Abstract
Cholecystokinin-positive (CCK+) basket cells are a major source of perisomatic GABAergic inputs to CA1 pyramidal cells. These interneurons express high levels of presynaptic cannabinoid type 1 (CB1) receptors that mediate short-term depression of GABA release following depolarization of postsynaptic cells. However, it is not known whether GABA release from CA1 CCK+ basket cells is under tonic endocannabinoid inhibition. In paired patch-clamp recordings, action potentials in presynaptic CCK+ basket cells evoked large IPSCs with fast kinetics in pyramidal cells. The proportion of action potentials that failed to evoke GABA release varied markedly between pairs, from highly reliable to virtually silent connections. Application of the CB1 receptor antagonist AM251 (10 μm) decreased the proportion of failures, revealing a persistent suppression of synaptic transmission by CB1 receptors. However, AM251 had no significant effect on the failure rate when the calcium chelator BAPTA (10 mm) was introduced into the postsynaptic cell, indicating that the tonic inhibition of GABA release by CB1 receptors is homosynaptically controlled by the postsynaptic cell, and that it is not due to constitutive CB1 receptor activity. Application of muscarinic or metabotropic glutamate receptor agonists inhibited synaptic transmission exclusively through the release of endocannabinoids from postsynaptic cells in a manner that could not be blocked by postsynaptic BAPTA, and had no direct effect on transmission. In contrast, GABAB receptor activation directly blocked GABA release, but there was no evidence for tonic inhibition of GABA release by GABAB receptors. Neither serotonergic nor μ-opioid agonists had significant influence on GABA release from CCK+ axon terminals. These results reveal that GABA release from CA1 CCK+ basket cells is under homosynaptic tonic inhibition by endocannabinoids, and it is subject to both direct and indirect modulation by various G-protein-dependent neuromodulators.
Cannabinoids are known to be powerful modulators of inhibition in neocortical and hippocampal networks. In particular, depolarization in postsynaptic cells results in a CB1 cannabinoid receptor-dependent, short-term inhibition of GABA release from cholecystokinin-positive (CCK+) interneurons, including basket cells, through a process known as depolarization-induced suppression of inhibition (DSI) (Llano et al. 1991; Pitler & Alger, 1992; Kreitzer & Regehr, 2001; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001). While considerable attention has focused on DSI-related, phasic cannabinoid actions on GABA release, less is known about how CB1 receptors may regulate GABAergic transmission in a tonic manner. However, there are interesting clues from several neuronal systems that indicate that a major function of CB1 receptors may be the tonic regulation of GABA release. For example, a recent study demonstrated that hypothalamic proopiomelanocortin neurons release endocannabinoids continuously under basal conditions, and that the released endocannabinoids significantly depress GABA release onto these cells (Hentges et al. 2005). There are also indications that such processes may be at play in the hippocampus as well, as a subpopulation of CCK+ interneurons in the stratum lucidum in the CA3 region was found to be tonically silenced by presynaptic CB1 cannabinoid receptors (Losonczy et al. 2004). However, it was not clear if tonic, CB1 receptor-dependent inhibition of GABA release in the hippocampus was a unique property of these ‘muted’ stratum lucidum interneuronal subpopulations, or whether other CCK+ interneurons outside the CA3 stratum lucidum were also under tonic control by CB1 receptors.
The mechanism of tonic CB1 receptor-dependent inhibition of GABA release in hippocampal circuits is also not known. GABA release may be tonically depressed because of the constitutive activity of the CB1 receptors that is thought to occur even in the absence of endocannabinoid ligands (Bouaboula et al. 1997; Pan et al. 1998; Vasquez & Lewis, 1999; Guo & Ikeda, 2004; Pertwee, 2005). In principle, such constitutive activity may be revealed using compounds that act as inverse agonists at the CB1 receptor. Alternatively, postsynaptic neurons may continuously release endocannabinoids resulting in a CB1 receptor-mediated basal depression of GABA release. The latter mechanism was shown to underlie the tonic inhibition of GABA release by CB1 receptors in the case of the hypothalamic proopiomelanocortin neurons (Hentges et al. 2005), as well as in the basolateral amygdala (Zhu & Lovinger, 2005), by demonstrating that CB1 receptor antagonists/inverse agonists do not enhance GABA release when the postsynaptic cells were filled with high concentrations of the calcium chelator BAPTA.
A better understanding of the tonic control of GABA release is also important in light of reports that several major neurotransmitter systems, including muscarinic acetylcholine and metabotropic glutamate receptors, exert their effects on GABA release from CCK+ cells indirectly, through the modulation of the synthesis and release of endocannabinoids from the postsynaptic pyramidal cells (Varma et al. 2001; Kim et al. 2002). For example, whereas low concentrations (<0.5 μm) of the muscarinic agonist carbachol enhance DSI without altering basal, stimulation-evoked IPSCs, higher concentrations of carbachol (≥1 μm) persistently depress evoked IPSCs mainly through the mobilization of endocannabinoids (Kim et al. 2002). However, because these studies were carried out using compound, electrical stimulation-evoked IPSCs that originate from a mixture of CB1 receptor-expressing and CB1 receptor-lacking fibres, it has not been unequivocally established whether either muscarinic or metabotropic glutamate receptors can directly modulate GABA release from CCK+ basket cells. For example, application of carbachol at 25 μm resulted in a significant depression of evoked IPSCs even in the presence of the CB1 receptor antagonist AM251 or in CB1 receptor knockout mice (Kim et al. 2002), but it is unclear whether this inhibition acts directly on presynaptic muscarinic receptors on the CCK+ basket cell terminals or only on the other components of the multifibre-evoked response. This issue is particularly relevant because parvalbumin-positive (PV+) terminals express muscarinic receptors (Hajos et al. 1998; Freund, 2003; Seeger et al. 2004).
In order to gain a better insight into the existence and mechanism of tonic CB1 receptor-mediated control of GABA release, we have performed paired, whole-cell patch-clamp recordings from identified CCK+ basket cells and postsynaptic pyramidal cells in the CA1 of the rat hippocampus. The results reveal a widespread, but highly variable influence of tonic, homosynaptic, CB1 receptor-dependent modulation of GABA release in these connections, and identify the postsynaptic release of endocannabinoid ligands as the main cause for the endocannabinoid tone. In addition, the data show that transmitter release at these connections is directly modulated by CB1 and GABAB receptors, but not by opioid, serotonergic, muscarinic or metabotropic glutamate receptors.
Methods
All protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Electrophysiology
Sprague-Dawley rats (16 to 20 days old) were decapitated under halothane anaesthesia. The brains were transferred rapidly to an ice-cold, sucrose-containing artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 85, NaHCO3 24, NaHPO4 1.25, CaCl2 0.5, MgCl2 4, KCl 2.5, sucrose 75 and glucose 25, and transverse hippocampal slices (350 μm) were cut using a 3000 Plus vibratome (Vibratome, St Louis, MO, USA). Slices were stored in sucrose-containing ACSF for at least 30 min at 33°C, and then transferred to a chamber continuously perfused with standard ACSF containing (mm): NaCl 126, NaHCO3 24, NaHPO4 1.25, CaCl2 2, MgCl2 2, KCl 2.5 and glucose 10 at 33°C for electrophysiological recordings.
Cells were visualized with an upright microscope (BX-50, Olympus, Tokyo, Japan) with differential interference contrast optics. Paired whole-cell patch-clamp recordings were obtained using MultiClamp 700A and 700B amplifiers and Clampfit software (Molecular Devices, Union City, CA, USA). Recordings were filtered at 4 kHz using a Bessel filter and digitized at 10 kHz with a Digidata 1320A analog–digital interface (Molecular Devices).
Recordings were obtained from 115 connected pairs between identified CCK+ basket cells and pyramidal cells. To obtain connected pairs, we first identified putative CCK+ basket cells by their typical electrophysiological phenotype (see Fig. 1) and then subsequently tested for connected partners in up to 10 pyramidal cells. The apparent connectivity observed in our experiments was relatively low (<10%). Presynaptic interneurons were held in current-clamp mode at −65 mV, and trains of 100 action potentials were elicited by brief current injections (3 ms). As previously described in detail (Földy et al. 2006), 10 Hz presynaptic stimulation frequency resulted in stable responses over time and also allowed us to obtain a sufficiently large number of events for reliable analysis. Depolarizing (carbachol, 3,5-dihydroxyphenylglycine (DHPG) and serotonin) or hyperpolarizing (baclofen) drug actions on the presynaptic cells were compensated by current injections. The intracellular solution for the presynaptic interneurons contained (mm): potassium gluconate 126, KCl 4, Hepes 10, MgATP 4, Na2GTP 0.3 and phosphocreatine 10, and 0.2% biocytin; pH was adjusted to 7.25 with KOH (270–290 mosmol l−1). The postsynaptic pyramidal neurons were held in voltage clamp at −70 mV, and recordings were discarded if the series resistance changed more than 15% during the recordings or exceeded 20 MΩ. The intracellular solution for postsynaptic cells contained (mm): potassium gluconate 90, CsCl 40, KCl 3.5, NaCl 1.8, MgCl2 1.7, Hepes 10, EGTA 0.1, MgATP 2, Na2GTP 0.4 and phosphocreatine 10, pH was adjusted to 7.25 with KOH (270–290 mosmol l−1).
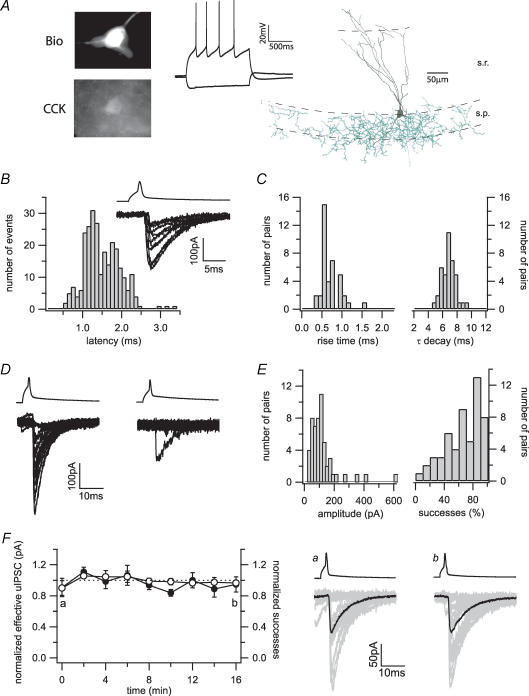
Figure 1. Basic properties of the synaptic transmission at the CCK+ interneuron to pyramidal cell synapse.
A, current-clamp recordings of a stratum radiatum interneuron following current injections of −80 and 120 pA from the resting membrane potential. Post hoc processing of the biocytin-filled cell (Bio) revealed immunoreactivity for CCK. The camera lucida drawing showed dendrites (black) mainly extending in the stratum radiatum (s.r.) towards the stratum lacunosum moleculare, and a basket cell-like dense axonal arborization (green) in the stratum pyramidale (s.p.). B, action potentials in a representative presynaptic CCK+ interneuron (upper trace) evoke uIPSCs with highly variable latencies in the postsynaptic pyramidal cell (bottom trace, 10 successful trials are shown). The histogram shows the distribution of synaptic latencies in 18 pairs (bin width, 0.1 ms). C, distribution of the average 10–90% rise time (bin width, 0.1 ms) and the decay time constant (τ) (bin width, 0.5 ms). D, 20 representative uIPSCs recorded from a low failure (left) and a high failure connection (right) in different pairs. E, the distributions of the average amplitudes clustered around 100 pA (bin width, 20 pA). In contrast, the distribution of the successes showed a continuum from highly reliable to highly unreliable transmissions (bin width, 10%). F, recordings from pairs over 16 min demonstrate the stability of the CCK+ interneuron to pyramidal cell synaptic transmission over time. The left plot shows averages of normalized effective uIPSC amplitudes (•) and success rates (○) of five cells over time. The right panels show 20 individual uIPSCs (grey) and averaged traces (black) taken from one pair at the indicated time points (a and b).
Clampfit 9.0 (Molecular Devices) and Excel (Microsoft) were used for data analysis and statistics. Values for average unitary IPSC (uIPSC) amplitudes do not include failures, whereas values for the so-called ‘effective’ uIPSC amplitudes include both successful events and failures. Successes were defined as events with amplitudes of at least twice the noise level that occurred in a time window between 0.5 and 3.5 ms after the peak of the action potential. To quantify drug effects, two series of 100 trials each were averaged immediately before drug application and at maximal block (when present) or 6–10 min after the start of drug application, respectively. Spontaneous synaptic input was analysed using Mini Analysis (Synaptosoft, Ducate, GA, USA). Data are given as means ± s.e.m. Statistical significance was assessed with Student's paired t tests.
Due to the difficulty of obtaining recordings from connected pairs, drugs were applied at relatively high concentrations that resulted in maximal but specific responses. AM251, DHPG, baclofen, DAMGO, (Tyr-D-Ala-Gly-NMe-Phe-Gly-Ol) naloxone hydrochloride, CGP55845, LY341495 and D-2-Amino-5-phosphonovaleric acid (D-APV) (all from Tocris) were dissolved according to the manufacturer's instructions, in 1 : 1000 stocks and stored at −20°C. Carbachol (Sigma) was dissolved in distilled water on the day of the experiment. Atropine and serotonin-creatine sulphate complex (Sigma) were dissolved in ethanol and 0.1 N HCl, respectively, stored at 4°C and used within days.
Immunohistochemistry
After termination of the recordings, slices were incubated in a fixative solution containing 4% paraformaldehyde and 0.2% picric acid in 0.1 m phosphate buffer for at least 24 h, and then resectioned into 50 μm thick sections. Immunoreactivity for CCK was revealed with a mouse monoclonal antibody (mAb 9303; generously provided by the Antibody/Radioimmunoassay Core of the Centre for Ulcer Research and Education (CURE)/Digestive Diseases Research Center, University of California, Los Angeles; National Institutes of Health Grant DK41301; diluted 1 : 1000). The reactions were visualized with a goat anti-mouse IgG conjugated to Alexa 594 for CCK (diluted 1 : 500 in Tris-buffered saline containing 2% normal goat serum; Invitrogen, Eugene, OR, USA) and streptavidin conjugated to Alexa 350 for biocytin (diluted 1 : 500). The sections were then mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and analysed with a fluorescent microscope. The staining for CCK with this antibody was specific as shown by (1) the lack of staining of parvalbumin positive processes (Földy et al. 2006), (2) the lack of staining of the so-called giant cells that reside in the stratum radiatum (not shown) and (3) controls carried out by Hefft & Jonas (2005). To reveal the axonal and dendritic arbors of the presynaptic basket cell in detail, the biocytin-filled cells were subsequently visualized with 3,3-diaminobenzidine tetrahydrochloride (0.015%) using a standard ABC kit (Vector Laboratories).
Results
Identification of CCK+ basket cells in the stratum radiatum of the CA1 area
To directly examine the transmission between CCK+ basket cells and pyramidal cells in the hippocampal CA1 region, patch-clamp recordings were obtained from neurons in the stratum radiatum close to the pyramidal layer, and expression of the marker protein CCK was assessed post hoc after fixation of the slices. Cells that were positive for CCK had a multipolar shape and a distinct electrophysiological phenotype. They had resting membrane potentials around −65 mV (−64.4 ± 0.8 mV, n = 15), high input resistances (206 ± 11 MΩ, n = 15) and broad action potentials (1.05 ± 0.04 ms, n = 15; Fig. 1A). During short spike trains, they showed considerable spike-frequency adaptation (Fig. 1A). Morphological analysis revealed axonal arborizations within or close to the pyramidal layer, defining these cells as basket cells (Fig. 1A) (Földy et al. 2006). These morphological and physiological criteria allowed us to obtain recordings from CCK+ interneurons in the stratum radiatum, and subsequently search for connected postsynaptic pyramidal neurons.
Basic properties of the CCK+ basket cell to pyramidal cell synapse
Action potentials in presynaptic CCK+ interneurons evoked inhibitory inputs in postsynaptic CA1 pyramidal cells with a mean synaptic latency of 1.46 ms (n = 18). In agreement with a previous report on CCK+ interneuron to granule cell connections in the dentate gyrus that identified a particularly loose coupling of calcium influx and transmitter release (Hefft & Jonas, 2005), there was a broad jitter in the synaptic latency even in the same pair with a standard deviation of 0.31 ± 0.09 ms (average of standard deviation of synaptic latency in n = 18 pairs ± s.e.m.) (Fig. 1B). As expected from a perisomatic input, the kinetics of both onset (10–90% rise time, 0.73 ± 0.05 ms, n = 40) and decay (τ decay, 6.8 ± 0.2 ms, n = 40) were rapid (Fig. 1C). Average uIPSC amplitudes were 118 ± 13 pA (n = 54), but a few average amplitudes exceeded 300 pA (Fig. 1D and E). There was a surprisingly broad distribution of the relative proportion of successful transmissions, from very reliable synaptic connections with almost no failures to apparently ‘muted’ pairs that displayed only extremely rare successes (Fig. 1D and E). The mean overall proportion of successeses was lower (0.65 ± 0.03, n = 54) than reported values in similar paired connections in the dentate gyrus (Hefft & Jonas, 2005). The synaptic transmission itself was stable over time, excluding a simple trial-to-trial scatter as cause for the variability of the transmission at the CCK+ interneuron to pyramidal cell synapse (Fig. 1F).
Postsynaptic origin of tonic inhibition of CCK+ terminals by CB1 receptors
As shown in Fig. 1E, the relative proportion of failures in a few identified CCK+ pairs was surprisingly high (Hefft & Jonas, 2005). These connections resembled the tonically ‘muted’ CCK+ interneuron to pyramidal cell connections described in the stratum lucidum layer in the CA3 area (Losonczy et al. 2004). Transmitter release from these virtually silent CCK+ interneurons in the CA3 had been shown to be inhibited by persistently active CB1 receptors. To test whether the same mechanism accounts for the extremely high failure rate in some of the CCK+ interneuron to pyramidal cell pairs in the CA1 region as well, we applied the CB1 receptor antagonist/inverse agonist AM251 (10 μm) onto one of these pairs. Indeed, application of AM251 transformed the connection into a highly reliable synapse (Fig. 2A). Such virtually silent CA1 connections that could be turned into a reliable synapse with AM251 have also been mentioned by Glickfeld & Scanziani (2006), indicating that their existence is not due to experimental methods that may be peculiar to specific laboratories.
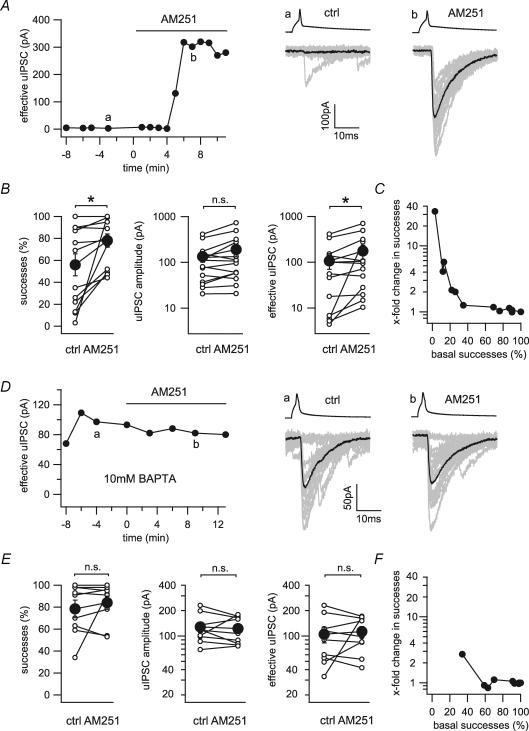
Figure 2. Tonic suppression of transmission at the CCK+ interneuron to pyramidal cell synapse.
A, time series of the effective uIPSC of a connected pair with a low basal success rate before and during the application of the CB1 antagonist AM251 (10 μm). The right panels show 20 representative uIPSCs (grey) and their averages (black) evoked by presynaptic action potentials (upper traces) taken from the time points indicated in the time series (a and b). B, line series of success rates, uIPSC amplitudes and effective uIPSC amplitudes before and during the application of AM251 for single connected pairs (○) and averages of 13 pairs (•). C, changes in success rate induced by application of AM251 plotted against the basal success rate. D, time series of the effective uIPSC of a connected pair under high intracellular calcium-buffering conditions (10 mm BAPTA) before and during the application of AM251. The right panels show 20 representative uIPSCs (grey) and their averages (black) taken from the time points indicated in the time series (a and b). E, line series of success rates, uIPSC amplitudes and effective uIPSC amplitudes before and during the application of AM251 under high calcium-buffering conditions for single connected pairs (○) and averages of nine pairs (•). F, changes in success rate induced by application of AM251 plotted against the basal success rate under high calcium-buffering conditions (10 mm BAPTA). *Statistical significance (P < 0.05); n.s., not significant.
We wondered whether basal CB1 receptor activity might contribute to the generally high failure rates at CCK+ interneuron to pyramidal cell synapses. Indeed, application of AM251 in 13 pairs significantly increased the proportion of successes (55.6 ± 10.4% to 77.5 ± 6.2%) and significantly increased the effective uIPSC amplitude (106.9 ± 36.5 to 176.3 ± 59.5 pA) (Fig. 2B). The effect of AM251 on the rate of successes was most prominent on connections with low basal (i.e. before AM251) successes (Fig. 2C). The synaptic latency, however, remained unaffected by the application of AM251 (1.53 ± 0.15 to 1.49 ± 0.17 ms).
The persistent activation of CB1 receptors on the CCK+ terminals might be explained by constitutive activity of the CB1 receptors in the absence of the agonist, or by elevated background levels of endocannabinoids. In order to differentiate between these two possibilities, baseline endocannabinoid mobilization from the postsynaptic pyramidal cell was depressed using intracellular calcium chelators (Hentges et al. 2005; Zhu & Lovinger, 2005). Specifically, we increased the calcium-buffering capacity in the postsynaptic neuron by adding 10 mm BAPTA to the intracellular solution. Increased calcium-buffering capacity abolished the facilitating effect of AM251 on both success rates (78.4 ± 7.7% to 84.1 ± 6.2%, n = 9) and effective uIPSC amplitudes (104.9 ± 22.5 to 112.7 ± 17.0 pA, n = 9; Fig. 2D and E).
Taken together, these data indicate that silenced CCK+ basket cell to pyramidal cell connections are not exclusive to subpopulations of CA3 stratum lucidum interneurons, but that they exist in the CA1 region as well. Furthermore, these data suggest that tonic endocannabinoid mobilization from postsynaptic cells contributes to the baseline suppression of uIPSCs. Finally, the fact that tonic CB1 receptor activation could be manipulated by calcium buffering in single postsynaptic cells indicates a homosynaptic mechanism that displays a surprisingly high degree of specificity.
Regulation of endocannabinoid release by metabotropic glutamate and muscarinic receptors
Previous reports demonstrated that activation of both the type 1 metabotropic glutamate receptor and muscarinic receptor can stimulate endocannabinoid release from CA1 pyramidal cells, and thereby indirectly reduce presynaptic GABA release (Maejima et al. 2001; Kim et al. 2002; Fukudome et al. 2004). However, it is not clear whether type 1 metabotropic glutamate receptors or muscarinic receptors directly act on presynaptic terminals of CCK+ basket cells, and whether they are involved in the control of tonic endocannabinoid release.
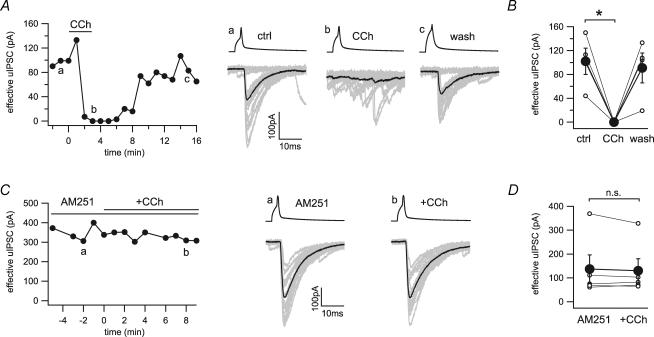
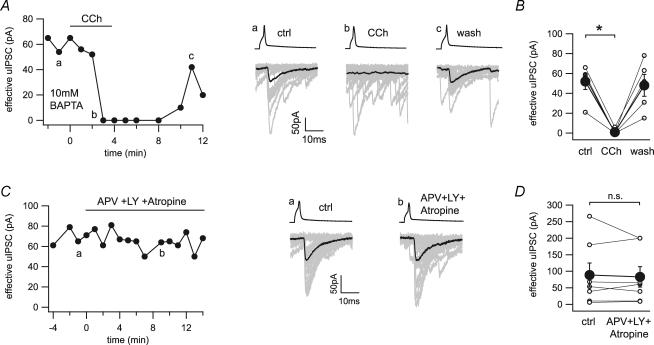
We applied agonists for type 1 metabotropic glutamate receptors and muscarinic receptors on connected pairs of CCK+ interneurons and pyramidal cells to directly examine their effects at these synapses. Application of the muscarinic agonist carbachol (25 μm) completely blocked the GABAergic transmission in a reversible manner (101.7 ± 22.0 to 0 pA, n = 4; Fig. 3A and B). Pre-application of the CB1 receptor antagonist AM251 (10 μm) fully blocked the effect of carbachol on uIPSCs (136.5 ± 58.8 to 129.6 ± 50.0 pA, n = 5; Fig. 3C and D). These data confirmed the previously reported indirect inhibition of GABA release by carbachol via the mobilization of endocannabinoids (Kim et al. 2002; Fukudome et al. 2004), and demonstrated the absence of functional muscarinic receptors on CCK+ basket cell terminals.
Figure 3. Indirect inhibition of GABA release by the cholinergic agonist carbachol at the CCK+ interneuron to pyramidal cell synapse.
A, time series and example traces of uIPSCs during application of carbachol (CCh, 25 μm) show a reversible block of synaptic transmission. Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a, b and c). B, line series of single pairs (○) and averages of four pairs (•) before and during application of carbachol. C, time series and example traces of uIPSCs during application of carbachol in the continuous presence of AM251 (10 μm). Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a and b). D, line series of single pairs (○) and averages of five pairs (•) before and during application of carbachol in the presence of AM251. *Statistical significance (P < 0.05); n.s., not significant.
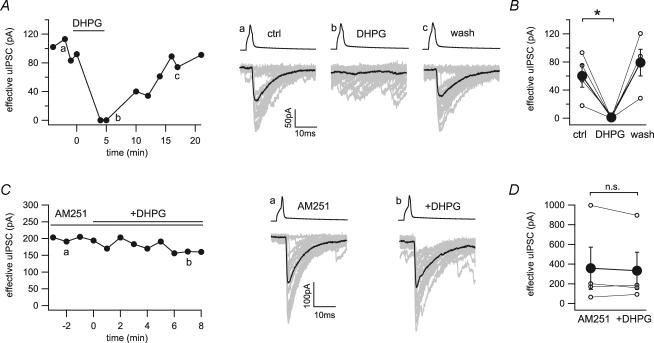
A similar effect could be observed with the type 1 metabotropic glutamate receptors agonist DHPG. Application of DHPG (25 μm) resulted in a suppression of uIPSCs that was fully reversible after washout (60.0 ± 16.2 to 1.1 ± 1.1 pA, n = 4; Fig. 4A and B). Again, preincubation of the slices with AM251 blocked the inhibitory effect of DHPG on the CCK+ basket cell to pyramidal cell transmission (357.7 ± 214.8 to 332.6 ± 188.5 pA, n = 4; Fig. 4C and D), excluding a direct effect of type 1 metabotropic glutamate receptors activation on the CCK+ presynaptic terminals. Note that during these experiments, AM251 did not significantly alter the increase in spontaneous synaptic input induced by either carbachol (control, 10.8 ± 1.9 to 53.0 ± 12.0 Hz; AM251, 6.1 ± 0.8 to 42.9 ± 7.8 Hz; P = 0.15) or DHPG (control, 5.3 ± 1.8 to 45.2 ± 11.0 Hz; AM251, 10.9 ± 5.5 to 53.9 ± 12.6 Hz; P = 0.60).
Figure 4. Indirect inhibition of GABA release by the metabotropic glutamate receptor agonist DHPG at the CCK+ interneuron to pyramidal cell synapse.
A, time series and example traces of uIPSCs during application of DHPG (25 μm) show a reversible block of synaptic transmission. Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a, b and c). B, line series of single pairs (○) and averages of four pairs (•) before and during application of DHPG. C, time series and example traces of uIPSCs during application of DHPG in the continuous presence of AM251 (10 μm). Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a and b). D, line series of single pairs (○) and averages of four pairs (•) before and during application of DHPG in the presence of AM251. *Statistical significance (P < 0.05); n.s., not significant.
We have shown that tonic inhibition of GABA release is homosynaptic and can be reduced by increasing the postsynaptic calcium-buffering capacity. We wondered whether the effect of endocannabinoid release driven by metabotropic receptor activation is blockable by postsynaptic calcium chelation. We included 10 mm BAPTA in the postsynaptic solution and tested the effect of carbachol (25 μm) on the synaptic transmission between CCK+ basket cells and pyramidal cells. As observed in pairs under low calcium-buffering conditions, carbachol completely blocked synaptic transmission in a reversible manner (51.8 ± 7.9 to 1.2 ± 1.2 pA, n = 5; Fig. 5A and B).
Figure 5. Effects of metabotropic receptor stimulation in the presence of BAPTA, and lack of effects of blockers of metabotropic receptors on synaptic transmission.
A, time series and example traces of uIPSCs during application of carbachol (CCh, 25 μm) show a reversible block of synaptic transmission even when the postsynaptic intracellular solution contained a high concentration of the calcium chelator BAPTA (10 mm). Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a, b and c). B, line series of single pairs (○) and averages of five pairs (•) before and during application of carbachol. C, time series and example traces of uIPSCs during application of D-APV (APV, 20 μm) + LY 341495 (LY, 100 μm) + atropine (2 μm). Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a and b). D, line series of single pairs (○) and averages of six pairs (•) before and during application of APV + LY + atropine. *Statistical significance (P < 0.05); n.s., not significant.
Next, we tested whether low background levels of acetylcholine and glutamate stimulate endocannabinoid release and therefore induce homosynaptic inhibition of GABA release. To address this issue, we applied antagonists for muscarinic (atropine, 2 μm) and metabotropic glutamate receptors (LY341495, 100 μm) on connected pairs. In addition, we blocked calcium influx through NMDA receptors by applying D-APV (20 μm). There was no effect on the synaptic transmission in seven pairs tested (effective uIPSC, 89.0 ± 36.8 to 83.4 ± 31.2 pA; Fig. 5C and D). These data indicate that the basal endocannabinoid release is neither due to tonic activation of metabotropic receptors nor to calcium influx through NMDA receptors.
Direct action of GABAB receptor activation
As both metabotropic glutamate and muscarinic receptor stimulation caused only indirect effects on GABA release from CCK+ basket cell to pyramidal cell connections, we sought to identify G-protein-coupled receptors that may exert their actions in a direct manner, without the involvement of CB1 receptors. GABAB receptors have been shown to control GABA release at many, but not all, perisomatic GABAergic terminals (Lambert & Wilson, 1993). Furthermore, there is also evidence from immunohistochemical studies that GABAB receptors are expressed in CCK+ interneurons in CA1 (Sloviter et al. 1999).
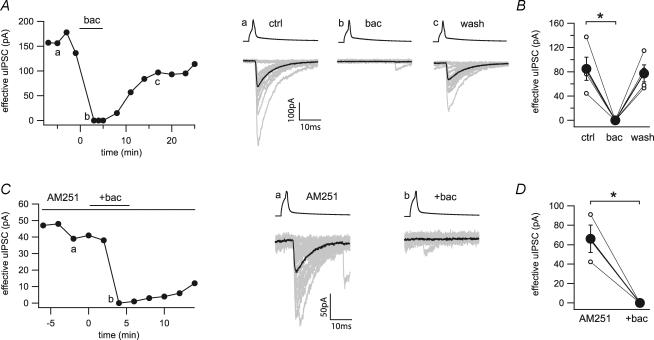
Application of the GABAB receptor agonist baclofen (100 μm) completely and reversibly inhibited uIPSCs (84.9 ± 19.4 to 0 pA, n = 4; Fig. 6A and B). The uIPSC suppression by baclofen was independent of CB1 receptors, because the inhibition was unaffected by pre-application of AM251 (66.1 ± 14.1 to 0 pA, n = 3; Fig. 6C and D). Therefore, these paired recording data show that CCK+ axonal terminals express, in addition to CB1 receptors, functional GABAB receptors that directly control GABA release.
Figure 6. Direct inhibition of GABA release by baclofen at the CCK+ interneuron to pyramidal cell synapse.
A, time series and example traces of uIPSCs during application of baclofen (bac, 100 μm) show a reversible block of synaptic transmission. Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a, b and c). B, line series of single pairs (○) and averages of four pairs (•) during application of baclofen. C, time series and example traces of uIPSCs during application of baclofen in the continuous presence of AM251 (10 μm). Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a and b). D, line series of single pairs (○) and averages of three pairs (•) before and during application of baclofen in the presence of AM251. *Statistical significance (P < 0.05).
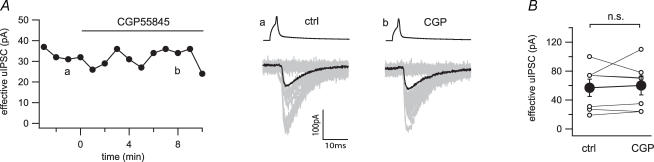
In a previous study, tonic inhibition of GABA release was described from immunocytochemically unidentified hippocampal basket cells by activation of presynaptic GABAB receptors (Buhl et al. 1995). We wondered whether this is the case in the specific subpopulation of CCK+ basket cell terminals and applied the GABAB receptor antagonist CGP55845 (2 μm). There was no effect of CGP55845 on the synaptic transmission in seven pairs tested (57.0 ± 11.7 to 60.0 ± 13.0 pA; Fig. 7A and B).
Figure 7. Absence of tonic GABAB receptor activation at the CCK+ interneuron to pyramidal cell synapse.
A, time series and example traces of uIPSCs before and during application of the GABAB receptor antagonist CGP55845 (CGP, 2 μm). Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a and b). B, line series of single pairs (○) and averages of seven pairs (•) during application of CGP. n.s., not significant.
Absence of functional receptors for serotonin and opioids on CCK+ basket cell terminals
Serotonergic receptors are expressed in cortical CCK+ interneurons (Ferezou et al. 2002), and serotonergic agonists have been shown to decrease inhibitory synaptic transmission onto CA1 pyramidal cells (Schmitz et al. 1995). In addition, μ-opioid receptors are expressed in hippocampal interneurons and inhibit perisomatic GABA release (Lambert & Wilson, 1993; Drake & Milner, 1999). However, it remains unclear whether there are modulatory effects of the abovementioned receptors directly on the CCK+ terminals.
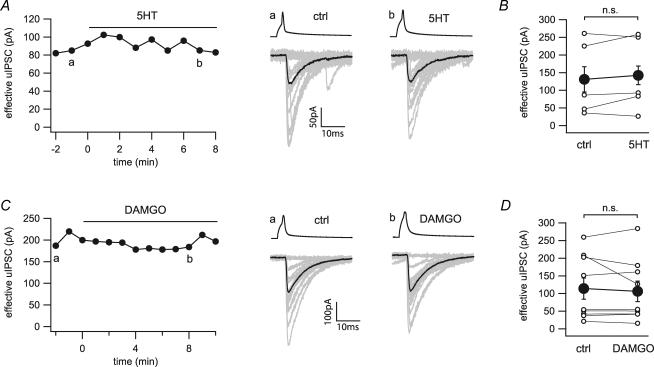
Application of serotonin (50 μm) had no effect on the failure rate or amplitude of the CCK+ basket cell to pyramidal cell transmission (131.0 ± 46.8 to 142.2 ± 47.2 pA, n = 5; Fig. 8A and B). As a positive control for serotonin, we verified that serotonin at the same concentration (50 μm) depressed evoked IPSCs in CA1 pyramidal cells (341.0 + 79.5 to 135.0 + 43.0 pA, n = 3; Supplementary Fig. 1A). Application of the selective μ-opioid receptor agonist DAMGO (1 μm) did not affect the transmission at the CCK+ basket cell to pyramidal cell synapse in eight out of nine connections (Fig. 8C and D). It is interesting that in one out of these nine pairs, the effective uIPSC amplitude was reduced by DAMGO (1 μm, 208 to 126 pA), which could then be restored to the control value by naloxone (10 μm, back to 209 pA; Supplementary Fig. 1B; note that this latter pair served as a positive control for DAMGO in these experiments). Statistical analysis on all nine connections revealed no significant decrease in effective uIPSC amplitude in response to application of DAMGO (114.0 ± 30.4 to 106.1 ± 29.5 pA, n = 9; Fig. 8D).
Figure 8. Lack of effects of serotonin and μ-opioid receptor stimulation on the CCK+ interneuron to pyramidal cell synapse.
A, time series and example traces of uIPSCs before and during application of serotonin (5HT, 50 μm). Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a and b). B, line series of single pairs (○) and averages of five pairs (•) during application of serotonin. C, time series and example traces of uIPSCs during application of DAMGO (1 μm). Twenty representative uIPSCs (grey) and their averages (black) were taken from the time points indicated in the time series (a and b). D, line series of single pairs (○) and averages of nine pairs (•) before and during application of DAMGO. n.s., not significant.
These data show that serotonin and μ-opioid receptors do not modulate, either directly or indirectly, GABA release from CCK+ basket cell terminals in the CA1 region.
Discussion
In the present study, we investigated the basic properties and the modulation of the inhibitory synaptic transmission between CCK+ basket cells and pyramidal cells in the hippocampal CA1 area. We found that action potentials in CCK+ interneurons evoked IPSCs with fast kinetics and variable latencies in CA1 pyramidal cells. The probability of failures varied greatly between pairs, and application of AM251 showed that these synapses are under tonic CB1 receptor-dependent control. Intracellular BAPTA in the postsynaptic cell abolished the ability of AM251 to enhance uIPSCs between CCK+ basket cells and pyramidal cells, demonstrating the ligand-driven nature and postsynaptic origin of tonic CB1 receptor-dependent control of GABA release. The fact that BAPTA in single postsynaptic neurons was sufficient to relieve the presynaptic terminals from CB1 receptor-mediated inhibition suggested the homosynaptic nature of endocannabinoid control at these synapses. In addition to CB1 receptors, GABAB receptors are also expressed on the presynaptic terminals and were able to inhibit GABA release in all pairs tested. In contrast, muscarinic and metabotropic glutamate receptors can only exert indirect effects through postsynaptic mobilization of endocannabinoids, but activation of these receptors does not directly modulate GABA release from CCK+ basket cells. Activation of metabotropic receptors by exogenously applied agonist mobilizes cannabinoids from the postsynaptic cells in a manner that cannot be blocked by BAPTA in the postsynaptic cell. Furthermore, basal endocannabinoid release is not due to tonic activation of metabotropic glutamate or acetylcholine receptors by their ligands, and it is also not due to calcium influx through NMDA receptors (of course, tonic stimulation of endocannabinoid release by metabotropic receptors other than muscarinic or metabotropic glutamate receptors may take place at these synapses). Serotonergic receptors are not involved in the regulation of transmission at these synapses, and μ-opioid receptors might be able to influence GABA release in a small subset of CCK+ basket cells, but have no significant effect on the majority of connections.
Endocannabinoid regulation of GABA release studied with paired recordings from CCK+ basket cells and pyramidal cells
In our paired recordings, we focused on the properties of perisomatic inhibitory input to CA1 pyramidal cells originating from single CCK+ basket cells. To avoid co-activation of inputs from CB1 receptor-lacking fibres, and to prevent changes in the relative contribution of CB1 receptor-positive and -negative fibres during drug applications, we selectively evoked action potentials in morphologically and immunohistochemically identified CCK+ basket cells (Wilson & Nicoll, 2001; Pawelzik et al. 2002; Losonczy et al. 2004; Hefft & Jonas, 2005; Glickfeld & Scanziani, 2006). Although this approach is difficult, especially when combined with prolonged recordings necessary for drug applications, the relative homogeneity of the synaptic connections leads to significant insights that could not be obtained with less direct techniques.
The CCK+ cells selected for our experiments all displayed a consistent electrophysiological phenotype (Fig. 1A) Although these CCK+ basket cells in the stratum radiatum do not comprise the whole phenotypic spectrum of CCK+ interneurons (Soltesz, 2005; Somogyi & Klausberger, 2005), they form a relatively homogeneous group of cells that allowed us to obtain connected pairs. The overall properties of the synaptic connections were in general agreement with previous data from CCK+ interneuron to principal cell connections in the hippocampal formation, making it unlikely that we selected an atypical subgroup with unusual properties (Cope et al. 2002; Pawelzik et al. 2002; Losonczy et al. 2004; Hefft & Jonas, 2005). The synaptic connections appeared rather homogenous regarding their biophysical and pharmacological properties, with two exceptions. First, the probability of failures showed a wide variability between connections, which is at least partly due to the varying amount of endocannabinoid mobilization from postsynaptic pyramidal cells. Second, in one out nine pairs, application of DAMGO caused a significant depression of uIPSCs that could be removed with μ-opioid receptor antagonist application. Although the one DAMGO-sensitive connection may represent a distinct subset of CCK+ basket cells that expresses functional μ-opioid receptors on their terminals, it is also possible that DAMGO affected GABA release indirectly. However, because of the low frequency of such opiate-sensitive CCK+ connections, this phenomenon could not be studied further in detail.
CB1 receptor-mediated tonic inhibition of GABA release is ligand driven from postsynaptic neurons
The regulation of transmitter release by endocannabinoids is a major hallmark of axon terminals originating from CCK+ hippocampal neurons (Katona et al. 1999). In our hands, all connections between CCK+ basket cells and CA1 pyramidal cells that have been tested previously were sensitive to the application of exogenous CB1 receptor agonists (Földy et al. 2006). Endocannabinoid release is usually thought to take place on demand, either in response to postsynaptic depolarization with a concomitant elevation in intracellular calcium levels or following activation of metabotropic receptors (Ohno-Shosaku et al. 2005). Our experiments showed rare instances of ‘muted’ connections in the CA1 region (see also Glickfeld & Scanziani, 2006), similar to the completely muted connections between CCK+, mossy fibre-associated interneurons in the CA3 stratum lucidum and pyramidal cells (Losonczy et al. 2004). As in the CA3 region, these muted connections also proved to be under CB1 receptor control, because the application of AM251 converted these tonically muted synapses to high-fidelity ones (Fig. 2A). But beyond these rare muted connections, a major result in our study is that tonic activity of CB1 receptors modulates not only the virtually muted connections, as AM251 had a significant overall effect across the CCK+ basket cell population.
Several mechanisms might explain the persistent CB1 receptor activity. First, CB1 receptors display constitutive activity (i.e. tonic endogenous activity observed even in the absence of the agonist; Bouaboula et al. 1997; Pan et al. 1998; Vasquez & Lewis, 1999; Pertwee, 2005). In principle, application of an inverse agonist such as AM251 should reduce the constitutive receptor activity and enhance synaptic transmission. Second, the tonic depression of GABA release by CB1 receptor activity may not be due to truly constitutive, ligand-free, intrinsic receptor activity, but may originate from basal endocannabinoid mobilization. The fact that postsynaptic application of 10 mm BAPTA abolished the ability of AM251 to enhance uIPSCs clearly points to a ligand-driven origin for the tonic CB1 receptor activity. This surprising result indicates that the manipulation of just a single postsynaptic cell is sufficient to result in an apparent decrease in the activity level of the CB1 receptors on the axon terminals of the presynaptic basket cell, suggesting that the endocannabinoid-dependent CB1 receptor-mediated tonic decrease in GABA release displays sufficient specificity to be called homosynaptic. Therefore, endocannabinoids released from the numerous other pyramidal cells adjacent to the recorded postsynaptic cell apparently cannot reach the CB1 receptors located on the GABAergic fibres forming synapses on the recorded pyramidal cell. It is interesting that this homosynaptic specificity is similar to that found in hypothalamic neurons (Hentges et al. 2005). Therefore, hippocampal CA1 pyramidal neurons do not only control their inhibitory input on a fast time scale on demand (as is the case with DSI), but they can apparently also suppress perisomatic inhibition in a tonic and spatially restricted, specific manner. The strategic positioning of the CB1 receptors close to the presynaptic release sites (Nyiri et al. 2005), together with the large number of CB1 receptors in the axon terminal and in the preterminal segment, may provide a not yet fully understood anatomical arrangement for such homosynaptic specificity in endocannabinoid control of GABA release.
A related issue is whether true constitutive CB1 activity exists in the mammalian brain. As pointed out previously (Guo & Ikeda, 2004), constitutive, intrinsic CB1 activity in heterologous expression systems is typically observed only with expression of large numbers of receptors. Furthermore, it is difficult to be certain that endocannabinoids are truly absent even in single-cell recordings used in heterologous expression system studies, because endocannabinoid ligands could be originating from the membrane of the cell under study.
The finding that the effects of carbachol and DHPG cannot be blocked by inclusion of BAPTA into the postsynaptic cell can be interpreted in two ways. First, the result may indicate that endocannabinoid release induced by metabotropic receptors is calcium independent (Maejima et al. 2001). Alternatively, these results may point to a breakdown of the homosynaptic specificity by massive release of endocannabinoids due to exogenously applied ligands of metabotropic receptors.
Functional relevance
Basket cells, due to their strategic innervation of perisomatic domains of postsynaptic neurons, perform a variety of key operations, including synchronization of neuronal assemblies, control of spike timing and synaptic integration (Cobb et al. 1995; Miles et al. 1996; Fricker & Miles, 2000; McBain & Fisahn, 2001; Pouille & Scanziani, 2001; Bartos et al. 2002; Mann et al. 2005). Recent in vivo and in vitro studies have made major advances in revealing the distinct properties and complementary roles of the two major basket cell classes, the PV+ and the CCK+ basket cells. In general, PV+ basket cells seem to be ideally suited for entraining network oscillations (Vreugdenhil et al. 2003). They fire fast, non-accommodating action potentials even at high frequencies, have relatively fast membrane time constants and low input resistances, respond reliably to excitation, and their GABA release is tightly coupled to rises in intracellular Ca2+ levels through P/Q-type Ca2+ channels (Wilson & Nicoll, 2001; Hefft & Jonas, 2005; Glickfeld & Scanziani, 2006). In contrast, CCK+ basket cells discharge accommodating action potentials, display slower membrane time constant and higher input resistance, seem to be better suited to integrate consecutive excitation by independent excitatory afferents as opposed to following repetitive incoming excitation, and GABA release from their terminals is regulated exclusively by N-type Ca2+ channels that may underlie a looser coupling of the Ca2+ source and sensor (Wilson & Nicoll, 2001; Guo & Ikeda, 2004; Hefft & Jonas, 2005; Glickfeld & Scanziani, 2006). In vivo, contrary to the stereotyped firing of PV+ cells, CCK+ interneurons appeared to fire in an episode-dependent manner during ripple oscillations, suggesting that PV+ and CCK+ hippocampal interneurons may play distinct roles in different brain states (Klausberger et al. 2005). Recently, it has been suggested that while PV+ basket cells provide a highly reliable clockwork that generates network oscillations, CCK+ basket cells might be specialized to act as modulators that adapt network activity to behavioural states (Freund, 2003). For example, there are a number of indications that the CCK+ basket cell to pyramidal cell synapses may be unusually rich targets for a number of neuromodulatory drugs with anxiolytic properties, including benzodiazepines (acting on α2-containing GABAA receptors), CCKB receptor antagonists, 5HT-3 receptor antagonists and cannabinoid ligands (for a review, see Freund, 2003). However, to date there has been no detailed study examining both the cannabinoid-dependent and -independent actions of various neurotransmitters and neuromodulators directly at the CCK+ basket cell to pyramidal cell connections using high-resolution paired recording techniques.
The present study revealed that CB1 receptors play a critical role in modulating the probability of release from these synapses in the CA1 area not only in a DSI-like, strictly activity-dependent mechanism, but also in a tonic manner. It is currently not known to what extent such tonic CB1-dependent inhibition of GABA release occurs in vivo. However, the apparently homosynaptic nature of the tonic endocannabinoid-mediated regulation of GABA release at CCK+ basket cell to pyramidal synapses suggests that it is unlikely that a general increase in endocannabinoid levels took place purely as a result of the in vitro slice protocol. It will be interesting to learn from future studies how tonic and phasic cannabinoid signalling affects brain state-dependent network oscillations (Klausberger et al. 2005).
Finally, future studies will need to address the tonic and phasic regulation of GABA release by CB1 receptors in pathological states as well, as retrograde endocannabinoid signalling has been shown to undergo robust, long-term changes after seizures (Chen et al. 2003; Wallace et al. 2003). It is interesting that both DSI and the tonic CB1 receptor-mediated inhibition of GABA release (as assessed by the increased ability of CB1 receptor antagonists/inverse agonists to enhance the amplitude of electrical stimulation-evoked, multifibre, compound IPSCs) are permanently augmented after developmental seizures at CCK+ perisomatic inputs (Chen et al. 2003). Future paired-recording experiments between CCK+ basket cells and pyramidal cells will need to be carried out to determine whether plasticity mechanisms of endocannabinoid-mediated processes differ between healthy and pathological states.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disordes and Stroke (NINDS) (NS38580 to I.S.) and the Deutsche Forschungsgemeinschaft (NE1185/1-1 to A.N.). We thank Rose Zhu for excellent help with immunostaining, and Drs Allyson Howard, Janos Szabadics and Gabor Nyiri for helpful discussions.
Supplemental material
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2006.121954 http://jp.physoc.org/cgi/content/full/jphysiol.2006.121954/DC1 and contains supplemental material consisting of a figure showing positive controls for Serotonin and DAMGO.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Cobb SR, Halasy K, Somogyi P. Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. Eur J Neurosci. 1995;7:1989–2004. doi: 10.1111/j.1460-9568.1995.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Ratzliff A, Hilgenberg L, Gulyas A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cope DW, Maccaferri G, Marton LF, Roberts JD, Cobden PM, Somogyi P. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience. 2002;109:63–80. doi: 10.1016/s0306-4522(01)00440-7. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res. 1999;849:203–215. doi: 10.1016/s0006-8993(99)01910-1. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Földy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Fricker D, Miles R. EPSP amplification and the precision of spike timing in hippocampal neurons. Neuron. 2000;28:559–569. doi: 10.1016/s0896-6273(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Ikeda SR. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol Pharmacol. 2004;65:665–674. doi: 10.1124/mol.65.3.665. [DOI] [PubMed] [Google Scholar]
- Hajos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA, Wilson WA. Heterogeneity in presynaptic regulation of GABA release from hippocampal inhibitory neurons. Neuron. 1993;11:1057–1067. doi: 10.1016/0896-6273(93)90219-h. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci U S A. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136:811–822. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Maejima T, Kano M. Calcium signaling and synaptic modulation: regulation of endocannabinoid-mediated synaptic modulation by calcium. Cell Calcium. 2005;38:369–374. doi: 10.1016/j.ceca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Pan X, Ikeda SR, Lewis DL. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI, Thomson AM. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. J Comp Neurol. 2002;443:346–367. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin and 8-OH-DPAT reduce excitatory transmission in rat hippocampal area CA1 via reduction in presumed presynaptic Ca2+ entry. Brain Res. 1995;701:249–254. doi: 10.1016/0006-8993(95)01005-5. [DOI] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci. 2004;24:10117–10127. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Ali-Akbarian L, Elliott RC, Bowery BJ, Bowery NG. Localization of GABA(B) (R1) receptors in the rat hippocampus by immunocytochemistry and high resolution autoradiography, with specific reference to its localization in identified hippocampal interneuron subpopulations. Neuropharmacology. 1999;38:1707–1721. doi: 10.1016/s0028-3908(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Soltesz I. Diversity in the Neuronal Machine. New York: Oxford University Press; 2005. [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurosci. 2005;25:6199–6207. doi: 10.1523/JNEUROSCI.1148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.