Abstract
Faced with mechanical inspiratory loading, awake animals and anaesthetized humans develop alveolar hypoventilation, whereas awake humans do defend ventilation. This points to a suprapontine compensatory mechanism instead of or in addition to the ‘traditional’ brainstem respiratory regulation. This study assesses the role of the cortical pre-motor representation of inspiratory muscles in this behaviour. Ten healthy subjects (age 19–34 years, three men) were studied during quiet breathing, CO2-stimulated breathing, inspiratory resistive loading, inspiratory threshold loading, and during self-paced voluntary sniffs. Pre-triggered ensemble averaging of Cz EEG epochs starting 2.5 s before the onset of inspiration was used to look for pre-motor activity. Pre-motor potentials were present during voluntary sniffs in all subjects (average latency (±s.d.): 1325 ± 521 ms), but also during inspiratory threshold loading (1427 ± 537 ms) and during inspiratory resistive loading (1109 ± 465 ms). Pre-motor potentials were systematically followed by motor potentials during inspiratory loading. Pre-motor potentials were lacking during quiet breathing (except in one case) and during CO2-stimulated breathing (except in two cases). The same pattern was observed during repeated experiments at an interval of several weeks in a subset of three subjects. The behavioural component of inspiratory loading compensation in awake humans could thus depend on higher cortical motor areas. Demonstrating a similar role of the cerebral cortex in the compensation of disease-related inspiratory loads (e.g. asthma attacks) would have important pathophysiological implications: it could for example contribute to explain why sleep is both altered and deleterious in such situations.
Faced with mechanical inspiratory loading, awake animals do not increase inspiratory effort to defend ventilation and thus develop alveolar hypoventilation. The ensuing hypercapnia eventually stimulates the chemosensitive brainstem respiratory neurones, which brings ventilation gradually back to baseline, but without complete compensation: Pa,CO2 rises in proportion to the load (review in Younes, 1995). The response of anaesthetized animals to inspiratory loading is roughly identical. Similarly, in anaesthetized humans, the respiratory control system does not react to an increase in respiratory resistance by increasing inspiratory effort (Whitelaw et al. 1976), and mechanical loading leads to hypoventilation. Conversely, awake humans do defend ventilation in the presence of a sustained inspiratory load (Axen et al. 1983). This bears witness to the activation of neural load compensating mechanisms, which are likely to include a behavioural component of suprapontine origin, in addition to a possible reflexive component (Younes, 1995). In this regard, it has recently been shown that inspiratory resistive breathing in awake humans is associated with a facilitated response of the diaphragm to transcranial magnetic stimulation, in spite of a reduced automatic drive to breathe (Locher et al. 2006). This has been attributed to load-related corticospinal inputs to the phrenic motoneurones. However, the precise suprapontine mechanisms possibly at play during inspiratory loading compensation are not known.
In addition to their automatic command arising from the brainstem and to their primary motor cortical representation (Foerster, 1936; Gandevia & Rothwell, 1987; Murphy et al. 1990; Colebatch et al. 1991; Similowski et al. 1996), the human respiratory muscles have a representation in the pre-motor cortex and in the supplementary motor area (Macefield & Gandevia, 1991; Sharshar et al. 2004). Neuronal activities are increased in these areas when a subject is asked to voluntarily overcome an inspiratory resistance (Fink et al. 1996). Of note, in awake patients with locked-in syndrome (preserved emotional influences on breathing but no voluntary control of respiratory movements), inspiratory resistive loading provokes hypoventilation as it does in animals or in humans during anaesthesia (Younes, 1995). The present study therefore tested the hypothesis of a higher cortex involvement in the compensation of sustained mechanical inspiratory loading in humans.
To this end, we studied respiratory pre-motor activity in healthy volunteers, through inspiratory pretriggered EEG averaging. In brief, this consisted of the ensemble averaging of a variable number of surface EEG epochs starting 2.5 s before the onset of inspiration (determined from a ventilatory flow signal) and ending 0.5 s after its end. The presence of a slow cortical negativity preceding inspiration was considered to be the sign of an activation of the pre-motor and supplementary motor areas (Birbaumer et al. 1990; Macefield & Gandevia, 1991; Hinterberger et al. 2003; Shibasaki & Hallett, 2006). The subjects were studied during unloaded quiet breathing and during mechanical inspiratory loading. Both inspiratory threshold and inspiratory resistive loading were tested, because their different nature could have implied different central adaptations. Indeed, subjects submitted to inspiratory threshold loading are obliged to develop and maintain the threshold pressure to breathe, whereas inspiratory resistive loading leaves room for breathing pattern adaptations that are apt to reduce the inspiratory efforts produced to overcome the load (e.g. slower inspirations). CO2-stimulated breathing was also studied for verification purpose. Indeed, CO2 stimulation has not been shown to activate the cortical areas involved in pre-motor activities and, in contrast, it tends to induce deactivation within the prefrontal cortex (Brannan et al. 2001): it was not expected to give rise to pre-motor potentials.
Methods
Subjects
Ten healthy subjects (age 19–34 years, three men, body mass index 21.5 ± 2.2 kg m−2) participated in the study after appropriate legal and ethical clearance (Comité Consultatif de Protection des Personnes se prêtant à des Recherches Biomédicales, Pitié-Salpêtrière, Paris, France). None of the subjects had previous experience with respiratory physiology experiments. They received detailed information about the methods used, but were naive concerning the purpose of the study. They gave written consent. None had a past history of respiratory or neurological disease. They were free of psychotropic treatment. They had been asked to refrain from alcohol consumption during the 24 h preceding the experiment and to avoid sleep deprivation.
Experimental conditions
The experiments took place in a warm and dark ambience. All the subjects were studied sitting in a comfortable easy chair that provided full support to the back, arms, neck and head. During the entire experiment, they watched a film continuously, without interruption, on a computer screen placed at the centre of their visual field, and were instructed to minimize eye movements. They were asked to perform stereotyped hand movements (finger snapping) at regular intervals. They wore headphones to ensure sound insulation from experimental auditory cues. The experimenters and the equipment were hidden from their view.
Respiratory measurements
The subjects, wearing a nose clip, breathed through a flanged mouthpiece, attached either to a heated low dead space pneumotachograph linear from 0 to 160 l min−1 (3700A series; Hans Rudolph, Kansas City, MO, USA; dead space 14 ml; flow resistance 0.02–0.04 cmH2O 1−1 s−1) or to a low resistance pneumotachograph linear from 0 to 1000 l min−1 (MLT 1000 l; ADInstruments; dead space 350 ml; flow resistance 0.002 cmH2O 1−1 s−1), connected to a ±2 cmH2O linear differential pressure transducer (DP-45-18; Validyne, Northridge, CA, USA) to measure the ventilatory flow. The low dead space pneumotachograph was assembled in series to a medium two-way non-rebreathing valve (2600 series; Hans Rudolph). The low resistance pneumotachograph was assembled in series to a large two-way non-rebreathing valve (2700 series; Hans Rudolph). The inspiratory ports of these valves could be connected either to a Douglas bag containing a mixture of 7% CO2 and 93% O2, to a 50 cmH2O l−1 s−1 linear resistance, or to a threshold loading device (Spring-to-stretch, Threshold Inspiratory Muscle Trainer N730; Health Scan, New Jersey, USA). The threshold load was initially set to 25 cmH2O. With this load, some subjects experienced severe respiratory discomfort. The load was then brought down within seconds after its application to allow the subjects to breathe without excessive trouble (median load value 20 cmH2O, interquartile range 17–25, 3 out of the 10 subjects breathing against the initial 25 cmH2O value). Tidal volume and minute ventilation were calculated from the integrated airflow.
Mouth pressure (PM) was measured from a side port of the mouthpiece, using a ±140 cmH2O differential pressure transducer (DP 15-32; Validyne, Northridge, CA, USA). End-tidal CO2 partial pressure (PET,CO2) was measured from another side port of the mouthpiece, using an infrared CO2 gas analyser (IR1505; Servomex, Plaine Saint Denis, France). The subjects self-evaluated their degree of respiratory discomfort at the end of each experimental condition on a 10 cm visual analogue scale on which they could displace a cursor between ‘no discomfort’ on the left to ‘intolerable discomfort’ on the right.
Electrophysiological measurements
EEG activities
EEG activities were recorded with a subcutaneous needle electrode inserted into the scalp at Cz (international EEG 10–20 system) (Rektor, 2002). Linked earlobes surface electrodes served as reference. The signal was fed to a Neuropack electroencephalograph (Nihon Kohden, Tokyo, Japan), amplified and filtered (0.1–500 Hz). They were digitized at 2 kHz (Chart v5.2, ADInstruments) and stored on an Apple Macintosh G4 computer for off-line analysis. During some confirmatory experiments, a 2000-fold gain preamplifier with a 0.05–500 Hz band-pass filter (Electronique du Mazet, Mazet Saint-Voy, France) was used to eliminate cable movement artefacts.
EMGs
Surface recordings of the activity of one scalene muscle were obtained with a pair of silver cup electrodes placed over the anatomical landmark of the middle body of this muscle, 2 cm above the clavicle (Aldrich et al. 2002; Hug et al. 2006). The EMG signals were fed to a Neuropack electromyograph (Nihon Kohden, Tokyo, Japan), amplified and filtered (20–3000 Hz). They were digitized at 2 kHz (Chart v5.2, ADInstruments) and stored on an Apple Macintosh G4 computer for off-line analysis.
Electro-oculograms (EOGs)
EOGs were also recorded using two skin taped silver cup electrodes placed at the external canthus of each eye.
Experimental sequences
Each subject underwent, in random order, two sets of measurements. The first, with the low dead space pneumotachograph, consisted of (1) a control period during quiet breathing, referred to as ‘control 1’; (2) inspiratory threshold loading; (3) 15 min rest following disconnection from the respiratory apparatus; (4) inspiratory resistive loading. The second set of measurements (low resistance pneumotachograph) consisted of (1) another control period during room air quiet breathing, referred to as ‘control 2’; (2) 7% inspired fraction of CO2(FI,CO2) breathing; (3) 15 min rest following disconnection from the respiratory apparatus; (4) self-paced sniffs through a nasal mask (ComfortClassic; Respironics Inc., USA). In each condition, a period of 5–10 min was allowed for stabilization of the respiratory pattern before respiratory discomfort assessment and actual recordings.
Data processing
In each condition, 120 respiratory cycles were recorded after the stabilization period. This 120 number was chosen as a safety margin after preliminary experiments conducted in a separate set of subjects (data not shown) had suggested that 80 cycles sufficed to describe respiratory pre-motor potentials. For the purpose of ensemble averaging, the EEG was split into 3 s epochs extending from 2.5 s before to 0.5 s after the onset of mechanical inspiration defined as the zero crossing by the rising flow signal. Each epoch exhibiting EEG artefacts, EEG spurious activity exceeding 20% of the baseline background signal or intense EOG activity was discarded.
On the averaged tracings, a slow negative shift starting between 2 and 0.5 s before inspiration was identified as pre-motor activity, first through visual inspection and then in an analytical manner, as follows. First-order least-square regression equations were fitted to data ranges subjectively identified as exhibiting a putative negativity. A pre-motor activity was considered present if, and only if, the slope of the corresponding equation was positive and significantly different from zero. A negativity increase synchronous with the onset of inspiration was identified as a motor potential (Shibasaki, 1992; Shibasaki & Hallett, 2006). It was considered present when fitted by a significant linear regression of order 1, if this slope was significantly different from the pre-motor slope (F test). The latencies of the pre-motor potentials were measured from the onset of neural inspiration, defined as the beginning of EMG scalene muscle activity. The potential amplitude was measured from baseline, at the start of neural inspiration.
Reproducibility
Three subjects were studied again at a 22–43 week intervals, with a simplified protocol (low-resistance pneumotachograph) comprising: (1) a control period during quiet breathing; (2) inspiratory threshold loading; (3) self-paced sniffs; (4) CO2 breathing.
Statistical analysis
Continuous variables were first tested for normality using the Kolmogorov-Smirnov test with Dallal and Wilkinson approximation (Dallal & Wilkinson, 1986) (Prism 4; GraphPad Software, Inc., USA). Because the normality condition was always verified, the results are henceforth expressed as means ± s.d. Comparisons between conditions were conducted with an analysis of variance for repeated measures, with the latencies and amplitudes of the potentials as dependent variables, a subject factor, and experimental condition as the between-subjects factor. Dunnett's post hoc test was used for comparisons with ‘control 1’ condition and Tukey's test was used for pairwise comparisons. The proportions of the subjects exhibiting pre-motor activity in the various conditions were compared with Friedman's test followed by post hoc Dunn's test, in order to make no assumptions concerning normality. Differences were considered significant when the probability P of a type I error was below 5%.
Results
Nine subjects underwent the full experimental sequence. In one case (subject no. 10), only the ‘control 1’ condition, inspiratory threshold loading and inspiratory resistive loading conditions, could be studied.
EEG activity
During quiet breathing under control conditions (control 1 and control 2), inspiration was never preceded by EEG changes suggestive of pre-motor activity, except in one subject (no. 8) in whom there was no motor potential. In contrast, during sniff manoeuvres, pre-motor activity was detected according to the criteria described in Methods in the nine subjects in whom this was studied. It consisted in a slow negative shift beginning 1325 ± 521 ms prior to the motor activity, thus corresponding to a typical pre-motor potential (PMP; Shibasaki, 1992; Shibasaki & Hallett, 2006) (Figs 1 and 2). Sniff pre-motor potentials were followed by motor potentials, except in one case.
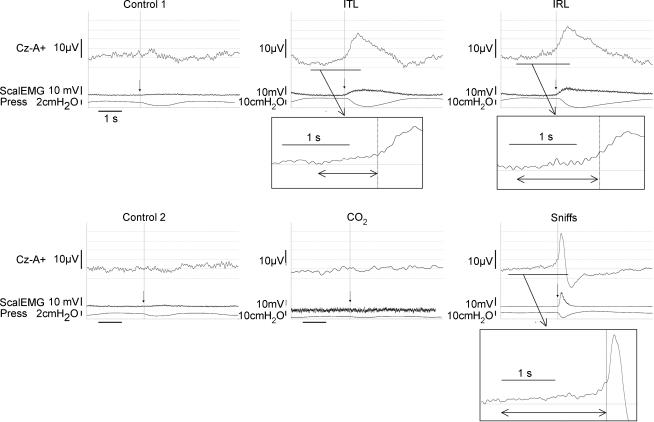
Figure 1. Representative example of the pre-inspiratory EEG activity recorded in one subject during quiet breathing with a low dead space pneumotachograph, inspiratory threshold loading, inspiratory resistive breathing, quiet breathing with a low resistance pneumotachograph, CO2 breathing, and self-paced sniffs.
Llow dead space pneumotachograph (control 1; top, left), inspiratory threshold loading (ITL; top, middle), inspiratory resistive breathing (IRL; top, right), quiet breathing with a low-resistance pneumotachograph (control 2; bottom, left), CO2 breathing (bottom, middle) and self-paced sniffs (bottom, right). The vertical arrows indicate the triggering point. The recording during CO2 breathing shown here was obtained using a preamplifier connected to the scalp electrode, whereas this was not the case for the other signals. Cz-A+, vertex EEG derivation; ScalEMG, root mean square scalene muscle EMG; Press, mouth or nasal pressure. The EEG positivity at the leftmost part of the ITL trace (and to a lesser extent of the IRL trace) corresponds to the end of the cortical activity related to the previous cycle.
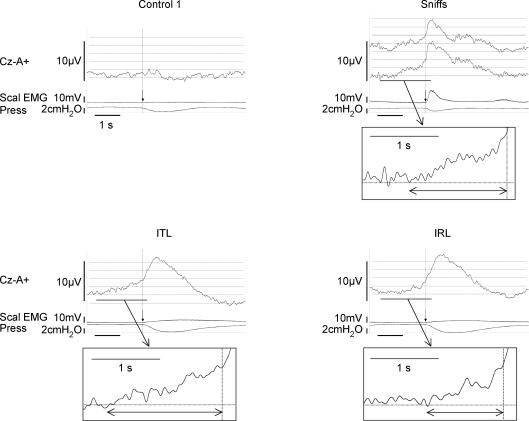
Figure 2. Ensemble averaging of the inspiratory pre-motor activities recorded in all the subjects, under the control 1 condition, self-paced sniffs and mechanical loading.
The vertical arrows indicate the triggering point. In the ‘sniffs’ panel, two EEG traces can be seen. The top one was obtained from all the subjects; it shows an initial positivity (also seen, to a lesser extent, on the IRL trace) that corresponds to the end of the cortical activity related to the previous cycle. The second EEG trace, that does not feature the initial positivity, corresponds to the ensemble averaging of the data gathered for the six subjects who performed low frequency sniffs.
Pre-motor activity was identified during the two types of inspiratory loading in all the subjects (Figs 1 and 2). The latencies and amplitudes of the corresponding potentials are given in Table 1. During inspiratory threshold and inspiratory resistive loading, motor potentials consistently followed the pre-motor negativities.
Table 1.
Amplitudes and latencies of the inspiratory pre-motor potentials according to the type of inspiratory activity
| Sniffs | ITL | IRL | |
|---|---|---|---|
| Amplitude (μV) | 9.9 ± 6.7 | 6.6 ± 2.9 | 6.9 ± 2.7 |
| Latency (ms) | 1325 ± 521 | 1427 ± 537 | 1109 ± 465 |
EEG recordings in Cz referenced to linked ear-lobes (International 10–20 system). ITL. inspiratory threshold loading; IRL, inspiratory resistive loading. Results are expressed as means ± s.d.
Breathing CO2 was not associated with EEG pre-motor activity, except in two cases (subject no. 5 and subject no. 9, among who only subject no. 5 also exhibited a motor potential). Of note, subject no. 8, who showed pre-motor activity during quiet breathing, did not show this during 7% CO2 breathing. During CO2 breathing, we often observed slow baseline oscillations on the averaged traces of individual subjects. We assigned this to cable movement artefacts due to the trunk oscillations associated with the intense respiratory stimulation. This hypothesis was verified by recording the EEG signal with a preamplifier connected to the Cz electrode and fixed on the scalp immediately beside it. This preamplifier had a high-pass filter frequency of 0.05 Hz that guards against filtering out pre-motor potentials even further than the 0.1 Hz value generally used in this experiment.
Figures 3 and 4 summarize the EEG results. The occurrence of pre-motor and motor activities was significantly more frequent during sniffs, inspiratory threshold loading and inspiratory resistive loading than during unloaded breathing (whatever the dead space) and during CO2 breathing.
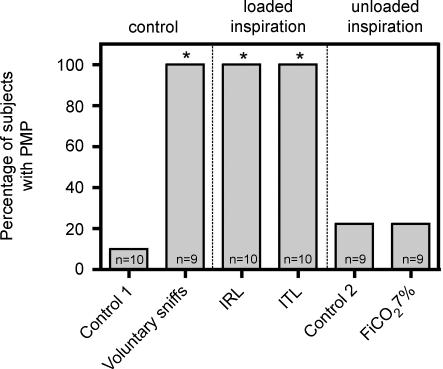
Figure 3. Proportion of subjects exhibiting pre-motor potentials in the various study conditions.
Control 1, quiet breathing with a low dead space pneumotachograph (see Methods); control 2, quiet breathing with a low resistance pneumotachograph (see Methods); FI,CO2 7%, 7% inspired fraction of CO2. *Significant difference versus the control 1 condition (P < 0.05).
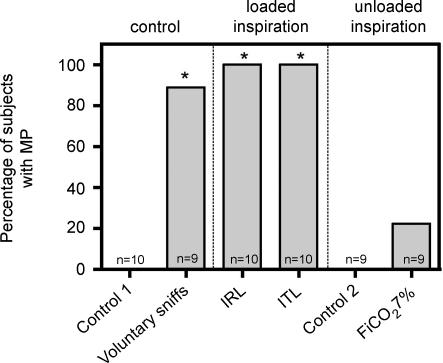
Figure 4. Proportion of subjects exhibiting motor potentials in the various study conditions.
Control 1, quiet breathing with a low dead space pneumotachograph (see Methods); control 2, quiet breathing with a low resistance pneumotachograph (see Methods); FI,CO2 7%, 7% inspired fraction of CO2. *Significant difference versus the control 1 condition (P < 0.05).
Respiratory discomfort
The respiratory discomfort was significantly increased during inspiratory threshold loading (ITL) and inspiratory resistive loading (IRL) as compared with the ‘control 1’ condition with the same apparatus (respectively 2.4 ± 1.8 and 2.6 ± 1.5 versus 0.4 ± 0.7, P < 0.05), but there was no difference between the two. Post hoc, the subjects described their respiratory discomfort during ITL and IRL as an ‘increased sensation of effort’. During 7% CO2 the intensity of respiratory discomfort was significantly superior to the corresponding control condition (4.1 ± 2.0 versus 0.8 ± 1.4, P < 0.05). Post hoc, the subjects described their respiratory discomfort during CO2-stimulated breathing as ‘air hunger’.
Respiratory pattern
ITL and IRL were associated with greater inspiratory pressure (PM) swings, and with a significant increase in cycle period (TT). Inspiratory time (TI) also increased, generally more than TT, hence a greater duty cycle (TI/TT) (particularly during resistive breathing). There was no significant increase in tidal volume (VT) and no significant change in mean inspiratory flow (VT/TI), an index of the respiratory drive (see review in Milic-Emili & Zin, 1986). Of note, no significant difference in breathing pattern was detected between threshold and resistive loading. Mechanical inspiratory loading did not affect the PET,CO2 value. In contrast, 7% CO2 significantly decreased TT and TI, increased VT and VT/TI with a fixed TI/TT. Of note, room air breathing with the low resistive pneumotachograph appeared to be stimulated as compared with room air breathing with the low volume pneumotachograph (dead space effect).
Reproducibility
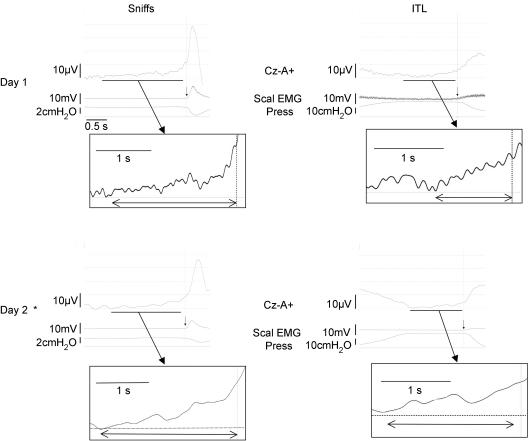
In the three subjects who were studied twice, room air breathing and CO2-stimulated breathing were not preceded by any discernible EEG pre-motor activity. Conversely, sniffs were preceded by pre-motor potentials as during the first session. EEG changes suggestive of pre-motor activity were also observed during repeated inspiratory threshold loading (Fig. 5).
Figure 5. Ensemble averaging of the inspiratory pre-motor activities recorded in one subject on two separate occasions (top and bottom) during self-paced sniffs (left) and ITL (right).
The vertical arrows indicate the triggering point. The EEG positivity at the leftmost part of the ITL traces corresponds to the end of the cortical activity related to the previous breath (magnified on the day 2 recording because of an increased respiratory frequency reducing the inter-breath period). *Denotes the use of a preamplifier shown to eliminate cable movement artefacts (see Methods).
Discussion
In this study, we observed a pre-motor EEG activity not only during voluntary ‘sniff’ manoeuvres, as previously described (Macefield & Gandevia, 1991), but also, systematically, during breathing against a sustained ITL or a sustained IRL. In contrast, no pre-motor EEG activity could be consistently identified during quiet breathing or when automatic breathing was stimulated by the inhalation of a CO2-enriched gas mixture. These observations provide a neurophysiological substratum to the behavioural component of inspiratory loading compensation in awake humans and point to the pre-motor cortex as its origin.
Methodological considerations
Although our subjects were naive to respiratory physiology and to the actual purpose of the study, they were studied wearing a nose clip and connected to respiratory measurement devices. In addition, they were asked to make judgements of their respiratory discomfort at the end of the different experimental sequences. Some degree of expectation of respiratory interventions must thus have occurred. This could explain why one subject exhibited a pre-motor activity during quiet breathing. However, we do not think that this constituted a serious confusion factor, for the following reasons. First, we did our best to provide the subjects with non-respiratory focuses during the experiments (watching a film, requests for finger snapping at regular intervals). Second, during the preliminary experiments that were used to fine tune the final study, we observed similar results even though the subjects were not asked about their respiratory sensations. Third, and more importantly, our subjects exhibited a higher level of respiratory activity during CO2-stimulated breathing than during loaded breathing, and reported similar levels of respiratory discomfort on the visual analogue scale. Yet pre-motor potentials occurred only twice in this condition (including in the subject who exhibited pre-motor activity during quiet breathing). Extra-respiratory muscle activation can occur during inspiratory loading and could also have been a confusing factor. We tried to minimize this, namely by instructing the subjects to relax when we observed that they tended to brace themselves on the armrests. Of note, the intense hyperventilation induced by 7% CO2 also resulted in body movements accompanying respiration, and, as mentioned before, was not associated with consistent pre-motor potentials.
Nature of the respiratory pre-motor activity
A pre-motor potential recorded before a motor action bears witness to its preparation. It is termed readiness potential or Bereitschaftspotential (BP; Deecke et al. 1969) in the case of a self-paced task, and contingent negative variation (CNV; Walter et al. 1964) in the case of movement expectancy during a ‘Go/NoGo’ protocol (where a ‘warning’ stimulus is followed or not by a planned task launched by a ‘Go’ stimulus). BPs originate within the mesial pre-motor cortex (Deecke, 1978; Knosche et al. 1996; Cui et al. 1999), either in the posterior part of the supplementary motor area or more anteriorly in the mesiofrontal cingular cortex (Ball et al. 1999). BPs are also recorded in deep subcortical structures (basal ganglia and posterior thalamus; Rektor, 2002; Paradiso et al. 2003; Rektor et al. 2005) projecting on the motor cortex. In our subjects, pre-motor potentials that were thus BPs occurred before self-paced inspiratory sniffs, with a 1451 ± 285 ms latency. They were followed by motor potentials. This result matches observations made during self-paced hand movements and is in exact accordance with the report by Macefield & Gandevia (1991) where the average latency was 1.2 ± 0.3 s. The lack of pre-motor activity during CO2-stimulated breathing is in keeping with the absence of cortical involvement in response to CO2 (Corfield et al. 1995) and even more with the corresponding prefrontal deactivation (Brannan et al. 2001). In contrast with the self-paced sniffs protocol, inspiratory loading does not correspond to any of the situations where pre-motor potentials have been observed. Indeed, our subjects had not been instructed to voluntarily breathe against the load (on the contrary, they had received minimal information on the objectives of the study and every effort was made to distract their attention from their breathing). Moreover, the motor action giving rise to the pre-motor potentials was mandatory given its inspiratory nature, and was associated with negative feedback in the form of respiratory discomfort. This could explain why the inspiratory-loading-associated pre-motor activity was not identical to that associated with self-paced sniffs (Figs 1, 2 and 5). Nevertheless, there are enough similarities between the pre-inspiratory EEG activities during loaded breathing, the pre-inspiratory activity preceding the sniffs, and data in the literature, for us to believe that the corresponding potentials are indeed BPs. This belief is further supported by the systematic presence of motor potentials after the pre-motor ones (Fig. 4). Of note, we did not observe significant differences in the respiratory patterns associated with threshold and resistive loading, and both situations were associated with pre-motor activities.
Source of the respiratory pre-motor activity
Our results suggest that the higher cortical sub regions implicated in the preparation and execution of movement are involved in inspiratory loading compensation. This could correspond to the functional significance of the cortico-diaphragmatic pathway described by Sharshar et al. (2004) that originates in the supplementary motor area. In addition, the pre-motor regions can be activated during an array of respiratory tasks (Colebatch et al. 1991; Ramsay et al. 1993; Fink et al. 1996; McKay et al. 2003). Fink et al. (1996) evidenced an activation of the supplementary motor area in subjects asked to voluntarily overcome a resistive load. Conversely, Isaev et al. (2002) failed to record enhanced metabolic activity in higher cortical structures in subjects exposed to an unexpected sustained IRL. This is in contrast with our findings. One explanation could lie in an insufficient resolution of the PET detection system used by Isaev et al. (2002). Another explanation could be the experimental paradigm. The subjects studied by Isaev et al. (2002) had been previously accustomed to resistive loads, whereas our subjects were fully naive to experimental respiratory loading. One could therefore argue that our findings mark the ‘discovery’ of loading by the subjects, the suprapontine compensation being transitory only (as previously advocated by Younes, 1995). However, we describe inspiratory pre-motor activities in subjects exposed to loading for more than 10 min, which is longer than the loading duration after which ventilatory changes are usually described (Younes, 1995). Above all, the subjects that we studied twice at an interval of several weeks still exhibited inspiratory pre-motor activity in response to ITL (Fig. 5). Naivety about the loading can therefore not explain the discrepancy between our results and the results of Isaev et al. (2002). This discrepancy could be due to the difference in the nature of the loading used: Isaev et al. (2002) used a resistance of 24 cmH2O l−1·s−1, versus 50 cmH2O l−1 s−1 in our subjects; this difference is likely to be associated with different behavioural responses.
Functional significance and perspectives
As mentioned in the introduction, awake humans generally do not hypoventilate in response to a mechanical inspiratory load. The pre-motor cortical activation described here could contribute to this particularity, as follows. In humans, the neural outflow to the respiratory muscle depends on the integration of motor inputs from brainstem respiratory pattern generators, the primary motor cortex (Foerster, 1936; Gandevia & Rothwell, 1987), and the pre-motor cortex (Sharshar et al. 2004). During sustained inspiratory loading, the brainstem respiratory drive is generally not augmented (Lopata et al. 1977; Ramonatxo et al. 1991; Clague et al. 1992; Locher et al. 2006). A purely volitional compensation is unlikely. In this respect, Locher et al. (2006) have shown that IRL could facilitate the diaphragm response to transcranial magnetic stimulation, but in a manner suggestive of a spinal rather than cortical mechanism. Because a pre-motor negativity can facilitate the response of the target muscle to transcranial magnetic stimulation (Zaaroor et al. 2003), Locher et al. (2006) hypothesized that their observations could reflect increased pre-motor inputs to the phrenic motoneurones. By confirming that the pre-motor cortex is most likely to be involved in the compensation of inspiratory loads, the present study lends support to this hypothesis. The participation of higher cortical structures in the maintenance of ventilation in inspiratory load generating diseases (e.g. asthma attacks) would have important pathophysiological implications, and could for example contribute to explain why sleep is both altered and deleterious in such situations.
Acknowledgments
The authors are grateful to the subjects for having accepted to participate in this study, and to Ms Marilyn Amouyal-Jones for her help with English style and grammar. They thank Professor Marc Zelter and Jean-Philippe Derenne for their input. This study was funded in part by a ‘Contrat de recherche triennal “Legs Poix” de la Chancellerie de l'Université de Paris’ and by the Association pour le Développement et l'Organization de la Recherche En Pneumologie (ADOREP), Paris, France. M.R. was a scholar of the ANTADIR association, Paris, France, and the recipient of a ‘Médaille d'Or’ grant from Assistance Publique-Hôpitaux de Paris, Paris, France. S.R. was a scholar of the Universita di Brescia, Brescia, Italy. C. M.-P. was the recipient of a ‘Médaille d'Or’ grant from Assistance Publique-Hôpitaux de Paris, Paris, France.
References
- Aldrich TK, Sinderby C, McKenzie DK, Estenne M, Gandevia SC. Electrophysiological techniques for the assessment of respiratory muscle function. Am J Respir Crit Care Med. 2002;166:548–558. [Google Scholar]
- Axen K, Haas SS, Haas F, Gaudino D, Haas A. Ventilatory adjustments during sustained mechanical loading in conscious humans. J Appl Physiol. 1983;55:1211–1218. doi: 10.1152/jappl.1983.55.4.1211. [DOI] [PubMed] [Google Scholar]
- Ball T, Schreiber A, Feige B, Wagner M, Lucking CH, Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage. 1999;10:682–694. doi: 10.1006/nimg.1999.0507. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox PT. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague JE, Carter J, Pearson MG, Calverley PM. Effort sensation, chemoresponsiveness, and breathing pattern during inspiratory resistive loading. J Appl Physiol. 1992;73:440–445. doi: 10.1152/jappl.1992.73.2.440. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Adams L, Murphy K, Martin AJ, Lammertsma AA, Tochon-Danguy HJ, Clark JC, Friston KJ, Guz A. Regional cerebral blood flow during volitional breathing in man. J Physiol. 1991;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield DR, Fink GR, Ramsay SC, Murphy K, Harty HR, Watson JD, Adams L, Frackowiak RS, Guz A. Evidence for limbic system activation during CO2-stimulated breathing in man. J Physiol. 1995;488:77–84. doi: 10.1113/jphysiol.1995.sp020947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui RQ, Huter D, Lang W, Deecke L. Neuroimage of voluntary movement: topography of the Bereitschafts-potential, a 64-channel DC current source density study. Neuroimage. 1999;9:124–134. doi: 10.1006/nimg.1998.0388. [DOI] [PubMed] [Google Scholar]
- Dallal GE, Wilkinson L. An analytic approximation to the distribution of Lilliefors's test statistic for normality. Am Stat. 1986;40:294–296. [Google Scholar]
- Deecke L. Dissociations between performance (time of movement onset) and slow potentials (Bereitschaftspotential and CNV) Electroencephalogr Clin Neurophysiol. 1978;(Suppl.):225–229. [PubMed] [Google Scholar]
- Deecke L, Scheid P, Kornhuber HH. Distribution of readiness potential, pre-motion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Exp Brain Res. 1969;7:158–168. doi: 10.1007/BF00235441. [DOI] [PubMed] [Google Scholar]
- Fink GR, Corfield DR, Murphy K, Kobayashi I, Dettmers C, Adams L, Frackowiak RS, Guz A. Human cerebral activity with increasing inspiratory force: a study using positron emission tomography. J Appl Physiol. 1996;81:1295–1305. doi: 10.1152/jappl.1996.81.3.1295. [DOI] [PubMed] [Google Scholar]
- Foerster O. Motorische Felden und Bähen. In: Bumke O, Foerster O, editors. Handbook der Neurologie. Vol. 6. Berlin: Springer; 1936. pp. 50–51. [Google Scholar]
- Gandevia SC, Rothwell JC. Activation of the human diaphragm from the motor cortex. J Physiol. 1987;384:109–118. doi: 10.1113/jphysiol.1987.sp016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger T, Veit R, Strehl U, Trevorrow T, Erb M, Kotchoubey B, Flor H, Birbaumer N. Brain areas activated in fMRI during self-regulation of slow cortical potentials (SCPs) Exp Brain Res. 2003;152:113–122. doi: 10.1007/s00221-003-1515-4. [DOI] [PubMed] [Google Scholar]
- Hug F, Raux M, Prella M, Morelot-Panzini C, Straus C, Similowski T. Optimized analysis of surface electromyograms of the scalenes during quiet breathing in humans. Respir Physiol Neurobiol. 2006;150:75–81. doi: 10.1016/j.resp.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Isaev G, Murphy K, Guz A, Adams L. Areas of the brain concerned with ventilatory load compensation in awake man. J Physiol. 2002;539:935–945. doi: 10.1113/jphysiol.2001.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knosche T, Praamstra P, Stegeman D, Peters M. Linear estimation discriminates midline sources and a motor cortex contribution to the readiness potential. Electroencephalogr Clin Neurophysiol. 1996;99:183–190. doi: 10.1016/0013-4694(96)95648-5. [DOI] [PubMed] [Google Scholar]
- Locher C, Raux M, Fiamma M-N, Morelot-Panzini C, Zelter M, Derenne JP, Similowski T, Straus C. Inspiratory resistances facilitate the diaphragm response to transcranial stimulation in humans. BMC Physiol. 2006;6:7. doi: 10.1186/1472-6793-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M, La Fata J, Evanich MJ, Lourenco RV. Effects of flow-resistive loading on mouth occlusion pressure during CO2 rebreathing. Am Rev Respir Dis. 1977;115:73–81. doi: 10.1164/arrd.1977.115.1.73. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC. The cortical drive to human respiratory muscles in the awake state assessed by premotor cerebral potentials. J Physiol. 1991;439:545–558. doi: 10.1113/jphysiol.1991.sp018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. J Appl Physiol. 2003;95:1170–1178. doi: 10.1152/japplphysiol.00641.2002. [DOI] [PubMed] [Google Scholar]
- Milic-Emili J, Zin WA. Relationship between neuromuscular respiratory drive and ventilatory output. In: Fishman AP, Macklem PT, Mead J, Geiger SR, editors. Handbook of Physiology, section 3, The Respiratory System, Mechanics of Breathing. Bethesda, MD: American Physiological Society; 1986. pp. 631–646. [Google Scholar]
- Murphy K, Mier A, Adams L, Guz A. Putative cerebral cortical involvement in the ventilatory response to inhaled CO2 in conscious man. J Physiol. 1990;420:1–18. doi: 10.1113/jphysiol.1990.sp017898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso G, Saint-Cyr JA, Lozano AM, Lang AE, Chen R. Involvement of the human subthalamic nucleus in movement preparation. Neurology. 2003;61:1538–1545. doi: 10.1212/01.wnl.0000096021.28967.57. [DOI] [PubMed] [Google Scholar]
- Ramonatxo M, Mercier J, Cohendy R, Prefaut C. Effect of resistive loads on pattern of respiratory muscle recruitment during exercise. J Appl Physiol. 1991;71:1941–1948. doi: 10.1152/jappl.1991.71.5.1941. [DOI] [PubMed] [Google Scholar]
- Ramsay SC, Adams L, Murphy K, Corfield DR, Grootoonk S, Bailey DL, Frackowiak RS, Guz A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. J Physiol. 1993;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rektor I. Scalp-recorded Bereitschaftspotential is the result of the activity of cortical and subcortical generators – a hypothesis. Clin Neurophysiol. 2002;113:1998–2005. doi: 10.1016/s1388-2457(02)00286-9. [DOI] [PubMed] [Google Scholar]
- Rektor I, Bares M, Brazdil M, Kanovsky P, Rektorova I, Sochurkova D, Kubova D, Kuba R, Daniel P. Cognitive- and movement-related potentials recorded in the human basal ganglia. Mov Disord. 2005;20:562–568. doi: 10.1002/mds.20368. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Hopkinson NS, Jonville S, Prigent H, Carlier R, Dayer MJ, Swallow EB, Lofaso F, Moxham J, Polkey MI. Demonstration of a second rapidly conducting cortico-diaphragmatic pathway in humans. J Physiol. 2004;560:897–908. doi: 10.1113/jphysiol.2004.061150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H. Movement-related cortical potentials. In: Halliday AM, editor. Evoked Potentials in Clinical Testing. 2. Edinburgh: Churchill Livingstone; 1992. pp. 523–537. [Google Scholar]
- Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–2356. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Similowski T, Straus C, Coic L, Derenne JP. Facilitation-independent response of the diaphragm to cortical magnetic stimulation. Am J Respir Crit Care Med. 1996;154:1771–1777. doi: 10.1164/ajrccm.154.6.8970369. [DOI] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Whitelaw WA, Derenne JP, Couture J, Milic-Emili J. Adaptation of anesthetized men to breathing through an inspiratory resistor. J Appl Physiol. 1976;41:285–291. doi: 10.1152/jappl.1976.41.3.285. [DOI] [PubMed] [Google Scholar]
- Younes M. Mechanisms of respiratory load compensation. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. Vol. 79. Philadelphia: Marcel Dekker; 1995. pp. 867–922. [Google Scholar]
- Zaaroor M, Pratt H, Starr A. Time course of motor excitability before and after a task-related movement. Neurophysiol Clin. 2003;33:130–137. doi: 10.1016/s0987-7053(03)00029-7. [DOI] [PubMed] [Google Scholar]