Abstract
We hypothesized that suppression of endogenous testosterone blunts mRNA expression post strength training (ST). Twenty-two young men were randomized for treatment with the GnRH analogue goserelin (3.6 mg every 4 weeks) or placebo for a period of 12 weeks. The ST period of 8 weeks started at week 4. Strength test, blood sampling, muscle biopsies, and whole-body dual-energy X-ray absorptiometry (DXA) scan were performed at weeks 4 and 12. Muscle biopsies were taken during the final ST session (pre, post 4 h, and post 24 h). Resting serum testosterone decreased significantly (P < 0.01) in the goserelin group from 22.6 ± 1.6 (mean ± s.e.m.) to 2.0 ± 0.1 nmol l−1 (week 4), whereas it remained unchanged in the placebo group. An acute increase of serum testosterone was observed during the final ST session in the placebo group (P < 0.05), whereas a decreased response was observed in the goserelin group (P < 0.05). mRNA expression of IGF-IE(bc) and myogenin increased, while expression of myostatin decreased (P < 0.01); however, no differences were observed between the groups. Muscle strength and muscle mass showed a tendency to increase more in the placebo group than in the goserelin group (P = 0.05). In conclusion, despite blocked acute responses of testosterone and 10- to 20-fold lower resting levels in the goserelin group, ST resulted in a similar mRNA expression of myoD, myogenin, IGF-IE(abc), myostatin and androgen receptor as observed in the placebo group. Therefore, in the present study, the molecular events were the same, despite divergent muscle hypertrophy and strength gains.
Skeletal muscle has an incredible potential for adaptation in response to strength training. The adaptation induced by strength training and producing muscle hypertrophy involves the orchestration of several anabolic mechanisms. Deeper insight into this process is needed to fully understand the signalling pathways that coordinate and regulate this adaptation. As pointed out by Haddad & Adams (2002), the majority of studies aimed at elucidating the adaptive processes to strength training have been dealing with end-point measurements on muscle strength and muscle hypertrophy. It is difficult, however, to precisely identify the signalling pathways and regulatory mechanisms which operate prior to the gain in muscle mass and muscle strength. A number of hormonal, cellular and molecular mechanisms involved in the anabolic process have been characterized, but their specific interactions are not understood. It appears that the first anabolic response is accumulation of specific proteins involved in the enlargement of muscle fibres. The second step seems to be proliferation and differentiation of satellite cells providing additional nuclei to the enlarging muscle fibres (Kadi & Thornell, 2000; Charge & Rudnicki, 2004; Ishido et al. 2004).
Different genes are expressed following strength training, and they might be important for the observed muscle hypertrophy (Cameron-Smith, 2002; Psilander et al. 2003; Fluck & Hoppeler, 2003; Kim et al. 2005). Myogenin and myoD, also called myogenic regulating factors (MRF), are expressed in satellite cells and muscle fibres, and they have been implicated in mediating the processes of cell proliferation and differentiation, as well as defining muscle phenotype (Charge & Rudnicki, 2004; Ishido et al. 2004). The expression of myoD and myogenin has been reported to increase after strength training in humans (Hespel et al. 2001; Willoughby & Nelson, 2002; Psilander et al. 2003; Willoughby & Rosene, 2003; Coffey et al. 2006). Unchanged expression of myoD (Hespel et al. 2001; Hameed et al. 2003; Bamman et al. 2004) and myogenin (Bamman et al. 2004), however, has also been reported. IGF-IEa, IGF-IEb and IGF-IEc are isoforms of IGF-I (insulin-like growth factor I) (Hameed et al. 2004). The IGF-IEc isoform, also called mechano growth factor (MGF), is thought to stimulate myofibrillar protein synthesis and satellite cell activation and proliferation (Adams, 1998; Goldspink, 1999; Yang & Goldspink, 2002; Hameed et al. 2004), whereas IGF-IEa promotes differentiation into muscle fibres (Hameed et al. 2003). Both increased (Hameed et al. 2003, 2004) and unchanged (Hameed et al. 2003; Psilander et al. 2003) expression of IGF-IEa and IGF-IEc have been reported in response to strength training. Myostatin is a transforming growth factor defined as a negative regulator of muscle mass (Doumit et al. 1996; McPherron & Lee, 1997; Reisz-Porszasz et al. 2003). Most of the previous studies have shown decreased myostatin expression following strength training (Roth et al. 2003; Kim et al. 2005; Coffey et al. 2006), although a single study showed increased expression (Willoughby, 2004).
Endogenous testosterone increases acutely in response to strength training (Kraemer et al. 1990, 1991, 1993, 1995, 1998, 1999; Hakkinen & Pakarinen, 1993; Hansen et al. 2001). The importance of testosterone in strength-training-induced muscle hypertrophy seems clear (Inoue et al. 1994; Hickson et al. 1994; Bhasin et al. 1996, 2001; Bamman et al. 2001; Hansen et al. 2001; Storer et al. 2003; Willoughby & Taylor, 2004; Kraemer & Ratamess, 2005). Moreover, previously published results from this study demonstrated that suppression of serum testosterone below 10% of normal levels attenuated the increase in lean mass and muscle strength during strength training (Kvorning et al. 2006).
However, the observation that muscle hypertrophy seems to occur only in the trained muscle, and not in the untrained muscle, tells us two things. First, this observation excludes a solely systemic mechanism. Secondly, the muscle ability to interact with the circulating levels of endogenous testosterone seems to be very important (Harridge, 2003). This suggests that the challenged muscles increase the sensitivity to this specific circulating anabolic hormone. Androgen receptor (AR) are expressed in myonuclei (Dorlochter et al. 1994) and satellite cells (Doumit et al. 1996). The importance of AR for the adaptation to electrical stimulation in rats has been investigated, and the increase in muscle mass was effectively suppressed by AR blockade (Inoue et al. 1994). In addition, Bamman et al. (2001) observed increased AR mRNA concentrations 48 h after a bout of leg training. Furthermore, Willoughby & Taylor (2004) reported that the mRNA expression for AR correlated to serum testosterone concentrations. It is not known if endogenous testosterone regulates the transcription of the above mentioned genes.
Therefore, the aim of the present study was to elucidate whether endogenous testosterone is involved in the regulation of genes proposed to be involved in strength-training-induced muscle hypertrophy in a randomized, placebo-controlled, and blinded intervention study. The endogenous production of testosterone was suppressed by the use of a GnRH analogue during the intervention period. We hypothesized that low testosterone levels blunt mRNA expression of myoD, myogenin, AR, IGF-IEa, IGF-IEb and IGF-IEc, and blunt the decrease in mRNA expression of myostatin, resulting in attenuation of the gain in muscle mass and muscle strength in response to strength training.
Methods
Subjects and study design
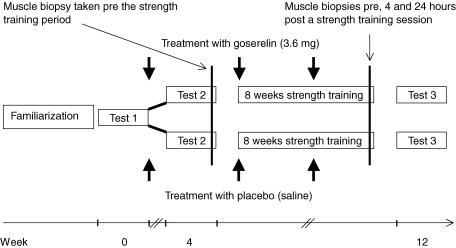
Details of this study design have been reported elsewhere (Kvorning et al. 2006). Briefly, 26 subjects volunteered to participate in the study. The subjects participated in leisure sport only once or twice per week, and previous experience with strength training did not exceed 1 h week−1. The study conformed to the guidelines in the Declaration of Helsinki and was approved by the local ethical committee (VF 20040173). All subjects were informed of the risks and purposes of the study before their written consent was obtained. The subjects were carefully matched in pairs with regard to isometric knee extension strength, body–mass index and age. Within each pair, the subjects were randomized to placebo (saline) or goserelin 3.6 mg (GnRH analogue) injections once every fourth week, three times in total. Clinical examination of the subjects was performed before the experiment and two subjects were disqualified due to exclusion criteria (metabolic disorders, low testosterone levels, angina pectoris, lower back disorders, prescription medication for heart or lung diseases, or any recent physical trauma). Moreover, two subjects did not complete the study due to an injury unrelated to the study and due to side-effects of the GnRH analogue treatment (hot flushes), respectively. Therefore, 22 young men completed the study (Table 1). The subjects and investigators involved in training and testing were blinded regarding the allocation of the subjects while two investigators (M.A. and K.B.) administering the study drugs and monitoring safety parameters were aware of the allocation. The schedule of study procedures are shown in Fig. 1.
Table 1.
Anthropometric measurements of the subjects before the strength training period
| Group | Age (years) | Height (cm) | Body mass (kg) | BMI (kg m−2) |
|---|---|---|---|---|
| Goserelin (n = 12) | 25 ± 1 | 179.5 ± 1.6 | 80.7 ± 3.7 | 25.3 ± 1.1 |
| Placebo (n = 10) | 23 ± 1 | 185.0 ± 1.4 | 83.4 ± 3.9 | 24.5 ± 1.1 |
BMI, body–mass index. Values are means ± s.e.m. None of the parameters differed significantly between the groups.
Figure 1. Overview of the study design.
After completion of Test 1 the subjects were randomized in to a goserelin group and a placebo group. Tests 1, 2 and 3 included blood sampling (resting levels and acute hormonal response to a strength training session), isometric strength testing and in addition whole-body dual-energy X-ray absorptiometry (DXA) scan and muscle biopsies were performed at Tests 2 and 3.
Testing procedures
The subjects underwent three test procedures during the study. Tests 1, 2, and 3 included measurements of hormonal resting levels, isometric strength testing, and measurements of acute hormonal responses to a strength training session. These measurements were completed in succession and on a separate day. Muscle biopsies and whole body dual-energy X-ray absorptiometry (DXA scan) were performed on separate days in relation to Test 2 and Test 3. In addition, at Tests 2 and 3, 2–3 days separated biopsies from the measurements of hormonal resting levels, isometric strength testing, etc. (Fig. 1). The subjects were familiarized with the study procedures approximately 2 weeks before entering Test 1. This included measuring of anthropometrics of the subjects and a careful introduction to the testing procedures. Furthermore, each subject completed the entire strength testing protocol and was introduced to the strength training exercises, where the subject was carefully corrected until proper technique was achieved. Subsequently, a 10 repetition maximum (RM) load was measured for all exercises in the training programme, to determine the initial training load.
Treatment with goserelin
Goserelin (Zoladex; AstraZeneca) 3.6 mg depot was injected subcutaneously in the abdomen once every fourth week, in order to reduce and maintain endogenous testosterone concentrations within castrate range. Goserelin prevents the reappearance of luteinising hormone releasing hormone (LHRH) receptors and consequently inhibits the secretion of luteinising hormone (LH) from the pituitary gland and thus testicular production of testosterone (Cockshott, 2000). All subjects received three injections in total, starting immediately after Test 1 (Fig. 1).
Training
A standardized warm-up was performed before training consisting of four sets of squats with 20 repetitions without load, with 1 min rest between sets. Subjects from both groups trained the same progressive strength training programme. The programme was designed in accordance with Kraemer et al. (2002). Previous studies with similar strength training programmes have demonstrated significant acute increases in the level of testosterone (Hakkinen & Pakarinen, 1993; Kraemer et al. 1998) and significant increases for muscle strength and muscle mass (Braith et al. 1989; Narici et al. 1996; Aagaard et al. 2002; Glowacki et al. 2004; Moore et al. 2005). The programmes were performed three times a week for 8 weeks and consisted of leg press, knee extension, leg curl, bench press, lat pull down, biceps curl and elbow extension. Subjects did four sets of each exercise for the legs, and three sets of each exercise for the upper body. The strength training period consisted of 24 training sessions comprising three periods of eight training sessions. In the first and third period, subjects trained 10 repetitions with corresponding 10 RM loads in all exercises, with 2 min rests between sets. In the second period, they trained six repetitions with corresponding 6 RM loads in all exercises, with 3 min rests between sets. The training loads were increased due to RM tests at the start of each of the three periods. The goserelin group increased the training load (10 RM) in the exercises leg press and bench press, measured before and after the training period, from 242 ± 10 to 320 ± 7 kg and 48 ± 3 to 56 ± 3 kg, respectively. The same measurements for the placebo group were 258 ± 17 to 327 ± 12 kg and 47 ± 2 to 55 ± 3 kg, respectively (n.s. between groups). Mean training volume (calculated as load multiplied by repetitions) for the leg press was 194 752 ± 8119 kg for the goserelin group and 205 913 ± 13 023 kg for the placebo group, and for bench press it was 26 443 ± 1335 kg for the goserelin group and 28 202 ± 1732 kg for the placebo group (n.s. between groups). All training sessions were supervised and both groups carried out the same number of training sessions (except for one training session); therefore, subjects in the goserelin group completed 23.7 training sessions on average, and the placebo group completed 23.6 training sessions on average. All subjects participated in a minimum of 22 training sessions.
Blood sampling (hormonal resting levels)
Subjects reported to the laboratory between 07.00 and 09.00 h, and were fasting from 24.00 h the day before, and refrained from strenuous physical activity for 48 h. Blood samples were drawn at the same time of the day for each subject during Tests 1, 2, and 3 after 30 min of supine rest from an antecubital vein for determination of serum endogenous total testosterone, free testosterone, sex hormone binding globulin (SHBG), growth hormone (GH) and cortisol. Blood (30 ml) was drawn for serum samples and immediately chilled on ice, and centrifuged at 3000 r.p.m. (1300 g) for 10 min at 20°C. All serum samples were then distributed to appropriate tubes and stored at −80°C until analysed. After blood sampling, a standardized breakfast was served for the subjects, followed by a 1 h rest before proceeding to isometric strength testing. The amount of food was adjusted in relation to body weight. Subjects were divided in three groups (e.g. light, medium and heavy body mass group) receiving different sizes of breakfast, containing in total 6.46 ± 0.10 kcal kg−1, consisting of 0.21 ± 0.01 (g protein) kg−1, 1.25 ± 0.02 (g carbohydrate) kg−1 and 0.10 ± 0.00 (g fat) kg−1.
Blood sampling (acute hormonal response to a strength training session)
Concurrent with the first (Test 2) and final (Test 3) strength training session, three blood samples were taken-before the strength training session (pre), immediately after the training session (post 0 min) and subsequently after 15 min of rest following the training session (post 15 min). Blood samples were drawn at the same time of the day for each subject during Tests 2 and 3. For analysis of testosterone, GH, SHBG and cortisol, 10 ml of blood was collected in pre-cooled tubes containing ethylendiaminetetraacetic acid (EDTA). The samples were immediately chilled on ice, centrifuged at 3000 r.p.m. (1300 g) for 10 min at 20°C, and plasma was stored at −80°C until assayed.
Analysis of hormones
Serum total testosterone was measured using an in-house assay based on extraction, chromatography, and radioimmunoassay (RIA), as described in Lykkesfeldt et al. (1985). Free testosterone (non protein bound) was calculated as described by Bartsch (1980). Serum GH, cortisol, and SHBG were measured by a time-resolved fluoroimmunoassay by AutoDelfia (Turku, Finland).
Muscle biopsies
In a resting condition, muscle biopsy samples (∼100 mg) from the middle portion of the vastus lateralis were obtained by using the Bergström needle technique (Bergström, 1962). Incisions were made through the skin and muscle fascia following the administration of local anaesthesia (2–3 ml 1% lidocaine (lignocaine)). Pre- and post-training biopsy samples were taken from the same region and depth of the muscle. The tissue was immediately freed from blood and visible connective tissue, rapidly frozen in liquid N2, and stored at −80°C for mRNA isolation. Biopsy samples were obtained at four time points to measure the mRNA expression of myoD, myogenin, myostatin, IGF-IEa, IGF-IEb, IGF-IEc and AR. The first biopsy was taken in the right leg (Test 2) and served as a pre-training period biopsy. In connection with the second, though last strength training session (Test 3), three biopsy samples were taken. One biopsy was taken in the right leg before the start of the training session and served both as a post-training period biopsy and a pre-training biopsy. This biopsy was taken 48 h after the previous strength training session. The subjects then completed the exercises, and another biopsy was taken in the left leg 4 h after completion of the strength training session. The final biopsy was taken in the right leg 24 h after the pre-training biopsy. Time points for all subjects were standardized and equal from day to day. The subjects had been fasting from 24.00 h the day before and had refrained from strenuous physical activity for 48 h. Two hours prior to all biopsies, subjects were served a standardized meal. Subjects were divided in three groups (e.g. light, medium and heavy body mass group) receiving different sizes of meals, containing in total 8.01 ± 0.14 kcal kg−1, consisting of 0.45 ± 0.01 (g protein) kg−1, 1.31 ± 0.03 (g carbohydrate) kg−1 and 0.10 ± 0.00 (g fat) kg−1.
RNA purification
Total RNA was isolated from muscle biopsy samples by phenol extraction (TriReagent; Molecular Research Center, OH, USA) as previously described (Kadi et al. 2004). Intact RNA was confirmed by denaturing agarose gel electrophoresis.
Real-time RT-PCR
mRNA expression of IGF-IEa, IGF-IEb, IGF-IEc, myostatin, AR and RPLP0 was analysed by real-time RT-PCR. Total RNA (500 ng) was converted into cDNA in 20 μl using the OmniScript reverse transcriptase (Qiagen, CA, USA) according to the manufacturer's protocol. For each target mRNA, 0.25 μl cDNA was amplified in a 25 μl SYBR Green PCR reaction containing 1× Quantitect SYBR Green Master Mix (Qiagen) and 100 nm of each primer (Table 2). The amplification was monitored real-time using the MX3000P real-time PCR machine (Stratagene, CA, USA). The threshold cycle (Ct) values were related to a standard curve made with the cloned PCR products and specificity ensured by melting curve analysis. The quantities were normalized to the GAPDH mRNA (Kadi et al. 2004).
Table 2.
Primers for real-time RT-PCR and Northern probes
| mRNA | Sense primer | Anti-sense primer |
|---|---|---|
| RT-PCR | ||
| IGF-IEa | GACATGCCCAAGACCCAGAAGGA | CGGTGGCATGTCACTCTTCACTC |
| IGF-IEb | GCCCCCATCTACCAACAAGAACAC | CAGACTTGCTTCTGTCCCCTCCTTC |
| IGF-IEc | GCCCCCATCTACCAACAAGAACAC | CGGTGGCATGTCACTCTTCACTC |
| Myostatin | TGCTGTAACCTTCCCAGGACCA | GCTCATCACAGTCAAGACCAAAATCC |
| AR | CAAGACGCTTCTACCAGCTCACCA | CGGAAAGTCCACGCTCACCA |
| RPLP0 | GGAAACTCTGCATTCTCGCTTCCT | CCAGGACTCGTTTGTACCCGTTG |
| GAPDHa | CCTCCTGCACCACCAACTGCTT | GAGGGGCCATCCACAGTCTTCT |
| Northern | ||
| Myogenin | GCAGGCTCAAGAAGGTGAAT | ATGGATGAGGAAGGGGATAG |
| MyoD | GCTCCGACGGCATGATGG | TAAAGCGCTGTTGGGAGG |
| GAPDHb | GAACATCATCCCTGCCTCTACT | GTCTACATGGCAACTGTGAGGA |
AR, androgen receptor; GAPDH, gylceraldehyde-3-phosphate dehydrogenase
GAPDH primers for RT-PCR
GAPDH primers for Northern probes.
Northern blotting
mRNA expression of myoD and myogenin was analysed by Northern Blotting. Northern analysis was performed as previously described (Kadi et al. 2004). Briefly, 350 ng total RNA was separated on a 1% denaturing formaldehyde agarose gel and blotted to a positively charged nylon membrane using alkaline transfer. Samples from the same subject were loaded together. The membrane was then hybridized with the specific single-stranded DNA probe (below) at 50°C (42°C for 28S) overnight in UltraHyb (Ambion, Austin, USA) followed by washing in 0.1× SSPE and 0.1% SDS at 60°C (42°C) to remove excess probe. The 32P-labelled probes were made from cloned PCR products (primers in Table 2) as previously described (Kadi et al. 2004). The 28S probe was made by 5′ phosphorylation of an oligonucleotide complementary to 28S rRNA (TCG CCG TTA CTG AGG GAA TCC TGG TTA GTT TCT TT) using T4 polynucleotide kinase and [γ-32P]ATP. The signals were detected and quantified on a PhosphorImager. The membranes were stripped for probe and hybridized with gylceraldehyde-3-phosphate dehydrogenase (GAPDH) for normalization succeeded by hybridization with the 28S rRNA oligo.
Changes in ‘housekeeping’ gene expression
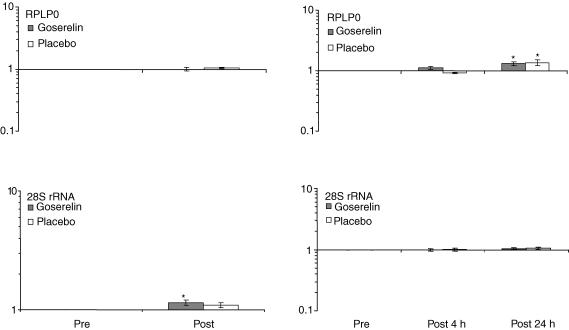
To test the stability of the ‘housekeeping’ gene, GAPDH, used for normalization of mRNA data, two other ‘housekeeping’ genes, 28S rRNA and RPLP0, were measured and normalized to GAPDH. There was a slight increase in 28S rRNA after the training period in the goserelin group, and RPLP0 mRNA increased slightly 24 h after the last training session in both groups (P < 0.05) (Fig. 2). If either of the apparent changes in 28S rRNA or RPLP0 mRNA expression was in reality due to a change in the normalization gene (encoding GAPDH) this would mean that GAPDH mRNA level would decrease. However, from a biological point of view, an increase in protein synthesis components (28S and RPLP0) is more likely than a decrease in a glycolytic enzyme following strength training. Furthermore, the changes seen in the other mRNAs were larger and can therefore not simply be an artefact of the chosen normalizing gene encoding GAPDH.
Figure 2. Changes in 28S rRNA and RPLP0 mRNA.
Changes in 28S rRNA and RPLP0 mRNA measured pre and post the strength training period and pre, 4 and 24 h post the strength training session, respectively (geomean ± s.e.m.) (n = 10 in the goserelin group, and n = 7 in the placebo group). * Significant difference compared with the corresponding pre value (P < 0.05).
Whole-body DXA scan
Subjects were DXA scanned (Hologic 4500 A, Waltham, MA, USA) before and after the training period (Tests 2 and 3). The DXA scan was conducted between 08.00 and 16.00 h and at least 24 h after training sessions (in order to avoid any impact of changes in hydration). Regional lean body mass was measured. The coefficient of variation (CV) for lean body mass is 0.5–1%.
Isometric strength testing
Subjects were strength tested at Tests 1, 2 and 3. After a 5 min standardized warm-up procedure on a bicycle ergometer, the dominant leg was tested in a KinCom dynamometer (KinCom 500H, software version 4.03; Chattecx Corp., USA). The protocol implicated isometric knee extensions performed at a locked position of 70° knee flexion (0° = full extension). Subjects were instructed to extend the knee as explosively and forcefully as possible and three attempts were performed with maximal contraction held for 3 s. A period of 45 s of recovery between trials was given and the highest absolute value for isometric measurements was used for further analysis. The isometric measurements were sampled on an external computer with a sampling rate of 1000 Hz and corrected for the influence of gravity (Aagaard et al. 1995). All measurements were filtered by a fourth-order zero-lag Butterworth low-pass filter (10 Hz cut off frequency) and analysed for peak torque. The isometric strength measurements at Test 1 were obtained to serve as control comparisons; however, for the ease of illustration, only Tests 2 and 3 are depicted.
Statistics
Differences in mean (pre and post the strength training period) within or between groups were tested using paired and unpaired t tests for mRNA expression (following logaritmic transformation) and for strength and DXA measurements and for training load and volume. Measurements of acute hormonal responses were analysed by two-way ANOVA repeated measurements. mRNA expression measured pre, 4th and 24th post the strength training session was analysed by two-way ANOVA repeated measurements on logarithmic values. mRNA expression values are presented as geometric means ± back transformed s.e.m. in figures. All other data are presented as means ± s.e.m. A significance level of P < 0.05 was chosen. Statistical analyses were performed using Stat View, SAS Institute 1998.
Results
Baseline values
No significant differences were observed between the groups regarding baseline values before the intervention period (Test 1) or before the strength training period (Test 2) in any of the variables measured.
Resting levels of serum testosterone, free testosterone, GH, SHBG and cortisol
As previously published (Kvorning et al. 2006), the change in serum endogenous testosterone levels differed significantly between the groups (P < 0.01). Testosterone remained constant in the placebo group throughout the intervention period, but decreased significantly in the goserelin group (P < 0.01). A similar difference between the groups was observed for the endogenous free testosterone levels (P < 0.01), with a decrease in the goserelin group from Test 1 to Tests 2 and 3 (P < 0.01), whereas it remained unchanged in the placebo group (Table 3). There were no changes observed in the resting levels of serum GH during the study (Table 3). The resting levels of cortisol and SHBG remained also unchanged throughout the intervention period (data not shown).
Table 3.
Resting levels of hormones
| Hormone | Group | Test 1 | Test 2 | Test 3 |
|---|---|---|---|---|
| Testosterone | Goserelin† | 22.6 ± 1.6 | 2.0 ± 0.1* | 1.1 ± 0.2* |
| (nmol l−1) | Placebo | 22.2 ± 1.4 | 24.7 ± 1.7 | 22.0 ± 1.5 |
| Free testosterone | Goserelin† | 0.62 ± 0.03 | 0.05 ± 0.00* | 0.02 ± 0.00* |
| (nmol l−1) | Placebo | 0.60 ± 0.03 | 0.69 ± 0.05 | 0.57 ± 0.03 |
| GH | Goserelin | 0.33 ± 0.17 (n = 11) | 0.14 ± 0.02 | 0.37 ± 0.11 (n = 11) |
| (mU l−1) | Placebo | 0.30 ± 0.12 | 0.27 ± 0.13 | 0.17 ± 0.09 |
GH, growth hormone. Values are means ± s.e.m. Test 1, before treatment; Test 2, after 3 weeks of treatment with either goserelin or placebo and before strength training; Test 3, after the strength training period.
Significant different from Test 1 (P < 0.01).
Significant treatment effect compared with placebo (P < 0.01).
Acute hormonal response to strength training sessions
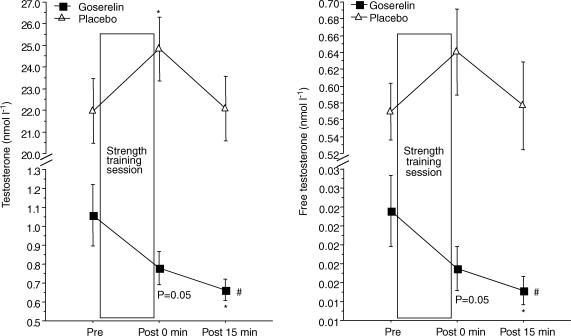
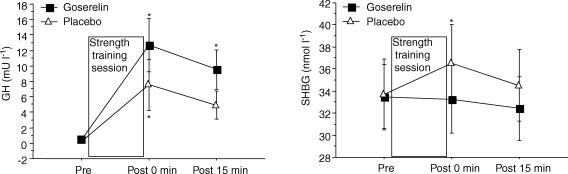
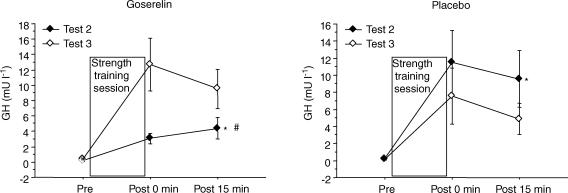
The acute response of testosterone, free testosterone and SHBG was similar at Tests 2 and 3. Only data from Test 3 are shown, since they corresponded to the measurements of acute mRNA expression. The placebo group responded to the final strength training session with a significant larger acute response in serum testosterone compared with the goserelin group (P < 0.01). The level of testosterone increased ∼15% immediately after the strength training session in the placebo group (P < 0.05). The goserelin group showed a decrease in testosterone and free testosterone 15 min post the strength training session (P < 0.05). In addition, the level trended to be below rest immediately after strength training (P = 0.05) (Fig. 3). A significant acute increase from rest in the level of SHBG was observed in the placebo group immediately after strength training (P < 0.05). The increase in serum SHBG in the placebo group, however, was not significantly different from the goserelin group. No changes were seen in serum SHBG in the goserelin group (Fig. 4). There was no significant difference between the groups regarding the acute response in serum GH at Test 3. Thus, a significant acute increase from rest in the level of GH was observed in the goserelin group immediately after strength training and 15 min post training (P < 0.05). The same picture was seen in the placebo group but the change was only significant immediately after the training session (P < 0.05) (Fig. 4). However, the goserelin group showed significantly lower GH response during Test 2 compared with Test 3 (P < 0.01). Conversely, the placebo group showed significantly higher GH response during Test 2 compared with Test 3 (P < 0.05) (Fig. 5). Serum cortisol showed no acute response at Test 2 or Test 3 for any of the two groups (data not shown).
Figure 3. Acute responses of testosterone and free testosterone measured during the final strength training session (Test 3).
Values are means ± s.e.m.*Significant difference from the corresponding pre value (P < 0.05). #Significant treatment effect compared with placebo (P < 0.01).
Figure 4. Acute responses of growth hormome (GH) and sex hormone binding globulin (SHBG) measured during the final strength training session (Test 3).
Values are means ±s.e.m. (GH, Test 3, n = 11 in the goserelin group). *Significant difference compared with the corresponding pre value (P < 0.05).
Figure 5. Acute response of growth hormone (GH) measured during the first (Test 2) and final strength training session (Test 3).
Values are means ±s.e.m. (Test 3, n = 11 in the goserelin group). *Test 2 significantly different from Test 3 (P < 0.05). #Significant treatment effect compared with placebo (P < 0.05).
Resting mRNA expression measured pre and post the strength training period
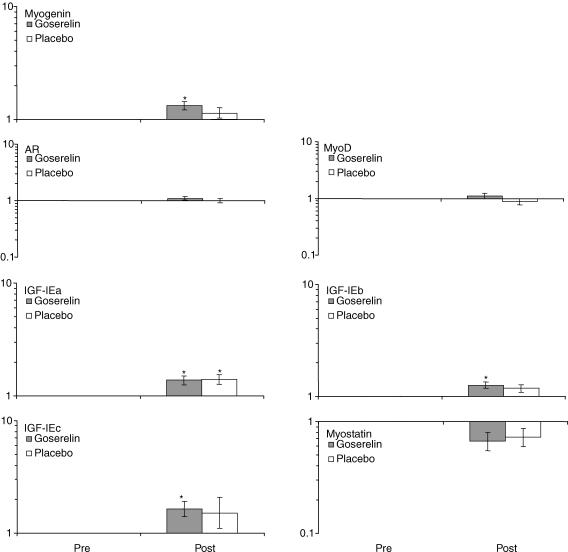
No differences were observed between the groups for the resting mRNA expression measured pre and post the strength training period. However, a significantly increased expression of IGF-IEa, IGF-IEb and IGF-IEc was seen in the goserelin group (P < 0.05). The placebo group showed a significant increase for IGF-IEa (P < 0.05) while IGF-IEb tended to increase (P = 0.07). There was a significant increased expression of myogenin following the strength training period in the goserelin group (P < 0.05), whereas a trend toward decreased mRNA expression of myostatin was observed (P = 0.06). Finally, no changes in the mRNA expression of AR or myoD were seen after the strength training period (Fig. 6).
Figure 6. Changes in resting mRNA expression.
Changes in resting mRNA expression measured pre and post the 8 weeks strength training period (geomean ± s.e.m.) (n = 10 in the goserelin group, and n = 7 in the placebo group). *Significant difference compared with the corresponding pre value (P < 0.05). No treatment effect (goserelin versus placebo) was observed in any of the genes.
Acute mRNA expression measured pre and post the strength training session
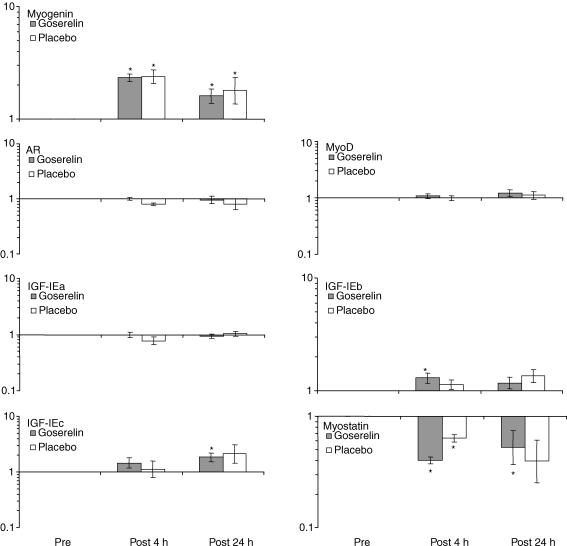
No differences were observed between the groups for the acute mRNA expression measured pre and post the strength training session. However, a significant increase was seen in the goserelin group 4 h post strength training regarding IGF-IEb and 24 h post training for IGF-IEc (P < 0.05). The placebo group showed a trend to increase 24 h post training for IGF-IEb and IGF-IEc, with P values of 0.07 and 0.05, respectively. Myostatin mRNA expression decreased in both groups 4 h post strength training (P < 0.05), and was still significantly reduced 24 h post training in the goserelin group (P < 0.05). Both groups showed increased mRNA expression for myogenin 4 h and 24 h post the strength training session (P < 0.05). There were no changes in the mRNA expression of AR and myoD after the strength training session (Fig. 7).
Figure 7. Changes in acute mRNA expression.
Changes in acute mRNA expression measured pre, 4 and 24 h post the strength training session (geomean ± s.e.m.) (n = 10 in the goserelin group, and n = 7 in the placebo group). *Significant difference compared with the corresponding pre value (P < 0.05). No treatment effect (goserelin versus placebo) was observed in any of the genes.
Isometric strength and lean leg mass
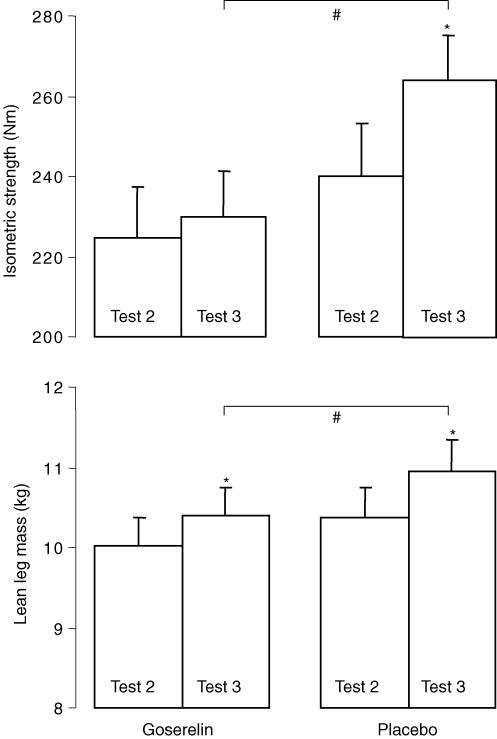
As previously published (Kvorning et al. 2006), only the placebo group showed a significant increase in isometric strength after 8 weeks of training (P < 0.05) and the change trended to be higher in the placebo group compared with goserelin group (P = 0.05). Lean leg mass increased significant in both groups (P < 0.05). However, the increase in the placebo group showed a trend to be larger than the increase in the goserelin group (P = 0.05) (Fig. 8).
Figure 8. Isometric strength and lean leg mass measured before (Test 2) and after (Test 3) the strength training period.
Values are means ± s.e.m.*Significant increase (P < 0.05). #P = 0.05 between groups.
Discussion
In the present study the use of a GnRH analogue effectively suppressed the resting levels and blocked the acute increase in serum testosterone in response to strength training. The absence of the acute increase of testosterone, however, had no influence on the acute mRNA expression of myoD, myogenin, myostatin, IGF-IEa, IGF-IEb, IGF-IEc and AR after the strength training session. Similarly, the lower resting level of testosterone had no effect on the resting mRNA expression before or after the strength training period. Therefore, endogenous testosterone does not seem to be involved in the transcriptional regulation of these particular genes, which are supposed to be involved in the adaptation to strength training. On the other hand, suppression of the level of testosterone attenuates the increase in lean mass and muscle strength. Therefore, the important news in the present study is that the molecular events were the same in spite of divergent muscle hypertrophy and strength gains.
The acute changes in mRNA expression seen in our study within the 24 h window are supported by previous studies (Willoughby & Nelson, 2002; Hameed et al. 2003, 2004; Psilander et al. 2003; Kim et al. 2005; Coffey et al. 2006). We found no changes, however, in the expression of IGF-IEa and myoD in agreement with previous observations (Hameed et al. 2003). The resting mRNA expression of IGF-IEa, IGF-IEb, IGF-IEc and myogenin increased after the strength training period and the mRNA expression of myostatin trended to decrease. Similar results have been obtained earlier (Roth et al. 2003; Willoughby & Rosene, 2003; Hameed et al. 2004; Bickel et al. 2005). The mRNA expression of myoD showed no changes as previously observed by Bamman et al. (2004).
In accordance with earlier studies with similar strength training programmes, the strength training session induced significant acute increases in the level of testosterone in the placebo group (Hakkinen & Pakarinen, 1993, Kraemer et al. 1998). The acute response of testosterone was parallel by and acute increase in the serum level of SHBG. On the other hand, the acute response in the goserelin group, showed a decreased level of testosterone. We can therefore relate the attenuated response to the strength training period (e.g. less gain in muscle mass and no gain in isometric muscle strength) seen in the goserelin group to endogenous testosterone. This implies that testosterone may regulate intracellular factors downstream from myoD, myogenin, myostatin, IGF-IEa, IGF-IEb and IGF-IEc mRNA transcription. In addition, testosterone could alter post-translational processes such as protein breakdown or efficiency of intracellular amino acid utilization. In support of this, a study without training intervention but with suppression of endogenous testosterone showed that the gene expression of actin and myosin were not altered; however, both lean mass and muscle strength decreased (Mauras et al. 1998). In addition, 4 weeks of functional overload in rats was shown to have no effect on myoD and myogenin expression even though lean mass was increased (Mozdziak et al. 1998). On the other hand, Mauras et al. (1998) reported decreases in IGF-I mRNA expression with suppression of endogenous testosterone and argued that androgens are necessary for local IGF-I production. The finding by Mauras et al. (1998) fits well with the observation of Urban et al. (1995) and Ferrando et al. (2002) where increasing testosterone levels by supplementation in elderly men were associated with increased IGF-I mRNA expression in skeletal muscle.
It was surprising to observe in the present study that no changes took place in the expression of AR either at rest or acute as a reaction to the dramatic changes of endogenous testosterone. Bamman et al. (2001) registered an increase in the expression of AR 48 h after a single strength training session. Furthermore, Willoughby & Taylor (2004) measured an increased mRNA expression of AR 48 h after two sequential strength training sessions. In both of the above-mentioned studies, biopsies were performed 48 h post the training session, whereas in the present study biopsies were taken 4 and 24 h post the training session. Therefore, the expression of AR seems to peak later than 24 h and timing of the biopsies may explain the divergent results. Finally, when comparing studies on gene expression, one must bear in mind that the impact on expression of exercise performed without prior familiarization or training is likely to differ markedly from the response to repeated exercise bouts or the trained response (Cameron-Smith, 2002; Coffey et al. 2006). The pre training session biopsy in the present study was taken 48 h after the previous training session in the present study, but we cannot be certain that this is a true baseline, since an elevated mRNA expression may be present in response to the preceding training session. We did not test whether the expression of myoD, myogenin, IGF-IEa, IGF-IEb, IGF-IEc, myostatin and AR was back to baseline 48 h post strength training. Previous studies have shown divergent results on this matter. Thus, studies demonstrate that expression of the respective genes seems to peak in a 24 h window post training (Psilander et al. 2003; Yang et al. 2005), whereas other studies show that the genes may continue to be upregulated 48 h post strength training (Roth et al. 2003; Bickel et al. 2005).
An important observation in the present study was that suppression of testosterone influenced the acute response of GH in the goserelin group. In addition, there seemed to be a trend towards a lower resting level of GH after 3 weeks of GnRH analogue treatment, but the level re-established after 8 weeks strength training. These findings are congruent with the previous finding by Mauras et al. (1987) where testosterone was shown to influence GH secretion. However, suppression of endogenous testosterone production had no significant influence on the resting level or acute response of serum cortisol. The trend towards a lower resting level and the lower acute response of GH was only present in the initial part of the strength training period, since the placebo and goserelin group showed identical resting levels and acute responses of GH at Test 3. In contrast, Mauras et al. (1998) found that suppression of testosterone by GnRH analogues was not accompanied by decreases in GH concentration. Instead the GH secretion increased after 10 weeks of hypogonadism. Ultimately, these findings are interesting since it has been postulated that GH and cortisol are involved in the regulation of the mRNA expression of IGF-I and myostatin (Rennie et al. 2004). In support of this, Hameed et al. (2003) reported that GH treatment increased IGF-IEa and IGF-IEc expression in the elderly and myostatin expression has been shown to increase in response to elevations in serum glucocorticoids (Lang et al. 2001; Ma et al. 2003). With these observations in mind, it could be speculated that the lower acute increase in the concentration of GH seen in the goserelin group during the first strength training session may have affected the acute IGF-IEa and IGF-IEc expression during the first part of the strength training period, thus leading to a lesser pronounced expression compared with the placebo group where a larger acute increase in the level of GH was present. However, similar serum cortisol levels in the placebo and goserelin groups may help to explain why there was no difference in the mRNA expression of myostatin between the groups. In contrast to the goserelin group, the placebo group showed a reduced acute response of GH at Test 3 compared with Test 2. This is in accordance with an earlier study (Ahtiainen et al. 2003), whereas Kraemer et al. (1998) reported unchanged acute response to training sessions after a training period.
Finally, it is important to stress that the relative contribution of transcriptional versus translational adaptations to strength training induced increase in muscle hypertrophy is not well understood (Cameron-Smith, 2002). Thus, increased protein synthesis could result from more mRNA molecules being translated or from an increased rate of translation of each molecule of mRNA. Chesley et al. (1992) demonstrated an increased protein synthesis after strength training without simultaneous increases in RNA content. In addition, Welle et al. (1999) concluded that the stimulation of protein synthesis by resistance exercise was mediated by more efficient translation of mRNA. Consequently, a translational mechanism may explain increased protein synthesis without increases in mRNA expression (Bolster et al. 2003). Therefore, caution must be applied to the analysis of adaptive changes in both mRNA responses to exercise and the impact of transcriptional compared with translational events (Cameron-Smith, 2002). If hormonal factors regulate the genes involved in the adaptation process through translational events or post-translational events, they were not detected in the present study. It may be speculated that a decreased translation was present in the goserelin group compared with the placebo group, induced by the lack of acute response of testosterone or/and by the low resting level of testosterone. Furthermore, the effect of testosterone on transcription may have occurred early in the training period and not been detected, since muscle biopsies were taken in relation to the final strength training session. Transcriptional events may have occurred after the first few training sessions and were attenuated later on when a new steady-state level of protein was attained. On the other hand, the present study does not exclude the possibility that endogenous testosterone may regulate other hypertrophic signalling genes besides the one measured, or affect other mechanisms responsible for gain in muscle mass and muscle strength. These speculations are supported by the observation that the goserelin group adapted to the strength training period by attenuated increases in both lean leg mass and isometric knee extension strength.
Conclusions
In spite of both blocked acute responses and very low resting levels of endogenous testosterone in the GnRH-analogue-treated group, strength training resulted in a similar mRNA expression of myoD, myogenin, IGF-IEa, IGF-IEb, IGF-IEc, myostatin and AR, as observed in a placebo group showing acute responses of testosterone to strength training and 10–20 times higher resting levels of testosterone. Therefore, endogenous testosterone does not seem to be involved in the regulation of the expression of these previously established signalling genes in the processes of strength-training-induced muscle hypertrophy. On the other hand, suppression of the level of endogenous testosterone attenuates the increase in lean mass and muscle strength. Therefore, the important finding in the present study is that the molecular events were the same despite divergent muscle hypertrophy and strength gains.
Acknowledgments
First of all we would like to thank the subjects who participated in the study. Secondly, we would like to thank the laboratory technicians Gitte Scheel Klemmensen, Bente Tøt, Donna Arbuckle-Lund, Kirsten Westermann and Anette Riis Madsen., engineer Cuno Rasmussen, Professor Per Aagaard, PhD student Anders Holsgaard Larsen, and the students Emil Pedersen and Jacob Søndergaard, for their helpful cooperation during the study. We would like to thank Anti Doping Denmark and the Team Denmark Foundation for their financial support.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- Aagaard P, Simonsen EB, Trolle M, Bangsbo J, Klausen K. Isokinetic hamstring/quadriceps strength ratio: influence from joint angular velocity, gravity correction and contraction mode. Acta Physiol Scand. 1995;4:421–427. doi: 10.1111/j.1748-1716.1995.tb09927.x. [DOI] [PubMed] [Google Scholar]
- Adams GR. Role of insulin-like growth factor-I in the regulation of skeletal muscle adaptation to increased loading. Exerc Sport Sci Rev. 1998;26:31–60. [PubMed] [Google Scholar]
- Ahtiainen JP, Pakarinen A, Alen M, Kraemer WJ, Hakkinen K. Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol. 2003;89:555–563. doi: 10.1007/s00421-003-0833-3. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26 and 64-yr-old men and women. J Appl Physiol. 2004;97:1329–1337. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, McLafferty CL, Jr, Urban RJ. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001;280:383–390. doi: 10.1152/ajpendo.2001.280.3.E383. [DOI] [PubMed] [Google Scholar]
- Bartsch W. Interrelationships between sex hormone-binding globulin and testosterone, 5 alpha-dihydrotestosterone and oestradiol-17 beta in blood of normal men. Maturitas. 1980;2:109–118. doi: 10.1016/0378-5122(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;68:1–110. [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose–response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:1172–1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR. Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol. 2005;98:482–488. doi: 10.1152/japplphysiol.00895.2004. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Kimball SR, Jefferson LS. Translational control mechanisms modulate skeletal muscle gene expression during hypertrophy. Exerc Sport Sci Rev. 2003;31:111–116. doi: 10.1097/00003677-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Braith RW, Graves JE, Pollock ML, Leggett SL, Carpenter DM, Colvin AB. Comparison of 2 vs 3 days/week of variable resistance training during 10- and 18-week programs. Int J Sports Med. 1989;10:450–454. doi: 10.1055/s-2007-1024942. [DOI] [PubMed] [Google Scholar]
- Cameron-Smith D. Exercise and skeletal muscle gene expression. Clin Exp Pharmacol Physiol. 2002;29:209–213. doi: 10.1046/j.1440-1681.2002.03621.x. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chesley A, MacDougall JD, Tarnapolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after exercise. J Appl Physiol. 1992;73:1383–1388. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- Cockshott ID. Clinical pharmacokinetics of goserelin. Clin Pharmacokinet. 2000;39:27–48. doi: 10.2165/00003088-200039010-00003. [DOI] [PubMed] [Google Scholar]
- Coffey VG, Shield A, Canny BJ, Carey KA, Cameron-Smith D, Hawley JA. Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab. 2006;290:849–855. doi: 10.1152/ajpendo.00299.2005. [DOI] [PubMed] [Google Scholar]
- Dorlochter M, Astrow SH, Herrera AA. Effects of testosterone on a sexually dimorphic frog muscle: repeated in vivo observations and androgen receptor distribution. J Neurobiol. 1994;25:897–916. doi: 10.1002/neu.480250802. [DOI] [PubMed] [Google Scholar]
- Doumit ME, Cook DR, Merkel RA. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology. 1996;137:1385–1394. doi: 10.1210/endo.137.4.8625915. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:601–607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity – from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- Glowacki SP, Martin SE, Maurer A, Baek W, Green JS, Crouse SF. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med Sci Sports Exerc. 2004;36:2119–2127. doi: 10.1249/01.mss.0000147629.74832.52. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194:323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Adams GR. Selected contribution: acute cellular and molecular responses to resistance exercise. J Appl Physiol. 2002;93:394–403. doi: 10.1152/japplphysiol.01153.2001. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Pakarinen A. Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol. 1993;74:882–887. doi: 10.1152/jappl.1993.74.2.882. [DOI] [PubMed] [Google Scholar]
- Hameed M, Lange KH, Andersen JL, Schjerling P, Kjaer M, Harridge SD, Goldspink G. The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol. 2004;555:231–240. doi: 10.1113/jphysiol.2003.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Kvorning T, Kjaer M, Sjoegaard G. The effect of short-term strength training on human skeletal muscle: the importance of physiologically elevated hormone levels. Scand J Med Sci Sports. 2001;11:347–354. doi: 10.1034/j.1600-0838.2001.110606.x. [DOI] [PubMed] [Google Scholar]
- Harridge SD. Ageing and local growth factors in muscle. Scand J Med Sci Sports. 2003;13:34–39. doi: 10.1034/j.1600-0838.2003.20235.x. [DOI] [PubMed] [Google Scholar]
- Hespel P, Op't Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol. 2001;536:625–633. doi: 10.1111/j.1469-7793.2001.0625c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson RC, Hidaka K, Foster C, Falduto MT, Chatterton RT., Jr Successive time courses of strength development and steroid hormone responses to heavy-resistance training. J Appl Physiol. 1994;76:663–670. doi: 10.1152/jappl.1994.76.2.663. [DOI] [PubMed] [Google Scholar]
- Inoue K, Yamasaki S, Fushiki T, Okada Y, Sugimoto E. Androgen receptor antagonist suppresses exercise-induced hypertrophy of skeletal muscle. Eur J Appl Physiol. 1994;69:88–91. doi: 10.1007/BF00867933. [DOI] [PubMed] [Google Scholar]
- Ishido M, Kami K, Masuhara M. Localization of MyoD, myogenin and cell cycle regulatory factors in hypertrophying rat skeletal muscles. Acta Physiol Scand. 2004;180:281–289. doi: 10.1046/j.0001-6772.2003.01238.x. [DOI] [PubMed] [Google Scholar]
- Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004;558:1005–1012. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113:99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005;288:1110–1119. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T. American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Häkkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW, Putukian M, Evans WJ. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol. 1999;87:982–992. doi: 10.1152/jappl.1999.87.3.982. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Marchitelli LJ, Gordon SE, Harman E, Dziados JE, Friedl K, Maresh C, Fry AC, Mello P, Fleck SJ. Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med. 1991;12:228–235. doi: 10.1055/s-2007-1024673. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Marchitelli LJ, Gordon SE, Harman E, Dziados JE, Frykman P, McCurry D, Fleck SJ. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol. 1990;69:1442–1450. doi: 10.1152/jappl.1990.69.4.1442. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Marchitelli LJ, Gordon SE, Harman E, Dziados JE, Frykman P, Mello P, Koziris LP, Triplett NT, Fleck SJ. Changes in hormonal concentrations after different heavy-resistance exercise protocols in women. J Appl Physiol. 1993;75:594–604. doi: 10.1152/jappl.1993.75.2.594. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Patton JF, Gordon SE, Harman EA, Deschenes MR, Reynolds K, Newton RU, Triplett NT, Dziados JE. Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol. 1995;78:976–989. doi: 10.1152/jappl.1995.78.3.976. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35:339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Staron RS, Hagerman FC, Hikida RS, Fry AC, Gordon SE, Nindl BC, Gothshalk LA, Volek JS, Marx JO, Newton RU, Häkkinen K. The effects of short-term resistance training on endocrine function in men and women. Eur J Appl Physiol. 1998;78:69–76. doi: 10.1007/s004210050389. [DOI] [PubMed] [Google Scholar]
- Kvorning T, Andersen M, Brixen K, Madsen K. Suppression of endogenous testosterone production attenuates the response to strength training; a randomized, placebo-controlled and blinded intervention study. Am J Physiol Endocrinol Metab. 2006;291:1325–1332. doi: 10.1152/ajpendo.00143.2006. [DOI] [PubMed] [Google Scholar]
- Lang CH, Silvis C, Nystrom G, Frost RA. Regulation of myostatin by glucocorticoids after thermal injury. FASEB J. 2001;15:1807–1809. doi: 10.1096/fj.00-0849fje. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt G, Bennett P, Lykkesfeldt AE, Micic S, Moller S, Svenstrup B. Abnormal androgen and oestrogen metabolism in men with steroid sulphatase deficiency and recessive X-linked ichthyosis. Clin Endocrinol. 1985;4:385–393. doi: 10.1111/j.1365-2265.1985.tb01096.x. [DOI] [PubMed] [Google Scholar]
- Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab. 2003;285:363–371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- Mauras N, Blizzard RM, Link K, Johnson ML, Rogol AD, Veldhuis JD. Augmentation of growth hormone secretion during puberty: evidence for a pulse amplitude-modulated phenomenon. J Clin Endocrinol Metab. 1987;64:596–601. doi: 10.1210/jcem-64-3-596. [DOI] [PubMed] [Google Scholar]
- Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis JD, Urban RJ. Testosterone deficiency in young men: Marked alterations in whole body protein kinetics, strength and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;6:1153–1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Mozdziak PE, Greaser ML, Schultz E. Myogenin, MyoD, and myosin expression after pharmacologically and surgically induced hypertrophy. J Appl Physiol. 1998;84:1359–1364. doi: 10.1152/jappl.1998.84.4.1359. [DOI] [PubMed] [Google Scholar]
- Narici MV, Hoppeler H, Kayser B, Landoni L, Claassen H, Gavardi C, Conti M, Cerretelli P. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol Scand. 1996;157:175–186. doi: 10.1046/j.1365-201X.1996.483230000.x. [DOI] [PubMed] [Google Scholar]
- Psilander N, Damsgaard R, Pilegaard H. Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol. 2003;95:1038–1044. doi: 10.1152/japplphysiol.00903.2002. [DOI] [PubMed] [Google Scholar]
- Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285:876–888. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA. Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med. 2003;228:706–709. doi: 10.1177/153537020322800609. [DOI] [PubMed] [Google Scholar]
- Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab. 1995;269:820–826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Thornton CA. Stimulation of myofibrillar synthesis by exercise is mediated by more efficient translation of mRNA. J Appl Physiol. 1999;86:1220–1225. doi: 10.1152/jappl.1999.86.4.1220. [DOI] [PubMed] [Google Scholar]
- Willoughby DS. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc. 2004;36:574–582. doi: 10.1249/01.mss.0000121952.71533.ea. [DOI] [PubMed] [Google Scholar]
- Willoughby DS, Nelson MJ. Myosin heavy-chain mRNA expression after a single session of heavy-resistance exercise. Med Sci Sports Exerc. 2002;34:1262–1269. doi: 10.1097/00005768-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Willoughby DS, Rosene JM. Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc. 2003;35:923–929. doi: 10.1249/01.MSS.0000069746.05241.F0. [DOI] [PubMed] [Google Scholar]
- Willoughby DS, Taylor L. Effects of sequential bouts of resistance exercise on androgen receptor expression. Med Sci Sports Exerc. 2004;36:1499–1506. doi: 10.1249/01.mss.0000139795.83030.d1. [DOI] [PubMed] [Google Scholar]
- Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol. 2005;5:1745–1752. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002;522:156–160. doi: 10.1016/s0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]