Abstract
The sigma receptor (σR), once considered a subtype of the opioid receptor, is now described as a distinct pharmacological entity. Modulation of N-methyl-d-aspartate receptor (NMDAR) functions by σR-1 ligands is well documented; however, its mechanism is not fully understood. Using patch-clamp whole-cell recordings in CA1 pyramidal cells of rat hippocampus and (+)pentazocine, a high-affinity σR-1 agonist, we found that σR-1 activation potentiates NMDAR responses and long-term potentiation (LTP) by preventing a small conductance Ca2+-activated K+ current (SK channels), known to shunt NMDAR responses, to open. Therefore, the block of SK channels and the resulting increased Ca2+ influx through the NMDAR enhances NMDAR responses and LTP. These results emphasize the importance of the σR-1 as postsynaptic regulator of synaptic transmission.
The sigma receptor (σR) was first described as a subtype of the opioid receptor (Martin et al. 1976). Further studies using ligands with high affinity and selectivity have demonstrated that it is a distinct pharmacological entity (Zukin & Zukin, 1979; Su, 1993). Two types of σRs have been described: σR type 1 (σR-1) and type 2 (σR-2) (Bowen, 2000). Molecular characterization has shown that the σR-1 is a novel protein with a molecular mass of 26 kDa (Hanner et al. 1996). This protein has a single putative membrane-spanning segment (Kekuda et al. 1996; Seth et al. 1997; Seth et al. 1998). The amino acid sequence of the σR-1 has no homology with known mammalian proteins, but a weak homology with fungal sterol isomerase has led some investigators to speculate that σRs-1 may be involved in steroid hormone biosynthesis (Jbilo et al. 1997; Moebius et al. 1997).
σRs-1 are widespread in the central nervous system and present in high levels in the prefrontal cortex, hippocampus and striatum (Hayashi & Su, 2004). Many studies have shown that σRs-1 can modulate several physiological and cellular events (Su & Hayashi, 2003). They have been implicated in the regulation of inosotol 1,4,5-triphosphate (IP3) receptors and Ca2+ signalling at the endoplasmic reticulum (Hayashi et al. 2000), mobilization of cytoskeletal adaptor proteins, modulation of nerve growth factor-induced neurite sprouting and alteration of psychostimulant-induced gene expression (Hayashi & Su, 2004). σR-1 ligands have also been described to regulate ion channels such as K+ channels (Wilke et al. 1999) and voltage-dependent Ca2+ channels (Zhang & Cuevas, 2002). Recently, Aydar and his co-workers (Aydar et al. 2002) have shown that the σRs modulate K+ channels as a regulatory subunit by a direct interaction. Importantly, the σRs-1 modulate N-methyl-d-aspartate receptor (NMDAR) functions in vivo and in vitro preparations (Hayashi & Su, 2004).
NMDARs are Ca2+-permeable ligand-gated channels that contribute to synaptic transmission and long-term events such as dendritic growth, synaptic modification, and control of gene expression (Waxman & Lynch, 2005). The Ca2+ influx through NMDARs is responsible for several forms of synaptic plasticity such as long-term potentiation (LTP) and depression (LTD; Collingridge et al. 2004). It is well documented that in the hippocampus σR-1 ligands increase the NMDAR response (Monnet et al. 1990; Ishihara & Sasa, 2002; Hayashi & Su, 2004); however, the mechanism through which the σR-1 modulates the NMDAR response is still not fully understood. Using patch-clamp whole-cell recordings in CA1 pyramidal cells of rat hippocampus, we show that σR-1 activation enhances NMDAR currents and LTP by preventing small conductance Ca2+-activated K+ channels to open.
Methods
Preparation of hippocampal slices
Coronal brain slices containing the hippocampus were obtained from Sprague-Dawley rats (21–28 days old). Prior to decapitation, the animals were anaesthetized using an isoflurane vaporizer (Stoelting, Wood Dale, IL, USA) in agreement with the guidelines of the Canadian Council of Animal Care. The concentration of isoflurane was 2–5% and the O2 flow rate was 1 l min−1. The brain was removed and placed in an oxygenated (95% O2–5% CO2) physiological solution, artificial cerebrospinal fluid (ACSF) at 4°C, containing (mm): 126 NaCl, 2.5 KCl, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2 and 10 glucose. The osmolarity of the ACSF was adjusted to 300 mosmol l−1 and the pH to 7.2. A block containing the region of interest was prepared, and sections (300 μm) were obtained with a vibrating microtome (Leica VT 1000S, Germany). The slices were stored for 1 h in an oxygenated chamber at room temperature before they were used for the experiments.
Data recording and analysis
Voltage-clamp experiments were performed with borosilicate pipettes filled with a solution containing (mm): 130 potassium gluconate, 10 Hepes, 10 KCl, 2 MgCl2, 5 lidocaine N-ethyl bromide (QX-314), 2 ATP-Mg and 0.2 GTP-tris(hydroxy-methil) aminomethane (pH 7.2 with KOH). When indicated in the text 0.2 mm ethylene glycol bis(2-aminoethyl ether)-N,N,N′N′-tetracetic acid (EGTA) or 10 mm caesium-BAPTA were added to this solution or a caesium-based solution was used. The caesium-based solution contained (mm): 130 caesium methanesulphonate, 10 Hepes, 10 CsCl, 2 MgCl2, 5 QX-314, 2 ATP-Mg and 0.2 GTP (pH 7.2 with CsOH). The osmolarity of both the solutions was adjusted to 280–290 mosmol l−1. With these solutions, the liquid junction potential was measured (∼10 mV) and the membrane potential (Vm) was corrected accordingly. The pipettes had a resistance of 3–6 MΩ. Recordings with series resistance higher than 20 MΩ were discarded. Bridge balance was monitored regularly during the recordings. Cells with a resting membrane potential > −60 mV were also discarded. To allow the drugs added in the pipette to induce their pharmacological action, a delay of 10–15 min was systematically observed prior to recording.
Whole-cell patch-clamp recordings were obtained with a Multiclamp 700A amplifier (Axon Instruments) under visual control using differential interference contrast and infrared video microscopy (IR-DIC; Leica DMLFSA). The recordings were performed at room temperature from individual pyramidal cells of the CA1 region of the hippocampus voltage-clamped at −65 mV.
Post-synaptic currents were evoked by electrical stimulation of the Schaffer collaterals with a bipolar microelectrode positioned in the stratum radiatum. The stimulation intensity consisted of 100 μs current pulses (10–200 μA) and was adjusted to evoke an EPSC amplitude in the range of 40–80 pA at Vm = −65 mV. Stimuli were delivered every 10 s.
To isolate the NMDAR-mediated component of evoked responses, we used ACSF containing a low concentration of MgCl2 (0.1 mm) with osmolarity maintained by CaCl2, and the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo-[f]quinoxaline-7-sulphonamide (NBQX, 20 μm), the GABAA receptor antagonist picrotoxin (50 μm), the GABAB receptor antagonist 3-[[(3,4-dichlorophenyl)methyl]amino]propyl]diethoxymethyl)-phosphinic acid (CGP 52432, 10 μm) and the glycine receptor antagonist strychnine (0.5 μm). Application of the NMDAR antagonist dl-2-amino-5-phosphonovaleric acid (AP-5, 50 μm) completely abolished the responses (n = 4; Supplementary Fig. 1).
Local drug injections were performed applying air pressure pulses (3–10 ms) with a picospritzer (Parker Hannifan Instrumentation, Fairfield, NJ, USA) to a patch pipette containing 100 μm NMDA. NMDA was dissolved in ACSF and applied every 30 s. The ejection pipette was positioned directly above the proximal dendrites. NMDA was then applied in the presence of TTX (0.5 μm) to avoid polysynaptic phenomena.
Kinetic analysis was performed on averaged EPSCs (usually 20–25 consecutive traces). The rise times of NMDAR currents were measured at peak to the end. Their decays were fitted with the exponential functions: y = Afe−t/τf + Ase−t/τs for double and y = A1exp−t/τ for single exponential decay, where A is the amplitude, t is the time, τ is the decay time constant, and the subscript f and s denote fast and slow components, respectively. Weighted time constants (τmean) were calculated using the equation:
Currents underlain by small-conductance voltage-insensitive Ca2+-activated K+ channels (SK channels; Shah & Haylett, 2002) sensitive to apamin (Sah, 1996) were evoked in voltage clamp by giving a 100 ms, 50 mV step from a holding potential of −50 mV in low Mg2+ ACSF. This procedure evoked unclamped Ca2+ spikes (Pedarzani & Storm, 1993) followed by outward tail currents. It has been shown that apamin abolished the early part of the outward tail currents (Stocker et al. 1999; Sailer et al. 2002; Gu et al. 2005). The current blocked using apamin, was examined by subtracting the current recorded in the presence of apamin from that recorded in absence (control) of apamin.
In the slices used for LTP experiments, the CA3 region of the hippocampus was removed by a surgical cut. Post-synaptic currents were evoked by electrical stimulation of the Schaffer collaterals with a bipolar microelectrode positioned in the stratum radiatum. Stimuli were delivered every 10 s. The recordings for the experiments using the pairing protocol to induce LTP were obtained in ACSF in the presence of picrotoxin (50 μm) at Vm = −65 mV. The pairing protocol used to induce LTP was composed of three brief high-frequency tetani (50 pulses at 100 Hz, 4 s intervals) given at the end of a long depolarization (3 min at 0 mV) (Chen et al. 1999; Martina et al. 2004). The pairing protocol was induced after 10–12 min of baseline in the absence or presence of drugs. This protocol induced an increase of the synaptic responses lasting for more than 40 min.
Data were collected using software pCLAMP 9 (Axon Instrument). Analyses were performed off-line with the software IGOR (WaveMetrics Inc., Lake Oswego, OR, USA). Statistical significance of the results was determined with unpaired t tests (two-tailed). All values are expressed as means ± s.e.m.
All drugs were obtained from Sigma-Aldrich, with the exception of CGP 52432, NBQX and ryanodine (Tocris, Bristol, UK). (+)-Cinnamyl-1-phenyl-1-N-methyl-N-cyclopropylene (igmesine or JO 1784) was a kind gift from Dr Guy Debonnel (McGill University, Canada). Stock solutions of haloperidol (1 mm), ryanodine (10 mm) and cyclopiazonic acid (CPA; 30 mm) were obtained dissolving the pharmacological agents in dimethyl sulfoxide (DMSO). A 1 mm of stock solution of nicardipine was made using HCl (1 n). A 1 mm solution of (+)-pentazocine was prepared by warming 2.85 mg of (+)pentazocine in 2 ml of HCl (0.1 n) with shaking. When all solids were dissolved, the solution was cooled to room temperature and diluted to 10 ml with a buffer at pH 7. The solutions were freshly prepared daily and kept at room temperature. The other drugs were dissolved in water to obtain stock solutions of 10 mm. All the drugs stock solutions were kept at −20°C.
Results
σR-1 activation increases NMDAR currents
To study the effect of σR-1 activation on NMDAR response, we measured the effect of (+)pentazocine (1 μm), a high-affinity and selective σR-1 agonist (Hayashi & Su, 2004), on isolated NMDAR currents recorded from CA1 pyramidal cells on rat hippocampal slices using the patch-clamp whole-cell technique. To evoke postsynaptic glutamatergic currents (PSCs), the Schaffer collaterals were stimulated with a bipolar electrode. The NMDAR-mediated component of the PSCs was pharmacologically isolated in a low-Mg2+ ACSF containing NBQX, picrotoxin, CGP 52432 and strychnine to block AMPA-, GABAA-, GABAB- and glycine receptor-mediated responses, respectively. We recorded NMDAR currents with a potassium-based solution in the recording pipette (see Methods) and applied (+)pentazocine. (+)Pentazocine (Steinfels et al. 1988) is most probably the more highly selective and potent σR-1 agonist (Quirion et al. 1992) and it has been widely used by many groups in different experimental protocols (mainly in vivo; for review see Hayashi & Su, 2004) where it dose-dependently potentiates the iontophoretic application of NMDA (Monnet et al. 1990; Bergeron et al. 1997). We used a concentration of (+)pentazocine of 1 μm because at this concentration the NMDAR currents were consistently and stably increased. The rise, decay, weighted time constants (τmean) as well as the relative portions (Af and As) of decay time constants of NMDAR currents were calculated (Table 1). (+)Pentazocine significantly increased the amplitude of NMDAR currents by 56.5 ± 4.87% (from an averaged amplitude of 20.24 ± 2.07 pA in control to 31.83 ± 3.73 pA in (+)pentazocine; n = 12; P < 0.05; Fig. 1A and B, while having no effect on their kinetic properties (Table 1).
Table 1.
Rise and decay time constants of NMDAR currents in CA1 pyramidal cells recorded in the absence and presence of (+)pentazocine (1 μm) at −65 mV
| Control | (+)Pentazocine (1 μm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampl (pA) | τact (ms) | τf (ms) | τs (ms) | τmean (ms) | Af (%) | As (%) | Ampl (pA) | τact (ms) | τf (ms) | τs (ms) | τmean (ms) | Af (%) | As (%) |
| Intracellular solution: potassium-based solution + 0.5 mm EGTA (n = 12) | |||||||||||||
| 20.24 | 10.6 | 85.0 | 728.7 | 177.2 | 82 | 18 | 31.83* | 11.4 | 95.3 | 795.5 | 201.6 | 82 | 18 |
| ± 2.07 | ± 1.47 | ± 3.93 | ± 96.6 | ± 15.3 | ± 2.68 | ± 2.68 | ± 3.73 | ± 1.26 | ± 6.72 | ± 115 | ± 25.2 | ± 2.52 | ± 2.52 |
| Intracellular solution: caesium-based solution + 0.5 mm EGTA (n = 9) | |||||||||||||
| 37.52 | 7.49 | 75.4 | 494.4 | 166.0 | 79 | 21 | 34.90 | 7.51 | 74.4 | 424.0 | 158.3 | 77 | 23 |
| ± 7.02 | ± 0.57 | ± 8.25 | ± 73.0 | ± 21.3 | ± 1.46 | ± 1.46 | ± 7.45 | ± 0.23 | ± 9.10 | ± 65.3 | ± 38.1 | ± 2.92 | ± 2.92 |
| Intracellular solution: potassium-based solution + 10 mm BAPTA (n = 5) | |||||||||||||
| 18.06 | 9.10 | 93.3 | 782.9 | 178.2 | 82 | 18 | 18.52 | 11.5 | 76.4 | 546.3 | 157.2 | 82 | 18 |
| ± 3.12 | ± 0.70 | ± 13.4 | ± 229 | ± 31.2 | ± 3.28 | ± 3.28 | ± 3.73 | ± 2.55 | ± 17.9 | ± 115 | ± 22.5 | ± 3.29 | ± 3.29 |
Values are mean ± s.e.m. Electrically evoked NMDAR currents were recorded in a low-Mg2+ ACSF (0.1 mm) in the presence of NBQX (20 μm), picrotoxin (50 μm), CGP 52432 (10 μm) and strychnine (0.5 μm). The fast and slow decay components are designated by τf and τs, and the weighted time constant by τmean. The amplitude of the NMDAR currents is also shown. Electrically evoked NMDAR currents were recorded in the absence (Control) and presence of (+)pentazocine (1 μm).
Significant difference between two values (P < 0.05).
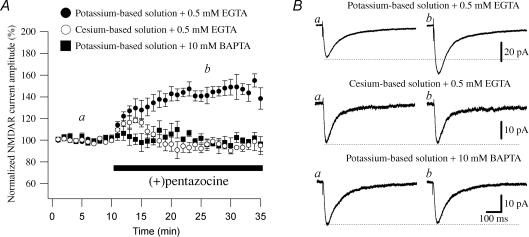
Figure 1. The effect of the σR-1 agonist (+)pentazocine on NMDAR currents is dependent on a K+ conductance and intracellular Ca2+ concentration.
A, normalized NMDAR current amplitudes (%) are plotted as a function of time. Each point (one every minute; mean ±s.e.m.) is the average of 6 points (stimulations every 10 s). The application of the σR-1 agonist (+)pentazocine (1 μm) caused an increase in the amplitude of the NMDAR currents when the CA1 pyramidal cells are recorded with a potassium-based solution including 0.5 mm EGTA (•; n = 12). When a caesium-based solution including 0.5 mm EGTA (○; n = 9) or potassium-based solution including 10 mm BAPTA (▪; n = 5) is used (+)pentazocine had no effect on the NMDAR current amplitude. B, examples of traces of NMDAR currents measured at the time points indicated in A (a and b) are shown. Each trace is an average of 20 traces.
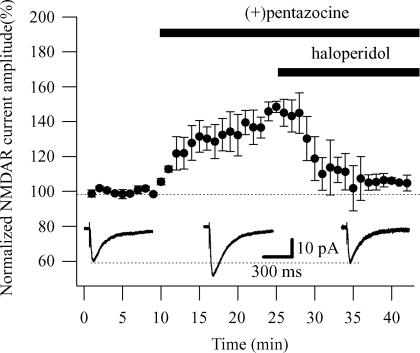
To test the specificity of the σR-1 activation, we observed the effect of haloperidol, a well-known and potent σR-1 antagonist (Hayashi & Su, 2004), when applied with (+)pentazocine. The addition of haloperidol (1 μm) completely reversed the effect of (+)pentazocine (1 μm), returning the NMDAR currents to 98.4 ± 3.7% of its initial amplitude (n = 3; Fig. 2). To rule out any effect of haloperidol on the NMDAR currents, we recorded NMDAR currents and applied haloperidol (1 μm) alone. Haloperidol reduced the NMDAR currents by 15.85 ± 5.43% (n = 3; Supplementary Fig. 2). This value is significantly smaller (P < 0.05) than that obtained in the presence of (+)pentazocine. Overall these results demonstrate the specificity of the σR-1 activation in enhancing the amplitude of NMDAR currents.
Figure 2. Haloperidol reverts the effect of (+)pentazocine on NMDAR currents.
Normalized NMDAR current amplitudes (%) are plotted as a function of time. Each point (one every minute; mean ± s.e.m.) is the average of 6 points (stimulations every 10 s). The application of the σR-1 agonist (+)pentazocine (1 μm) caused an increase in the amplitude of the NMDAR currents (n = 3). The subsequent addition of haloperidol (1 μm) completely reversed the effect of (+)pentazocine (1 μm), returning the NMDAR currents to their initial amplitude (n = 3). Insets show examples of traces of the NMDAR currents measured in absence (right) and presence of (+)pentazocine (centre) and (+)pentazocine plus haloperidol (left), respectively. Each trace is an average of 20 traces.
To rule out a presynaptic effect of (+)pentazocine, we performed experiments using paired pulses. If the enhancement of the NMDAR currents by (+)pentazocine were due to a presynaptic increase in the transmitter release probability then (+)pentazocine would be expected to reduce paired pulse facilitation. Paired pulses were delivered with an interpulse interval of 50 ms, the second response showing facilitation in CA1 pyramidal cells. Consistent with a postsynaptic effect of (+)pentazocine, stimuli-induced NMDAR currents showed similar ratios (peak 2/peak 1; P > 0.05) in the absence (control, 2.74 ± 0.34; n = 6) and in the presence of (+)pentazocine (2.93 ± 0.46; n = 6; Supplementary Fig. 3).
The effect of the σR-1 agonist (+)pentazocine on NMDAR currents depends on a K+ conductance
Since several σR ligands modulate and interact with K+ channels (Wilke et al. 1999; Aydar et al. 2002), we tested the implication of a K+ conductance in the potentiation of the NMDAR responses by the σR-1 agonist. We examined the effect of blocking postsynaptic K+ currents by replacing intracellular K+ with caesium ions (see Methods). With this caesium-based internal solution, the application of (+)pentazocine (1 μm) did not increase (P > 0.05) the amplitude (from 37.52 ± 7.02 to 34.90 ± 7.45 pA; n = 9; Fig. 1A and B) of the NMDAR currents (Table 1). The kinetics of the NMDAR currents recorded with this solution in the absence or presence of (+)pentazocine were similar to those recorded with the potassium-based solution (Table 1). This finding strongly suggests the involvement of a K+ conductance in the potentiating effect observed on NMDAR currents following the application of (+)pentazocine.
Intracellular Ca2+ is required for the σR-1 agonist to exert its effect on NMDAR currents
σRs-1 have been described to regulate intracellular Ca2+ concentration via the inositol 1,4,5-triphosphate (IP3) receptor on the endoplasmatic reticulum (Hayashi et al. 2000) and voltage-dependent Ca2+ channels (Zhang & Cuevas, 2002). To evaluate whether intracellular Ca2+ is required for the σR-1 agonist to exercise its action on NMDAR currents, we recorded CA1 pyramidal cells with a potassium-based solution with the addition of 10 mm BAPTA in the recording pipette to buffer cytosolic Ca2+. We found that in the cells recorded with this solution, the application of (+)pentazocine (1 μm) did not increase the amplitude of NMDAR currents (from 18.03 ± 3.12 to 18.52 ± 3.73 pA; n = 5; P > 0.05; Fig. 1A and B) or change their kinetics (Table 1), suggesting that intracellular Ca2+ is required for the σR-1 agonist to exert its effect on NMDAR currents. The lack of effect of (+)pentazocine in experiments where a caesium-based intracellular solution including 0.5 mm EGTA instead of 10 mm BAPTA was used, ruled out any effect of the σR-1 agonist on the concentration of Ca2+ alone in its action on NMDAR currents.
Ca2+ influx through NMDARs is required for σR-1 agonist modulation of NMDAR currents
The Ca2+ and K+ dependence of the enhancing effect of σR-1 activation on NMDAR currents suggests the involvement of a Ca2+-activated K+ conductance. It has been reported that NMDAR-mediated rise in Ca2+ concentration results in the activation of Ca2+-activated K+ channels (Shah & Haylett, 2002; Ngo-Anh et al. 2005). It has also been found that σRs-1 modulate Ca2+ release from intracellular Ca2+ storage sites (Hayashi et al. 2000) and voltage-dependent Ca2+ channels (Zhang & Cuevas, 2002).
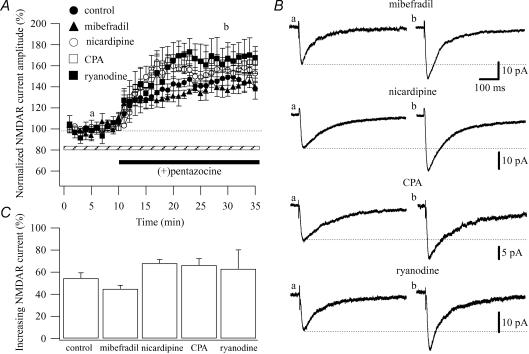
To rule out the possibility that a rise in Ca2+ concentration originates from voltage-dependent Ca2+ channels, we examined the effect of (+)pentazocine (1 μm) on NMDAR currents in the presence of blockers of the different types of Ca2+ channels: mibefradil dihydrochloride hydrate (mibefradil; T-type Ca2+ channel blocker), nicardipine (L-type Ca2+ channel blocker), ù-conotoxin GVIA (N-type Ca2+ channel blocker) and ω-agatoxin IVA (P/Q-type Ca2+ channel blocker). When (+)pentazocine was applied after mibefradil (10 μm), the amplitude of the NMDAR currents was significantly increased by 44.94 ± 3.26% (n = 5; P < 0.05; from 18.93 ± 2.37 pA in control to 27.50 ± 3.63 pA; Fig. 3A and B). In the same way, the application of (+)pentazocine after nicardipine (5 μm) increased the amplitude of the NMDAR currents by 68.15 ± 3.46% (n = 4; P < 0.05; from 18.68 ± 5.89 pA in control to 30.97 ± 9.18 pA; Fig. 3A and B). Since, N- and P/Q-type Ca2+ channels are the predominant species of Ca2+ channels in presynaptic nerve terminals (Westenbroek et al. 1992, 1995) and that these channels couple physically to proteins that form the release machinery for synaptic vesicles (Stanley, 1997), to test the ω-conotoxin GVIA (N-type Ca2+ channel blocker) and ω-agatoxin IVA (P/Q-type Ca2+ channel blocker) we first observed these drugs alone on the NMDAR currents and then applied (+)pentazocine. Following 10 min of ω-conotoxin GVIA (3 μm) and ω-agatoxin IVA (200 nm) application, the amplitude of NMDAR currents was reduced by 13.85 ± 4.85% (n = 3) and 19.21 ± 3.79% (n = 3), respectively. The additional application of (+)pentazocine significantly increased the NMDAR currents by 46.7 ± 9.18% (n = 3) and 41.08 ± 4.66% (n = 3), respectively (Supplementary Fig. 4). These values were similar to that obtained when (+)pentazocine was applied alone (56.5 ± 4.87%, n = 12; Fig. 3C). Overall, these results ruled out a role for Ca2+ entering through voltage-dependent Ca2+ channels in the enhancing effect of the σR-1 agonist on NMDAR currents.
Figure 3. σR-1 agonist modulation of NMDAR currents does not depend on Ca2+ influx via T- and L-type Ca2+ channels or Ca2+ release from the intracellular stores.
A, normalized NMDAR current amplitudes (%) are plotted as a function of time. Each point (one every minute; mean ± s.e.m.) is the average of 6 points (stimulations every 10 s). The application of (+)pentazocine (1 μm; black bar) caused an increase in the amplitude of the NMDAR currents when the CA1 pyramidal cells were recorded in presence of mibefradil (10 μm; ▴ n = 5), nicardipine (5 μm; ○; n = 4), CPA (30 μm; □; n = 4) and ryanodine (10 μm; ▪; n = 4), dotted bar. B, examples of traces of the NMDAR currents measured at the time points indicated in A (a and b) are shown for mibefradil, nicardipine, CPA and ryanodine. Each trace is an average of 20 traces. C, histogram showing the average of the enhancing effect of (+)pentazocine on NMDAR currents when the CA1 pyramidal cells are recorded in control (no additional drug; n = 12), in presence of mibefradil (n = 5), nicardipine (n = 4), CPA (n = 4) and ryanodine (n = 4). All the values are means ± s.e.m.
To rule out the possibility that a rise in Ca2+ concentration originates from intracellular Ca2+ storage sites, we tested the effect of (+)pentazocine (1 μm) on NMDAR currents in presence of two Ca2+ ATPase inhibitors that cause the depletion of intracellular Ca2+ stores (cyclopiazonic acid (CPA) and ryanodine). When (+)pentazocine was applied after CPA (30 μm) and ryanodine (10 μm), the amplitude of the NMDAR currents was significantly increased by 66.36 ± 6.01% (n = 4; P < 0.05; from 15.26 ± 2.37 pA in control to 25.14 ± 3.58 pA; Fig. 3A and B) and 63.03 ± 17.21% (n = 4; P < 0.05; from 19.10 ± 2.29 pA in control to 29.99 ± 2.64 pA; Fig. 3A and B), respectively. These data were similar (P > 0.05) to those observed with (+)pentazocine alone, ruling out a role for Ca2+ freed from intracellular Ca2+ stores in the potentiating effect of the σR-1 agonist on NMDAR currents (Fig. 3C). Experiments with CPA and ryanodine also ruled out the involvement of the σR-1 action on the Ca2+ store in the regulation of NMDAR currents.
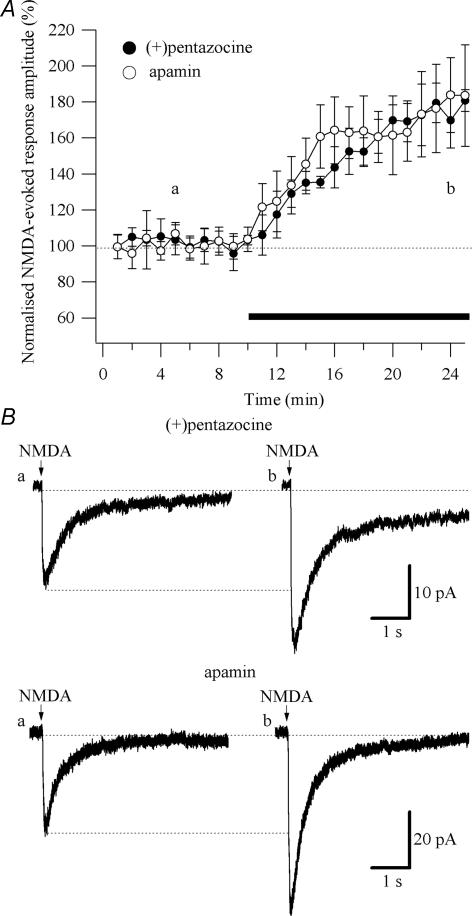
To test whether Ca2+ influx through post-synaptic NMDARs is required for (+)pentazocine to exert the enhancement of the NMDAR currents, we measured the effect of (+)pentazocine (1 μm) on the response to local application of NMDA (100 μm). NMDA (100 μm) was applied in a low Mg2+ ACSF (0.1 mm) in the presence of tetrodotoxin (TTX, 0.5 μm). The amplitude of the currents in response to NMDA application was 18.32 ± 4.46 pA (n = 5; Fig. 4B). Application of (+)pentazocine significantly (P < 0.05) enhanced the response evoked by NMDA by 67.29 ± 12.68% (n = 5; Fig. 4A). Application of AP-5 (50 μm) completely abolished the responses (n = 4; data not shown).
Figure 4. Effect of (+)pentazocine and apamin on the responses evoked by local pressure application of NMDA.
NMDA (100 μm) was applied through a patch pipette positioned directly above the proximal dendrites. TTX (0.5 μm) was present throughout the experiments. The cells were voltage-clamped at Vm = −65 mV. A, normalized amplitudes of responses evoked by NMDA (%) are plotted as a function of time. Each point (one every minute; mean ± s.e.m.) is the average of 2 points (pressure application every 30 s). The application of (+)pentazocine (1 μm; •; n = 5) and apamin (100 nm; ○; n = 6) caused an increase in the amplitude of the responses evoked by NMDA. The black bar indicates the duration of the drugs application. B, examples of responses evoked by NMDA observed in low-Mg2+ ACSF (a) and during application of (+)pentazocine and apamin (b). Each trace is an average of 10 traces.
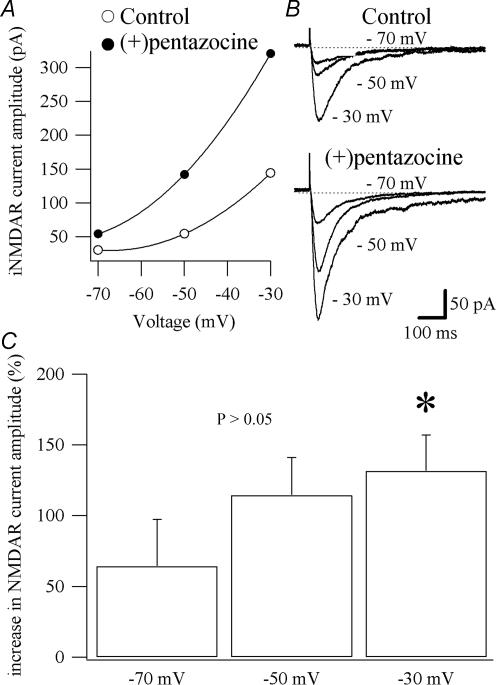
NMDARs are highly permeable to Ca2+ and are blocked by Mg2+ at resting membrane potential in a voltage-dependent manner (Nowak et al. 1984). Their activation requires binding of glutamate and its coagonist glycine, as well as membrane depolarization (Cull-Candy & Leszkiewicz, 2004). If the σR-1 activation potentiated NMDAR currents through its action on Ca2+-activated K+ channels activated by Ca2+ entering the cells through the NMDARs, then the effect of (+)pentazocine would be greater at more depolarized potentials where the Mg2+ block is relieved and the driving force for the K+ ion larger. To test this hypothesis, we recorded CA1 pyramidal cells at different potentials (−30, −50 and −70 mV; Fig. 5A). The NMDAR currents were pharmacologically isolated using a normal ACSF (normal Mg2+; see Methods) containing NBQX, picrotoxin, CGP 52432 and strychnine (Fig. 5B). The amplitude of NMDAR currents was significantly more increased (P < 0.05) by (+)pentazocine (1 μm) at a holding potential of −30 mV (131.6 ± 25.3%; n = 6) compared with −70 mV (64.3 ± 33.06%; n = 6; Fig. 5A and C).
Figure 5. Voltage-dependent effect of (+)pentazocine on NMDAR currents.
A, current–voltage relationships for the NMDAR currents shown in B. The application of (+)pentazocine (1 μm) caused a voltage-dependent enhancement of the NMDAR currents. B, examples of traces of NMDAR currents recorded in voltage clamp obtained from CA1 pyramidal cells held at −70, −50 and −30 mV. Each trace is an average of 6 traces. C, histogram showing the average of the enhancing effect of (+)pentazocine application on NMDAR currents at different holding potential in 6 CA1 pyramidal cells (mean ±s.e.m.). *Significant difference between the percentages of augmentation of the NMDAR currents recorded at −70 and −30 mV.
Overall these results indicate that Ca2+ influx through post-synaptic NMDARs is required for the σR-1 activation to exert the enhancement of the NMDAR currents.
(+)Pentazocine enhancement of NMDAR currents is similar to that of apamin
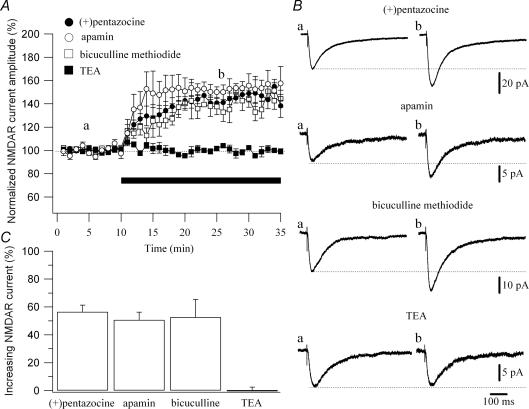
Ca2+ influx through NMDARs can open Ca2+-activated K+ channels in hippocampal slices and cultured neurons (Zorumski et al. 1989; Shah & Haylett, 2002; Ngo-Anh et al. 2005). In CA1 pyramidal cells, these channels are small-conductance voltage-insensitive Ca2+-activated K+ channels (SK channels; Shah & Haylett, 2002), sensitive to apamin (Sah, 1996). In these cells, action potentials are followed by a rise in intracellular Ca2+ that leads to an after-hyperpolarization (AHP) of the membrane. The AHP is comprised of three components, fast AHP, medium AHP and slow AHP, reflecting the activation of different K+ currents (Storm, 1987, 1990). The fast AHP is due to large-conductance Ca2+-activated K+ channels (BK channels). The type of current that contributes to the medium AHP in CA1 pyramidal cells is a matter of controversy. Several groups have observed an apamin-sensitive component of the medium AHP in CA1 pyramidal neurons (Stocker et al. 1999; Empson & Jefferys, 2001; Oh et al. 2003; Kramar et al. 2004), suggesting that the medium AHP is underlain by the opening of SK channels (see Sah, 1996; Stocker et al. 1999). Recently, it has been proposed that SK channels, although available for activation, are not activated by spike train in CA1 pyramidal cells, and consequently do not affect the medium AHP or spike frequency adaptation (Gu et al. 2005), excluding the contribution of SK channel to the medium AHP (Storm, 1989; Gu et al. 2005). The slow AHP is underlain by an apamin-, tetraethylammonium (TEA)- and 4-aminopyridine-insensitive Ca2+-activated K+ conductance (Sah, 1996). It has been shown that apamin-sensitive SK channels respond to rapid increases in Ca2+ concentration and reduce the amplitude of NMDAR currents (Ngo-Anh et al. 2005). To test whether the effect of the σR-1 agonist was mimicked by the block of SK channels, we recorded CA1 pyramidal cells with a potassium-based solution and compared the effect of two SK channel blockers (apamin and bicuculline methiodide) to that of (+)pentazocine on NMDAR currents (Fig. 6B). The percentage increment of NMDAR currents amplitude caused by (+)pentazocine (1 μm; 56.5 ± 4.87%; n = 12; Fig. 6A and C) was similar (P > 0.05) to that induced by apamin (100 nm; 50.7 ± 5.60%, n = 8; Fig. 6A and C) and bicuculline methiodide (10 μm; 52.5 ± 12.94%, n = 5; Fig. 6A and C). Contrary to this, the application of a low concentration of TEA (1 mm), which blocks BK channels, did not increase the amplitude of NMDAR currents (0.05 ± 2.43%, n = 3; Fig. 6A and C).
Figure 6. (+)Pentazocine modulation of the NMDAR current is similar to that caused by blockers of SK channels.
A, normalized NMDAR current amplitudes (%) are plotted as a function of time. Each point (one every minute; mean ± s.e.m.) is the average of 6 points (stimulations every 10 s). The application of drugs (black bar) caused an increase in the amplitude of the NMDAR currents when the CA1 pyramidal cells were recorded in presence of (+)pentazocine (1 μm; •; n = 12), apamin (100 nm; ○; n = 8) and bicuculline methiodide (10 μm; □; n = 5). The application of TEA (1 mm; ▪; n = 3) did not increase the amplitude of the NMDAR currents. B, examples of traces of the NMDAR currents measured at the time points indicated in A (a and b) are shown for (+)pentazocine, apamin, bicuculline methiodide and TEA. Each trace is an average of 20 traces. C, histogram showing the average of the enhancing effect of (+)pentazocine (n = 12), apamin (n = 8), bicuculline methiodide (n = 5) and TEA (n = 3) application on NMDAR currents.
To test whether (+)pentazocine was able to occlude the effect of apamin on NMDAR currents, we recorded CA1 pyramidal cells with a potassium-based solution and measured the effect of apamin (100 nm) on the amplitude of NMDAR currents after the application of (+)pentazocine (1 μm). The NMDAR currents were pharmacologically isolated from EPSCs elicited by stimulation of Schaffer collaterals using a low-Mg2+ ACSF containing NBQX, picrotoxin, CGP 52432 and strychnine (see Methods). The application of (+)pentazocine increased the amplitude of the NMDAR currents by 65.14 ± 9.88% (P < 0.05; n = 4). The subsequent application of apamin did not change the amplitude of the NMDAR currents (35.0 ± 9.29 pA in (+)pentazocine and 34.7 ± 8.66 pA in (+)pentazocine plus apamin; n = 4; supplementary Fig. 5A and B).
To further test whether another σR-1 ligand with a chemical structure unrelated to (+)pentazocine can occlude the effect of apamin, we recorded CA1 pyramidal cells with a potassium-based solution and measured the effect of apamin (100 nm) on the amplitude of NMDAR currents after the application of (+) - cinnamyl - 1-phenyl-1-N-methyl-N-cyclopropylene (igmesine or JO 1784; 1 μm). Igmesine is a potent and selective ligand for rat and mouse sigma sites (Roman et al. 1990). Igmesine has an IC50 = 39 ± 8 nm (Roman et al. 1990), consequently a concentration of 1 μm was used to ensure the activation of all the σRs-1. It has also been shown that igmesine enhanced the neuronal activation induced by NMDA application in vivo (Monnet et al. 1990). As for (+)pentazocine, the application of igmesine (1 μm) significantly and consistently increased the amplitude of the NMDAR currents by 77.52 ± 16.22% (P < 0.05; n = 5). The additional application of apamin did not change the amplitude of the NMDAR currents (26.5 ± 5.91 pA in igmesine and 26.2 ± 5.60 pA in igmesine plus apamin; n = 5; Supplementary Fig. 5C and D).
Overall, these results strongly suggest that σR-1 activation with (+)pentazocine or igmesine prevents SK channels opening.
To test whether SK channels inhibitors mimicked the effect of (+)pentazocine on currents evoked by NMDA, we measured the effect of apamin (100 nm) on the response to local application of NMDA (100 μm; Fig. 4). NMDA (100 μm) was applied in a low-Mg2+ ACSF (0.1 mm) in presence of tetrodotoxin (TTX, 0.5 μm). The amplitude of the currents in response to NMDA application was 18.54 ± 2.37 pA (n = 6; Fig. 4B). Application of apamin significantly (P < 0.05) enhanced the currents evoked by NMDA by 85.13 ± 38.7% (n = 6; Fig. 4A and B). To test if (+)pentazocine was able to occlude the effect that apamin had on currents evoked by NMDA, we measured the effect of apamin (100 nm) on the response to local application of NMDA (100 μm) after the application of (+)pentazocine (1 μm). The application of (+)pentazocine increases the amplitude of the currents evoked by NMDA by 88.77 ± 16.81% (P < 0.05; n = 3). The additional application of apamin did not change the amplitude of the currents evoked by NMDA (40.31 ± 13.91 pA in (+)pentazocine and 44.80 ± 16.87 pA in (+)pentazocine plus apamin; n = 3; Supplementary Fig. 6).
σR-1 activation prevents SK channels opening
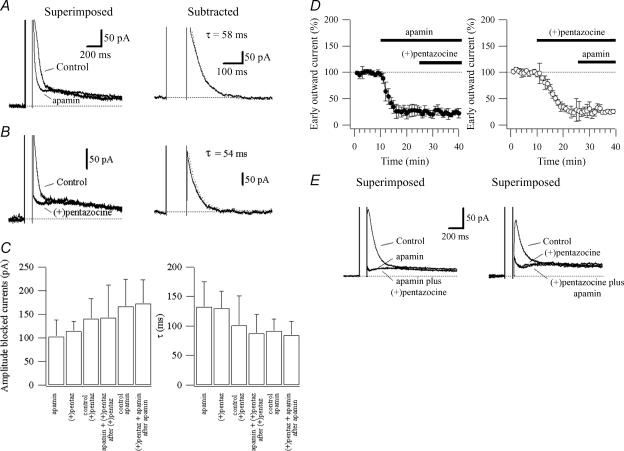
To test the effect of σR-1 activation on SK channels, we recorded the Ca2+-activated K+ currents underlain by SK channels in voltage clamp. These currents were evoked in voltage clamp by giving a 100 ms, 50 mV step from a holding potential of −50 mV in low-Mg2+ ACSF. This voltage step evoked unclamped Ca2+ spikes (Pedarzani & Storm, 1993; Stocker et al. 1999; Gu et al. 2005) followed by outward tail currents (Fig. 7A and B). In CA1 pyramidal cells, the SK channel blocker apamin (100 nm) abolished the early part of the outward tail current (n = 8; Fig. 7A; see also Stocker et al. 1999; Sailer et al. 2002; Gu et al. 2005). Similarly to apamin, the σR-1 activation with (+)pentazocine (1 μm) blocked the early part of the outward tail current (n = 11; Fig. 7B). The blocked currents were extracted by subtraction of the current before and after application of apamin or (+)pentazocine (Fig. 7A and B). These currents decayed with a similar (P > 0.05) time constant: 132.7 ± 42.5 ms (n = 8) for apamin and 130.1 ± 29.2 ms (n = 11) for (+)pentazocine (Fig. 7A, B and C). The currents had a peak amplitude of 101.0 ± 35.0 pA (n = 8) and 114.1 ± 21.3 pA (n = 11) for apamin and (+)pentazocine, respectively (Fig. 7C). These values were not significantly different (P > 0.05). In three CA1 pyramidal cells, apamin (100 nm) was applied after (+)pentazocine (1 μm) to test if apamin blocked an additional current. The blocked currents were extracted by subtraction of the current before and after application of (+)pentazocine, and before the application of (+)pentazocine and after the application of (+)pentazocine plus apamin. The currents blocked by σR-1 activation with (+)pentazocine had an amplitude of 140.66 ± 42.2 pA (n = 3) and decayed with a time constant of 100.9 ± 49.7 ms (n = 3; Fig. 7C). The additional application of apamin did not significantly change the amplitude (143.0 ± 68.6 pA; n = 3) nor the decay of the blocked current (87.7 ± 32.6 ms; n = 3, P > 0.05; Fig. 7C and E).
Figure 7. Activation of σR-1 with (+)pentazocine prevents SK channels opening.
Currents recorded in voltage clamp in response to a 100 ms step from a holding potential of −50 mV. The currents recorded in absence (control) and presence of apamin (100 nm; A) or (+)pentazocine (1 μm; B) are superimposed. The current obtained by subtraction is shown on the right for apamin (A) and (+)pentazocine (B). C, histograms summarizing the data for the amplitude (left) and decayed time constant (right; τ) of the currents blocked by apamin (n = 8) and (+)pentazocine (n = 11). Control (+)pentaz (n = 3) represent the currents blocked by (+)pentazocine for occlusion experiments of the effect of apamin on (+)pentazocine (apamin + (+)pentaz after (+)pentaz; n = 3). Control apamin (n = 5) represent the currents blocked by apamin for occlusion experiments of the effect of (+)pentazocine on apamin ((+)pentaz + apamin after apamin; n = 5). D, summary data showing the time course of the effect of apamin (left, n = 5) and (+)pentazocine (right, n = 3) on the early tail currents (normalized; control = 100%) and their respective occlusion effect. E, currents recorded in voltage clamp in response to a 100 ms step from a holding potential of −50 mV. Left: currents recorded in absence (control) and presence of apamin and apamin plus (+)pentazocine. Right: currents recorded in absence (control) and presence of (+)pentazocine and (+)pentazocine plus apamin.
To test whether apamin was able to occlude the effect of (+)pentazocine on SK channels, we recorded the Ca2+-activated K+ currents as described above and measured the effect of (+)pentazocine (1 μm) on the amplitude and kinetics of the currents blocked by apamin (100 nm). The blocked currents were extracted by subtraction of the current before and after application of apamin, and before the application of apamin and after the application of apamin plus (+)pentazocine. The currents blocked by apamin had an amplitude of 166.92 ± 56.7 pA and decayed with a time constant of 91.7 ± 20.3 ms (n = 5). The additional application of (+)pentazocine did not significantly change the amplitude (172.28 ± 50.8 pA; n = 5) nor the decay of the blocked current (84.4 ± 23.9 ms; n = 5, P > 0.05; Fig. 7C and E).
Figure 7D shows the time course of the effect of apamin (n = 5) and (+)pentazocine (n = 3) and their respective occlusion effects on the early tail currents.
These data strongly suggest that the current blocked by apamin and that blocked by σR-1 activation with (+)pentazocine is the same. The fitting values of the decays were similar to that previously described (Stocker et al. 1999; Faber & Sah, 2002; Sailer et al. 2002), suggesting that the activation of σR-1 with (+)pentazocine prevents SK channels opening.
σR-1 activation enhances LTP
Finally, we sought to examine the functional role of σR-1 activation in regulating the synaptic transmission in the CA1 region of the hippocampus. Since in CA1 pyramidal cells application of apamin enhances LTP (Behnisch & Reymann, 1998; Stackman et al. 2002; Ngo-Anh et al. 2005; Hammond et al. 2006), then, if we assume that activation of σR-1 with (+)pentazocine prevents SK channels opening, (+)pentazocine should enhance LTP.
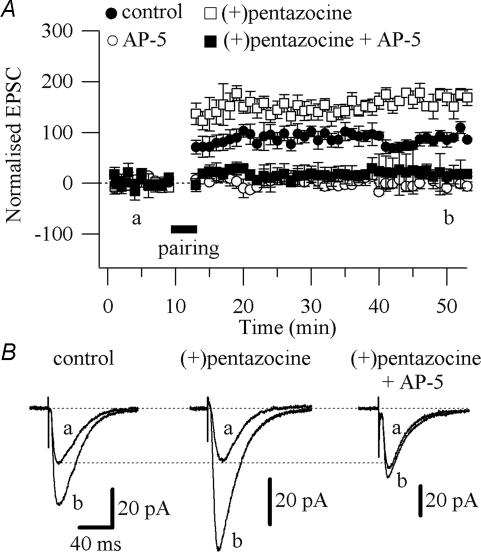
We recorded CA1 pyramidal cells and induced LTP with a pairing protocol comprised of three brief high-frequency tetani (50 pulses at 100 Hz, 4 s intervals) given at the end of a 3 min long depolarization at 0 mV. This protocol induced a 90.9 ± 11.9% (control, n = 13; P < 0.005; Fig. 8A and B) increase above baseline of the synaptic responses, lasting for more than 40 min. This LTP was NMDAR dependent since it was prevented by application of AP-5 (50 μm; −9.50 ± 8.77% above baseline; n = 4; P > 0.05; Fig. 8A). LTP in the presence of (+)pentazocine (1 μm) was significantly enhanced (154.26 ± 17.4% above baseline; n = 5; P < 0.05; Fig. 8A and B) compared with control conditions, and was abolished by the concomitant application of (+)pentazocine (1 μm) and AP-5 (50 μm; 8.82 ± 15.6% above baseline; n = 5; Fig. 8A and B). Since the increase of NMDAR currents would primarily affect the induction of LTP, we analysed the summation during the tetanic stimulation in the absence (control) and presence of (+)pentazocine. We found that in the presence of (+)pentazocine the summation was significantly larger (P < 0.05) compared with control (tetanus 1: 50.51 ± 97.26 pA, n = 6 in control versus 394.4 ± 83.86 pA, n = 4 in (+)pentazocine; tetanus 2: 6.19 ± 63.10 pA, n = 6 in control versus 167.54 ± 50.07 pA, n = 4 in (+)pentazocine; tetanus 3: −20.61 ± 50.68 pA, n = 6 in control versus 113.80 ± 52.48 pA, n = 4 in (+)pentazocine; Supplementary Fig. 7). Overall these results suggest that the enhanced LTP observed in the presence of (+)pentazocine was probably the result of the enhanced Ca2+ influx through the NMDAR due to the prevention of SK channels activation.
Figure 8. Effect of (+)pentazocine on LTP.
A, pooled data of the effect of AP-5 (50 μm; ○; n = 4), (+)pentazocine (1 μm; □; n = 5) and (+)pentazocine (1 μm) plus AP-5 (50 μm; ▪; n = 5) on the LTP, compared with the LTP in absence of drugs (control; •; n = 13) All values are mean ± s.e.m.B, examples of traces of EPSCs measured at the time points indicated in A (a and b) are shown for control, (+)pentazocine and (+)pentazocine plus AP-5. Each trace is an average of 20 traces.
Discussion
In this paper we show that the potentiation of NMDAR currents following the activation of the σR-1 by (+)pentazocine is due to the prevention of SK channel activation. Ca2+ entering the cells through the NMDAR activates a Ca2+-activated K+ current, underlain by SK channels, which in turn shunts the NMDAR responses. Consequently, the prevention of SK channel opening by σR-1 activation increases the NMDAR response and LTP.
The existence of a functional interaction between σRs and NMDARs has been suggested by several studies using biochemical, neuroendocrinological and behavioural models (Su & Hayashi, 2003). In the hippocampus, σRs-1 have been shown to play a role in the modulation of the glutamatergic neurotransmission via NMDARs (Monnet et al. 1990; Bergeron et al. 1997). Several σR-1 ligands have also been shown to increase the NMDAR response (Bergeron et al. 1993; Yamamoto et al. 1995; Bergeron et al. 1996; Karasawa et al. 2002). In addition, σRs have been described to modulate the excitability of peptidergic nerve terminals in the neurohypophysis by inhibiting voltage-dependent K+ channels (Wilke et al. 1999). The activation of σRs by a variety of ligands reduces current flow through two distinct K+ channel types: the A current channel and the Ca2+-activated K+ channel (Wilke et al. 1999). It has also been found that σRs and voltage-gated K+ channels have a protein–protein interaction and that ligands binding to σRs modulate channel activity through this interaction (Aydar et al. 2002). Indeed, none of the classical mechanisms of ion channel modulation, such as G protein or phosphorylation, were found to be involved in the mechanism of transduction through which the σRs modulate K+ channels (Lupardus et al. 2000).
Very recently it has been demonstrated that in dendritic spines of hippocampal CA1 pyramidal neurons, Ca2+ entry after synaptic activation opens SK channels that act to limit the amplitude of synaptic potentials and reduce Ca2+ influx through NMDARs (Ngo-Anh et al. 2005). It has also been established that Ca2+ influx through NMDARs could open Ca2+-activated K+ channels in several systems. In hippocampal slices, glutamate-evoked membrane depolarization could be followed by a Ca2+-dependent and K+-mediated AHP (Nicoll & Alger, 1981). Similarly, NMDA application evokes a Ca2+-dependent K+ current in cultured hippocampal neurons (Zorumski et al. 1989). Evidence of an interaction between NMDARs and Ca2+-activated K+ channels has been reported by Isaacson and Murphy in olfactory bulb granule cells (Isaacson & Murphy, 2001). They showed that NMDAR-mediated Ca2+ influx was coupled to large-conductance (BK) Ca2+-activated K+ channels. However, in cultured hippocampal neurons, NMDA application was observed to activate SK channels, but not BK channels (Shah & Haylett, 2002). Indeed, the NMDAR-mediated rise in Ca2+ concentration results in the activation of an apamin-sensitive current (Shah & Haylett, 2002; Ngo-Anh et al. 2005). The Ca2+ dependence, as well as the blockade by intracellular caesium of the enhancing effect of σR-1 activation on NMDAR currents, suggests the involvement of a Ca2+-activated K+ conductance. The enhancing effect of (+)pentazocine on NMDAR currents was mimicked by apamin and bicuculline methiodide but not by a low concentration of TEA, suggesting the implication of SK channels in the modulation of the NMDAR currents. This hypothesis is further supported by the fact that the activation of σR-1 with (+)pentazocine prevented SK channel activation. The voltage dependence of the effect of (+)pentazocine on the NMDAR currents strengthens the evidence for a relationship between the NMDAR-mediated rise in the Ca2+ concentration and K+ current. This was also supported by experiments using local application of NMDA confirming that the Ca2+ influx through postsynaptic NMDARs is required to activate Ca2+-activated K+ channels. We ruled out a direct block of SK channels by (+)pentazocine because igmesine, a σR-1 agonist structurally unrelated to (+)pentazocine, also occluded the effect of apamin on NMDAR currents.
SK channels are activated by submicromolar concentration of intracellular Ca2+ and behave as high-affinity Ca2+ sensors that convert fluctuation of intracellular Ca2+ concentrations into changes in membrane potential (Xia et al. 1998). All the SK channel subtypes exhibit a similar Ca2+ dose–response relationship with Ca2+ concentration required for half-maximal activation (K0.5) of ∼0.3 μm and an onset of the currents that commence within 1 ms with a time constant of 5–12 ms (Xia et al. 1998). The intracellular concentration of Ca2+ is maintained very low (10–100 nm) by channels, pumps and exchangers, allowing rapid metabolic response to Ca2+ changes. In the spines of CA1 pyramidal cells, the synaptic Ca2+ signals are primarily caused by Ca2+ influx through NMDARs (Kovalchuk et al. 2000), with a 20–80% rise time of the Ca2+ transient, evoked by uncaging of glutamate, of 13 ± 2 ms (Ngo-Anh et al. 2005). In the spine of the CA1 pyramidal cells, Ca2+ signals (subthreshold Ca2+ signals) are generated by NMDARs that are not completely blocked by Mg2+ at resting membrane potential (subthreshold NMDARs). Consequently, when the glutamate is released from a single vesicle, it partially activates NMDARs (subthreshold NMDARs; Kovalchuk et al. 2000). Since the activation kinetics of the NMDAR currents recorded in our experiments is ∼7–11 ms (Table 1) and a concentration of 0.7 μm could be reached in the spine of CA1 pyramidal cells just after subthreshold NMDAR activation and/or single spontaneous synaptic vesicle release (Kovalchuk et al. 2000), the Ca2+ influx to the spine can easily activate SK channels (from the first millisecond) and affect the peak amplitude of the NMDAR currents (7–11 ms). We suggest that both subthreshold-activated NMDAR channels and evoked NMDAR currents (NMDAR currents evoked by electrical stimulation) may provide the source of Ca2+ that rapidly activates the SK channels and consequently influences the peak amplitude of NMDAR currents.
Time course experiments showed that the amplitude of NMDAR currents were stably increased by (+)pentazocine after 12–15 min of drug application, while apamin stably increased the amplitude of NMDAR currents after 5–7 min (Fig. 6). This is supported by the time course experiments on SK currents showing that apamin and (+)pentazocine stably blocked the currents after 5–7 min and 12–15 min, respectively (Fig. 7). Previous reports have shown that the amplitude of the current underlain by SK channels was stably blocked by the application of apamin after ∼5–7 min (Sailer et al. 2002; Gu et al. 2005). The differences in time course between apamin and (+)pentazocine could be due to the difference in their mechanism of action in blocking or preventing the opening of SK channels.
σRs-1 have been described to regulate Ca2+ release and signalling from intracellular Ca2+ storage sites via inositol 1,4,5-triphosphate (IP3) receptor on the endoplasmatic reticulum (Hayashi et al. 2000). Indeed, the σRs-1 have been described to be localized on the endoplasmatic reticulum. The effect of σR-1 agonists on the Ca2+ store in regulating NMDAR currents is unlikely because of the inability of CPA and ryanodine, two Ca2+ ATPase inhibitors that cause the depletion of intracellular Ca2+ stores, to reverse the effect of (+)pentazocine on NMDAR currents. σRs-1 have also been described to regulate voltage-dependent Ca2+ channels (Zhang & Cuevas, 2002). There are multiple types of Ca2+ channels with unique physiological roles in the central nervous system. These have been classified by their distinct electrophysiological and pharmacological profiles into T-, N-, L-, Q-, P- and R-types (McCleskey, 1994). Mibefradil, ω-conotoxin GVIA, nicardipine and ω-agatoxin IVA, which block T-, N-, L- and P/Q-type Ca2+ channels, respectively, had no effect in abolishing the increasing effect caused by (+)pentazocine on NMDAR currents, excluding the implication of voltage-dependent Ca2+ channels in this effect. The lack of positive control experiments ensuring that CPA, ryanodine, mibefradil, ω-conotoxin GVIA, nicardipine and ω-agatoxin IVA inhibited SERCA pumps, ryanodine receptors and voltage-dependent T-, N-, L- and P/Q-type Ca2+ channels, respectively, limits our capacity to affirm that these sources of Ca2+ did not participate in the effect of σR-1 activation on NMDAR currents. However, previous reports showing no effect of CPA, ryanodine and nicardipine in blocking the increasing effect of apamin on NMDAR EPSPs strongly support our hypothesis (Faber et al. 2005).
LTP of the Schaffer collateral synapses is NMDAR dependent and requires pre-synaptic activity and post-synaptic depolarization. The post-synaptic depolarization is necessary due to the properties of NMDARs, which require the relief of the Mg2+ block to open (Nowak et al. 1984). Once NMDARs are open, Ca2+ influx triggers synaptic plasticity (Bliss & Collingridge, 1993). Indeed, the competitive NMDAR antagonist AP-5 prevented the induction of LTP. Since (+)pentazocine enhances NMDAR currents through the prevention of SK channels activation, the enhanced LTP observed in the presence of the σR-1 agonist is likely to be the result of the enhanced Ca2+ influx through the NMDAR. In the CA1 region of the hippocampus, the induction of LTP can also be influenced by the Ca2+ entering the cell through Ca2+ channels or by release from the intracellular Ca2+ stores (Chen et al. 1999; Kovalchuk et al. 2000). Since σRs-1 have been described to influence Ca2+ release and signalling from intracellular Ca2+ storage sites (Hayashi et al. 2000) as well as voltage-dependent Ca2+ channels (Zhang & Cuevas, 2002), the abolition of the LTP when AP-5 was applied with (+)pentazocine suggested that (+)pentazocine did not influence the induction of LTP through other mechanisms than NMDARs. Our findings are also supported by previous reports showing that in CA1 pyramidal cells, application of apamin enhances LTP (Behnisch & Reymann, 1998; Stackman et al. 2002; Hammond et al. 2006) and that blocking SK channels facilitates the induction of LTP by enhancing NMDAR-dependent Ca2+ signals within dendritic spines (Ngo-Anh et al. 2005). The effect of σR-1 activation on SK channels and NMDARs supports the role that σRs-1 play on synaptic transmission.
Acknowledgments
This work was supported by the Natural Sciences Engineering Research Council of Canada (NSERC). We thank Drs Joseph T. Coyle, Leo P. Renaud and Peter Stys for reading the manuscript. We also thank C. Metivier for technical assistance.
Supplementary material
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2006.116178 http://jp.physoc.org/cgi/content/full/jphysiol.2006.116178/DC1 and contains six supplemental figures.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Behnisch T, Reymann KG. Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci Lett. 1998;253:91–94. doi: 10.1016/s0304-3940(98)00612-0. [DOI] [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Effect of short-term and long-term treatments with sigma ligands on the N-methyl-D-aspartate response in the CA3 region of the rat dorsal hippocampus. Br J Pharmacol. 1997;120:1351–1359. doi: 10.1038/sj.bjp.0701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R, Debonnel G, de Montigny C. Modification of the N-methyl-D-aspartate response by antidepressant sigma receptor ligands. Eur J Pharmacol. 1993;240:319–323. doi: 10.1016/0014-2999(93)90918-8. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bowen WD. Sigma receptors: recent advances and new clinical potentials. Pharm Acta Helv. 2000;74:211–218. doi: 10.1016/s0031-6865(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Chen HX, Otmakhov N, Lisman J. Requirements for LTP induction by pairing in hippocampal CA1 pyramidal cells. J Neurophysiol. 1999;82:526–532. doi: 10.1152/jn.1999.82.2.526. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Empson RM, Jefferys JGR. Ca2+ entry through L-type Ca2+ channels helps terminate epileptiform activity by activation of a Ca2+ dependent afterhyperpolarisation in hippocampal CA3. Neuroscience. 2001;102:297–306. doi: 10.1016/s0306-4522(00)00494-2. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566:689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su TP. Ca2+ signaling via σ1-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. J Pharmacol Exp Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Murphy GJ. Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca2+-activated K+ channels. Neuron. 2001;31:1027–1034. doi: 10.1016/s0896-6273(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Sasa M. Modulation of neuronal activities in the central nervous system via sigma receptors (in Japanese) Nihon Shinkei Seishin Yakurigaku Zasshi. 2002;22:23–30. [PubMed] [Google Scholar]
- Jbilo O, Vidal H, Paul R, De Nys N, Bensaid M, Silve S, et al. Purification and characterization of the human SR 31747A-binding protein. A nuclear membrane protein related to yeast sterol isomerase. J Biol Chem. 1997;272:27107–27115. doi: 10.1074/jbc.272.43.27107. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Yamamoto H, Yamamoto T, Sagi N, Horikomi K, Sora I. MS-377, a selective sigma receptor ligand, indirectly blocks the action of PCP in the N-methyl-D-aspartate receptor ion-channel complex in primary cultured rat neuronal cells. Life Sci. 2002;70:1631–1642. doi: 10.1016/s0024-3205(01)01549-1. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Eilers J, Lisman J, Konnerth A. NMDA receptor-mediated subthreshold Ca2+ signals in spines of hippocampal neurons. J Neurosci. 2000;20:1791–1799. doi: 10.1523/JNEUROSCI.20-05-01791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin C-Y, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol. 2000;526:527–539. doi: 10.1111/j.1469-7793.2000.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey EW. Calcium channels: cellular roles and molecular mechanisms. Curr Opin Neurobiol. 1994;4:304–312. doi: 10.1016/0959-4388(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, Schmidt CJ, Bergeron R. Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiol. 2004;557:489–500. doi: 10.1113/jphysiol.2004.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moebius FF, Reiter RJ, Hanner M, Glossmann H. High affinity of sigma 1-binding sites for sterol isomerization inhibitors: evidence for a pharmacological relationship with the yeast sterol C8–C7 isomerase. Br J Pharmacol. 1997;121:1–6. doi: 10.1038/sj.bjp.0701079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, Junien JL, de Montigny C. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur J Pharmacol. 1990;179:441–445. doi: 10.1016/0014-2999(90)90186-a. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Alger BE. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981;212:957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophisiol. 2003;90:2171–2179. doi: 10.1152/jn.01177.2002. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993;11:1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Roman FJ, Pascaud X, Martin B, Vauche D, Junien JL. JO 1784, a potent and selective ligand for rat and mouse brain sigma-sites. J Pharm Pharmacol. 1990;42:439–440. doi: 10.1111/j.2042-7158.1990.tb06588.x. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22:9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- Shah MM, Haylett DG. K+ currents generated by NMDA receptor activation in rat hippocampal pyramidal neurons. J Neurophysiol. 2002;87:2983–2989. doi: 10.1152/jn.2002.87.6.2983. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF. The calcium channel and the organization of the presynaptic transmitter release face. Trends Neurosci. 1997;20:404–409. doi: 10.1016/s0166-2236(97)01091-6. [DOI] [PubMed] [Google Scholar]
- Steinfels GF, Alberici GP, Tam SW, Cook L. Biochemical, behavioral, and electrophysiologic actions of the selective sigma receptor ligand (+)-pentazocine. Neuropsychopharmacology. 1988;1:321–327. [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Su TP. Delineating biochemical and functional properties of sigma receptors: emerging concepts. Crit Rev Neurobiol. 1993;7:187–203. [PubMed] [Google Scholar]
- Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke RA, Lupardus PJ, Grandy DK, Rubinstein M, Low MJ, Jackson MB. K+ channel modulation in rodent neurohypophysial nerve terminals by sigma receptors and not by dopamine receptors. J Physiol. 1999;517:391–406. doi: 10.1111/j.1469-7793.1999.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yamamoto T, Sagi N, Klenerova V, Goji K, Kawai N, Baba A, Takamori E, Moroji T. Sigma ligands indirectly modulate the NMDA receptor-ion channel complex on intact neuronal cells via sigma 1 site. J Neurosci. 1995;15:731–736. doi: 10.1523/JNEUROSCI.15-01-00731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol. 2002;87:2867–2879. doi: 10.1152/jn.2002.87.6.2867. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Thio LL, Clark GD, Clifford DB. Calcium influx through N-methyl-D-aspartate channels activates a potassium current in postnatal rat hippocampal neurons. Neurosci Lett. 1989;99:293–299. doi: 10.1016/0304-3940(89)90462-x. [DOI] [PubMed] [Google Scholar]
- Zukin SR, Zukin RS. Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci U S A. 1979;76:5372–5376. doi: 10.1073/pnas.76.10.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.