Abstract
A single TMS pulse (110% resting motor threshold, RMT) to the left dorsal premotor cortex (PMd) (CS2) suppresses the amplitude of motor evoked potentials (MEPs) from a test pulse (TS) over the right motor cortex (M1), and facilitates MEPs from the left motor cortex. We probed how this interaction was changed by a prior conditioning pulse over PMd (CS1) using a paired pulse TMS design. In the main experiments, the intensity of CS1 was 80% RMT. Basal suppression of right M1 was removed when CS1–CS2 was 1 ms or 5 ms with a similar tendency at 15 ms. Basal facilitation of left M1 was suppressed at CS1–CS2 of 5 ms. A similar time course was seen if CS2 was increased to 100% RMT, but there was no significant effect if CS1 was 70% RMT. Preconditioning PMd with continuous or intermittent theta burst repetitive TMS (cTBS, iTBS) abolished the basal CS2–TS interaction between premotor and motor cortices. Finally, if very short interstimulus intervals between CS1 and CS2 were explored to detect interactions similar to I-wave facilitation in M1, we found that the basal suppression of right M1 was abolished at CS1–CS2 intervals of 1.8 and 2.8 ms. We suggest that paired pulse TMS may be capable of investigating properties of intrinsic circuits in PMd and that their properties differ from those in the nearby M1. Paired TMS may be a useful method of studying the excitability of intrinsic circuits in non-primary areas of the motor system.
Paired pulse protocols are commonly used in neurophysiological studies to examine the time course of interactions within a neural circuit. In particular, they have been used successfully in experiments employing transcranial magnetic stimulation (TMS) to examine the excitability of circuits intrinsic to the cerebral cortex itself. The most well known of the paradigms is that of Kujirai et al. (1993) in which a subthreshold conditioning stimulus (S1) is applied to the M1 at different times before a suprathreshold test stimulus (S2). When the interval between S1 and S2 is 1–4 ms, then the EMG response evoked by S2 is suppressed, whereas if the interval is 8–20 ms, it is facilitated (Kujirai et al. 1993; Ridding et al. 1995; Ziemann et al. 1996; Rothwell, 1997; Roshan et al. 2003; Chen, 2004). Since the intensity of S1 is too small to evoke any output from M1 (Kujirai et al. 1993; Nakamura et al. 1997; Di Lazzaro et al. 1998; Chen et al. 1998), the interaction between S1 and S2 must be caused by activity in intrinsic cortical connections. This has given rise to the terms short interval intracortical inhibition (SICI) to describe initial inhibition and intracortical facilitation (ICF) to describe the later facilitation. Later investigations added to the range of interactions that could be studied with this method by showing that by varying the interstimulus interval (ISI) and stimulus intensities of S1 and S2 it was possible to explore other intracortical systems such as those responsible for the facilitatory I-wave interaction (short intracortical facilitation, SICF) and long interval intracortical inhibition (LICI) (Valls-Sole et al. 1992; Wassermann et al. 1996; Ziemann et al. 1998; Hanajima et al. 2002).
Recent studies have used the same approach to show that similar interactions occur in other cortical areas. For example, Oliveri et al. (2000) examined the interaction between pairs of pulses over the right posterior parietal cortex. A single suprathreshold TMS pulse (S2) can, if correctly timed, suppress perception of weak somatosensory stimuli applied to the opposite hand. This effect can be modulated by a preceding subthreshold stimulus (S1). Thus, tactile suppression is increased if the S1–S2 interval is 1 ms whereas there is less suppression if the interval is 5 ms. Paired TMS applied over the same parietal area is also able to modulate visual awareness, again with a similar temporal profile of inhibition at S1–S2 intervals of 5 ms (Koch et al. 2005). In contrast, when the stimuli are given over the primary somatosensory cortex, there is no interaction between S1 and S1 at short intervals, although suppression of tactile perception is facilitated when S1–S2 is 10 or 15 ms (Koch et al. 2006a). In an experiment on the primary visual cortex, Sparing et al. (2005) found that phosphenes elicited by single TMS pulse were facilitated if a smaller conditioning pulse was applied 2–12 ms earlier. The conclusion from such studies is that interactions between pairs of TMS pulses can be demonstrated in many areas of cerebral cortex, but that the time course of the effects differs from that in the M1. This implies that there are differences in the organization or excitability of neural circuits in different areas of cortex.
The aim of the present study was to test whether a similar approach could be used to study intracortical interactions within the dorsal premotor cortex (PMd). To do this we made use of the fact that effects of a single TMS pulse over PMd can be monitored indirectly by its effect on excitability of primary motor cortex (M1). Thus, Civardi et al. (2001) found that a low intensity (90% AMT) stimulus to PMd reduced the amplitude of MEPs evoked by a test pulse over M1 if the interstimulus interval was 6 ms. If the intensity of the PMd stimulus was increased to 110–120% AMT, the effect became facilitatory. A stimulus over PMd also has effects on the excitability of the contralateral M1 that are in some respects the mirror image of those seen by Civardi et al. (2001) in the ipsilateral cortex. Thus, Baumer et al. (2006) and Mochizuki et al. (2004) showed that a low intensity conditioning stimulus (80% AMT) facilitated contralateral MEPs at an ISI of 8 ms, whereas a higher intensity stimulus (90–110% RMT) at ISI = 8–10 ms was inhibitory.
In the present experiments we gave a low intensity conditioning stimulus (CS1) to PMd that on its own had no effect on excitability of M1 and tested its effect on the ipsi- and contralateral paired pulse interactions between PMd (CS2) and M1 that were evoked by stimuli at 110% RMT. The effects observed on these ‘basal’ ipsilateral or transcallosal interactions when the CS2 was preceded by CS1 at different ISIs were then assumed to reflect changes in the intracortical excitability of PMd itself. This paradigm was then tested following the application of repetitive (rTMS) trains, with the recently developed theta burst stimulation (TBS) protocols (Huang et al. 2005). Finally it should be noted that we specifically examined these interactions in the left PMd, which is known to have a larger role in movements of either hand than the right PMd (Rushworth et al. 2003).
Methods
Subjects
Twenty-one healthy volunteers (10 men and 11 women, 22–35 years old) participated in this study. All subjects were right handed based on the Edinburgh Handedness Inventory. Written informed consent was obtained from all subjects. The experimental procedures used here were approved by the local Ethics Committee and were carried out in accordance with the Declaration of Helsinki.
Transcranial magnetic stimulation
Motor-evoked potentials were recorded bilaterally from the first dorsal interosseous (FDI) muscles using 9 mm diameter, Ag–AgCl surface cup electrodes. The active electrode was placed over the muscle belly and the reference electrode over the metacarpophalangeal joint of the index finger. Responses were amplified with a Digitimer D360 amplifier (Digitimer Ltd, Welwyn Garden City, Herts, UK) through filters set at 20 Hz and 2 kHz, then recorded by a computer using SIGNAL software (Cambridge Electronic Design, Cambridge, UK) with a sampling rate of 5 kHz per channel.
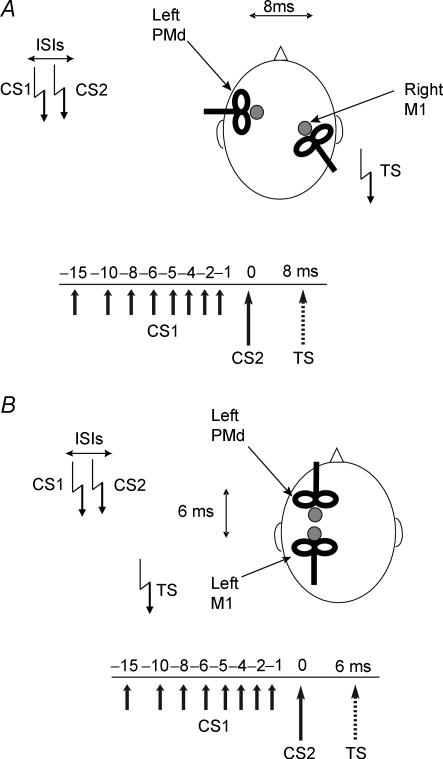
Experiment 1: effects on interhemispheric interaction between left PMd and right M1 (Fig. 1)
Figure 1. Schematic representation of the experimental procedure used in experiments 1–4.
Paired pulse stimulation was applied at different interstimulus intervals (ISIs) over the left PMd. The first conditioning TMS pulse (CS1) was delivered at 80% of RMT to left PMd while the second conditioning TMS pulse (CS2) was applied at suprathreshold intensity (110% RMT). In the main experiments, the CS intensity was adjusted to be 80 or 110% of resting motor threshold (RMT) as defined for the M1 hand area of the same hemisphere. The test TMS pulse (TS) was given over right M1 after a fixed delay of 8 ms from CS2 over the contralateral PMd cortex (A), or over left M1 after 6 ms from CS2 over the ipsilateral PMd cortex (B). The orientation of the coil positioned over left PMd varied in the two experiments due to space considerations.
Twelve subjects took part in this experiment. We used a triple pulse stimulation technique with three high-power Magstim 200 machines (Magstim Co., Whitland, Dyfed, UK) to test how a low intensity conditioning stimulus affected the interhemispheric interaction between left PMd and right motor cortex (M1). The magnetic stimuli had a nearly monophasic pulse configuration, with a rise time of 100 μs, decaying back to zero over 0.8 ms. Baseline interhemispheric interactions were examined with an ISI of 8 ms between PMd and M1; the intensity of the conditioning stimulus over PMd (CS2) was set at 110% RMT, and the intensity of the test stimulus (TS) over M1 was adjusted to produce an MEP in the resting contralateral FDI muscle of about 1 mV peak to peak amplitude. This interaction was conditioned by a low intensity conditioning pulse at 80% RMT (CS1) over PMd that preceded CS2 by ISI = 1, 2, 4, 5, 6, 8, 10 and 15 ms. RMT was defined as the lowest intensity that evoked five small responses (about 50 μV) in the contralateral FDI muscle in a series of 10 stimuli when the subject kept the FDI muscles relaxed in both hands (Rossini et al. 1994). The conditioning stimulator was connected to a small custom-made figure-of-eight-shaped coil (external diameter 5.5 cm) in order to reduce the effective area of stimulation, and the test stimulator was connected to a standard, larger, figure-of-eight-shaped coil (external diameter 7 cm). A randomized conditioning-test design was used. Various conditions (the test stimulus given alone, or the test stimulus preceded by the single conditioning stimulus or paired conditioning pulses at various ISIs) were intermixed randomly in one session. In each session 10 conditions were randomly intermingled: TS alone (MEP); CS2 + TS (conditioned MEP); CS1 + CS2 + TS (paired conditioned MEP for each of eight different ISIs). Ten responses were collected for the paired conditioned MEP for each ISI, 20 responses for the conditioned MEP and 20 for the test stimulus alone. A total number of 120 trials were performed in each session. The intertrial interval was set at 5 s (± 10%), for a total duration of 10 min. Measurements were made on each individual trial. The mean peak-to-peak amplitude of the conditioned MEP and of the paired conditioned MEP at each ISI was expressed as a percentage of the mean peak-to-peak amplitude of the unconditioned test pulse in that block.
The coil position for premotor TMS was defined relative to the position of the motor hot spot for the FDI. A positron emission tomographic (PET) study showed that the dorsal premotor cortex is located ∼2 cm anterior to the M1 hand area (Fink et al. 1997). To minimize M1 activation during premotor TMS, we calculated for each subject 8% of the distance between the nasion and inion (typically ∼3 cm) and defined the premotor area as this distance anterior to the hot spot of the M1 hand area (Munchau et al. 2002; Rizzo et al. 2004; Koch et al. 2006b). The coil was held with the handle pointing laterally so as to induce a medially directed current in the stimulated cortex (experiment 1). In their previous study, Mochizuki et al. (2004) showed that different orientations did not induce dissimilar effects. We chose this orientation since it allowed a more comfortable experimental setting.
Experiment 2: effect of CS1 intensity
In two separate sessions we tested the effect of a lower intensity of 70% RMT and a higher of 100% RMT using the same protocol as in the main experiment. Eight subjects participated in this experiment. Paired pulse stimulation was applied at different inter stimulus intervals (ISI = 1, 2, 4, 5, 6, 8, 10 and 15 ms) over the left PMd. The first conditioning TMS pulse (CS1) was followed by a second conditioning TMS pulse (CS2) at suprathreshold intensity (110% RMT); the test TMS pulse (TS) was given over right M1 after a fixed delay of 8 ms following CS2 over the left PMd cortex (experiment 2).
Experiment 3: control experiment to test the effect of CS1 alone
To test possible transcallosal effects of CS1 alone on the amplitude of the test MEP we performed another control experiment. In eight subjects CS1 was delivered at different intensities (70 or 80% RMT) over the left PMd at ISIs of 8, 13 and 25 ms before the test TMS pulse over right M1. We did not test the effects of CS1 alone at 9 ms since this was relative to the 1 ms CS1–CS2 interactions. At this interval, any interpretation of the interactions is difficult because of potential refractoriness of neurones activated by CS1 when CS2 was applied. In each session four conditions were randomly intermingled: TS alone (MEP) and CS1 + TS (conditioned MEP) for different ISIs of 8, 13 and 25 ms. Ten responses were collected for conditioned MEPs at each ISI and 20 for test stimulus alone. A total number of 50 trials were performed for each session. Two sessions were run for each subject with CS1 70% or 80% RMT. The order of presentation was randomized across subjects.
Experiment 4: control experiment to test the site of interaction between pairs of PMd conditioning stimuli: CS1 applied over PMd; CS2 and TS applied over M1
Although the interaction between CS1 and CS2 seems likely to take place in PMd, it is possible that CS1 produces a volley to M1 that then interacts with a volley evoked by CS2 and changes its effect on TS. To test for this possibility we performed a further control experiment in six subjects. As in experiment 1, we applied CS2 at 110% RMT over the left PMd, 8 ms before a TS was applied over the contralateral M1. However, in the new experiment, CS1 was given over M1. The timing of CS1 was adjusted by 8 ms to take account of the conduction time from PMd to M1 (see time line in Fig. 3C). Thus, CS2 occurred first over PMd; CS1 was given 3 ms later over M1; TS was given 5 ms after CS1. Ten responses were collected for the conditioned MEP for each condition (CS1 right M1 + TS right M1; CS2 left PMd+ TS right M1; CS1 right M1 + CS2 left PMd + TS right M1) and 20 for TS alone. A total number of 50 trials was performed for each session.
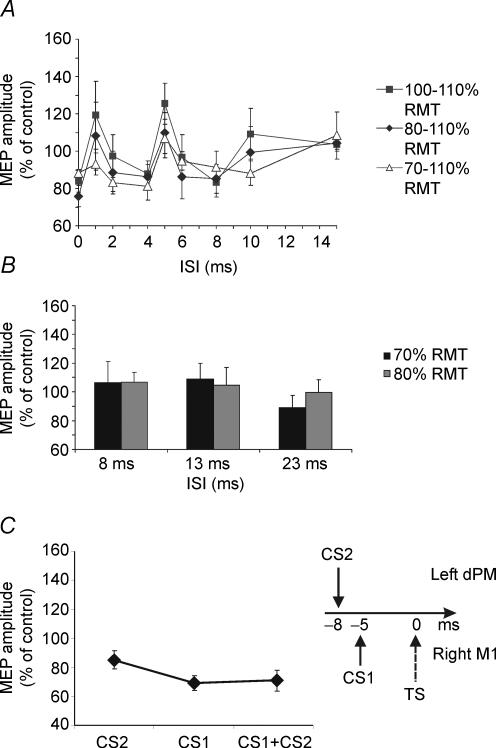
Figure 3.
A, effects of different intensities of the CS1 while the CS2 was fixed at supra-threshold intensity (110% RMT). Similar profiles were obtained when the CS1 intensity is increased to 100% of RMT in comparison with the intensity of 80% RMT. Conversely, no significant effect was observed when the intensity was 70% of RMT, although a trend towards a similar profile is found. Error bars represent s.e.m.B, when the subthreshold CS1 was not followed by the CS2, no effect was found on the MEP evoked by the contralateral M1, for both intensities of 70% (black bars) and 80% (grey bars) of RMT. C, when CS1 at 80% RMT was applied over right M1 and not left PMd at adjusted timing (corresponding to 5 ms ISI), CS1 did not induce any inhibition of the effects of CS2 applied over left PMd as in experiment 1, but instead induced significant further inhibition of MEP evoked from right M1. Error bars represent s.e.m.
Experiment 5: effects on the interaction between PMd and M1 within the left hemisphere (intrahemispheric interaction)
The same protocol was tested for ipsilateral PMd–M1 connectivity within the left hemisphere of 11 subjects. Paired pulse stimulation was applied at different interstimulus intervals (ISIs) over the left PMd. The first conditioning TMS pulse (CS1) was delivered at 80% RMT to left PMd while the second conditioning TMS pulse (CS2) was applied at suprathreshold intensity (110% RMT); the test TMS pulse (TS) was given over the left M1 6 ms after CS2 over the PMd cortex as described by Civardi et al. (2001). In this experiment two small figure-of-eight coils (5.5 cm) were used. Because of space considerations, the conditioning coil was orientated with the handle pointing forward to induce anterior to posterior (AP) currents in the underlying PMd, while the TS coil had an opposite orientation with the handle pointing backward inducing a posterior to anterior (PA) current in M1. The coil position for TMS over PMd was adjusted relative to the position of the hot spot for the FDI as in experiment 1 (approximately 3 cm anterior to the motor hot spot). In each session 10 conditions were randomly intermingled: TS alone (MEP); CS2 + TS (conditioned MEP); CS1 + CS2 + TS (paired conditioned MEP at eight different ISIs). Ten responses were collected for paired conditioned MEP at each ISI, 20 responses for conditioned MEP and 20 for test stimulus alone. A total number of 120 trials were recorded.
Experiment 6: effects of rTMS on connectivity between left PMd and right M1
The effects of repetitive TMS (rTMS) on PMd intracortical circuits were tested in this experiment. We adopted the recently developed theta burst TMS, to induce focal long lasting modulation of PMd cortical excitability. Three-pulse bursts at 50 Hz repeated every 200 ms for 20 s (equivalent to ‘continuous theta burst stimulation’ (cTBS) in Huang et al. 2005) were delivered at 80% AMT over left PMd (300 pulses) (n = 9). In the intermittent theta burst stimulation pattern (iTBS), a 2 s train of TBS is repeated every 10 s for a total of 90 s (300 pulses) (n = 8). Immediately after the rTMS session, subjects were tested with the same protocol as in experiment 1.
Experiment 7: possible short interval facilitation between CS1 and CS2
A protocol for short intracortical facilitation (SICF) was used in this experiment in 10 subjects. In this protocol a suprathreshold (110% RMT) CS1 was followed by a second CS2 at different intensities (ranging from 80 to 110% RMT), in order to mimic the SICF procedure used to test I wave interaction in M1 (Ziemann et al. 1998; Ilic et al. 2002). Paired pulse stimulation was applied at different ISIs over the left PMd (1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, 3.0 ms). The first conditioning TMS pulse (CS1) was delivered at 110% RMT to left PMd while the second conditioning TMS pulse (CS2) was applied at different intensities (80% RMT, 100% RMT, 110% RMT) in different blocks. The CS intensity was adjusted to be between 80 or 110% of RMT as defined for the M1 hand area of the same hemisphere. The test TS was given over right M1 after a fixed delay of 8 ms following CS1 over the left PMd cortex. Ten responses were collected for paired conditioned MEP for each ISI, 20 responses for conditioned MEP and 20 for test stimulus alone. A total number of 150 trials were recorded in each block.
Statistical analysis
The effects of single and paired stimulation of the left PMd on the size of MEPs evoked from right or left M1 were expressed as a percentage of the mean peak-to-peak amplitude of the unconditioned test pulse. In experiment 1, the mean percentage values were analysed with a repeated measures analyses of variance (ANOVA) with paired TMS CONDITION (MEP conditioned by CS2 alone, or with CS1-CS2 ISI = 1,2,4,5,6,8,10, 15 ms) as within-subjects main factor. The same analysis was conducted for experiments 2 and 5. In experiment 3 an ANOVA with paired TMS CONDITION (conditioned MEP at 8, 13 or 25 ms) was performed on the mean percentage for each condition in respect of the of the mean peak-to-peak amplitude of the unconditioned test pulse. In experiment 4 an ANOVA with paired TMS CONDITION (CS2 + TS versus CS1 + TS versus CS1 + CS2) was performed on the mean percentage for each condition in respect of the mean peak-to-peak amplitude of the unconditioned test pulse. In experiment 6 the TBS effects on PMd intracortical circuits were analysed through two different ANOVAs for each protocol (iTBS or cTBS) with rTMS (basal versus post TBS) and ISI (CS1-CS2 ISI = 1, 2, 4, 5, 6, 8, 10, 15 ms) as main factors. In experiment 7 the mean percentage values were analysed using different ANOVAs with paired TMS CONDITION (1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, 3.0 ms) as within-subjects main factor for each intensity of the CS2.
When a significant main effect was reached, post hoc tests with Bonferroni's correction were employed to characterize the different effects of the specific ISIs. For all statistical analyses, a P-value of < 0.05 was considered to be significant. Mauchley's test examined for sphericity. The Greenhouse-Geisser correction was used for non-spherical data.
Results
The mean RMT of the left M1 was 42 ± 4.8% of maximal stimulator output. The procedure was well tolerated by all subjects.
Effects of single and paired pulse conditioning of the left PMd at different ISIs on MEPs evoked from right M1 (experiment 1 and 2)
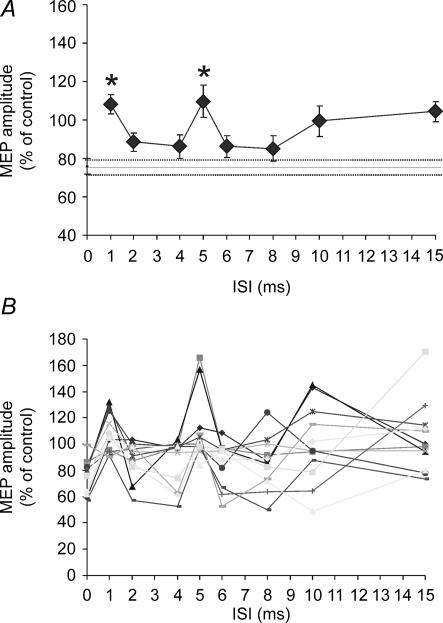
Confirming the results obtained by Mochizuki et al. (2004) who used the same parameters, we found that CS2 alone (110% RMT) reduced MEPs evoked by the test shock to 75.9 ± 12.8% of their control size. This baseline effect was modulated by a preceding CS1 (F1,11 = 5.44; P = 0.0001) depending on the interval between CS1 and CS2. In comparison with the effect of the single CS2, post hoc analysis revealed a significant reduction at ISI = 1 (P = 0.005) and 5 ms (P = 0.01), with a trend also for a slight reduction at ISI = 15 ms (uncorrected Student's paired t test, P = 0.003; Bonferroni correction P = 0.09) (Fig. 2).
Figure 2. Effects of single and paired pulse TMS of the left PMd on the excitability of contralateral M1.
A single CS2 induced an inhibition of approximately 20% in the amplitude of the MEP evoked by contralateral M1 stimulation (depicted as a continuous line on the graph with the dotted line representing the s.e.m.). Paired pulse stimulation of left PMd modulated the interhemispheric inhibition towards the contralateral M1 depending on the ISI between the subthreshold CS1 and the supra-threshold CS2 conditioning stimuli. Intracortical inhibition was found for the ISIs of 1 ms and 5 ms. A, group result for the effects of the single CS2 and the paired pulse at different ISIs. B, individual results. Each line represent a single subject (n = 12). Error bars represent s.e.m. Asterisks indicate P-values < 0.05.
This pattern was repeated in experiment 2 when the CS1 intensity was increased to 100% RMT (F1,7 = 3.14; P < 0.05). Again, compared with the effect of CS2 alone, post hoc analysis revealed a significant reduction of inhibition for an ISI of 5 ms (P = 0.01) (Fig. 3A). There was no significant effect if the intensity of CS1 was reduced to 70% RMT, although there was a trend towards a similar temporal profile (F1,7 = 1.82; P = 0.09) (Fig. 3A).
Effects of CS1 alone (left PMd) on MEPs from right M1 (experiment 3)
When CS1 was given alone at intervals that had previously reduced the effect of CS2 it had no influence on the amplitude of MEPs evoked from right M1. These intervals were 13 ms (= 5 ms between the two CSs, + 8 ms between the CS2 and the TS) and 23 ms (= 15 ms between the two CSs, + 8 ms between the CS2 and the TS) (Fig. 3B). This was true whether the intensity of CS1 was 70% (F1,7 = 1.15; n.s) or 80% of RMT (ANOVA F1,7 = 0.39; n.s) analysed.
Effects of CS applied over left PMd on paired pulses applied over right M1 (experiment 4)
There was a significant effect of condition when CS1 was given with adjusted timing over right M1 in comparison with the effects induced by CS2 alone and when paired pulses were applied with 5 ms ISI on right M1 (ANOVA F1,5 = 4,94; P < 0.05). Post hoc analysis showed that CS1 did not suppress the effects of CS2 applied over left PMd as in experiment 1, but instead induced significant further inhibition of the MEP evoked from right M1 (CS2 + TS: 85.3 ± 15.1% versus CS1 + CS2 + TS: 69.3 ± 12.7%; P < 0.05). Indeed, when CS1 alone preceded TS by 5 ms there was stronger inhibition than when CS2 was applied over left PMd (CS2 + TS: 85.3 ± 15.1% versus CS1 + TS: 70.9 ± 15.5%; P < 0.05) (Fig. 3C).
Effects of single and paired pulse conditioning of the left PMd on MEPs evoked from left M1 (experiment 5)
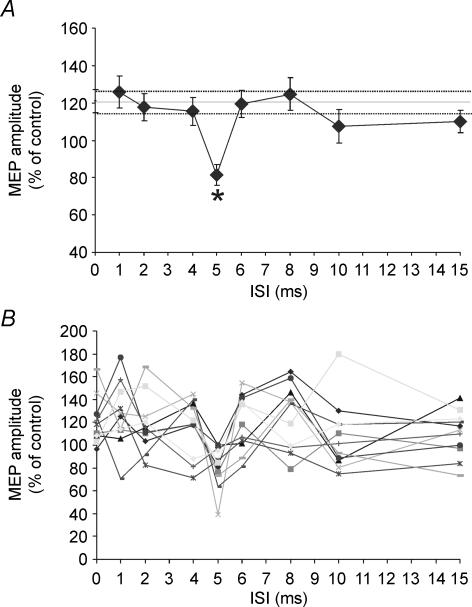
As reported by Civardi et al. (2001), a single supra-threshold CS2 facilitated MEPs to 120.1 ± 18.8% of their control values. Consistent with the data of experiment 1, application of CS1 could suppress this effect if the ISI = 5 ms (F1,10 = 3.55; P < 0.001; P < 0.001 at post hoc analysis in comparison with single conditioned MEP) (Fig. 4). Although the data suggest that the facilitation may even have reversed to inhibition, this was not significant as revealed by paired t test analysis on mean MEP amplitude values (TS: 1.05 ± 0.52 mV; CS1 + CS2 at 5 ms ISI: 0.91 ± 0.55 mV; n.s.). There was no effect of CS1 at ISI = 1 ms.
Figure 4. Effects of single and paired pulse TMS of the left PMd on the ipsilateral M1.
A single CS2 induced a facilitation of approximately 20% on the amplitude of the MEP evoked by ipsilateral M1 stimulation (depicted as a continuous line on the graph with the dotted line representing the s.e.m.). Paired pulse stimulation of left PMd suppressed the facilitation towards the ipsilateral M1 depending on the ISI between the subthreshold CS1 and the supra-threshold CS2 conditioning stimuli. Intracortical inhibition was found for the ISIs of 5 ms. A, group result for the effects of the single CS2 and the paired pulse at different ISIs. B, individual results. Each line represents a single subject (n = 11). Error bars represent s.e.m. Asterisks indicate P-values < 0.05.
Effects of cTBS and iTBS of the left PMd on single and paired pulse conditioning on the amplitude of MEPs evoked from right M1 (experiment 6)
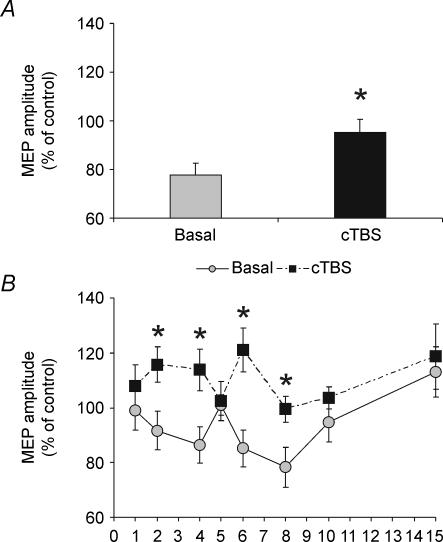
The usual inhibitory effect of CS2 alone was abolished immediately after application of cTBS (Student's paired t test, t = 3.1; P = 0.01). cTBS also changed the time course of the CS1–CS2 interaction. A two factor ANOVA revealed a significant effect of the main factor rTMS (F1,8 = 12.68; P < 0.001) and an interaction rTMS × ISI (F = 2.41; P < 0.05). Post hoc paired t tests showed significant differences for ISIs of 2 ms (t = 2.5; P = 0.036), 4 ms (t = 3.2; P = 0.012), 6 ms (t = 5.2; P = 0.001) and 8 ms (t = 2.3; P = 0.045) (Fig. 5).
Figure 5. Effects of cTBS on single (A) and paired pulse (B) conditioning of the left PMd on the contralateral M1 excitability.
cTBS suppressed the transcallosal inihibition induced by the single CS2 (A) and was effective in modulating the interaction between paired of stimuli applied on PMd (B).
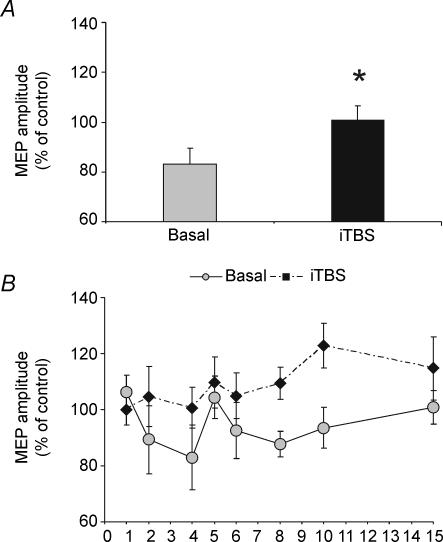
Immediately after iTBS of left PMd, a single CS applied over the same area at 110% RMT intensity failed to induce consistent inhibition of the contralateral M1 (Student's paired t test, t = 3.0; P = 0.04). Although the effect of iTBS on the time course of CS1–CS2 interaction appeared similar to that after cTBS, the ANOVA in this instance failed to detect any significant rTMS–ISI interaction (Fig. 6). We were unable to conduct a detailed comparison between the effects of iTBS and cTBS since not all the subjects were the same for each intervention.
Figure 6.
iTBS suppressed the transcallosal inihibition induced by the single CS2 (A) but did not significantly modify the interaction between paired of stimuli applied on PMd (B).
Effects of paired pulses over the left PMd at very short ISIs on MEPs evoked from left M1 (experiment 7)
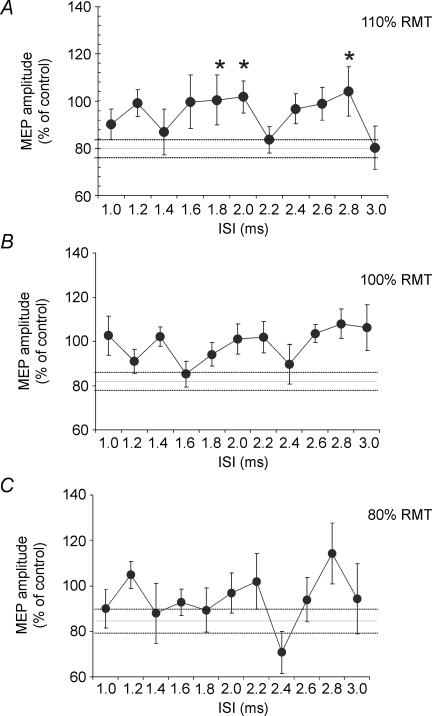
When a suprathreshold CS1 was followed by CS2 at the same intensity (110% RMT) using very short ISIs ranging from 1 to 3 ms, we observed a specific time course of the combined effects on left M1 (F = 2.43; P < 0.05). Paired t tests showed that the effects of paired TMS differed from those using a single CS2 for ISI = 1.8 ms (P = 0.009), 2.0 ms (P = 0.007) and 2.8 ms (P = 0.04). No significant effect was found when the intensity of CS2 was reduced to 100% RMT or 80% RMT (Fig. 7).
Figure 7. Short interval intracortical facilitation (SICF) in PMd.
In this protocol a suprathreshold (110% RMT) CS1 was followed by a second CS2 at different intensities (ranging from 80 to 110% RMT), in order to mimic the SICF procedure used to test I wave interaction in M1 (Ziemann et al. 1998; Ilic et al. 2002). Paired pulse stimulation was applied at different interstimulus intervals (ISIs) over the left PMd (1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, 3.0 ms). The first conditioning TMS pulse (CS1) was delivered at 110% RMT to left PMd while the second conditioning TMS pulse (CS2) was applied at different intensities (110% RMT, 100% RMT, 80% RMT) in different blocks. The test TS was given over right M1 after a fixed delay of 8 ms following CS1 over the left PMd cortex. Significant interactions between CS1 and CS2 were found when the intensity of CS2 was set at 110% RMT (A) at ISIs of 1.8 ms, 2.0 ms and 2.8 ms, while no significant effect was found for lower CS2 intensities of 100% RMT (B) and 80% RMT (C). Error bars represent s.e.m. Asterisks indicate P-values < 0.05.
Discussion
The main finding of the current study is that paired pulse interaction between PMd (CS2) and ipsilateral or contralateral M1 is modulated by a preceding conditioning pulse (CS1) over PMd. We argue below that this effect may have been due to CS1–CS2 interaction within the PMd, and hence may be a way of testing intracortical connectivity in a secondary motor area of cortex. The results also showed that the interhemispheric interaction was abolished by a period of cTBS over PMd, which is reminiscent of the effect of cTBS over M1 on MEPs and SICI.
The experiments focused on the effects of a CS2 using an intensity of 110% RMT, which facilitates ipsilateral and suppresses contralateral M1. Opposite effects can be obtained by using smaller intensities of CS2 (90% or 80% AMT for ipsilateral and contralateral projections, respectively) but these are more variable than the effects with a larger CS2 (Civardi et al. 2001; Baumer et al. 2006). Since the variance of the MEP data is likely to increase with increasing numbers of conditioning pulses, we focused on the higher intensity in order to keep the variance of the data as low as possible.
It should also be noted that all experiments involved stimulation of left PMd. Since it is known that the functions of left and right PMd differ, with left PMd having a greater influence on movement selection in both left and right hands (Rushworth et al. 2003), it may well be that CS1–CS2 interactions may differ in the right PMd. These need to be examined in future experiments.
Evidence supporting the hypothesis that interaction takes place in PMd
Previous papers have provided strong evidence that the basal PMd–M1 interaction in the present experiments is likely to take place in the M1, implying that CS2 activates outputs to M1 that interact with the test pulse that evokes the MEP (Civardi et al. 2001; Mochizuki et al. 2004; Baumer et al. 2006; Koch et al. 2006b). In the present experiments, the question arises as to the location of the CS1–CS2 interaction even though both stimuli were applied over the same point in PMd.
The main evidence in favour of an interaction in PMd is that CS1 alone, at intervals equivalent to those at which significant interaction occurred with CS2, had no effect on the response of M1. However, the argument is not water-tight: it can be argued that CS1 activated an input to M1 that had no effect on the response to a M1 test pulse, but nevertheless suppressed the circuits recruited by input from PMd evoked by CS2. In this case, the CS1–CS2 interaction would have occurred within the M1 even though the two stimuli were applied over PMd. We tried to test this hypothesis in a further control experiment (experiment 4) where we assumed that the CS1–CS2 interaction occurred within right M1. When CS1 was applied with adjusted timing (corresponding to 5 ms ISI) over M1 rather than PMd, it did not inhibit the effects of CS2 applied over left PMd. In fact there was a trend toward stronger inhibition of M1 excitability, perhaps reflecting activation of SICI circuits (Kujirai et al. 1993) by CS1.
There is one other piece of evidence that suggests that CS1–CS2 interaction occurred within left PMd and not within right M1. When we used a range of CS1 intensities, we found that all of them had the same time course of effects on CS2. Since we know that single PMd stimuli of intensities in the same range (70–100% RMT) evoke opposite effects on M1 (ipsilateral inhibition/contralateral facilitation at low intensities and ipsilateral facilitation/contralateral inhibition at higher intensities (see Introduction)), it seems unlikely that they would have maintained the same temporal profile of CS1–CS2 interaction. In fact, we observed that CS1 suppressed the effect of CS2 at an ISI of 5 ms whether we tested the effect of PMd on left or right M1. Since the timing between CS2 and TS was 6 ms for left M1 and 8 ms for right M1, we may argue that the delay between the CS1 and the CS2, but not between the CS2 and the TS was critical in inducing the specific effect. We suggest therefore that the most likely location of CS1–CS2 interaction is within the intrinsic circuits of PMd.
The location of the interaction between the pairs of pulses used in experiment 6, in which the intensities of CS1 and CS2 were equal (110% RMT), is less certain. Clearly, there could have been interaction both within PMd and M1. However, the time course of the interaction is quite rapid with peaks occurring at around 1.2, 2 and 2.8 ms (although the peak at 1.2 ms was not statistically significant). The equivalent rapid interaction between I-waves in M1 (SICF) has peaks at approx. 1.3, 2.8 and 4.4 ms. Thus it may be more likely that the interaction is within PMd rather than M1.
Time course of CS1–CS2 interaction in PMd
There have now been many reports of paired pulse interactions in various areas of the cerebral cortex, and it appears that their time course differs according to the stimulus intensities used and the area of cortex that is tested. In the M1, the time course of paired pulse interaction between a small CS1 and a later test pulse has a characteristic early phase of inhibition at ISIs of 1–5 ms and a later facilitation at ISIs > 5 ms (SICI/ICF). However, the time course differs if the intensity of the pulses is changed: for example, facilitatory I-wave interactions are observed at very short intervals (1–3 ms, SICF) when CS1 and CS2 are around 100% RMT. This is thought to reflect the fact that different circuits are activated by different intensities of pulse and that each has a particular time course of synaptic interaction. Indeed, as noted in the Introduction, the time course of paired pulse interaction is different in parietal and visual cortex.
In PMd we found that CS1 suppressed the effect of CS2 on both the contralateral and ipsilateral M1 when the ISI was 5 ms. However, there was additional inhibition of the contralateral projection at ISI = 1 ms with a tendency for inhibition at ISI = 15 ms. The implication is that even though the intensity of CS2 was the same, the effects that it produces on contralateral and ipsilateral M1 are not mediated by exactly the same sets of neurons. It could be, for example, that the projection involved in the effect at 5 ms is the same for both ipsilateral and contralateral effects, whereas the projection involved in the contralateral effects at 1 and 15 ms is not present ipsilaterally. Alternatively it is possible that the difference observed may depend on different coil orientation used in the two experiments. Similarly to what has been observed in the M1 (Hanajima et al. 1998, 2002) different coil orientations may activate preferentially different intracortical circuits. For instance, the 5 ms circuit would be more susceptible to cortical excitability changes than the 1 ms circuit, irrespective of whether the orientation of the coil is latero-medial or anterior to posterior directed.
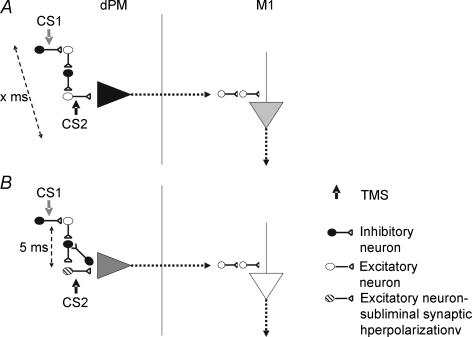
It is less clear why the timings of the interaction are so specific, particularly at 5 ms, in all individuals tested. Interestingly a similar specific effect at an interstimulus interval of 5 ms was found for paired pulse interactions in posterior parietal cortex where the presence of the conditioning stimulus reduced the usual amount of somatosensory or visual suppression seen after a single pulse (Oliveri et al. 2000; Koch et al. 2005, 2006a). It may be that this reflects the resonant frequency of an inhibitory circuit in certain cortical areas (Fig. 8), but until more information is available, further speculation about mechanism is not justified. The effect observed at CS1–CS2 of 1 ms is difficult to interpret since it may involve refractoriness of neurons activated by CS1, at least in the projection from left PMd to right M1.
Figure 8. Hypothetical mechanism for the specific intracortical inhibition in PMd.
The CS2 activates the CS2 the initial segment of the excitatory interneurons responsible for the activation of the pyramidal output neurons. The projection originating from the pyramidal neurons likely interacts with the I waves system in the primary motor cortex (A). The subthreshold CS1 activates an oligosynaptic circuit of inhibitory and excitatory interneurons that present similar characteristics of tight synchronization, inducing IPSPs at the excitatory interneurons that lead to a reduced number of action potentials by the subsequent suprathreshold CS2. This pathway is ineffective and does not alter the CS2 (A) unless a specific ISI of 5 ms occurs (B). Intracortical inhibition may be observed only when the excitatory interneuron activated by the CS2 is in a state of hyperpolarization due to IPSPs resulting from the activation of the inhibitory pathway by the CS1 (B). The circuit responsible for ICI in PMd may be comprise a chain of three or four inhibitory and excitatory interneurons that present very high synchronization of discharge.
Effect of preconditioning with TBS
In M1, cTBS suppresses corticospinal output and reduces SICI whereas iTBS has the opposite effect. In contrast, both forms of conditioning stimulation had very similar effects on PMd. In particular, both of them abolished the baseline suppression of contralateral M1 by CS2. There may also have been a reduction in CS1–CS2 interaction, but since this could have been due to a ‘floor’ effect caused by the absence of the baseline PMd–MCx interaction we cannot comment further. The difference with the effects of TBS over M1 would be consistent with the hypothesis that the interaction between CS1 and CS2 did not occur in M1.
The effect of cTBS on PMd is similar to its effect on M1 where it reduces the cortical output produced by a test TMS pulse. Thus it may be that after cTBS to PMd the output normally evoked by CS2 is reduced and hence its baseline effect on M1 is smaller than expected or even abolished. It is not clear why iTBS had the same effect on PMd as cTBS whereas it has opposite effects on the output from M1. Presumably, like the time course of CS1–CS2 interaction, this is another aspect of the cortical circuitry that differs between the two areas.
Short interval interactions between CS1–CS2 over PMd
We applied equal intensity CS1–CS2 over PMd at very short intervals to test whether there might be circuits equivalent to the I-wave generating circuits that can be observed in M1 using the SICF technique. The data revealed interactions at short intervals, although the frequency was faster than that over M1 (approx. peaks of interaction every 0.8–1 ms, compared with every 1.5 ms over M1). As noted above, we cannot be certain that the PMd interaction occurred within PMd itself, but the difference in frequency from that in M1 suggests that this is the case.
It should be noted that the interaction reduced rather than reinforced the usual PMd suppression of M1, perhaps indicating that the CS1–CS2 interaction suppressed inhibitory output from PMd. If so, then this would be opposite to what occurs in M1, where I-wave interaction facilitates corticospinal output. One possible explanation might relate to the fact that a CS over PMd recruits a mixture of excitatory and inhibitory connections from PMd to contralateral M1; facilitation occurs at low intensities, whereas suppression is seen at high intensities (Mochizuki et al. 2004; Baumer et al. 2006; Koch et al. 2006b). In the present experiments we used relatively high intensities that would recruit mainly inhibitory effects but also facilitatory interhemispheric projections. Perhaps the reduction in suppression that occurred every 0.8 ms was due to interactions in the facilitatory interhemispheric projections that cancelled out the usually stronger inhibitory effects recruited at high intensities of CS.
Anatomo-functional differences between M1 and PMd
It is well known that primary motor and premotor cortices differ between human and non-human primates in both anatomical and functional features (Barbas & Pandya, 1987; Matelli et al. 1991; Johnson et al. 1996; Rizzolatti & Luppino, 2001; Picard & Strick, 2001; Dum & Strick, 2005; Chouinard & Paus, 2006). There is general agreement that while M1 plays a major role in generation of segmented distal movements, PMd is involved in different complex functions such as selecting motor programmes based on sensory information, or in decisional processes that depend on previously learned arbitrary associations (see Wise et al. 1997 for a review). Classically, the main cytoarchitectonic features of M1 are (1) presence of giant pyramidal cells organized in multiple rows, (2) columnar pattern extending from the white matter to the superficial layers, and (3) low cellular density in the lower part of layer III. Conversely the dorsal premotor cortex is characterized by (1) a thin row of medium-size pyramids in the lowest part of layer III, (2) a columnar pattern extending to the superficial layers, (3) dense and darker layer V, and (4) few scattered giant pyramids in layer Vb (Matelli et al. 1991; Johnson et al. 1996). Furthermore to date, the Brodmann area 6 appears as a complex mosaic of separate area reciprocally connected, involved in different aspects of motor control (Marconi et al. 2001; Dum & Strick, 2005; see Rizzolatti et al. 1998 for a review) with different subfields within PMd forming different parieto-frontal circuits (Matelli et al. 1998; Marconi et al. 2001). Given the difference in the time courses of activation of inhibitory intracortical circuits in PMd and M1 that emerged from our data, it is possible this phenomenon may rely on such anatomo-functional differences. Furthermore we might speculate that the specific inhibitory intracortical circuits observed in our study may be crucial to mediate interactions among the several parallel parieto-frontal pathways.
In conclusion our results demonstrate that pairs of TMS pulses over left PMd interact with the excitability of M1 with a different time course from that seen after a single pulse. We suggest that this is due to activation of intracortical circuits within PMd by CS1 and that these change the excitability of circuits normally probed by CS2. The time course of the CS1–CS2 interaction differs from that seen in M1, and may reflect differences in the basic circuitry of the two areas of brain. Finally, conditioning of PMd with repetitive TMS in the theta burst protocol (TBS) led to changes in the interaction for several minutes after application of TBS. However, unlike the situation in the M1 the effects of cTBS were similar to those of iTBS, consistent with the idea that the intrinsic circuits of the two motor areas differ.
If further studies should confirm these results, this new triple pulse TMS approach may usefully provide a fundamental insight into role of the other non-primary motor areas in several aspects of motor control and in the pathophysiology of various neurological disorders.
Acknowledgments
This work was funded by the Medical Research Council, UK and by grants from the Wellington Hospital.
References
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Baumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, Munchau A. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol. 2006;572:857–868. doi: 10.1113/jphysiol.2006.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching. Physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Albrecht UV, Caltagirone C, Rothwell JC. Effects of paired pulse TMS of primary somatosensory cortex on perception of a peripheral electrical stimulus. Exp Brain Res. 2006a;172:416–424. doi: 10.1007/s00221-006-0359-0. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006b;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Caltagirone C. Modulation of excitatory and inhibitory circuits for visual awareness in the human right parietal cortex. Exp Brain Res. 2005;160:510–516. doi: 10.1007/s00221-004-2039-2. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex. 2001;11:513–527. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J Comp Neurol. 1998;21:327–352. [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol. 1991;311:445–462. doi: 10.1002/cne.903110402. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci. 2002;22:554–561. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Caltagirone C, Filippi MM, Traversa R, Cicinelli P, Pasqualetti P, Rossini PM. Paired transcranial magnetic stimulation protocols reveal a pattern of inhibition and facilitation in the human parietal cortex. J Physiol. 2000;529:461–468. doi: 10.1111/j.1469-7793.2000.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. Review. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry. 1995;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V, Siebner HR, Modugno N, Pesenti A, Munchau A, Gerschlager W, Webb RM, Rothwell JC. Shaping the excitability of human motor cortex with premotor rTMS. J Physiol. 2004;554:483–495. doi: 10.1113/jphysiol.2003.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Meth. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices. Motor attention and selection. Neuroimage. 2003;20:S89–S100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sparing R, Dambeck N, Stock K, Meister IG, Huetter D, Boroojerdi B. Investigation of the primary visual cortex using short-interval paired-pulse transcranial magnetic stimulation (TMS) Neurosci Lett. 2005;382:312–316. doi: 10.1016/j.neulet.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex. Corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]