Abstract
The electrogenic Na+–HCO3− cotransporter (NBCe1) plays a central role in intracellular pH (pHi) regulation as well as HCO3− secretion by pancreatic ducts and HCO3− reabsorption by renal proximal tubules. To understand the structural requirements for the electrogenicity of NBCe1, we constructed chimeras of NBCe1-A and the electroneutral NBCn1-B, and used two-electrode voltage clamp to measure electrogenic transporter current in Xenopus oocytes exposed to 5% CO2–26 mm HCO3−(pH 7.40). The chimera consisting of NBCe1-A (i.e. NBCe1-A ‘background’) with the cytoplasmic N-terminal domain (Nt) of NBCn1-B had a reversal potential of −156.3 mV (compared with a membrane potential Vm of −43.1 mV in a HCO3−-free solution) and a slope conductance of 3.0 μS (compared with 12.5 μS for NBCe1-A). Also electrogenic were chimeras with an NBCe1-A background but with NBCn1-B contributing the extracellular loop (L) between transmembrane segment (TM) 5 and 6 (−140.9 mV/11.1 μS), the cytoplasmic C-terminal domain (Ct; −123.8 mV/9.7 μS) or Nt + L + Ct (−120.9 mV/3.7 μS). Reciprocal chimeras (with an NBCn1 background but with NBCe1 contributing Nt, L, Ct or Nt + L + Ct) produced no measurable electrogenic transporter currents in the presence of CO2–HCO3−. pHi recovered from an acid load, but without the negative shift of Vm that is characteristic of electrogenic Na+–HCO3− cotransporters. Thus, these chimeras were electroneutral, as were two others consisting of NBCe1(Nt–L)/NBCn1(TM6–Ct) and NBCn1(Nt–L)/NBCe1(TM6–Ct). We propose that the electrogenicity of NBCe1 requires interactions between TM1–5 and TM6–13.
Cells regulate their internal ionic environment via membrane proteins that mediate the transmembrane movement of ions. The SLC4A gene family, which has 10 members, consists mainly of transporters that are specialized to move HCO3− (or in some cases, CO32−) across plasma membranes (for review see Romero et al. 2004). For many SLC4 family members, major physiological roles include the regulation of intracellular pH (pHi) and/or the transepithelial movement of HCO3−. Regarding pHi regulation, some SLC4 members mediate the efflux of HCO3− (or CO32−) and thus act to lower pHi (acid loaders), whereas others mediate the influx of HCO3− (or CO32−) and thus act to raise pHi (acid extruders). The balance between the activities of acid extruders and loaders, as well as metabolic production and consumption of H+ ions, determines the steady-state pHi (Boron, 1989). For example, the electrogenic Na+–HCO3− cotransporter NBCe1 (SLC4A4) (Bevensee et al. 1995) – operating with a Na+:HCO3− stoichiometry of 1 : 2 in cells such as astrocytes – is an acid extruder. This HCO3−-mediated acid extrusion helps to avoid pHi in astrocytes falling below normal levels during cerebral acidification (Giffard et al. 2000).
In HCO3−-transporting epithelia, at least one member of the SLC4A gene family is usually present in the basolateral membranes, mediating a crucial step in the transepithelial movement of HCO3−. For example, NBCe1, apparently operating with a 1 : 2 stoichiometry, mediates HCO3− uptake across the basolateral membrane of the pancreatic duct for secretion into the lumen (Marino et al. 1999). In the renal proximal tubule, which reclaims more than 80% of filtered HCO3−, NBCe1 mediates the efflux of HCO3− across the basolateral membrane for delivery to the blood. An essential requirement for this HCO3− exit is that NBCe1 in the proximal tubule operates with an apparent Na+:HCO3− stoichiometry of 1 : 3, which makes the reversal potential for the transporter more positive than the basolateral membrane potential of the proximal tubule cell (Soleimani et al. 1987; Boron & Boulpaep, 1989). By moving Na+ out of the cell, NBCe1 in the proximal tubule may be unique in moving Na+ against the Na+ electrochemical gradient without utilizing ATP directly.
In spite of the importance of the electrogenicity of NBCe1 for its physiological function, the protein domains responsible for electrogenic ion translocation are not known. Sequence analysis reveals a high level of amino acid identity (> 50%) among the five known Na+–HCO3− transporters of the SLC4 family (Romero et al. 2004), two of which are electrogenic (NBCe1/SLC4A4 and NBCe2/SLC4A5) (Burnham et al. 1997; Romero et al. 1997; Sassani et al. 2002; Virkki et al. 2002) and three of which are electroneutral (NBCn1/SLC4A7, NDCBE/SLC4A8 and NCBE/SLC4A10) (Pushkin et al. 1999; Choi et al. 2000; Wang et al. 2000; Grichtchenko et al. 2001). It is not surprising that the identity level is generally higher in the transmembrane domains of the proteins than in the cytoplasmic N- and C-terminal domains and the large, third extracellular domain between transmembrane segment (TM) 5 and TM6. This relationship is consistent with the hypothesis that the transmembrane domains mediate the actual transport event, but that differences in these less-conserved regions somehow modulate the transmembrane domains and thereby control the stoichiometry of ion transport. Several studies have shed light on the structure–function relationships of NBCe1. A large-scale mutagenesis study targeted acidic and basic amino acids near or outside transmembrane segments, and identified several that block transport (Abuladze et al. 2005). However, it remains unclear whether those residues directly participate in ion translocation or determine stoichiometry.
In the present study, we systematically constructed a series of chimeric transporters between NBCe1 and the electroneutral Na+–HCO3− cotransporter NBCn1 (for a sequence comparison of the two transporters, see Choi et al. 2000). We swapped transmembrane and non-transmembrane domains of NBCe1 and NBCn1, expressed them in Xenopus oocytes, and analysed the HCO3−-dependent currents mediated by chimeras in two-electrode voltage clamp. We found that neither the cytoplasmic N-terminal domain (Nt), the large, third extracellular loop (L) nor the cytoplasmic C-terminal domain (Ct) affects electrogenicity. However, both the transmembrane domain before the third extracellular loop (TM1–5) and the transmembrane domain after the third extracellular loop (TM6 to the last TM) are necessary for electrogenicity.
Methods
Construction of chimeras
The ‘renal’ splice variant of rat NBCe1 (NBCe1-A, GenBank accession number NM_053424) and NBCn1 (NBCe1-B, GenBank accession number NM_058211) were used for constructing chimeric proteins. Table 1 summarizes the regions that we swapped. To swap regions of interest, we introduced restriction endonuclease sites (generating silent mutations that did not affect amino acid composition) at both ends of the corresponding regions of both transporters, using the QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA). Details are available upon request. PCR was carried out using Pfu DNA polymerase with high proofreading capability. Chimeric constructs were sequenced by the Biomedical Facility at Emory University or the Keck Center at Yale University. The constructs were inserted in the oocyte expression vector pGH19 (Choi et al. 1999), which contains the 5′ and 3′ untranslated regions of the Xenopus β-globin gene. For wild-type NBCe1 and chimeras containing the N-terminal domain of NBCe1, we used the pTLN2 vector (Romero et al. 1998) because of incompatibility of the restriction enzyme sites. pTLN2 is virtually identical to pGH19 except for the polycloning sites.
Table 1.
Summary of regions swapped between NBCe1-A and NBCn1-B
| Region | NBCe1-A | NBCn1-B |
|---|---|---|
| Nt | MSTEN…ALNIQ (424) | MEADG…ALSLQ (611) |
| Loop | ADYYP…CNFVP (83) | GEIYA…GPYVP (76) |
| Ct | KGMDY…RHTSC (92) | KLMDL…AETSL (95) |
| Front (start–Loop) | MSTEN…CNFVP (646) | MEADG…GPYVP (826) |
| Back (TM6–end) | DITLM…RHTSC (389) | DVLFW…AETSL (392) |
Each cell contains the single-letter codes of the first five and last five amino acids of the fragment; the total number of amino acids is in parentheses.
Transporter expression in Xenopus oocytes
The DNAs encoding chimeric transporters were linearized with Not I or Nhe I for constructs in pGH19 and in vitro transcribed with T7 RNA polymerase using the mMessage/mMachine transcription kit (Ambion, Austin, TX, USA). For constructs in pTLN2, Mlu I was used for linearization and SP6 RNA polymerase for transcription. Oocytes (stages V and VI) were prepared from Xenopus laevis. A piece of ovary containing oocytes was excised, rinsed five times with Ca2+-free ND96 solution containing (mm): NaCl 96, KCl 2, MgCl2 1 and Hepes 5 (pH 7.5), for 20 min each. The tissue was then agitated in sterile-filtered Ca2+-free ND96 with collagenase (Type 1 A, Sigma-Aldrich, St Louis, MO, USA) for 40 min. Defolliculated oocytes were washed with Ca2+-free ND96, sorted and stored at 18°C in OR3 media (50% Leibovitz L-15 media in 5 mm Hepes, pH 7.5) supplemented with 5 U ml−1 penicillin-streptomycin. Oocytes were injected with 50 nl of either RNA (0.4 μg μl−1). Injected oocytes were maintained for 3–7 days at 18°C.
Two-electrode voltage clamp
Two-electrode voltage clamp was performed using the oocyte clamp OC-725C (Warner, Hamden, CT, USA). The electrodes were filled with 3 m KCl with a resistance of less than 1.5 MΩ. Oocytes were clamped at a holding potential of −60 mV and subjected to staircase voltage commands stepping from −140 to +60 mV (20 mV increment) for 100 ms. Measurements of the electrogenic current were made at 1 min after switching bath solutions from modified amphibian ND96 solution containing (mm): NaCl 96, KCl 2, CaCl2 1.8, MgCl2 2 and Hepes 5 (pH 7.4), to one buffered with 5% CO2–26 mm HCO3−(pH 7.4; NaCl replaced by NaHCO3). Measurements of the ionic currents associated with NBCn1 were done in ND96, as previously described (Cooper et al. 2005). In Na+-free solutions, Na+ was replaced by NMDG+. The voltage signal was sampled by the Digidata 1322 interface (Axon Instruments, Union City, CA, USA) connected to an IBM-compatible PC. Data were acquired and analysed using pClamp 8 (Axon Instruments).
Measurements of pHi in oocytes
We impaled oocytes with two electrodes, one sensitive to pH and the other a conventional voltage electrode, as previously described (Siebens & Boron, 1987; Romero et al. 1997; Lu et al. 2006). The pH electrode was made with borosilicate fibre capillaries, silanized, and filled with the proton ionophore 1 cocktail B (Sigma-Aldrich), and back filled with a phosphate buffer at pH 7.0. The voltage electrode was filled with 3 m KCl and had a resistance of 0.7–2.0 MΩ. Electrodes were connected to a high-impedance electrometer FD-223 (WPI, Sarasota, FL, USA). The voltage signal was subtracted from the voltage from the pH electrode, which yields a voltage due solely to pH. The data were sampled by an A/D converter interfaced to a computer. Measurements of pHi were first done in the nominally HCO3−-free ND96 solution (pH 7.5) and then in a solution equilibrated with 1.5% CO2–10 mm HCO3− (pH 7.5). NaCl was replaced by NaHCO3. The osmolality was adjusted to 196–200 mosmol kg−1. All experiments were performed at room temperature (∼22°C).
Statistical analysis
Data were reported as means ± s.e.m. Levels of significance were assessed using the unpaired, two-tailed Student t test. P < 0.05 was considered significant. Rates of pHi change were fitted by a line using a least-squares method.
Results
Electrogenic HCO3− currents mediated by NBCe1
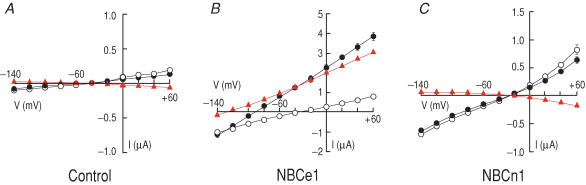
In analysing the electrogenicity of chimeric transporters, our experimental approach was to express the transporters in Xenopus oocytes and determine steady-state current–voltage (I – V) relationships with the oocyte first exposed to the CO2–HCO3−-free ND96 solution and then to the solution buffered with 5% CO2–26 mm HCO3− (pH 7.4). Figure 1A shows average data for water-injected control oocytes. In the ND96 solution, they had a mean reversal potential (END96) around −41 mV, low background currents (IND96) and a low slope conductance (GND96). After the switch to CO2–HCO3−, the reversal potential (EHCO3), currents (IHCO3) and slope conductance (GHCO3) were not substantially different from those in the presence of ND96 solution. The difference current IHCO3 – IND96 (red triangles) nearly lies on the voltage axis. Table 2 summarizes the data from control oocytes as well as oocytes (discussed below) expressing NBCe1-A, NBCn1-B and various other chimeras.
Figure 1. Mean steady-state current–voltage (I–V) relationships in control oocytes and oocytes expressing wild-type NBCe1 or NBCn1.
A, control oocytes injected with water (n = 5). B, oocytes expressing NBCe1 (n = 7). C, oocytes expressing NBCn1 (n = 7). Oocytes were clamped at −60 mV and voltage was stepped from −140 to +60 mV for 100 ms in 20 mV increments. The recordings were done in the pH 7.40 HCO3−-free ND96 solution (○) or in a comparable solution buffered to pH 7.40 with 5% CO2–26 mm HCO3−(•). The difference IHCO3 – IND96 is an estimate of the electrogenic cotransport current (▴).
Table 2.
Reversal potentials and slope conductances of oocytes expressing chimeric transporters
| Chimera name | Figure | n | ND96 | HCO3–CO2 | Cotransport current | |||
|---|---|---|---|---|---|---|---|---|
| END96 | GND96 (μS) | EHCO3 | GHCO3 (μS) | ENBC | GNBC (μS) | |||
| water | Fig. 1A | 5 | −41.0 ± 5.6 | 1.1 ± 0.1 | −47.4 ± 6.6 | 0.8 ± 0.1 | −27.7 ± 2.8 | −0.4 ± 0.1 |
| NBCe1-A | Fig. 1B | 7 | −36.5 ± 1.2 | 7.7 ± 0.9 | −91.5 ± 2.8 | 24.0 ± 1.4 | −129.3 ± 4.5* | 12.5 ± 0.9** |
| NBCn1-B | Fig. 1C | 7 | −33.4 ± 5.1 | 5.9 ± 0.3 | −26.2 ± 0.5 | 4.7 ± 0.2 | −23.7 ± 1.8 | −1.3 ± 0.3 |
| n1(Nt)/e1 | Fig. 3 | 7 | −43.2 ± 2.4 | 3.2 ± 0.4 | −98.9 ± 4.8 | 6.0 ± 0.6 | −156.3 ± 9.2* | 3.0 ± 0.4** |
| n1(L)/e1 | — | 6 | −37.9 ± 2.2 | 4.9 ± 0.5 | −100.3 ± 2.5 | 14.3 ± 0.9 | −140.9 ± 6.4* | 11.1 ± 0.7** |
| n1(Ct)/e1 | — | 7 | −43.2 ± 2.1 | 3.0 ± 2.1 | −113.1 ± 1.9 | 9.8 ± 1.1 | −123.8 ± 1.6* | 9.7 ± 1.2** |
| n1(NtLCt)/e1 | Fig. 4 | 5 | −38.0 ± 2.5 | 1.9 ± 0.1 | −94.0 ± 3.9 | 5.7 ± 0.6 | −120.9 ± 3.3* | 3.7 ± 0.6** |
| e1(Nt)/n1 | Fig. 5 | 7 | −27.2 ± 0.4 | 6.1 ± 0.3 | −27.9 ± 0.4 | 5.2 ± 0.3 | −24.9 ± 2.0 | −0.9 ± 0.9 |
| e1(L)/n1 | — | 7 | −30.9 ± 1.1 | 6.3 ± 0.5 | −33.0 ± 1.1 | 4.9 ± 0.3 | −22.6 ± 1.2 | −1.3 ± 0.2 |
| e1(Ct)/n1 | — | 6 | −30.4 ± 1.3 | 4.1 ± 0.3 | −33.6 ± 3.0 | 3.1 ± 0.5 | −23.5 ± 3.3 | −0.9 ± 0.3 |
| e1(NtLCt)/n1 | Fig. 6 | 7 | −33.3 ± 0.9 | 5.6 ± 1.1 | −37.8 ± 1.0 | 3.9 ± 0.9 | −23.2 ± 0.8 | −1.7 ± 0.3 |
| n1(1–821)/e1 | Fig. 7 | 8 | −30.5 ± 2.2 | 1.3 ± 0.2 | −32.3 ± 2.5 | 0.9 ± 0.2 | −26.4 ± 1.6 | −0.4 ± 0.1 |
| n1(822–1218)/e1 | Fig. 8 | 7 | −30.3 ± 0.6 | 7.7 ± 1.0 | −32.5 ± 0.8 | 6.4 ± 0.9 | −20.8 ± 1.2 | −1.3 ± 0.2 |
Conductance (G) was computed from current–voltage (I–V) values crossing the zero currents.
Significant compared to water-injected controls (P < 0.05).
Figure 1B shows average data for an oocyte expressing NBCe1-A. IND96 values (at positive voltages) as well as GND96 values were somewhat higher than in H2O-injected control oocytes, consistent with the report by Sciortino & Romero (1999). IHCO3 values at positive voltages as well as GHCO3 values were much larger than for H2O-injected control oocytes, with a substantial negative shift of EHCO3. These changes are due to the cotransport activity of NBCe1-A as it moves net negative charge (i.e. 1 Na+ and the equivalent of 2 HCO3− ions) into the cell. The difference IHCO3 – IND96 (red symbols) is a good estimate of the electrogenic cotransport current (INBC), particularly for the larger outward currents (Lu et al. 2006). The slope conductance of INBC (GNBC) was substantially greater than GND96, and the reversal potential of INBC (ENBC) was about −129.3 mV so that INBC was outwardly directed at all voltages more positive than this value.
Figure 1C shows average data for an oocyte expressing NBCn1-B. IND96 values (at positive voltages) as well as GND96 values were higher than in H2O-injected oocytes, and END96 was relatively positive, reflecting the background Na+ current apparently carried by NBCn1 (Choi et al. 2000; Cooper et al. 2005). However, IHCO3 (at positive voltages) was no higher than IND96, and EHCO3 was not appreciably different from END96. In other words, NBCn1 did not produce HCO3−-dependent currents. Nevertheless, compared to water-injected controls, oocytes expressing NBCn1-B had a greater GND96 (5.9 versus 1.1 μS). Previous work showed that this additional ionic conductance is mostly due to a Na+ current but does not require HCO3−. This Na+ current is mediated by NBCn1 expressed both in Xenopus oocytes and human embryonic kidney (HEK) cells, indicating that the conductance is probably intrinsic to the transporter (Choi et al. 2000; Cooper et al. 2005).
Design of chimeric transporters
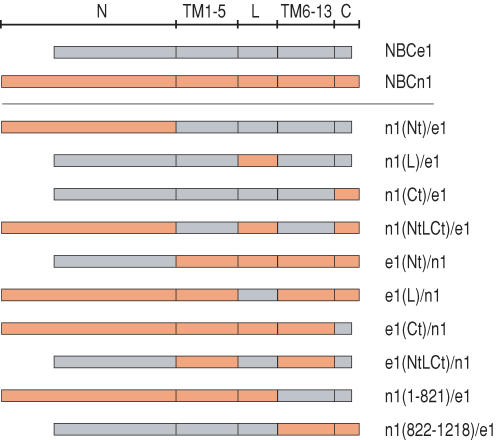
Rat NBCe1-A and NBCn1-B share overall ∼55% amino acid identity (more than 65% in the transmembrane domains), and have similar hydropathy plots (Choi et al. 2000). We presumed that this similarity would facilitate the construction of chimeric proteins between the two transporters without major problems in retaining protein conformation. Although both NBCe1 and NBCn1 exist as multiple splice variants, we are aware of no marked functional differences among variants of NBCn1. In the absence of protein binding partners such as IRBIT (Shirakabe et al. 2006), NBCe1-A, with its unique Nt, is substantially more active than either NBCe1-B or NBCe1-C (McAlear et al. 2006). Therefore, we made our constructs with rat NBCe1-A, which is expressed in the proximal tubules of the kidney (Romero et al. 1998), and rat NBCn1-B, which is predominantly present in the cardiovascular system (Cooper et al. 2006). One specific prediction is that replacing the Nt of NBCe1-A with that of NBCn1-B may be analogous to replacing the Nt of NBCe1-A with that of NBCe1-B, and thus render an electrogenic transporter less active. Chimeras might also be less active than the wild-type molecules because of ‘mismatches’ of various domains, which might lead to decreased surface expression, or to decreased activity of individual molecules. Here we use the shorthand terminology ‘e1’ for NBCe1-A and ‘n1’ for NBCn1-B. We made 10 chimeric constructs by swapping different regions of NBCe1-A with the corresponding ones of NBCn1-B (Fig. 2 and Table 1). In establishing boundaries of regions to be swapped, we followed the 13-TM topology model proposed for the Cl−–HCO3− exchanger AE1 (Fujinaga et al. 1999; Kuma et al. 2002).
Figure 2. Cotransporters used in the present study.
We generated chimeras between NBCe1-A (grey areas) and NBCn1-B (orange areas). N, N-terminal domain; TM1–5, transmembrane segments 1–5; L, third extracellular loop; TM6–13, transmembrane segments 6–13; C, C-terminal domain.
The cytoplasmic N and C termini and the third extracellular loop of NBCe1-A are not important for electrogenicity
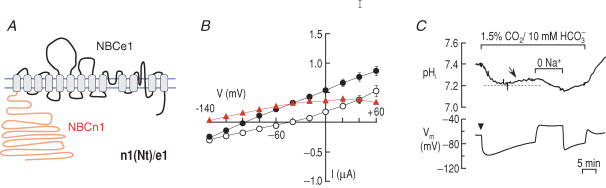
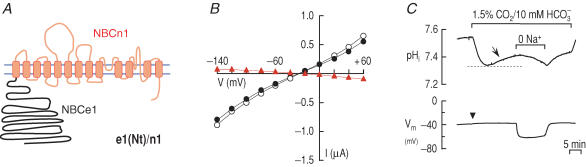
Figure 3 summarizes the function of the chimeric transporter n1(Nt)/e1 (Fig. 3A), which has the cytoplasmic N-terminal domain of NBCn1-B, with the remainder of the molecule contributed by NBCe1 (i.e. NBCe1 ‘background’). In voltage-clamp experiments (e.g. Figure 3B), mean IND96 (at positive voltages) and GND96 (Table 2) were relatively low compared to NBCe1-A (Fig. 1B), but higher than for H2O-injected controls (Fig. 1A). END96 for n1(Nt)/e1 was about −43 mV. In the presence of 5% CO2–26 mm HCO3−, IHCO3 (at positive voltages) and GHCO3 were much higher than the corresponding values in the presence of ND96. Moreover, EHCO3 was markedly more negative than END96. From the difference IHCO3 – IND96, we see that INBC (at positive voltages) and GNBC were much greater than the corresponding values for the chimera in ND96, but much less than GNBC for wild-type NBCe1-A. For the chimera, ENBC was more negative than −150 mV. These results indicate that replacing the N-terminal domain of NBCe1-A with the corresponding region of NBCn1-B results in a transporter that is electrogenic. The relatively low GNBC of the chimera, which presumably reflects mismatching the N terminus to the rest of the transporter, could be due to either reduced protein delivery to the cell surface or to reduced activity of individual transporters.
Figure 3. Replacing the N-terminal domain of NBCe1-A with the N-terminal domain of NBCn1-B.
A, chimeric transporter n1(Nt)/e1. B, (I–V) relationships obtained from oocytes expressing n1(Nt)/e1 (n = 7) in the absence (○) and presence (•) of 5% CO2–26 mm HCO3− (pH 7.4). The difference IHCO3 – IND96 is an estimate of the electrogenic cotransport current (▴). C, representative recordings of pHi and membrane potential (Vm) in an oocyte expressing n1(Nt)/e1. The pHi recovery from a CO2-induced acidification (arrow) and the hyperpolarization upon application of 1.5%CO2–10 mm HCO3− (arrowhead) are hallmarks of electrogenic Na+–HCO3− transport. One of four experiments.
To assess the acid–base transport characteristics of n1(N)/e1, we used microelectrodes to monitor pHi and membrane potential (Vm) of oocytes (Fig. 3C). After an initial CO2-induced acidification in the solution containing 1.5% CO2–10 mm HCO3−, the pHi of oocytes expressing n1(Nt)/e1 recovered as a result of the influx of HCO3− (or CO32−). Na+ removal in the continued presence of CO2–HCO3− reversed the pHi recovery, indicating that the recovery process is Na+-dependent. Application of CO2–HCO3− also caused a hyperpolarization, indicating that the chimera mediates the flux of 1 Na+ and at least 2 HCO3− (or equivalent) ions. Thus, the chimera n1(Nt)/e1 is an electrogenic Na+–HCO3− cotransporter.
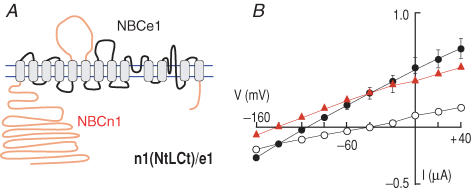
In this series of experiments, we analysed three other chimeras. The first, n1(L)/e1, had the third extracellular loop of NBCn1-B on a background of NBCe1-A (not shown). The second, n1(Ct)/e1, had the cytoplasmic C-terminal domain of NBCn1-B on a background of NBCe1-A (not shown). The third chimera, n1(NtLCt)/e1, had the N terminus, extracellular loop and C terminus from NBCn1-B on a background of NBCe1 (Fig. 4A). Voltage-clamp data for all three chimeras (summarized in Table 2) were similar to those shown for the triple chimera in Fig. 4B. GHCO3 was substantially higher than GND96, GNBC was much higher than GND96 in H2O-injected oocytes (Fig. 1A), and ENBC for n1(NtLCt)/e1 was more negative. In other words, all three had the functional characteristics of an electrogenic transporter. These data show that the major non-transmembrane domains (i.e. cytoplasmic N and C termini and third extracellular loop, which are less-well conserved than the transmembrane domains) of NBCe1-A are not critical for electrogenicity. In other words, the transmembrane domains of NBCe1-A are the only components required for electrogenic function.
Figure 4. Replacing the major non-transmembrane domains of NBCe1-A with the corresponding domains of NBCn1-B.
A, chimeric transporter n1(NtLCt)/e1. B, (I–V) relationships obtained from oocytes expressing n1(NtLCt)/e1 in the absence (○) and presence (•) of HCO3−–CO2 (n = 5).
Replacing the major non-transmembrane domains of NBCn1-B with the homologous domains of NBCe1-A does not induce electrogenic function
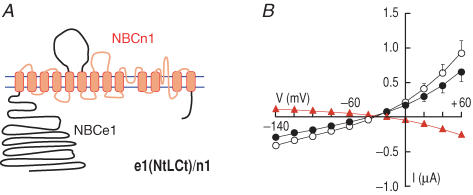
To further confirm the above findings, we constructed the four opposite chimeras in which the major non-transmembrane domains of NBCe1-A replaced the homologous domains of NBCn1-B. The first, e1(Nt)/n1, had the cytoplasmic N terminus of NBCe1-A on a background of NBCn1-B (Fig. 5A). The second and third, e1(L)/n1 and e1(Ct)/n1, respectively, had the third extracellular loop or cytoplasmic C terminus of NBCe1-A on a background of NBCn1-B (not shown). Finally, e1(NtLCt)/n1 had the N and C termini as well as the third extracellular loop of NBCe1-A on a background of NBCn1-B (Fig. 6A). Voltage-clamp data for all four chimeras (summarized in Table 2) were similar to data shown in Figs 5B and 6B. IND96 (at positive voltages) and GND96 values were higher than for H2O-injected oocytes. END96 values were around −30 mV. The chimeras share these characteristics with wild-type NBCn1-B, which has a background conductance. IHCO3 (at positive voltages) and GHCO3 for the chimeras were somewhat lower than corresponding values in the presence of ND96; this is also a characteristic of wild-type NBCn1-B (Fig. 1C). The I–V relationship of the triple chimera e1(NtLCt)n1 exhibited slight outward rectification (Fig. 6B). Clearly, none of these four chimeras exhibited the currents characteristic of electrogenic cotransport. Conversely, the presence of the background conductance in all four chimeras is consistent with previous conclusions that the ionic conductance is likely to be a property of the transmembrane domains of NBCn1-B.
Figure 5. Replacing the N-terminal domain of NBCn1-B with the N-terminal domain of NBCe1-A.
A, chimeric transporter e1(Nt)/n1. B, (I–V) relationships obtained from oocytes expressing e1(Nt)/n1 in the absence (○) and presence (•) of HCO3−–CO2 (n = 7). C, representative recordings of pHi and Vm in an oocyte expressing e1(Nt)/n1. The pHi recovery from a CO2-induced acidification (arrow) without affecting membrane potentials (arrowhead) are hallmarks of electroneutral Na+–HCO3− transport. A substantial hyperpolarization caused by Na+ removal is due to an associated Na+ conductance. One of four experiments.
Figure 6. Replacing the major non-transmembrane domains of NBCn1-B with the corresponding domains of NBCe1-A.
A, chimeric transporter e1(NtLCt)/n1. B, (I–V) relationships obtained from oocytes expressing e1(NtLCt)/n1 in the absence (○) and presence (•) of HCO3−–CO2 (n = 7).
In the experiments on pHi recovery, the initial CO2-induced acidification was followed by a robust pHi recovery in oocytes expressing e1(Nt)/n1 (Fig. 5C). Na+ removal in the continued presence of HCO3− reversed the pHi recovery. Note that the pHi recovery was not accompanied by the hyperpolarization characteristic of electrogenic cotransport. Moreover, Na+ removal caused a substantial hyperpolarization, reflecting the background Na+ conductance of wild-type NBCn1-B.
Taken together, the data in this section demonstrate that the major non-transmembrane domains (i.e. cytoplasmic N and C termini and third extracellular loop) of NBCe1-A do not confer electrogenicity on chimeras in which the transmembrane domains come from NBCn1-B. Conversely, the transmembrane domains of NBCn1-B are the only components required for the background conductance.
Replacing either the front or back half of NBCe1-A with the homologous region of NBCn1-B abolishes electrogenic Transport
Front half n1
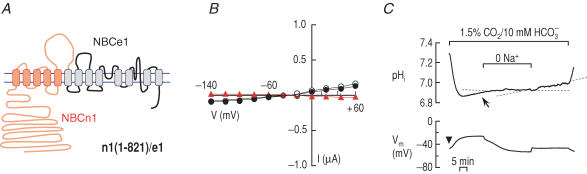
Our data described above show that the major non-transmembrane domains of NBCe1-A, despite their unique amino acid composition, are not necessary for, nor do they by themselves confer, electrogenicity of Na+–HCO3− cotransport. Thus, the better-conserved transmembrane domains must determine whether cotransport is electrogenic. Based on the work of others on AE1 (for review see Alper et al. 2001), it is reasonable to divide the transmembrane domains of NBCe1-A into two regions: TM1–5 and TM6–13. Separating the two regions is the third extracellular loop that, as demonstrated by the above data, has no significant role in electrogenicity of NBCe1-A or the background conductance of NBCn1-B. To examine the functional role of these two transmembrane regions, we made two final chimeras. The first, n1(1–821)/e1, had the composition of NBCn1-B from the N terminus to the end of the third extracellular loop (‘front half’), and the composition of NBCe1-A from TM6 to the C terminus (‘back half’, Fig. 7A). Voltage-clamp data for oocytes expressing this chimera (summarized in Table 2) show that GND96 was very low, suggesting that the chimera does not have the background conductance characteristic of NBCn1-B (Fig. 7B). Moreover, GHCO3 was slightly lower than GND96 and EHCO3 was not shifted to negative values, suggesting that the chimera does not have the electrogenic cotransport characteristic of NBCe1-A.
Figure 7. Replacing the front half (N-terminus, TM1–5 and loop) of NBCe1-A with the homologous parts of NBCn1-B.
A, chimeric transporter n1(1–821)/e1. B, (I–V) relationships obtained from oocytes expressing n1(1–821)/e1 in the absence (○) and presence (•) of HCO3−–CO2 (n = 5). C, representative recordings of pHi and Vm in an oocyte expressing n1(1–821)/e1. The number in parenthesis (1–821) represents amino acids of NBCn1-B. The pHi recovery from an acidification (arrow) without hyperpolarization (arrowhead) is shown. One of three experiments.
In the experiments on pHi (Fig. 7C), oocytes expressing n1(1–821)/e1 recovered from a CO2-induced acid load at about half the rate (4.8 ± 0.5 × 10−5 pH units s−1, n = 5) of those expressing the n1(Nt)/e1 chimera as shown in Fig. 3C (10.3 ± 3.6 × 10−5 pH units s−1, n = 4). The difference between these two values is statistically insignificant (P > 0.05) Immunocytochemistry (see Supplemental Material) indicates that the surface expression of the n1(1–821)/e1 chimera is less than that of the wild-type NBCe1-A. The slower pHi recovery of n1(1–821)/e1 (Fig. 7C) compared with that of n1(Nt)/e1 (Fig. 3C) could be due to lower surface expression or to lower activity of individual molecules. Because both n1(1–821)/e1 and n1(Nt)/e1 lack the Nt of NBCe1-A, the part of the molecule responsible for the relative slowness of n1(1–821)/e1 must be TM1–5 (i.e. a mismatch between the front and back halves of the chimeric cotransporter).
Nevertheless, oocytes expressing n1(1–821)/e1 clearly exhibited a more rapid pHi recovery than water-injected controls (−0.6 ± 0.5 × 10−5 pH units s−1, n = 5), and the difference is highly significant (P = 0.001, unpaired two-tailed t test). Thus, n1(1–821)/e1 engages in significant acid–base transport. Because even very slow pHi recovery rates produce large changes in Vm (Romero et al. 1997) (i.e. Vm is a very sensitive indicator of electrogenicity), we are in a position to judge the electrogenicity of the n1(1–821)/e1 chimera. However, application of CO2–HCO3− did not cause the large, abrupt hyperpolarization characteristic of NBCe1-A or even of the n1(Nt)/e1 chimera shown in Fig. 3C (change in Vm, −33.3 ± 2.8 mV, n = 4), but instead led to a slow, small depolarization (change in Vm, +14.7 ± 1.7 mV, n = 5) that we often observed in H2O-injected, control oocytes. Moreover, Na+ removal caused neither the large abrupt depolarization characteristic of NBCe1-A or even of the n1(Nt)/e1 chimera (+38.3 ± 10.0 mV, n = 4), nor the large hyperpolarization (reflecting the background Na+ conductance) of the n1(822–1218)/e1 chimera (−29.1 ± 4.9, n = 3), but only a small hyperpolarization (−6.4 ± 0.7, n = 5) similar to the observation in H2O-injected, control oocytes (−2.9 ± 1.6, n = 5). These data show that the chimera n1(1–821)/e1, although active in terms of pHi changes, has neither electrogenic cotransport nor a background Na+ conductance.
Back half n1
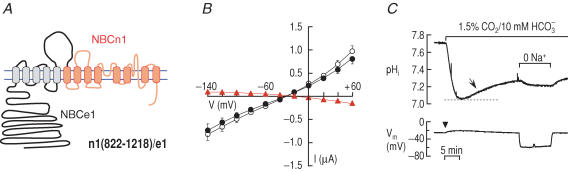
The absence of electrogenic cotransport in n1(1–821)/e1 shows that TM1–5 of NBCe1 is essential for electrogenicity. To test whether TM6–13 is also involved, we then made the reciprocal chimera n1(822–1218)/e1, which had the composition of NBCe1-A from the N terminus to the end of the third extracellular loop (‘front half’), and the composition of NBCn1-B from TM6 to the C terminus (‘back half’, Fig. 8A). Voltage-clamp data for oocytes expressing n1(822–1218)/e1 (summarized in Table 2) show that GND96 was relatively high, suggesting that the chimera has the background conductance characteristic of NBCn1-B (Fig. 8B). On the other hand, GHCO3 was slightly lower than GND96 and EHCO3 was not shifted towards more negative values, suggesting that this chimera lacks the electrogenic cotransport characteristic of NBCe1-A.
Figure 8. Replacing the second half (TM6-13 and C-terminus) of NBCe1-A with the homologous parts of NBCn1-B.
A, chimeric transporter n1(822–1218)/e1. B, I–V relationships obtained from oocytes expressing n1(822–1218)/e1 in the absence (○) and presence (•) of HCO3−–CO2 (n = 7). C, representative recordings of pHi and Vm. The pHi recovery from an acidification (arrow) without hyperpolarization (arrowhead) is shown. One of three experiments.
The chimera mediated a robust pHi recovery from a CO2-induced acid load, but without the abrupt hyperpolarization characteristic of NBCe1-A (Fig. 8C). The removal of Na+ eliminated the pHi recovery and evoked a large hyperpolarization. These observations confirm that the chimera is an electroneutral Na+–HCO3− cotransporter, complete with the background Na+ conductance.
In conclusion, our chimera studies indicate that the electrogenicity of NBCe1-A simultaneously requires both TM1–5 and TM6–13. On the other hand, the background Na+ conductance of NBCn1-B requires only TM6–13.
Discussion
Overview
In this study, we provide the first molecular details of the structural requirements for electrogenic transport of Na+–HCO3− cotransporters. By exchanging elements between NBCe1 and NBCn1, we determined that the relatively well-conserved transmembrane domains of NBCe1 are necessary and sufficient for electrogenic cotransport. That is, electrogenicity requires the simultaneous presence of TM1–5 and TM6–13 of NBCe1-A. However, electrogenicity is independent of the three, large, less-well conserved hydrophilic domains, namely, the cytoplasmic N terminus, the third extracellular loop and the cytoplasmic C terminus. We also found that the background Na+ conductance of NBCn1-B requires TM6–13 but not TM1–5 or any of the three large hydrophilic elements. These conclusions could be a valuable staring point for future studies aimed at determining the domains responsible for electrogenic versus electroneutral function as well as the background Na+ conductance of NBCn1-B.
Structural domains and motifs
Electrogenic transport
Among the chimeras we constructed, those containing, and only those containing, both TM1–5 and TM6–13 of NBCe1 were electrogenic. Breaking the interaction between TM1–5 and TM6–13 of NBCe1-A (i.e. pairing an electroneutral TM1–5 with an electrogenic TM6–13, or vice versa) abolishes electrogenicity and yet maintains cotransport. In other words, both TM1–5 and TM6–13 of NBCe1-A have the machinery necessary for performing electroneutral transport, which is perhaps the basal state of NBCe1.
Electrogenic cotransport requires an interaction between at least one motif in TM1–5 and at least one motif in TM6–13. The identity of these motifs remains unclear. Up to now, only a handful of studies have focused on structure–function relationships of NBCe1. Abuladze et al. (2005) recently mutated a large number of acidic and basic amino acids in NBCe1 – in putative transmembrane segments and intra- or extracellular loops – to oppositely charged and/or neutral amino acids. Examining the effects on the Na+-dependent recovery of pHi from an acid load in HEK-293T cells, they found that many mutations caused substantial decreases in acid-extrusion rate, some resulting in severe functional defects. Whereas the report by Abuladze et al. (2005) provides useful information on residues that are important for transport activity, it was not intended to address the electrogenicity of Na+–HCO3− transport and, indeed, the assays did not involve electrophysiological measurements. Moreover, the authors focused on amino acid residues that are conserved among members of the SLC4A family including Cl−–HCO3− exchangers and electroneutral Na+-coupled HCO3− transporters. Thus, it is unlikely that these conserved residues are critical for determining the electrogenicity of NBCe1.
Inferences from the AE subfamily
It is possible that some insight into the structural elements of Na+–HCO3− transporters may be inferred from the AE-type Cl−–HCO3− exchangers, which have been more thoroughly studied than the NBCs. Traditionally, AE was divided into two structurally and functionally distinct domains: the cytoplasmic N-terminal domain (43 kDa) that binds a variety of other proteins (Low, 1986) and the transmembrane domain (55 kDa) that mediates anion transport (Passow, 1986). Studies of AE1/AE2 chimeras reveal that the transmembrane domain determines the fundamental transport characteristics (i.e, Cl−–HCO3− exchange and chloride–nitrate exchange), whereas the cytoplasmic N-terminal domain modulates the transport (i.e. pH dependence) (Alper et al. 2001). The membrane domain works as a ‘sensor’, whereas the cytoplasmic N-terminus is a ‘modifier’. The study of AE2/AE3 chimeras supports this functional compartmentalization (Fujinaga et al. 2003). Muller-Berger et al. (1995b) have proposed that TM5, 8, 9, 10 and 13 of AE1 are functional segments for anion translocation. Several amino acids in these TMs have been proposed to serve as a proton coupling site (Jennings & Smith, 1992; Sekler et al. 1995), an anion selectivity filter (Zhu & Casey, 2004) or a pore-entry site (Muller-Berger et al. 1995a; Tang et al. 1998, 1999; Kuma et al. 2002; Jennings, 2005). These reports emphasized the structural importance of multiple amino acids in several TMs. It is possible that homologous amino acids in NBCs may also play similar roles in ion translocation (McAlear & Bevensee, 2006).
Natural NBCe1 mutations
At least eight naturally occurring mutations in human NBCe1 have been reported (Igarashi et al. 1999, 2002; Inatomi et al. 2004; Horita et al. 2005): Q29, R298S, S427L, R510H, T485S, A799V, R881C and a frameshift at codon 721. Q29 is a non-sense mutation, whereas the others, with the exception of the frameshift at 721, are missense. All of these mutations have no more than 50% of the wild-type activity, causing a severe proximal renal tubular acidosis with the arterial blood pH of below 7.10. Additional symptoms may include ocular abnormalities such as glaucoma, cataracts, band keratopathy and blindness. Many of these point mutations cause targeting defects of the transporters, whereas others appear to have normal function (Inatomi et al. 2004; Horita et al. 2005; Toye et al. 2006). Dinour et al. (2004) has recently reported that S427L mutation in TM1 alters voltage- and Na+-dependent HCO3− movement, resulting in approximately 10% of HCO3− currents compared to wild-type renal NBCe1-A. The change in voltage- and Na+-dependence in the S427L mutant may be due to the absence of the ‘voltage-sensing’ process of the transporter. Li et al. (2005) reported that S427L has an apical targeting process in polarized kidney cells. It is interesting to note that all electroneutral Na+–HCO3− transporters have an alanine residue at the site corresponding to S427 in NBCe1-A.
The Na+ conductance of NBCn1
Among the chimeras we constructed, only those containing TM6–13 of NBCn1 exhibited the Na+ conductance typical of NBCn1 (Choi et al. 2000; Cooper et al. 2005). Thus, only TM6–13 is required for the Na+ conductance. However, the identity of the critical motifs remains unclear.
Another electroneutral member of the SLC4 family, AE1, can under some circumstances exhibit an ion conductance. AE1 may be responsible for a small, DIDS-sensitive anion conductance observed in human erythrocytes (Knauf et al. 1977; Kaplan et al. 1983). Although normally electroneutral in its natural environment, trout AE1 (tAE1) responds to cell swelling by exhibiting an anion conductance (for review see Motais et al. 1997) that contributes to regulated volume decrease. When this same tAE1 is expressed in Xenopus oocytes, it exhibits a Cl− conductance even in the absence of cell swelling (Fievet et al. 1995). Studies with trout–mouse AE1 chimeras indicate that this anion conductance of tAE1 requires residues in the extracellular loop between TM5 and TM6, as well as residues in the remaining C-terminal portion of the molecule (i.e. the ‘back half’ of tAE1) (Fievet et al. 1995; Borgese et al. 2004). It is interesting to note that, when expressed in Xenopus oocytes, the human AE1 construct AE1Δ(6:7), which lacks transmembrane segments 6 and 7, also mediates a large anion conductance. In addition, oocytes expressing AE1Δ(6:7) exhibit a Na+ conductance (Parker et al. 2006). As suggested by Parker et al. (2006), the TM6 and 7 module in human AE1 may be necessary to ensure that transport is electroneutral.
Thus, as is the case for AE1, data on NBCn1 point to the ‘back half’ of the molecule as being critical for ion conductance.
Preservation of cotransport activity
Our study was faciitated by the strong homology between NBCe1 and NBCn1, which permitted us to construct functional chimeras. Improper folding often results in failure of membrane proteins to target to the plasma membrane and instead to accumulate in the endoplasmic reticulum or Golgi. The C-terminal domains of NBCe1 and NBCn1 play an essential role in targeting and sorting of the protein. Using a series of deletion mutants with the C-terminal end of NBCe1-A, Li et al. (2004) have identified the sequence QQPFLS (amino acid residues 1010–1015) which contributes to the targeting of NBCe1-A to the basolateral membrane of the kidney epithelial cells. Nonetheless, the deletion of this motif does not abolish the targeting of the transporter to the plasma membrane in Xenopus oocytes. It is thus likely that QQPFLS would play a role in membrane polarity of the transporter. Our sequence analysis shows no significant amino acid homology in this motif among the SLC4A family. For NBCn1-B, the PDZ motif at the C-terminal domain is more important for targeting to the membrane (Loiselle et al. 2003). Deletion of the PDZ motif of the human homologue inhibited the transporter from being targeted to the plasma membrane in HEK cells and instead caused an intracellular accumulation of the transporter (Loiselle et al. 2003; Li et al. 2004). The information on the targeting role of the N-terminal domain is very rare. Despite this, our success in producing functionally active chimeras may be due at least in part to the presence of the native cytoplasmic N- and C-terminal domains of NBCe1 and NBCn1 in our chimeras.
Limitations
Strictly speaking, our results pertain only to NBCe1-A and NBCn1-B expressed in Xenopus oocytes, where NBCe1-A has a stoichiometry for Na+:HCO3− of 1 : 2 (Heyer et al. 1999). It is impossible, from the present work, to draw conclusions about the structural requirements for the 1 : 3 stoichiometry that is believed to prevail in the renal proximal tubule.
Acknowledgments
The work was supported by NIH grant DK30344 (W.F.B.) and an NKF Young Investigator Grant (I.C.).
Supplementary material
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2006.114959 http://jp.physoc.org/cgi/content/full/jphysiol.2006.114959/DC1 and contains supplemental material.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Abuladze N, Azimov R, Newman D, Liu W, Tatishchev S, Pushkin A, Kurtz I. Critical amino acid residues involved in the electrogenic sodium bicarbonate cotransporter kNBC1-mediated transport. J Physiol. 2005;565:717–730. doi: 10.1113/jphysiol.2005.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper SL, Chernova MN, Stewart AK. Regulation of Na+-independent Cl−/HCO3 exchangers by pH. JOP. 2001;2:171–175. [PubMed] [Google Scholar]
- Bevensee MO, Boron WF, Apkon M. Electrogenic Na+/HCO3− cotransport in single hippocampal astrocytes from rat. FASEB J. 1995;9:A308. [Google Scholar]
- Borgese F, Renard C, Gabillat N, Pellissier B, Guizouarn H. Molecular mapping of the conductance activity linked to tAE1 expressed in Xenopus oocyte. Biochim Biophys Acta. 2004;1664:80–87. doi: 10.1016/j.bbamem.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Boron WF. Cellular buffering and intracellular pH. In: Seldin DW, Giebisch G, editors. The Regulation of Acid-Base Balance. New York: Raven Press; 1989. pp. 33–56. [Google Scholar]
- Boron WF, Boulpaep EL. The electrogenic Na/HCO3 cotransporter. Kidney Int. 1989;36:392–402. doi: 10.1038/ki.1989.208. [DOI] [PubMed] [Google Scholar]
- Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+: HCO3− cotransporter. J Biol Chem. 1997;272:19111–19114. doi: 10.1074/jbc.272.31.19111. [DOI] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC) Am J Physiol Cell Physiol. 1999;276:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Lee HJ, Yang HS, Kippen J, Yun CC, Choi I. The electroneutral sodium/bicarbonate cotransporter containing an amino terminal 123-amino-acid cassette is expressed predominantly in the heart. J Biomed Sci. 2006;13:593–595. doi: 10.1007/s11373-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem. 2005;280:17823–17830. doi: 10.1074/jbc.M408646200. [DOI] [PubMed] [Google Scholar]
- Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem. 2004;279:52238–52246. doi: 10.1074/jbc.M406591200. [DOI] [PubMed] [Google Scholar]
- Fievet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO J. 1995;14:5158–5169. doi: 10.1002/j.1460-2075.1995.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga J, Loiselle FB, Casey JR. Transport activity of chimaeric AE2-AE3 chloride/bicarbonate anion exchange proteins. Biochem J. 2003;371:687–696. doi: 10.1042/BJ20030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga J, Tang XB, Casey JR. Topology of the membrane domain of human erythrocyte anion exchange protein, AE1. J Biol Chem. 1999;274:6626–6633. doi: 10.1074/jbc.274.10.6626. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Papadopoulos MC, van Hooft JA, Xu L, Giuffrida R, Monyer H. The electrogenic sodium bicarbonate cotransporter: developmental expression in rat brain and possible role in acid vulnerability. J Neurosci. 2000;20:1001–1008. doi: 10.1523/JNEUROSCI.20-03-01001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem. 2001;276:8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- Heyer M, Muller-Berger S, Romero MF, Boron WF, Frömter E. Stoichiometry of the rat kidney Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes. Pflugers Arch. 1999;438:322–329. doi: 10.1007/s004240050916. [DOI] [PubMed] [Google Scholar]
- Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, et al. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol. 2005;16:2270–2278. doi: 10.1681/ASN.2004080667. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, et al. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Sekine T, Inatomi J, Seki G. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol. 2002;13:2171–2177. doi: 10.1097/01.asn.0000025281.70901.30. [DOI] [PubMed] [Google Scholar]
- Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, et al. Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch. 2004;448:438–444. doi: 10.1007/s00424-004-1278-1. [DOI] [PubMed] [Google Scholar]
- Jennings ML. Evidence for a second binding/transport site for chloride in erythrocyte anion transporter AE1 modified at glutamate 681. Biophys J. 2005;88:2681–2691. doi: 10.1529/biophysj.104.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML, Smith JS. Anion-proton cotransport through the human red blood cell band 3 protein. J Biol Chem. 1992;267:13964–13971. [PubMed] [Google Scholar]
- Kaplan JH, Pring M, Passow H. Band-3 protein-mediated anion conductance of the red cell membrane. Slippage vs ionic diffusion. FEBS Lett. 1983;156:175–179. doi: 10.1016/0014-5793(83)80272-5. [DOI] [PubMed] [Google Scholar]
- Knauf PA, Fuhrmann GF, Rothstein S, Rothstein A. The relationship between anion exchange and net anion flow across the human red blood cell membrane. J Gen Physiol. 1977;69:363–386. doi: 10.1085/jgp.69.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma H, Shinde AA, Howren TR, Jennings ML. Topology of the anion exchange protein AE1: the controversial sidedness of lysine 743. Biochemistry. 2002;41:3380–3388. doi: 10.1021/bi015879p. [DOI] [PubMed] [Google Scholar]
- Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L, Soleimani M. Missense mutations in Na+: HCO3− cotransporter NBC1 show abnormal trafficking in polarized kidney cells: a basis of proximal renal tubular acidosis. Am J Physiol Renal Physiol. 2005;289:F61–F71. doi: 10.1152/ajprenal.00032.2005. [DOI] [PubMed] [Google Scholar]
- Li HC, Worrell RT, Matthews JB, Husseinzadeh H, Neumeier L, Petrovic S, Conforti L, Soleimani M. Identification of a carboxyl-terminal motif essential for the targeting of Na+-HCO3− cotransporter NBC1 to the basolateral membrane. J Biol Chem. 2004;279:43190–43197. doi: 10.1074/jbc.M405780200. [DOI] [PubMed] [Google Scholar]
- Loiselle FB, Jaschke P, Casey JR. Structural and functional characterization of the human NBC3 sodium/bicarbonate co-transporter carboxyl-terminal cytoplasmic domain. Mol Membr Biol. 2003;20:307–317. doi: 10.1080/0968768031000122520. [DOI] [PubMed] [Google Scholar]
- Low PS. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane–peripheral protein interactions. Biochim Biophys Acta. 1986;864:145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem. 2006;281:19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- McAlear SD, Bevensee MO. A cysteine-scanning mutagenesis study of transmembrane domain 8 of the electrogenic Na/bicarbonate cotransporter NBCe1. J Biol Chem. 2006;281:32417–27. doi: 10.1074/jbc.M607253200. [DOI] [PubMed] [Google Scholar]
- McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol. 2006;127:639–658. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na+-HCO3− cotransporter in human pancreas. Am J Physiol Gastrointest Liver Physiol. 1999;277:G487–G494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- Motais R, Fievet B, Borgese F, Garcia-Romeu F. Association of the band 3 protein with a volume-activated, anion and amino acid channel: a molecular approach. J Exp Biol. 1997;200:361–367. doi: 10.1242/jeb.200.2.361. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Karbach D, Kang D, Aranibar N, Wood PG, Ruterjans H, Passow H. Roles of histidine 752 and glutamate 699 in the pH dependence of mouse band 3 protein-mediated anion transport. Biochemistry. 1995a;34:9325–9332. doi: 10.1021/bi00029a007. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Karbach D, Konig J, Lepke S, Wood PG, Appelhans H, Passow H. Inhibition of mouse erythroid band 3-mediated chloride transport by site-directed mutagenesis of histidine residues and its reversal by second site mutation of Lys 558, the locus of covalent H2DIDS binding. Biochemistry. 1995b;34:9315–9324. doi: 10.1021/bi00029a006. [DOI] [PubMed] [Google Scholar]
- Parker MD, Young MT, Daly CM, Meech RW, Boron WF, Tanner MJA. A conductive pathway generated from fragments of the human red cell anion exchanger, AE1. J Physiol. 2006 doi: 10.1113/jphysiol.2007.128389. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passow H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol. 1986;103:61–223. doi: 10.1007/3540153330_2. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol. 1998;274:F425–F432. doi: 10.1152/ajprenal.1998.274.2.F425. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflugers Arch. 2004;447:495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4: a new electrogenic sodium-bicarbonate cotransporter. Am J Physiol Cell Physiol. 2002;282:C408–C416. doi: 10.1152/ajpcell.00409.2001. [DOI] [PubMed] [Google Scholar]
- Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+-HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol. 1999;277:F611–F623. doi: 10.1152/ajprenal.1999.277.4.F611. [DOI] [PubMed] [Google Scholar]
- Sekler I, Lo RS, Kopito RR. A conserved glutamate is responsible for ion selectivity and pH dependence of the mammalian anion exchangers AE1 and AE2. J Biol Chem. 1995;270:28751–28758. doi: 10.1074/jbc.270.48.28751. [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter (pNBC) Proc Natl Acad Sci U S A. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebens AW, Boron WF. Effect of electroneutral luminal and basolateral lactate transport on intracellular pH in salamander proximal tubules. J Gen Physiol. 1987;90:799–831. doi: 10.1085/jgp.90.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Grassl SM, Aronson PS. Stoichiometry of Na+-HCO3− cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987;79:1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XB, Fujinaga J, Kopito R, Casey JR. Topology of the region surrounding Glu681 of human AE1 protein, the erythrocyte anion exchanger. J Biol Chem. 1998;273:22545–22553. doi: 10.1074/jbc.273.35.22545. [DOI] [PubMed] [Google Scholar]
- Tang XB, Kovacs M, Sterling D, Casey JR. Identification of residues lining the translocation pore of human AE1, plasma membrane anion exchange protein. J Biol Chem. 1999;274:3557–3564. doi: 10.1074/jbc.274.6.3557. [DOI] [PubMed] [Google Scholar]
- Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol. 2006;291:C788–C801. doi: 10.1152/ajpcell.00094.2006. [DOI] [PubMed] [Google Scholar]
- Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO cotransporter (NBCe2) Am J Physiol Cell Physiol. 2002;282:C1278–C1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl−/HCO3− exchanger: cloning, tissue distribution, and functional characterization. J Biol Chem. 2000;275:35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Casey JR. The substrate anion selectivity filter in the human erythrocyte Cl−/HCO3− exchange protein, AE1. J Biol Chem. 2004;279:23565–23573. doi: 10.1074/jbc.M401380200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.