Abstract
The specific role of vasopressin in colonic crypt function and its possible synergistic action with aldosterone were studied. Sprague-Dawley rats fed a high-Na+ (HS; 150 mm NaCl) or a low-Na+ (LS; 150 μm NaCl) diet were deprived of water or infused with vasopressin, and some animals were treated with specific vasopressin receptor subtype V1 and V2 antagonists. The expression of the epithelial Na+ channel (ENaC), α-smooth muscle actin (α-SMA) and aquaporin-2 (AQP-2) were determined by immunolocalization in distal colonic mucosa. The pericryptal Na+ concentration was determined by confocal microscopy, using a low-affinity Na+-sensitive fluorescent dye (sodium red) and crypt permeability was measured by the rate of escape of fluorescein isothiocyanate-labelled dextran (10 kDa) from the crypt lumen into the pericryptal space in isolated rat distal colonic mucosa. A high plasma concentration of vasopressin raised α-SMA expression in the pericryptal sheath (P < 0.05), increased the pericryptal Na+ accumulation in this space (P < 0.01) and caused a reduction of crypt wall permeability (P < 0.01). All these effects were reversed by selective blockade of V1 and V2 receptors. No synergistic effects with aldosterone were observed. Dehydration and vasopressin infusion increased AQP-2 expression in distal colonic mucosa (P < 0.05). This action of vasopressin was prevented by tolvaptan, a specific V2 receptor antagonist (P < 0.05). It is concluded that vasopressin has trophic effects in the rat distal colon, increasing pericryptal myofibroblast growth which affects crypt absorption, and these effects are independent of the presence of aldosterone.
The antidiuretic hormone vasopressin (AVP) plays a major role in the regulation of body water and electrolyte homeostasis. AVP acts by two different G-protein-coupled receptor subtypes, V1 and V2 (Morel et al. 1992; Lolait et al. 1992). V1 receptors are involved in the regulation of blood pressure and V2 receptors are responsible for the antidiuretic effect of AVP (Nonoguchi et al. 1995), essentially regulating aquaporin-2 (AQP-2) expression (Nielsen et al. 1995). AVP appears to stimulate the synthesis of AQP-2 mRNA and to regulate the insertion of AQP-2 into luminal membranes through fast exocytosis (Fushimi et al. 1993; Saito et al. 1997).
Colonic epithelium can modulate both the absorption and secretion of a variety of electrolytes and therefore has a role in water and salt homeostasis. This function may be particularly important in animals retaining a cloaca (Balment et al. 2006), such as fish, amphibians and birds, and is regulated by many endocrine, neurocrine and paracrine agents. In earlier studies, we described the role of aldosterone in colon crypt permeability and pericryptal myofibroblast growth during adaptation to a low-Na+ diet (Moretó et al. 2005; Cristià et al. 2005). Our results support the view that aldosterone stimulates myofibroblast growth in the distal colon crypts and this effect is responsible for both increased Na+ absorption and decreased tissue permeability. Aldosterone increased the expression of the epithelial sodium channel (ENaC) and α-smooth muscle actin (α-SMA), increasing Na+ accumulation in the pericryptal space and decreasing dextran permeability across the mucosa.
There is evidence that the colon could be a target of action for AVP, as it has been shown in the rat distal colon that AVP stimulates water absorption (Bridges et al. 1983, 1984) and AQP-2 has been found in both rat (Gallardo et al. 2001) and human (Mobasheri et al. 2005) distal colon. AVP, like aldosterone, has been implicated in extracellular matrix remodelling and growth of the heart (Harada et al. 1998; Goldsmith & Gheorghiade, 2005), and both show similar fibrotic effects in heart and kidney (Zannad & Radauceanu, 2005; Nagai et al. 2005). Similar actions of AVP and aldosterone have been described. AVP potentiates the aldosterone-mediated activation in the colon of prostasin, a membrane-bound serine protease that plays a crucial role in Na+ transport, and of 11β-hydorxysteroid dehydrogenase type 2, the enzyme that inactivates glucocorticoids (Fukushima et al. 2004, 2005). In the kidney, AVP stimulates sodium reabsorption in the renal collecting duct (Schafer & Hawk, 1992) and can exert synergistic effects with aldosterone (Verrey, 1994; Hawk et al. 1996). In this organ, both aldosterone and AVP can enhance ENaC expression (Machida et al. 2003). Moreover, both aldosterone and AVP secretion are inhibited by fluid and electrolyte homeostasis regulators such as adrenomedullin (AM) and atrial natriuretic peptide (ANP) (Nonoguchi et al. 1988; Szalay et al. 1998; Taylor & Samson, 2002).
The aim of this study was to determine a possible role for AVP in collagen formation in the distal colon and to clarify its role in water absorption and permeability. This was done by investigating the action of the two AVP receptor subtypes to determine whether there are synergistic or similar effects of AVP and aldosterone on these actions.
Methods
Experimental animals
Studies were performed on adult male Sprague-Dawley rats (Harlan Iberica, Barcelona, Spain) weighing 200–250 g on the day of experiment. They were housed one per cage under a 12 h light–12 h dark cycle. The Ethical Committee for Animal Experimentation of the Universitat de Barcelona approved the experimental procedures.
Experimental protocol
Diets
All animals were fed wheat and barley and had access to food ad libitum throughout the experiments. Sodium was given in the drinking water: the high-Na+ diet (HS) contained 150 mm NaCl whereas the low-Na+ diet (LS) contained 150 μm NaCl. The HS group were fed this diet for 7 days. To study adaptation to a LS diet, after 4 days of HS diet, the LS group was changed to the LS diet for 3 days.
AVP study
Two strategies were used to increase plasma AVP concentration: 24 h water restriction and AVP infusion. Animals given either HS or LS were deprived of water for 24 h on the last day of the experiment (i.e. HSD and LSD groups). Osmotic minipumps (model 2002, Alzet, Palo Alto, CA, USA) filled with AVP (Sigma Chemicals, St Louis, MO, USA) dissolved in saline, were implanted subcutaneously in the neck in both HS and LS groups. AVP was delivered at a rate of 1 ng kg−1 min−1 for 3 days. To inhibit the production of aldosterone and angiotensin II, some rats given LS received the angiotensin-converting enzyme inhibitor, captopril (CAP; Sigma), administered in drinking water at a dose of 65 mg kg−1 day−1 for 3 days. Moreover, some animals were treated with AVP receptor antagonists. Manning peptide (d(CH2)51Tyr(Me)2Arg8)-vasopressin, Sigma), a V1 receptor antagonist, was administered at 1 μg kg−1i.p. for 3 days (VR1 group). Tolvaptan (OPC-41061, gift from Otsuka Pharmaceutical Co, Japan), a V2 receptor antagonist, was used at a dose of 5 mg kg−1 for 3 days, dissolved in 1% hydroxypropylmethylcellulose (Sigma) as described by Yamamura et al. (1998) (VR2 group). A summary of all groups with their plasma hormonal profiles is shown in Table 1.
Table 1.
Experimental groups and their hormonal profiles
| Group | Aldosterone | Vasopressin |
|---|---|---|
| HS | ↓ | ↓ |
| HSD | ↓ | ↑ |
| HS + AVP | ↓ | ↑ |
| HS + AVP + VR2 | ↓ | ↑ |
| LS | ↑ | ↓ |
| LSD | ↑ | ↑ |
| LSD + CAP | ↓ | ↑ |
| LSD + CAP + VR1 | ↓ | ↑ |
| LSD + CAP + VR2 | ↓ | ↑ |
| LSD + AVP + CAP | ↓ | ↑ |
Control groups, high-Na+ diet (HS) and low-Na+ diet (LS); D, rats were deprived of water for 24 h; AVP, animals were infused with AVP for 7 days; VR1, rats were treated with a V1 receptor antagonist; VR2, rats were treated with a V2 receptor antagonist; CAP, rats were administered with captopril. ↑, increased; ↓, decreased.
Tissue preparation
Rats were maintained in metabolic cages for the last 3 days of the experiment, and 24 h urinary output, water intake, food consumption and weight changes were measured daily. Urinary volume, osmolality and Na+, K+ and AVP concentrations were also measured. To prevent urinary bacterial growth, azlocillin (Sigma) was added to the collection tube. Thereafter, rats were anaesthetized with ketamine–xylacine (100 and 10 mg kg−1, respectively) i.p.; blood samples were obtained by heart puncture and the descending colon was removed rapidly and washed with PBS. Animals were killed by cervical dislocation. Colonic mucosa was isolated from the underlaying serosa by scraping with a glass slide and maintained in Earle's medium for use in in vitro functional studies (pericryptal Na+ accumulation and dextran permeability), or fixed in 4% paraformaldehyde in PBS at 4°C for 24 h for immunohistochemistry studies. Blood was collected in tubes containing EDTA (1.5 mg (ml blood)−1) and a protease inhibitor cocktail (Sigma) and kept on ice until centrifugation at 4°C. Plasma was divided into aliquots and stored at −80°C for subsequent assay of plasma aldosterone and ion concentrations.
Hormone and ion determinations
Hormone levels were determined by radioimmunoassay (RIA) using commercially available kits: aldosterone RIA kit (Immunotech, France) for plasma aldosterone and RIA I125 Bühlmann Laboratories (Switzerland) for urine vasopressin. Plasma Na+ and K+ concentrations were measured using potentiometric direct dry chemistry (Beckman Coulter Corporation, USA). Their urinary concentrations were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES Thermo Jarrell Ash model ICAP 61E, Genesis Laboratories Systems Inc., CO, USA).
Immunocytochemistry
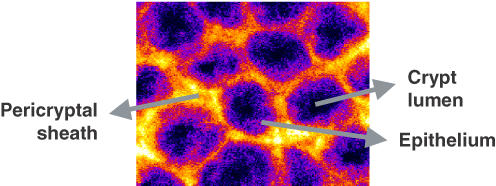
Colonic mucosal tissues (0.5 cm × 0.5 cm pieces) were stained according to the following protocol. The procedure was the same for all antibodies used. Tissues were permeabilized in 0.2% Triton X-100 in blocking buffer (1% BSA in PBS and glycine) for 30 min. Samples were washed three times in PBS and incubated for 90 min with primary antibody (1 : 100 for ENaC and α-SMA, 1 : 50 for AQP-2). The antibodies used were monoclonal anti-α-SMA clone IA4 and rabbit anti-water channel AQP-2 from Sigma, and rabbit anti-rat γ-ENaC antibody from Alpha Diagnostics International Inc. (San Antonio, TX, USA). After washing three times in PBS, tissues were incubated for 60 min with either secondary antibody (1 : 100). Both secondary antibodies were purchased from Molecular Probes (Eugene, OR, USA), anti-αSMA staining Texas Red goat anti-mouse IgG and anti-rabbit Alexa-488 antibody for ENaC and AQP-2. Finally, tissues were again washed three times in PBS. Representative distal colon mucosa seen under the confocal microscope is shown in Fig. 1, indicating the localization of the crypt lumen, the epithelium and the pericryptal space.
Figure 1. Representative distal colon mucosa seen under the confocal microscope.
The location of the crypt lumen, the epithelium and the pericryptal space is indicated. Myofibroblasts are localized in the pericryptal space surrounding the crypts.
Pericryptal Na+ accumulation
Na+ concentration in isolated rat distal colonic mucosa was determined as described previously (Moretó et al. 2005) using a modification of a dual-wavelength laser scanning confocal microscopic method in which low-affinity Na+-sensitive dye (sodium red and BODIPY-fl) is bound to microscopic polystyrene beads (Jayaraman et al. 2001). Briefly, colonic mucosal pieces were loaded with the dye-containing beads and viewed under confocal microscopy. The local Na+ concentration was estimated by ratiometric imaging of the beads using the sodium red signal, which increases linearly in the concentration range 0–500 mm, and the green signal from BODIPY-fl.
Dextran permeability
Crypt permeability to dextran was monitored by the rate of escape of fluorescein isothiocyanate (FITC)-labelled dextran (FITC-dextran; molecular weight, 10 000; Sigma) from the crypt lumen into the pericryptal space at 37°C. Crypt luminal and pericryptal concentration of FITC-dextran was estimated by monitoring the ratio of fluorescence intensity of each zone. The procedures performed were as described previously (Naftalin et al. 1999; Moretó et al. 2005).
Confocal images
Each piece of tissue was viewed from the mucosal side with the confocal microscope using a × 20 or × 40 Leica SPII oil immersion lens. The focal plane was taken to the surface of the tissue and images were captured in 2.5 or 5 μm steps using the automatic Z-step motor. Images were taken from 0 μm down to 40 μm below the surface. For measurements of dextran permeability, the scans were repeated at 5 min intervals for 20 min. For sodium red ratio imaging, the tissues were pre-incubated at 37°C in a humidified hood for 30 min with the dye prior to viewing with the confocal microscope. This allows the dye beads to penetrate into the pericryptal and interstitial spaces. The captured images represent the general level of staining throughout the whole tissue and were obtained with the same optical conditions, gain and section size.
Image analysis
The captured images were analysed using the program Image J 1.32 (http://rsb.info.nih.gov/ij) to quantify the fluorescence from each antibody (Abramoff et al. 2004). The fluorescence was quantified by areas, which were divided into either crypt or intercrypt and were taken by selecting a region of interest within the relevant part of the image. Three areas of both crypt and intercrypt regions were taken for image analyses and there were 30 measurements per tissue at the various depths. The mean (± s.e.m.) of the measurements after background subtraction from each image was calculated. To obtain averaged fluorescence intensities within the crypt lumen and pericryptal sheath, projected images using maximal intensity averaging over the depths 5–30 μm were obtained for each rat and results compared for each condition. For the sodium ratio analysis, image pairs of sodium red and BODIPY-fl were acquired from the same field. Background images were obtained under the same conditions, but without loading with the dye. Ratio images (red-to-green fluorescence) were obtained by pixel-by-pixel division of background-subtracted images. Background values were < 10% of signal. Averaged ratios were obtained by integration of red and green fluorescence intensities over specified regions of interest.
Statistical analyses
Data were expressed as means ± s.e.m. Comparisons between groups were made by ANOVA using SPSS-10.0 software (SPSS) followed by Scheffé's post hoc test. Three different effects were analysed: dietary effect (HS versus LS), AVP effect (AVP-infused and water-deprived groups versus HS and LS) and effects of the antagonists (VR1 and VR2 versus AVP). In the case of plasma aldosterone concentrations, the captopril effect was analysed versus the LS group. Differences were considered statistically significant when the test yielded P < 0.05.
Results
Physiological data
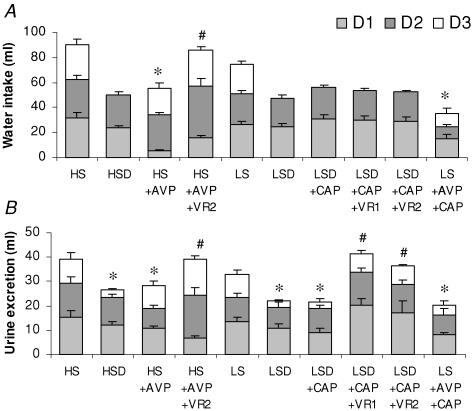
All physiological findings indicated both the consistency and efficacy of dietary and pharmacological treatments. Figure 2 shows the 24 h water and urine excretion during the 3 days in the metabolic cages, where an appreciable antidiuretic effect of AVP was observed. Total water intake was reduced by AVP administration (HS + AVP and LS + AVP + CAP groups) but this effect was prevented by the V2 receptor antagonist. Urine excretion was reduced in groups with high plasma AVP concentration, resulting from 24 h water deprivation and AVP infusion. Both receptor subtype antagonists prevented the antidiuretic AVP effect on urine excretion.
Figure 2. Water intake and urine excretion.
Twenty-four hour water intake and urine excretion during the last 3 days of diet. Control groups, high-Na+ diet (HS) and low-Na+ diet (LS); D, rats were deprived of water for 24 h; AVP, animals were infused with AVP for 7 days; VR1, rats were treated with a V1 receptor antagonist; VR2, rats were treated with a V2 receptor antagonist; CAP, rats were administered with captopril. Values are means ± s.e.m. (n = 7–11 rats). *P < 0.05, control versus AVP; #P < 0.05, antagonist versus AVP.
The effect of 24 h water deprivation on urine osmolality, haematocrit and plasma osmolality is shown in Table 2. The effects on plasma osmolality and haematocrit were consistent with dehydration; both were significantly increased after 24 h water deprivation. The urine osmolality, urine excretion rate and AVP measurements were also consistent with dehydration, and similar to those reported previously (Desai et al. 2005; Gottlieb et al. 2006). Urine osmolality increased after 24 h water deprivation but in both VR1 and VR2 groups, the receptor antagonists reversed the antidiuretic response to AVP.
Table 2.
Physiological data for control and water-deprived groups
| Urine osmolality (mosmol kg−1) | Haematocrit (%) | Plasma osmolality (mosmol kg−1) | |
|---|---|---|---|
| HS | 1171 ± 149 (8) | 42.3 ± 0.4 (5) | 288 ± 7 (5) |
| HSD | 1668 ± 230 (8)* | 45.5 ± 0.7 (6)* | 316 ± 4 (5)* |
| LS | 894 ± 153 (7)† | 43.3 ± 0.1 (5) | 286 ± 6 (5) |
| LSD | 1680 ± 222 (8)* | 45.9 ± 0.7 (5)* | 319 ± 3 (6)* |
| LSD + CAP | 1466 ± 145 (7)* | 45.8 ± 0.6 (6)* | 314 ± 6 (6)* |
| LSD + CAP + VR1 | 918 ± 67 (8)# | 45.9 ± 0.3 (5)* | 315 ± 7 (5)* |
| LSD + CAP + VR2 | 871 ± 71 (8)# | 45.7 ± 0.4 (8)* | 314 ± 2 (5)* |
Urine and plasma osmolality and haematocrit data from all groups on the last day on the diet. Control groups, high-Na+ diet (HS) and low-Na+ diet (LS); D, rats were deprived of water for 24 h; AVP, animals were infused with AVP for 7 days; VR1, rats were treated with a V1 receptor antagonist; VR2, rats were treated with a V2 receptor antagonist; CAP, rats were administered with captopril. Values are means ± s.e.m. (number of rats).
P < 0.05, LS versus HS
P < 0.05, control versus D
P < 0.05, antagonist effect.
Hormonal and Na+ and K+ determinations in the treatment groups are shown in Table 3. AVP excretion was significantly increased after both water deprivation and AVP infusion (P < 0.01). Dietary NaCl intake had no effect on AVP plasma concentration. The plasma aldosterone concentration in LS groups was much higher than in HS groups. The HS condition is well suited to independent study of AVP action, as aldosterone levels are very low. The animals given LS, either with normal hydration or deprived of water (LSD), had similar plasma aldosterone concentrations. CAP administration to animals given LS lowered plasma aldosterone concentration (P < 0.01) and resulted in an increased Na+ excretion, as expected. The V2 receptor antagonist prevented the reduction in Na+ excretion induced by AVP in the HS conditions. Plasma Na+ concentration was maintained in the physiological range in all experimental groups.
Table 3.
Hormone and ion concentration for all experimental groups
| Plasma aldosterone (nm) | Urinary vasopressin (pg ml−1) | Urinary Na+ (mmol, 3 days) | Urinary K+ (mmol, 3 days) | Plasma Na+ (mm, at day 3) | Plasma K+ (mm, at day 3) | |
|---|---|---|---|---|---|---|
| HS | 0.15 ± 0.03 (7) | 1.87 ± 0.40 (6) | 9.63 ± 0.57 (7) | 3.01 ± 0.07 (7) | 141 ± 1.05 (7) | 5.38 ± 0.22 (7) |
| HSD | 0.45 ± 0.14 (5) | 45.2 ± 2.1 (5)* | 5.65 ± 0.57 (6)* | 3.65 ± 0.26 (6) | 144 ± 1.4 (6) | 5.05 ± 0.20 (6) |
| HS + AVP | 0.18 ± 0.03 (5) | 123 ± 21 (5)* | 5.44 ± 0.58 (4)* | 2.83 ± 0.14 (4) | 146 ± 2.1 (5) | 4.82 ± 0.22 (5) |
| HS + AVP + VR2 | 0.26 ± 0.08 (5) | 132 ± 33 (5)* | 9.68 ± 0.54 (4)# | 2.75 ± 0.06 (4) | 142 ± 2.4 (5) | 4.98 ± 0.27 (5) |
| LS | 1.4 ± 0.29 (7)† | 2.05 ± 0.41 (6) | 0.31 ± 0.06 (7)† | 2.71 ± 0.22 (7) | 142 ± 2.1 (6) | 5.29 ± 0.15 (6) |
| LSD | 1.2 ± 0.42 (6)† | 42.4 ± 2.7 (5)* | 0.34 ± 0.06 (6)† | 2.98 ± 0.18 (6) | 145 ± 2.4 (8) | 5.07 ± 0.10 (8) |
| LSD + CAP | 0.04 ± 0.01 (6)‡ | 47.6 ± 7.0 (5)* | 0.64 ± 0.05 (8) ‡ | 3.10 ± 0.35 (8) | 141 ± 0.9 (8) | 5.04 ± 0.09 (8) |
| LSD + CAP + VR1 | 0.22 ± 0.07 (6)‡ | 39.8 ± 2.3 (5)* | 0.83 ± 0.10 (8)‡ | 3.21 ± 0.16 (8) | 140 ± 0.9 (8) | 5.21 ± 0.15 (8) |
| LSD + CAP + VR2 | 0.14 ± 0.05 (6)‡ | 43.0 ± 5.3 (5)* | 0.86 ± 0.09 (8)‡ | 2.67 ± 0.23 (8) | 143 ± 1.8 (8) | 5.12 ± 0.19 (8) |
| LS + AVP + CAP | 0.23 ± 0.12 (5)‡ | 153 ± 41 (5)* | 2.24 ± 0.09 (4)‡ | 2.16 ± 0.09 (4) | 140 ± 2.9 (4) | 4.93 ± 0.07 (4) |
Plasma aldosterone, Na+ and K+ levels and urinary vasopressin, Na+ and K+ excretion in all experimental groups. Abbreviations as in Table 2. ‘3 days’ refers to the total amount of ions excreted during the 3 days rats were kept in metabolic cages; ‘at day 3’ refers to analysis done on the last day animals were in the metabolic cage. Values are means ± s.e.m. (number of rats).
P < 0.01, LS versus HS
P < 0.05, control versus D and AVP
P < 0.05, antagonist effect
P < 0.01, CAP effect.
AVP effect on pericryptal myofibroblasts
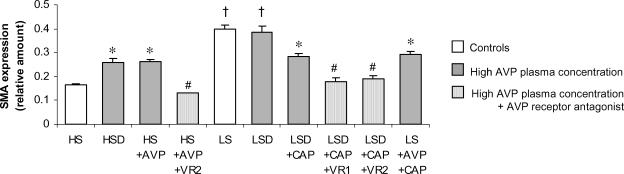
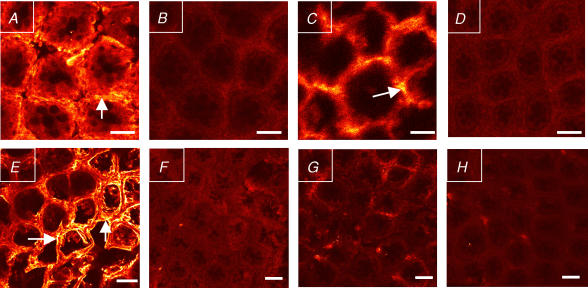
The effect of AVP on pericryptal myofibroblast growth was studied by looking at α-SMA expression, as it is the protein used to identify myofibroblasts (Jain et al. 1998). As reported previously, during the transition from an HS to an LS diet myofibroblasts surrounding colonic crypts proliferate (LS and LSD groups versus HS, P < 0.01; Fig. 3) and this effect has been ascribed to aldosterone (Cristià et al. 2005). In this series of experiments, the presence of high AVP levels increased by 2-fold the α-SMA expression versus the control HS (P < 0.05) in both HS and LS (HSD, HS + AVP, LSD + CAP and LS + AVP + CAP) groups. In the absence of aldosterone, the action of AVP on α-SMA expression, although less than that observed with aldosterone alone (LS) or with both aldosterone and AVP together (LSD) where the increase is 4-fold higher, is still 2-fold higher than in the HS group (Fig. 3). The effect of AVP on α-SMA expression was confirmed with AVP receptor antagonists, which prevented the AVP-induced increase in α-SMA. Thus, both receptor subtypes, V1 and V2, are implicated in this trophic stimulation. Confocal images can be seen in Fig. 4.
Figure 3. α-smooth muscle actin (α-SMA) expression in colonic crypts.
Values are means ± s.e.m. (n = 8–12 crypts). Experimental groups are described in legend to Fig. 2. †P < 0.01, LS diet versus HS; *P < 0.05, AVP effect versus HS; #P < 0.05, antagonist effect.
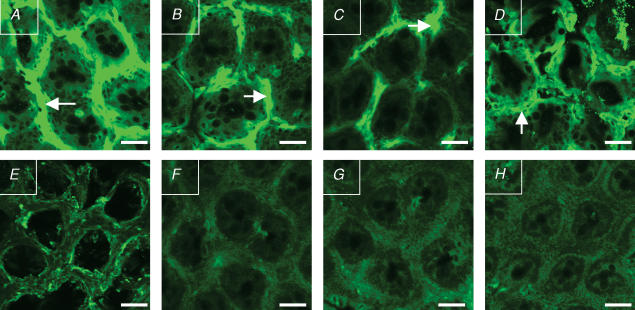
Figure 4. Confocal images of α-smooth muscle actin (α-SMA) staining in colonic crypts.
A, LSD group, with high aldosterone and vasopressin plasma concentrations. B, HSD group. C, LS + AVP + CAP group. D, HS + AVP group. B and C are groups with high plasma vasopressin concentration and low aldosterone. E, HS group, with low plasma concentration of both aldosterone and vasopressin. F, LSD + CAP + VR1 group, with high vasopressin plasma concentration but administered with a selective V1 receptor antagonist. G, LSD + CAP + VR2 group. H, HS + AVP + VR2 group. G and H have high vasopressin plasma concentration but were treated with a selective V2 receptor antagonist. The primary antibody used was monoclonal anti-α-SMA clone IA4 and the secondary antibody was anti-α-SMA staining Texas Red goat anti-mouse IgG. α-SMA immunostaining in the pericryptal space is indicated by arrows. All images were taken at a depth of 20 μm from the tissue surface. For abbreviations see legend to Fig. 2.
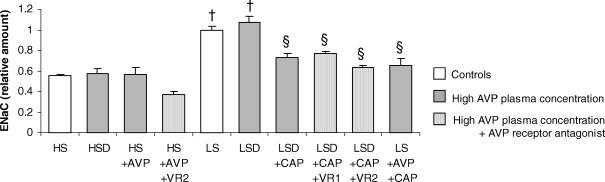
The pericryptal sheath myofibroblasts create a barrier to diffusion behind which Na+ accumulates. Thus, quantification of pericryptal Na+ accumulation is an indirect measurement of myofibroblast barrier function and development. Significant differences were found in pericryptal Na+ accumulation between all the LS groups and the HS group (P < 0.01); it was higher in the LS condition. In groups with high plasma concentration of AVP and low levels or no aldosterone (HSD, HS + AVP, LSD + CAP, LS + AVP + CAP), Na+ accumulation in the pericryptal space was greatly increased. Raised pericryptal Na+ was also found in the LSD group, where both plasma aldosterone and AVP concentrations were high, but no synergistic effects were observed. Administration of the two AVP receptor antagonists prevented pericryptal Na+ accumulation, with similar values to those observed in the HS group. Pericryptal Na+ concentration for each group can be seen in Fig. 5A.
Figure 5. Pericryptal sodium accumulation and dextran permeability.
A, quantification of Na+ accumulation in the pericryptal space in all experimental groups; pericryptal Na+versus AVP concentration in HS, HS + AVP and HS + AVP + VR2 groups. B, dextran permeability in all experimental groups; dextran permeability versus AVP concentration in HS, HS + AVP and HS + AVP + VR2 groups. Experimental groups are described in legend to Fig. 2. Values are means ± s.e.m. (n = 8–12 crypts). †P < 0.01, LS versus HS; *P < 0.01, AVP effect; #P < 0.01, antagonist effect.
Effect of AVP on colonic permeability and transport
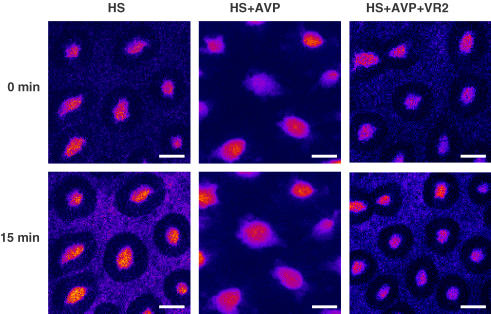
The dextran permeability of the crypt wall was measured by the rate of escape of FITC-dextran from the crypt lumen into the pericryptal space. Dextran permeability between the crypt lumen and the pericryptal space is an indicator of paracellular permeability of the crypt epithelium and it is also a reflection of the permeability of the pericryptal sheath (Thiagarajah et al. 2001a,b). In the HS group, aldosterone perfusion converted the leaky colon (typical of the HS condition) into a tight tissue (Moretó et al. 2005). In the present experiments, AVP had similar effects to aldosterone in reducing dextran permeability. AVP-perfused groups, without aldosterone, had similar dextran permeabilities as in the LS or LSD groups, where aldosterone was present, and were significantly different from the HS group (P < 0.01; Fig. 5B). Both receptor subtype antagonists reversed the action of AVP on dextran permeability (i.e. increased permeability). Images of FITC-dextran diffusion to the pericryptal space in HS, HS + AVP and HS + AVP + VR2 groups can be seen in Fig. 6.
Figure 6. Illustration of dextran permeability in colonic crypts.
Images show fluorescein isothiocyanate-labelled dextran diffusing across crypt wall into the interstitial space 15 min after addition of the label. Note that dextran (10 kDa) is less permeable across HS + AVP crypts than across the HS and HS + AVP + VR2 crypts, showing that AVP reduces crypt permeability. Images were taken at 15 μm from surface. For abbreviations see legend to Fig. 2.
The effect of AVP on the capacity to take up Na+ was studied by quantification of γENaC subunit expression. The γENaC subunit was strongly induced in the distal colon in the LS groups, when aldosterone was present (LS and LSD, P < 0.01; Fig. 7). However, when the LSD group was treated with CAP to inhibit aldosterone synthesis, the rise in the channel expression was abrogated. Similarly, none of the other groups with high plasma AVP concentration, and without aldosterone, showed an increase in γENaC immunohistochemical staining, indicating that ENaC expression in the distal colon is independent of AVP levels.
Figure 7. Epithelial Na+ channel (ENaC) expression in colonic crypts.
Values are means ± s.e.m. (n = 8–12 crypts). Experimental groups are described in legend to Fig. 2. †P < 0.01 LS versus HS; §P < 0.05 CAP effect.
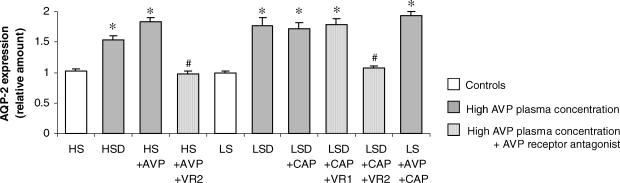
Effect of AVP on AQP-2 expression
The amount of AQP-2 was quantified by using confocal imaging of the fluorescence. These results showed that both dehydration and AVP infusion increased AQP-2 expression in colonic distal mucosa (Fig. 8). Immunohistochemical staining of AQP-2 is present only on the surface mucosa epithelium, not in crypt epithelial cells. Aldosterone did not affect AQP-2 expression, as the LS group did not show any staining. The V2 receptor antagonist tolvaptan prevented the stimulatory effect of AVP on AQP-2 expression in both HS and LS groups, showing the participation of the V2 receptor in this AVP action. The V1 receptor antagonist was ineffective in reducing AVP-induced effects on AQP-2 expression. A selection of representative AQP-2 and γENaC staining confocal images is shown in Fig. 9.
Figure 8. Aquaporin-2 (AQP-2) expression in colonic mucosa.
Values are means ± s.e.m. Experimental groups are described in legend to Fig. 2. (n = 9–12 crypts). *P < 0.05, AVP effect; #P < 0.05, antagonist effect.
Figure 9. Confocal images of AQP-2 and ENaC staining in colonic crypts.
A–D, AQP-2 expression. A (LSD group) and C (HS + AVP group) show increased AQP-2 expression due to raised vasopressin. B (LSD + CAP + VR2 group) and D (HS + AVP + VR2 group) show the antagonist effects of tolvaptan. E–H, γ-ENaC expression. E (LSD group; with high plasma aldosterone concentration) shows increased ENaC expression. F, LSD + CAP group; G, LSD + CAP + VR2 group; and H, HS + AVP group, all with low plasma aldosterone concentration, show very low ENaC labelling. Primary antibodies were rabbit anti-AQP-2 and rabbit anti-γ-ENaC and the secondary antibody was anti-rabbit Alexa-488. AQP-2 and γ-ENaC immunostaining are indicated with arrows. All images were taken at a depth of 20 μm from the tissue surface. For abbreviations see legend to Fig. 2.
Discussion
The effects of AVP on colonic crypt function have been studied in rats fed HS or LS in order to explore further the role of this hormone in the physiological adaptations to LS intake (Naftalin & Pedley, 1999; Moretó et al. 2005). The new findings presented here indicate that the effects of aldosterone and AVP are complementary.
By monitoring pericryptal myofibroblast growth, Na+ accumulation in the pericryptal sheath, dextran permeability and ENaC and AQP-2 expression in colonocytes we have obtained a broad view of the interrelated factors regulating the transport and permeability properties of the crypt wall. Proliferation of the pericryptal myofibroblast layer is illustrated by enhanced α-SMA expression. It appears that AVP can mimic the action of aldosterone in stimulating myofibroblast growth as α-SMA expression is raised by V1 and V2 receptor activation. This trophic effect of AVP has been corroborated by measuring dextran permeability across the mucosa and Na+ accumulation in the pericryptal sheath. AVP enhances Na+ accumulation in the pericryptal sheath and creates a hyperosmotic gradient across the crypt wall similar to that induced by aldosterone (Moretó et al. 2005). The growth of the myofibroblast layer also decreases dextran permeability across the crypt wall. These effects cannot be ascribed to aldosterone action, because its plasma concentration in the groups with high plasma AVP concentration is very low, except in the LSD condition, where a complementary effect of both hormones is observed. It seems therefore that AVP and aldosterone have similar effects in modulating pericryptal myofibroblast growth.
Trophic effects of AVP on the extracellular matrix have been previously observed. Harada et al. (1998) reported that chronic AVP infusion in rats caused a significant increase in transforming growth factor-beta 1 (TGF-β1) and its mRNA expression in kidney. TGF-β1 increases the extracellular matrix by stimulating the production of collagen and non-collagenous protein components and by inhibiting their degradation (Roberts et al. 1986; Campbell & Katwa, 1997; Thiagarajah et al. 2002). Moreover, patients with heart failure had a significantly higher plasma concentration of AVP compared with control groups (Sanghi et al. 2005), suggesting that AVP may be involved in glomerular proliferation and in the expansion of the mesangial matrix in vivo thus playing an important role in the pathophysiology of heart failure. In rats, AVP has been shown to stimulate myocardial cell hypertrophy, an effect that shares many of the features seen in the typical hypertrophy response of the adult heart to other hormones, such as aldosterone or angiotensin II (Tahara et al. 1998). In the heart, both AVP receptors are involved in organ remodelling. The stimulation of V1 receptors is thought to contribute directly to myocardial hypertrophy and aggravate adverse remodelling, and the stimulation of V2 receptors may contribute to increased cardiac preload which, in turn, exacerbates diastolic wall stress which decreases eccentric remodelling (Goldsmith & Gheorghiade, 2005). Our experiments show that both receptors are implicated in the trophic effects of AVP in the distal colon as specific antagonists of both receptors prevent the effects of AVP.
Both aldosterone and AVP increase Na+ accumulation in the pericryptal space as this effect has been observed in conditions with high levels of plasma aldosterone and low AVP (LS condition), with high aldosterone and AVP (LSD condition) and with low aldosterone and high AVP (HSD condition). Pericryptal Na+ concentrations depend on three variables (1) electrogenic Na+ transport across the epithelium (which depends on ENaC expression and ATPase function), (2) tight junction permeability (which controls Na+ backflux to the crypt lumen), and (3) the permeability of the pericryptal sheath (which controls the diffusion of Na+ to the crypt capillaries). Aldosterone affects the three variables (Moretó et al. 2005; Cristià et al. 2005); however, AVP has no effects on ENaC expression. Hormonal regulation of ENaC varies between organs and tissues (Stokes & Sigmund, 1998). In the kidney, both aldosterone and AVP have a role in enhancing ENaC expression but in the distal colon only aldosterone is involved (Machida et al. 2003). Infusion of 1-deamino-8-D-arginine-vasopressin, a selective V2 receptor agonist, did not modify mRNA expression of any ENaC subunit in the distal colon (Nicco et al. 2001). It has been suggested that inhibition by AVP of electrogenic Na+ absorption depends mainly on V1 receptor activation (Sato et al. 1999). Our results show that in the LSD group (with high aldosterone and AVP concentrations), ENaC expression is similar to that in the LS group (with high aldosterone and low AVP concentrations). From the results of our study, we cannot rule out an effect of AVP to up-regulate the Na+–H+ exchanger (NHE). However, there is no evidence that AVP affects NHE expression in other tissues; on the contrary, studies in guinea-pig colon show that AVP inhibits electroneutral Na+ absorption rather than electrogenic Na+ transport (Sato et al. 1999) and no effect of AVP was observed on NHE activity in chicken colon (De la Horra et al. 2001). Our findings support the view that the effects of AVP on Na+ accumulation are the result of its trophic effects on myofibroblast growth by regulation of paracellular Na+ permeability across the crypt epithelium.
Both water deprivation (Nielsen et al. 1995; Kishore et al. 2005) and AVP infusion (Kishore et al. 1996; Terris et al. 1996) increase AQP-2 expression in the kidney, an effect mediated by the basolateral V2 receptors. AVP regulates AQP-2, acutely controlling intracellular AQP-2 trafficking (Nielsen et al. 1995; Noda & Sasaki, 2005). A slower effect at the transcriptional level has been observed in rats after 24 h of water restriction (DiGiovanni et al. 1994; Saito et al. 1997). Gallardo et al. (2001) were the first to observe the presence of AQP-2 in the dehydrated rat colon. They suggested that AQP-2 could provide an apical membrane route for regulated transepithelial water transport in the colon as they showed that water absorption in the distal colon is increased by water deprivation and inhibited by AQP-2 blockade.
Our results confirm that dehydration enhances colonic expression of AQP-2 and demonstrate that this is a direct response to AVP infusion. AQP-2 is expressed only on the surface mucosa epithelium, not in crypt epithelial cells, with a similar distribution to that described by Gallardo et al. (2001) who found AQP-2 protein only in the apical membrane of columnar absorptive cells of the mucosal surface, although the AQP-2 mRNA was mainly found in crypt epithelial colonocytes. As in the kidney (Hayashi et al. 1996), the effect of AVP on AQP-2 regulation in the colon is a response to V2 receptor activation. This is corroborated by our finding that tolvaptan prevents colonic AQP-2 expression.
The dual effects of AVP in the distal colon in stimulating myofibroblast growth and increasing AQP-2 are consistent with its antidiuretic role. In the distal colon, water is removed from luminal faeces both by the surface mucosa, which can exert very little suction tension but has a high transport capacity, and by crypts, which have a low water transport capacity but can exert a high tension (Bleakman & Naftalin, 1990; McKie et al. 1990; Zammit et al. 1994). One of the roles attributed to pericryptal sheath myofibroblasts in the distal colon is to maintain a hypertonic NaCl concentration in the pericryptal space. This generates the large hydraulic tension within the crypt lumen needed to dehydrate faeces (Naftalin, 2004). AVP could facilitate water absorption in the distal colon by two mechanisms: (1) by stimulating myofibroblast growth, which enhances pericryptal NaCl hypertonicity by retarding NaCl leakage into the submucosa, and (2) by regulating AQP-2 expression in the apical membrane, which increases the rate of fluid absorbed from watery faeces.
These results clarify the mechanisms of regulation of colonic function and show that aldosterone and AVP induce trophic responses that are also observed in pathological alterations of kidney and heart. This suggests that the colon can be used as a model to explore the mechanisms involved in the regulation of myofibroblast growth.
Acknowledgments
This work was supported by projects BFI2003-05124 and BFU2006-08410/BFI (Ministerio de Ciencia y Tecnología, Spain) and 2005SGR00632 (Generalitat de Catalunya, Spain). E.C. was recipient of an Formación de Profesorado Universitario (FPU) grant from Ministerio de Educación y Clencia (MEC) (Spain). We are grateful to Otsuka Pharmaceutical Co. (Japan) for the gift of tolvaptan and to Dr Carme Villà for plasma ion determinations. The support of the Confocal Service and the Spectroscopy Service, Serveis Científicotècnics, Universitat de Barcelona are also acknowledged.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Balment RJ, Lu W, Weybourne E, Warne JM. Arginine vasotocin a key hormone in fish physiology and behaviour: a review with insights from mammalian models. Gen Comp Endocrinol. 2006;147:9–16. doi: 10.1016/j.ygcen.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Naftalin RJ. Hypertonic fluid absorption from rabbit descending colon in vitro. Am J Physiol Gastrointest Liver Physiol. 1990;258:G377–G390. doi: 10.1152/ajpgi.1990.258.3.G377. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Nell G, Rummel W. Influence of vasopressin and calcium on electrolyte transport across isolated colon from normal and dexamethasone-treated rats. J Physiol. 1983;338:463–475. doi: 10.1113/jphysiol.1983.sp014684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Rummel W, Wollenberg P. Effects of vasopressin on electrolyte transport across isolated colon from normal and dexamethasone-treated rats. J Physiol. 1984;355:11–23. doi: 10.1113/jphysiol.1984.sp015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta 1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- Cristià E, Afzal-Ahmed I, Pérez-Bosque A, Amat C, Naftalin RJ, Moretó M. Pericryptal myofibroblast growth in rat descending colon induced by low-sodium diets is mediated by aldosterone and not by angiotensin II. J Membr Biol. 2005;206:53–59. doi: 10.1007/s00232-005-0773-4. [DOI] [PubMed] [Google Scholar]
- De la Horra MC, Cano M, Peral MJ, Calonge ML, Ilundain AA. Hormonal regulation of chicken intestinal NHE and SGLT-1 activities. Am J Physiol Regul Integr Comp Physiol. 2001;280:R655–R660. doi: 10.1152/ajpregu.2001.280.3.R655. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle D, Kallichanda N, Ross MG. Gender specificity of programmed plasma hypertonicity and hemoconcentration in adult offspring of water-restricted rat dams. J Soc Gynecol Investig. 2005;12:409–415. doi: 10.1016/j.jsgi.2005.04.007. [DOI] [PubMed] [Google Scholar]
- DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci U S A. 1994;91:8984–8988. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Funayama Y, Yonezawa H, Takahashi K, Haneda S, Suzuki T, Sasano H, Naito H, Shibata C, Krozowski ZS, Sasaki I. Aldosterone enhances 11beta-hydroxysteroid dehydrogenase type 2 expression in colonic epithelial cells in vivo. Scand J Gastroenterol. 2005;40:850–857. doi: 10.1080/00365520510015700. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Naito H, Funayama Y, Yonezawa H, Haneda S, Shibata C, Sasaki I. In vivo induction of prostasin mRNA in colonic epithelial cells by dietary sodium depletion and aldosterone infusion in rats. J Gastroenterol. 2004;39:940–947. doi: 10.1007/s00535-004-1425-7. [DOI] [PubMed] [Google Scholar]
- Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993;361:549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Gallardo P, Cid LP, Vio CP, Sepulveda F. Aquaporin-2, a regulated water channel, is expressed in apical membranes of rat distal colon epithelium. Am J Physiol Gastrointest Liver Physiol. 2001;281:G856–G863. doi: 10.1152/ajpgi.2001.281.3.G856. [DOI] [PubMed] [Google Scholar]
- Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46:1785–1791. doi: 10.1016/j.jacc.2005.02.095. [DOI] [PubMed] [Google Scholar]
- Gottlieb HB, Ji LL, Jones H, Penny ML, Fleming T, Cunningham JT. Differential effects of water and saline intake on water deprivation-induced c-Fos staining in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1251–R1261. doi: 10.1152/ajpregu.00727.2005. [DOI] [PubMed] [Google Scholar]
- Harada K, Ogura T, Yamauchi T, Otsuka F, Mimura Y, Hashimoto M, Oishi T, Makino H. Effect of continuous infusion of vasopressin on glomerular growth response in spontaneously hypertensive rats. Regul Pept. 1998;74:11–18. doi: 10.1016/s0167-0115(98)00009-3. [DOI] [PubMed] [Google Scholar]
- Hawk CT, Li L, Schafer JA. AVP and aldosterone at physiological concentrations have synergistic effects on Na+ transport in rat CCD. Kidney Int Suppl. 1996;57:S35–S41. [PubMed] [Google Scholar]
- Hayashi M, Sasaki S, Tsuganezawa H, Monkawa T, Kitajima W, Konishi K, Fushimi K, Marumo F, Saruta T. Role of vasopressin V2 receptor in acute regulation of aquaporin-2. Kidney Blood Press Res. 1996;19:32–37. doi: 10.1159/000174043. [DOI] [PubMed] [Google Scholar]
- Jain MK, Layne MD, Watanabe M, Chin MT, Feinberg MW, Sibinga NE, Hsieh CM, Yet SF, Stemple DL, Lee ME. In vitro system for differentiating pluripotent neural crest cells into smooth muscle cells. J Biol Chem. 1998;273:5993–5996. doi: 10.1074/jbc.273.11.5993. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Non-invasive fluorescence measurement of salt concentration in the airway surface liquid. J Clin Invest. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore BK, Krane CM, Miller RL, Shi H, Zhang P, Hemmert A, Sun R, Nelson RD. P2Y2 receptor mRNA and protein expression is altered in inner medullas of hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol. 2005;288:F1164–F1172. doi: 10.1152/ajprenal.00199.2004. [DOI] [PubMed] [Google Scholar]
- Kishore BK, Terris JM, Knepper MA. Quantitation of aquaporin-2 abundance in microdissected collecting ducts: axial distribution and control by AVP. Am J Physiol Renal Physiol. 1996;271:F61–F70. doi: 10.1152/ajprenal.1996.271.1.F62. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, O'Carroll AM, McBride OW, Konig M, Morel A, Brownstein MJ. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- McKie AT, Powrie W, Naftalin RJ. Mechanical aspects of fecal dehydration. Am J Gastrointest Liver Physiol. 1990;258:G391–G394. doi: 10.1152/ajpgi.1990.258.3.G391. [DOI] [PubMed] [Google Scholar]
- Machida K, Nonoguchi H, Wakamatsu S, Inoue H, Yosifovska T, Inoue T, Tomita K. Acute regulation of the epithelial sodium channel gene by vasopressin and hyperosmolality. Hypertens Res. 2003;26:629–634. doi: 10.1291/hypres.26.629. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Wray S, Marples D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J Mol Histol. 2005;36:1–14. doi: 10.1007/s10735-004-2633-4. [DOI] [PubMed] [Google Scholar]
- Morel A, O'Carroll AM, Brownstein MJ, Lolait SJ. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992;356:523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Moretó M, Cristià E, Pérez-Bosque A, Afzal-Ahmed I, Amat C, Naftalin RJ. Aldosterone reduces crypt colon permeability during low-sodium adaptation. J Membr Biol. 2005;206:43–51. doi: 10.1007/s00232-005-0772-5. [DOI] [PubMed] [Google Scholar]
- Naftalin RJ. Alterations in colonic barrier function caused by a low sodium diet or ionizing radiation. J Environ Pathol Toxicol Oncol. 2004;23:79–97. doi: 10.1615/jenvpathtoxoncol.v23.i2.10. [DOI] [PubMed] [Google Scholar]
- Naftalin RJ, Pedley KC. Regional crypt function in rat large intestine in relation to fluid absorption and growth of the pericryptal sheath. J Physiol. 1999;514:211–227. doi: 10.1111/j.1469-7793.1999.211af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin RJ, Zammit PS, Pedley KC. Regional differences in rat large intestinal crypt function in relation to dehydrating capacity in vivo. J Physiol. 1999;514:201–210. doi: 10.1111/j.1469-7793.1999.201af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Miyata K, Sun GP, Rahman M, Kimura S, Miyatake A, Kiyomoto H, Kohno M, Abe Y, Yoshizumi M, Nishiyama A. Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension. 2005;46:1039–1045. doi: 10.1161/01.HYP.0000174593.88899.68. [DOI] [PubMed] [Google Scholar]
- Nicco C, Wittner M, DiStefano A, Jounier S, Bankir L, Bouby N. Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension. 2001;38:1143–1149. doi: 10.1161/hy1001.092641. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Sasaki S. Trafficking mechanism of water channel aquaporin-2. Biol Cell. 2005;97:885–892. doi: 10.1042/BC20040120. [DOI] [PubMed] [Google Scholar]
- Nonoguchi H, Owada A, Kobayashi N, Takayama M, Terada Y, Koike J, Ujiie K, Marumo F, Sakai T, Tomita K. Immunohistochemical localization of V2 vasopressin receptor along the nephron and functional role of luminal V2 receptor in terminal inner medullary collecting ducts. J Clin Invest. 1995;96:1768–1778. doi: 10.1172/JCI118222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoguchi H, Sands JM, Knepper MA. Atrial natriuretic factor inhibits vasopressin-stimulated osmotic water permeability in rat inner medullary collecting duct. J Clin Invest. 1988;82:1383–1390. doi: 10.1172/JCI113742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Ishikawa SE, Sasaki S, Fujita N, Fushimi K, Okada K, Takeuchi K, Sakamoto A, Ookawara S, Kaneko T, Marumo F. Alteration in water channel AQP2 by removal of AVP stimulation in collecting duct cells of dehydrated rats. Am J Physiol Renal Physiol. 1997;272:F183–F191. doi: 10.1152/ajprenal.1997.272.2.F183. [DOI] [PubMed] [Google Scholar]
- Sanghi P, Uretsky BF, Schwarz ER. Vasopressin antagonism: a future treatment option in heart failure. Eur Heart J. 2005;26:538–543. doi: 10.1093/eurheartj/ehi145. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hanai H, Nogaki A, Hirasawa K, Kaneko E, Hayashi H, Suzuki Y. Role of the vasopressin V1 receptor in regulating the epithelial functions of the guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 1999;277:G819–G828. doi: 10.1152/ajpgi.1999.277.4.G819. [DOI] [PubMed] [Google Scholar]
- Schafer JA, Hawk CT. Regulation of Na+ channels in the cortical collecting duct by AVP and mineralcorticoids. Kidney Int. 1992;41:255–268. doi: 10.1038/ki.1992.37. [DOI] [PubMed] [Google Scholar]
- Stokes JB, Sigmund RD. Regulation of rENaC mRNA by dietary NaCl and steroids: organ, tissue and steroid heterogeneity. Am J Physiol Cell Physiol. 1998;274:C1699–C1707. doi: 10.1152/ajpcell.1998.274.6.C1699. [DOI] [PubMed] [Google Scholar]
- Szalay KS, Beck M, Toth M, de Chatel R. Interactions between ouabain, atrial natriuretic peptide, angiotensin II and potassium: effects on rat zona glomerulosa aldosterone production. Life Sci. 1998;62:1845–1852. doi: 10.1016/s0024-3205(98)00150-7. [DOI] [PubMed] [Google Scholar]
- Tahara A, Tomura Y, Wada K, Kusayama T, Tsukada J, Ishii N, Yatsu T, Uchida W, Tanaka A. Effect of YM087, a potent nonpeptide vasopressin antagonist, on vasopressin-induced protein synthesis in neonatal rat cardiomyocyte. Cardiovasc Res. 1998;38:198–205. doi: 10.1016/s0008-6363(97)00324-6. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Samson WK. Adrenomedullin and the integrative physiology of fluid and electrolyte balance. Microsc Res Tech. 2002;57:105–109. doi: 10.1002/jemt.10055. [DOI] [PubMed] [Google Scholar]
- Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol Renal Physiol. 1996;271:F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- Thiagarajah JR, Griffiths NM, Pedley KC, Naftalin RJ. Evidence for modulation of pericryptal sheath myofibroblasts in rat descending colon by transforming growth factor beta and angiotensin. BMC Gastroenterol. 2002;2:4–15. doi: 10.1186/1471-230X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah JR, Jayaraman S, Naftalin RJ, Verkman AS. In vivo fluorescence measurement of Na+ concentration in the pericryptal space of mouse descending colon. Am J Physiol Cell Physiol. 2001a;281:C1898–C1903. doi: 10.1152/ajpcell.2001.281.6.C1898. [DOI] [PubMed] [Google Scholar]
- Thiagarajah JR, Pedley KC, Naftalin RJ. Evidence of amiloride-sensitive fluid absorption in rat descending colonic crypts from fluorescence recovery of FITC-labeled dextran after photobleaching. J Physiol. 2001b;536:541–553. doi: 10.1111/j.1469-7793.2001.0541c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrey F. Antidiuretic hormone action in A6 cells: effect on apical Cl and Na conductances and synergism with aldosterone for NaCl reabsorption. J Membr Biol. 1994;138:65–76. doi: 10.1007/BF00211070. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral doping in rats. J Pharmacol Exp Ther. 1998;287:860–867. [PubMed] [Google Scholar]
- Zammit PS, Mendizabal M, Naftalin RJ. Effects on fluid and Na+ flux of varying luminal hydraulic resistance in rat colon in vivo. J Physiol. 1994;477:539–548. doi: 10.1113/jphysiol.1994.sp020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannad F, Radauceanu A. Effect of MR blockade on collagen formation and cardiovascular disease with a specific emphasis on heart failure. Heart Fail Rev. 2005;10:71–78. doi: 10.1007/s10741-005-2351-3. [DOI] [PubMed] [Google Scholar]