Abstract
Prolonged, moderate cerebral hypothermia is consistently neuroprotective after experimental hypoxia–ischaemia; however, it has not been tested in the preterm brain. Preterm (0.7 gestation) fetal sheep received complete umbilical cord occlusion for 25 min followed by cerebral hypothermia (fetal extradural temperature reduced from 39.4 ± 0.3 to 29.5 ± 2.6°C) from 90 min to 70 h after the end of occlusion or sham cooling. Occlusion led to severe acidosis and profound hypotension, which recovered rapidly after release of occlusion. After 3 days recovery the EEG spectral frequency, but not total intensity, was increased in the hypothermia-occlusion group compared with normothermia-occlusion. Hypothermia was associated with a significant overall reduction in loss of immature oligodendrocytes in the periventricular white matter (P < 0.001), and neuronal loss in the hippocampus and basal ganglia (P < 0.001), with suppression of activated caspase-3 and microglia (isolectin-B4 positive). Proliferation was significantly reduced in periventricular white matter after occlusion (P < 0.05), but not improved after hypothermia. In conclusion, delayed, prolonged head cooling after a profound hypoxic insult in the preterm fetus was associated with a significant reduction in loss of neurons and immature oligodendroglia, with evidence of EEG and haemodynamic improvement after 3 days recovery, but also with a persisting reduction in proliferation of cells in the periventricular region. Further studies are required to evaluate the long-term impact of cooling on brain growth and maturation.
There is now strong clinical and experimental evidence that a prolonged period of moderate cerebral hypothermia initiated within a few hours after severe hypoxia–ischaemia can reduce subsequent neuronal loss and improve behavioural recovery (Colbourne & Corbett, 1995; Gunn et al. 1997, 1998b; Bernard et al. 2002; The Hypothermia after Cardiac Arrest Study Group, 2002; Tooley et al. 2003; Gluckman et al. 2005; Shankaran et al. 2005). However, these studies have focused on neuroprotection with hypothermia at term and in adults. Preterm infants have a very high burden of neurological injury and subsequent neurodevelopmental handicap (du Plessis & Volpe, 2002; Marlow et al. 2005). It is unknown whether hypothermia is protective in the very immature brain, while there is some evidence that preterm infants may be particularly vulnerable to adverse systemic effects during even mild systemic hypothermia (Silverman et al. 1958; Buetow & Klein, 1964; Day et al. 1964).
The characteristic pattern of injury also differs in preterm infants compared with term, with a much higher rate of selective damage to the periventricular white matter (periventricular leucomalacia, PVL) (Barkovich & Truwit, 1990), with loss of immature, premyelinating oligodendrocytes (Haynes et al. 2003). However, imaging and post-mortem studies show that there is also an appreciable incidence of acute neuronal cell injury, mainly in subcortical nuclei (Paneth et al. 1990; Barkovich & Sargent, 1995; de Vries et al. 1998; Gilles et al. 1998; Leijser et al. 2004; Bell et al. 2005). Neuronal loss can be associated both with cystic (Volpe, 2005) and diffuse PVL (Bell et al. 2005), and after recovery to term equivalent or older, premature infants show reduced volumes of grey matter regions compared with infants born at term (Yokochi, 1997; Lin et al. 2001; Argyropoulou et al. 2003; Inder et al. 2005). In turn, although the pathogenesis of brain injury in preterm infants is multifactorial, and not fully defined, adverse outcomes are associated with exposure to hypoxia as shown by metabolic acidosis, active labour, abnormal heart rate traces in labour and subsequent low Apgar scores (Low et al. 1995; de Vries et al. 1998; Weinberger et al. 2000; Osborn et al. 2003; Bell et al. 2005). Consistent with this evidence that prenatal and intrapartum hypoxia contribute to a significant subset of preterm brain injury, larger premature infants, from 31 to 36 weeks gestation, with acidaemia on cord blood have a high rate of evolving clinical encephalopathy after birth (Salhab & Perlman, 2005). Thus, the authors suggested that this group of premature infants might benefit from potential neuroprotective treatments such as hypothermia.
The aim of this study was therefore to test the hypothesis that following profound hypoxia induced by complete umbilical cord occlusion in 0.7 gestation fetal sheep (Dean et al. 2006), treatment with cerebral hypothermia, initiated 90 min after reperfusion and continued for 3 days, would improve neural outcome. In terms of cerebral maturity the 0.7 gestation fetal sheep is broadly comparable to the human brain at 28–32 weeks of gestation, prior to the onset of cortical myelination (Barlow, 1969).
Methods
Experimental preparation
All procedures were approved by the Animal Ethics Committee of The University of Auckland. Twenty-three time-mated singleton Romney/Suffolk fetal sheep were instrumented at 97–99 days of gestation (term = 147 days) under general anaesthesia (2% halothane in O2) using sterile techniques.
Food, but not water was withdrawn 18 h before surgery. Ewes were given 5 ml of Streptocin (procaine penicillin (250 000 IU) and dihydrostreptomycin (250 mg ml−1); Stockguard Laboratories Ltd, Hamilton, New Zealand) intramuscularly for prophylaxis 30 min prior to the start of surgery. Anaesthesia was induced by i.v. injection of Alfaxan (alphaxalone, 3 mg kg−1; Jurox, Rutherford, Australia), and general anaesthesia maintained using 2–3% halothane in O2. Ewes were allowed to breathe spontaneously and the depth of anaesthesia, maternal heart rate and respiration were constantly monitored by trained anaesthetic staff. Under anaesthesia a 20 gauge catheter was placed in a maternal front leg vein for the duration of surgery, and the ewes were placed on a constant infusion isotonic saline drip (at an infusion rate of approximately 250 ml h−1) to maintain maternal fluid balance.
All surgical procedures were performed using sterile techniques (Bennet et al. 1999; Quaedackers et al. 2004; Dean et al. 2006). The fetal head and upper chest were exposed through a midline Caesarean incision, and a small incision in the uterus. Fetal catheters were placed in the left femoral artery and vein, right brachial artery and vein, and the amniotic sac. Left carotid artery blood flow (CaBF) was measured by a 3S ultrasound blood flow probe (Transonic Systems Inc., Ithaca, NY, USA). Two pairs of electroencephalogram (EEG) electrodes (AS633-5SSF, Cooner wire Co., Chatsworth, CA, USA) were placed on the dura over the parasagittal parietal cortex (5 mm and 15 mm anterior to bregma and 10 mm lateral), with a reference electrode sewn over the occiput. A thermistor (Incutemp-1, Mallinckrodt Medical Inc., St Louis, MO, USA) was placed over the parasagittal dura 20 mm anterior to bregma, to measure extradural temperature, and the burr holes were sealed and the skin over the fetal skull was secured with cyanoacrylate glue. Electrocardiogram (ECG) electrodes were sewn across the chest to record the fetal heart rate (FHR). A second thermistor (to measure fetal core body temperature) was placed in the fetal oesophagus at the level of the right atrium (Gunn et al. 1997). An inflatable silicone occluder was placed around the umbilical cord of all fetuses (In Vivo Metric, Healdsburg, CA, USA). A cooling coil made from silicone tubing (external diameter 7.9 mm, internal diameter 4.8 mm; Silclear, Degania Silicone, Degania Bet, Israel) was attached over the dorsal surface of the scalp and extended over the lateral surface of the cranium down to the level of the external auditory meatus.
All fetal catheters and leads were exteriorized through the maternal flank. After surgery, all exteriorized catheters and leads were kept in an enclosed perspex box suspended from the side of the ewes' metabolic cage. The maternal long saphenous vein was catheterized to provide access for post-operative maternal care and killing. Antibiotics were administered into the amniotic sac prior to closure of the uterus (80 mg Gentamicin, Pharmacia and Upjohn, Perth, Australia). The maternal skin incision was infiltrated with a long-acting local anaesthetic, Marcain (bupivacaine hydrochloride 0.25% with adrenaline 1 : 400 000; Astra Zeneca, North Ryde, NSW, Australia).
Following surgery, sheep were housed together in separate metabolic cages with access to water and food ad libitum. They were kept in a temperature-controlled room (16 ± 1°C, humidity 50 ± 10%), in a 12 h light–dark cycle. A period of 4–5 days post-operative recovery was allowed before experiments commenced, during which time antibiotics were administered daily for 4 days i.v. to the ewe (600 mg benzylpencillin Sodium, Novartis Ltd, Auckland, New Zealand, and 80 mg gentamicin). The health and welfare of all animals was closely monitored by the researchers daily, and supervised by the University veterinarian. Fetal brachial arterial blood was taken daily for blood gas analysis for the assessment of fetal health. Catheters were maintained patent by continuous infusion of heparinized isotonic saline (20 U ml−1, 0.2 ml h−1).
Experimental design and recordings
Experiments were conducted at 103–104 days gestation. Fetal mean arterial pressure (MAP), corrected for maternal movement by subtraction of amniotic fluid pressure, FHR, CaBF and EEG activity were recorded continuously from 12 h before the experiment until 72 h afterwards. Data were collected by computer and stored to disk for off-line analysis (Labview for Windows, National Instruments Ltd, Austin, Texas, USA).
Fetuses were randomly assigned to either normothermia occlusion (n = 8), hypothermia occlusion (n = 8) or normothermia-sham occlusion (sham control; n = 7) groups. Fetal asphyxia was induced in both occlusion groups by rapid inflation of the umbilical cord occluder for 25 min with sterile saline of a defined volume known to completely inflate the occluder (Quaedackers et al. 2004; Dean et al. 2006). Successful occlusion was confirmed by observation of a rapid flattening of the fetal EEG. In all groups, fetal arterial blood was taken at 15 min prior to occlusion, 20 min during occlusion, and 0.5, 2, 4, 6, 24, 48 and 72 h post-occlusion for blood gas, acid–base balance (Ciba-Corning Diagnostics 845 blood gas analyser and co-oximeter, East Walpole, MA, USA) and for glucose and lactate determination (YSI model 2300, Yellow Springs, OH, USA). Intrauterine cooling was performed from 90 min to 70 h after the end of occlusion. Delayed initiation of cooling was chosen to allow a clinically realistic delay after the end of the insult before starting treatment. As previously reviewed, extensive data in older animals suggest that prolonged cooling of up to 72 h is required for optimal protection with delayed cooling (Gunn et al. 2005). Fetuses were then allowed to spontaneously rewarm for 2 h. Cooling was induced by circulating cold water (10°C) through a coil around the fetal head. Cooling was titrated in the first 2 h to reduce fetal extradural temperature from 39.4 ± 0.1°C to between 29 and 33°C. At 72 h after occlusion the ewes and fetuses were killed by an overdose of sodium pentobarbitone (9 g, i.v. to the ewe; Pentobarb 300, Chemstock International, Christchurch, New Zealand).
Histology tissue preparation
Fetal brains were perfusion fixed in situ with normal saline followed by 500 ml of 10% phosphate buffered formalin with the perfusates suspended 1 m above the brain. Following removal from the skull, tissue was fixed for a further 5–6 days before processing and embedding using a standard paraffin tissue preparation (Gunn et al. 1997). Activated microglia were labelled on serial sections using the lectin, Bandeiraea simplicifolia isolectin-B4 (Sigma, St Louis, MO, USA) (Roelfsema et al. 2004).
Immunohistochemistry
Stainings were performed on coronal serial sections (6 μm) at the level of the mid-striatum and thalamus cut immediately adjacent to sections used above, and mounted on chrome alum-coated slides. The following primary antibodies were used: mouse anti-NeuN (neuronal-specific nuclear protein) (Chemicon International Inc., Temecula, CA, USA; 1 : 1000), mouse anti-oligodendrocyte marker O4 (Chemicon International Inc.; 1 : 50), rabbit anti-caspase-3 Asp175 (Cell Signalling Technology, Beverly, MA, USA; 1 : 1000), mouse anti-glial fibrillary acidic protein (Chemicon International Inc.; 1 : 500), and mouse anti-proliferating cell nuclear antigen (PCNA; Dako Corporation, CA, USA; 1 : 100).
Prior to specific staining, all the slides were deparaffinized in xylene twice for 15 min, rehydrated in a series of ethanol (100%, 100%, 95%, 95%, 70%, for 2 min each), and incubated in 0.1 m phosphate-buffered saline (PBS) twice for 5 min. Sections were then pre-treated with either 1% H2O2 in 100% methanol (for NeuN, cleaved caspase-3, GFAP, and PCNA antibodies) or 1% H2O2 in PBS (for anti-oligodendrocyte O4 sulphatide marker antibody) for 10 min, and then washed in dH2O for 1 × 5 min and PBS for 2 × 5 min. Sections were then boiled in 10 mm sodium citrate buffer (pH 6.0) for 1 min for antigen unmasking, left to cool at room temperature for 20 min, blocked in PBS plus 2.5% normal goat serum (NGS; for caspase-3) or 2.5% normal horse serum (NHS; for NeuN, GFAP, PCNA, and O4) for 1 h at room temperature, and then incubated in primary antibody for 3 days at 4°C.
The primary antibodies were washed off with PBS (3 × 5 min), and then incubated with either anti-mouse biotintylated immunoglobulin G (Vector Laboratories, Burlingame, CA, USA; diluted 1 : 200) for NeuN, GFAP, or PCNA antibody; with anti-rabbit biotintylated immunoglobulin G (Vector laboratories, diluted 1 : 200) for caspase-3 antibody; or with anti-mouse biotintylated immunoglobulin M (Serotec, Raleigh, NC, USA; diluted 1 : 200) for O4 antibody, overnight at 4°C. The sections were washed in PBS for 3 × 5 min, incubated in avidin–biotin complex (ABC, Vector Laboratories, Peterborough, UK) in the ratio 1 : 50 for 3 h at room temperature, washed again in PBS for 3 × 5 min, and then reacted with diaminobenzidine tetra hydrochloride (DAB, Sigma). Sections were then dehydrated in a series of alcohol to xylene washes and cover-slipped with mounting medium. Control sections were processed in the same way except that the primary antibodies were omitted from the incubation solution.
Data analysis and statistics
Off-line analysis of electronic data was performed using an analysis program written using Labview for Windows. The EEG signal was low-pass filtered with a 6th order low-pass filter, with a cut-off frequency of 50 Hz. A power spectrum was then calculated from this 256 Hz sampled signal. The EEG intensity and spectral edge frequency (SEF) were then calculated on the portion of the power spectrum between 1 Hz and 20 Hz, and stored as 1 min averages for the analysis of average EEG changes over time. The total EEG intensity (power) was normalized by log transformation (dB, 20 × log (intensity)), and data from left and right EEG electrodes were averaged to give mean total EEG activity. The 90% spectral edge of the EEG, i.e. the frequency below which lay 90% of the EEG intensity, was calculated from the spectra. Additionally, the raw EEG signal was processed through a digital low-pass filter with a cut-off frequency of 30 Hz and stored at a sampling rate of 64 Hz for analysis of seizures. Electrographic seizures were identified visually and defined as the concurrent appearance of sudden, repetitive, evolving stereotyped waveforms in the EEG signal lasting more than 10 s (Scher, 2002).
Numbers of cells labelled by immunohistochemistry were counted by light microscopy at × 10 magnification on coronal sections in multiple 0.8 mm × 0.55 mm (0.44 mm2) pre-selected areas of the dorso-lateral aspect of the caudate nucleus, the putamen, the cornu ammonis (CA) regions of the hippocampus, and the periventricular white matter (Fig. 1) (Dean et al. 2006). The periventricular area was taken from the midpoint between the lateral ventricle and the adjacent sulcus. Values from the left and right hemisphere were averaged. As there was no significant difference in values between the caudate nucleus and the putamen, the mean of these values was calculated to give a single striatal value. The cross-sectional area of the nuclei was measured (Sigmascan, SPSS Inc, Chicago, IL, USA) to verify that there was no significant difference in area between groups (Roelfsema et al. 2004).
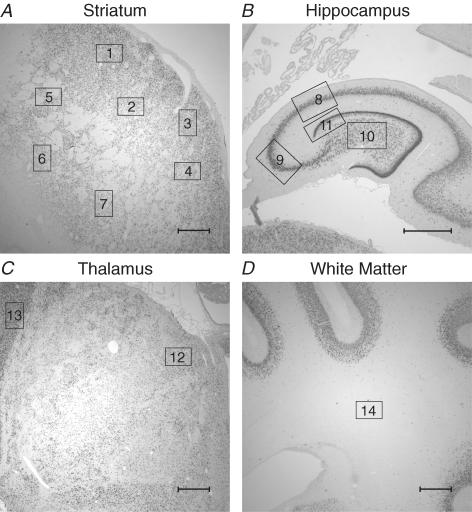
Figure 1. Photomicrographs showing the fields of view used for histological analysis of brain injury in the striatum (A), the hippocampus (B), the thalamus (C), and the subcortical white matter (D).
Seven fields of view in the striatum (4 caudate, 3 putamen; squares 1–7), one field of view in each of the CA1/2, CA3, CA4 and dentate gyrus (squares 8, 9, 10 and 11, respectively), 2 fields of view in the thalamus (1 medial nucleus, 1 reticular formation; squares 12 and 13, respectively), and one field of view in the periventricular white matter (square 14), in both hemispheres, were used to count numbers of labelled cells. Other regions used for measurement of cortical neuronal survival are not shown. Scale bar, 1 mm.
Treatment effects were evaluated by analysis of variance (ANOVA, SPSS v10, SPSS Inc.), followed by Tukey's honestly significant difference post hoc test when a significant overall effect was found. For comparisons over time, baseline levels were used as a covariate (ANCOVA). The baseline period was taken as the mean of the 6 h before occlusion. Cerebral region and time series data, respectively, were treated as repeated measures to allow for repeated sampling. Statistical significance was accepted when P < 0.05. Data are mean ± s.d.
Results
All fetuses had normal blood gases, acid–base, glucose and lactate status (Table 1), FHR, MAP and EEG recordings before each experiment, according to the standards of our laboratory. At post-mortem, body weights were similar between groups: sham control group 1622 ± 145 g, normothermia-occlusion group 1782 ± 268 g, hypothermia-occlusion group 1707 ± 269 g (not significant).
Table 1.
Fetal arterial pH, blood gases, glucose and lactate values for sham control (C), normothermia-occlusion (N) and hypothermia-occlusion (H) groups 15 min before (baseline), at 20 min during (occl), and 30 min, 2 h, 4 h, 6 h, 24 h, 48 h and 72 h after the end of either sham occlusion or 25 min of umbilical cord occlusion
| Control | 20 min occl | 30 min | 2 h | 4 h | 6 h | 24 h | 48 h | 72 h | ||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | C | 7.39 ± 0.0 | 7.38 ± 0.0 | 7.38 ± 0.0 | 7.39 ± 0.0 | 7.39 ± 0.0 | 7.38 ± 0.0 | 7.38 ± 0.0 | 7.37 ± 0.0 | 7.37 ± 0.0 |
| N | 7.37 ± 0.0 | 6.82 ± 0.0* | 7.25 ± 0.0* | 7.34 ± 0.0* | 7.40 ± 0.0 | 7.41 ± 0.0* | 7.37 ± 0.0 | 7.38 ± 0.0 | 7.38 ± 0.0 | |
| H | 7.39 ± 0.0 | 6.85 ± 0.0* | 7.27 ± 0.0* | 7.39 ± 0.0# | 7.46 ± 0.0*# | 7.44 ± 0.0**# | 7.43 ± 0.0*# | 7.41 ± 0.0*# | 7.43 ± 0.0*# | |
| Pa,CO2 (mmHg) | C | 48.8 ± 4.5 | 49.1 ± 4.1 | 47.8 ± 4.1 | 48.1 ± 4.3 | 49.2 ± 5.2 | 49.4 ± 5.0 | 47.5 ± 6.3 | 49.2 ± 4.1 | 48.2 ± 6.4 |
| N | 48.2 ± 2.6 | 142.2 ± 16* | 45.8 ± 2.2 | 46.8 ± 2.6 | 47.7 ± 3.2 | 45.6 ± 4.0 | 47.4 ± 2.9 | 47.1 ± 2.5 | 46.4 ± 3.2 | |
| H | 47.8 ± 4.3 | 138.4 ± 24* | 43.8 ± 3.5 | 37.9 ± 2.9*# | 39.8 ± 4.0*# | 39.5 ± 3.0*# | 38.8 ± 7.3*# | 41.5 ± 2.9*# | 42.9 ± 5.1 | |
| Pa,O2 (mmHg) | C | 22.5 ± 2.6 | 22.7 ± 2.3 | 22.3 ± 2.1 | 22.1 ± 2.6 | 22.6 ± 2.6 | 22.3 ± 2.5 | 22.2 ± 4.8 | 23.4 ± 4.0 | 23.5 ± 3.7 |
| N | 23.0 ± 2.6 | 8.7 ± 2.2* | 27.9 ± 3.8* | 26.2 ± 3.8* | 24.8 ± 2.8 | 24.8 ± 2.5 | 26.6 ± 3.7* | 28.0 ± 3.2* | 28.3 ± 3.6 | |
| H | 24.7 ± 3.0 | 9.1 ± 4.3* | 27.3 ± 2.0* | 24.7 ± 3.7 | 25.1 ± 3.7 | 24.4 ± 3.2 | 23.6 ± 2.6 | 24.5 ± 4.3 | 27.0 ± 7.1 | |
| O2ct | C | 3.6 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.4 ± 0.6 | 3.4 ± 0.6 | 3.4 ± 0.6 | 3.2 ± 0.6 | 3.4 ± 0.7 | 3.7 ± 0.6 |
| N | 3.5 ± 0.5 | 0.5 ± 0.1* | 3.9 ± 0.5 | 4.1 ± 0.5 | 3.9 ± 0.8 | 4.0 ± 0.7 | 4.1 ± 0.8 | 4.1 ± 0.7 | 4.2 ± 0.7 | |
| H | 3.7 ± 0.4 | 0.5 ± 0.2* | 3.9 ± 0.6 | 4.2 ± 0.8* | 4.5 ± 0.6* | 4.5 ± 0.8* | 4.2 ± 0.5 | 3.9 ± 0.6 | 4.3 ± 0.5 | |
| Glucose (mM) | C | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.3 | 1.2 ± 0.2 | 1.1 ± 0.3 |
| N | 1.0 ± 0.2 | 0.7 ± 0.3* | 1.3 ± 0.4 | 1.2 ± 0.2 | 1.3 ± 0.3 | 1.4 ± 0.3* | 1.5 ± 0.4 | 1.3 ± 0.2 | 1.2 ± 0.2 | |
| H | 0.9 ± 0.2 | 0.5 ± 0.4* | 1.4 ± 0.5 | 1.3 ± 0.3 | 1.4 ± 0.3* | 1.5 ± 0.3* | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.3 | |
| Lactate (mM) | C | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.4 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.7 ± 0.1 | 0.8 ± 0.3 | 0.9 ± 0.3 |
| N | 0.6 ± 0.2 | 6.5 ± 0.5* | 4.8 ± 0.5* | 3.6 ± 1.5* | 2.4 ± 1.3* | 1.9 ± 0.7* | 1.0 ± 0.3* | 0.8 ± 0.3 | 0.9 ± 0.2 | |
| H | 0.7 ± 0.1 | 5.8± 1.4* | 4.7 ± 1.3* | 2.9 ± 1.6* | 1.4 ± 0.8# | 1.2 ± 0.5*# | 0.9 ± 0.3 | 0.7 ± 0.2 | 0.8 ± 0.2 |
Pa,CO2, fetal arterial partial pressure of carbon dioxide; Pa,O2, fetal arterial partial pressure of oxygen; O2ct, fetal arterial oxygen content. Data are mean ± s.d.
P < 0.05 versus sham control group
P < 0.05 normothermia-occlusion group versus hypothermia-occlusion group (ANOVA, followed by the least-significant difference post hoc test for between group comparisons).
Blood composition
Values and statistical comparisons for arterial pH, blood gases, and glucose and lactate levels for the sham control, normothermia-occlusion and hypothermia-occlusion groups are presented in Table 1. Following release of the umbilical occluder there was rapid recovery of blood gases, pH and glucose and lactate levels. Arterial Pa,O2 was significantly elevated compared with the sham control group in the two occlusion groups 30 min after the end of occlusion, with a subsequent persistent elevation in the normothermia-occlusion group but not the hypothermia-occlusion group. The hypothermia-occlusion group showed a small reduction in arterial Pa,CO2 (P < 0.05) and a mild elevation of pH (P < 0.05) compared with both the sham control group and the normothermia-occlusion group from 2 h to 48 h after the end of occlusion.
Effect of umbilical cord occlusion
Umbilical cord occlusion led to bradycardia, hypotension and cerebral hypoperfusion. EEG intensity was rapidly and profoundly depressed during the entire period of occlusion (P < 0.05 for each variable versus the sham control group; data not shown). There were no significant differences for any of these measures between the animals allocated to the normothermia-occlusion and the hypothermia-occlusion groups during the occlusion period.
Effect of cooling on brain and body temperature
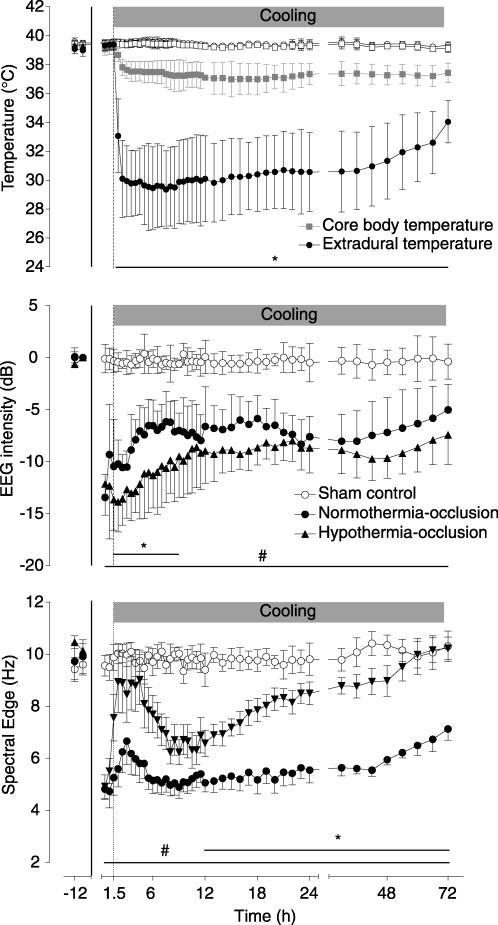
Head cooling was initiated 90 min after the end of the umbilical cord occlusion and was associated with a significant fall in extradural temperature starting within an hour after the onset of cooling, reaching a nadir of 29.5 ± 2.6°C (versus 39.3 ± 0.3°C normothermia-occlusion group; P < 0.05, Fig. 2 top panel). Extradural temperature gradually increased during the second and third day of cooling to a mean of 32.3 ± 2.2°C in the last 6 h. During cooling, core body temperature dropped mildly to a nadir of 37.2 ± 0.8°C (versus 39.0 ± 0.4°C normothermia-occlusion group; P < 0.05), which was sustained over the whole cooling period.
Figure 2. Time sequence of changes in fetal temperature, electroencephalogram (EEG) intensity (power) and spectral edge (frequency).
The 25 min period of umbilical cord occlusion is shown by the continuous vertical lines, while cooling is shown by the shaded bar. The top panel shows changes in extradural and core body (oesophageal) temperatures in the hypothermia-occlusion and normothermia-occlusion groups; sham control group data are not shown for clarity. Bar plus *P < 0.05 hypothermia-occlusion versus normothermia-occlusion group. Bar plus #P < 0.05 normothermia-occlusion versus sham control group. Data are mean ±s.d.
EEG intensity and spectral edge frequency
EEG intensity was rapidly suppressed, within 30–60 s, at the onset of occlusion in both occlusion groups, and remained significantly suppressed compared with the sham control group following reperfusion for the remainder of the experiment (P < 0.01, Fig. 2, middle panel). Hypothermia was associated with an immediate fall in EEG intensity, which remained significantly less than in the normothermia-occlusion group until 9 h after occlusion (P < 0.05); thereafter there was no significant difference between the two groups.
EEG SEF was also markedly suppressed in both occlusion groups during and after reperfusion, with a modest rise in the first 4 h after reperfusion, followed by a fall reaching its nadir at approximately 7 h (Fig. 2, bottom panel). There was no significant difference in SEF in the hypothermia-occlusion group compared with the normothermia-occlusion group during the first 12 h after reperfusion. From 12 h onward the hypothermia-occlusion group showed an increase in SEF compared with the normothermia-occlusion group (P < 0.001, repeated measures ANOVA), and SEF values in the hypothermia-occlusion group were not different from the sham control group in the final 24 h of the study.
Seizures
High-amplitude, low-frequency seizures developed from 9.1 ± 5.3 h after the end of occlusion in the normothermia-occlusion group versus 10.1 ± 4.7 h in the hypothermia group (not significant), with a median (25th, 75th percentile) of 59 (18, 98) versus 57 (42, 130) seizure events, respectively (not significant). No seizures were seen in either occlusion group in the 2 h rewarming period, and no seizures were observed in sham control fetuses at any time.
FHR, MAP and CaBF
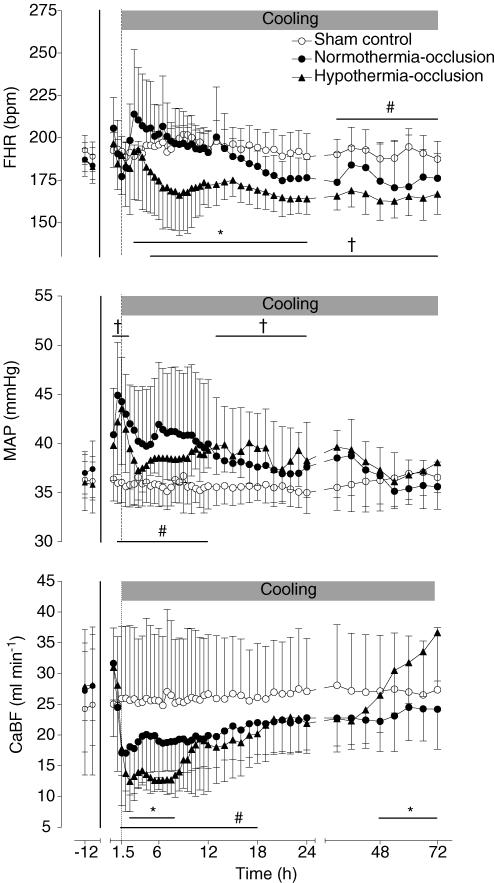
Following reperfusion, there was an initial rapid recovery in MAP, FHR and CaBF. The normothermia-occlusion group showed a transient tachycardia (maximal at 3 h after occlusion; P < 0.01, ANOVA, Fig. 3, top panel), followed by a gradual fall, and became significantly lower than the sham control group in the final 48 h of the study (P < 0.05, ANOVA). In contrast, cooling was associated with a significant reduction in FHR compared with both the sham control group (P < 0.001, from 5 h after the end of occlusion until the end of cooling) and the normothermia-occlusion group (P < 0.05, from 3 h to 24 h).
Figure 3. Time sequence of changes in fetal heart rate (FHR, bpm), mean arterial blood pressure (MAP, mmHg), and total carotid blood flow (CaBF, ml min−1) in the sham control, normothermia-occlusion, and the hypothermia-occlusion groups.
The 25 min period of umbilical cord occlusion is shown by the continuous vertical line, while cooling is shown by the shaded bar. Bar plus *P < 0.05 hypothermia-occlusion versus normothermia-occlusion group. Bar plus † P < 0.05 hypothermia-occlusion versus sham control group. Bar plus # P < 0.05 normothermia-occlusion versus sham control group. Data are mean ± s.d.
MAP was significantly elevated in the first 12 h after the end of occlusion in the normothermia-occlusion group compared with the sham control group (P < 0.005, ANOVA, Fig. 3, middle panel). The elevation was maximal at 1 h. The hypothermia-occlusion group was not significantly different from the normothermia-occlusion group overall (ANOVA); however, there was an apparently more rapid resolution of the initial elevation of MAP, such that on post hoc tests the hypothermia group was not significantly different from the sham control group from 3 h to 12 h after occlusion.
Following reperfusion CaBF initially returned to sham control group values in both the occlusion groups, followed by a secondary fall to levels that were significantly less than the sham control group from 90 min to 18 h after the end of occlusion (Fig. 3, bottom panel). Thereafter, CaBF was not significantly different from the sham control group. Hypothermia was associated with a greater reduction in CaBF than in the normothermia-occlusion group from 2.5 to 8 h after reperfusion (P < 0.05). In the final 42 h of the experiment the hypothermia group showed a progressive increase in CaBF, reaching values greater than the occlusion-normothermia group in the final 24 h (P < 0.05, ANOVA).
Grey matter NeuN expression
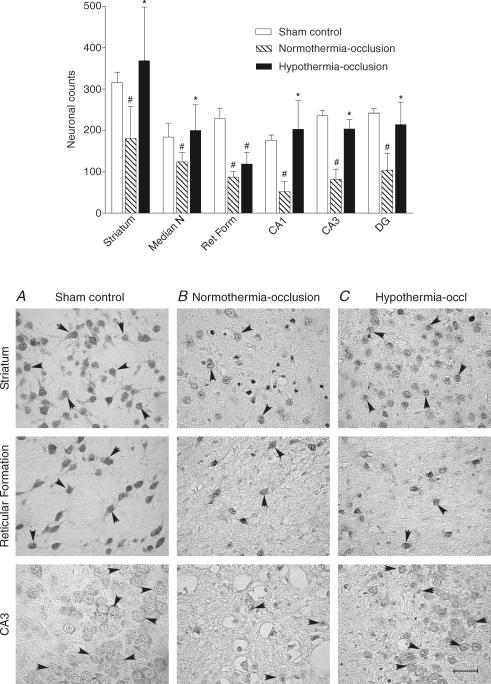
NeuN staining was primarily localized within neuronal nuclei, with lighter staining in the cytoplasm and occasional staining of proximal processes. Only cells with normal nuclear staining and morphology were counted. The normothermia-occlusion group showed a significant overall decrease in numbers of NeuN-positive neurons in all regions counted versus the sham-occlusion group (P < 0.001; Fig. 4), while there was significant overall increase in NeuN-positive neurons in the hypothermia-occlusion group versus the normothermia-occlusion group (P < 0.001). Post hoc testing indicated that there was a significant improvement in numbers of neurons in all hippocampal and striatal regions with hypothermia and in the median nucleus of the thalamus (P < 0.05), but not in the reticular nucleus of the thalamus (P = 0.08, Tukey test).
Figure 4. The effect of hypothermia on neuronal survival assessed with neuronal specific nuclear protein (NeuN) after 3 days recovery from 25 min of umbilical cord occlusion Top.
panel, the effect of hypothermia on numbers of NeuN-positive cells in the sham control, normothermia-occlusion, and the hypothermia-occlusion groups. #P < 0.05 versus sham control group; *P < 0.05 versus normothermia-occlusion group. Data are mean ± s.d. Striatum, Th (thalamus), CA (cornu ammonis of the hippocampus), DG (dentate gyrus), thalamic nuclei: Median N (median nucleus), Ret Form (reticular formation) Bottom panel, photomicrographs of the striatum, reticular formation of the thalamus, and CA3 showing NeuN-positive cells (arrowheads) in the sham control group (A), the normothermia-occlusion group (B), and the hypothermia-occlusion group (C). Scale bar, 40 μm.
Grey matter caspase-3 and activated microglia expression
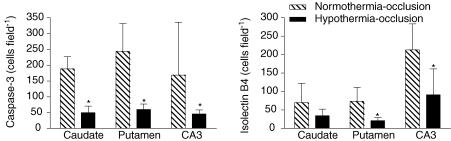
In order to further explore the mechanisms of hypothermic neuronal protection we quantified numbers of cleaved 17 kDa caspase-3-positive cells and isolectin-B4-positive microglia in a severely damaged region (the CA3 region of the hippocampus) and a more moderately damaged region, the striatum. The density of caspase-3 staining varied between cells due to the timing of cleavage of caspase-3. Cells were counted that had evidence of newly cleaved caspase-3, as shown by morphologically dense caspase-3 (Asp175) staining evenly distributed within the ectoplasm. Only occasional activated caspase-3-positive cells were seen in some sham control group animals. Following umbilical cord occlusion in the normothermia-occlusion group, marked activated caspase-3 expression was seen (CA3: 169 ± 167 cells area−1, striatum: 216 ± 55 cells area−1; P < 0.001 versus sham control; Figs 5 and 6). Hypothermia was associated with significantly reduced numbers of cells expressing activated caspase-3 compared with the normothermia-occlusion group in both regions (CA3: 46 ± 12 cells area−1, striatum: 55 ± 17 cells area−1; P < 0.001, ANOVA).
Figure 5. The effect of hypothermia on numbers of activated caspase-3 (Asp175)- and isolectin-B4-stained cells in subcortical neuronal nuclei after 3 days recovery from 25 min of umbilical cord occlusion.
*P < 0.05 versus normothermia-occlusion group. Data are mean ± s.d.
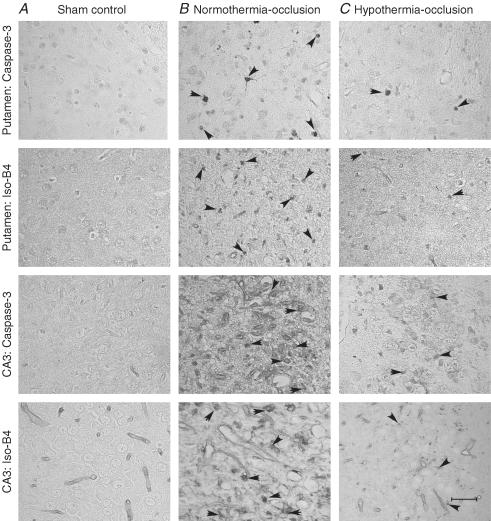
Figure 6. Caspase-3 and isolectin B4 expression in selected neuronal nuclei.
Photomicrographs of selected subcortical neuronal nuclei showing activated caspase-3 (Asp175)- and isolectin-B4-stained cells (arrowheads) in the sham control group (A), the normothermia-occlusion group (B), and the hypothermia-occlusion group (C). Scale bar, 40 μm.
The sham control group showed no isolectin-B4 staining in any region. Occlusion was associated with induction of isolectin-B4-stained activated microglia (CA3: 213 ± 71 cells area−1, striatum: 119.7 ± 34.3 cells area−1; P < 0.001 versus sham control; Figs 5 and 6), which was significantly reduced in the hypothermia-occlusion group (CA3: 91 ± 70 cells area−1, striatum: 20 ± 16 cells area−1, P < 0.01).
White matter O4 expression
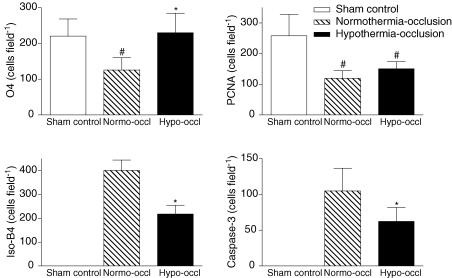
Dense O4-positive staining was present in the periventricular white matter in the sham control group. There was a significant loss of O4-positive cells in the periventricular white matter in the normothermia-occlusion group (P < 0.01; Figs 7 and 8). Hypothermia was associated with a significant increase in numbers of O4-positive cells compared with the normothermia-occlusion group (P < 0.01).
Figure 7. The effect of hypothermia on periventricular white matter.
The effect of hypothermia on numbers of O4-positive immature oligodendrocytes, activated caspase-3 (Asp175)-, isolectin-B4-, and proliferating cell nuclear antigen (PCNA)-positive cells in the periventricular white matter after 3 days recovery from 25 min of umbilical cord occlusion. #P < 0.05 versus sham control group; *P < 0.05 versus normothermia-occlusion group. Data are mean ± s.d.
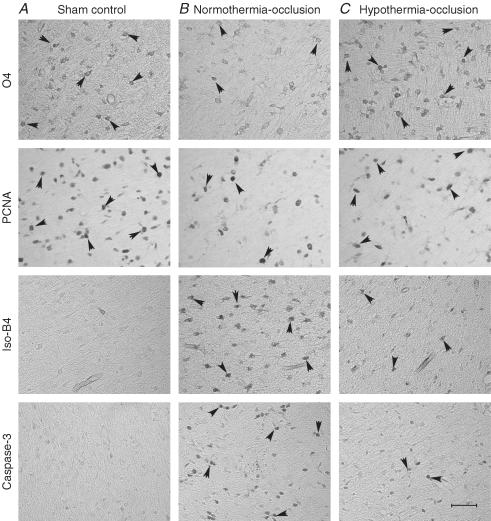
Figure 8. Periventricular white matter.
Photomicrographs of periventricular white matter showing O4-positive immature oligodendrocytes, activated caspase-3- (Asp175), isolectin-B4-, and proliferating cell nuclear antigen (PCNA)-positive cells (arrowheads) in the sham-control group (A), the normothermia-occlusion group (B), and the hypothermia-occlusion group (C). Scale bar, 40 μm.
White matter caspase-3 and activated microglia expression
Only occasional cleaved 17 kDa caspase-3-positive cells were seen in the periventricular white matter in a few sham control group animals. Both occlusion groups showed a significant increase in activated caspase-3 expression in the periventricular white matter (P < 0.001; Figs 7 and 8). Hypothermia was associated with a significant reduction in numbers of cells expressing activated caspase-3 compared with the normothermia-occlusion group in the periventricular white matter (P < 0.05).
No isolectin-B4-positive cells were seen in the periventricular white matter in the sham control group. Both occlusion groups showed a significant increase in isolectin B4-positive cells in the periventricular white matter (P < 0.001, Figs 7 and 8). Hypothermia was associated with a significant reduction in numbers of isolectin-B4-positive cells compared with the normothermia-occlusion group (P < 0.05).
White matter PCNA expression
Cell proliferation in the white matter tracts was assessed by PCNA immunohistochemistry. There were abundant PCNA-labelled cells in the sham control group animals. Umbilical cord occlusion was associated with a significant reduction in the number of PCNA-labelled cells compared with the sham control group (P < 0.05; Figs 7 and 8). The number of PCNA-labelled cells was not significantly different between the normothermia-occlusion and the hypothermia-occlusion groups.
Discussion
The present study demonstrates that moderate, delayed cerebral hypothermia started 90 min after a prolonged episode of umbilical cord occlusion, and continued for 3 days, is associated with markedly reduced loss of neurons and immature oligodendrocytes. After 3 days recovery from umbilical cord occlusion, carotid blood flow was significantly greater in the hypothermia-occlusion group than in the normothermia-occlusion group, and EEG frequency, although not amplitude, was significantly improved and reached sham control levels. These haemodynamic and electrophysiological improvements were reflected in greater histological neuronal survival in many nuclei of the basal ganglia and the cornu ammonis regions of the hippocampus, with suppression of induction of activated caspase-3 and of microglia.
It is now well established that hypoxic–ischaemic injury in term infants is an evolving process, with initial, transient recovery of cerebral oxidative metabolism after birth in a ‘latent’ phase, followed by secondary deterioration with cerebral energy failure from approximately 6 h or even later after birth (Azzopardi et al. 1989; Roth et al. 1992). As recently reviewed (Gunn et al. 2005), a sufficiently prolonged interval of moderate hypothermia can significantly improve neural outcomes, provided it is initiated in this latent phase, before the onset of secondary deterioration (Thoresen et al. 1995; Gunn et al. 1997; Gunn et al. 1999). At term this deterioration may be marked by the delayed onset of seizures, cytotoxic oedema, accumulation of excitotoxins and redox deterioration (Marks et al. 1996; Tan et al. 1996; Gunn et al. 1997; Nedelcu et al. 1999).
Despite the encouraging results of induced cooling in encephalopathic term infants (Gluckman et al. 2005; Shankaran et al. 2005), considerable caution is needed in evaluating to what extent these findings may be applicable to preterm infants. The pathogenesis of injury is incompletely understood, and the pattern of brain injury in this group is typically quite different from term infants, with a predominance of PVL (Barkovich & Truwit, 1990). We have previously shown that delayed initiation of a prolonged interval of hypothermia is associated with a time-of-initiation-dependent amelioration of post-ischaemic loss of mature, myelinating oligodendrocytes and demyelination in the near-term fetal sheep (Roelfsema et al. 2004); however, there are no data on the effects of delayed hypothermia on preterm post-hypoxic white matter damage.
Consistent with previous reports (Mallard et al. 2003; Welin et al. 2005; Dean et al. 2006), in the present study there was a marked loss of these premyelinating oligodendroglia after prolonged umbilical cord occlusion. Immature oligodendrocytes were identified using the O4 surface antigen, which may be present on both late oligodendrocyte progenitors and immature oligodendrocytes (Back et al. 2002), but not on myelinating cells. The present study demonstrates for the first time that delayed hypothermia can reduce post-hypoxic loss of these immature oligodendrocytes in the periventricular white matter.
Despite this improvement, the number of proliferating cells in the periventricular region remained suppressed in both occlusion groups. The reduction in proliferation in the normothermia-occlusion group presumably reflects the balance between profound loss of proliferating immature oligodendrocytes, but an increase in proliferation by microglia (Si et al. 1997; Guan et al. 2001). However, the improved survival of O4-positive cells in the hypothermia-occlusion group and marked reduction in numbers of reactive microglia were not accompanied by recovery in the numbers of proliferating cells. This continued reduction probably reflects suppression by hypothermia of glial proliferation in general (Lee et al. 2002), as well as microglia (Si et al. 1997). Further studies are required to evaluate whether oligodendroglial proliferation fully recovers in the long term, so as to allow the development of normal myelination.
A relatively neglected aspect of preterm brain injury is damage to subcortical grey matter. Post-mortem and early imaging studies suggest that this is a common complication of severe perinatal hypoxia, accompanying periventricular white matter damage (Paneth et al. 1990; Barkovich & Sargent, 1995; Gilles et al. 1998; Lin et al. 2001; Leijser et al. 2004). It is likely that the presence of such injury has considerable importance since, in the long term, impairment of grey matter growth is independently associated with functional impairment, after adjustment for white matter injury (Woodward et al. 2005). Similar to previous studies of prolonged hypoxia in the preterm fetal sheep (George et al. 2004; Welin et al. 2005; Dean et al. 2006), in the present study we observed severe subcortical neuronal loss. Hypothermia significantly increased numbers of surviving, NeuN-positive, neurons. Intriguingly, there appeared to be less benefit of hypothermia on neuronal survival in thalamic nuclei, and in particular no significant improvement in the reticular formation, whereas the median nucleus showed marked improvement. The reasons for this difference are unknown. It could reflect reduced temperatures in the deeper regions of the brain during head cooling. There is some evidence from piglets that the optimal degree of cooling can vary between cerebral regions (Iwata et al. 2005), and thus, it is possible that some thalamic regions require greater cooling than attained in the present study.
The improved survival of neurons and immature oligodendrocytes was associated with suppression of caspase-3 and microglial induction. These findings are consistent with previous reports suggesting that the mechanisms of action of hypothermia may include suppression of apoptotic events (Edwards et al. 1995; Roelfsema et al. 2004) and local inflammation (Silverstein et al. 1997; Roelfsema et al. 2004; Welin et al. 2005).
Hypothermia was associated with a transient exaggeration of the secondary fall in carotid blood flow from 2 to 8 h after the end of umbilical cord occlusion compared with the normothermia-occlusion group, similar to its effect after reversible cerebral ischaemia at term (Gunn et al. 1997). Although cerebral metabolism was not measured in the present study, it is likely that this reflects an autoregulatory adjustment to the well-established linear suppression of cerebral metabolism during hypothermia (Laptook et al. 1995), as demonstrated by the significant suppression of EEG activity during cooling. Over the last 2 days of the present study, carotid blood flow progressively increased despite continued cooling, and became significantly greater than in the normothermia-occlusion group in the final 24 h. It is likely that this increase in blood flow accounts for the observed relative increase in extradural temperature over time despite continued cooling. In turn, this increase probably reflects increased brain activity, as shown by normalization of the spectral edge frequency of EEG activity to sham control values during cooling, whereas EEG intensity remained suppressed in the normothermia-occlusion group. Together, these data suggest that cerebral function and activity significantly improved during continued cooling.
There was no significant change in numbers of post-hypoxia seizures, consistent with previous data which showed either no significant effect of cooling on secondary seizures (Gunn et al. 1997, 1998b) or a modest reduction (Tooley et al. 2003). Further, Yager and colleagues have shown in the neonatal rat that preventing spontaneous hyperthermia after hypoxia–ischaemia prevented injury associated with kainic acid-induced seizures without reducing the numbers of seizures (Yager et al. 2004). These findings are consistent with data in term models of injury that highlight the relatively late onset of post-ischaemic seizures, after the onset of secondary mitochondrial failure (Marks et al. 1996), suggesting that they are unlikely to play a major role in extending injury.
A potential limitation of the present study is that evolving neuronal and white matter injury may extend for longer than the 3 days recovery from ischaemia in the present study (Geddes et al. 2001). The continued presence of activated caspase-3 at this time also suggests ongoing injury, and thus, it is possible that the observed protection may reduce over time. Nevertheless, hypothermia was associated with markedly reduced caspase-3 expression as well as greater oligodendrocyte and neuronal survival after 72 h recovery, suggesting a reduction in ongoing damage. Consistent with this, in adult rodents persistent neuronal protection with hypothermia has been found 2–6 months after ischaemia (Colbourne & Corbett, 1995; Corbett et al. 2000), although further longer term outcome studies are necessary to confirm the present findings.
The use of a chronically instrumented fetal preparation to test the effect of post-insult cooling is clearly a compromise as fetal and neonatal metabolism differ in several aspects, although the results of previous studies using this approach at term (Gunn et al. 1997) have been able to be effectively applied clinically (Gluckman et al. 2005). This approach provides an insult which occurs under fetal conditions consistent with the potential target population of premature infants exposed to perinatal hypoxia (Salhab & Perlman, 2005), ensures stable control temperatures (Gunn & Gunn, 1996), and avoids the major confounding effects of anaesthesia (Nakashima & Todd, 1996).
There is some evidence from before modern neonatal intensive care that premature infants may be particularly vulnerable to death or other complications during cooling (Silverman et al. 1958). Although it is not practical to cool only the brain, partially selective cerebral cooling can be obtained using a cooling cap applied to the scalp (Gunn et al. 1998a; Tooley et al. 2003; Gluckman et al. 2005). Studies in the term piglet using this approach have shown that a significant gradient between core brain temperature and rectal temperature can be achieved and maintained for at least 24 h (Tooley et al. 2003). The current study confirms that a significant gradient can be established and maintained between the extradural space and core body temperature using this technique in the preterm fetal sheep. The present study can only address in part whether selective head cooling can prevent deleterious systemic effects in preterm infants and it is unknown whether the brain temperatures achieved are optimal for protection; further studies in the newborns of large species will be required to fully assess the safety of cooling. Nevertheless, we observed only minimal systemic effects. FHR was mildly reduced by cooling compared with the normothermia group, consistent with its well-established effects on cardiac conduction in adults (Solomon et al. 1989), and at term (Azzopardi et al. 2000; Gluckman et al. 2005). MAP was reduced in the first 10 h in hypothermic compared with normothermic-occlusion animals; however, importantly, hypotension did not develop. Furthermore, there was no evidence of significant metabolic acidosis or other adverse changes with cooling, except for a mild respiratory alkalosis.
Finally, the results of the present study cannot and should not be extrapolated to all preterm infants, since preterm neural injury is a multifactorial disorder that may be associated with both antenatal and postnatal events including systemic inflammation, as well as exposure to hypoxia–ischaemia (du Plessis & Volpe, 2002). Nevertheless, data from serial EEG recordings and early MRI imaging suggest that many cases are related to events in the immediate peripartum period (de Vries et al. 1998; Hayakawa et al. 1999; Okumura et al. 2001). Pathologically, the pattern of neural injury seen in the present study is consistent with the clinical occurrence of diffuse periventricular white matter loss and severe damage in subcortical nuclei, but cortical sparing, after acute hypoxia at birth (Barkovich & Sargent, 1995; de Vries et al. 1998). In particular, ‘older’ premature infants (31–36 weeks gestation) with metabolic acidaemia on cord blood have a high risk of evolving neural injury after birth (Salhab & Perlman, 2005). Thus, the present study of fetal sheep at comparable maturation stage (McIntosh et al. 1979) supports the concept that this group may be potential candidates for treatment with neuroprotective strategies such as hypothermia (Salhab & Perlman, 2005).
In summary, these data demonstrate that selective mild to moderate cerebral hypothermia after profound hypoxia–ischaemia induced by complete occlusion of the umbilical cord can reduce short-term injury of immature, premyelinating oligodendroglia, and subcortical neuronal structures in the preterm brain. These histological changes were associated with progressive normalization of carotid blood flow and EEG frequency, although not intensity (power), consistent with functional improvement. Hypothermia was not associated with hypotension or metabolic acidosis, suggesting it may be safe in the preterm infant if carefully controlled. However, it is of potential concern that cooling was not associated with an improvement in post-hypoxic suppression of cell proliferation in periventricular white matter. Given the high levels of proliferation at this stage of development (Kinney & Back, 1998), further studies are essential to evaluate how or whether hypothermia may affect subsequent brain development.
Acknowledgments
The present study was supported by the Health Research Council of New Zealand, the Auckland Medical Research Foundation, the Lottery Grants Board of New Zealand, and the March of Dimes Birth Defects Foundation.
References
- Argyropoulou MI, Xydis V, Drougia A, Argyropoulou PI, Tzoufi M, Bassounas A, Andronikou S, Efremidis SC. MRI measurements of the pons and cerebellum in children born preterm; associations with the severity of periventricular leukomalacia and perinatal risk factors. Neuroradiology. 2003;45:730–734. doi: 10.1007/s00234-003-1067-0. [DOI] [PubMed] [Google Scholar]
- Azzopardi D, Robertson NJ, Cowan FM, Rutherford MA, Rampling M, Edwards AD. Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics. 2000;106:684–694. doi: 10.1542/peds.106.4.684. [DOI] [PubMed] [Google Scholar]
- Azzopardi D, Wyatt JS, Cady EB, Delpy DT, Baudin J, Stewart AL, Hope PL, Hamilton PA, Reynolds EO. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1989;25:445–451. doi: 10.1203/00006450-198905000-00004. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Sargent SK. Profound asphyxia in the premature infant: imaging findings. AJNR Am J Neuroradiol. 1995;16:1837–1846. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Truwit CL. Brain damage from perinatal asphyxia: correlation of MR findings with gestational age. AJNR Am J Neuroradiol. 1990;11:1087–1096. [PMC free article] [PubMed] [Google Scholar]
- Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol. 1969;135:249–262. doi: 10.1002/cne.901350302. [DOI] [PubMed] [Google Scholar]
- Bell JE, Becher JC, Wyatt B, Keeling JW, McIntosh N. Brain damage and axonal injury in a Scottish cohort of neonatal deaths. Brain. 2005;128:1070–1081. doi: 10.1093/brain/awh436. [DOI] [PubMed] [Google Scholar]
- Bennet L, Rossenrode S, Gunning MI, Gluckman PD, Gunn AJ. The cardiovascular and cerebrovascular responses of the immature fetal sheep to acute umbilical cord occlusion. J Physiol. 1999;517:247–257. doi: 10.1111/j.1469-7793.1999.0247z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Buetow KC, Klein SW. Effect of maintenance of ‘normal’ skin temperature on survival of infants of low birth weight. Pediatrics. 1964;34:163–170. [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Hamilton M, Colbourne F. Persistent neuroprotection with prolonged postischemic hypothermia in adult rats subjected to transient middle cerebral artery occlusion. Exp Neurol. 2000;163:200–206. doi: 10.1006/exnr.2000.7369. [DOI] [PubMed] [Google Scholar]
- Day RL, Caliguiri L, Kamenski C, Ehrlich F. Body temperature and survival of premature infants. Pediatrics. 1964;34:171–181. [PubMed] [Google Scholar]
- Dean JM, George SA, Wassink G, Gunn AJ, Bennet L. Suppression of post hypoxic-ischemic EEG transients with dizocilpine is associated with partial striatal protection in the preterm fetal sheep. Neuropharmacology. 2006;50:491–503. doi: 10.1016/j.neuropharm.2005.10.017. [DOI] [PubMed] [Google Scholar]
- de Vries LS, Eken P, Groenendaal F, Rademaker KJ, Hoogervorst B, Bruinse HW. Antenatal onset of haemorrhagic and/or ischaemic lesions in preterm infants: prevalence and associated obstetric variables. Arch Dis Child Fetal Neonatal Ed. 1998;78:F51–F56. doi: 10.1136/fn.78.1.f51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol. 2002;15:151–157. doi: 10.1097/00019052-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Yue X, Squier MV, Thoresen M, Cady EB, Penrice J, Cooper CE, Wyatt JS, Reynolds EO, Mehmet H. Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Commun. 1995;217:1193–1199. doi: 10.1006/bbrc.1995.2895. [DOI] [PubMed] [Google Scholar]
- Geddes R, Vannucci RC, Vannucci SJ. Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev Neurosci. 2001;23:180–185. doi: 10.1159/000046140. [DOI] [PubMed] [Google Scholar]
- George S, Gunn AJ, Westgate JA, Brabyn C, Guan J, Bennet L. Fetal heart rate variability and brainstem injury after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;287:R925–R933. doi: 10.1152/ajpregu.00263.2004. [DOI] [PubMed] [Google Scholar]
- Gilles FH, Leviton A, Golden JA, Paneth N, Rudelli RD. Groups of histopathologic abnormalities in brains of very low birthweight infants. J Neuropathol Exp Neurol. 1998;57:1026–1034. doi: 10.1097/00005072-199811000-00005. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard D, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ, on behalf of the CooICap study Group Selective head cooling with mild systemic hypothermia to improve neurodevelopmental outcome following neonatal encephalopathy. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Guan J, Bennet L, George S, Wu D, Waldvogel HJ, Gluckman PD, Faull RL, Crosier PS, Gunn AJ. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21:493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Battin M, Gluckman PD, Gunn TR, Bennet L. Therapeutic hypothermia: from lab to NICU. J Perinat Med. 2005;33:340–346. doi: 10.1515/JPM.2005.061. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46:274–280. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998a;102:885–892. doi: 10.1542/peds.102.4.885. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR. Effect of radiant heat on head temperature gradient in term infants. Arch Dis Child Fetal Neonatal Ed. 1996;74:F200–F203. doi: 10.1136/fn.74.3.f200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998b;102:1098–1106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- Hayakawa F, Okumura A, Kato T, Kuno K, Watanabe K. Determination of timing of brain injury in preterm infants with periventricular leukomalacia with serial neonatal electroencephalography. Pediatrics. 1999;104:1077–1081. doi: 10.1542/peds.104.5.1077. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Iwata O, Thornton JS, Sellwood MW, Iwata S, Sakata Y, Noone MA, O'Brien FE, Bainbridge A, De Vita E, Raivich G, Peebles D, Scaravilli F, Cady EB, Ordidge R, Wyatt JS, Robertson NJ. Depth of delayed cooling alters neuroprotection pattern after hypoxia-ischemia. Ann Neurol. 2005;58:75–87. doi: 10.1002/ana.20528. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Back SA. Human oligodendroglial development: relationship to periventricular leukomalacia. Semin Pediatr Neurol. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- Laptook AR, Corbett RJ, Sterett R, Garcia D, Tollefsbol G. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using 31P and 1H magnetic resonance spectroscopy. Pediatr Res. 1995;38:919–925. doi: 10.1203/00006450-199512000-00015. [DOI] [PubMed] [Google Scholar]
- Lee KS, Lim BV, Jang MH, Shin MC, Lee TH, Kim YP, Shin HS, Cho SY, Kim H, Shin MS, Kim EH, Kim CJ. Hypothermia inhibits cell proliferation and nitric oxide synthase expression in rats. Neurosci Lett. 2002;329:53–56. doi: 10.1016/s0304-3940(02)00591-8. [DOI] [PubMed] [Google Scholar]
- Leijser LM, Klein RH, Veen S, Liauw L, Van Wezel-Meijler G. Hyperechogenicity of the thalamus and basal ganglia in very preterm infants: radiological findings and short-term neurological outcome. Neuropediatrics. 2004;35:283–289. doi: 10.1055/s-2004-830364. [DOI] [PubMed] [Google Scholar]
- Lin Y, Okumura A, Hayakawa F, Kato K, Kuno T, Watanabe K. Quantitative evaluation of thalami and basal ganglia in infants with periventricular leukomalacia. Dev Med Child Neurol. 2001;43:481–485. doi: 10.1017/s0012162201000883. [DOI] [PubMed] [Google Scholar]
- Low JA, Panagiotopoulos C, Derrick EJ. Newborn complications after intrapartum asphyxia with metabolic acidosis in the preterm fetus. Am J Obstet Gynecol. 1995;172:805–810. doi: 10.1016/0002-9378(95)90003-9. [DOI] [PubMed] [Google Scholar]
- McIntosh GH, Baghurst KI, Potter BJ, Hetzel BS. Foetal brain development in the sheep. Neuropathol Appl Neurobiol. 1979;5:103–114. doi: 10.1111/j.1365-2990.1979.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28:215–223. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- Marks KA, Mallard EC, Roberts I, Williams CE, Sirimanne ES, Johnston B, Gluckman PD, Edwards AD. Delayed vasodilation and altered oxygenation after cerebral ischemia in fetal sheep. Pediatr Res. 1996;39:48–54. doi: 10.1203/00006450-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Todd MM. Effects of hypothermia, pentobarbital, and isoflurane on postdepolarization amino acid release during complete global cerebral ischemia. Anesthesiology. 1996;85:161–168. doi: 10.1097/00000542-199607000-00022. [DOI] [PubMed] [Google Scholar]
- Nedelcu J, Klein MA, Aguzzi A, Boesiger P, Martin E. Biphasic edema after hypoxic-ischemic brain injury in neonatal rats reflects early neuronal and late glial damage. Pediatr Res. 1999;46:297–304. doi: 10.1203/00006450-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Okumura A, Watanabe K, Hayakawa F, Kato T. The timing of brain insults in preterm infants who later developed West syndrome. Neuropediatrics. 2001;32:245–249. doi: 10.1055/s-2001-19118. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics. 2003;112:33–39. doi: 10.1542/peds.112.1.33. [DOI] [PubMed] [Google Scholar]
- Paneth N, Rudelli R, Monte W, Rodriguez E, Pinto J, Kairam R, Kazam E. White matter necrosis in very low birth weight infants: neuropathologic and ultrasonographic findings in infants surviving six days or longer. J Pediatr. 1990;116:975–984. doi: 10.1016/s0022-3476(05)80664-x. [DOI] [PubMed] [Google Scholar]
- Quaedackers JS, Roelfsema V, Heineman E, Gunn AJ, Bennet L. The role of the sympathetic nervous system in post-asphyxial intestinal hypoperfusion in the preterm sheep fetus. J Physiol. 2004;557:1033–1044. doi: 10.1113/jphysiol.2004.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema V, Bennet L, George S, Wu D, Guan J, Veerman M, Gunn AJ. The window of opportunity for cerebral hypothermia and white matter injury after cerebral ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2004;24:877–886. doi: 10.1097/01.WCB.0000123904.17746.92. [DOI] [PubMed] [Google Scholar]
- Roth SC, Edwards AD, Cady EB, Delpy DT, Wyatt JS, Azzopardi D, Baudin J, Townsend J, Stewart AL, Reynolds EO. Relation between cerebral oxidative metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Dev Med Child Neurol. 1992;34:285–295. doi: 10.1111/j.1469-8749.1992.tb11432.x. [DOI] [PubMed] [Google Scholar]
- Salhab WA, Perlman JM. Severe fetal acidemia and subsequent neonatal encephalopathy in the larger premature infant. Pediatr Neurol. 2005;32:25–29. doi: 10.1016/j.pediatrneurol.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Scher MS. Controversies regarding neonatal seizure recognition. Epileptic Disord. 2002;4:139–158. [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Si QS, Nakamura Y, Kataoka K. Hypothermic suppression of microglial activation in culture: inhibition of cell proliferation and production of nitric oxide and superoxide. Neuroscience. 1997;81:223–229. doi: 10.1016/s0306-4522(97)00172-3. [DOI] [PubMed] [Google Scholar]
- Silverman WA, Fertig JW, Berger AP. The influence of the thermal environment upon the survival of newly born premature infants. Pediatrics. 1958;22:876–886. [PubMed] [Google Scholar]
- Silverstein FS, Barks JD, Hagan P, Liu XH, Ivacko J, Szaflarski J. Cytokines and perinatal brain injury. Neurochem Int. 1997;30:375–383. doi: 10.1016/s0197-0186(96)00072-1. [DOI] [PubMed] [Google Scholar]
- Solomon A, Barish RA, Browne B, Tso E. The electrocardiographic features of hypothermia. J Emerg Med. 1989;7:169–173. doi: 10.1016/0736-4679(89)90265-5. [DOI] [PubMed] [Google Scholar]
- Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, Gluckman PD. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res. 1996;39:791–797. doi: 10.1203/00006450-199605000-00008. [DOI] [PubMed] [Google Scholar]
- The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, Cooper CE, Brown GC, Edwards AD, Wyatt JS. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37:667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- Tooley JR, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol. 2003;53:65–72. doi: 10.1002/ana.10402. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Anwar M, Hegyi T, Hiatt M, Koons A, Paneth N. Antecedents and neonatal consequences of low Apgar scores in preterm newborns: a population study. Arch Pediatr Adolesc Med. 2000;154:294–300. doi: 10.1001/archpedi.154.3.294. [DOI] [PubMed] [Google Scholar]
- Welin AK, Sandberg M, Lindblom A, Arvidsson P, Nilsson UA, Kjellmer I, Mallard C. White matter injury following prolonged free radical formation in the 0.65 gestation fetal sheep brain. Pediatr Res. 2005;58:100–105. doi: 10.1203/01.PDR.0000163388.04017.26. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57. doi: 10.1016/j.brainres.2004.02.070. [DOI] [PubMed] [Google Scholar]
- Yokochi K. Thalamic lesions revealed by MR associated with periventricular leukomalacia and clinical profiles of subjects. Acta Paediatr. 1997;86:493–496. doi: 10.1111/j.1651-2227.1997.tb08919.x. [DOI] [PubMed] [Google Scholar]