Abstract
The behavioural consequences of prenatal glucocorticoid exposure are not well understood, though emerging studies in humans indicate hyperactivity and altered cognitive development can occur. Further, recent reports indicate that N-methyl-d-aspartate receptors (NMDARs) may mediate the development of postnatal stress behaviours. We hypothesized that prenatal betamethasone (Beta) administration would alter behaviour and the expression of hippocampal NMDAR subunits NR1, NR2A and NR2B in juvenile guinea pig offspring. We found that repeated maternal Beta (1 mg kg−1) treatment on gestational days (gd) 40/41, 50/51 and 60/61 (term∼70 days) had no significant effect on birthweight or early growth. However, Beta produced sex-specific effects on open-field activity and hippocampal NMDAR subunit expression compared with controls. Female Beta offspring exhibited significantly increased locomotor activity while there was no effect in Beta males. Beta males exhibited a tendency for decreased anxiety-like behaviour. With respect to NMDAR subunit expression, Beta-exposed females exhibited significantly reduced NR1 mRNA in CA1/2 and CA3 subfields of the hippocampus; there were no effects in Beta males. In conclusion, repeated maternal treatment with Beta, in a similar regimen to that administered to pregnant women at risk of delivering preterm, has profound consequences on behaviour and development of crucial neurotransmitter systems in postnatal life.
Approximately 7–10% of pregnant women in the developed world are at risk of preterm delivery (Gilstrap, 2001). The majority of these women are prescribed antenatal glucocorticoid therapy in order to develop the fetal lungs. Indeed, surveys of obstetrical practice indicate that, until recently, repeated dose regimens have been common (Quinlivan et al. 1998; Brocklehurst et al. 1999). However, the long-term behavioural consequences of repeated prenatal glucocorticoid exposure on behaviour are largely unknown. Evidence is beginning to emerge indicating that children who were exposed to either prenatal synthetic glucocorticoid administration or endogenous glucocorticoid, because of maternal stress, may be at higher risk of emotional and behavioural abnormalities. A recent study conducted in school-aged children who received three or more courses of antenatal betamethasone showed that such treatment was associated with an increased risk of postnatal aggressive/destructive behaviour, increased distractibility, and hyperactivity (French et al. 2004). Accordingly, studies of pre-adolescent children whose mothers experienced high anxiety during pregnancy have shown that they are significantly more prone to developing attention deficit and hyperactivity disorder (ADHD) and other behavioural problems (O'Connor et al. 2002, 2003; Rodriguez & Bohlin, 2005).
Young and adult rats born to mothers treated with dexamethasone displayed sex-specific changes in locomotor and habituation activity in anxiety behaviour tests (Kreider et al. 2005). Prenatally stressed rats also displayed significantly more depressive and anxious behaviours (Patin et al. 2005; Van den Hove et al. 2005). Goats that underwent transport stress during pregnancy produced kids with greater exploratory responses to novelty and reaction to startling stimuli (Roussel et al. 2005). In the guinea pig, we have shown altered wall-seeking behaviour (thigmotaxis) in adult male guinea pigs that had been exposed to prenatal stress (Kapoor & Matthews, 2005). However, the mechanisms underlying these behavioural sequelae are poorly understood.
The N-methyl-d-aspartate receptor (NMDAR) has been implicated in the programming of behaviour. NMDARs are excitatory ligand-gated ion channels comprised of four or five subunits: at least two NR1 subunits, which are central to glutamate binding and NR function, and any of the NR2 (A, B, C, D) or NR3 (A, B, C) subunits, which confer distinct pharmacological and electrophysiological properties (Feldmeyer & Cull-Candy, 1996; Cull-Candy et al. 2001). In animal studies, mice with mild disruption of the NMDAR exhibited increased startle reactivity but decreased aversion to a novel environment such as an open-field (Mohn et al. 1999; Kew et al. 2000). We have also previously shown that prenatal dexamethasone exposure markedly alters NMDAR subunit expression in the late-gestation guinea pig fetus in a sex-specific and dose-dependent manner (Owen et al. 2004). Recently, studies in the spinal cord have shown direct regulation of NMDAR subunit expression by glucocorticoids (Lim et al. 2005; Wang et al. 2005).
In the present study, we hypothesized that repeated prenatal glucocorticoid exposure during critical periods of fetal brain development would impact open-field behaviour in juvenile guinea pig offspring. We also postulated that alterations in NMDAR expression may underlie behavioural changes observed in these juvenile offspring. The guinea pig, like the human, and unlike the rat, initiates the most rapid phase of brain growth during late fetal life (Dobbing & Sands, 1970, 1979). It is in this phase of brain development that the human fetal brain can be exposed to very high levels of synthetic glucocorticoid during treatment of suspected preterm labour (Owen et al. 2005).
Methods
Prenatal betamethasone treatment
Pregnant guinea pigs were subcutaneously injected with betamethasone (Beta; Betaject, Sabex, Boucherville, QC, Canada; 1 mg kg−1; 6 mg ml−1; n = 13) or vehicle (Veh; saline; 166 μl kg−1; n = 15) on gestational days (gd) 40/41 (period of rapid neurogenesis), 50/51 (peak brain growth), and 60/61 (period of myelination) (Dobbing & Sands, 1970). The dose (1 mg kg−1) of synthetic glucocorticoid used in this study in the guinea pig is comparable to the dose used in pregnant women (approximately 0.25 mg kg−1), since the guinea pig glucocorticoid receptor has a fourfold lower affinity for dexamethasone (Keightley & Fuller, 1995). Beta is becoming the synthetic glucocorticoid of choice for clinical management of preterm labour. Pregnant animals were allowed to deliver undisturbed. Normal litter size is 2–3 offspring. On postnatal day (pnd) 10, one group of offspring were rapidly decapitated on removal from the mother (females: Veh = 8(7), Beta = 8(8); males: Veh = 8(8), Beta = 10(9); number of offspring (number of litters)). These animals were used for analysis of growth, organ weights and molecular analysis of NMDAR subunit expression. Another group was tested in an open-field arena (females: Veh = 8(8), Beta = 8(8); males: Veh = 8(7), Beta = 8(6)). In the majority of cases, one male and one female from each litter were placed into the growth/molecular analysis groups, and one male and one female from each litter were placed into the behavioural analysis groups. However, if more than a single male or single female from the same litter was present in any experimental group (i.e. two female offspring from the same litter), a mean value for the specific measurement was calculated from the two offspring, and this was used to produce group means and for all statistical analyses.
Open-field behaviour
Locomotor activity was monitored for 30 min (OptoMax, Columbus Instruments, Columbus, OH, USA; box dimensions: 42.5 × 42.5 × 23 cm), as previously described for the guinea pig (Kapoor & Matthews, 2005). Behavioural data (30 min open-field exposure) are presented as activity for 5 min intervals across a 30 min test period, and as cumulative activity for the entire 30 min period. Variables measured in the open-field were: total activity (all activity in all directions, horizontal and vertical combined; defined as Optomax units (OU)) and time spent in the centre of the arena (32.5 cm × 32.5 cm; 10 cm border from edge of arena).
Tissue collection and analysis
The left hemispheres were frozen for in situ hybridization analysis of NR1, NR2A and NR2B mRNA. Whole hippocampi were dissected from the right cerebral hemispheres and frozen for Western blot analysis of NR1 and NR2A protein.
In situ hybridization
The method for in situ hybridization has been described in detail elsewhere (Matthews, 1998). Briefly, coronal cryosections (10 μm) were mounted onto poly-l-lysine-coated slides, dried and fixed in paraformaldehyde (4%). Previously characterized oligonucleotide probes for NR1, NR2A and NR2B (Owen et al. 2004) were labelled using terminal deoxynucleotidyl transferase (Gibco, Burlington, ON, Canada) and [35S]dATP (1300 Ci mmol−1; Perkin Elmer, Woodbridge, ON, Canada) to a specific activity of 1.0 × 109 c.p.m. μg−1 (Owen et al. 2004). Labelled probe in hybridization buffer (200 μl) was applied to slides at a concentration of 1.0 × 103 c.p.m. μl−1. Slides were incubated overnight in a moist chamber at 42.5°C. After washing in 1× saline–sodium citrate buffer (SSC), the slides were rinsed and dehydrated in ethanol. The slides were dried and exposed to autoradiographic film (Biomax MR, Kodak, Perkin Elmer, Woodbridge, ON, Canada). Films were developed using an automatic processor (exposure: NR1, 14 days; NR2A, 9 days; NR2B, 6 days).
Brain sections were processed simultaneously to allow direct comparison between groups and exposed together with 14C standards (American Radiochemical Company, St Louis, MO, USA) to ensure analysis in the linear range of the autoradiographic film. The relative optical density (ROD) of the signal on autoradiographic film was quantified, after subtraction of background values, using a computerized image analysis system (Imaging Research Inc., St Catharines, ON, Canada) (Owen & Matthews, 2003). Levels of NMDAR subunit mRNA expression were measured in the hippocampus and cortex, as previously described (Owen et al. 2004).
Western blot analysis
The method for Western blot analysis has been described in detail previously (Owen & Matthews, 2003). Briefly, hippocampi were homogenized in ice cold Radioimmunoprecipitation (RIPA) lysis buffer and centrifuged. Protein concentration of the resultant supernatant was determined by the Bradford method (Bradford, 1976). Laemmli sample buffer (2× concentration; 15 μl; Sigma, Oakville, ON, Canada) was added to each sample (50 μg protein), which was then denatured (boiled 5 min at 95°C). Samples were separated by SDS-PAGE (8% resolving polyacrylamide gel) and transferred electrophoretically to a nitrocellulose membrane (Bio-Rad, Mississauga, ON, Canada).
Nitrocellulose membranes were blocked overnight (4°C) and incubated with NR1 antibody (1:500; Chemicon International, Temecula, CA, USA; AB1516, 1 h, 23°C) prior to incubation with horseradish-peroxidase-conjugated goat anti-rabbit IgG (1:5000; 1 h, 23°C; Perkin Elmer, Woodbridge, ON, Canada). Blots were washed and exposed to Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer, Woodbridge, ON, Canada) and bands were visualized by exposure to Kodak Blue X-OMAT (Perkin Elmer). Membranes were stripped in Restore Western Blot Stripping buffer (Pierce, MJS Biolynx, Mississauga, ON, Canada) and subsequently probed with anti-NR2A (1:500; Chemicon International; AB1555) and tubulin (1:5000; antitubulin; Sigma, Oakville, ON, Canada). NR2B protein levels were not assessed, as an antibody that specifically detects guinea pig NR2B was not available. Western blots were performed a minimum of four times. Data were pooled to derive a mean value for each animal. Expression levels are presented as a ratio of NR1 or NR2A to tubulin signal.
Statistical analysis
Group data are presented as means ± s.e.m. Behavioural data are graphed at 5 min intervals, and these were analysed by repeated measures ANOVA in which the effects of time, sex and prenatal treatment were considered. Cumulative behavioural data were analysed by two-way ANOVA (prenatal treatment × sex). Gene expression data were analysed by three-way ANOVA (prenatal treatment × sex × region). All ANOVAs were followed by Duncan's method of post hoc comparison (Statistica, Statsoft, Inc., Tulsa, OK, USA). Statistical significance was set at P < 0.05.
Results
Birth weights, litter size and organ weights
There was no significant effect of repeated betamethasone treatment on litter size (Veh 2.9 ± 0.2; Beta 3.0 ± 0.3) and birthweight (females: Veh 110.5 ± 5.6; Beta 98.0 ± 5.8; males: Veh 109.75 ± 4.32; Beta 106.8 ± 3.92). At pnd10, there were no differences in pup weights or any organ weights, including brain, hippocampus, pituitary, lungs, heart, liver, kidneys, adrenals or gonads (data not shown).
Open-field activity
In female offspring, prenatal betamethasone exposure resulted in a significant increase in total activity (repeated measures ANOVA (5 min intervals) P < 0.02; 30 min cumulative activity P < 0.02; Fig. 1A. There was a significant reduction in activity over the 30 min test period in Beta female (P < 0.0001) and Veh offspring (P < 0.0001). Post hoc analysis revealed that differences between the Beta and Veh female offspring were greatest between 15 and 20 min (P < 0.002) and 20–25 min (P < 0.007) exposure to the open-field. In male offspring, while there was a significant decrease in activity over time (P < 0.0001), there was no significant difference in activity between Beta and Veh offspring (Fig. 1B). Given the significant effects of sex on open-field activity, all subsequent analyses were undertaken separately in males and females.
Figure 1. Open field activity.
Total activity (A and B) and time spent in cage centre (C and D) in female and male offspring born to mothers treated with betamethasone (Beta; 1 mg kg−1; filled circles/bars) or vehicle (Veh; open circles/bars) on gestational days (gd) 40/41, 50/51 and 60/61. Insets in A and B are the cumulative totals for the entire 30 min period. OU, OptoMax activity units. Females: Veh n = 8, Beta n = 8; males: Veh n = 7, Beta n = 6. *Significant difference from Veh group (P < 0.05).
While most animals exhibit thigmotactic behaviour, remaining at the edge of the open-field, exploration of the centre area provides an index of anxiety. Increased exploration of the centre region is suggestive of reduced anxiety (Choleris et al. 2001; Kapoor & Matthews, 2005). In the present study, there were no significant effects of prenatal betamethasone treatment on time spent in the cage centre in either male or female offspring (Fig. 1C and D). However, there was a tendency for male Beta offspring to spend more time in the cage centre.
Hippocampal and cortical NMDAR subunit expression
NR1, NR2A and NR2B mRNAs were expressed in all regions of the hippocampus (CA1/2, CA3 and CA4) as well as the dentate gyrus and cerebral cortex at pnd10 (Fig. 2).
Figure 2. Representative images of NR1, NR2A and NR2B mRNA expression in the hippocampus and cortex after in situ hybridization and autoradiography.
Bar, 2.5 mm. A section was also hybridized with a sense control yielding no signal over the brain section.
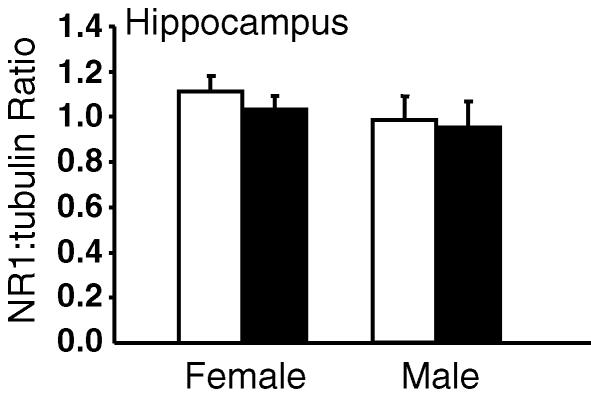
In female offspring, two-way ANOVA revealed a significant effect of prenatal betamethasone treatment (P < 0.002) on NR1 mRNA levels when all regions of the limbic system were considered together. However, there were also significant regional differences (P < 0.0001) in expression. Post hoc analysis revealed a significant reduction in NR1 mRNA in the CA1/2 (P < 0.03) and CA3 (P < 0.004) regions of the hippocampus in female Beta offspring (Fig. 3A and B). There was no significant effect of prenatal betamethasone exposure on NR1 mRNA in the CA4 and dentate gyrus (Fig. 3C and D). In male offspring, there was no significant effect of prenatal betamethasone treatment on NR1 mRNA levels in any region of the limbic system measured. Overall, there were significant sex differences (P < 0.02) in NR1 mRNA levels and this was most pronounced in the CA1/2 (P < 0.05) and CA3 (P = 0.058) regions of the hippocampus in control offspring. There was no sex difference in the betamethasone-exposed groups (Fig. 3A and B). Western analysis of NR1 protein in the entire right hippocampus failed to reveal a significant effect of prenatal betamethasone treatment. However, there was a tendency for a reduction in NR1 protein in female Beta offspring (Fig. 4).
Figure 3. Hippocampal NR1 mRNA expression.
NR1 mRNA expression in the CA1/2 (A), CA3 (B) and CA4 (C) region of the hippocampus, and the dentate gyrus (DG; D) in vehicle (open bars), and betamethasone-exposed (filled bars) animals. Data are expressed as mean relative optical density (ROD) ±s.e.m.*Significant difference from Veh group (P < 0.05). †Significant sex difference between Veh animals; n = 7–9 in each group.
Figure 4. Hippocampal NR1 protein expression in vehicle and betamethasone-exposed offspring.
Vehicle, open bars; betamethasone-exposed, filled bars. Data are expressed as mean NR1:tubulin ratio ±s.e.m;n = 7–9 in each group.
There were no significant effects of prenatal betamethasone treatment on NR2A mRNA (Table 1) or NR2B mRNA (Table 2) in the hippocampus or dentate gyrus. However, there were significant regional differences in NR2A mRNA (P < 0.0001; CA1/2 > CA3 > dentate gyrus > CA4) and NR2B mRNA (P < 0.0001; CA1/2 > CA3 > dentate gyrus > CA4). There were also no significant effects of prenatal treatment on NR2A protein (females: Veh 1.19 ± 0.09; Beta 1.18 ± 0.08; males: Veh 1.46 ± 0.09; Beta 1.65 ± 0.08 NR2A:tubulin ratio). There were no significant effects of prenatal betamethasone on NR1, NR2A and NR2B mRNA levels in the cerebral cortex of either female or male offspring (Table 3).
Table 1.
NR2A mRNA expression in the hippocampus of postnatal day 10 offspring born to mothers treated with vehicle (Veh) or betamethasone (Beta) in late gestation
| Region | Sex | Veh | Beta |
|---|---|---|---|
| CA1/2 | Female | 0.262 ± 0.012 | 0.256 ± 0.009 |
| Male | 0.244 ± 0.010 | 0.261 ± 0.006 | |
| CA3 | Female | 0.232 ± 0.009 | 0.226 ± 0.009 |
| Male | 0.235 ± 0.013 | 0.241 ± 0.006 | |
| CA4 | Female | 0.174 ± 0.006 | 0.168 ± 0.011 |
| Male | 0.170 ± 0.010 | 0.177 ± 0.005 | |
| DG | Female | 0.229 ± 0.004 | 0.224 ± 0.008 |
| Male | 0.223 ± 0.007 | 0.227 ± 0.003 |
DG, dentate gyrus. Data are expressed as mean relative optical density (ROD) ±s.e.m.;n = 7–9 in each group.
Table 2.
NR2B mRNA expression in the hippocampus of postnatal day 10 offspring born to mothers treated with Veh or Beta in late gestation
| Region | Sex | Veh | Beta |
|---|---|---|---|
| CA1/2 | Female | 0.403 ± 0.016 | 0.398 ± 0.013 |
| Male | 0.402 ± 0.035 | 0.421 ± 0.010 | |
| CA3 | Female | 0.330 ± 0.009 | 0.319 ± 0.013 |
| Male | 0.371 ± 0.013 | 0.360 ± 0.007 | |
| CA4 | Female | 0.235 ± 0.007 | 0.215 ± 0.003 |
| Male | 0.236 ± 0.009 | 0.234 ± 0.009 | |
| DG | Female | 0.334 ± 0.012 | 0.327 ± 0.013 |
| Male | 0.329 ± 0.020 | 0.344 ± 0.008 |
Data are expressed as mean ROD ±s.e.m.;n = 7–9 in each group.
Table 3.
Cortical NR1, NR2A, NR2B mRNA expression in postnatal day 10 offspring born to mothers treated with Veh or Beta in late gestation
| Subunit | Sex | Veh | Beta |
|---|---|---|---|
| NR1 | Female | 0.162 ± 0.008 | 0.151 ± 0.010 |
| Male | 0.138 ± 0.007 | 0.150 ± 0.007 | |
| NR2A | Female | 0.147 ± 0.007 | 0.137 ± 0.008 |
| Male | 0.138 ± 0.007 | 0.147 ± 0.007 | |
| NR2B | Female | 0.193 ± 0.003 | 0.187 ± 0.004 |
| Male | 0.189 ± 0.010 | 0.197 ± 0.006 |
Data are expressed as mean ROD ±s.e.m.;n = 7–9 in each group.
Discussion
In the current study, we have shown that repeated maternal treatment with betamethasone in late gestation can alter activity in an open-field and hippocampal NMDAR expression in early juvenile life in a sex-specific manner. Female offspring born to mothers treated with betamethasone exhibited increased activity in an open-field. This hyperactivity was associated with reduced NR1 subunit mRNA levels in CA1/2 and CA3 regions of the hippocampus.
The trend in activity over the 30 min period as well as cumulative activities is more reflective of adaptation to the novel environment. Habituation/adaptation (lessened anxiety) to an open-field can be defined as decreased activity as a function of the duration of time that the animal is exposed to the open-field (Crawley, 1999). In all groups, there was an effect of time on activity, indicating that habituation was occurring. The Beta females showed statistically increased locomotor behaviour across the entire 30 min test period. However, this effect did not begin to emerge until after the first 10 min of exposure to the open-field, suggesting that while these animals initially began to adapt to the environment they did not adapt as rapidly as the control groups and remained hyperactive. Interestingly, this effect of prenatal betamethasone is confined to females as there was no difference in total activity in males. In the present study, we also assessed time spent in the centre of the open-field arena. Increased exploration in the centre of the arena is considered to indicate reduced anxiety, while wall-seeking behaviour (thigmotaxis) is considered an indicator of increased anxiety. We found no significant effect of prenatal betamethasone treatment on time spent in the centre of the arena in juvenile females; however, there was a tendency for increased exploration in the male offspring born to mothers treated with glucocorticoid, suggestive of decreased anxiety in response to the open-field in this group. In previous studies, we have shown that prenatal stress in late gestation leads to increased anxiety behaviour in young male (25 days of age), but not female guinea pigs (Kapoor & Matthews, 2005). Further studies are required to investigate whether increased anxiety emerges as a function of age in animals exposed to prenatal glucocorticoid.
Rats and mice that received dexamethasone or betamethasone in the last third of pregnancy gave birth to offspring with reduced exploratory behaviour in an open-field in adulthood (Rayburn et al. 1997; Welberg et al. 2001). Various prenatal stress protocols in rats, which expose fetuses to elevated endogenous glucocorticoids in utero, have also been shown to reduce activity in an open-field in adult offspring, indicative of elevated anxiety (Lehmann et al. 2000; Welberg et al. 2000; Fujioka et al. 2001). However, our finding that Beta female offspring are hyperactive in juvenile life is consistent with recent assessments of behaviour in school-aged children whose mothers were highly anxious or who received more than three courses of synthetic glucocorticoids during pregnancy (O'Connor et al. 2002, 2003; French et al. 2004). These children were more distractible, aggressive and hyperkinetic, and showed a greater tendency to develop attention deficit disorder. Similarly, another recent study has shown that exposure to maternal stress during pregnancy is associated with a significantly greater incidence of restless/disruptive behaviour and emotional and conduct disorders in 27-month-old toddlers (Gutteling et al. 2005). Further studies are required to determine whether the effects of prenatal glucocorticoid exposure on activity are maintained into adulthood. Differences between the effects of prenatal glucocorticoid exposure in guinea pigs and rats/mice are likely to be attributable to the stage of brain development at which glucocorticoid exposure occurs. The guinea pig, like the human, and unlike the rat, initiates the most rapid phase of brain growth during late fetal life (Dobbing & Sands, 1970, 1979).
In the present study, we have shown a reduction in NR1 mRNA in female offspring born to Beta-treated mothers, and this was associated with increased locomotor activity in the open field. The NMDAR has been implicated in modulating the expression of locomotor and anxiety behaviour. Pharmacological blockade of NMDARs has been shown to produce anxiolysis and to increase exploratory activity in an open-field (Plaznik et al. 1994; Jessa et al. 1995, 1996). Mutation of the NR1 subunit at the glycine-binding site resulted in mice that spent more time in the cage centre in an open-field (Kew et al. 2000). Another more recent study has shown that mutant mice lacking the NR1 subunit exhibit increased spontaneous locomotor activity in an open-field (Miyamoto et al. 2004). This is consistent with the fact that genetic disruption of the NR1 subunit is linked to hyperfunction of central dopamine systems (Miyamoto et al. 2001). Given the fact that the NR1 subunit is fundamental to the core structure of all functional NMDARs, reduction in NR1 expression or NR1 mutation will have profound effects on function of the NMDAR (Moriyoshi et al. 1991). The latter observations in the mouse support our present data linking reduced hippocampal NR1 expression with increased spontaneous locomotor activity in young female offspring that have been prenatally exposed to synthetic glucocorticoid. However, we did not identify increased exploratory activity in the cage centre as described in NR1 mutant mice by Kew et al. (2000). Whether such behaviour emerges in later life, or whether the reduction in NR1 expression following synthetic glucocorticoid, in the present study, was not sufficient to produce such an effect, remains to be determined.
There is also evidence emerging that glucocorticoids may regulate central NMDAR expression. Adult rats that were exposed to dexamethasone in neonatal life exhibited reduced synaptic NR2B expression in the hippocampus (Kamphuis et al. 2003). We have shown that repeated maternal treatment with synthetic glucocorticoid significantly downregulates NR1 expression and upregulates NR2A mRNA levels in the hippocampus of female guinea pig fetuses (Owen et al. 2004). The reduction in NR1 mRNA was dose-dependent, and was confined to limbic structures (CA1/2, CA3, CA4 and dentate gyrus), and did not occur in the cortex. Intriguingly, there were no effects of prenatal glucocorticoid exposure on NR1 mRNA levels in male fetuses. These observations, taken together with our present data, suggest that prenatal glucocorticoid exposure downregulates NR1 mRNA levels in female fetuses and that these effects are maintained into postnatal life. Further, there was a trend towards a reduction in NR1 protein (by Western analysis) in the entire hippocampus, indicating that changes in mRNA are probably reflected by changes in protein. As it is not possible to accurately microdissect the hippocampus, the hippocampal structure analysed for NR1 protein by Western blotting in the present study contained all subfields of the hippocampus (CA1–CA4) as well as the dentate gyrus and amygdala. This probably accounts for the lack of an overall significant reduction in NR1 protein (NR1 mRNA was only decreased in CA1/2 and CA3 regions of the hippocampus).
In summary, we have shown that repeated maternal treatment with betamethasone can affect activity and NMDAR expression. These effects are confined to females indicating that effects are highly sex-specific. The reduction in hippocampal NR1 mRNA expression in juvenile offspring following prenatal glucocorticoid exposure is consistent with acute effects that we have reported in female fetuses following a similar maternal treatment regimen. This suggests that prenatal effects of glucocorticoid on the NMDAR system extend into the postnatal period. It remains to be determined whether effects extend into adulthood. This demonstration of altered behaviour and hippocampal NMDAR expression is important given that these changes have been implicated in the development of schizophrenia and cognitive impairment in humans (Qin et al. 2005; Rowland et al. 2005; Northoff et al. 2005). That such changes are already detectable in early life may indicate potentially adverse outcomes for the psychiatric health and learning ability of children whose mothers experienced high levels of anxiety during pregnancy or were treated with repeated courses of synthetic glucocorticoid.
Acknowledgments
We would like to thank Ms Sonja Banjanin, Ms Elaine Setiawan, Ms Alice Kostaki and Dr Marcus H. Andrews for their assistance with these experiments. This study was funded by the Canadian Institute for Health Research — SGM: MOP-49511 and DO: MD/PhD Studentship.
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brocklehurst P, Gates S, McKenzie-McHard K, Alfirevic, Chamberlain G. Are we prescribing multiple courses of antenatal corticosteroids? A survey of practice in the UK. Br J Obstet Gynaecol. 1999;106:977–979. doi: 10.1111/j.1471-0528.1999.tb08440.x. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Cull-Candy S. Functional consequences of changes in NMDA receptor subunit expression during development. J Neurocytol. 1996;25:857–867. doi: 10.1007/BF02284847. [DOI] [PubMed] [Google Scholar]
- French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190:588–595. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Tan N, Chowdhury GM, Mouri H, Sakata Y, Nakamura S. Mild prenatal stress enhances learning performance in the non-adopted rat offspring. Neuroscience. 2001;103:301–307. doi: 10.1016/s0306-4522(00)00582-0. [DOI] [PubMed] [Google Scholar]
- Gilstrap LC. Antenatal corticosteroids revisited: Repeat courses – National Institutes of Health consensus development conference statement, August 17–18, 2000. Obstet Gynecol. 2001;98:144–150. doi: 10.1016/s0029-7844(01)01410-7. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry. 2005;14:41–51. doi: 10.1007/s00787-005-0435-1. [DOI] [PubMed] [Google Scholar]
- Jessa M, Nazar M, Bidzinski A, Plaznik A. The effects of repeated administration of diazepam, MK-801 and CGP 37849 on rat behavior in two models of anxiety. Eur Neuropsychopharmacol. 1996;6:55–61. doi: 10.1016/0924-977x(95)00068-z. [DOI] [PubMed] [Google Scholar]
- Jessa M, Nazar M, Plaznik A. Anxiolytic-like action of intra-hippocampally administered NMDA antagonists in rats. Pol J Pharmacol. 1995;47:81–84. [PubMed] [Google Scholar]
- Kamphuis PJ, Gardoni F, Kamal A, Croiset G, Bakker JM, Cattabeni F, Gispen WH, van Bel F, Di Luca M, Wiegant VM. Long-lasting effects of neonatal dexamethasone treatment on spatial learning and hippocampal synaptic plasticity: involvement of the NMDA receptor complex. FASEB J. 2003;17:911–913. doi: 10.1096/fj.02-0333fje. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley M-C, Fuller PJ. Cortisol resistance and the guinea pig glucocorticoid receptor. Steroids. 1995;60:87–92. doi: 10.1016/0039-128x(94)00014-4. [DOI] [PubMed] [Google Scholar]
- Kew JN, Koester A, Moreau JL, Jenck F, Ouagazzal AM, Mutel V, Richards JG, Trube G, Fischer G, Montkowski A, Hundt W, Reinscheid RK, Pauly-Evers M, Kemp JA, Bluethmann H. Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci. 2000;20:4037–4049. doi: 10.1523/JNEUROSCI.20-11-04037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider ML, Levin ED, Seidler FJ, Slotkin TA. Gestational dexamethasone treatment elicits sex-dependent alterations in locomotor activity, reward-based memory and hippocampal cholinergic function in adolescent and adult rats. Neuropsychopharmacology. 2005;30:1617–1623. doi: 10.1038/sj.npp.1300716. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res. 2000;107:133–144. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Lim G, Wang S, Zeng Q, Sung B, Yang L, Mao J. Expression of spinal NMDA receptor and PKC gamma after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci. 2005;25:11145–11154. doi: 10.1523/JNEUROSCI.3768-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SG. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Dev Brain Res. 1998;107:123–132. doi: 10.1016/s0165-3806(98)00008-x. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamada K, Nagai T, Mori H, Mishina M, Furukawa H, Noda Y, Nabeshima T. Behavioural adaptations to addictive drugs in mice lacking the NMDA receptor epsilon1 subunit. Eur J Neurosci. 2004;19:151–158. doi: 10.1111/j.1460-9568.2004.03086.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, Nabeshima T. Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor epsilon1 subunit. J Neurosci. 2001;21:750–757. doi: 10.1523/JNEUROSCI.21-02-00750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Northoff G, Richter A, Bermpohl F, Grimm S, Martin E, Marcar VL, Wahl C, Hell D, Boeker H. NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophr Res. 2005;72:235–248. doi: 10.1016/j.schres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children (see comments) Br J Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Owen D, Setiawan E, Li A, McCabe L, Matthews SG. Regulation of N-methyl-d-aspartate receptor subunit expression in the fetal guinea pig brain. Biol Reprod. 2004;71:676–683. doi: 10.1095/biolreprod.104.027946. [DOI] [PubMed] [Google Scholar]
- Patin V, Lordi B, Vincent A, Caston J. Effects of prenatal stress on anxiety and social interactions in adult rats. Brain Res Dev Brain Res. 2005;160:265–274. doi: 10.1016/j.devbrainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Plaznik A, Palejko W, Nazar M, Jessa M. Effects of antagonists at the NMDA receptor complex in two models of anxiety. Eur Neuropsychopharmacol. 1994;4:503–512. doi: 10.1016/0924-977x(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Qin S, Zhao X, Pan Y, Liu J, Feng G, Fu J, Bao J, Zhang Z, He L. An association study of the N-methyl-d-aspartate receptor NR1 subunit gene (GRIN1) and NR2B subunit gene (GRIN2B) in schizophrenia with universal DNA microarray. Eur J Hum Genet. 2005;13:807–814. doi: 10.1038/sj.ejhg.5201418. [DOI] [PubMed] [Google Scholar]
- Quinlivan JA, Evans SF, Dunlop SA, Beazley LD, Newnham JP. Use of corticosteroids by Australian obstetricians – a survey of clinical practice. Aust NZ J Obstet Gynaecol. 1998;38:1–7. doi: 10.1111/j.1479-828x.1998.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Rayburn WF, Christensen HD, Gonzalez CL. A placebo-controlled comparison between betamethasone and dexamethasone for fetal maturation: Differences in neurobehavioral development of mice offspring. Am J Obstet Gynecol. 1997;176:842–850. doi: 10.1016/s0002-9378(97)70609-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry. 2005;46:246–254. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- Roussel S, Boissy A, Montigny D, Hemsworth PH, Duvaux-Ponter C. Gender-specific effects of prenatal stress on emotional reactivity and stress physiology of goat kids. Horm Behav. 2005;47:256–266. doi: 10.1016/j.yhbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30:633–639. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Blanco CE, Aendekerk B, Desbonnet L, Bruschettini M, Steinbusch HP, Prickaerts J, Steinbusch HW. Prenatal restraint stress and long-term affective consequences. Dev Neurosci. 2005;27:313–320. doi: 10.1159/000086711. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Zeng Q, Sung B, Yang L, Mao J. Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. J Neurosci. 2005;25:488–495. doi: 10.1523/JNEUROSCI.4127-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11β-hydroxysteroid dehydrogenase, the foeto–placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]