Abstract
Hyaluronan (HA) retention inside the synovial cavity of joints serves diverse protective roles. We tested the hypothesis that HA retention is mediated by the network of extracellular matrix proteins in the synovial lining. Cannulated rabbit knee joints were infused with HA solution with or without pretreatment by chymopapain, a collagen-sparing protease. Trans-synovial fluid escape rate was measured and, after a period of trans-synovial filtration, samples of intra-articular fluid and subsynovial fluid were analysed for HA to assess its trans-synovial ultrafiltration. In control joints, HA ultrafiltration was confirmed by postfiltration increases in intra-articular HA concentration (259 ± 17% of infused concentration) and reduced subsynovial concentration (30 ± 8%; n = 11). The proportion of HA molecules reflected by the synovium was 57–75%. Chymopapain treatment increased the hydraulic permeability of the synovial lining ∼13-fold, almost abolished the trans-synovial difference in HA concentration and reduced the HA reflected fraction to 3–7% (n = 6; P < 0.001, ANOVA). Structural studies confirmed that chymopapain treatment depleted the matrix of proteoglycans but preserved its collagen. The findings thus demonstrate that HA ultrafiltration and synovial hydraulic permeability are determined by the network of non-collagen, extracellular matrix proteins. This may be important clinically, since protease activity is raised in rheumatoid arthritis, as are HA and fluid escape.
In healthy joints, the synovial lining (synovium) serves as a leaky, confining pseudomembrane, with sufficient resistance to retain a normal quantity of lubricating synovial fluid in the joint cavity. Yet synovium, unlike a true membrane of epithelium or mesothelium, is perforated by extensive, micrometre-wide intercellular gaps. Since the gaps are occupied by extracellular matrix, it is likely that the matrix is largely responsible for the resistance of the lining to synovial fluid escape (Scott et al. 2003). The hydraulic resistance of any extracellular matrix is determined by the concentration of glycosaminoglycans, proteoglycans and glycoproteins between the collagen fibrils, because fluid passes through the narrow spaces between the polymer chains (Levick, 1987). In keeping with its high hydraulic resistance, synovial matrix has a high glycosaminoglycan concentration (Price et al. 1996), depletion of which raises synovial hydraulic permeability (Scott et al. 1998a). Synovium resists not only water escape, however, but also hyaluronan (HA) escape to a much greater degree. Hyaluronan, a lubricating glycosaminoglycan of molecular mass 2 × 106 Da, permeates the synovium much more slowly than water or plasma proteins and thus undergoes partial ultrafiltration or ‘molecular sieving’ during fluid drainage (Scott et al. 1998b; Sabaratnam et al. 2003). This selective trapping of HA in the cavity is physiologically important because it generates a high concentration of viscous, lubricating HA in the synovial fluid. It also reduces the required replacement rate, thus reducing the metabolic burden on the HA-secreting synoviocytes (Coleman et al. 1997).
The high HA concentration in synovial fluid has two important physiological roles. It contributes to low-load lubrication (Murakami et al. 1998) and it is crucial to retaining synovial fluid in the joint cavity when the pressure is raised, for example by a maintained flexion (Coleman et al. 1999). When fluid is driven out of the joint cavity across the synovial lining by a raised pressure, the ultrafiltration of HA causes it to accumulate on the synovial surface, and the osmotic pressure of this concentration polarization layer buffers the rate of fluid escape (‘outflow buffering’; Lu et al. 2004, 2005). If either the chain length or the concentration of the HA is reduced (both being common features of arthritides), the HA escapes across the synovium more freely (Asari et al. 1998; Sabaratnam et al. 2005, 2006). Consequently, the outflow buffering mechanism is impaired (Coleman et al. 2000; Lu et al. 2005).
The radius of the HA molecular domain is 0.1–0.2 μm, whereas the synovial intercellular gaps are 1–2 μm wide, so the gaps themselves are clearly not responsible for the molecular sieving. Indeed, synovium has sieving properties equivalent to those of cylindrical pores of radius only 33–59 nm (Sabaratnam et al. 2005). This led us to consider the intercellular matrix as the likely site of HA ultrafiltration. Our working hypothesis was that the irregular network of synovial proteoglycan and glycoprotein chains not only generates a high hydraulic resistance but also sieves out and reflects a high proportion of HA in the fluid draining from the joint cavity. Although we have shown previously that matrix depletion raises synovial hydraulic permeability (Scott et al. 1998a; Coleman, 2002, 2005), the role of the matrix proteins in the selective retention of HA has never previously been investigated. To test this, we examined the effect of enzymatic depletion of the proteoglycans and glycoproteins, while sparing the structural collagen, on HA reflection and hydraulic permeability in vivo. Since there is extensive activation of metalloproteinases and other matrix-degrading enzymes in chronic human inflammatory arthritides such as rheumatoid arthritis (Ishiguro et al. 2001; Jones & Riley, 2005), the findings may also contribute to our understanding of raised serum HA, joint HA loss and fluid escape in certain inflammatory arthritides.
Methods
Overview
The knee joint cavity of an anaesthetized rabbit was infused continuously with HA and fluorescein dextran (FD), with or without prior treatment with a collagen-sparing protease, chymopapain. Trans-synovial fluid escape rate was measured at a known pressure to estimate synovial hydraulic permeability. A standardized trans-synovial filtration rate was then maintained for several hours, with subsynovial fluid sampling at intervals and intra-articular fluid sampling at the end. Hyaluronan ultrafiltration was quantified by changes in the sample [HA]:[FD] ratio.
Materials
Rooster comb HA (0.2 mg ml−1, 2230 kDa, domain radius 101–171 nm, polydispersity ratio 2.3; Coleman et al. 1999) and fluorescein dextran (30 μg ml−1, 20 kDa, radius 3.1 nm) from Sigma (Poole, UK) were coadministered in Ringer solution (Baxter Healthcare Ltd, Thetford, UK). The HA concentration was dictated by technical considerations and equalled the lowest concentration in rheumatoid effusions (Dahl et al. 1985). Chymopapain (Sigma; EC3.4.22.6; 0.1 i.u. ml−1) is a protease with a usefully restricted specificity and has been used clinically for intervertebral disc chemonucleolysis. This selective proteolytic agent spares fibrillar collagens as assessed by hydroxyproline release (Bradford et al. 1984) but degrades extrafibrillar proteoglycans and glycoproteins (Oegema et al. 1988) and rapidly depletes synovium of proteoglycan-bound glycosaminoglycans (Scott et al. 1998a). Chymopapain was dissolved in Ringer solution containing the chymopapain activating agents l-cysteine hydrochloride (1 mm) and EDTA (1 mm), which do not affect synovial permeability (Scott et al. 1998a). Based on the stated activity of the Sigma preparation at pH 6.2, 0.1 i.u. should degrade the estimated synovial interstitial protein mass in ∼6 min (perhaps longer at pH 7.4 in vivo), in keeping with the observed loss of immunohistochemical staining for proteoglycans (Scott et al. 1998a).
Joint preparation and trans-synovial filtration
New Zealand white rabbits (∼3 kg) were anaesthetized by intravenous pentobarbitone (30 mg kg−1) and urethane (500 mg kg−1) and maintained by intravenous injection of 15 mg pentobarbitone and 250 mg urethane every 30 min. The anaesthetic depth was sufficient to abolish the corneal blink and paw pinch reflexes. One knee was double-cannulated (Coleman et al. 1999) per animal. One intra-articular cannula was connected to a pressure transducer to record fluid pressure, Pj (± 0.1 cmH2O). A second cannula was connected to an infusion reservoir, the height of which regulated Pj, the force driving trans-synovial filtration (Coleman et al. 1999). Flow into the joint cavity from the reservoir at constant Pj depends mainly on the rate of trans-synovial fluid escape, and was measured using a photoelectric drop-counter. A small correction, derived from earlier oil infusion data, was applied for residual viscoelastic creep (Coleman et al. 1999). At the end of experiments, animals were killed by intravenous pentobarbitone. All procedures conformed to UK animal legislation and local institution animal welfare guidelines.
Filtrate sampling
Protocol A
Initially we attempted to collect not only subsynovial fluid but also joint-derived lymph, as in previous work (Sabaratnam et al. 2003). The joint was pretreated with intra-articular chymopapain (0.1 i.u. in 1 mml-cysteine, 1 mm EDTA, 1 ml total volume) or Ringer solution (control) for 90 min and then infused with the HA–FD solution for several hours while lymph collection was attempted from a cannulated femoral lymphatic vessel, into which the subsynovial fluid drains. At the end of the study, the circulation was arrested by an intravenous pentobarbitone overdose and the subsynovial fluid sampled post mortem by aspiration of fluid from the dissected periarticular space (Sabaratnam et al. 2003). Also, the intra-articular fluid was aspirated after mixing by flexion–extension cycles. Control joints yielded a good lymph harvest, but the lymph flow was unexpectedly low from two chymopapain-treated joints, possibly owing to lymphatic collapse following chymopapain treatment. In light of this problem, the protocol for chymopapain studies was adapted to obtain repeated subsynovial fluid samples, as follows.
Protocol B
Pretreatment of the synovium with intra-articular chymopapain solution (0.l i.u. in 1 ml) for 20 min was followed by 10 × 1 ml joint washouts with HA–FD solution. Trans-synovial filtration rate was measured at Pj = 13 cmH2O for 10 min to assess changes in synovial hydraulic permeability. Pressure was then adjusted to generate similar trans-synovial filtration rates in the chymopapain-treated joints (32 ± 6 μl min−1) and control joints (32 ± 3 μl min−1). Filtration rate rather than pressure was standardized because the HA reflected fraction varies with filtration rate (Sabaratnam et al. 2004). Chymopapain causes marked microvascular plasma leakage (Scott et al. 1998a), so to prevent contamination of the trans-synovial filtrate by continuous plasma filtration from chymopapain-damaged capillaries, the circulation was arrested by intravenous pentobarbitone. (In the absence of chymopapain, capillary filtration is too slow to influence the composition of the subsynovial filtrate significantly, as demonstrated by the uniform FD concentration in vivo; see Fig. 2.) Previous studies show that circulatory arrest per se does not alter HA molecular sieving (Coleman et al. 1999). The subsynovial space was approached by dissection from the postero-lateral surface, and a collection well was constructed there from para-film (American Can Co., Greenwich, CT, USA). The trans-synovial filtrate accumulated in the well and was aspirated every ∼30 min for 2–3 h. Finally the intra-articular fluid was mixed by flexion–extension cycles and sampled. A total of 25 subsynovial samples from six chymopapain-treated rabbits (protocol B) was compared with 11 subsynovial samples from 11 control rabbits (protocol A).
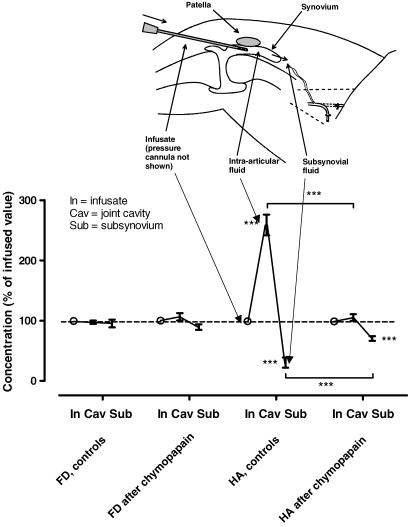
Figure 2. Effect of chymopapain treatment on hyaluronan and fluorescein dextran concentration profiles across synovium during fluid drainage in situ.
Concentration normalized as a percentage of the infused value (mean ± s.e.m.) is plotted as a function of anatomical location following filtration (In, infusion line; Cav, joint cavity; Sub, subsynovial fluid). The infusate contained 20 kDa fluorescein dextran and 2230 kDa hyaluronan (0.2 mg ml−1). One group of knees received 0.1 i.u. intra-articular chymopapain in Ringer solution before the filtration period (n = 6). The control group received Ringer solution without chymopapain (n = 11). ***P < 0.001 compared with infusate or comparison indicated by brackets (Student's paired t test).
Sample analysis
Samples were analysed by size exclusion, high-performance liquid chromatography (HPLC) using a Waters 2690 separation module (Waters Ltd, Watford, UK) and TosoHaas TSK G6000 PWXL column (Anachem Ltd, Luton, UK). A Waters 486 ultraviolet absorbance detector was set at 206 nm for HA analysis. The molecular weight was interpolated from retention time (Coleman et al. 1997). Fluorescein dextran was measured using the same column and an in-line Waters 474 SATIN fluorimeter (excitation 475 nm, emission 530 nm). Filtrate composition was close to a steady state over 2–3 h (Sabaratnam et al. 2003), and subsynovial concentration did not change significantly over 3 h (regression analysis, not shown), so a mean value was calculated for each preparation, as follows.
Trans-synovial transmitted fraction (1 – Rsyn)
The fraction of HA molecules transmitted through synovium into the subsynovial fluid is (1 – Rsyn), where Rsyn is the reflected fraction. The transmitted fraction was calculated as the HA concentration in the subsynovial fluid, normalized as a percentage of the infused HA concentration, divided by the concentration of FD in the same sample, likewise normalized as a percentage of the infused concentration (Sabaratnam et al. 2003). For example, if the subsynovial HA concentration was 20% of the infused concentration and the subsynovial FD concentration was 100%, the HA transmitted fraction was 0.2 and Rsyn was 0.8.
Lymph transmitted fraction (1 –Rlymph)
The fraction of molecules reflected by the combined synovium–lymphatic barrier is Rlymph. All the control studies, but only one of the chymopapain studies, yielded sufficient lymph for analysis. Transmission from joint to lymph (1 – Rlymph), was again calculated using the ratio of normalized HA and FD concentrations (Sabaratnam et al. 2003).
Hyaluronan accumulation in joint cavity (Rcav)
Hyaluronan reflection raises the concentration of HA in the joint cavity. Calculation of the reflected fraction based on intracavity accumulation (Rcav) requires an estimate of the cavity fluid volume, to calculate the accumulated HA mass from the measured concentration. Volume at the recorded Pj was estimated from the average pressure–volume relation of rabbit knee joints (Knight & Levick, 1982). Reflection Rcav was then calculated as the fraction of HA in the filtered fluid that had been retained in the joint cavity (Scott et al. 1998b).
Synovial structure after chymopapain treatment
It is known from immunohistochemical studies that 0.1 i.u. intra-articular chymopapain severely depletes the synovium of chondroitin sulphate proteoglycan, keratan sulphate proteoglycan and proteoglycan-bound matrix hyaluronan (Scott et al. 1998a). In the present study, transmission electron microscopy was used to confirm the collagen fibril-sparing action of chymopapain in rabbit synovium. The synovium was excised from the anterior joint cavity of chymopapain-treated and control knees and processed for transmission electron microscopy as described previously (Coleman, 2002).
Statistical methods
Mean values ± s.e.m. are cited throughout. Student's t test was used for unpaired and one-sample comparisons on results that passed the Kolmogorov–Smirnov normality test. One-way ANOVA with Tukey's post hoc test was used for multiple comparisons as implemented in Graphpad Prism (San Diego, CA, USA). Significance was accepted at P ≤ 0.05.
Results
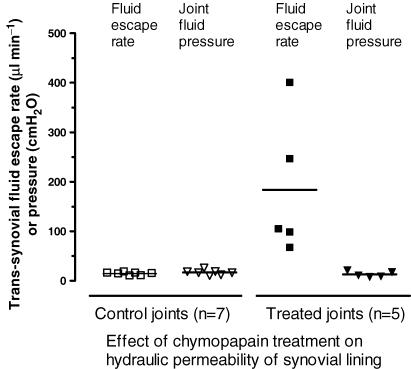
Trans-synovial flows were measured at the start of each study in order to confirm the activity of the chymopapain in vivo (Fig. 1). The trans-synovial filtration rate at 13 cmH2O pressure, namely 14 ± 1 μl min−1 in the Ringer-treated control joints (n = 7), increased to 184 ± 62 μl min−1 in the chymopapain-treated joints (P = 0.008, n = 5, Student's unpaired t test). Chymopapain thus increased the hydraulic permeability of the synovial lining by more than an order of magnitude. The increases spanned a wide range (68–401 μl min−1), though the coefficient of variation, 0.75, was slightly less than that observed by Scott et al. (1998a), 0.89; see Discussion.
Figure 1. Effect of chymopapain on hydraulic permeability of synovial lining in vivo.
Rabbit knee joints were treated with 0.1 i.u. intra-articular activated chymopapain for 20 min before measuring the rate of trans-synovial escape of an infused solution of 0.2 mg ml−1 hyaluronan. Control joints received no chymopapain. Horizontal bar indicates mean.
Hyaluronan concentration gradients across synovium
In control joints, the HA concentration gradient across synovium was very steep (Fig. 2), confirming previous findings (Sabaratnam et al. 2003). The intra-articular HA concentration increased to 259 ± 17% of the infused concentration (P < 0.0001, Student's one-sample t test, n = 11 joints), and the subsynovial concentration fell to 30 ± 8% (P = 0.0002, Student's one-sample t test). The FD profile was flat, as expected for a freely permeable membrane; the intra-articular concentration was not increased (98 ± 3% of infused concentration, n = 11) and the subsynovial concentration was not significantly decreased (95 ± 7%; P = 0.5, Student's one-sample t test).
The HA concentration profile across chymopapain-treated synovium was markedly different; the profile was much flatter, approaching that of FD (Fig. 2). Intra-articular accumulation of HA was negligible (105 ± 6% of infused concentration; P = 0.41, Student's one-sample t test, n = 6 joints), and the fall in subsynovial HA concentration to 70 ± 4% was attenuated compared with the control values (30 ± 8%, P < 0.0001, Student's unpaired t test) The FD profile after chymopapain treatment again showed no significant change in intra-articular concentration, in keeping with the free permeation of FD. The subsynovial FD concentration was a little lower, by 5.9%, than in the control studies, possibly owing to a slight dilution of the well fluid by extra-articular fluid.
Chymopapain treatment thus substantially downgraded the molecular selectivity of the synovium. To quantify the loss of selectivity, the reflected fractions were evaluated, as follows.
Hyaluronan reflected fractions
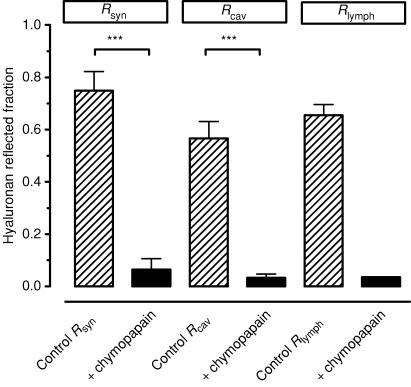
The reflected fraction Rsyn calculated from subsynovial fluid composition was 0.75 ± 0.07 in the control joints under the prevailing filtration conditions (n = 6). The reflected fraction Rcav estimated from the intra-articular accumulation of HA in control joints was 0.57 ± 0.06 (n = 11) and the reflected fraction Rlymph calculated from the lymph samples was 0.65 ± 0.04 (n = 11). Differences between these reflection estimates in control joints were not statistically significant (P = 0.41, one-way ANOVA).
Chymopapain treatment greatly reduced the HA reflected fraction (Fig. 3) (P < 0.001, one-way ANOVA, Tukey's post hoc comparison). The value of Rsyn fell to 0.065 ± 0.04, less than a tenth of its control value, and was not significantly different from zero (P = 0.13, Student's one-sample t test, n = 6 joints). The value of Rcav likewise fell by an order of magnitude to 0.03 ± 0.01 (borderline difference from zero, P = 0.06, Student's one-sample t test, n = 6) and was not significantly different from Rsyn(P = 0.7, Student's unpaired t test). Although only one pooled lymph sample was obtained in sufficient quantity for analysis from chymopapain-treated joints subjected to protocol A, the result was important because no subsynovial surgery was involved in this protocol. Chymopapain treatment reduced the Rlymph to 0.04, an order of magnitude smaller than Rlymph in 11 control lymphatic preparations, 0.65 ± 0.04 (lowest value 0.42).
Figure 3. Effect of intra-articular chymopapain on hyaluronan reflected fraction.
Fraction of hyaluronan molecules reflected during filtration across synovial lining in situ was calculated from subsynovial fluid concentration (Rsyn; n = 6 control, n = 6 after chymopapain), or intra-articular fluid aspirated at the end of the study (Rcav; n = 11 control, n = 6 after chymopapain) or lymph concentration (Rlymph; n = 11 control, n = 1 after chymopapain). ***P ≤ 0.001, one-way ANOVA with Tukey's post hoc comparison.
Molecular size of HA after chymopapain treatment
Shortening of the HA chain length reduces the reflected fraction (Sabaratnam et al. 2005). To test for HA degradation, the HA chromatogram retention time, tret, was analysed; tret is negatively related to HA chain length (Coleman et al. 1997). In 11 control joints, tret was not significantly different between HA in the infusate (7.36 ± 0.05 min), final intra-articular aspirate (7.23 ± 0.05 min), subsynovial fluid (7.39 ± 0.17 min) and lymph (7.45 ± 0.12 min; P = 0.43, one-way ANOVA), although the fall in intra-articular aspirate tret reached significance in an earlier study, indicating selective retention of the longer chains of the heterodisperse sample (Sabaratnam et al. 2005). Hyaluronan from chymopapain-treated joints showed no increase in tret (infusate 7.16 ± 0.03 min, aspirate 7.05 ± 0.01 min, subsynovium 6.99 ± 0.06 min, lymph 7.10 ± 0.03 min). Thus the HA was not degraded in the chymopapain-treated joints.
Synovial ultrastructure following chymopapain treatment
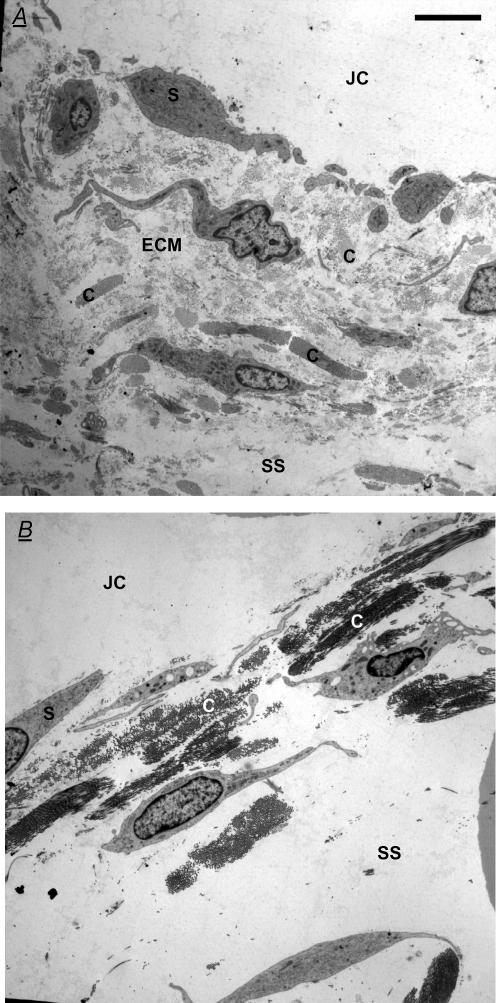
Electron micrographs of chymopapain-treated synovium showed that the network of synovial collagen bundles remained intact, confirming the collagen-sparing action of this protease (Fig. 4). Many collagen bundles appeared to have collapsed together, in keeping with the depletion of the intervening proteoglycan and glycoprotein matrix (Scott et al. 1998a), which normally exerts a swelling pressure.
Figure 4. Effect of intra-articular chymopapain on synovial ultrastructure.
A, electron micrograph of control rabbit knee synovium in transverse section B, synovium from a knee treated with 0.1 i.u. intra-articular chymopapain for 20 min. Both panels are ‘Gold’ sections (50–90 nm) of suprapatellar synovium normal to the surface stained with uranyl acetate and lead citrate. JC, joint cavity; S, synovial lining cell; SS, subsynovium; C, collagen bundle; and ECM, extrafibrillar matrix. Note the persistence of collagen fibrils after chymopapain treatment and the increased distinctness of collagen bundles owing to loss of the lightly staining extrafibrillar matrix. Scale bars represent 5 μm.
Discussion
The principal new finding was that the removal of non-collagenous proteins from the synovial matrix virtually abolished the ability of synovium to retain hyaluronan molecules within the joint cavity. This is the first study, to our knowledge, that defines the effect of a protease on the molecular sieving properties of interstitial matrix. The fall in reflected fraction cannot be attributed to the subsynovial well dissection (protocol B), because control joints with wells had normal reflected fractions. Moreover, Rlymph was reduced by chymopapain even in the absence of subsynovial surgery (protocol A). The results thus support the hypothesis that HA reflection is governed by a sieving network of extrafibrillar proteins.
Comparison with previous work
The steep HA gradient across normal synovium (Fig. 2) reinforced the histological evidence (Asari et al. 1998) and biophysical evidence (Coleman et al. 1997, 1999, 2000; Sabaratnam et al. 2003) that ∼2000 kDa HA experiences ultrafiltration across synovium. Proteoglycans and radio-colloids too show restricted permeation relative to albumin (Page-Thomas et al. 1987; Reimann et al. 1989). The magnitude of the reflection depends on the filtration velocity (Sabaratnam et al. 2004), chain length (Sabaratnam et al. 2005) and concentration (Sabaratnam et al. 2006).
Quantitative biochemistry (Price et al. 1996), immunohistochemistry (Coleman et al. 1998) and ultrastructure (Levick & McDonald, 1989; Price et al. 1995) combine to show that the trans-synovial drainage pathway is a complex, radially ordered matrix containing chondroitin sulphate proteoglycan, decorin, biglycan, heparan sulphate proteoglycan, hyaluronan, keratan sulphate, fibromodulin, fibronectin, laminin, entactin, tenascin, fibrillin, type IV collagen, superficial type VI collagen and, at increasing depths, abundant I–III–V collagen bundles (Worral et al. 1994; Price et al. 1995; Revell et al. 1995; Levick et al. 1996). Chymopapain treatment depletes interstitium of its permeability-regulating, swelling elements, the proteoglycans and glycoproteins (Oegema et al. 1988; Scott et al. 1998a) while preserving the tensile, structural elements, the collagen fibrils (Fig. 4). It is possible that minor variations in the completeness of proteoglycan removal contributed to the wide range of increase in hydraulic conductance (Fig. 1); matrix conductance is a highly non-linear function of polymer concentration and becomes exquisitely sensitive to concentration as the latter approaches zero, as modelled by Scott et al. (1998a; their Fig. 7).
Physiological importance
The selective retention of HA during fluid turnover prolongs the intra-articular half-life of HA (Coleman et al. 1997) and enables a high concentration of HA to accumulate in the synovial fluid (Price et al. 1996). This creates a high viscoelasticity (Balazs & Denlinger, 1985) and provides hydrodynamic lubrication of low-load areas such as synovium-on-synovium, synovium-on-cartilage and, under some conditions, cartilage-on-cartilage (Murakami et al. 1998), complementing the boundary lubricant lubricin. The relatively small amount of HA that escapes from the joint cavity (Brown et al. 1991) is replaced by synovial synthesis/secretion (Coleman et al. 1997; Momberger et al. 2006).
Hyaluronan reflection by interstitial matrix proteins is also important for the conservation of synovial fluid. Although joints evolved for movement, they also experience long periods of maintained static flexion. This raises the intra-articular fluid pressure (Jayson & Dixon, 1970; Knight & Levick, 1982), which begins to drive fluid out of the cavity. Hyaluronan massively attenuates this pressure-induced fluid loss (Coleman et al. 1999), because the reflected HA forms a concentrated layer on the surface of the synovial matrix. The osmotic pressure of this concentrated layer opposes further fluid loss (‘outflow buffering’; Lu et al. 2004, 2005). Although the evidence for concentration polarization was obtained in static joints, a mathematical model of cyclically moving joints predicts that an analogous, oscillating concentration polarization layer will buffer trans-synovial fluid movement in moving joints too.
Change in interstitial pore radius or matrix density
Interstitial proteoglycans and glycoproteins form an irregular network of polymer chains permeated by interstitial fluid (Levick, 1987). In normal synovial matrix, the fluid spaces have exclusion properties equivalent to cylindrical pores of radius 33–59 nm (Sabaratnam et al. 2005). The equivalent pore radius was calculated to increase 5.7-fold to 192–336 nm after chymopapain treatment, using Rsyn (0.065) as an approximate measure of the reduced reflection coefficient. Although the equivalent pore radius has an intuitive appeal, fibre matrix theory may offer a closer approach to biophysical reality. Here, polymer concentration is the key parameter governing molecular sieving (Ogston, 1970; Curry, 1984). Application of Ogston's polymer exclusion theory (Sabaratnam et al. 2005) indicates that a reduction in extrafibrillar biopolymer concentration from ∼11.5 mg ml−1 (control) to ≤ 0.5 mg ml−1 would reduce the synovial reflection coefficient to 0.065. In agreement with this, synovial immunohistology shows that matrix is severely depleted of proteoglycans after chymopapain treatment (Scott et al. 1998a). The average 13-fold rise in hydraulic permeability can likewise be explained by the severe depletion of extrafibrillar biopolymer. Application of fibre matrix theory to the hydraulic permeability results predicts a fall in extrafibrillar biopolymer concentration to 3 mg ml−1, if the collagen distribution remains unchanged (Levick, 1987; Scott et al. 1998a). The difference of 2.5 mg ml−1 between the residual extrafibrillar concentrations estimated from the reflection results and that estimated from the flow result may be due in part to the re-organization of the collagen bundles (Fig. 4), which affects the area and tortuosity of the flow pathway.
Pathological importance: protease activity in arthritides
There are large increases in active metalloproteinase concentration in the synovial fluid and articular tissues in inflammatory arthritides such as rheumatoid arthritis (Jones & Riley, 2005). The consequences for arthritic synovial permeability have never been studied directly, but it is known that trans-synovial flow increases in human inflammatory arthritis (Wallis et al. 1987) and that serum HA rises, especially with movement (Engström-Laurent & Hällgren, 1987; Criscione et al. 2005). Our results may help to explain these clinical findings. Synovial matrix degradation by proteases raises the synovial hydraulic conductivity, reduces HA reflection and attenuates concentration polarization-induced outflow buffering, and should thus enhance arthritic fluid outflow, as observed by Wallis et al. (1987). Protease-induced increases in HA leakage, along with the stimulation of HA secretion by pro-inflammatory agents (Stuhlmeier, 2006), can be expected to contribute to the elevation of serum HA, a feature of rheumatoid arthritis. The fall in HA chain length and concentration in arthritis (Balazs et al. 1967; Bjelle et al. 1982; Dahl et al. 1985) will further reduce the reflection of HA by synovium (Sabaratnam et al. 2005, 2006). Positive feedback may operate under these conditions, since increased HA escape reduces HA concentration, while reduction of HA concentration increases HA escape (Sabaratnam et al. 2006). Animal studies thus reveal a multiplicity of interacting factors that may exacerbate HA loss from arthritic joints.
Reduced HA reflection and osmotic buffering in arthritic joints may be biologically useful in that they promote trans-synovial fluid escape, which should attenuate the accumulation of an effusion and hasten its resolution. The downside, however, is increased HA loss, reduced HA lubrication, peri-articular fluid accumulation leading to morning stiffness, and the metabolic burden of a compensatory increase in HA synthesis driven by pro-inflammatory cytokines (Tanimoto et al. 2004; Momberger et al. 2005; Stuhlmeier, 2006). Even in unstimulated rabbit synovium, the synthesis of HA (380 000 molecules per synoviocyte per hour in vivo) accounts for at least 16–25% of synovial glucose consumption, HA being a polymer of N-acetylglucosamine and d-glucuronic acid.
To summarize, the retention of hyaluronan inside synovial joints and the synovial hydraulic resistance both depend on the presence of non-collagen extracellular matrix proteins. When these are degraded by protease, high rates of fluid and hyaluronan loss ensue. In addition to serving as a proof of principle, the findings have important implications for the pathophysiology of inflammatory arthritides.
Acknowledgments
We thank Vaitha Arunan for technical assistance. The work was funded by Wellcome Trust grant 056983/Z/99.
References
- Asari A, Miyauchi S, Matsuzaka S, Ito T, Kominami E, Uchiyama Y. Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol. 1998;61:125–135. doi: 10.1679/aohc.61.125. [DOI] [PubMed] [Google Scholar]
- Balazs EA, Denlinger JL. Sodium hyaluronate and joint function. J Equine Vet Sci. 1985;5:217–228. [Google Scholar]
- Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 1967;10:357–376. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- Bjelle A, Anderson T, Granath K. Molecular weight distribution of hyaluronic acid of human synovial fluid in rheumatic diseases. Scand J Rheumatol. 1982;12:133–138. doi: 10.3109/03009748309102899. [DOI] [PubMed] [Google Scholar]
- Bradford DS, Oegema TR, Cooper KM, Wakano K, Chao EY. Chymopapain, chemonucleolysis and nucleus pulposus regeneration. A biochemical and biomechanical study. Spine. 1984;9:135–147. doi: 10.1097/00007632-198403000-00004. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Laurent UBG, Fraser JRE. Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit. Exp Physiol. 1991;76:125–134. doi: 10.1113/expphysiol.1991.sp003474. [DOI] [PubMed] [Google Scholar]
- Coleman PJ. Evidence for a role of hyaluronan in the spacing of fibrils within collagen bundles in rabbit synovium. Biochim Biophys Acta. 2002;1571:173–182. doi: 10.1016/s0304-4165(02)00213-1. [DOI] [PubMed] [Google Scholar]
- Coleman PJ. A role for hyaluronan in the preservation of interstitial structure. Microcirc. 2005;12:1–13. doi: 10.1080/10739680590905143. [DOI] [PubMed] [Google Scholar]
- Coleman P, Kavanagh E, Mason RM, Levick JR, Ashhurst DE. The proteoglycans and glycosaminoglycan chains of rabbit synovium. Histochem J. 1998;30:519–524. doi: 10.1023/a:1003291303380. [DOI] [PubMed] [Google Scholar]
- Coleman PJ, Scott D, Mason RM, Levick JR. Characterization of the effect of high molecular weight hyaluronan on trans-synovial flow in rabbit knees. J Physiol. 1999;514:265–282. doi: 10.1111/j.1469-7793.1999.265af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PJ, Scott D, Mason RM, Levick JR. Role of hyaluronan chain length in buffering interstitial flow across synovium in rabbits. J Physiol. 2000;526:425–434. doi: 10.1111/j.1469-7793.2000.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PJ, Scott D, Ray J, Mason RM, Levick JR. Hyaluronan secretion into the synovial cavity of rabbit knees and comparison with albumin turnover. J Physiol. 1997;503:645–656. doi: 10.1111/j.1469-7793.1997.645bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscione LG, Elliott AL, Stabler T, Jordan JM, Pieper CF, Kraus B. Variation in serum hyaluronan with activity in knee osteoarthritis. Osteoarthritis Cartilage. 2005;13:837–840. doi: 10.1016/j.joca.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Curry FE. Mechanics and thermodynamics of transcapillary exchange. In: Renkin EM, Michel CC, editors. Handbook of Physiology, section 2, The Cardiovascular System, The Microcirculation. IV. Bethesda: American Physiological Society; 1984. pp. 309–374. [Google Scholar]
- Dahl LB, Dahl IM, Engström-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheumatic Dis. 1985;44:817–822. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström-Laurent A, Hällgren R. Circulating hyaluronic acid levels vary with physical activity in healthy subjects and in rheumatoid arthritis patients. Arthritis Rheum. 1987;30:1333–1338. doi: 10.1002/art.1780301203. [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Ito T, Oguchi T, Kojima T, Iwata H, Ionescu M, Poole AR. Relationships of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover and inflammation as revealed by analyses of synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 2001;44:2503–2511. doi: 10.1002/1529-0131(200111)44:11<2503::aid-art430>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Jayson MIV, Dixon AStJ. Intra-articular pressure in rheumatoid arthritis of the knee. III. Pressure changes during joint use. Ann Rheumatic Dis. 1970;29:401–408. doi: 10.1136/ard.29.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GC, Riley GP. ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res Ther. 2005;7:160–169. doi: 10.1186/ar1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AD, Levick JR. Pressure–volume relationships above and below atmospheric pressure in the synovial cavity of the rabbit knee. J Physiol. 1982;328:403–420. doi: 10.1113/jphysiol.1982.sp014273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick JR. Flow through interstitium and other fibrous matrices. Exp Physiol. 1987;72:409–438. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- Levick JR, McDonald JN. Ultrastructure of transport pathways in stressed synovium of the knee in anaesthetized rabbits. J Physiol. 1989;419:493–508. doi: 10.1113/jphysiol.1989.sp017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick JR, Price FM, Mason RM. Synovial matrix–synovial fluid system of joints. In: Comper WD, editor. Extracellular Matrix, Tissue Function. Vol. 1. Amsterdam: Harwood Academic Publishers; 1996. pp. 328–377. [Google Scholar]
- Lu Y, Levick JR, Wang W. Concentration polarization of hyaluronan on the surface of the synovial lining of infused joints. J Physiol. 2004;561:559–573. doi: 10.1113/jphysiol.2004.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Levick JR, Wang W. Synovial fluid retention in pressurised joint cavities is achieved by hyaluronan concentration polarisation. Microcirc. 2005;12:581–595. doi: 10.1080/10739680500253527. [DOI] [PubMed] [Google Scholar]
- Momberger TS, Levick JR, Mason RM. Hyaluronan secretion by synoviocytes is mechanosensitive. Matrix Biol. 2005;24:510–519. doi: 10.1016/j.matbio.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momberger TS, Levick JR, Mason RM. Mechanosensitive synoviocytes: a Ca2+-PKCα-MAP kinase pathway contributes to stretch-induced hyaluronan synthesis in vitro. Matrix Biol. 2006;25:306–316. doi: 10.1016/j.matbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Murakami T, Higaki H, Sawae Y, Ohtsuki N, Moriyama S, Nakanishi Y. Adaptive multimode lubrication in natural synovial joints and artificial joints. Proc Inst Mech Eng. 1998;212:23–35. doi: 10.1243/0954411981533791. [DOI] [PubMed] [Google Scholar]
- Oegema TR, Swedenburg SM, Bradford DS, Thonar EJM. Levels of keratan sulphate-bearing fragments rise predictably following chemonucleolysis of dog intervertebral discs with chymopapain. Spine. 1988;13:707–711. [PubMed] [Google Scholar]
- Ogston AG. On the interaction of solute molecules with porous networks. J Phys Chem. 1970;74:668–669. [Google Scholar]
- Page-Thomas DP, Bard D, King B, Dingle JT. Clearance of proteoglycan from joint cavities. Ann Rheumatic Dis. 1987;46:934–937. doi: 10.1136/ard.46.12.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FM, Levick JR, Mason RM. Glycosaminoglycan concentration in synovium and other tissues of rabbit knee in relation to hydraulic resistance. J Physiol. 1996;495:803–820. doi: 10.1113/jphysiol.1996.sp021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FM, Mason RM, Levick JR. Radial organization of interstitial exchange pathway and influence of collagen in synovium. Biophys J. 1995;69:1429–1439. doi: 10.1016/S0006-3495(95)80012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann I, Vittas D, Nielsen SL, Svalastoga E. Lymphatic transport from normal and synovitic knees in rabbits. Acta Orthopaedica Scand. 1989;60:185–187. doi: 10.3109/17453678909149250. [DOI] [PubMed] [Google Scholar]
- Revell PA, Al-Saffar N, Fish S, Osei D. Extracellular matrix of the synovial intimal cell layer. Ann Rheumatic Dis. 1995;54:404–407. doi: 10.1136/ard.54.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaratnam S, Arunan V, Coleman PJ, Mason RM, Levick JR. Size-selectivity of hyaluronan molecular sieving by extracellular matrix in rabbit synovial joints. J Physiol. 2005;567:569–581. doi: 10.1113/jphysiol.2005.088906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaratnam S, Mason RM, Levick JR. Molecular sieving of hyaluronan by synovial interstitial matrix and lymphatic capillary endothelium evaluated by lymph analysis in rabbits. Microvasc Res. 2003;66:227–236. doi: 10.1016/j.mvr.2003.08.003. with Figures erratum in Microvasc Res67, 245–246. [DOI] [PubMed] [Google Scholar]
- Sabaratnam S, Mason RM, Levick JR. Filtration rate dependence of hyaluronan reflection by joint-to-lymph barrier in rabbit knees: evidence for concentration polarisation. J Physiol. 2004;557:909–922. doi: 10.1113/jphysiol.2004.063529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaratnam S, Mason RM, Levick JR. Hyaluronan molecular reflection by synovial lining is concentration dependent and reduced in dilute effusions in a rabbit model. Arthritis Rheum. 2006;54:1673–1681. doi: 10.1002/art.21803. [DOI] [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Abiona A, Ashhurst DE, Mason RM, Levick JR. Effect of depletion of glycosaminoglycans and non-collagenous proteins on interstitial hydraulic permeability in rabbit synovium. J Physiol. 1998a;511:629–643. doi: 10.1111/j.1469-7793.1998.629bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Coleman PJ, Mason RM, Levick JR. Direct evidence for the partial reflection of hyaluronan molecules by the lining of joints during trans-synovial flow. J Physiol. 1998b;508:610–623. doi: 10.1111/j.1469-7793.1998.619bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Levick JR, Miserocchi G. Non-linear dependence of interstitial fluid pressure on joint cavity pressure and implications for interstitial resistance in rabbit knee. Acta Physiol Scand. 2003;179:93–101. doi: 10.1046/j.1365-201X.2003.01148.x. [DOI] [PubMed] [Google Scholar]
- Stuhlmeier KM. Prostaglandin E2: a potent activator of hyaluronan synthase 1 in type-B-synoviocytes. Biophys Biochim Acta. 2006 doi: 10.1016/j.bbagen.2006.07.001. Epub (July) [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Suzuki A, Ohno S, Honda K, Tanaka N, Doi T, Yoneno K, Ohno-Nakahara M, Nakatani Y, Ueki M, Tanne K. Effects of TGF-β on hyaluronan anabolism in fibroblasts derived from the synovial membrane of the rabbit temporomandibular joint. J Dent Res. 2004;83:40–44. doi: 10.1177/154405910408300108. [DOI] [PubMed] [Google Scholar]
- Wallis WJ, Simkin PA, Nelp WB. Protein traffic in human synovial effusions. Arthritis Rheum. 1987;30:57–63. doi: 10.1002/art.1780300108. [DOI] [PubMed] [Google Scholar]
- Worrall JG, Wilkinson LS, Bayliss MT, Edwards JCW. Zonal distribution of chondroitin-4-sulphate/dermatan sulphate and chondroitin-6-sulphate in normal and diseased human synovium. Ann Rheumatic Dis. 1994;53:35–38. doi: 10.1136/ard.53.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]