both groups, but no statistically significant difference was observed in oxygen uptake or in FFA uptake between the groups during exercise.
Figure 2. Skeletal muscle free fatty acid (FFA) (A) and oxygen (O2) uptake (B) at rest and during exercise and myocardial (C) and hepatic (D) FFA uptake between more (grey bars) and less active group (white bars) .
Hepatic and cardiac FFA uptake
Hepatic FFA uptake was 33% lower (P = 0.04) in the more active compared to the less active group (Fig. 2D). Hepatic FFA uptake was associated significantly with myocardial and skeletal muscle FFA uptake at rest and during exercise (Table 3). Hepatic FFA uptake was also associated with the whole body fat percentage in the whole study group (β = 0.093, standard error of the regression coefficient = 0.041, P = 0.05). When the whole-body fat percentage was used as the covariate in ancova, the difference in hepatic FFA uptake between the groups was not so strong (P = 0.08). Myocardial FFA uptake was not different between the groups (Fig. 2C).
Table 3.
Hepatic free fatty acid (FFA) uptake (μmol 100 ml−1 min−1) association with skeletal muscle and myocardial FFA uptake
| Whole study group | Less active | More active | |||||
|---|---|---|---|---|---|---|---|
| β | s.e. | P | r | P | r | P | |
| Myocardial FFA uptake (μmol (100 g)−1 min−1) | 0.185 | 0.029 | < 0.001 | 0.969 | < 0.001 | 0.751 | 0.02 |
| Skeletal muscle FFA uptake at rest (μmol (100 g)−1 min−1) | 2.852 | 0.563 | 0.001 | 0.677 | 0.05 | 0.889 | 0.001 |
| Skeletal muscle FFA uptake during exercise (μmol (100 g)−1 min−1) | 0.605 | 0.095 | < 0.001 | 0.794 | 0.01 | 0.895 | 0.001 |
β, regression coefficient (linear mixed model); s.e., standard error of regression coefficient; β, the slope of the relationship determining how much and to what direction the second parameter changes when the first is changing one unit.
Discussion
The results in the present study show that in monozygotic twins discordant for physical activity and fitness, the more active twins have ∼30% lower hepatic FFA uptake but similar skeletal muscle FFA uptake at rest and during low-intensity exercise and myocardial FFA uptake compared with the less active cotwins. Furthermore, hepatic FFA uptake was significantly associated with the whole body fat percentage, and myocardial and skeletal muscle FFA uptake.
The finding that resting muscle FFA uptake was not different between the twin groups is in agreement with the previous exercise training studies in a fasting state (Turcotte et al. 1992; Kiens et al. 1993; Bergman et al. 1999). Some evidence exists that during hyperinsulinaemia, muscle FFA uptake is enhanced in endurance-trained compared to untrained men (Iozzo et al. 2004), but it should be noted that fat oxidation and FFA uptake are both strongly suppressed during insulin stimulation.
During exercise skeletal muscle FFA uptake is dependent on the exercise mode, intensity and duration, and the nutritional and fitness status of the subject. In relation to other energy sources the proportion of FFA taken up from the blood for energy production is highest when large muscle groups are doing long-lasting low- or moderate-intensity exercise in a fasting state. Previous studies have shown both similar (Turcotte et al. 1992; Kiens et al. 1993) and higher (Turcotte et al. 1992; Kiens et al. 1993; Bergman et al. 1999) muscle FFA uptake during acute submaximal exercise after exercise training. When FFA uptake has been measured soon after the start of and after 1 h of one-legged knee extension exercise, no differences have been observed between trained and untrained muscle (Turcotte et al. 1992; Kiens et al. 1993). However, with prolonged exercise in these same studies increased FFA uptake was observed after 2 h (Kiens et al. 1993) and 3 h (Turcotte et al. 1992) of exercise. In the present study, the one-legged knee extension exercise had lasted ∼50 min at the beginning of skeletal muscle FFA measurement (20 min scanning) and no differences were observed between the study groups, which fits well with previous findings recorded after 1 h of exercise (Turcotte et al. 1992; Kiens et al. 1993).
According to the crossover concept (Brooks & Mercier, 1994) substrate utilization depends on the interaction between exercise intensity-induced responses and endurance training-induced responses, and at the crossover point and with further higher exercise intensities, energy from carbohydrate predominates over energy derived from fats. Exercise training enhances lipid oxidation and decreases sympathetic nervous system activity and thus lipid oxidation should be increased after exercise training at the same submaximal exercise intensity than before training. Increased FFA oxidation has been suggested to be due to exercise training induced muscle tissue adaptations (increased capillary density, reduced diffusion distance, and enhanced blood mean capillary transit time and FFA extraction fraction), all of which might prevent the net saturation of FFA uptake in endurance exercise (Kiens et al. 1993).
Contrary to the previous findings with local exercise (Turcotte et al. 1992; Kiens et al. 1993), FFA uptake was increased already in the early phase of the acute bicycle exercise (Bergman et al. 1999). This might be due to the more strenuous exercise mode (bicycle versus one-legged knee extension exercise) with higher energy demands and larger changes in the internal milieu (increased plasma catecholamine and decreased insulin levels), which may increase the rate of lipolysis and affect muscle FFA uptake.
In human and animal studies it has also been shown that endurance exercise training either decreases (Coggan et al. 1995) or increases (Donovan & Brooks, 1983; Donovan & Pagliassotti, 1989; Sumida et al. 1993; Podolin et al. 1994; Sumida & Donovan, 1995; Bergman et al. 2000) gluconeogenesis at rest and during exercise. If the latter case is true, it is possible that the similar or unchanged FFA uptake in the present and two previous knee-extension exercise studies after 1 and 2 h of exercise is due to higher availability of glucose in trained subjects, enhancing the shunting of glucose to working muscles. As the availability of substrates plays an important role in the energy utilization, it might be that in the present study more active twins used more glucose than less active twins and the expected difference in substrate utilization was in the glucose and not in the FFA uptake as supposed.
We estimated the amount of oxygen needed to oxidize the amount of FFA taken up during exercise in the present study based on the knowledge that 1 μmol of the medium-sized FFA palmitate consumes 1.748 ml O2 (Frayn, 1983) assuming that ∼36% of 18F-labelled FTHA is entering mitochondria for β-oxidation (Takala et al. 2002). These calculations show that the more active group used 40% and less active group 66% (P = 0.04) of oxygen to oxidize FFAs taken up by the muscle during exercise. This suggests that the more active group oxidized more of the other energy substrates in the exercising muscle than the less active group. There is evidence that the total lipid oxidation in skeletal muscle is increased after endurance training (Turcotte et al. 1992; Kiens et al. 1993; Bergman et al. 1999), and thus one of the implications from our results is that the utilization of intramyocellular triglycerides would possibly have been higher in the more than in the less active group. This assumption is supported by many of the previous studies (Hurley et al. 1986; Kiens & Richter, 1998; Guo et al. 2000; Brechtel et al. 2001; van Loon et al. 2001; van Loon et al. 2003; Schrauwen-Hinderling et al. 2003), but not all (Kiens & Richter, 1998; Guo et al. 2000).
One limitation of the aforementioned calculations is that the amount of 18F-labelled FTHA entering β-oxidation during exercise is probably higher than the ∼36% observed at rest. Thus, this mean that also the calculated percentages of oxygen used for FFA oxidation would most probably be higher in the present study. It has been previously shown that at an exercise intensity of 25% of V˙O2,max, plasma FFA oxidation accounts for ∼80% of the energy consumption in skeletal muscle in the postabsorptive state (Romijn et al. 1993), which fits quite nicely with our results.
The main methodological advance in the present study compared to previous studies is that FFA uptake was measured directly from the muscle tissue. Previously, FFA uptake has been measured from arteriovenous differences across the whole leg (Turcotte et al. 1992; Kiens et al. 1993; Bergman et al. 1999), which means that confounding factors such as lipolysis on adipose tissue during exercise (Stallknecht et al. 2001) could not be ruled out. In the present study FFA uptake was measured using 18F-labelled FTHA and PET. 18F-labelled FTHA is a radiolabelled long-chain fatty acid analogue and it enters cells in proportion to the oxidative rate of FFA. In β-oxidation, after formation of two acetyl-CoA it cannot be further oxidized and it is trapped within the mitochondria (DeGrado et al. 1991). With the FTHA–PET method the fractional rate of the total amount of FFA entering mitochondria can be non-invasively quantified in muscle, myocardial and hepatic tissues (Takala et al. 2002). In resting skeletal muscle, ∼36% of accumulated 18F-labelled FTHA appears to directly enter mitochondria and a major fraction of 18F-labelled FTHA is taken up into other cell fractions (Takala et al. 2002).
Skeletal muscle oxygen uptake was similar between the groups at rest and during exercise, which agrees with previous studies (Kiens et al. 1993; Putman et al. 1998; Bergman et al. 1999; Beere et al. 1999; Kalliokoski et al. 2001; Kemppainen et al. 2003). In the present study, exercise load during the knee-extension exercise was set according to the subjects' QF mass (Saltin, 1985) and the intention was to strain each gram of muscle by the same load in each subject. It was hypothesized that the more active twins would have a larger QF mass. Although the mean muscle mass values differed between groups, the difference was not statistically significant and thus no difference was found in exercise loads between the groups (more active group 3.4 ± 0.5 kg and less active group 3.2 ± 0.4 kg, P = 0.21). As both groups had a similar exercise load but the more active twins had better whole body V˙O2,max it can be estimated that the relative workload was lower in the more active group. On the other hand, if the measured skeletal muscle oxygen uptake during exercise was related to the V˙O2,max both groups seemed to have the same relative workload in the present study. Thus, the more active group should have had lower or similar muscle oxygen uptake compared to the less active group.
Exercise intensity in previous studies has been 65% of the whole body V˙O2,peak (Bergman et al. 1999), 65% of V˙O2,peak for leg extensors (Kiens et al. 1993), and 60% of the leg maximal working capacity (Turcotte et al. 1992). Exercise intensity was not measured in the present study. However, based on the increase in oxygen uptake due to exercise, it can be estimated that the intensity could have been at the same level as during cycling at 20–30% of whole body V˙O2,max (Grimby et al. 1967).
The earlier invasive study of Heiss et al. (1976) showed that postprandial myocardial FFA uptake was decreased in trained compared to untrained subjects. Using PET, we were not able to find differences in myocardial FFA uptake between endurance-trained and untrained men during euglycaemic hyperinsulaemic clamp (Takala et al. 1999). Correspondingly, the lipid oxidation rate was not significantly altered in endurance athletes in the previous single positron emission computed tomography study in a fasting state (Turpeinen et al. 1996). In agreement with these studies, the amount of FFA taken up by the myocardium was not different between twin groups in the present study. However, based on the trend of ∼20% lower mean myocardial FFA uptake in the more active groups observed in the present and previous studies (Turpeinen et al. 1996; Takala et al. 1999), it is possible that with larger study groups the difference would have been significant.
In a fasting state, the liver mainly utilizes FFAs and amino acids as fuels (Muller, 1995, 1998). In the whole body lipid metabolism liver converts carbohydrates into FFAs, and consumes, stores and releases lipids as part of lipoproteins, and thus controls blood lipid levels. In rats, acute prolonged exercise has been shown to increase liver FFA oxidation while exercise training has been suggested to reduce liver triglyceride production. In humans exercise training has been suggested to increase high density lipoprotein cholesterol production (Gorski et al. 1990). To the best of our knowledge the heredity-independent effects of long-term volitionally increased physical activity and fitness on hepatic FFA uptake have not been previously studied.
Hepatic FFA uptake was significantly lower in the more active compared to the less active group. However, it was not associated with the amount of physical activity or with fitness state, but correlated significantly with the whole body fat percentage. Although both the more and less active twins had normal weight, the more active twins had 10% lower whole body fat percentage. This was mainly due to the lower abdominal subcutaneous fat although the difference in visceral fat mass between groups was statistically more significant (Table 1). When the difference in whole body fat percentage was taken into account in ancova, the difference in hepatic FFA uptake between the groups decreased (P = 0.08) suggesting that hepatic FFA uptake is at least partly influenced by body adiposity. The lower hepatic FFA uptake in the more active twins is in agreement with the suggestion that with decreased body adiposity, especially in the intra-abdominal area (Montague & O'Rahilly, 2000), the rate of adipose tissue lipolysis is lower, thus decreasing the FFA load to the liver (Arner, 2002).
Exercise training increases whole body and skeletal muscle insulin sensitivity (Nuutila et al. 1994) and also hepatic insulin stimulated glucose uptake (Iozzo et al. 2004). Exercise training increases insulin-stimulated suppression of adipose tissue lipolysis. This has been demonstrated in recent human studies during euglycaemic–hyperinsulinaemic clamp (Stallknecht et al. 2000; Polak et al. 2005; DiPietro et al. 2006). Exercise training does not seem to modify expression of genes involved in the control of lipolysis on α2- and β-adrenergic receptor sensitivity to adrenaline in subcutaneous adipose tissue, but training increases the functional balance between α2- and β-adrenergic pathways in subcutaneous adipose tissue of obese subjects (Richterova et al. 2004).
As discussed with skeletal muscle, with energy flux being the major determinant of energy utilization it is possible that the lower lipolysis rate (due to decreased body adiposity and increased insulin-stimulated suppression of adipose tissue lipolysis) and probably increased gluconeogenesis (Bergman et al. 2000) in the more active twins leads to metabolic shunting of glucose to the liver. This reduces hepatic FFA uptake compared to the less active twins.
Wahren et al. (1984) have shown using catheter technique that splanchnic FFA uptake directly relates to FFA delivery and further FFA delivery depends on blood FFA concentration and the amount of blood flow. They have also shown that cycle ergometer exercise increases splanchnic oleic acid uptake by 70%. In the present study exercise was local one-legged knee extension exercise and the exercise intensity only submaximal, and thus it was considered not to strain the liver; on the contrary, the liver was considered to be in a resting state. There sem to have been no previous studies on the effects of exercise training on hepatic FFA delivery. In previous studies no differences have been observed in the more and less active men in resting splanchnic blood flow (Ho et al. 1997) or in women in portal vein blood flow (Clapp et al. 2000). Therefore, it is unlikely that decreased FFA uptake in the more active twins found in the present study is due to changes in blood flow, but it is possibly due to changes in hepatic FFA extraction.
It is always a challenge to find monozygotic twin pairs who are volitionally discordant for physical activity and fitness to an extent that any significant changes in the function of human body can be observed. The difference in V˙O2,max (50.9 versus 43.4 ml kg−1 min−1) is at the same level as in previous studies investigating the effects of 6 months of intensive endurance training on healthy subjects (Prud'homme et al. 1984; Schwartz et al. 1991; Suter et al. 1995; Skinner et al. 2000). Although the difference is not large, it is clear and has remained larger than the usual training period in most previous studies. We especially wanted to have subjects who are volitionally more or less active to avoid the problems of pushing one to train and prohibiting physical activity from the other. If we had taken subjects with a small difference in physical activity and fitness to begin with, it would have required a quite long period of training to get the brothers to have a similar difference in aerobic fitness as was observed in the present subjects. However, the more active group cannot be classified as athletes or the less active twins as sedentary in the present study. Thus, it is possible that greater differences would have been observed if the difference in physical activity and aerobic fitness within the pairs and the number of twin pairs had been larger. This needs to be further studied but it may be difficult to find MZ twin pairs with a larger difference in physical activity and fitness as they are both strongly influenced by genetic factors.
In conclusion, in the absence of the confounding effects of genetic factors, we found that moderately increased physical activity and aerobic fitness decrease hepatic FFA uptake but have no significant effects on skeletal muscle or myocardial FFA uptake. The decrease in hepatic uptake in a fasting state seems to be partly explained by decreased intra-abdominal adiposity. As the liver is metabolically the most versatile organ and covers most of its energy demands by oxidizing FFAs, it is reasonable to presume that liver is more sensitive to the changes in FFA availability compared to skeletal muscle and the myocardium.
Acknowledgments
The authors want to thank the personnel of the Turku PET Centre for help during the study. The present study was financially supported by the Academy of Finland (grants 206970 and 204240), the Ministry of Education (grants 143/722/2002, 51/722/2003 and 40/627/2005), the Juho Vainio Foundation, the Turku University Foundation, the Finnish Cultural Foundation, the South Western Finland Cultural Foundation, the Finnish Sports Institute Foundation, and the Finnish Sports Research Foundation. The FinnTwin16 study has been supported by the National Institute on Alcohol Abuse and Alcoholism (grants AA08315 and AA12502), the Academy of Finland (grants 44069 and 100499) and the European Union Fifth Framework Programme (QLG2-CT-2002-01254).
References
- Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65:403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- Alenius S, Ruotsalainen U, Astola J. Using local median as the location of the prior distribution in iterative emission tomography image reconstruction. IEEE Trans Nuclear Sci. 1998;45:3097–3104. [Google Scholar]
- Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev. 2002;18(Suppl. 2):S5–S9. doi: 10.1002/dmrr.254. [DOI] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol Endocrinol Metab. 1999;276:E106–E117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab. 2000;278:E244–E251. doi: 10.1152/ajpendo.2000.278.2.E244. [DOI] [PubMed] [Google Scholar]
- Blaak EE, Wagenmakers AJ, Glatz JF, Wolffenbuttel BH, Kemerink GJ, Langenberg CJ, Heidendal GA, Saris WH. Plasma FFA utilization and fatty acid-binding protein content are diminished in type 2 diabetic muscle. Am J Physiol Endocrinol Metab. 2000;279:E146–E154. doi: 10.1152/ajpendo.2000.279.1.E146. [DOI] [PubMed] [Google Scholar]
- Brechtel K, Niess AM, Machann J, Rett K, Schick F, Claussen CD, Dickhuth HH, Haering HU, Jacob S. Utilisation of intramyocellular lipids (IMCLs) during exercise as assessed by proton magnetic resonance spectroscopy (1H-MRS) Horm Metab Res. 2001;33:63–66. doi: 10.1055/s-2001-12407. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise – the crossover concept. J Appl Physiol. 1994;76:2253–2261. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- Clapp JF, Stepanchak W, Tomaselli J, Kortan M, Faneslow S. Portal vein blood flow – Effects of pregnancy, gravity, and exercise. Am J Obstetrics Gynecol. 2000;183:167–172. doi: 10.1067/mob.2000.105902. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Swanson SC, Mendenhall LA, Habash DL, Kien CL. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. Am J Physiol Endocrinol Metab. 1995;31:E375–E383. doi: 10.1152/ajpendo.1995.268.3.E375. [DOI] [PubMed] [Google Scholar]
- Crouzel C, Clark JC, Brihaye C, et al. Radiochemistry automation for PET. In: Stocklin G, Pike VW, editors. Radiopharmaceuticals for Positron Emission Tomography. Kluwer Academic Publishers; 1993. pp. 45–90. [Google Scholar]
- De Glisezinski I, Moro C, Pillard F, Marion-Latard F, Harant I, Meste M, Berlan M, Crampes F, Riviere D. Aerobic training improves exercise-induced lipolysis in SCAT and lipid utilization in overweight men. Am J Physiol Endocrinol Metab. 2003;285:E984–E990. doi: 10.1152/ajpendo.00152.2003. [DOI] [PubMed] [Google Scholar]
- DeGrado TR, Coenen HH, Stocklin G. 14(R,S)-[F-18]Fluoro-6-Thia-Heptadecanoic Acid (Ftha) – Evaluation in Mouse of A new Probe of Myocardial utilization of long-chain fatty-acids. J Nucl Med. 1991;32:1888–1896. [PubMed] [Google Scholar]
- DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol. 2006;100:142–149. doi: 10.1152/japplphysiol.00474.2005. [DOI] [PubMed] [Google Scholar]
- Donovan CM, Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol. 1983;244:E83–E92. doi: 10.1152/ajpendo.1983.244.1.E83. [DOI] [PubMed] [Google Scholar]
- Donovan CM, Pagliassotti MJ. Endurance training enhances lactate clearance during hyperlactatemia. Am J Physiol. 1989;257:E782–E789. doi: 10.1152/ajpendo.1989.257.5.E782. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- Gorski J, Oscai LB, Palmer WK. Hepatic lipid-metabolism in exercise and training. Med Sci Sports Exerc. 1990;22:213–221. [PubMed] [Google Scholar]
- Grimby G, Haggenda E, Saltin B. Local xenon 133 clearance from quadriceps muscle during exercise in man. J Appl Physiol. 1967;22:305–310. doi: 10.1152/jappl.1967.22.2.305. [DOI] [PubMed] [Google Scholar]
- Guo Z, Burguera B, Jensen MD. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol. 2000;89:2057–2064. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- Hannukainen JC, Kujala UM, Toikka J, Heinonen OJ, Kapanen J, Vahlberg T, Kaprio J, Kalliokoski KK. Cardiac structure and function in monozygotic twin pairs discordant for physical fitness. J Appl Physiol. 2005;99:535–541. doi: 10.1152/japplphysiol.00107.2005. [DOI] [PubMed] [Google Scholar]
- Heiss HW, Barmeyer J, Wink K, Hell G, Cerny FJ, Keul J, Reindell H. Studies on the regulation of myocardial blood flow in man. I. Training effects on blood flow and metabolism of the healthy heart at rest and during standardized heavy exercise. Basic Res Cardiol. 1976;71:658–675. doi: 10.1007/BF01906411. [DOI] [PubMed] [Google Scholar]
- Henriksson J. Training induced adaptation of skeletal-muscle and metabolism during submaximal exercise. J Physiol. 1977;270:661–675. doi: 10.1113/jphysiol.1977.sp011974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CW, Beard JL, Farrell PA, Minson CT, Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl Physiol. 1997;82:1126–1135. doi: 10.1152/jappl.1997.82.4.1126. [DOI] [PubMed] [Google Scholar]
- Hurley BF, Nemeth PM, Martin WH, III, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol. 1986;60:562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- Iozzo P, Takala T, Oikonen V, Bergman J, Grönroos T, Ferrannini E, Nuutila P, Knuuti J. Effect of training status on regional disposal of circulating free fatty acids in the liver and skeletal muscle during physiological hyperinsulinemia. Diabetes Care. 2004;27:2172–2177. doi: 10.2337/diacare.27.9.2172. [DOI] [PubMed] [Google Scholar]
- Jansson E, Kaijser L. Substrate utilization and enzymes in skeletal-muscle of extremely endurance-trained men. J Appl Physiol. 1987;62:999–1005. doi: 10.1152/jappl.1987.62.3.999. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Kemppainen J, Larmola K, Takala TO, Peltoniemi P, Oksanen A, Ruotsalainen U, Cobelli C, Knuuti J, Nuutila P. Muscle blood flow and flow heterogeneity during exercise studied with positron emission tomography in humans. Eur J Appl Physiol. 2000;83:395–401. doi: 10.1007/s004210000267. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Oikonen V, Takala TO, Sipilä H, Knuuti J, Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab. 2001;280:E1015–E1021. doi: 10.1152/ajpendo.2001.280.6.E1015. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kemppainen J, Stolen K, Kalliokoski KK, Salo T, Karanko H, Viljanen T, Airaksinen J, Nuutila P, Knuuti J. Exercise training improves insulin stimulated skeletal muscle glucose uptake independent of changes in perfusion in patients with dilated cardiomyopathy. J Card Fail. 2003;9:286–295. doi: 10.1054/jcaf.2003.35. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol Endocrinol Metab. 1998;275:E332–E337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- Klein S, Coyle EF, Wolfe RR. Fat metabolism during low-intensity exercise in endurance-trained and untrained men. Am J Physiol Endocrinol Metab. 1994;267:E934–E940. doi: 10.1152/ajpendo.1994.267.6.E934. [DOI] [PubMed] [Google Scholar]
- Laaksonen MS, Kalliokoski KK, Kyröläinen H, Kemppainen J, Teräs M, Sipilä H, Nuutila P, Knuuti J. Skeletal muscle blood flow and flow heterogeneity during dynamic and isometric exercise in humans. Am J Physiol Heart Circ Physiol. 2003;284:H979–H986. doi: 10.1152/ajpheart.00714.2002. [DOI] [PubMed] [Google Scholar]
- van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol. 2003;553:611–625. doi: 10.1113/jphysiol.2003.052431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäki MT, Haaparanta M, Nuutila P, Oikonen V, Luotolahti M, Eskola O, Knuuti JM. Free fatty acid uptake in the myocardium and skeletal muscle using fluorine-18-fluoro-6-thia-heptadecanoic acid. J Nucl Med. 1998;39:1320–1327. [PubMed] [Google Scholar]
- Martin WHI, Dalsky GP, Hurley BF, Matthews DE, Bier DM, Hagberg JM, Rogers MA, King DS, Holloszy JO. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol Endocrinol Metab. 1993;265:E708–E714. doi: 10.1152/ajpendo.1993.265.5.E708. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, Defronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;283:E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- Montague CT, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- Muller MJ. Hepatic fuel selection. Proc Nutr Soc. 1995;54:139–150. doi: 10.1079/pns19950043. [DOI] [PubMed] [Google Scholar]
- Muller MJ. Hepatic energy and substrate metabolism: a possible metabolic basis for early nutritional support in cirrhotic patients. Nutrition. 1998;14:30–38. doi: 10.1016/s0899-9007(97)00390-0. [DOI] [PubMed] [Google Scholar]
- Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284:E1065–E1071. doi: 10.1152/ajpendo.00442.2002. [DOI] [PubMed] [Google Scholar]
- Nuutila P, Knuuti MJ, Heinonen OJ, Ruotsalainen U, Teräs M, Bergman J, Solin O, Yki-Järvinen H, Voipio-Pulkki LM, Wegelius U, Koivisto VA. Different alterations in the insulin-stimulated glucose-uptake in the athletes heart and skeletal-muscle. J Clin Invest. 1994;93:2267–2274. doi: 10.1172/JCI117226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutila P, Peltoniemi P, Oikonen V, Larmola K, Kemppainen J, Takala T, Sipilä H, Oksanen A, Ruotsalainen U, Bolli GB, Yki-Järvinen H. Enhanced stimulation of glucose uptake by insulin increases exercise-stimulated glucose uptake in skeletal muscle in humans – Studies using [15O]O2, [15O]H2O, [18F]fluoro-deoxy-glucose and positron emission tomography. Diabetes. 2000;49:1084–1091. doi: 10.2337/diabetes.49.7.1084. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data – generalizations. J Cereb Blood Flow Metab. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Pietiläinen KH, Rissanen A, Kaprio J, Mäkimattila S, Häkkinen AM, Westerbacka J, Sutinen J, Vehkavaara S, Yki-Järvinen H. Acquired obesity is associated with increased liver fat, intra-abdominal fat, and insulin resistance in young adult monozygotic twins. Am J Physiol Endocrinol Metab. 2005;288:E768–E774. doi: 10.1152/ajpendo.00381.2004. [DOI] [PubMed] [Google Scholar]
- Podolin DA, Gleeson TT, Mazzeo RS. Role of norepinephrine in hepatic gluconeogenesis – Evidence of aging and training effects. Am J Physiol Endocrinol Metab. 1994;30:E680–E686. doi: 10.1152/ajpendo.1994.267.5.E680. [DOI] [PubMed] [Google Scholar]
- Polak J, Moro C, Klimcakova E, Hejnova J, Majercik M, Viguerie N, Langin D, Lafontan M, Stich V, Berlan M. Dynamic strength training improves insulin sensitivity and functional balance between adrenergic alpha 2A and beta pathways in subcutaneous adipose tissue of obese subjects. Diabetologia. 2005;48:2631–2640. doi: 10.1007/s00125-005-0003-8. [DOI] [PubMed] [Google Scholar]
- Prud'homme D, Bouchard C, Leblanc C, Landry F, Fontaine E. Sensitivity of maximal aerobic power to training is genotype-dependent. Med Sci Sports Exerc. 1984;16:489–493. doi: 10.1249/00005768-198410000-00012. [DOI] [PubMed] [Google Scholar]
- Putman CT, Jones NL, Hultman E, Hollidge-Horvat MG, Bonen A, Mcconachie DR, Heigenhauser GJ. Effects of short-term submaximal training in humans on muscle metabolism in exercise. Am J Physiol Endocrinol Metab. 1998;275:E132–E139. doi: 10.1152/ajpendo.1998.275.1.E132. [DOI] [PubMed] [Google Scholar]
- Richterova B, Stich V, Moro C, Polak J, Klimcakova E, Majercik M, Harant I, Viguerie N, Crampes F, Langin D, Lafontan M, Berlan M. Effect of endurance training on adrenergic control of lipolysis in adipose tissue of obese women. J Clin Endocrinol Metab. 2004;89:1325–1331. doi: 10.1210/jc.2003-031001. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate-metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Saltin B. Hemodynamic adaptations to exercise. Am J Cardiol. 1985;55:D42–D47. doi: 10.1016/0002-9149(85)91054-9. [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Schrauwen P, Hesselink MK, van Engelshoven JM, Nicolay K, Saris WH, Kessels AG, Kooi ME. The increase in intramyocellular lipid content is a very early response to training. J Clin Endocrinol Metab. 2003;88:1610–1616. doi: 10.1210/jc.2002-021464. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Shuman WP, Larson V, Cain KC, Fellingham GW, Beard JC, Kahn SE, Stratton JR, Cerqueira MD, Abrass IB. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism. 1991;40:545–551. doi: 10.1016/0026-0495(91)90239-s. [DOI] [PubMed] [Google Scholar]
- Skinner JS, Wilmore KM, Krasnoff JB, Jaskolski A, Jaskolska A, Gagnon J, Province MA, Leon AS, Rao DC, Wilmore JH, Bouchard C. Adaptation to a standardized training program and changes in fitness in a large, heterogeneous population: the HERITAGE Family Study. Med Sci Sports Exerc. 2000;32:157–161. doi: 10.1097/00005768-200001000-00023. [DOI] [PubMed] [Google Scholar]
- Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, Duscha BD, Kraus WE. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99:1613–1618. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Larsen JJ, Mikines KJ, Simonsen L, Bulow J, Galbo H. Effect of training on insulin sensitivity of glucose uptake and lipolysis in human adipose tissue. Am J Physiol Endocrinol Metab. 2000;279:E376–E385. doi: 10.1152/ajpendo.2000.279.2.E376. [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Lorentsen J, Enevoldsen LH, Bulow J, Sorensen FB, Galbo H, Kjaer M. Role of the sympathoadrenergic system in adipose tissue metabolism during exercise in humans. J Physiol. 2001;536:283–294. doi: 10.1111/j.1469-7793.2001.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolen KQ, Kemppainen J, Kalliokoski KK, Hällsten K, Luotolahti M, Karanko H, Lehikoinen P, Viljanen T, Salo T, Airaksinen KEJ, Nuutila P, Knuuti J. Myocardial perfusion reserve and oxidative metabolism contribute to exercise capacity in patients with dilated cardiomyopathy. J Card Fail. 2004;10:132–140. doi: 10.1016/j.cardfail.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Strijckmans K, Vandecasteele C, Sambre J. Production and quality control of 15O2 and C15O2 for medical use. Int J Appl Radiat Isot. 1985;36:279–283. doi: 10.1016/0020-708x(85)90085-7. [DOI] [PubMed] [Google Scholar]
- Sumida KD, Donovan CM. Enhanced hepatic gluconeogenic capacity for selected precursors after endurance training. J Appl Physiol. 1995;79:1883–1888. doi: 10.1152/jappl.1995.79.6.1883. [DOI] [PubMed] [Google Scholar]
- Sumida KD, Urdiales JH, Donovan CM. Enhanced gluconeogenesis from lactate in perfused livers after endurance training. J Appl Physiol. 1993;74:782–787. doi: 10.1152/jappl.1993.74.2.782. [DOI] [PubMed] [Google Scholar]
- Suter E, Hoppeler H, Claassen H, Billeter R, Aebi U, Horber F, Jaeger P, Marti B. Ultrastructural modification of human skeletal-muscle tissue with 6-month moderate-intensity exercise training. Int J Sports Med. 1995;16:160–166. doi: 10.1055/s-2007-972985. [DOI] [PubMed] [Google Scholar]
- Takala TO, Nuutila P, Katoh C, Luotolahti M, Bergman J, Mäki M, Oikonen V, Ruotsalainen U, Grönroos T, Haaparanta M, Kapanen S, Knuuti J. Myocardial blood flow, oxygen consumption, and fatty acid uptake in endurance athletes during insulin stimulation. Am J Physiol Endocrinol Metab. 1999;277:E585–E590. doi: 10.1152/ajpendo.1999.277.4.E585. [DOI] [PubMed] [Google Scholar]
- Takala TO, Nuutila P, Pulkki K, Oikonen V, Grönroos T, Savunen T, Vahasilta T, Luotolahti M, Kallajoki M, Bergman J, Forsback S, Knuuti J. 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid as a tracer of free fatty acid uptake and oxidation in myocardium and skeletal muscle. Eur J Nuclear Med Mol Imaging. 2002;29:1617–1622. doi: 10.1007/s00259-002-0979-y. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–3196. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol Endocrinol Metab. 1992;262:E791–E799. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]
- Turpeinen AK, Kuikka JT, Vanninen E, Vainio P, Vanninen R, Litmanen H, Koivisto VA, Bergström K, Uusitupa MI. Athletic heart: a metabolic, anatomical, and functional study. Med Sci Sports Exerc. 1996;28:33–40. doi: 10.1097/00005768-199601000-00011. [DOI] [PubMed] [Google Scholar]
- Turpeinen AK, Takala TO, Nuutila P, Axelin T, Luotolahti M, Haaparanta M, Bergman J, Hämäläinen H, Iida H, Mäki M, Uusitupa MI, Knuuti J. Impaired free fatty acid uptake in skeletal muscle but not in myocardium in patients with impaired glucose tolerance: studies with PET and 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid. Diabetes. 1999;48:1245–1250. doi: 10.2337/diabetes.48.6.1245. [DOI] [PubMed] [Google Scholar]
- Vauhkonen I, Niskanen L, Vanninen E, Kainulainen S, Uusitupa M, Laakso M. Defects in insulin secretion and insulin action in non-insulin-dependent diabetes mellitus are inherited – Metabolic studies on offspring of diabetic probands. J Clin Invest. 1998;101:86–96. doi: 10.1172/JCI716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J, Sato Y, Ostman J, Hagenfeldt L, Felig P. Turnover and splanchnic metabolism of free fatty acids and ketones in insulin-dependent diabetics at rest and in response to exercise. J Clin Invest. 1984;73:1367–1376. doi: 10.1172/JCI111340. [DOI] [PMC free article] [PubMed] [Google Scholar]