Abstract
While idiopathic generalized epilepsies are thought to evolve from temporal highly synchronized oscillations between thalamic and cortical networks, their cellular basis remains poorly understood. Here we show in a genetic rat model of absence epilepsy (WAG/Rij) that a rapid decline in expression of hyperpolarization-activated cyclic-nucleotide gated (HCN1) channels (Ih) precedes the onset of seizures, suggesting that the loss of HCN1 channel expression is inherited rather than acquired. Loss of HCN1 occurs primarily in the apical dendrites of layer 5 pyramidal neurons in the cortex, leading to a spatially uniform 2-fold reduction in dendritic HCN current throughout the entire somato-dendritic axis. Dual whole-cell recordings from the soma and apical dendrites demonstrate that loss of HCN1 increases somato-dendritic coupling and significantly reduces the frequency threshold for generation of dendritic Ca2+ spikes by backpropagating action potentials. As a result of increased dendritic Ca2+ electrogenesis a large population of WAG/Rij layer 5 neurons showed intrinsic high-frequency burst firing. Using morphologically realistic models of layer 5 pyramidal neurons from control Wistar and WAG/Rij animals we show that the experimentally observed loss of dendritic Ih recruits dendritic Ca2+ channels to amplify action potential-triggered dendritic Ca2+ spikes and increase burst firing. Thus, loss of function of dendritic HCN1 channels in layer 5 pyramidal neurons provides a somato-dendritic mechanism for increasing the synchronization of cortical output, and is therefore likely to play an important role in the generation of absence seizures.
Absence seizures are characterized in the electroencephalogram (EEG) by bi-laterally spreading 3–4 Hz rhythmic large amplitude spike-wave discharges (SWDs), driven by the hypersynchronized discharging of reciprocally connected corticothalamic and thalamocortical circuits (Avoli et al. 1983; Crunelli & Leresche, 2002; Meeren et al. 2002; Steriade & Amzica, 2003; Pinault et al. 2006). While previous work traditionally ascribes the generation of absence seizures to disturbances of intrinsic thalamic neuronal properties or synaptic interactions between thalamic circuits (Crunelli & Leresche, 2002; Ludwig et al. 2003; Budde et al. 2005), there is now mounting evidence that the cortex exerts a leading role in the initial phase of seizure onset (Avoli et al. 1983; Meeren et al. 2002; Steriade & Amzica, 2003; Holmes et al. 2004; Pinault et al. 2006) and governs the synchronization of thalamocortical networks (Avoli et al. 1983; Nicolelis & Fanselow, 2002; Pinault, 2003; Steriade & Amzica, 2003; Pinault et al. 2006). The cellular, intrinsic and synaptic mechanisms underlying the cortical origin of absence seizure initiation are, however, not well understood.
Recently, in a genetic rat model of generalized absence epilepsy, hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, coding for the h-current (Ih), were found to be reduced in the cortex (Strauss et al. 2004). Although the Ih reduction in this rat model is consistent with the epileptic phenotype of the HCN2 knockout mice (Ludwig et al. 2003), Ihincreases have also been associated with SWDs based on experimental (Di Pasquale et al. 1997; Budde et al. 2005) and computational studies (Timofeev et al. 2002). In addition, inference about a causal relationship between HCN channel function and epilepsy is complicated by reports that changes in HCN channel isoforms can be acquired in models where focal epileptic seizures are generated by kainic acid or hyperthermia (Chen et al. 2001; Shah et al. 2004; Brewster et al. 2005), indicating that activity-dependent HCN modifications can evolve secondarily to seizure activity. These and other studies have led to a plethora of viewpoints on the role of HCN in epileptogenesis, which is currently the subject of much debate (Poolos, 2004).
An important step in establishing whether modifications in Ih could play a role in the generation of generalized epilepsies is to track changes in Ih expression with respect to seizure onset. In the well-established WAG/Rij genetic rat model of absence seizures, 7–11 Hz SWDs evolve as a function of age and seizure onset occurs around 3 months (Coenen & van Luijtelaar, 2003). In this animal model, we focused on the very high densities of HCN channels in the apical dendrites of layer 5 (L5) pyramidal neurons (Stuart & Spruston, 1998; Williams & Stuart, 2000; Lorincz et al. 2002; Kole et al. 2006) where Ih acts to control somatodendritic excitability (Williams & Stuart, 2000; Berger et al. 2003). We report that a rapid loss of dendritic HCN1 channels in WAG/Rij cortex precedes seizure onset. Further, we identify that dendritic HCN1 channel loss plays a direct role in frequency-dependent amplification of backpropagating action potentials, and enhances high-frequency burst firing in L5 pyramidal neurons, suggesting that HCN1 loss facilitates the initiation and propagation of spontaneous generalized seizures.
Methods
Animals
Male WAG/Rij rats (Harlan, Horst, the Netherlands) and Wistar rats (Animal Service Division, JCSMR, Canberra, Australia) were used between 2 weeks and 11 months of age, as stated in the text. All experiments were carried out according to guidelines approved by the Animal Ethics Committee of the Australian National University and German law (in congruence with 86/609/EEC). All WAG/Rij rats older than ∼140 days (Coenen & van Luijtelaar, 1987) suffer from genetically determined seizure disorder, characterized by hundreds of spontaneous bi-lateral spike-wave discharges in the frequency range of 7–11 Hz (Meeren et al. 2002; Coenen & van Luijtelaar, 2003), which can be suppressed by anti-absence drugs, but aggravated by drugs effective against tonic-clonic seizures (Coenen & van Luijtelaar, 2003). A low percentage of Wistar rats may show SWD seizures when older than 6 months (Vergnes et al. 1982). Animals were always age-matched for comparisons.
Patch-clamp recordings
Rats were deeply anaesthetized by isoflurane inhalation and quickly decapitated. One brain hemisphere was removed and parasagital brain slices (300 μm) were prepared from S1 cortex including barrel, hind- and forelimb areas. Throughout the preparation of brain slices the brain was maintained in ice-cold sucrose-based artificial cerebrospinal fluid (ACSF) of the following composition (mm): 87.0 NaCl, 25.0 NaHCO3, 2.5 KCl, 25.0 NaH2PO4, 75.0 sucrose, 25.0 glucose, 0.5 CaCl2 and 7.0 MgCl2 (pH 7.4; oxygenated with 5% CO2–95% O2). After cutting, slices were transferred to a holding chamber filled with oxygenated sucrose–ACSF maintained at 35°C for 45 min, and then subsequently transferred to oxygenated ACSF consisting of (mm): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 25 glucose, 2.0 CaCl2 and 3 MgCl2 (pH 7.4; 5% CO2–95% O2), and thereafter stored at room temperature.
For recording, individual slices were transferred to the stage of an upright microscope (Olympus, Japan) equipped with DIC optics. The microscope bath was perfused with oxygenated (95% O2, 5% CO2) ACSF consisting of (mm): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 25 glucose, 2 CaCl2 and 1 MgCl2. Somatic and dendritic cell-attached recordings from visualized L5 pyramidal neuron dendrites were performed with borosilicate glass electrodes (Harvard, Edenbridge, Kent, UK) pulled to a resistance of 5–6 MΩ for somatic recordings and 10–12 MΩ for dendritic recordings. For somatic and dendritic whole-cell voltage recordings pipettes contained (mm) 120 potassium gluconate, 20 KCl, 4 Mg-ATP, 0.3 Na-GTP, 10 Hepes and 10 Na2-phosphocreatine (pH 7.2 with KOH). Dual current-clamp recordings were made with two identical Dagan BVC-700A amplifiers (Dagan Corporation, Minneapolis, MN, USA). The bridge was monitored and corrected regularly. Cell-attached recordings of macroscopic Ih currents were done with pipettes filled with (mm): 120 KCl, 20 TEA-Cl, 10 Hepes, 5 EGTA, 5 4-AP, 1 MgCl2, 1 BaCl2, 1 NiCl2, 0.5 CdCl2 and 0.001 TTX (pH 7.4, 285 mosmol l−1). Cell-attached patch-clamp recordings were made with an Axopatch 200B amplifier (Molecular Devices Corp., Sunnyvale, CA, USA) in patch mode. All signals were analog low-pass filtered at 10 kHz (Bessel) and digitally sampled at 20 kHz using the data acquisition software Axograph (v. 4.9.1, Axon Instruments Inc.). All data were obtained at 32 ± 1°C.
Some neurons were filled by adding 0.2% biocytin (Molecular Probes, Eugene, OR, USA) to the pipette solution. Slices were fixed in 4% paraformaldehyde and processed with an avidin–biotin peroxidase reaction (Vectastain ABC kit, Vector laboratories, Burlingame, CA, USA) and diaminobenzidine treatment. Slices were mounted in Moviol and cells analysed and reconstructed with the aid of a three-dimensional computerized system (Neurolucida, Microbrightfield Inc, Williston, VT, USA).
The patch holding potential for cell-attached recordings was estimated by correcting for a liquid junction potential (LJP) of the cell-attached solution of −3 mV (Williams & Stuart, 2000), assuming a membrane potential (Vm) at the soma of −79 mV (corrected for Donnan potenial and LJP). In addition, we corrected for a distance-dependent depolarization of the local Vm of the dendrites by ∼1 mV per 100 μm (Stuart et al. 1997; Kole et al. 2006).
The rising phase of Ih was fitted either with a single exponential [A exp(−t/τ)] or the sum of two exponentials [A exp(−t/τfast) + B exp(−t/τslow) + C] using a simplex algorithm. Analysis of the sum-of-squared errors (SSE) after fitting with single or double exponentials showed that double-exponential fits described the current transient significantly better than single exponential fits (n = 13). Data are presented as the amplitude weighted mean of both components [τw = (A τfast+ Bτslow)/(A + B)]. Steady-state activation curves were constructed from the amplitude of tail currents following voltage steps of different amplitude. These data were normalized to the maximum tail amplitude and fitted with a single Boltzmann equation (Fig. 2B).
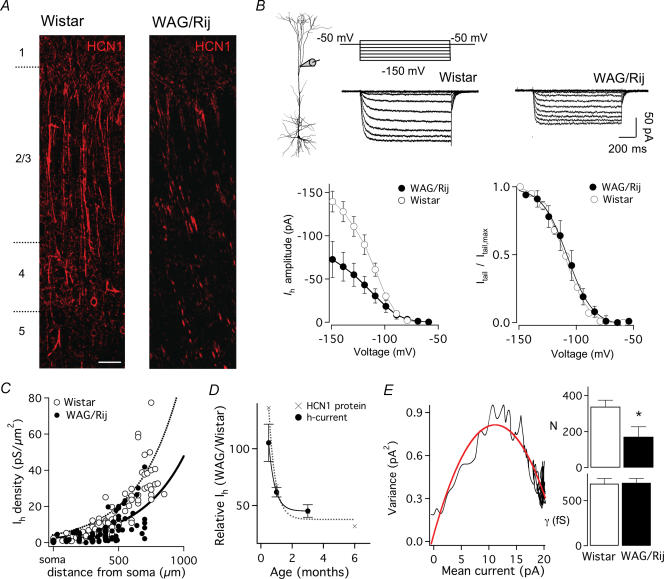
Figure 2. Properties and spatial distribution of HCN1 and Ih current and single channels in absence epilepsy.
A, immunofluorescence of the HCN1 protein in columns of somatosensory cortex from Wistar (left) and weaker labelling in WAG/Rij (right). Scale bar, 100 μm. B, top, voltage-clamp command potentials used for dendritic cell-attached pach-clamp recordings of Ih in dendrites. In WAG/Rij patches (right traces, 700 μm) significantly lower Ih amplitudes were obtained compared with Wistar (left traces, 660 μm) observed as a significant reduction in the steady-state current–voltage relationship (left plot). Tail current analysis indicated that Ih was activated with a similar voltage dependence (right plot). C, the Ih amplitude inhibition in WAG/Rij dendrites is similar at all dendritic locations and scales single-exponentially with distance from the soma with a similar length constant of ∼330 μm but smaller exponential constants (Wistar; A = 4.3; WAG/Rij A = 2.5). D, development of changes in Ih density plotted as mean WAG/Rij Ih current amplitude relative to mean Wistar Ih current amplitude at similar dendritic locations (450–550 μm from the soma; filled circles). Developmental changes in Ih paralleled changes in HCN1 protein expression (grey crosses, same data as in Fig. 1E). Lines represent single-exponential fits to the data (e-fold change, 0.4 months) E, results from non-stationary fluctuation analysis of dendritic sites at ∼400 μm distance from soma was consistent with a loss in single-channel number (N) (Student's t test, *P < 0.05, n = 4) without change in single-channel conductance (γ). Data shown as mean ± s.e.m.
The somato-dendritic coupling coefficient (Carnevale & Johnston, 1982) (Fig. 3B), kds, was determined by kds =Vd/Vs, where Vd is the steady-state dendritic voltage and Vs the steady-state somatic voltage generated by −1 nA current injections. Sag ratio was calculated as the steady-state voltage divided by the peak voltage (at around −94 mV) evoked by a hyperpolarizing current injection.
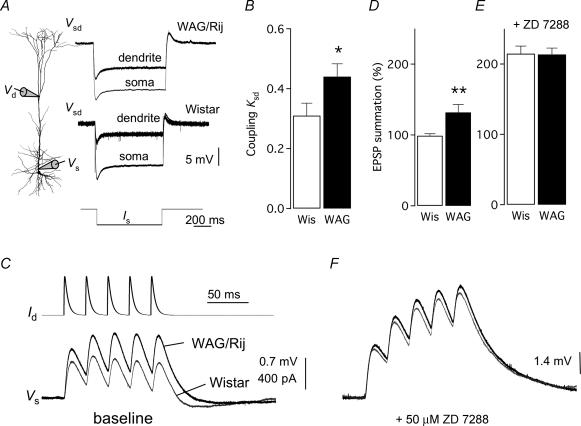
Figure 3. Ih-dependent increases in somato-dendritic coupling and synaptic drive in absence epilepsy.
A, experimental configuration of dual whole-cell recording in a L5 pyramidal neuron. Step hyperpolarization current injection into the soma (lower trace, −1 nA) induces time-dependent somatic voltage (Vs) responses and attenuated voltage in dendrites (Vd). Note the reduced steady-state attenuation in WAG/Rij (top panel) compared with Wistar (middle panel). B, population data showing increased coupling coefficient in WAG/Rij neurons (Student's t test, *P < 0.05). C, bi-expontential current injections in the dendrite (Id, top traces) simulating EPSPs at 50 Hz. Note the increased summation in WAG/Rij neurons (black trace) compared with no amplitude summation at the soma in Wistar (grey trace). D, summary columns show significant larger summation of EPSP-induced voltage in WAG/Rij (Student's t test, **P < 0.01). E, strain differences in EPSP were removed by blocking Ih conductance with 50 μm ZD 7288. F, similarity in EPSP summation comparing Wistar (grey) and WAG/Rij (black) in ZD 7288. For clarity, voltage traces are shown superimposed on a normalized resting Vm. Data shown as mean ± s.e.m.
Non-stationary fluctuation analysis was performed as described in detail previously (Kole et al. 2006). In brief, ensemble recordings of ∼100 consecutively Ih traces were recorded with 10 kHz filtering (internal low-pass Bessel filter). Traces were digitally filtered at 100 Hz, analysed for variance (σ2), and plotted versus the averaged mean amplitude. Variance–mean plots were fitted with a least-square algorithm to a parabolic equation in Igor Pro 5.01 (Wavemetrics, Inc., Lake Oswego, OR, USA). The single-channel conductance, γ, was estimated by i/(V − Vrev), and the open probability, Po, determined by Po = Imax/(i N), where Imax is the average current amplitude at steady state. The Vrev with 120 mm K+ outside was estimated to be 0 mV (Kole et al. 2006).
ECoG recording
For electrocorticogram (ECoG) recording animals were anaesthetized with i.p. injections of ketamine–xylazine (80 and 12 mg kg−1) and fixed in a stereotaxic frame. During the recording period the depth of anaesthesia was regularly monitored by respiration rate, response to foot pinch and eyelid reflexes. Body temperature was maintained at 37°C with a heating pad. A part of the skull was made accessible over the frontal cortex and a low-resistance silver-ball electrode was positioned at anteroposterior +3 and mediolateral L +3 (mm from Bregma), a site where maximum amplitudes of the SWD can be found (Meeren et al. 2002). A ground electrode was positioned in muscle near the recording site. ECoG signals were obtained at 500-fold gain, analog filtered at 500 Hz, digitally sampled at 100 Hz. To minimize the known impact of ketamine on SWDs (Kandel & Buzsaki, 1997; Midzyanovskaya et al. 2004) we commenced recordings approximately 1 h after the last anaesthesia application. The appearance of SWDs was always associated with eyelid and/or whisker twitching, characteristic for seizures in this strain (Meeren et al. 2002; van Luijtelaar & Sitnikova, 2006). ECoGs were analysed using a Fourier transformation and the peaks of the power spectra determined for frequency bands between 4 and 8 and 8–12 Hz. The duration of SWDs was defined by the first and last peak of large-amplitude (> 600 μV) rhythmic spike and slow-wave discharges, lasting longer than 1 s (Meeren et al. 2002). At the end of the experiments animals were killed by decapitation.
Western blot densitometry
For Western blot analysis total protein extracts of the neocortex were subjected to SDS–polyacrylamide gel electrophoresis on 12% polyacrylamide gel and 40 μg of the soluble fraction was loaded per lane. The separated proteins were then electroblotted onto a Hybond ECL nitrocellulose membrane (Schleicher and Schuell, Germany). Immunoanalysis was performed with anti-HCN1 polyclonal antibody from Alomone Laboratories (Jerusalem, Israel), and anti-HCN2 polyclonal antibody kindly provided by Dr R. Shigemoto (National Institute for Physiological Sciences, Okazaki, Japan). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Sigma-Aldrich, Steinheim, Germany) expression was used as an internal control for standardization of protein amounts. Quantitatively similar results were obtained using β-actin for standardization (Sigma-Aldrich). Myosin 212 kDa, MBP-β-galactosidase (158 kDa), and maltose binding protein 2 (42 kDa) were used as protein molecular size references.
Western blot band density measurements were performed using a computerized videodensitometry system (Metamorph, Universal Imaging, Downingtown, PA, USA). The autoradiographs were digitized using a scanner (Micotek ScanMaker 636). For analysis of the intensity of each band, the computerized video densitometry system was used to determine the signal intensity on a pixel level. A visually established pixel intensity threshold was set to remove the unlabelled portion of the image and a standard rectangle (1.5 mm2) was defined and placed at three different positions over the band, and kept constant during analysis across different ages. For each position, the percentage of pixels within the rectangle representing signal intensities higher than the threshold was determined without further discriminating between signal intensities above this value. Threshold values were set differently for GAPDH and HCN.
Immunocytochemistry
Animals were deeply anaesthetized as earlier and perfused by vascular injection with 0.9% NaCl solution, followed by 4% paraformaldehyde in 0.1 m sodium phosphate buffer (pH 7.4). Brains were removed and cut on a vibratome. Immunocytochemistry buffers and procedures have been described in detail before (Bräuer et al. 2001). In brief, in coronal brain sections (40 μm) the endogenous peroxidase was quenched in 0.5% hydrogen peroxide diluted in PBS. After washing, brain sections were quenched again in 50 mm NH4Cl–PBS for 30 min. Sections were blocked with 10% goat serum–0.1% Saponin in PBS for 1 h at room temperature and exposed to anti-HCN1 polyclonal antibody (Alomone Laboratories), anti-HCN2 polyclonal (Dr Shigemoto), or MAP2 monoclonal antibody (Sigma) (diluted 1 : 200 or 1 : 500 in blocking solution) at 4°C over night. After washing in PBS–0.1% saponin, sections were incubated with anti-rabbit or anti-mouse biotinylated antisera overnight at 4°C and then in avidin–biotin peroxidase complex reagent (Vector Laboratories) for 2 h at room temperature. Immunoreaction was visualized with 3,3′-diaminobenzidine as a chromogen. For fluorescence detection the first antibodies were detected with Alexa-488-labelled goat anti-rabbit antibody or Alexa-564-labelled goat anti-mouse antibody (diluted 1 : 500, Molecular Probes, Invitrogen, Karlsruhe, Germany). The sections were mounted with Immu-Mount (Shandon, Waltham, MA, USA). Sections were imaged using an upright Leica confocal microscope (TCS) or with a CCD camera on a Leica DM LB microscope.
NEURON simulation
Three-dimensional reconstructions of L5 pyramidal neurons from adult Wistar and WAG/Rij animals (4–5 months of age) were converted into compartmental models using NEURON v. 5.7 for Mac OS X (Hines & Carnevale, 1997). The Wistar neuron contained 1322 segments (Fig. 7A) and the WAG/Rij neuron 1396 segments of 20 μm length (Fig. 7B). Spines were incorporated by decreasing the specific Rm (20 000 Ωcm2) and increasing Cm (1 μF cm−2) 2-fold in all dendritic compartments. Specific axial resistivity Ri was 100 Ωcm and Vm at the soma set to −79 mV, equivalent to the experimentally recorded control Vm (Supplementary Table S1). A synthetic axon was attached to the soma with a tapering diameter from 6 μm at the axon hillock to 0.9 μm in the main axon (length = 100 μm). To account for active properties models of the following voltage-gated channels were distributed with the following densities (in pS μm−2): soma: gNa = 100, gKa = 0.06, gKv = 500, gKm = 2.2, gKca = 2.5, gCa = 3, and gCa,T = 0.0008; dendrites: gNa = 100, gKa = 0.03, gKv = 54, gKm = 0.3, gKca = 2.5, gCa = 1.2, gCa,T = 0.0008. In the axon, sodium and potassium channel densities were gNa = 50 000 and gKv = 2000. The conductance density of Ih (gh) in Wistar neurons was distributed across compartments using the exponential function: gh = y0 + A exp(d/l), where y0 = −2 pS μm−2, A = 4.29 pS μm−2, λ = 324 μm and d = distance from soma (in μm) (Kole et al. 2006), obtained from an exponential fit to non-binned experimental data points which were post hoc corrected for a 5.1-fold Ih current increase due to high external K+ (Kole et al. 2006), and a free membrane area of 4.5 μm2 at the pipette tip. The kinetic Ih model together with the implementation details are available at http://senselab.med.yale.edu/senselab/modeldb/ShowModel.asp?model=64195. Experimental fitting of WAG/Rij amplitudes revealed parameters for y0 = −2 pS μm−2, A = 2.53 pS μm−2 and λ = 340 μm. Thus, we modelled the gh changes in WAG/Rij by varying the exponential constant A over a range of 0.1–8.0 pS μm−2 (Fig. 7D and F).
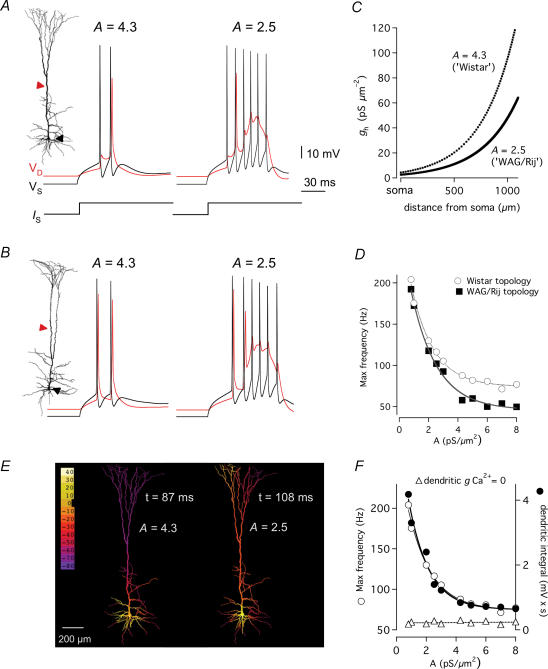
Figure 7. Non-linear dependence of AP rates on dendritic Ih channel densities in L5 model neurons.
A, model of a Wistar L5 neuron activated with somatic current injections (1.4 nA, lower trace). Implementing the Ih distribution with scaling values of A = 4.3, as observed for the Wistar density distribution (cf. Fig. 2C) elicits low-frequency firing. With A = 2.5, mimicking the WAG/Rij channel distribution, firing was converted into high-frequency bursts. B, model with a WAG/Rij neuron morphology shows that changes in the Ih distribution, as in A, leads to similar conversion in AP firing patterns. C, distance dependence of the somatodendritic distribution of Ih conductance in the model neurons, fitted with single-exponential scaling function gh = y0 + A exp(d/λ), as in Fig. 2C. D, comparison of maximum somatic firing frequencies between Wistar (○) and WAG/Rij topologies (▪) as a function of a range exponential scaling factors (A = 0.1–8.0). Note the steep non-linear increase with channel densities lower than control, with a near-complete channel block A = 0.1 inducing > 200 Hz firing frequency, mimicking the experimentally recorded ZD 7288-induced AP rates (cf. Fig. 5D). Lines represent single exponential fits to the data. E, spatial extent of somato-dendritic voltage distribution in the Wistar model neuron. Voltage scaling as indicated in colour bar. Whereas the step depolarizations induce only local depolarization in the proximal dendrites with control Ih densities (> 4.3), with low Ih densities (< 2.5) voltage spreads far into apical tuft branches due to increased somato-dendritic coupling. Images are stills from Supplemental Video S1 and S2. F, coupling between the somatic AP rates (○) and dendritic voltage integral (•) suggests a role of dendritic Ca2+ conductance. Removal of dendritic Ca2+ channels (▵) induces stable AP rates for the entire range of Ih densities. Data fitted with a linear regression.
Statistical testing
The statistical significance of differences between groups was tested using paired or unpaired two-tailed Student's t tests against a cut-off significance level (P) of 0.05. In case of multiple replicates for different age groups (e.g. Fig. 1C) we analysed the main and interaction effects with a two-way ANOVA. If significant, this was followed by Bonferroni post hoc tests for comparison of separate time points (Prism 4.0, GraphPad Software Inc., San Diego, CA, USA).
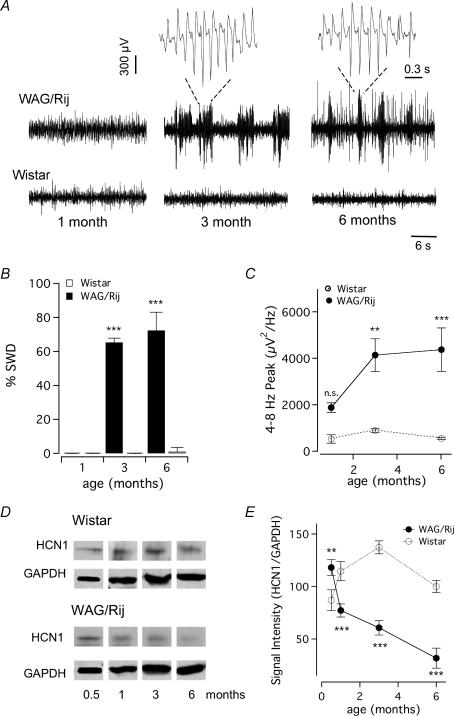
Figure 1. Ontogeny of electrocorticogram (ECoG) activity and neocortical HCN protein levels.
A, ECoG recordings of WAG/Rij (top panels) and Wistar animals (bottom panels) of 1, 3 and 6 months of age. The spike-wave discharges (SWDs) in the ECoG, shown on an expanded time scale, occur only late in development. B, analysis of percentage time spent in SWD shows that the onset occurs at ∼3 months of age (Bonferroni post hoc test, ***P < 0.0001). C, peak in power densities shows that WAG/Rij animals have pronounced large-amplitude 4–8 Hz SWD activities, starting at 3 months of age (Bonferroni post hoc test, **P < 0.01). D, Western blot examples of the HCN1 subunit and control protein (GADPH) intensity levels from equal amounts of protein from neocortex of Wistar and WAG/Rij at 0.5, 1, 3 and 6 months of age. Note the inverse age-dependent developmental pattern of HCN1. E, quantification of HCN1 protein levels was performed by plotting the optical density of HCN1/GAPDH versus age. HCN1 expression depends dynamically on age, being larger at 15 days (Bonferroni post hoc test, **P < 0.01) and then reducing from 1 month onwards compared with Wistar HCN1 (for 1, 3 and 6 months, post hoc test, ***P < 0.001). Data shown as mean ± s.e.m.
Results
Temporal development of spike-wave discharges and HCN isoforms
To investigate HCN channel expression in relation to the developmental onset of seizures we first recorded frontal electrocorticogram (ECoG) in WAG/Rij animals, and in the Wistar strain from which WAG/Rij is inbred (Coenen & van Luijtelaar, 2003), at the ages of 1, 3 and 6 months (Fig. 1A). Analysis of SWD occurrence showed that ageing was associated with the appearance of periods of large-voltage (> 600 μV; ∼5 s duration) rhythmic SWD activity (2-way ANOVA, P < 0.001) in WAG/Rij rats 3 months of age and older (Fig. 1A and B, n = 4 in each group, post hoc test, P < 0.001). This was further supported by an alternative analysis using the peaks of the power spectra for the two frequency bandwidths 4–8 Hz and 8–12 Hz, which both showed significant strain- and age-dependent differences (for each: 2-way-ANOVA, P < 0.05). Whereas the average peak amplitude in the 4–8 Hz bandwidth in WAG/Rij and Wistar animals at 1 month was similar (Fig. 1C, n = 4 in each group, post hoc test P > 0.05), at 3 and 6 months the power was greatly increased in WAG/Rij animals (Fig. 1C, 3 months: post hoc test P < 0.01; 6 months: post hoc test P < 0.001, n = 4 per group). At 6 months also the power of the ECoG in the 8–12 Hz bandwidth was increased in WAG/Rij compared with Wistar animals (n = 4, post hoc test P < 0.001, data not shown). This ontogeny of SWD occurrence and frequency shows that the inbred WAG/Rij animals from our colony follow a similar development as previously described for rats inbred for spontaneous SWDs (Vergnes et al. 1986; Coenen & van Luijtelaar, 1987; Klein et al. 2004).
We performed parallel studies to map the development of HCN1 and HCN2 expression in the cortex, the main HCN isoforms coding for Ih in the neocortex (Notomi & Shigemoto, 2004), between 2 weeks and 6 months of age. Figure 1D shows that the HCN1 immunoblot intensity, normalized to GAPDH, followed strikingly inverse developmental patterns in WAG/Rij and Wistar rats. Whereas in Wistar cortex HCN1 protein levels steadily increased in the first 3 months of age, in the WAG/Rij cortex the levels declined (Fig. 1E, 2-way ANOVA, P < 0.0001, n = 3 in each group). When compared with Wistar rats, the HCN1 protein levels decreased by 33, 56 and 68% at 1, 3 and 6 months of age, respectively (Fig. 1E; post hoc test P < 0.0001). In contrast, the HCN2 subunit protein levels were similar in WAG/Rij and control Wistar cortex for the entire age period examined (1–6 months, 3 animals per group, 2-way-ANOVA, P > 0.56, data not shown). These results show that while HCN1 protein expression in the neocortex of Wistar follows a similar developmental expression as shown previously for the hippocampus (Brewster et al. 2006), an inverse pattern is observed in WAG/Rij rats (Fig. 1D and E). Thus, the HCN1 subunit loss occurs early in development and precedes the developmental onset of SWDs.
Sub-cellular HCN distribution in WAG/Rij L5 dendrites
Cell surface expression of HCN isoforms was examined by immunolabelling in somatosensory cortex of 3-month-old Wistar and WAG/Rij animals. In Wistar cortex the immunoreactivity for HCN1 had an identical regional and subcellular distribution to previous reports (Lorincz et al. 2002; Notomi & Shigemoto, 2004), with strong immunopositive labelling in the apical dendrites of L5 cells crossing layers 2/3 and 1 (Fig. 2A, left panel). In contrast, in WAG/Rij cortex only weak labelling was found in a few apical dendrites (Fig. 2A, right panel). Immunolabelling with the HCN2 antibody showed more interspersed staining of cell somata in layers 2/3, which was similar in Wistar and WAG/Rij cortex (data not shown). Similar results were obtained in three other animals for each group.
To assess functional changes in HCN channel activity we made cell-attached patch-clamp recordings at dendritic sites up to 900 μm from the soma of large L5 pyramidal neurons in Wistar and WAG/Rij rats 2 weeks, 1 month or 3 months old. Ih current was evoked by negative voltage steps from a holding potential 25 mV depolarized to the resting membrane potential, and was blocked 82 ± 7% by bath application of 50 μm ZD 7288 (n = 3, not shown). In 1- to 3-month-old animals dendritic recordings 600–700 μm from the soma revealed a nearly 2-fold reduction in Ih amplitude over a large range of hyperpolarizing steps (Fig. 2B, bottom left; −110 to −150 mV; P < 0.05; n = 6 patches) without a change in the voltage dependence of Ih activation (Fig. 2B, bottom right). Steady-state voltage activation had a half-maximum (Vhalf) of on average −110 ± 1.5 mV, with slope constant of 10.1 ± 0.5 mV, in Wistar L5 neurons (600–700 μm; n = 9 patches). At similar distances from the soma in WAG/Rij L5 neurons Vhalf was −108.3 ± 4.9 mV (corrected for −5 mV difference in dendritic resting membrane potential; see Supplementary Table S1), with a slope factor of 9.9 ± 0.6 (n = 5, P > 0.23). The time course of Ih current onset with steps approximately to −150 mV was best fitted with a double-exponential function. In pooled data from patches 400–800 μm from the soma Ih activated with a τweighted of 36.4 ± 2.2 ms (n = 54) in control Wistar L5 dendrites, but was significantly slower in WAG/Rij dendrites (τweighted = 50.3 ± 3.9 ms, n = 36, P < 0.01, data not shown). Taken together, these data are consistent with a reduction in the faster HCN1 channel subunit.
We next investigated changes in Ih density at different locations along the apical dendrite of L5 pyramidal neurons. Current amplitudes were converted into conductance densities (pS μm−2) based on a reversal potential of 0 mV (Kole et al. 2006), assumed patch membrane area of 4.5 μm2 (see Engel & Jonas, 2005), and corrected for the 5.1-fold difference in Ih amplitude due to high external K+ (Kole et al. 2006). The dendritic distribution of conductance density (gh) in Wistar was plotted against distance from the soma (Fig. 2C, 1–3 months of age; n = 88 patches, data taken from Kole et al. (2006)) and could be well fitted by a single-exponential function (see Methods) from which it can be inferred that Ih conductance density increases e-fold for every 324 μm (λ). In patches from WAG/Rij dendrites (1–3 months of age; n = 78, 0–700 μm) Ih conductance density increased with distance with a similar e-fold scaling (λ = 340 μm), but significantly smaller exponential scaling factor (A) (2.5 in WAG/Rij versus 4.3 in Wistar), indicating that the reduction in Ih in Wag/Rij dendrites is similar at all dendritic locations (Fig. 2C). We subsequently analysed the developmental reduction in Ih by comparing the magnitude of dendritic Ih at a given distance from the soma (450–550 μm) in WAG/Rij relative to Wistar animals at 2 weeks, 1 month and 3 months of age (Fig. 2D, black circles). Consistent with the relative reduction in HCN1 protein expression in WAG/Rij cortex compared with Wistar cortex (Fig. 2D, grey crosses and exponential fit; based on data from Fig. 1E), the reduction in Ih is significant at 1 month of age, and stabilizes after 3 months.
Ih single-channel properties in Wistar and WAG/Rij dendrites
To investigate changes in single-channel properties of Ih we used non-stationary fluctuation analysis (Kole et al. 2006). We recorded approximately 100 consecutive sweeps during 100 mV hyperpolarizations in dendritic cell-attached patches from WAG/Rij and Wistar L5 pyramidal neurons (n = 4 patches each; 400–500 μm from the soma). Figure 2E shows an example of variance–mean plot for Ih in a WAG/Rij dendritic patch (∼400 μm) fitted with a parabolic function. The data yielded identical estimates for Ih single-channel conductance (γ; Wistar: 0.69 ± 0.08 pS versus WAG/Rij: 0.68 ± 0.06 pS, P > 0.2) and open probability (Wistar: 1.0 ± 0.07; WAG/Rij: 0.9 ± 0.03). In contrast, the estimated channel number (N) was ∼2-fold lower in WAG/Rij compared with control Wistar dendrites (Fig. 2E, top right panel; P < 0.05, n = 4), indicating a significant loss in number of functional Ih channels in L5 pyramidal neuron dendrites in WAG/Rij rats. Taken together, the data above indicate that seizure onset in WAG/Rij animals is temporally preceded by a loss in cell-surface expression of HCN1 channels, which due to its steep distance dependency (Kole et al. 2006) (Fig. 2C) will primarily impact on distal apical dendritic sites.
Resting properties of Wistar and WAG/Rij L5 neurons
To understand the impact of HCN1 changes on somato-dendritic integrative properties, we made somatic and dendritic whole-cell patch-clamp recordings from L5 pyramidal neurons in 3- to 5-month-old WAG/Rij and Wistar rats. Consistent with a reduction in Ih, the somatic resting membrane potential (Vm) in L5 neurons was on average ∼3 mV more hyperpolarized in WAG/Rij (−82 mV, n = 62) compared with Wistar L5 neurons (−79 mV, n = 29, P < 0.01, see Supplemental Table S1). Furthermore, the local dendritic Vm (400–500 μm from the soma) was more hyperpolarized in WAG/Rij compared with Wistar layer 5 dendrites (Supplemental Table S1), consistent with a reduction in dendritic Ih channels. In addition, WAG/Rij L5 pyramidal neurons had a significantly larger somatic input resistance (RN) (P < 0.01), and smaller sag ratio (P < 0.001, Supplemental Table S1). These strain-specific differences in resting properties were abolished by bath application of the Ih blocker ZD 7288 (50 μm; Supplemental Table S1), indicating that they are caused by differential Ih expression. Finally, these Ih-associated changes in resting membrane properties became significant at 3 months of age, and persisted up to 11 months of age in chronic epileptic WAG/Rij animals (the oldest animals examined in this study; Supplemental Fig. S1).
Increased somato-dendritic coupling and EPSP summation in WAG/Rij dendrites
Using dual whole-cell recordings from L5 neurons from 3- to 5-month-old animals we examined the consequences of dendritic Ih loss on synaptic integration. Figure 3A shows that long (800 ms) hyperpolarizing somatic current injections (1 nA) evoked from resting Vm generate time-dependent voltage responses that are attenuated at the dendritic recording site. For Wistar L5 neurons the coupling coefficient, kds, for steady-state voltage signals from soma to the dendrite (Vd/Vs) was on average 0.31 ± 0.04 (Fig. 3A and B; n = 7; ∼460 μm from the soma). Recordings at similar dendritic locations in WAG/Rij L5 neurons (on average 470 μm; n = 11) showed that kds was increased to 0.44 ± 0.04 (P < 0.02). These alterations were directly related to a reduction in dendritic Ih as blocking HCN channels with 50 μm ZD 7288 increased kds to similar levels in WAG/Rij (0.75 ± 0.0, n = 3) and Wistar (0.73 ± 0.02, n = 5, P > 0.36) L5 neurons.
The loss of a dendritic conductance, which is active at rest, would be expected to change temporal summation of dendritic excitatory postsynaptic potentials (Williams & Stuart, 2000). This was tested using a train (50 Hz) of five simulated excitatory postsynaptic potentials (sEPSP) generated using bi-expotential current waveforms injected at similar dendritic distances (Wistar: 410 ± 47.4 μm, WAG/Rij: 390 ± 19.8 μm) and recorded at the soma at resting Vm (Fig. 3C). Whereas there was no appreciable summation of successive sEPSP waveforms in control Wistar L5 neurons (Fig. 3C and D; sEPSP5/sEPSP1 = 98 ± 2.9%, n = 10), in WAG/Rij L5 recordings we observed significant temporal summation (Fig. 3C and D; 131 ± 12.7%, n = 19, P < 0.02), and a larger time integral of the somatic voltage (Wistar: 84 ± 8 μV s and WAG/Rij: 147 ± 15 μV s, P < 0.05). The pharmacological block of HCN channels normalized the difference in temporal summation (Fig. 3E and F; WAG/Rij: 214 ± 9.2%, n = 3, Wistar: 215 ± 12.4%, n = 4), indicating that differences in HCN channel expression underlie increased temporal summation in WAG/Rij L5 neurons.
Enhanced dendritic calcium electrogenesis in WAG/Rij dendrites
We next characterized the active properties of L5 neurons in WAG/Rij rats. Action potentials (APs) were elicited by brief (3 ms) somatic current steps evoked from resting potential. Analysis of back propagating APs (bAPs) indicated that the amplitude of bAPs was slightly larger, and the rate of rise of faster, in WAG/Rij compared with Wistar L5 pyramidal neuron dendrites (Fig. 4A; P < 0.05; Supplemental Table S2).
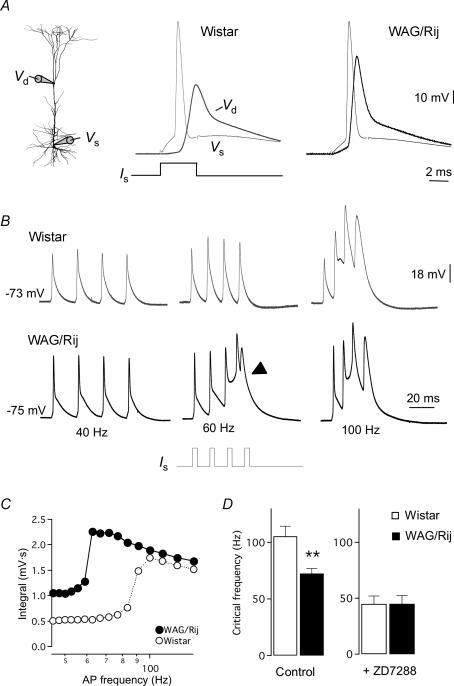
Figure 4. Enhanced Ih-mediated dendritic electrogenesis in WAG/Rij cortical L5 pyramidal neurons.
A, experimental configuration for dual whole-cell recording and injection of depolarizing current steps at the soma (lower trace, 3 nA; 3 ms, Is) to evoke a single AP with dendritic bAP. Single APs in WAG/Rij dendrites showed increased amplitude and dV/dt (right panel). Voltage traces normalized to local resting Vm. B, four consecutive somatic current injections were applied to examine the frequency dependence of dendritic calcium electrogenesis. Examples are shown for 40, 60 and 100 Hz. Note the large dendritic voltage in WAG/Rij L5 dendrites during AP trains at 60 Hz, inducing a burst (lower panel, marked with arrow). C, frequency dependence of dendritic electrogenesis of the examples shown in B, exemplifying the negative shift in critical frequency for WAG/Rij somato-dendritic recordings. D, population data of critical frequency values shows a significant reduction in WAG/Rij dendrites (Student's t test, **P < 0.01) that could be blocked after application of 50 μm ZD 7288 (t test, P > 0.5). Data shown as mean ± s.e.m.
The frequency dependence of AP backpropagation was investigated using AP trains, which showed that the generation of dendritic Ca2+ spikes occurs at significantly lower frequencies in WAG/Rij dendrites (Fig. 4B–D). The frequency threshold for induction of dendritic Ca2+ electrogenesis (critical frequency) is defined as the AP frequency where a supralinear increase in the dendritic integral occurs (Larkum et al. 1999). On average, in control Wistar L5 neurons the critical frequency was 105 Hz (Fig. 4D, left; range: 76–161 Hz, n = 13), consistent with previous findings (Larkum et al. 1999; Berger et al. 2003). In contrast, WAG/Rij L5 neurons had a significant lower critical frequency of ∼75 Hz (Fig. 4C and D; range: 53–106 Hz, n = 21, P < 0.003). Bath application of 50 μm ZD 7288 further reduced the critical frequency to ∼50 Hz for both groups (Fig. 4D; right; Wistar; n = 5, WAG/Rij; n = 3, P > 0.64), indicating that the difference in critical frequency for generation of dendritic calcium electrogenesis in Wistar and WAG/Rij L5 neurons is attributable to reduced HCN expression in WAG/Rij dendrites.
Increased burst discharges in WAG/Rij L5 neurons
Dendritically initiated calcium spikes lead to depolarizing current flow back to the soma, and can provide a source for axo-somatic burst firing (Larkum et al. 1999; Williams & Stuart, 1999). Therefore, we analysed the firing properties of L5 pyramidal neurons in Wistar and WAG/Rij animals (3 to 5 months old) during somatic current injection at resting Vm. The results showed that somatic current injection induced regular firing patterns in 20/26 (77%) of Wistar and 35/61 (57%) of WAG/Rij L5 neurons. Of these regular firing cells, the input/output (I/f) curves were not significantly different (Supplemental Fig. S2) presumably indicating that the more hyperpolarized resting Vm and increased RN in WAG/Rij neurons (Supplemental Table S1) counter-balance one another. Somatic current injection also induced burst firing in a minority (23%, 6/29 neurons) of Wistar L5 neurons (Fig. 5A). The type of burst firing observed in Wistar neurons was weak (Williams & Stuart, 1999) with an initial high-frequency discharge of two to three APs rapidly adapting to regular firing (Fig. 5A; 6/6 neurons). In sharp contrast, burst firing was observed in a significantly larger proportion (43%, 26/61 neurons) of WAG/Rij neurons. Across the entire population of recorded L5 neurons burst firing was significantly greater in WAG/Rij animals (Fig. 5C; χ2 test, P < 0.05). While there was no difference in the firing rate within a burst (Wistar; 1st interspike interval (ISI) = 218 ± 24 Hz, n = 6; WAG/Rij 1st ISI = 232 ± 12 Hz, n = 26), the burst firing patterns in Wistar and WAG/Rij L5 neurons were markedly different. In 50% (13/26) of intrinsically bursting WAG/Rij neurons the first burst discharge was followed by a repetitive series of bursts indicative of strong burst firing (Williams & Stuart, 1999) (Fig. 5B). When comparing 1- to 11-month-old WAG/Rij animals, burst firing was found to develop between 1 and 3 months of age and persisted throughout the chronic phase of epilepsy in these animals (up to 11 months of age; Supplemental Fig. S1C).
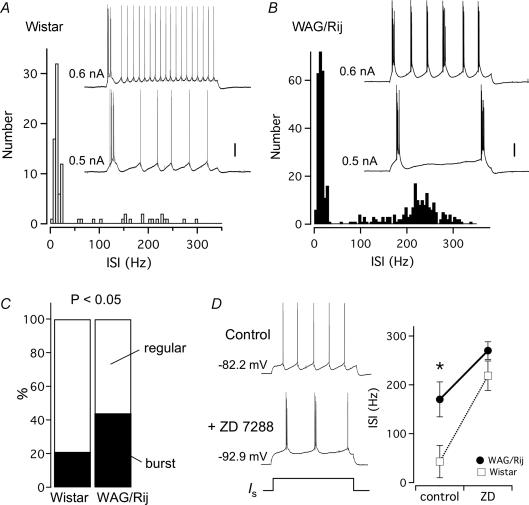
Figure 5. Increased population of intrinsically strong-bursting L5 neurons in absence epilepsy.
A, ISI histograms from bursting L5 neurons in Wistar rats (n = 6) showing the relative absence of high-frequency AP rates (> 200 Hz) (inset; scale 10 mV, Vm = −77.6 mV). B, histogram of ISI for WAG/Rij population of bursting neurons (n = 27) shows a large incidence of high-frequency action potential rates > 200 Hz, and strong bursting firing patterns (inset; scale 10 mV, Vm = −80.4 mV). C, population data for the observed percentage of regular and intrinsically bursting L5 neurons in the somatosensory cortex of WAG/Rij and Wistar rats (χ2 test, *P < 0.05). D, differences in AP rates between Wistar and WAG/Rij ISI (Student's t test, *P < 0.05) are removed after blocking Ih channels, suggesting that differences in HCN channel expression underlie AP firing differences between strains. Left, example of a control Wistar neuron before and after bath application of 50 μm ZD 7288. Data shown as mean ± s.e.m.
Previous studies have shown that activation of dendritic Na+ and Ca2+ channels is required for L5 cortical burst firing (Williams & Stuart, 1999), which is likely to be influenced by the extent of somato-dendritic coupling by Ih (Berger et al. 2003). This was examined by determining the AP firing patterns before and after ZD 7288 application, without correcting for the ZD 7288-induced hyperpolarization. Figure 5D shows that in Wistar L5 neurons regular spiking could be converted into burst firing as measured by a 5-fold increase in the frequency of the 1st ISI (n = 5, paired t test P < 0.01) but for WAG/Rij cells only a moderate shift was observed (Fig. 5D; 1.6-fold, paired t test, P > 0.12, n = 5). These data support the idea that loss of dendritic Ih in WAG/Rij L5 pyramidal neurons leads to the increase in intrinsic burst firing in these neurons.
We next investigated the impact of increased burst firing on cortical synchronization. In these experiments we recorded from pairs of L5 pyramidal neurons during spontaneous paroxysmal discharges (PDS) generated in the presence of bicuculline and low magnesium in WAG/Rij and control cortical slices (Supplemental Fig. S3). Network driven depolarizations during PSDs were significantly larger in amplitude in WAG/Rij L5 neurons; a finding that could be mimicked in control slices by the internal application of Ih blockers. These data extend our finding of increased burst firing in WAG/Rij L5 neurons to show that down-regulation of dendritic Ih also leads to increased network-driven responses under experimental seizure conditions.
Morphological characteristics of WAG/Rij L5 neurons
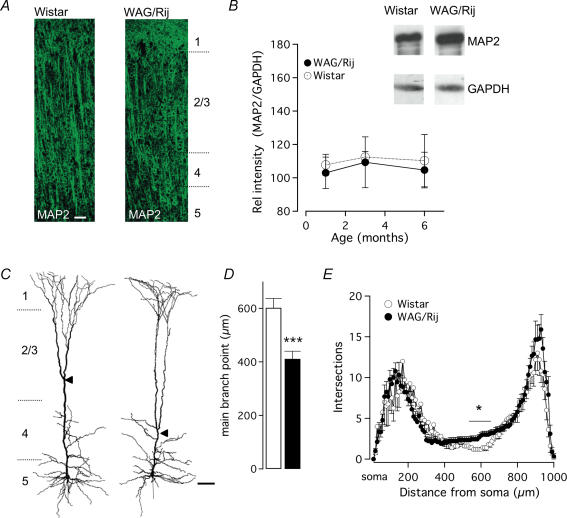
Diversity in neocortical firing patterns has previously been associated with variations in morphology (Mainen & Sejnowski, 1996). Furthermore, morphological differences in classes of L5 pyramidal neurons have been correlated with differences in the propensity to show burst firing, with thick-tufted L5 pyramidal subtypes generating intrinsic burst firing, while smaller and less arborized neurons usually showing regular spiking patterns (Chagnac-Amitai et al. 1990; Schubert et al. 2001). Morphological changes in WAG/Rij upper layer cortical cells were recently observed (Karpova et al. 2005). To determine the potential contribution of changes in dendritic structure to the observed differences in firing patterns in Wistar and WAG/Rij L5 neurons, we first examined the distribution and development of the dendritic marker MAP2 (Fig. 6A and B). The total MAP2 protein measured by immunoblot intensity was not affected by onset of epilepsy (Fig. 6B; n = 3 for Wistar and WAG/Rij in each group, P > 0.23). Next, we made three-dimensional reconstructions of biocytin-labelled L5 pyramidal neurons from WAG/Rij and Wistar cortex (Fig. 6C). The results indicated a striking change in the subcellular architecture of WAG/Rij L5 pyramidal neurons with the main bifurcation point located ∼200 μm more proximal to the soma compared with Wistar L5 neurons (Fig. 6D; WAG/Rij average: 411 μm, n = 24 cells; Wistar average: 602 μm, n = 16 cells; P < 0.001). This difference in branch point location was already present at 1 month of age (data not shown). Sholl analysis (10 μm bins) of the total number of intersections indicated that early branch splitting was associated with one to two more dendritic intersections in WAG/Rij L5 dendrites (n = 9 cells) compared with Wistar L5 dendrites (n = 4) for distances between 550 and 650 μm from the soma (Fig. 6E; P < 0.05). However, the overall size of L5 pyramidal neurons was not different based on general morphological properties (Supplemental Table S3, for all P > 0.5), consistent with the MAP2 data.
Figure 6. Morphological properties of L5 pyramidal neuron dendrites from Wistar and WAG/Rij cortex.
A, similarity in MAP2 distribution in columns of the somatosensory cortex between Wistar (left) and WAG/Rij (right). Scale bar, 100 μm. B, densitometry of MAP2 in WAG/Rij, normalized to GAPDH indicates stable levels of MAP2 without strain differences (2-way ANOVA, P > 0.23). C, three-dimensional reconstructions of biocytin-labelled L5 neurons reveal a subcellular architectural change in the location of the main bifurcation (arrowheads). Scale bar, 100 μm. D, the primary bifurcation point is located closer to the soma in WAG/Rij L5 neurons (Student's t test, ***P < 0.001). E, Sholl plot of apical dendritic intersections from Wistar and WAG/Rij L5 pyramidal neurons reveals more intersections locally between 550 and 650 μm from the soma (for all: t test, P < 0.05). Data shown as mean ± s.e.m.
Modelling the role of Ih on somato-dendritic excitability
To distinguish between the role of the observed changes in dendritic Ih and branching architecture on AP firing patterns in WAG/Rij L5 pyramidal neurons, we examined their contribution separately in multicompartmental models (Hines & Carnevale, 1997). Voltage-dependent Na+, K+ and Ca2+ channel densities were uniformly distributed throughout dendritic compartments in models generated from biocytin-labelled and reconstructed L5 pyramidal neurons from 4-month-old Wistar and WAG/Rij animals (Fig. 7A and B). The mathematical model, kinetics and distribution of Ih conductance was implemented as previously described (Kole et al. 2006). Reducing the scaling factor (A) of the exponential distribution of Ih from 4.3 to 2.5 reliably mimicked the epilepsy-related shift in Ih distribution (Fig. 7C; compare with Fig. 2C, see Methods). This led to a shift in the somatic membrane potential (Vm) and input resistance (RN) in models with the two different Ih distributions that mimicked the experimentally observed differences in resting properties in Wistar and WAG/Rij L5 neurons (Supplemental Table S1). For example, the somatic resting Vm was reduced from −79.0 to −81.6 mV, while somatic RN was increased by 18% in models with reduced dendritic Ih.
APs were generated by somatic current injections from the resting potential in the model Wistar and WAG/Rij L5 neurons. Both Wistar and WAG/Rij L5 models showed enhanced AP burst firing following a reduction in dendritic Ih (Fig. 7A and B), indicating that differences in Ih conductance rather than morphology account for the observed increased bursting firing in WAG/Rij L5 neurons. We next varied the steepness of the Ih distribution using a range of scaling factors. For Ih scaling factors between 8 and 4 (the control Wistar value) the maximum AP firing frequency was relatively constant, but higher in Wistar L5 models (Fig. 7D). In contrast, as the steepness of the exponential Ih distribution was reduced below 4 there was a significant increase in the maximum AP firing frequency in Wistar and WAG/Rij L5 models, with both models showing AP burst firing up to 200 Hz (Fig. 7D).
We next investigated the dendritic mechanisms leading to increased high-frequency burst firing in models with similar morphology but different Ih distributions. Models with a reduced Ih scaling factor of 2.5, as observed in WAG/Rij L5 neurons, showed significantly larger and spatially more distributed depolarization across the apical trunk and tuft during AP firing (Fig. 7E, Supplemental Video S1) compared with control Ih conductance densities (Fig. 7E, Supplemental Video S2), consistent with the idea that reducing dendritic Ih makes distal apical dendrites less isolated from the soma. Dendritic integral and the maximum frequency of somatic AP firing were strongly correlated and non-linearly related to dendritic Ih density (Fig. 7F, circles). This non-linear behaviour was absent in models that lacked low- and high-voltage-activated dendritic Ca2+ channels (Fig. 7F; open triangles). These simulations indicate that reductions in dendritic Ih, rather than changes in dendritic morphology, enhance the activation of dendritic voltage-activated Ca2+ channels by bAPs, increasing high-frequency burst firing in WAG/Rij L5 pyramidal neurons.
Discussion
Generalized spike-and-wave discharges (SWDs) reflect aberrant synchronicity between cortical and thalamic circuits, and are a common hallmark of pathological ECoG rhythms both in idiopathic generalized epilepsies in human (∼3 Hz) and animal models (∼7 Hz) (Crunelli & Leresche, 2002; Coenen & van Luijtelaar, 2003; Steriade & Amzica, 2003; Holmes et al. 2004). Here, we provide direct experimental evidence supported by simulations that in a genetic rodent model of absence epilepsy HCN1 channel down-regulation in the dendrites of cortical L5 neurons occurs temporally before the developmental onset of SWDs and at a cellular level plays a direct role in promoting dendritic Ca2+ electrogenesis and burst firing. These specific changes in L5 excitability are likely to have an important role in strengthening the corticothalamic drive during initiation of SWDs.
Developmental loss of HCN1 in WAG/Rij cortex
Ih channels in L5 dendrites are located in a spatially non-uniform manner and at high densities at distal dendritic locations (Kole et al. 2006) increasing the local membrane conductance and electrically isolating distal dendrites from the axonal AP initiation site. As we show here, in WAG/Rij cortex HCN1 loss decreases dendritic current density and is associated with a slowing in activation kinetics with no observed change in voltage dependence (Fig. 2). These data are consistent with Ih in layer 5 neurons being primarily mediated by HCN1 subunits (Kole et al. 2006). Based on a combined Ih which is 80% HCN1 and 20% HCN2 (Kole et al. 2006), one can predict the outcome of a reduction in HCN1 alone on both the voltage dependence of Ih activation and the weighted time constant of Ih kinetics. These predictions indicate that the observed ∼50% reduction in HCN1 will have a significantly larger impact on the kinetics of Ih activation with little effect on the half-voltage of Ih activation (G. J. Stuart & M. H. P. Kole, unpublished observations), consistent with our experimental observations. The absence of a significant effect on the voltage dependence of Ih activation in WAG/Rij L5 pyramidal neurons differs from observations in L2/3 neurons in WAG/Rij animals, which show significantly slower activation kinetics, and a depolarizing shift in Vhalf compared with control Wistar rats (Strauss et al. 2004). This difference can presumably be explained by a larger fraction of slowly activating HCN2 subunits mediating Ih in L2/3 neurons.
The molecular mechanisms involved in the developmental reduction in HCN1 cell-surface expression in WAG/Rij animals remain to be established. Given the genetic basis of the appearance and properties of SWDs (Peeters et al. 1992; Gauguier et al. 2004), and the observed early down-regulation of HCN1 cell-surface expression during ontogeny it seems possible that mutations are present in the WAG/Rij HCN1 gene sequence, or in accessory proteins involved in post-translational regulation of HCN1 channel trafficking and/or dendritic targeting such as the recently identified TRIP8b protein (Santoro et al. 2004). Based on our Ih single-channel analysis both gating conductance and kinetics appear normal (Fig. 2B and E), which suggests that in WAG/Rij animals sites downstream from the channel itself such as the regulation of HCN1 cell-surface expression or channel assembly could be impaired. In future work it will be important to determine which of these interactions are affected in the WAG/Rij model.
A dendritic origin of high-frequency firing in absence epilepsy
APs attenuate and broaden as they backpropagate into the apical dendrites of L5 neurons in vitro and in vivo (Stuart et al. 1997; Helmchen et al. 1999). In contrast, AP bursts at relatively high frequencies can lead to generation of dendritic Ca2+ electrogenesis (Stuart et al. 1997; Helmchen et al. 1999; Larkum et al. 1999; Berger et al. 2003), which provides an essential feed-forward mechanism for somatic burst firing. The reduction in HCN1 channels, partially open at rest, induces both hyperpolarization and increases in input resistance in the soma and dendrites of WAG/Rij L5 neurons. In addition, the age-dependent loss of HCN1 channels increases electric coupling and reduces the threshold for dendritic Ca2+ spikes by about ∼30 Hz, providing a broader frequency range for generation of dendritic Ca2+ spikes during axo-somatic AP firing in WAG/Rij L5 neurons (Figs 3 and 4). Consistent with this idea, we observed a significantly larger population of intrinsically bursting L5 neurons in WAG/Rij somatosensory cortex compared with Wistar controls. Given that previous data indicate that dendritic depolarization, rather than hyperpolarization, promotes burst firing in L5 pyramidal neurons (Williams & Stuart, 1999), it seems likely that the HCN1-related reduction in dendritic leak conductance underlies the increase in somato-dendritic excitability, ultimately leading to increased burst firing. Furthermore, our computational model showed that increased AP burst firing in WAG/Rij L5 neurons was not the result of differences in dendritic morphology, but rather due to increased activation of dendritic Ca2+ channels by bAPs as a consequence of reduced dendritic Ih. Thus, these findings suggest a key role of active dendritic properties in modifying AP output of L5 pyramidal neurons in this model of absence epilepsy.
Proconvulsive action of HCN1 loss
The present results extend previous work on the role of HCN in epilepsy (Chen et al. 2001; Timofeev et al. 2002; Ludwig et al. 2003; Shah et al. 2004; Strauss et al. 2004; Budde et al. 2005; Kuisle et al. 2006; Schridde et al. 2006) and argue for a proconvulsive action of HCN1 loss in the neocortex. This conclusion is based on the developmental loss of HCN1, which is significant at 1 month and stabilizes at approximately 3 months of age; a developmental stage in WAG/Rij animals associated with the onset of SWDs (see Fig. 1A–C). In contrast, cortical HCN1 protein levels reach their maximum in control Wistar animals at this time (Fig. 1) (Brewster et al. 2006). These opposing temporal patterns of cortical HCN1 channel regulation in WAG/Rij and Wistar animals are inconsistent with the concept of ‘seizure-induced’ down-regulation of HCN1, as observed in provoked epilepsy models (Chen et al. 2001; Shah et al. 2004; Brewster et al. 2005). Indirect evidence against a seizure-induced HCN1 down-regulation during absence seizures also comes from both the WAG/Rij and GAERS (the Genetic Absence Epilepsy Rat from Strasbourg) animal models in which recent work indicates that thalamocortical neurons are in fact characterized by increased HCN1 levels, continuing into the chronic epilepsy state (Budde et al. 2005; Kuisle et al. 2006). In contrast to a recent study, we did not find evidence for compensatory mechanisms opposing increased excitability of cortical layer 5 pyramidal neurons in WAG/Rij animals, as seen in thalamocortical relay neurons in GAERS rats (Kuisle et al. 2006). The Ih-dependent changes in active and passive membrane properties only became significant at 3 months of age and persisted up to 11 months in WAG/Rij L5 neurons (Supplemental Fig. S1), and were mimicked in our simulations by loss of HCN conductance alone (Fig. 7).
Given the known polygenic basis of absence epilepsy in rodents (Peeters et al. 1992; Gauguier et al. 2004) and human idiopathic epilepsies (Mulley et al. 2003) it seems likely that HCN1 channel loss in the WAG/Rij model acts in concert with other changes in voltage- or ligand-gated channels (van Luijtelaar & Sitnikova, 2006) to ultimately lead to epileptogenesis. For example, among the channels studied in the WAG/Rij cortex so far higher amounts of the protein levels of Nav1.1 and Nav1.6 sodium channel isoforms were observed in the somatosensory area (Klein et al. 2004). Up-regulation of Na+ channels may account for the observed increase in amplitude and dV/dt of the bAP in WAG/Rij L5 dendrites (Fig. 4A, Supplemental Table S2). Against this idea, however, we observed that somato-dendritic Na+ current densities in L5 neurons were similar in age-matched Wistar and WAG/Rij animals (M. H. P. Kole, unpublished observations). Previous work also indicates that dendritic HCN1 interacts with both excitatory and inhibitory synaptic potentials in L5 pyramidal neurons, reducing their amplitude and duration (Williams & Stuart, 2000, 2003). Loss of distal dendritic HCN1 may therefore underlie or act synergistically with the reported increase in N-methyl-d-aspartate (NMDA)-dependent synaptic excitability in deep layer pyramidal neurons in vivo in the WAG/Rij cortex (D'Antuono et al. 2006).
A cortical focus of seizure initiation
It is well known that SWDs require the bi-lateral network activity of reciprocally connected thalamic and cortical structures. Recent recordings at high temporal resolution of cortical and thalamic activity during SWDs have found that the first ∼0.5 s of SWDs is dominated by cortical activity (Seidenbecher et al. 1998; Meeren et al. 2002; Pinault, 2003; Pinault et al. 2006) with a focal origin in the oro-facial region of primary sensory cortex (Meeren et al. 2002). One reason this may be the case is that the normal wake-related 7–12 Hz rhythm of the trigeminal somatosensory system during whisking shares features with the activity associated with SWDs (Meeren et al. 2002; Nicolelis & Fanselow, 2002; Wiest et al. 2005; van Luijtelaar & Sitnikova, 2006). During whisking, a behaviour implicated in sensory discrimination processing (Fanselow et al. 2001; Nicolelis & Fanselow, 2002), the somatosensory system expresses oscillatory activity, initiated by burst firing of corticothalamic neurons in S1 cortex, and spreading seconds later to the thalamus (Nicolelis & Fanselow, 2002). These physiologically relevant whisker rhythms could launch paroxysmal oscillation patterns by engaging large ensembles of rhythmically burst-firing L5 cortical neurons, which we found to be intrinsically primed for high-frequency firing in the WAG/Rij animal. Indeed, in the GAERS rat model absence epilepsy L5 neurons show a high degree of burst firing in vivo during all cycles of the SWD (Pinault, 2003). Such a role of L5 neurons in SWD initiation would be consistent with these neurons acting as a driver in relaying information within corticothalamic networks by evoking large amplitude all-or-none postsynaptic responses in the thalamic posterior medial nucleus (Reichova & Sherman, 2004). In contrast, corticothalamic layer 6 neurons, although sending far more abundant projections to the thalamus, generate modulating small-amplitude synaptic responses in thalamic relay cells (Reichova & Sherman, 2004).
Our identification of a cellular source of cortical burst firing also has important implications for the evaluation of anti-absence drugs. For example, the application of the first-choice anti-absence drug ethosuximide directly to S1 cortex suppresses SWD as efficiently as systemic application (Manning et al. 2004). Although the molecular mechanism by which this is achieved is controversial, the supposed inactivation of low-threshold Ca2+ channels (Gomora et al. 2001) would be compatible with inhibiting the local interplay of dendritic Ih and Ca2+ channels, and thereby suppressing dendritic electrogenesis (Fig. 7D–F). Interestingly, the anti-convulsant lamotrigine, which shifts the voltage activation of Ih positively by ∼10 mV (Poolos et al. 2002), is ineffective in reducing SWD activity in WAG/Rij rats (Coenen & van Luijtelaar, 2003). This suggests that increases in cortical HCN1 channel density, rather than changes in voltage dependence, are required to reduce SWDs associated with absence epilepsy. Consistent with this prediction, early postnatal handling of WAG/Rij animals reduces the number of SWDs later in life, and is accompanied by a persistent up-regulation of HCN1 (Schridde et al. 2006).
Taken together, the present identification of the cellular mechanisms underlying intrinsic cortical burst firing in a genetic rat model of absence epilepsy contributes to an understanding of the cortical basis of idiopathic generalized epilepsies and lends support to the idea that the mechanisms and accessory molecules involved in HCN1 expression and targeting are promising therapeutic targets for the treatment of absence seizures.
Acknowledgments
We thank Stephen R. Williams, Stefan Hallermann, and members of the lab for helpful discussion and comments. This work was supported by the National Health and Medical Research Council (NH and MRC) of Australia (G.J.S and M.H.P.K).
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.122028
http://jp.physoc.org/cgi/content/full/jphysiol.2006.122028/DC1 and contains three figures, three tables and two videos (voltage space plots of a NEURONsimulation) as supplemental material.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Avoli M, Gloor P, Kostopoulos G, Gotman J. An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. J Neurophysiol. 1983;50:819–837. doi: 10.1152/jn.1983.50.4.819. [DOI] [PubMed] [Google Scholar]
- Berger T, Senn W, Luscher HR. Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J Neurophysiol. 2003;90:2428–2437. doi: 10.1152/jn.00377.2003. [DOI] [PubMed] [Google Scholar]
- Bräuer AU, Savaskan NE, Kole MHP, Plaschke M, Monteggia LM, Nestler EJ, Simburger E, Deisz RA, Ninnemann O, Nitsch R. Molecular and functional analysis of hyperpolarization-activated pacemaker channels in the hippocampus after entorhinal cortex lesion. Faseb J. 2001;15:2689–2701. doi: 10.1096/fj.01-0235com. [DOI] [PubMed] [Google Scholar]
- Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb Cortex. 2006 doi: 10.1093/cercor/bhk021. 10.1093/cercor/bhk021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Caputi L, Kanyshkova T, Staak R, Abrahamczik C, Munsch T, Pape HC. Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. J Neurosci. 2005;25:9871–9882. doi: 10.1523/JNEUROSCI.2590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale NT, Johnston D. Electrophysiological characterization of remote chemical synapses. J Neurophysiol. 1982;47:606–621. doi: 10.1152/jn.1982.47.4.606. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol. 1990;296:598–613. doi: 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen AM, van Luijtelaar EL. The WAG/Rij rat model for absence epilepsy: age and sex factors. Epilepsy Res. 1987;1:297–301. doi: 10.1016/0920-1211(87)90005-2. [DOI] [PubMed] [Google Scholar]
- Coenen AM, van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- D'Antuono M, Inaba Y, Biagini G, D'Arcangelo G, Tancredi V, Avoli M. Synaptic hyperexcitability of deep layer neocortical cells in a genetic model of absence seizures. Genes Brain Behav. 2006;5:73–84. doi: 10.1111/j.1601-183X.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Keegan KD, Noebels JL. Increased excitability and inward rectification in layer V cortical pyramidal neurons in the epileptic mutant mouse Stargazer. J Neurophysiol. 1997;77:621–631. doi: 10.1152/jn.1997.77.2.621. [DOI] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Sameshima K, Baccala LA, Nicolelis MA. Thalamic bursting in rats during different awake behavioral states. Proc Natl Acad Sci U S A. 2001;98:15330–15335. doi: 10.1073/pnas.261273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauguier D, van Luijtelaar G, Bihoreau MT, Wilder SP, Godfrey RF, Vossen J, Coenen A, Cox RD. Chromosomal mapping of genetic loci controlling absence epilepsy phenotypes in the WAG/Rij rat. Epilepsia. 2004;45:908–915. doi: 10.1111/j.0013-9580.2004.13104.x. [DOI] [PubMed] [Google Scholar]
- Gomora JC, Daud AN, Weiergraber M, Perez-Reyes E. Block of cloned human T-type calcium channels by succinimide antiepileptic drugs. Mol Pharmacol. 2001;60:1121–1132. [PubMed] [Google Scholar]
- Helmchen F, Svoboda K, Denk W, Tank DW. In vivo dendritic calcium dynamics in deep-layer cortical pyramidal neurons. Nat Neurosci. 1999;2:989–996. doi: 10.1038/14788. [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Brown M, Tucker DM. Are ‘generalized’ seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45:1568–1579. doi: 10.1111/j.0013-9580.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci. 1997;17:6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova AV, Bikbaev AF, Coenen AM, van Luijtelaar G. Morphometric Golgi study of cortical locations in WAG/Rij rats: the cortical focus theory. Neurosci Res. 2005;51:119–128. doi: 10.1016/j.neures.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Kole MHP, Hallermann S, Stuart GJ. Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci. 2006;26:1677–1687. doi: 10.1523/JNEUROSCI.3664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuisle M, Wanaverbecq N, Brewster AL, Frere SG, Pinault D, Baram TZ, Luthi A. Functional stabilization of weakened thalamic pacemaker channel regulation in rat absence epilepsy. J Physiol. 2006;575:83–100. doi: 10.1113/jphysiol.2006.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Kaiser KM, Sakmann B. Calcium electrogenesis in distal apical dendrites of layer 5 pyramidal cells at a critical frequency of back-propagating action potentials. Proc Natl Acad Sci U S A. 1999;96:14600–14604. doi: 10.1073/pnas.96.25.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Manning JP, Richards DA, Leresche N, Crunelli V, Bowery NG. Cortical-area specific block of genetically determined absence seizures by ethosuximide. Neuroscience. 2004;123:5–9. doi: 10.1016/j.neuroscience.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzyanovskaya IS, Salonin DV, Bosnyakova DY, Kuznetsova GD, van Luijtelaar EL. The multiple effects of ketamine on electroencephalographic activity and behavior in WAG/Rij rats. Pharmacol Biochem Behav. 2004;79:83–91. doi: 10.1016/j.pbb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Mulley JC, Scheffer IE, Petrou S, Berkovic SF. Channelopathies as a genetic cause of epilepsy. Curr Opin Neurol. 2003;16:171–176. doi: 10.1097/01.wco.0000063767.15877.c7. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Fanselow EE. Thalamocortical optimization of tactile processing according to behavioral state. Nat Neurosci. 2002;5:517–523. doi: 10.1038/nn0602-517. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Peeters BW, Kerbusch JM, Coenen AM, Vossen JM, van Luijtelaar EL. Genetics of spike-wave discharges in the electroencephalogram (EEG) of the WAG/Rij inbred rat strain: a classical Mendelian crossbreeding study. Behav Genet. 1992;22:361–368. doi: 10.1007/BF01066667. [DOI] [PubMed] [Google Scholar]
- Pinault D. Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5–9 Hz oscillations. J Physiol. 2003;552:881–905. doi: 10.1113/jphysiol.2003.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D, Slezia A, Acsady L. Corticothalamic 5–9 Hz oscillations are more pro-epileptogenic than sleep spindles in rats. J Physiol. 2006;574:209–227. doi: 10.1113/jphysiol.2006.108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP. The yin and yang of the H-channel and its role in epilepsy. Epilepsy Curr. 2004;4:3–6. doi: 10.1111/j.1535-7597.2004.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel c-terminal protein–protein interaction. J Neurosci. 2004;24:10750–10762. doi: 10.1523/JNEUROSCI.3300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, Strauss U, Bräuer AU, van Luijtelaar EL. Environmental manipulations early in development alter seizure activity, Ih and HCN1 protein expression later in life. Eur J Neurosci. 2006;23:3346–3358. doi: 10.1111/j.1460-9568.2006.04865.x. [DOI] [PubMed] [Google Scholar]
- Schubert D, Staiger JF, Cho N, Kotter R, Zilles K, Luhmann HJ. Layer-specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci. 2001;21:3580–3592. doi: 10.1523/JNEUROSCI.21-10-03580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Staak R, Pape HC. Relations between cortical and thalamic cellular activities during absence seizures in rats. Eur J Neurosci. 1998;10:1103–1112. doi: 10.1046/j.1460-9568.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- Shah MM, Anderson AE, Leung V, Lin X, Johnston D. Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons. Neuron. 2004;44:495–508. doi: 10.1016/j.neuron.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Sleep oscillations developing into seizures in corticothalamic systems. Epilepsia. 2003;44(Suppl. 12):9–20. doi: 10.1111/j.0013-9580.2003.12006.x. [DOI] [PubMed] [Google Scholar]
- Strauss U, Kole MHP, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol. 1997;505:617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci. 1998;18:3501–3510. doi: 10.1523/JNEUROSCI.18-10-03501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Bazhenov M, Sejnowski T, Steriade M. Cortical hyperpolarization-activated depolarizing current takes part in the generation of focal paroxysmal activities. Proc Natl Acad Sci U S A. 2002;99:9533–9537. doi: 10.1073/pnas.132259899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luijtelaar G, Sitnikova E. Global and focal aspects of absence epilepsy: The contribution of genetic models. Neurosci Biobehav Rev. 2006;30:983–1003. doi: 10.1016/j.neubiorev.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Depaulis A, Micheletti G, Warter JM. Ontogeny of spontaneous petit mal-like seizures in Wistar rats. Brain Res. 1986;395:85–87. doi: 10.1016/s0006-8993(86)80011-7. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Micheletti G, Reis J, Depaulis A, Rumbach L, Warter JM. Spontaneous paroxysmal electroclinical patterns in rat: a model of generalized non-convulsive epilepsy. Neurosci Lett. 1982;33:97–101. doi: 10.1016/0304-3940(82)90136-7. [DOI] [PubMed] [Google Scholar]
- Wiest MC, Bentley N, Nicolelis MA. Heterogeneous integration of bilateral whisker signals by neurons in primary somatosensory cortex of awake rats. J Neurophysiol. 2005;93:2966–2973. doi: 10.1152/jn.00556.2004. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J Physiol. 1999;521:467–482. doi: 10.1111/j.1469-7793.1999.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic Ih in neocortical pyramidal neurons. J Neurophysiol. 2000;83:3177–3182. doi: 10.1152/jn.2000.83.5.3177. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Voltage- and site-dependent control of the somatic impact of dendritic IPSPs. J Neurosci. 2003;23:7358–7367. doi: 10.1523/JNEUROSCI.23-19-07358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.