Abstract
For almost one hundred years, the exact role of human brain structures controlling the cardiorespiratory response to exercise (‘central command’) has been sought. Animal experiments and functional imaging studies have provided clues, but the underlying electrophysiological activity of proposed relevant neural sites in humans has never been measured. In this study, local field potentials were directly recorded in a number of ‘deep’ brain nuclei during an exercise task designed to dissociate the exercise from peripheral feedback mechanisms. Several patient groups had electrodes implanted sterotaxically for the treatment of movement disorder or chronic pain. Fast Fourier transform analysis was applied to the neurograms to identify the power of fundamental spectral frequencies. Anticipation of exercise resulted in increases in heart rate, blood pressure and ventilation. The greatest neural changes were found in the periaqueductal grey area (PAG) where anticipation of exercise was accompanied by an increase of 43% in the power of the 12–25 Hz frequency band (P = 0.007). Exercise increased the activity by 87% compared to rest (P = 0.006). Changes were also seen in the 60–90 Hz band when anticipation or exercise increased power by 32% (P = 0.006) and 109% (P < 0.001), respectively. In the subthalamic nucleus there was a reduction in the power of the beta frequency during both anticipation (7.6 ± 0.68% P = 0.001) and exercise (17.3 ± 0.96% P < 0.001), whereas an increase was seen with exercise only at higher frequencies (93 ± 1.8% P = 0.007). No significant changes were seen in the globus pallidus during anticipation of exercise. We provide direct electrophysiological evidence highlighting the PAG as an important subcortical area in the neural circuitry of the cardiorespiratory response to exercise, since stimulation of this structure is known to alter blood pressure in awake humans.
The precise role the brain plays in setting the cardiorespiratory response to dynamic exercise so that the body's metabolic demands are met is still not fully understood. Krogh & Lindhard (1913) proposed a role for higher brain centres (‘central command’) where heart rate (HR), arterial blood pressure (ABP) and pulmonary ventilation (V˙E) could be altered by manipulating the subject's perception of exercise. This resulted in a cardiorespiratory response that anticipated the perceived work rate and provided the first, albeit circumstantial, evidence, for the concept of ‘central command’. Others have reported similar results using hypnotic suggestion of exercise which focuses attention on the motor task whilst uncoupling the task from the movement itself; modest cardiovascular changes were reported, whereas the respiratory responses were more dramatic (Morgan et al. 1976; Thornton et al. 2001; Williamson et al. 2001).
The neurocircuitry underpinning the cardiorespiratory response to exercise is not established although there are several target areas from previous work that may provide clues. Using positron emission tomography (PET) and one-legged exercise, a role for the contralateral primary motor cortex in the area concerned with motor control of the leg and nearby diaphragm has been identified, together with the supplementary and premotor areas (Fink et al. 1995). These areas are also activated when V˙E is increased as a result of subjects imagining exercise without the movement itself (Thornton et al. 2001). In addition, activation was seen in the dorsolateral prefrontal cortex, and it was suggested that these areas, which are involved in storing motor tasks based on working memory, may provide a site for initiating a behavioural or learnt component of exercise hyperpnoea. The small increase in HR associated with imagination of exercise may be associated with activation of the insular cortex, a site that projects to cardiac vagal motor neurons (Williamson et al. 2001).
Although higher cortical sites are involved in setting the command signal to exercise, the role of midbrain nuclei in driving both locomotion and cardiorespiratory responses is less clear. Whether subcortical structures act as relay stations or are important synaptic sites for generating the cardiorespiratory response to exercise is not known. Electrical stimulation of the hypothalamus, basal ganglia, and mesencephalic locomotor region (MLR) in decorticate preparations drives locomotion in association with cardiorespiratory responses similar to those occurring during volitional exercise (Smith et al. 1960; Eldridge et al. 1981; Eldridge et al. 1985). The cardio-respiratory responses persist following paralysis (‘fictive locomotion’), indicating that they are not necessarily related to afferent feedback from exercising muscle (Eldridge et al. 1981, 1985). In awake humans, we have reported that direct electrical stimulation of basal ganglia nuclei, in particular the subthalamic nucleus and thalamus can cause HR and ABP to increase without muscle movement (Thornton et al. 2002). More recently we have seen that stimulation of the periaqueductal grey area (PAG) in humans produces profound increases or decreases in ABP. This response is dependent on the precise location of the electrode within the PAG (Green et al. 2005). Although the PAG is not regarded as a site for central command in exercise, the above studies indicate that descending activity from subcortical structures could be involved in ‘parallel activation’ of both locomotor and cardiorespiratory systems during exercise and contribute to the neurocircuitry of ‘central command’.
We have re-visited the paradigm of Krogh & Lindhard (1913) to test the hypothesis that neural activity in subcortical structures recorded from humans who have deep brain stimulating electrodes chronically implanted is directly related to changes in HR, ABP and V˙E when they are altered by anticipation of exercise and actual exercise. By uncoupling peripheral feedback from the movement itself, we seek to establish whether the subcortical structures provide neural circuitry that is involved in the anticipatory cardiorespiratory response to exercise in humans.
Methods
Twelve patients (10 male, 2 female, mean age 47.5 years) undergoing deep brain stimulation (DBS) were selected for this study (Table 1). Five patients underwent stimulation of the subthalamic nucleus (STN) for Parkinson's disease (mean age 58.4 years); four had globus pallidus interna (GPi) stimulation for generalized dystonia (mean age 37.8 years) and three had periaqueductal grey (PAG) stimulation for the treatment of chronic neuropathic pain (mean age 42.3 years). Patients were excluded if they were (a) considered unable to exercise for any prolonged length of time, (b) hypertensive, or (c) on medication that was likely to affect HR and ABP, such as beta blockers. Fully informed consent was obtained prior to both surgery and the study, and permission was obtained from the local ethics committee (Oxfordshire REC C: 05/Q1605/47); the study conformed with the Declaration of Helsinki.
Table 1.
Patient Characteristics
| Nucleus | Patient | Age (year) | Sex | Diagnosis | Medication (total/24 h) |
|---|---|---|---|---|---|
| STN | 1 | 55 | M | Parkinson's disease | Apomorphine 7 mg, Cabergoline 3 mg |
| 2 | 57 | F | Parkinson's disease | Sinemet 500 mg, Pramipexole 5.6 mg, Thyroxine 150 μg | |
| 3 | 58 | M | Parkinson's disease | Trihexphenidyl 10 mg | |
| 4 | 58 | M | Parkinson's disease | Madopar 62 mg, Madopar CR 250 mg, Selegiline 10 mg, Amantadine 100 mg, Cabergoline 6 mg, Amitriptylline 10 mg, Gabapentin 500 mg, Co-codamol | |
| 5 | 65 | M | Parkinson's disease | Sinemet 100 mg, Sinemet CR 800 mg, Entacapone 800 mg, Ropinirole 24 mg, Amitriptylline 25 mg, Co-codamol | |
| GPi | 6 | 25 | M | Dystonic tremor | Mysoline 750 mg |
| 7 | 53 | M | Generalized dystonia | Nil | |
| 8 | 26 | M | Spasmodic torticollis | Nil | |
| 9 | 53 | M | Generalized dystonia | Atenolol 50 mg | |
| PAG | 10 | 29 | M | Neuropathic pain (shoulder) | Paracetamol/Ibuprofen as needed |
| 11 | 58 | M | Neuropathic pain (oral) | Gabapentin 800 mg bd | |
| 12 | 35 | F | Trigeminal neuralgia | Gabapentin 500 mg bd |
Surgical procedure
Our surgical procedure for localization of electrodes in the PAG has been described (Green et al. 2005). In brief, preoperative magnetic resonance (MR) images of the patient's brain were fused to a stereotactic computed tomography (CT) scan on the day of surgery using Image Plan software (Integra, Radionics, Burlington, MA, USA) (Papanastassiou et al. 1998). PAG (pain) patients and STN (Parkinson's disease) patients had their surgery performed whilst awake under local anaesthetic and GPi (dystonia) patients were implanted under general anaesthetic. Electrodes (Medtronic 3387, Medtronic, Minneapolis, MN, USA) consisted of four cylindrical contacts of 1.5 mm, spaced at 1 mm. Targets were calculated visually as follows: the PAG at the level of the superior colliculus, 2–4 mm lateral to the cerebral aqueduct on the side contralateral to the pain; STN with the middle two of the four contacts within the dorsal part of the nucleus, bilaterally; GPi (posteroventral part) with the lowest contact just touching the optic tract, bilaterally. The electrodes were tunnelled laterally and externalized for a week of testing, and if clinical effect was shown, were internalized and connected to the Kinetra (Medtronic, Minneapolis, MN, USA) implantable pulse generator that was positioned in a subcutaneous pocket in the chest wall 1 week later under general anaesthetic.
Experimental protocol
Experiments took place at least 2 h after any meal. Patients took their usual anti-Parkinson's medication, where appropriate, but pain and dystonia patients refrained from taking any medication on the day of testing. Exercise took place in the semirecumbent position on a custom made examination couch, with a pedal ergometer attached to one end. The load was fixed at 15 W. After resting for approximately 4 min, subjects were alerted to exercise by an oral cue. One of the investigators counted a 10 s ‘anticipatory’ interval, the end of which, ‘Go’, was the signal to start exercising. Patients then performed ‘light exercise’ (60–80 revolutions per minute) for at least 30 s (up to 1 min), at which time another oral cue and countdown signalled them to rest. This was repeated five times, with approximately 1 min of rest in between each exercise session.
Experimental measurements
Non-invasive continuous finger ABP was measured with a Portapres (Finapres Medical Systems, Amsterdam, the Netherlands). Although we used the height correction unit, the pressure transducer and finger cuff were positioned at heart level to increase accuracy. Lead II electrocardiogram was recorded using disposable adhesive Ag/AgCl electrodes (H207PT, Kendall-LTP, MA, USA) and amplified ×1000 (CED 1902, Cambridge Electronic Design, Cambridge, UK). The finger pressure and ECG were digitized at 4 kHz with 16-bit resolution (CED 1401 Mark II, Cambridge Electronic Design, Cambridge, UK) using Spike II software (version 5.0, Cambridge Electronic Design). Measurement of respiratory frequency was made using a custom built detector of thoracic expansion/contraction (based on changes of pressure in a convoluted tube as the patient breathed in and out), and digitized as above.
Local field potentials (LFPs) were simultaneously recorded with bipolar configuration from the adjacent four circumferential contacts of each DBS macroelectrode. Signals were filtered at 0.5–500 Hz and amplified (×10 000) using isolated CED (1902) amplifiers and digitized using CED 1401 Mark II at a rate of 4 kHz (Cambridge Electronic Design). LFPs were then displayed online and saved onto hard disk using Spike II.
Data analysis
The postoperative MRI or CT scan (fused to the preoperative MRI) for each patient was used to determine the electrode location in each nucleus. For each of the five trials for each patient, LFP data were broken into epochs of 10 s duration from each of rest, anticipation of exercise, exercise and recovery. To identify the fundamental spectral frequencies, a transformation of each of the 10 s epochs was performed from the time domain into the frequency domain by applying a fast Fourier Transform (FFT) algorithm offline using a digital spectrum analyser (Matlab, v 6.5 MathWorks Inc., Natick, MA, USA). Signals were re-sampled at a rate of 1000 Hz and 50 Hz frequencies were filtered to avoid the inclusion of mains artefact. A Hanning window of 1.5 s in width was selected so that the signal could be carefully examined. The area under each power spectrum for each condition (e.g. rest, anticipation, etc.) was calculated for the following frequency bands: 4–8, 8–12, 12–25, 25–60 and 60–90 Hz. Mean arterial pressure (MAP) was calculated from the blood pressure trace and heart rate from the ECG trace, for the same time data segments used in the LFP analysis. For statistical purposes, n refers to the number of observations per patient, taking into account that STN and GPi patients had bilateral electrodes. Therefore, five STN patients had a total of 50 observations (5 trials, 2 electrodes each), four GPi patients had 40 observations, and three PAG patients had 15 observations. There were a total of 60 cardiorespiratory observations (5 trials in 12 patients). Any significant differences between the five conditions were assessed by one-way analysis of variance (ANOVA) in SPSS (v 13, SPSS Inc., Chicago, IL, USA). Post hoc analysis of each condition was performed using Tukey's test of the power values for the condition (e.g. anticipation) versus the resting condition in each patient, followed by a Bonferroni correction for small sample size. All results are displayed as the mean ± standard error of the mean.
Results
Cardiorespiratory parameters
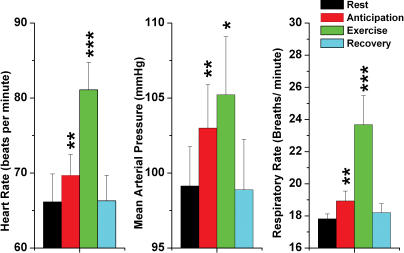
Figure 1 summarizes the changes in cardiovascular parameters and respiratory frequency consequent upon anticipation of exercise, actual exercise and recovery. HR increased during anticipation (P = 0.006, n = 60, t test), and during exercise (P < 0.001, n = 60, t test). Recovery showed a return to resting parameters. Similarly, MAP increased during anticipation (P = 0.009, n = 60, t test), and during exercise (P = 0.017, n = 60, t test), also returning to resting levels during recovery. Respiratory frequency also increased during anticipation (P = 0.008, n = 60, t test) and during exercise (P < 0.001, n = 60, t test), returning to baseline levels after the cessation of exercise. Analysis of cardiorespiratory changes by ANOVA did not reveal significant differences in any of the patients during imagined or actual exercise.
Figure 1. Changes in cardiorespiratory parameters with exercise and anticipation of exercise.
Note that all three parameters, i.e. heart rate, mean arterial pressure and respiratory rate, increased with anticipation, and further with exercise. Similarly, all parameters recovered after exercise was stopped. Error bars show 1 standard error of the mean; **P < 0.01, ***P < 0.001.
Subthalamic nuclei local field potentials
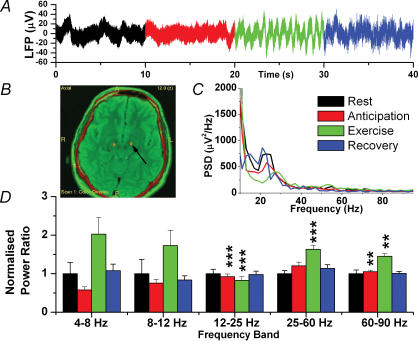
Figure 2 shows a typical electrode placement in a patient with bilateral STN electrodes, and changes in power spectral density (PSD) averaged across all five patients. ANOVA revealed that there was a difference among the conditions (rest, anticipation of exercise, actual exercise, and recovery, P < 0.001) for all patients. However, recovery did not significantly differ from rest.
Figure 2. Local Field Potential Changes in the STN.
A, raw data trace. B, postoperative image (postoperative CT fused to preoperative MR) showing bilateral electrodes in STN (arrow). C, mean power spectral density for all five patients (recovery not shown for clarity). D, normalized spectral changes (rest = 1.0) divided into frequency bands. **P < 0.01, ***P < 0.001.
The major changes in the STN occurred in the higher frequency bands. In the 12–25 Hz (beta) frequency band, anticipation was associated with a reduction in PSD of −7.6 ± 0.68%, and in exercise an even greater reduction of −17.3 ± 0.96% (P = 0.001 and P < 0.001, respectively, n = 50). In the 25–60 Hz (lower gamma) band, exercise was associated with a 93 ± 1.8% increase in PSD (P = 0.007, n = 50). In the highest band (60–90 Hz), both anticipation and exercise were associated with an increase in PSD (5.1 ± 0.7%, P = 0.002 and 45.0 ± 7.2%, P = 0.005, respectively).
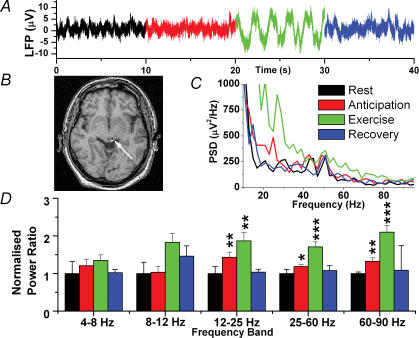
Periaqueductal grey area local field potentials
Figure 3 shows the changes in the PAG and a postoperative image. Again, changes only occurred in the upper three frequency bands but the results were fundamentally different from STN in that changes on anticipation were more prominent and PSD always increased with both anticipation and exercise. The largest change in anticipation was in the 12–25 Hz frequency band where PSD increased by 43.2 ± 13% (P = 0.007, n = 15). Exercise increased the activity by 87 ± 21% compared to resting (P = 0.006, n = 15). The second largest changes were seen in the 60–90 Hz band where anticipation and exercise increased PSD by 32 ± 8% (P = 0.006, n = 15) and 109 ± 18% (P < 0.001, n = 15), respectively. Increases were also seen in the 25–60 Hz band.
Figure 3. Local field potential changes in the PAG.
A, raw data trace. B, postoperative MR image showing a unilateral electrode in the left PAG (arrow). C, mean power spectral density for all three patients (recovery not shown for clarity). D, normalized spectral changes (rest = 1.0) divided into frequency bands. *P < 0.05, **P < 0.01, ***P < 0.001.
Globus pallidus interna local field potentials
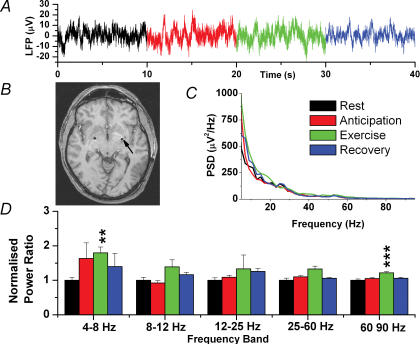
Figure 4 shows that, in contrast to both STN and PAG, there were few changes in the GPi in any of the conditions. In fact, there were no significant differences between rest and anticipation. However, exercise did increase the PSD of the 4–8 Hz band by 79.5 ± 17.1% (P = 0.009, n = 40) and the 60–90 Hz band by 21.5 ± 3.9% (P < 0.001, n = 40).
Figure 4. Local field potential changes in the GPi.
A, raw data trace. B, postoperative MR image showing bilateral electrodes in the GPi (arrow). C, mean power spectral density for all four patients (recovery not shown for clarity). D, normalized spectral changes (rest = 1.0) divided into frequency bands. **P < 0.01, ***P < 0.001.
Discussion
Anticipation of exercise, with associated increases in cardiorespiratory variables, is correlated with an increase in periaqueductal grey (PAG) activity suggesting that it is directly involved in the neurocircuitry of central command before the actual onset of movement, whereas subthalamic nucleus (STN) activity decreased. During exercise itself, PAG activity further increases alongside increases in STN activity. When taken together with animal data, albeit in decorticate preparations, we provide direct neurophysiological evidence in the human that these structures are involved in an aspect of the central command response to anticipation of exercise, and actual exercise.
Role of the subthalamic nucleus during exercise
We have shown that an exercise related depression in the beta band occurs in the STN with anticipation of exercise and with exercise itself. Since STN LFPs are likely to be a consequence of local synchronous neuronal population activity, suppression of the beta band reflects reductions in network synchrony or network size. This observation is consistent with a host of recent evidence that indicates STN suppression in the 8–10 Hz band following behaviourally relevant stimuli such as after warning and ‘go’ cues (Cassidy et al. 2002; Levy et al. 2002; Priori et al. 2002; Williams et al. 2003; Kuhn et al. 2004; Doyle et al. 2005). Movement-related suppression in the 11–30 Hz band does not seem to be related to the Parkinsonian state per se, as it is also manifest in the striatum of healthy monkeys (Courtemanche et al. 2003) and in healthy human putamen (Sochurkova & Rektor, 2003).
Several observations have provided a basis for the hypothesis that modulation of frequency oscillations with movement may be involved in the mediation or facilitation of preparatory responses or attentional shifts in response to a warning stimulus. This circumstantial association between reduction in 8–30 Hz LFP activity and the preparation of the motor response is strengthened by a study demonstrating a positive correlation between the drop in 8–30 Hz activity following a go-cue and reaction time across Parkinsonian patients (Levy et al. 2002).
Neural responses in the basal ganglia in response to cue stimulus have been demonstrated in non-human primates and rodents using multiple-channel, single-unit recording techniques. In primates, studies have been carried out while performing a delayed conditional sequence of cocoordinated pull and grasp movements in response to a cue presentation (Smith et al. 2000). Similarly in rats, studies involve the presentation of an auditory cue prior to the start of a treadmill (Tsubokawa & Sutin, 1972; Goto & O'Donnell, 2001). In such cases, neurons in major basal ganglia regions (STR, GP, STN, SNr) exhibit characteristics of anticipatory activity.
Evidence of STN involvement in the parallel activation of cardiorespiratory and locomotor systems comes from studies involving decorticate animals, neuromuscularly blocked to eliminate peripheral feedback from motor activity (Smith et al. 1960; Eldridge et al. 1981; Angyan, 1991; Bedford et al. 1992). High frequency electrical stimulation of the STN in awake humans results in a cardiovascular response resembling that seen in exercise (Thornton et al. 2002). Indeed, the basal ganglia project to a number of nuclei which affect cardiovascular function in addition to their projections to the thalamus via which they control movement. (Dampney et al. 1984; Verberne & Owens, 1998).
The performance of electrophysiological recordings from this subcortical nucleus goes some way to defining the relationship between activity in the STN and activation of locomotion and cardiovascular systems in parallel. As our study shows, increase in STN activity with exercise (but not with anticipation) at frequencies > 30 Hz and < 15 Hz may be involved not only in the control of movement but also in the mediation of appropriate cardiorespiratory responses. Amplification of the activity in this area may also be mediated by feedback from the movement itself (Waldrop & Stremmel, 1989).
Role of the periaqueductal grey area during exercise
Our findings demonstrate marked increases in neural activity in the PAG of awake humans during anticipation of exercise and with exercise itself across the 25–90 Hz frequency bands. Moreover, the pattern of increased activity with anticipation and even more activity with exercise mirrors the changes in HR and MAP.
The midbrain PAG is an important neural structure for autonomic regulation and modulation of the cardiovascular changes that are associated with integrated behavioural ‘defence’ responses (Bandler & Carrive, 1988). The organization of the PAG into four linear, functionally distinct columns is well recognized (Carrive & Bandler, 1991b). The cardiovascular responses to PAG stimulation are well characterized. For example, activation of the dorsomedial and dorsolateral columns elicits ‘fight or flight’ responses such as hypertension and tachycardia (Duggan & Morton, 1983; Lovick, 1985; Carrive & Bandler, 1991a). As such, these autonomic responses can prepare the animal for high intensity exercise. Stimulation of the lateral and venterolateral columns, on the other hand, produces passive coping responses such as bradycardia and hypotension as demonstrated in both animals (Kabat et al. 1935) and awake humans (Green et al. 2005).
The role of the PAG in the exercise pressor reflex has been demonstrated further in animal studies. For example, muscle contraction increases PAG neuropeptide Y and enkephalin release in cats (Williams et al. 1992; Williams, 1996). In addition, c-Fos expression identified the activation of PAG neurons during dynamic treadmill exercise in rats (Iwamoto et al. 1996), and muscle contraction increases PAG neuron discharge (Kramer et al. 1996). However, the PAG is not a site that is regarded as a centre for central command such as the cortical areas described earlier. The results of this study suggest that it is an important area for the supraspinal control of exercise-related cardiorespiratory changes but there is no evidence that it is involved in control of locomotion. Therefore, although we can suggest a putative role of command signals emanating from the PAG in mediating the cardiovascular responses associated with anticipation of exercise – compatible with the classical ‘parallel activation’ of locomotor and cardiovascular systems – it is unclear whether it is a ‘central command’ site as such. Rather, its role may be important in preparing the body for action.
Role of the GPi during exercise
We have found increased activity in the GPi with exercise in some frequency bands. This is consistent with rat studies that show increased firing rates during locomotion (Chang et al. 2005), although these are single-cell recording studies. These are thought to be behavioural context-dependent changes and related to movement itself. This would fit with our findings that there were no significant changes with anticipation of exercise. PET studies have shown increased cerebral blood flow in the globus pallidus associated with the intention of relaxation (Critchley et al. 2001). This would agree with the notion that the GPi provides the major inhibitory output of the basal ganglia but is something that could not be measured in this study as intent to stop exercise was masked by the changes occurring during that exercise.
Limitations of this study
The main limitation of this study is that we are dealing with human subjects that have pathological conditions. This is particularly the case in the dystonia and PD patients who may have pathology relating to the nucleus that we are testing. Evidence for dysfunction in the PAG in patients with chronic pain is lacking and therefore the PAG recordings are less likely to be affected by this phenomenon. However, the increases in HR and MAP on anticipation and during exercise in all subgroups suggests that central command systems are functioning normally. The PD patients present a further challenge in that autonomic function is commonly poor (Netten et al. 1995), and therefore larger cardiovascular responses may be observed in subjects with normal autonomic function. In addition, anti-Parkinsonian medication can have cardiovascular effects (Pollak, 1997).
A second limitation is the presence of possible exercise ‘artefact’, i.e. electrical activity associated with the movement itself. Obviously this would not affect the anticipation recordings but might lead to some of the large increases seen in the exercise recordings. However, if this was the case, we would expect to see artefact over a relatively narrow frequency band which we did not observe. Furthermore, we saw a reduction of beta band activity in the STN during exercise, consistent with previous reports.
In conclusion, we have shown, in humans, alteration of electrical activity in deep brain nuclei associated with both exercise and its anticipation. The changes in Periaqueductal Grey, especially the large increases of activity during anticipation and actual exercise, probably reflect the importance of this area in the integration of the cardiorespiratory response to movement.
Acknowledgments
Shouyan Wang is supported by the Norman Collison Foundation, and Tipu Aziz by the Medical Research Council, Wellcome Trust and Templeton Foundation. We would also like to thank the participants of this study for their kind support.
References
- Angyan L. Substantia nigra stimulation and blood pressure effects of locally applied kainic acid. Neuroreport. 1991;2:785–788. [PubMed] [Google Scholar]
- Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bedford TG, Loi PK, Crandall CC. A model of dynamic exercise: the decerebrate rat locomotor preparation. J Appl Physiol. 1992;72:121–127. doi: 10.1152/jappl.1992.72.1.121. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R. Control of extracranial and hindlimb blood flow by the midbrain periaqueductal grey of the cat. Exp Brain Res. 1991a;84:599–606. doi: 10.1007/BF00230972. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal grey: a correlative functional and anatomical study. Brain Res. 1991b;541:206–215. doi: 10.1016/0006-8993(91)91020-2. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Chang JY, Shi LH, Luo F, Woodward DJ. Neural responses in multiple basal ganglia regions following unilateral dopamine depletion in behaving rats performing a treadmill locomotion task. Exp Brain Res. 2005;172:193–207. doi: 10.1007/s00221-005-0312-7. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Brain activity during biofeedback relaxation: a functional neuroimaging investigation. Brain. 2001;124:1003–1012. doi: 10.1093/brain/124.5.1003. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Goodchild AK, Tan E. Identification of cardiovascular cell groups in the brain stem. Clin Exp Hypertens A. 1984;6:205–220. doi: 10.3109/10641968409062561. [DOI] [PubMed] [Google Scholar]
- Doyle LM, Kuhn AA, Hariz M, Kupsch A, Schneider GH, Brown P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson's disease. Eur J Neurosci. 2005;21:1403–1412. doi: 10.1111/j.1460-9568.2005.03969.x. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Morton CR. Periaqueductal grey stimulation: an association between selective inhibition of dorsal horn neurones and changes in peripheral circulation. Pain. 1983;15:237–248. doi: 10.1016/0304-3959(83)90059-3. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol. 1985;59:313–337. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981;211:844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- Fink GR, Adams L, Watson JD, Innes JA, Wuyam B, Kobayashi I, Corfield DR, Murphy K, Jones I, Frackowiak RS, et al. Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. J Physiol. 1995;489:663–675. doi: 10.1113/jphysiol.1995.sp021081. Erratum in: J Physiol (1996) 494, 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O'Donnell P. Network synchrony in the nucleus accumbens in vivo. J Neurosci. 2001;21:4498–4504. doi: 10.1523/JNEUROSCI.21-12-04498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Wang S, Owen SLF, Xie K, Liu X, Paterson DJ, Stein JF, Bain PG, Aziz T. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport. 2005;16:1741–1745. doi: 10.1097/01.wnr.0000183904.15773.47. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Wappel SM, Fox GM, Buetow KA, Waldrop TG. Identification of diencephalic and brainstem cardiorespiratory areas activated during exercise. Brain Res. 1996;726:109–122. [PubMed] [Google Scholar]
- Kabat H, Magoun HW, Ranson SW. Electrical stimulation of points in the forebrain and midbrain. The resultant alteration in blood pressure. Arch Neurol Psych. 1935;34:931–955. [Google Scholar]
- Kramer JM, Jarboe MO, Waldrop TG. Periaqueductal gray neuronal responses to hindlimb muscle contraction in the cat. Soc Neurosci Abstr. 1996;22:89. [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain. 2002;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Ventrolateral medullary lesions block the antinociceptive and cardiovascular responses elicited by stimulating the dorsal periaqueductal grey matter in rats. Pain. 1985;21:241–252. doi: 10.1016/0304-3959(85)90088-0. [DOI] [PubMed] [Google Scholar]
- Morgan WP, Hirta K, Weitz GA, Balke B. Hypnotic peturbation of perceived exertion: ventilatory consequences. Am J Clin Hypn. 1976;18:182–190. doi: 10.1080/00029157.1976.10403796. [DOI] [PubMed] [Google Scholar]
- Netten PM, De Vos K, Horstink MW, Hoefnagels WH. Autonomic dysfunction in Parkinson's disease, tested with a computerized method using a Finapres device. Clin Auton Res. 1995;5:85–89. doi: 10.1007/BF01827468. [DOI] [PubMed] [Google Scholar]
- Papanastassiou V, Rowe J, Scott R, Silburn P, Davies L, Aziz T. Use of the Radionics Image Fusion™ and Stereoplan™ programs for target localisation in functional neurosurgery. J Clin Neurosci. 1998;5:28–32. doi: 10.1016/s0967-5868(98)90197-7. [DOI] [PubMed] [Google Scholar]
- Pollak P. Mechanisms and treatments of Parkinson disease. Rev Prat. 1997;47:1068–1076. [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Bianchi A, Chiesa V, Bselli G, Caputo E, Tamma F, Rampini P, Egidi M, Locatelli M, Barbieri S, Scarlato G. Movement-related modulation of neural activity in human basal ganglia and its L-DOPA dependency: recordings from deep brain stimulation electrodes in patients with Parkinson's disease. Neurol Sci. 2002;23:S101–S102. doi: 10.1007/s100720200089. [DOI] [PubMed] [Google Scholar]
- Smith OA, Astley CA, Spelman FA, Golanov EV, Bowden DM, Chesney MA, Chalyan V. Cardiovascular responses in anticipation of changes in posture and locomotion. Brain Res Bull. 2000;53:69–76. doi: 10.1016/s0361-9230(00)00310-5. [DOI] [PubMed] [Google Scholar]
- Smith OA, Jr, Rushmer RF, Lasher EP. Similarity of cardiovascular responses to exercise and to diencephalic stimulation. Am J Physiol. 1960;198:1139–1142. doi: 10.1152/ajplegacy.1960.198.6.1139. [DOI] [PubMed] [Google Scholar]
- Sochurkova D, Rektor I. Event-related desynchronization/synchronization in the putamen. An SEEG case study. Exp Brain Res. 2003;149:401–404. doi: 10.1007/s00221-003-1371-2. [DOI] [PubMed] [Google Scholar]
- Thornton JM, Aziz T, Shlugman D, Paterson DJ. Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol. 2002;539:615–621. doi: 10.1113/jphysiol.2001.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B, Paterson DJ. Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol. 2001;533:823–836. doi: 10.1111/j.1469-7793.2001.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubokawa T, Sutin J. Pallidal and tegmental inhibition of oscillatory slow waves and unit activity in the subthalamic nucleus. Brain Res. 1972;41:101–118. doi: 10.1016/0006-8993(72)90619-1. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Stremmel RW. Muscular contraction stimulates posterior hypothalamic neurons. Am J Physiol. 1989;256:R348–R356. doi: 10.1152/ajpregu.1989.256.2.R348. [DOI] [PubMed] [Google Scholar]
- Williams CA. Neuropeptide Y-like substances are released from the rostral brainstem of cats during the muscle pressor response. J Physiol. 1996;495:267–277. doi: 10.1113/jphysiol.1996.sp021591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CA, Holtsclaw LI, Chiverton JA. Release of immunoreactive enkephalinergic substances in the periaqueductal grey of the cat during fatiguing isometric contractions. Neurosci Lett. 1992;139:19–23. doi: 10.1016/0304-3940(92)90848-2. [DOI] [PubMed] [Google Scholar]
- Williams D, Kuhn A, Kupsch A, Tijssen M, Van Bruggen G, Speelman H, Hotton G, Yarrow K, Brown P. Behavioural cues are associated with modulations of synchronous oscillations in the human subthalamic nucleus. Brain. 2003;126:1975–1985. doi: 10.1093/brain/awg194. [DOI] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Matthews D, Mitchell JH, Raven PB, Morgan WP. Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol. 2001;90:1392–1399. doi: 10.1152/jappl.2001.90.4.1392. [DOI] [PubMed] [Google Scholar]