Abstract
During wakefulness and sleep, neurons in the neocortex emit action potentials tonically or in rhythmic bursts, respectively. However, the role of synchronized discharge patterns is largely unknown. We have recently shown that pairings of excitatory postsynaptic potentials (EPSPs) and action potential bursts or single spikes lead to long-term depression (burst-LTD) or long-term potentiation, respectively. In this study, we elucidate the cellular mechanisms of burst-LTD and characterize its functional properties. Whole-cell patch-clamp recordings were obtained from layer V pyramidal cells in somatosensory cortex of juvenile rats in vitro and composite EPSPs and EPSCs were evoked extracellularly in layers II/III. Repetitive burst-pairings led to a long-lasting depression of EPSPs and EPSCs that was blocked by inhibitors of metabotropic glutamate group 1 receptors, phospholipase C, protein kinase C (PKC) and calcium release from the endoplasmic reticulum, and that required an intact machinery for endocytosis. Thus, burst-LTD is induced via a Ca2+- and phosphatidylinositol-dependent activation of PKC and expressed through phosphorylation-triggered endocytosis of AMPA receptors. Functionally, burst-LTD is inversely related to EPSP size and bursts dominate single spikes in determining the sign of synaptic plasticity. Thus burst-firing constitutes a signal by which coincident synaptic inputs are proportionally downsized. Overall, our data thus suggest a mechanism by which synaptic weights can be reconfigured during non-rapid eye movement sleep.
Long-term potentiation (LTP) and depression (LTD) are lasting increases or decreases in synaptic strength, respectively, that are considered important cellular processes underlying learning and memory formation (Rioult-Pedotti et al. 2000). However, the physiological stimuli that lead to permanent synaptic changes are not precisely known. Experimentally, LTP (or LTD) can be induced by repetitive application of high (or low) frequency stimulation (Artola & Singer, 1993). Yet, with more physiological spike patterns LTP dominates LTD, probably due to the omnipresence of high-frequency discharge episodes in stochastic spike trains (Dobrunz & Stevens, 1999; Perrett et al. 2001). Nevertheless, to prevent synaptic weights from saturating, LTP and LTD eventually have to be kept in balance.
It was recently shown that the relative timing of pre- versus postsynaptic action potentials can lead to selective induction of LTP/LTD: EPSPs followed by an antidromic spike underwent LTP whereas in the reverse order LTD was induced (Debanne et al. 1994; Markram et al. 1997; Feldman, 2000). But again, subsequent experiments revealed that spike order-dependent induction of LTP/LTD is limited because LTP dominates LTD at higher firing rates as often seen in vivo (Sjöström et al. 2001).
Another way to balance LTP/LTD may be the recently described firing mode-dependency of LTP/LTD (Birtoli & Ulrich, 2004). Many neuronal cell types are capable of firing action potentials in two different modes: either as individual action potentials (tonic mode) or as spike bursts (burst mode; Connors et al. 1982; Llinás, 1988). Tonic discharges are prevalent during wakefulness and paradoxical sleep whereas bursts occur predominantly during slow-wave sleep (SWS) (Steriade et al. 2001). It has been hypothesized that synaptic strength may be downsized during SWS as part of a homeostatic or memory-consolidating process (Tononi & Cirelli, 2003) for which our data suggest a cellular mechanism.
Methods
Tissue preparation
Parasagittal slices of 300 μm thickness were prepared at 4°C from 3- to 4-week-old Wistar rats after decapitation, and incubated at 35°C in standard artificial cerebrospinal fluid containing (mm): NaCl 125, NaH2PO4 1.25, NaHCO3 25, KCl 2.5, MgCl2 1, CaCl2 2 and glucose 19; equilibrated with 5% CO2–95% O2. All experimental procedures were approved by our local animal care committee (Veterinary Services, Office of Agriculture, Bern).
Electrophysiology
Patch pipettes were filled with solution containing (mm): potassium gluconate 130, NaCl 10, EGTA 0.1, Hepes 10 and ATP 5. Pyramidal cells in layer V of somatosensory cortex were visualized with infrared differential interference contrast video microscopy (Dodt & Zieglgänsberger, 1990). Whole-cell voltage- or current-clamp recordings were obtained with Axoprobe 1A or Axoclamp 2B amplifiers (Molecular Devices, Union City, CA, USA), low-pass filtered at 1 kHz and digitized at 3 kHz with a Labmaster analog-to-digital converter (Scientific Solutions, Solon, OH, USA). Series resistance (∼10 MΩ) was compensated for by adjusting the bridge and recordings with unstable (i.e. ± 10%) membrane potential and/or access resistance were discarded. A liquid-junction potential of −10 mV was left uncorrected. EPSPs/EPSCs were evoked by brief extracellular voltage pulses (0.02–0.08 ms, 10–100 V) with a Grass SD9 stimulator (West Warwick, RI, USA) through insulated bipolar nickel–chromium electrodes of 0.025 mm diameter (Goodfellow Corporation, Devon, PA, USA) placed in layers II/III. Stimuli were applied at 0.2 Hz. Bursts of three or four action potentials were elicited by brief somatic current injections (14–18 ms, 1–3 nA) with a delay of 10 ms. Heparin and ruthenium red were from Sigma (Buchs, Switzerland). All other drugs were from Tocris (Bristol, UK) and bath-applied unless stated otherwise.
Data analysis
EPSP/EPSC amplitudes were measured by subtracting time-averaged voltage or current values at baseline from peak. Data are presented as means ± s.e.m. Statistical significance of EPSP/EPSC changes was assessed with the two-tailed Wilcoxon test on normalized EPSP/EPSC amplitudes. The significance level was 0.05.
Results
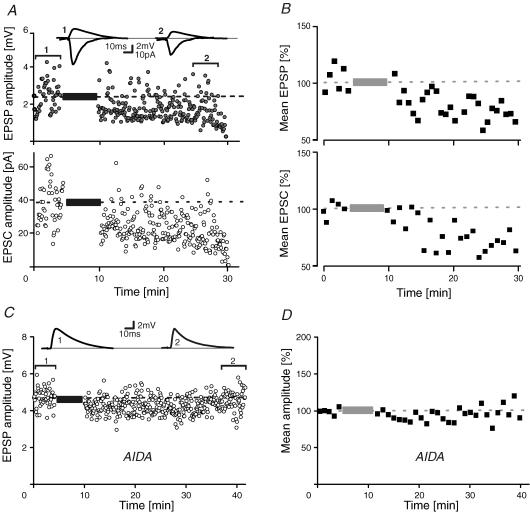
To characterize burst-LTD, whole-cell patch-clamp recordings were obtained from visually identified layer V pyramidal cells of somatosensory cortex (Stuart et al. 1993). Composite EPSPs and EPSCs were evoked alternately within layers II/III at 0.2 Hz (Fig. 1A) by computer-controlled switching between current-clamp bridge mode and continuous single-electrode voltage clamp. After a control period, EPSPs were paired with a 10 ms delayed burst of three action potentials. The intraburst firing rate was ∼150–300 Hz. After 70 pairings, intracellular stimuli were turned off and EPSPs/EPSCs again triggered in isolation. As shown in Fig. 1A and B, the conditioning EPSPs and EPSCs diminished in parallel to a common new steady-state level of 63 ± 9% and 68 ± 11% (n = 11) of control, respectively (P < 0.05, Fig. 1B). In two previous studies (Birtoli & Ulrich, 2004; Rosanova & Ulrich, 2005) we found that under identical recording conditions without conditioning, EPSPs remained stable over the same time interval (93 ± 4%, P > 0.05, n = 12). Thus, repetitive temporal associations of EPSPs and intrinsic bursts induce LTD. A similar decline of EPSPs was seen with pairing frequencies of 0.1 and 1 Hz (data not shown). The fact that synaptic potentials and currents are similarly depressed by the conditioning protocol suggests that burst-LTD affects mainly synaptic rather than intrinsic ionic conductances (Anwyl, 1999).
Figure 1. Burst-LTD of EPSPs/EPSCs.
A, single-cell EPSP/EPSC amplitude–time series and sample traces in control (1) and after burst-conditioning (2). B, mean time course of normalized EPSP/EPSC time-averaged (1 min) amplitudes (n = 11). C, sample EPSPs and amplitude–time series of a pairing experiment in the presence of the type 1 metabotropic glutamate receptor-specific antagonist 1-aminoindan-1,5-dicarboxylic acid (AIDA; 0.2 mm). D, population data of all AIDA experiments (n = 7). Dashed lines and bars represent average values in control and the pairing period, respectively.
Mechanisms of burst-LTD induction
In a former study (Birtoli & Ulrich, 2004), we showed that burst-LTD is blocked by (α-methyl-4-carboxyphenylglycine (MCPG), a non-selective antagonist of metabotropic glutamate receptors (mGluRs). To discriminate the mGluR subtypes involved, we performed another set of burst-pairing experiments (Fig. 1C and D) in which group 1 mGluRs were specifically blocked with the selective antagonist 1-aminoindan-1,5-dicarboxylic acid (AIDA; 0.2 mm). In the presence of AIDA, burst-LTD was clearly abolished (102 ± 25%, n = 7, P > 0.5, Fig. 1C and D) suggesting a role for mGluRs coupled to phospholipase C (PLC) in burst-LTD.
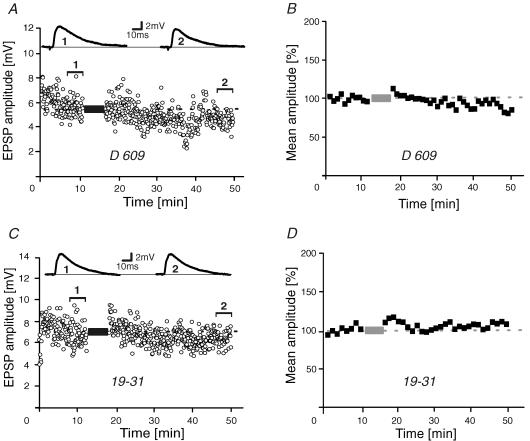
To characterize the signal transduction cascade involved in burst-LTD, a series of burst-pairing experiments was carried out with the PLC inhibitor O-(octahydro-4,7-methano-1H-inden-5-yl) carbonopotassium dithioate (D609; 30 μm) added to the pipette solution (Fig. 2A). In the presence of D609, EPSPs were still 89 ± 7% (n = 6) of control after burst-conditioning (Fig. 2A and B), (i.e. not significantly reduced; P > 0.5). PLC catalyses the breakdown of phosphatidylinositol 4,5-bisphosphate into its constituent components 1,2-diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG, together with Ca2+, in turn can activate protein kinase C (PKC). Compatible with this signalling pathway, intracellular application of the PKC inhibitor peptide 19-31 (RFARKGALRQKNV; 0.5 μm; House & Kemp, 1987) rendered burst-conditioning ineffective as conditioned EPSPs were still 103 ± 13% of control (n = 8, Fig. 2C and D). To assess whether burst-LTD requires rises in intracellular Ca2+ concentration, we performed a series of pairing experiments with the Ca2+ chelator BAPTA (30 mm) added to the pipette solution (Fig. 3A and B). In the presence of BAPTA, burst-LTD was again abolished (100 ± 6%, n = 8). IP3 may trigger Ca2+ release from the endoplasmic reticulum (ER), an internal Ca2+ store that is known to participate in synaptic plasticity (Rose & Konnerth, 2001). To assess an involvement of the ER in burst-LTD, IP3 receptors were blocked by intracellularly applying heparin (1 mg ml−1). Burst-pairings were again ineffective as EPSPs were still 86 ± 6% (n = 7, P > 0.2) after the conditioning (i.e. not significantly diminished; Fig. 3C and D). We have previously shown that burst-LTD depends on Ca2+ influx via T-type channels (Birtoli & Ulrich, 2004). Influx of Ca2+ may secondarily lead to Ca2+-induced Ca2+ release (CICR) from the ER. In agreement with a role of CICR in burst-LTD, blockade of ryanodine receptors with intracellularly applied ruthenium red (0.2 mm) abolished burst-LTD (91 ± 6%, n = 6, P > 0.7, Fig. 3E and F). Together, these data suggest an involvement of the type 1 mGluR–PLC–PKC signalling cascade in inducing burst-LTD involving Ca2+ release from the ER. Our conclusion is corroborated further by the additional finding that blocking the protein phosphatase calcineurin, another enzyme frequently involved in LTD, did not prevent burst-LTD (see Fig. 5).
Figure 2. Induction of burst-LTD.
A and B, EPSP amplitude–time series and sample traces (A) and normalized time-averaged (1 min) population data (B) of burst-pairing experiments in the presence of the phospholipase C inhibitor O-(octahydro-4,7-methano-1H-inden-5-yl) carbonopotassium dithioate (D609) added to the patch pipette (30 μm; n = 6). C and D, similar experiments during intracellular application of the protein kinase C pseudosubstrate 19-31 (0.5 μm; n = 8). Dashed lines and bars represent average control values and the pairing period, respectively.
Figure 3. Burst-LTD and intracellular Ca2+.
A and B, EPSP amplitude–time series with sample traces (A) and normalized population data (B) of burst-pairing experiments during intracellular application of BAPTA (30 mm, n = 8). C and D, EPSP amplitude–time series with sample traces (C) and normalized 1 min time-averaged population data (D) of burst-pairing experiments in the presence of the inositol 1,4,5-trisphosphate receptor blocker heparin added to the pipette solution (1 mg ml−1, n = 7). E and F, comparable experiments in the presence of ruthenium red (0.2 mm) to block ryanodine receptors (n = 6). Dashed lines and bars represent average control values and the pairing period, respectively.
Figure 5. Summary graph of pharmacology of burst-LTD.
A and B, histograms of EPSP amplitudes after burst-conditioning. A, antagonists that successfully prevented burst-LTD. B, inhibitors that did not interfere with burst-LTD. Dotted and dashed line, average control and burst-conditioned EPSP amplitude, respectively. AIDA, 1-aminoindan-1,5-dicarboxylic acid; AM-251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; CDC, cinnamyl 3,4,-dihydroxy-α-cyanocinnamate; CN, calcineurin autoinhibitory peptide (ITSFEEAKGLDRINERMPPR); D15, PPPQVPSRPNRAPPG; D609, O-(octahydro-4,7-methano-1H-inden-5-yl) carbonopotassium dithioate; 19-31, RFARKGALRQKNV; Hep, heparin; Nif, nifedipine; L-NNA, NG.-nitro-l-arginine; RR, ruthenium red; ext, extracellularly applied; int, intracellularly applied.
Mechanisms of burst-LTD expression
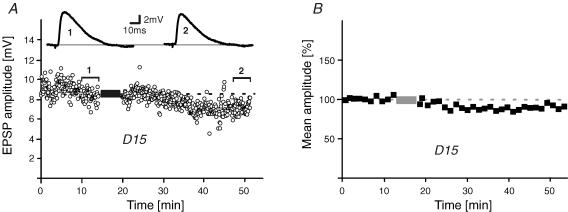
We next investigated the expression mechanism of burst-LTD. In general, a diminution of synaptic responses can either be caused by a reduced transmitter release or a decreased responsiveness of the postsynaptic membrane. Among the mechanisms for the latter is receptor endocytosis which has been shown to be important for LTD expression (Malinow & Malenka, 2002). To assess a role for receptor internalization in burst-LTD, burst-pairing experiments were repeated with intracellularly applied D15 (PPPQVPSRPNRAPPG; 1 mm), a peptide that interferes with clathrin-dependent endocytosis (Lüscher et al. 1999). As shown in Fig. 4A and B, burst-LTD was blocked by D15 as conditioned EPSPs were now not significantly diminished (86 ± 6%, n = 7, P > 0.2). Thus, this result indicates primarily a postsynaptic locus for burst-LTD expression involving endocytosis of AMPA receptors, although a presynaptic contribution cannot be ruled out completely. This conclusion is further supported by the fact that burst-LTD was not abolished by different inhibitors of common retrograde messengers, which would need to be present to communicate synaptic changes to presynaptic elements (see Discussion). Figure 5 shows a summary graph of all pharmacological interventions performed in this study.
Figure 4. Expression of burst-LTD.
A and B, burst-LTD is blocked after intracellular application of the endocytosis inhibitor peptide D15 (1 mm). EPSP amplitude–time series in an individual experiment (A) and 1 min time-averaged population data (B) (n = 7). Dashed lines and bars represent average control values and the pairing period, respectively.
Functional properties of burst-LTD
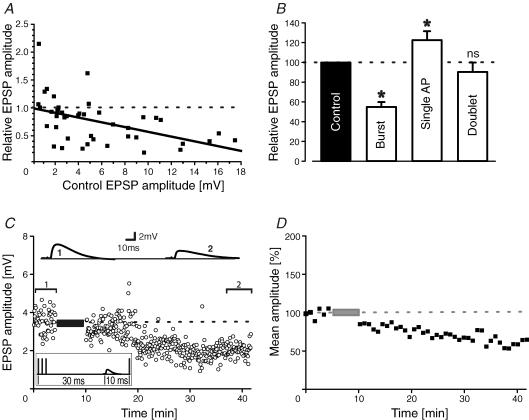
Figure 6A depicts a summary graph of burst-pairing experiments where the relative change of synaptic strength is related to the initial amplitude of the EPSP. The amount of burst-LTD is inversely related to EPSP size in control; that is, the larger an EPSP the more it will become depressed. With the size of the EPSPs usually evoked in this study of 2–10 mV, associations with single action potentials, spike doublets or bursts of three spikes predominantly led to LTP, no change or LTD, respectively (Fig. 6B). In addition, conditionings with longer bursts (i.e. more than three spikes) were comparable to three spike–burst pairings (data not shown). Bursts of action potentials in neocortical pyramidal cells largely occur during SWS intermingled with individual action potentials (Steriade et al. 2001). To investigate the interplay between firing modes and synaptic plasticity we designed a pairing protocol consisting of bursts and single spikes. We have previously shown that single spikes following an EPSP by 10 ms lead to LTP whereas burst-LTD was significant over a longer interval ranging from −100 to +200 ms (Birtoli & Ulrich, 2004). Figure 6C shows a pairing experiment with a −20 ms burst and a +10 ms single spike leading to LTD despite the single spike occurring in its LTP time window. Overall, burst–single spike pairings reduced EPSPs to 61 ± 9% (n = 6), i.e. to a value similar to bursts alone. This result suggests that bursts dominate over single spikes in determining the sign of synaptic plasticity.
Figure 6. Burst and single spikes.
A, summary graph of relative EPSP size after conditioning versus the initial EPSP amplitude. A straight line was fitted to the data points by linear regression (r = 0.49; P < 0.0001). B, summary histogram of pairing experiments with bursts (three to four spikes), single action potentials and spike doublets (*P < 0.05; ns, not significant). C, sample traces and EPSP amplitude–time series of an experiment where EPSPs were simultaneously paired with a −20 ms burst and a +10 ms single action potential. D, 1 min averaged time course of normalized EPSP amplitudes with concomitant burst–single spike pairings (n = 6 cells).
Discussion
In this study we show that burst-LTD is blocked by inhibitors of class 1 mGluRs, PLC, PKC and calcium release from internal stores, and involves endocytosis of AMPA receptors. Additionally, we show that burst-LTD depends on EPSP size and that bursts dominate single spikes in determining the sign of synaptic plasticity.
Endo- and exocytosis of AMPA receptors are important cellular processes in LTD and LTP, respectively (Malinow & Malenka, 2002). Among the established signalling cascades involved in endocytosis of AMPA receptors is the PKC-mediated phosphorylation of the GLuR2 of ANPA-R receptors. This modification changes the affinity of AMPA receptors for the associated proteins GRIP and PICK, eventually leading to receptor internalization (Seidenman et al. 2003). Our results with inhibitors of type 1 mGluRs, PLC, PKC and clathrin-mediated endocytosis suggest a similar mechanism for burst-LTD. In contrast, in some previous studies in the same cell type LTD was found to be of presynaptic origin, involving endocannabinoids as retrograde messengers (Sjöström et al. 2003). However, we have ruled out experimentally an involvement of endocannabinoids as well as NO (Volgushev et al. 2000) and 12(S)-hydroxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid (Feinmark et al. 2003) in burst-LTD (Fig. 5). A postsynaptic locus for burst-LTD is also compatible with the known postsynaptic localization of type 1 mGluRs (Nusser, 1999). The conflicting results in allocating synaptic changes during LTD may indicate that several variants of LTD coexist in pyramidal neurons probably each depending on particular stimulation patterns (Malenka & Bear, 2004).
CICR usually depends on Ca2+ influx via L-type channels (Chavis et al. 1996) whereas burst-LTD could still be elicited in presence of the L-type channel blocker nifedipine (Fig. 5). However, we have previously shown the participation of T-type Ca2+ channels in burst-LTD (Birtoli & Ulrich, 2004). It is thus likely that CICR may also be coupled to T-type channels in pyramidal cells as recently shown for certain thalamic neurons (Richter et al. 2005). In line with our findings, an involvement of T-channels and CICR in LTD has also been demonstrated previously in the dentate gyrus (Wang et al. 1997).
What could be the particular role of burst-firing in LTD? Presynaptically, high-frequency discharges were shown to be efficient in activating mGluRs (Batchelor & Garthwaite, 1997). Postsynaptically, burst-firing may lead to a prolonged Ca2+ influx that favours LTD over LTP (Yang et al. 1999). In support of the latter, LTP was consecutively shifted to no change and LTD by pairings with single action potentials, spike doublets and bursts, and the latter dominated single spikes in determining the sign of synaptic changes, thus indicating an important role for the duration of Ca2+ concentration changes. A similar transition from LTP to LTD was also found in pyramidal cells of entorhinal cortex by artificially prolonging Ca2+ influx during single spike pairings (Zhou et al. 2005).
In contrast, in a recent study in the same cells, associations of unitary EPSPs and spike bursts lead to NMDA receptor-mediated LTP (Kampa et al. 2006). This discrepancy may be due to the different size of the EPSPs involved. Unitary synaptic connections between layer V pyramidal cells are usually < 1 mV, whereas the composite EPSPs in our experiments were in the range of 2–10 mV, which is comparable to EPSPs seen in vivo (Petersen et al. 2003). Indeed our data show that the amount of burst-LTD is related to EPSP size (Fig. 6A). This may be due to the observation that mGluRs are mainly perisynaptically localized (Nusser, 1999). Their activation may thus require higher glutamate levels than produced by an individual release site (cf. Charpak & Gähwiler, 1991).
Burst-LTD described in this study has some resemblance to synaptic plasticity in the cerebellum. There, associations of parallel and climbing fibre inputs lead to complex spike-dependent homo- and heterosynaptic depression of parallel fibre inputs (Ito, 2001). Also, at least for homosynaptic LTD, a type 1 mGluR-dependent internalization of AMPA receptors is responsible for the long-lasting diminution of synaptic transmission while heterosynaptic LTD seems to depend on NO-mediated interactions between pre- and postsynaptic elements. Although we have ruled out NO as a signalling molecule in burst-LTD for homosynaptic inputs (see above), further experiments will be needed to assess its role in heterosynaptic LTD. Whereas burst-LTD in the cerebellum is thought to be part of motor learning, much less is known about the impact of burst-firing in the cortex. There, bursts are believed to act as feature detectors or attention signals (Krahe & Gabbiani, 2004). But because action potential bursts in neocortex predominantly occur during sleep (Steriade et al. 2001), they are thought to participate in sleep-associated memory formation (Stickgold et al. 2001; Tononi & Cirelli, 2003). Our data would be compatible with this hypothesis.
Acknowledgments
This work was supported by the Swiss National Science Foundation grant 3100AO-100574. We thank Drs B. Bettler (University Basel) and A. Lüthi (Friedrich Miescher Institute, Basel) for helpful comments on the manuscript.
References
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 1999;366:151–158. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. doi: 10.1038/385074a0. [DOI] [PubMed] [Google Scholar]
- Birtoli B, Ulrich D. Firing mode-dependent synaptic plasticity in rat neocortical pyramidal neurons. J Neurosci. 2004;24:4935–4940. doi: 10.1523/JNEUROSCI.0795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S, Gähwiler B. Glutamate mediates a slow synaptic repsonse in hippocampal slices cultures. Proc R Soc Lond B Biol Sci. 1991;243:221–226. doi: 10.1098/rspb.1991.0035. [DOI] [PubMed] [Google Scholar]
- Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ, Prince DA. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982;48:1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Asynchronous pre- and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proc Natl Acad Sci U S A. 1994;91:1148–1152. doi: 10.1073/pnas.91.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Response of hippocampal synapses to natural stimulation patterns. Neuron. 1999;22:157–166. doi: 10.1016/s0896-6273(00)80687-x. [DOI] [PubMed] [Google Scholar]
- Dodt H-U, Zieglgänsberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- Feinmark SJ, Begum R, Tsvetkov E, Goussakov I, Funk CD, Siegelbaum SA, Bolshakov VY. 12-lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3-CA1 synapses. J Neurosci. 2003;23:11427–11435. doi: 10.1523/JNEUROSCI.23-36-11427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototype in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Requirement of dendritic calcium spikes for induction of spike-timing dependent synaptic plasticity. J Physiol. 2006;574:283–290. doi: 10.1113/jphysiol.2006.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci. 2004;5:13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;30:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Nusser Z. Subcellular distribution of neurotransmitter receptors and voltage-gated ion channels. In: Stuart G, Spruston N, Häusser M, editors. Dendrites. Oxford: Oxford University Press; 1999. pp. 85–113. [Google Scholar]
- Perrett SP, Dudek SM, Eagleman D, Montague PR, Friedlander MJ. LTD induction in adult visual cortex: role of stimulus timing and inhibition. J Neurosci. 2001;21:2308–2319. doi: 10.1523/JNEUROSCI.21-07-02308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Kolaj M, Renaud LP. Low voltage-activated Ca2+ channels are coupled to Ca2+-induced Ca2+ release in rat thalamic midline neurons. J Neurosci. 2005;25:8267–8271. doi: 10.1523/JNEUROSCI.1942-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti M-S, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. Stores not just for storage: intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning and dreams: off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Baraban P, Chistiakova M, Eysel UT. Retrograde signaling with nitric oxide at neocortical synapses. Eur J Neurosci. 2000;12:4255–4267. doi: 10.1046/j.0953-816x.2000.01322.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rowan MJ, Anwyl R. Induction of LTD in the dentate gyrus in vitro is NMDA receptor independent, but dependent on Ca2+ influx via low-voltage-activated Ca2+ channels and release of Ca2+ from intracellular stores. J Neurophysiol. 1997;77:812–825. doi: 10.1152/jn.1997.77.2.812. [DOI] [PubMed] [Google Scholar]
- Yang S-N, Tang Y-G, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Acker CD, Netoff TI, Sen K, White JA. Increasing Ca2+ transients by broadening postsynaptic action potentials enhances timing-dependent synaptic depression. Proc Natl Acad Sci U S A. 2005;102:19121–19125. doi: 10.1073/pnas.0509856103. [DOI] [PMC free article] [PubMed] [Google Scholar]