Abstract
Cerebellar granule cell to Purkinje cell synapses have been reported to show plasticity when stimulating the parallel fibres, but not when granule cell axons are stimulated in the granular layer. The latter absence of plasticity has been attributed either to the synapses made by ascending granule cell axons lacking some feature needed to evoke plasticity, such as metabotropic glutamate receptors, or to spillover of glutamate between adjacent active synapses being essential for plasticity to occur and having a greater effect for parallel fibre stimulation than for granular layer stimulation. Here we show that both long-term depression (LTD) and endocannabinoid plasticity can depend on interaction between adjacent synapses. These results focus attention on the need to characterize the spatial pattern of parallel fibre activity evoked by physiological stimuli, in order to assess the conditions under which synaptic plasticity will occur in vivo.
Alterations in the strength of cerebellar synapses are thought to underlie the storage of motor patterns by the cerebellum. Changes of synaptic strength mediated by retrograde endocannabinoid signalling and during long-term depression (LTD) at the cerebellar granule cell to Purkinje cell synapse are triggered by activation of postsynaptic type 1 metabotropic glutamate receptors (mGluR1s) (Linden et al. 1991; Hartell, 1994; Conquet et al. 1994; Ichise et al. 2000; Neale et al. 2001; Brown et al. 2003; Safo & Regehr, 2005). Both types of plasticity occur when trains of stimuli are applied to the parallel fibres (the axons of granule cells) in the molecular layer, which is the commonest way to study granule cell to Purkinje cell synaptic transmission. However, neither type of plasticity is evoked when granule cell axons are stimulated near the recorded Purkinje cell in the granular layer (Fig. 1A; Sims & Hartell, 2005; Marcaggi & Attwell, 2005). Two explanations have been proposed for the lack of plasticity evoked by granular layer stimulation.
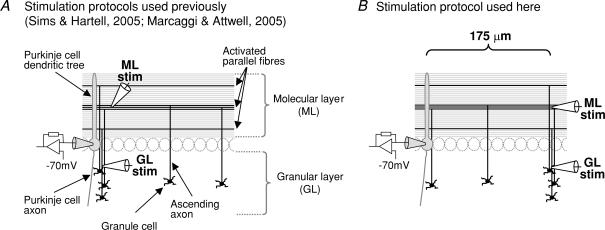
Figure 1. Stimulation in the molecular layer and granular layer to activate the same number (shown as three here) of granule cell to Purkinje cell synapses, that are either spatially adjacent or dispersed.
A, protocols of stimulation used previously to evoke synaptic plasticity. Sims & Hartell (2005) stimulated at different distances from the recorded Purkinje cell in the molecular layer (ML), or in the granular layer (GL) in the plane of the dendritic tree of the recorded Purkinje cell. Marcaggi & Attwell (2005) stimulated in either layer about 50 μm away from the plane of the recorded Purkinje cell. In these two studies, stimulation in the granular layer may have activated synapses produced by the ascending axons of granule cells. B, protocol of stimulation used in this study. The stimulation in the granular layer is 175 μm away from the plane of the dendritic tree of the recorded Purkinje cell. At such a distance, none of the activated ascending axons would make synapses from the ascending axon onto the recorded Purkinje cell; only their parallel fibre synapses will produce synaptic input. The stimulation in the molecular layer is also 175 μm away from the plane of the dendritic tree of the recorded Purkinje cell.
First, it has been debated (Gundappa-Sulur et al. 1999; Isope & Barbour, 2002) whether the synapses made by ascending granule cell axons, which may have been preferentially activated by granular layer stimulation near a recorded Purkinje cell (Fig. 1A) in the studies of Sims & Hartell (2005) and Marcaggi & Attwell (2005), may differ from parallel fibre synapses, perhaps lacking mGluR1s or (for LTD) being unaffected by climbing fibre stimulation (Sims & Hartell, 2005). Alternatively, we have shown that, in contrast to stimulation in the granular layer, stimulation of the parallel fibres in the molecular layer leads to glutamate diffusion between adjacent activated synapses. This enhances activation of AMPA receptors (Marcaggi et al. 2003), presumably because the effect on surrounding receptors of glutamate diffusing out of a synapse is larger when glutamate is also released at other synapses nearby because of non-linear summation of the effects of glutamate, either via the dose–response curve of the receptors or via local saturation of glutamate uptake. Activation of the mGluR1s, which initiate retrograde cannabinoid signalling and LTD, is also enhanced by crosstalk between adjacent active synapses evoked by parallel fibre stimulation (Marcaggi & Attwell, 2005). Thus, activation of spatially dispersed synapses by granular layer stimulation (Fig. 1), leading to less activation of mGluR1s than for parallel fibre stimulation, might also explain the lack of plasticity evoked by granular layer stimulation.
Resolving this controversy is important for understanding the conditions under which synaptic plasticity and information storage can occur in the cerebellar cortex. Studies of the plasticity that is evoked by stimulating in the molecular layer to activate nearby parallel fibre synapses are widely assumed to provide an understanding of cerebellar learning that occurs in vivo. Yet, because of the massive number of parallel fibres contacting a Purkinje cell (∼150 000), one might imagine that physiological input to the granular layer of the cerebellar cortex would result in the activation of synapses that are more spatially dispersed on the dendritic tree.
We therefore took advantage of the geometrical architecture of the cerebellum to compare the effects of activating the same number of parallel fibre synapses with either a sparse or a dense spatial distribution on the Purkinje cell dendritic tree, while ruling out contaminant activation of synapses made by ascending granule cell axons when stimulating in the granular layer (Fig. 1B). Our data suggest that the lack of endocannabinoid-mediated plasticity (Marcaggi & Attwell, 2005) and LTD (Sims & Hartell, 2005) observed for granular layer stimulation is due to a more spatially dispersed pattern of synapse activation, rather than recruitment of ascending axon synapses with properties differing from those of the parallel fibre synapses.
Methods
Electrophysiology
Rats (18 days old) were killed by cervical dislocation followed by decapitation in accordance with the UK Animals (Scientific Procedures) Act 1986. For most experiments, the cerebellum was rapidly removed and sliced in the coronal plane (using a Leica VT1000 S slicer) in dissection medium containing (mm): NaCl 124, KCl 2.5, NaH2PO4 1, NaHCO3 26, CaCl2 3, MgCl2 1, glucose 10 and kynurenic acid 1; bubbled with 95% O2–5% CO2 at ∼4°C. The slices (200 μm thick) were then stored in that medium for up to 5 h. Purkinje cells were whole-cell voltage or current clamped at 33–35°C. The external solution contained (mm): NaCl 124, KCl 2.5, NaH2PO4 1, NaHCO3 26, CaCl2 3, MgCl2 1, glucose 10 and gabazine 0.01 (to block GABAA receptors); bubbled with 95% O2–5% CO2. For the LTD experiments in Fig. 4A, CaCl2 was 2 mm and 5 μm DPCPX (8-cyclopentyl-1, 3-dipropylxanthine), and 1 μm CGP55845 were present to block adenosine (A1) and GABAB receptors respectively (as used previously to block possible K+ currents evoked by GABA or adenosine release, by Casado et al. (2002), who also studied LTD in coronal slices). The pipette solution contained (mm): potassium gluconate 140, NaCl 4, Hepes 10, MgATP 4, Na3GTP 0.5, K2.5EGTA 0.5 and CaCl2 0.1; pH adjusted to 7.3 with KOH. The electrode junction potential was compensated. During LTD experiments the series resistance was monitored every 10 s; the effect on the EPSC amplitude of moderate variations in series resistance was compensated for (by measuring the effect on EPSC amplitude of spontaneous rapid changes of series resistance that occurred), but if the series resistance changed by more than 50% then the data were discarded. In voltage-clamp mode, cells were clamped at −70 mV. In current-clamp mode, current was injected to set the resting potential to −70 mV.
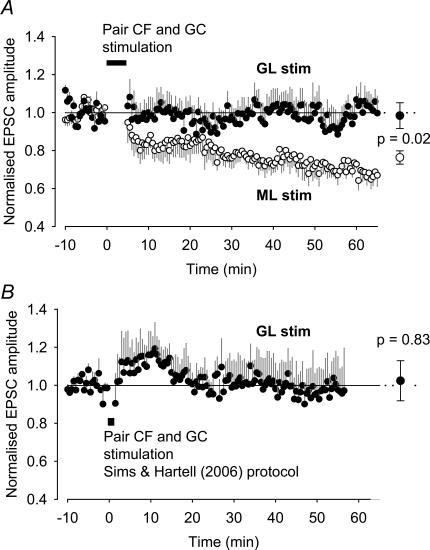
Figure 4. Long-term depression (LTD) is absent when stimulating the granular layer 175 μm from the recorded Purkinje cell.
A, effect on the normalized EPSC (mean ± s.e.m.), evoked by single molecular (ML) or granular layer (GL) stimulation, of pairing climbing fibre (CF) stimulation (two pulses at 10 Hz) with stimulation of the granule cell (GC) axons in the molecular or granular layer (five pulses at 200 Hz, starting with the climbing fibre stimulation). This protocol was repeated at 10 s intervals for 5 mins. Difference between ML and GL stimulation is significant with P = 0.02 from 10 to 65 min (mean data shown at right, n = 6 cells each). B, effect on the normalized EPSC (mean ± s.e.m.), evoked by single GL stimulation, with the LTD induction protocol used by Sims & Hartell (2006): stimulation of the GC axons (five pulses at 100 Hz) paired with stimulation of the CF (one pulse, 100 ms after the start of the GC axon stimulation). This protocol was repeated at 2 s intervals 50 times. The normalized EPSC amplitude from 7 to 55 mins (mean data shown at right, n = 5 cells) is not significantly different from unity (P = 0.83).
For the LTD experiments in Fig. 4B, the experimental procedure mimicked that used by Sims & Hartell (2006). (The main differences are that they used a different brain slicing solution with NaCl replaced by sucrose, they recorded at room temperature, and had stronger [H+] buffering in their internal solution; in addition, their internal solution recipe did not specify the GTP salt used (so we used Tris3-GTP) or pH (we adjusted the pH to 7.3), and had less Mg than ATP (but we added the same Mg concentration as ATP).) Purkinje cells were whole-cell voltage or current clamped at room temperature (22°C). The dissection and slicing medium contained (mm): sucrose 250, KCl 2.5, NaH2PO4 1.25, NaHCO3 26, CaCl2 2, MgCl2 1 and glucose 10. After slicing, the slices were stored for up to 3 h in the external solution used for recording which contained (mm): NaCl 120, KCl 2.7, NaH2PO4 1.2, NaHCO3 25, CaCl2 2.5, MgCl2 1.2, glucose 11 and picrotoxin 0.02 (to block GABAA receptors); bubbled with 95% O2–5% CO2. The pipette solution contained (mm): potassium gluconate 132, NaCl 8, Hepes 30, MgATP 4, Tris3GTP 0.3 and K2.5EGTA 0.5; pH adjusted to 7.3 with KOH.
Stimulation
Stimuli were applied from a glass pipette placed at a distance of 175 μm from the recorded Purkinje cell (Fig. 1B), either in the molecular layer to activate parallel fibres directly, or in the granular layer (which will also activate parallel fibres; Coutinho et al. 2004). For studying mGluR1-mediated currents or endocannabinoid-mediated plasticity, stimulus trains were applied every minute. To evoke LTD, climbing fibre and granule cell stimulation were paired as described in the legend to Fig. 4, using either a protocol similar to that used by Sims & Hartell (2005) and Safo & Regehr (2005) (Fig. 4A), or a protocol identical to that of Sims & Hartell (2006) (Fig. 4B). The size of the AMPA receptor-mediated EPSC was monitored by stimulating once every 10 s.
Statistics
Data are presented as means ± s.e.m. Statistical comparisons were by unpaired two tailed Student's t test.
Results
Stimulating in the granular layer without activating synapses produced by the ascending axons
We studied the mGluR1-mediated postsynaptic current (Tempia et al. 1998), and the plasticity of AMPA receptor-mediated synaptic signalling, evoked by stimulating either the granular layer or the molecular layer at a distance of 175 μm perpendicular to the plane of the dendritic tree of the recorded Purkinje cell in coronal cerebellar slices (Fig. 1B). This is far larger than the thickness of the planar dendritic tree of the Purkinje cell (< 15 μm) or the separation between Purkinje cells (34 μm) (Takacs & Hamori, 1994), ensuring that granular layer stimulation evokes parallel fibre activity without activity at synapses from ascending granule cell axons onto the recorded Purkinje cell (Fig. 1B). Furthermore, when stimulating in the granular layer, because the stimulated granule cell axons rise to different levels in the molecular layer (Cajal, 1911), the synapses that are activated will be spatially dispersed across the Purkinje cell dendritic tree (Fig. 1B) and no interaction between active synapses is likely. By contrast, when stimulating the parallel fibres in the molecular layer, adjacent parallel fibres are activated (Fig. 1B) and interaction between active synapses mediated by glutamate spillover is likely.
The stimuli for each location were adjusted to produce the same size of fast AMPA receptor-mediated EPSC (441 ± 51 and 448 ± 54 pA, for stimulation in the molecular layer and in the granular layer, respectively, at −70 mV for a single stimulus in n = 29 cells). Previous work has shown that glutamate spillover between adjacent synapses produced by parallel fibre stimulation has little effect on the peak EPSC produced (Marcaggi et al. 2003), so matching the EPSC amplitudes implies matching the number of synapses activated.
Endocannabinoid-mediated short-term plasticity is absent for granular layer stimulation-evoked activation of spatially dispersed parallel fibres
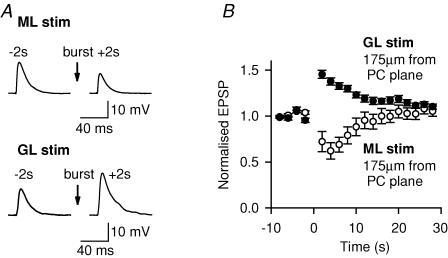
With the Purkinje cell recorded in current-clamp mode, a 10 pulse train of stimuli at 200 Hz transiently depressed the AMPA receptor-mediated fast EPSP produced by single stimulation of the parallel fibres, but potentiated the EPSP produced by granular layer stimulation (Fig. 2A and B). This difference in plasticity in response to stimulation of the molecular and granular layers is similar to that reported for stimulation close to the Purkinje cell (Marcaggi & Attwell, 2005), for which the mGluR1- and endocannabinoid-mediated depression (Neale et al. 2001; Brown et al. 2003) that is evoked by parallel fibre stimulation is abolished by blocking type 1 cannabinoid receptors (CB1) (while the potentiation seen for granular layer stimulation is unaffected; Marcaggi & Attwell, 2005).
Figure 2. Endocannabinoid-mediated plasticity is absent when stimulating the granular layer 175 μm from the recorded Purkinje cell.
A, effect of a 10 pulse train at 200 Hz (burst) on the fast EPSP evoked at −70 mV by a single stimulus 2 s before (−2 s) and 2 s after (+2 s) the train, using molecular layer (ML) and granular layer (GL) stimulation in the same rat Purkinje cell (PC). B, effect of the train on the fast EPSP in 10 cells (mean ± s.e.m.).
The mGluR1-mediated slow EPSC is reduced for granular layer stimulation-evoked activation of spatially dispersed parallel fibres
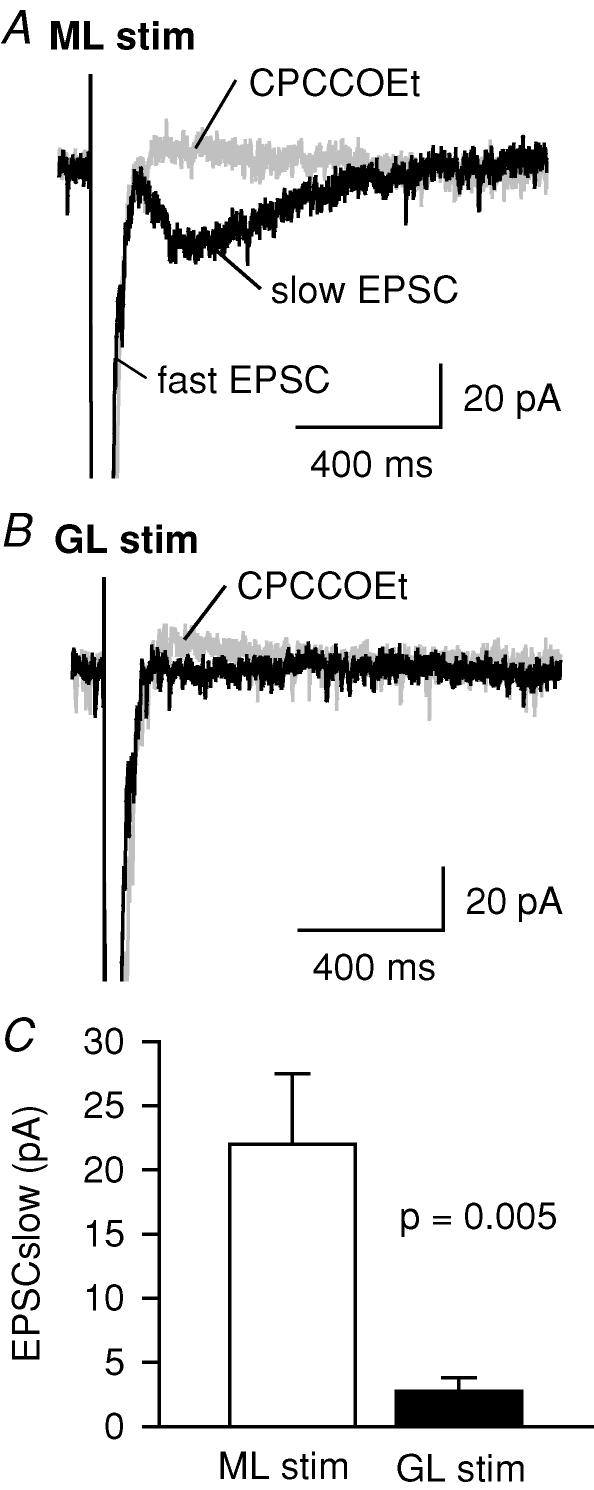
Applying a short burst of stimuli (four pulses at 200 Hz) with the Purkinje cell voltage clamped revealed that parallel fibre stimulation evoked an AMPA receptor-mediated fast EPSC followed by a robust mGluR1-mediated slow EPSC that was abolished by the mGluR1-specific antagonist CPCCOEt, while granular layer stimulation evoked the fast EPSC followed by a much smaller or absent slow EPSC (Fig. 3A–C). Thus, there is minimal activation of the mGluR1s which trigger endocannabinoid release when stimulating in the granular layer.
Figure 3. The slow type 1 metabotropic glutamate receptor (mGluR1)-mediated EPSC is absent when stimulating the granular layer 175 μm from the recorded Purkinje cell.
A-B, current evoked at −70 mV by four stimuli at 200 Hz shows a fast EPSC (black traces), followed by a slow mGluR1-mediated EPSC (blocked by CPCCOEt, 200 μm, grey traces) The slow mGluR1 mediated EPSC is large for molecular layer (ML), but small for granular layer (GL) stimulation. C, mean size of slow EPSC (13 cells).
When stimulating in the granular layer 175 μm from the plane of the dendritic tree of the Purkinje cell, the only synapses activated onto the Purkinje cell will be parallel fibre synapses (Fig. 1B). Therefore the lack of a slow EPSC and lack of endocannabinoid-mediated synaptic plasticity seen for granular layer stimulation cannot reflect a preferential recruitment of ascending synapses which might lack mGluR1s. Instead, it presumably reflects the fact that mGluR1s are only activated when many nearby synapses release glutamate (Marcaggi & Attwell, 2005); a condition which occurs for parallel fibre stimulation (Fig. 3A), but not for the more spatially dispersed activation of synapses produced by granular layer stimulation (Fig. 3B). For parallel fibre stimulation, the post-tetanic depression (∼40%) seen in Fig. 2B is smaller than when stimulating near the Purkinje cell (∼80%; Marcaggi & Attwell, 2005) This is probably because the parallel fibres are not in fact completely parallel, and the activated fibres impinge on a more spatially spread out area of the Purkinje cell than is the case when stimulating near the cell, resulting in less interaction between activated synapses.
LTD is absent for granular layer stimulation-evoked activation of spatially dispersed parallel fibres
Pairing parallel fibre stimulation with climbing fibre stimulation produces LTD of the parallel fibre fast EPSC, that is dependent on mGluR1 activation (Linden et al. 1991; Hartell, 1994; Conquet et al. 1994; Ichise et al. 2000) and also on endocannabinoid release (Safo & Regehr, 2005). However, this LTD is not seen when pairing climbing fibre stimulation with stimulation of the granular layer under the recorded Purkinje cell (Sims & Hartell, 2005). We stimulated 175 μm away from the plane of the denditic tree of the Purkinje cell, so that granular layer stimulation activates only parallel fibre synapses and not ascending synapses (Fig. 1B). In this case, although pairing parallel fibre with climbing fibre stimulation evoked LTD, pairing granular layer stimulation with climbing fibre stimulation did not (Fig. 4A).
Sims & Hartell (2006) reported that a slowly developing LTD could still be observed when climbing fibre activation was paired with activation of dispersed parallel fibre inputs (activated by stimulating in the granular layer 150–300 μm from the Purkinje cell dendritic plane). As this appears to be in conflict with our observations, we tried to reproduce the result of Sims & Hartell (2006) by mimicking their conditions (i.e. experiments at room temperature, and with a different composition for the dissecting, extracellular and intracellular media. Also, the stimulation protocol differed slightly from the one used in Fig. 4A). However, when parallel fibre synapses were activated by granular layer stimulation 175 μm lateral to the Purkinje cell dendritic plane, on average no significant LTD was observed (in five recordings lasting more than 55 min following the induction protocol; Fig. 4B).
Discussion
The work reported here clarifies the pattern of activity in cerebellar granule cell axons that is needed to produce synaptic plasticity at the granule cell to Purkinje cell synapse. These experiments differ from those in the earlier work of Sims & Hartell (2005) and Marcaggi & Attwell (2005) because for granular layer stimulation the present experiments ensure that the granule cell synapses activated are those on the parallel fibres, and eliminate any possible activation of synapses from ascending granule cell axons to the recorded Purkinje cell (cf. Fig. 1A and 1B). Our data show that the previously reported absence of both endocannabinoid-mediated plasticity and LTD when stimulating the granule cell axons in the granular layer is not a result of synapses on the ascending granule cell axon lacking mGluR1s or being unaffected by climbing fibre stimulation (Sims & Hartell, 2005), because the same lack of plasticity is observed when activating only parallel fibre synapses from the granular layer. However, mGluR1s are activated much more strongly when the parallel fibres are stimulated than when the granular layer is stimulated, reflecting these receptors acting as detectors of glutamate spillover between adjacent active synapses (Marcaggi & Attwell, 2005). Plasticity dependent on mGluR1 activation is expected therefore to be far stronger when stimulating the parallel fibres than when stimulating the granular layer, as is indeed observed. In vivo one would expect plasticity to occur most readily when adjacent parallel fibres are active.
In contrast to our results, Sims & Hartell (2006) very recently reported that LTD could be evoked by stimulating in the granular layer at a large distance from the recorded Purkinje cell, even though this did not evoke an mGluR1-mediated current in the cell (Fig. 3). This discrepancy with our results could be due to some difference in the protocols used for the preparation of the slices, the recording of the cells, or the induction of LTD. In particular, Sims & Hartell (2006) performed experiments at room temperature which is expected to reduce glutamate transporter activity and hence promote glutamate spillover. However, even using the same protocols as Sims & Hartell (2006), we did not observe LTD for stimulation in the granular layer at a large distance from the Purkinje cell (Fig. 4B). At present we have no explanation for this discrepancy.
In addition to the supralinear dependence of the mGluR1-mediated slow EPSC on the number of nearby activated synapses which, as mentioned above, is due to the effects of glutamate spillover (Marcaggi & Attwell, 2005), a non-linear dependence of the postsynaptic rise in calcium or sodium concentration on the number of nearby activated synapses has also been reported (Eilers et al. 1995; Callaway & Ross, 1997). When paired with climbing fibre activation in order to evoke LTD, increasing the number of activated parallel fibre synapses produces an amplification of the postsynaptic calcium rise, mediated by voltage-gated calcium channels (Wang et al. 2000). We cannot rule out the possibility that such voltage amplification may contribute to favouring the induction of LTD when the activated synapses are in close proximity.
As both LTD and endocannabinoid-mediated plasticity depend critically on the proximity of the activated synapses, the commonly used experimental protocol of stimulating the parallel fibres may evoke plasticity that would not occur in vivo with a more spatially dispersed pattern of synaptic inputs. However, some protocols may induce plasticity even when spatially dispersed synapses are activated (Casado et al. 2002) or when very few nearby synapses are activated (Wang et al. 2000). These data highlight the need for a better understanding of the spatial distribution of granule cell axon activity that physiologically impinges on Purkinje cells, to understand under exactly what conditions plasticity will occur in vivo.
Acknowledgments
Supported by the Wellcome Trust and a Wolfson-Royal Society award. We thank Angus Silver, Alasdair Gibb and Philippe Isope for comments on the paper.
References
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Cajal Ramón y S. Histologie Du Système Nerveux de L'homme et Des Vertébré. Maloine, Paris: 1911. [Google Scholar]
- Callaway JC, Ross WN. Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J Neurophysiol. 1997;77:145–152. doi: 10.1152/jn.1997.77.1.145. [DOI] [PubMed] [Google Scholar]
- Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33:123–130. doi: 10.1016/s0896-6273(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crepel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:218–219. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Coutinho V, Mutoh H, Knopfel T. Functional topology of the mossy fibre-granule cell–Purkinje cell system revealed by imaging of intrinsic fluorescence in mouse cerebellum. Eur J Neurosci. 2004;20:740–748. doi: 10.1111/j.1460-9568.2004.03533.x. [DOI] [PubMed] [Google Scholar]
- Eilers J, Augustine GJ, Konnerth A. Subthreshold synaptic Ca2+ signalling in fine dendrites and spines of cerebellar Purkinje neurons. Nature. 1995;373:155–158. doi: 10.1038/373155a0. [DOI] [PubMed] [Google Scholar]
- Gundappa-Sulur G, De Schutter E, Bower JM. Ascending granule cell axon: an important component of cerebellar cortical circuitry. J Comp Neurol. 1999;408:580–596. doi: 10.1002/(sici)1096-9861(19990614)408:4<580::aid-cne11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hartell NA. Induction of cerebellar long-term depression requires activation of glutamate metabotropic receptors. Neuroreport. 1994;5:913–916. doi: 10.1097/00001756-199404000-00015. [DOI] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Isope P, Barbour B. Properties of unitary granule cell→Purkinje cell synapses in adult rat cerebellar slices. J Neurosci. 2002;22:9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–89. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:776–781. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaggi P, Billups D, Attwell D. The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fibre synapses. J Physiol. 2003;552:89–107. doi: 10.1113/jphysiol.2003.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale SA, Garthwaite J, Batchelor AM. mGlu1 receptors mediate a post-tetanic depression at parallel fibre-Purkinje cell synapses in rat cerebellum. Eur J Neurosci. 2001;14:1313–1319. doi: 10.1046/j.0953-816x.2001.01769.x. [DOI] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Sims RE, Hartell NA. Differences in transmission properties and susceptibility to long-term depression reveal functional specialization of ascending axon and parallel fiber synapses to Purkinje cells. J Neurosci. 2005;25:3246–3257. doi: 10.1523/JNEUROSCI.0073-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RE, Hartell NA. Differential susceptibility to synaptic plasticity reveals a functional specialization of ascending axon and parallel fiber synapses to cerebellar Purkinje cells. J Neurosci. 2006;26:5153–5159. doi: 10.1523/JNEUROSCI.4121-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs J, Hamori J. Developmental dynamics of Purkinje cells and dendritic spines in rat cerebellar cortex. J Neurosci Res. 1994;38:515–530. doi: 10.1002/jnr.490380505. [DOI] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- Wang SS, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]