Abstract
The intracellular second messenger, cyclic guanosine monophosphate (cGMP), a soluble guanylate cyclase (GC) product, is a primary mechanism for the transduction of a nitric oxide (NO)-initiated signal in the central nervous system. NO is produced from l-arginine by neuronal nitric oxide synthase (NOS), which is found in sympathetic preganglionic neurons of the intermediolateral cell column. This suggests the possibility that NO is a modulator of sympathetic nervous activity (SNA) through a cGMP-mediated mechanism. The aim of this study was to determine the effects of intrathecally injected membrane-permeant 8-bromo-cGMP and 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ), a selective inhibitor of the soluble form of GC, on arterial pressure in urethane anaesthetized (1.4 g kg−1i.p.) rats. The effects of intrathecal cGMP and ODQ on haemodynamic responses to haemorrhage were also investigated. Finally, l-arginine, the NO precursor, was also injected intrathecally, alone and in the presence of ODQ. Baseline mean arterial pressure (MAP) increased significantly after intrathecal 8-Br-cGMP injection (10 μl, 1, 3, 10, 30, 100 μm). A dose–effect relationship (1 μm to 100 μm) was also established (EC50= 6.03 μm). During continuous haemorrhage, MAP was maintained in animals injected with 8-Br-cGMP, relative to the control group. Although no change in baseline MAP was observed as a result of intrathecal ODQ injection (10 μl, 100 mm), a greater rate of fall in MAP was observed during haemorrhage. Injecting l-arginine (10, 100, 1000 μm, 10 μl) showed a pressor effect that was consistent with the effect of the downstream messenger, cGMP. Furthermore, its pressor effect was blocked by ODQ pre-administration. The results indicate that cGMP increases blood pressure, and thus suggest that cGMP increases SNA. This supports the hypothesis that the sympathoexcitatory effects of spinal delivery of NO are mediated by a cGMP-dependent mechanism.
The nitric oxide (NO)–cyclic guanosine monophosphate (cGMP) signalling pathway has been observed to modulate the neural control of cardiovascular function (Garthwaite, 1991; Zanzinger, 1999). NO synthesis, from l-arginine, is catalysed by nitric oxide synthase (NOS). In the central nervous system (CNS), NOS is activated by the action of the neurotransmitter glutamate on N-methyl-d-aspartate (NMDA) receptors, including NMDA receptors found on sympathetic preganglionic neurons (SPN) of the intermediolateral cell column (IML) of the spinal cord (Arnolda et al. 2000). The mechanism, as proposed by Garthwaite et al. (1988), depends on the increased permeability of the NMDA receptor to calcium during glutamate-mediated NMDA receptor channel opening. NOS activation relies on raised levels of intracellular calcium, leading to increased intracellular NO. The primary means for NO signal transduction is via activation of the soluble form of guanylate cyclase (sGC) (Ahern et al. 2002), which in turn, catalyses the conversion of guanosine triphosphate (GTP) to cGMP.
It is known that NO elicits raised levels of cyclic GMP in the CNS (Garthwaite et al. 1988), as a result of sGC activation. Cyclic GMP signalling is also activated by membrane-bound GCs, which are controlled by natriuretic peptides and are thought to have a significant effect on postsynaptic modulation in the CNS. The mechanisms and the effects of natriuretic peptides on the neural control of cardiovascular function have been described (Calaresu & McKitrick, 1991), but the actions of cGMP within the CNS in the control of arterial pressure are not well understood (Siegel et al. 1999).
Experiments have localized NOS in sites where SPN are found, including the IML of the spinal cord (Anderson, 1992; Blottner & Baumgarten, 1992; Dampney, 1994; Saito et al. 1994; Reuss & Reuss, 2001). In the thoracic region of the spinal cord these SPN control arterial pressure and heart rate. In these neurons the actions of cGMP in blood pressure control need to be elucidated to appreciate the effects of neural modulation of cardiovascular function by NO (Garthwaite, 1991). The effects of intrathecally delivered cGMP on cardiovascular parameters have not been described to date.
This study was designed to observe the effects on blood pressure of cGMP. This was done at first, broadly, by injecting an exogenous form into the spinal cord, and then more specifically by assessing the effects of endogenous cGMP production by NO via blockade of soluble guanylate cyclase. Cyclic GMP is the downstream effector molecule of the NO–cGMP signalling pathway, and as cGMP, NOS and NO have been located in the IML of the spinal cord, which controls spinal sympathetic outflow to the heart and blood vessels, we speculate that any changes in blood pressure achieved by cGMP may arise from its effect on sympathetic outflow from the spinal cord. To this end, 8-Br-cGMP, a membrane-permeable analogue of cGMP, was injected intrathecally (1, 3, 10, 30, 100 μm) into urethane-anaesthetized rats during continuous monitoring of mean arterial pressure (MAP). A selective inhibitor of sGC, 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ; 100 mm) was also injected intrathecally, in order to observe the cardiovascular effects of inhibiting endogenous cGMP formation. Thus blocking endogenous cGMP should produce an opposite effect on arterial pressure to that observed when 8-Br-cGMP is administered. The effects of cGMP and ODQ on sympathetic cardiovascular reflexes, specifically the effects on the haemodynamic responses to haemorrhage, were also investigated. Finally, l-arginine (10, 100, 1000 μm, 10 μl), the precursor of NO, was administered intrathecally, in order to observe whether its effects correlate with those observed when the downstream effector of the NO–cGMP pathway was studied. In order to see whether the cardiovascular effects of l-arginine are blocked by a selective sGC inhibitor, some animals were pretreated with ODQ (100 mm) prior to l-arginine (1 mm) administration.
Cardiovascular responses to haemorrhage have been extensively studied in experiments on humans (Barcroft et al. 1944; Warren et al. 1945), rabbits (Schadt & Gladdis, 1986; Ludbrook et al. 1988), dogs (Shen et al. 1990) and rats, including the rat strain used in this study (Holobotvskyy et al. 2004). All studies have reported a biphasic response in variables such as MAP, heart rate (HR) and vascular resistance. The first part of acute blood loss is characterized by sympathoexcitation. This occurs before 25–35% of blood has been lost (Schadt & Ludbrook, 1991), and MAP is maintained by an increase in vascular resistance, despite the linear decrease of cardiac output. HR is also maintained, contributing to the support of MAP by rising gradually to a peak throughout the early phase of haemorrhage. Thus, the effects of haemorrhage on cardiovascular homeostasis are compensated by the increased activity (driven by the baroreceptor reflex) of the sympathetic nervous system during this phase, thus termed the compensatory phase of haemorrhage. The second phase is characterized by rapid hypotension driven by bradycardia and a profound decrease in vascular resistance. Active sympathoinhibition is involved in this second response to acute blood loss, termed the decompensatory phase of haemorrhage. It is not well known what mechanisms result in inhibition of the sympathetic nervous system (Ang et al. 1999). However, the effects of cGMP on sympathetic (compensatory) responses to haemorrhage can be used to assess how pathways that elicit the production of cGMP within the IML of the spinal cord, such as the NO–cGMP pathway, can modulate SNA in response to perturbations of cardiovascular homeostasis, such as haemorrhage. In addition, the use of ODQ during haemorrhage allowed us to investigate what effect endogenous cGMP has on sympathetic activation elicited by this physiological stimulus.
Methods
General methods
The experiment was divided into two parts. The first part was designed to provide information regarding the cardiovascular effects of the membrane-permeant analogue of cGMP, 8-Br-cGMP (1, 3, 10, 30, 100 μm, 10 μl), in the spinal cord of rats. Intrathecal injection of the sGC inhibitor; ODQ (100 mm, 10 μl) was included in this part of the experiment in order to study the effects of blocking endogenous cGMP formation. Intrathecal l-arginine (10, 100, 1000 μm, 10 μl) administration was also included in order to see whether the NO precursor has similar effects on blood pressure to its downstream effector; cGMP. The second part was designed to explore the effects of 8-Br-cGMP (2.5 μm, 10 μl, intrathecal) and ODQ (100 mm, 10 μl, intrathecal) on the haemodynamic responses to haemorrhage. All procedures were performed in the Research Centre of Royal Perth Hospital (Perth, Western Australia) in accordance with the National Health and Medical Research Council of Australia guidelines on the use of animals for scientific purposes. Experiments were approved by the Animal Ethics Committee of Royal Perth Hospital.
Animals used for all parts of the experiment were anaesthetized with urethane (ethyl carbamate, Sigma, St Louis, MO, USA) injected intraperitoneally (i.p., 1.4 g (kg body weight)−1). The depth of the anaesthesia was monitored using the corneal or the paw- and tail-pinch reflex. The experiment was terminated by injecting an overdose of barbiturate anaesthetic (Lethabarb, pentobarbitone sodium, 100–150 mg kg−1, i.p.).
Drugs used for the experiments
A membrane-permeable analogue of cGMP, 8-Br-cGMP (Sigma-Aldrich, USA), was used. Doses of 1, 3, 10, 30 and 100 μm dissolved in PBS (phosphate-buffered saline, 10 μl) were employed in the experimental (cGMP-treated) group. The group that was to be haemorrhaged received a dose of 2.5 μm 8-Br-cGMP dissolved in PBS (10 μl). The cGMP control group received PBS (10 μl, 1.54 mm KH2PO4, 155.17 mm NaCl, 2.71 mm Na2HPO4–7H2O, pH 7.2). A dose of 100 mm of the selective sGC inhibitor, ODQ, was dissolved in 10 μl dimethyl sulfoxide (DMSO). The ODQ control group received DMSO (10 μl) only. l-Arginine (Sigma-Aldrich, USA) was dissolved in PBS (10 μl) in doses of 10 μm, 100 μm and 1000 μm. The l-arginine control group received PBS (10 μl).
The effects of intrathecal cGMP/ODQ/L-arginine ± ODQ on MAP
Animals and surgical preparation
Sprague–Dawley rats (n = 35) were used for the study. Five rats were assigned to each group; the cGMP-treated group, the PBS-treated (cGMP vehicle) group, the ODQ-treated group, the DMSO-treated (ODQ vehicle) group, the l-arginine-treated group, the PBS-treated (l-arginine vehicle) group and the ODQ and l-arginine-treated group.
A saline-filled arterial line (polyethylene, i.d. 0.50 mm, o.d. 1.50 mm, Dural Plastic Engineering, NSW, Australia) was introduced into the femoral artery and the cannula was connected to a pressure transducer (Bell and Howell, CA, USA). The animal was then placed in a stereotaxic frame and the cervical spinal region was exposed to the atlanto-occipital membrane. The membrane and the underlying layer of dura mater were pierced and a cannula (polyethylene, i.d. 0.28 mm, o.d. 0.61 mm, Dural Plastic Engineering, NSW, Australia) was introduced into the subarachnoid space. The cannula had been filled with 8-Br-cGMP, PBS, ODQ, DMSO or l-arginine prior to insertion and was threaded 7.0 cm to the level of the T12 vertebra. Correct placement of the cannula was confirmed by injecting methylene blue intrathecally, at the end of the experiment and confirming blue staining of the exposed spinal cord.
Experimental procedures
MAP values were monitored for two minutes prior to the intrathecal injection. At 2 min, 8-Br-cGMP (10 μl, 1, 3, 10, 30, 100 μm, n = 5), PBS (10 μl, n = 5), ODQ (100 mm, 10 μl, n = 5), DMSO (10 μl, n = 5), l-arginine (10 μl, 10, 100, 1000 μm, n = 5), l-arginine vehicle (PBS, 10 μl, n = 5) and ODQ (100 mm, 10 μl) followed five minutes later by l-arginine (1 mm, 10 μl, n = 5), assigned without prearranged order over the 35 rats, was injected into the subarachnoid space and the haemodynamic effects monitored for a further 10 min. Apparently depending on dose, changes in MAP generally returned to baseline within 2–8 min after injecting 8-Br-cGMP. In order to plot a dose–effect relationship for 8-Br-cGMP, incremental doses of cGMP were given after MAP returned to baseline. As MAP post-l-arginine injection generally returned to baseline in a similar time-frame, an incremental regimen was also employed for l-arginine administration.
The effects of intrathecal cGMP/ODQ on MAP during haemorrhage
Animals and surgical preparation
The haemorrhage experiment involved Sprague-Dawley rats (n = 20), and, as in the first part, five rats were assigned to each group; cGMP-treated, PBS-treated, ODQ-treated or DMSO-treated. These animals were subjected to haemorrhage after intrathecal injection of cGMP or ODQ, or their respective vehicle.
Saline-filled arterial cannulas were introduced into the femoral arteries of both hindlimbs. One line was connected to a 3 ml syringe, and the other to the pressure transducer. An intrathecal catheter was inserted through an incision in the atlanto-occipital membrane and the tip advanced to the level of T12.
Experimental procedures
MAP was monitored for 2 min prior to intrathecal injection. Within 5 min after the cGMP, PBS, ODQ or DMSO injection, when MAP had returned to baseline, 1.5% of the body weight of the animal was withdrawn as millilitres of blood from the syringe connected to the second arterial line, at a constant rate over 180 s.
Data acquisition and statistical analysis
A pressure transducer was connected to an IBM-compatible computer equipped with PowerLab hardware and software (ADInstruments, NSW, Australia). The digitized output was presented as pulsatile pressure (measured in mmHg), which was then used to calculate MAP (in mmHg). HR (beats min−1) was derived from the pulsatile pressure signal.
MAP/HR values were recorded at 0.01 s intervals. Representative samples were averaged into mean ±s.e.m. MAP/HR per 1 s bin. MAP/HR values before and after drug administration were compared using two-way ANOVA. Where multiple doses were given (8-Br-cGMP, l-arginine), MAP values prior to each injection were compared to the MAP prior to commencing the experiment using one-way ANOVA and Dunnett's post hoc test, in order to test the stability of baseline MAP values (significant changes were considered to have P < 0.05). Baseline values across both groups did not change. As there was no significant change in baseline MAP values across the cGMP experiment, an average baseline MAP ±s.e.m. was calculated from the above data points and was used to compare to the changes in MAP elicited by the doses of cGMP delivered into the spinal cord. The variable-slope dose–response (Hill) equation was then used to plot a line that was fitted to the plotted data points, and the EC50 was calculated. To determine an effective dose of cGMP to use in the haemorrhage studies, the points on the fitted curve between the lowest dose and the EC50 were analysed using a program for the determination of significant changes in the slopes of lines (Jones & Dey, 1995). The inflection point at which the slope of the dose–response curve changed from the relatively flat low-dose portion of the curve to the relatively steep portion of the curve that passed through the EC50 was determined.
The course of haemorrhage in the second set of experiments was broken down into tenths of the total blood volume lost. Samples from each rat at each stage of haemorrhage were averaged into 10% bins, and the mean values ±s.e.m. were used to form lines of regression for the course of blood loss. The slopes of the linear regressions were calculated in order to determine trends in second-order relationships between the two groups. Statistical significance between baseline MAP (MAP immediately prior to the start of haemorrhage) and MAP values at subsequent stages of blood loss was assessed using one-way ANOVA and Dunnett's post hoc test, in order to determine the extent of MAP maintenance. Analysis of variance was also used to test the significance of changes in HR.
Results
Effects of intrathecal 8-Br-cGMP/ODQ/L-arginine ± ODQ on resting MAP values
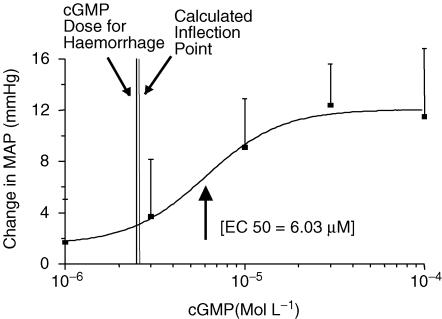
Baseline values in the cGMP-treated group and the PBS-treated group were 96 ± 5.8 mmHg and 95 ± 4.8 mmHg, respectively, and were not significantly different (P > 0.05). Intrathecal injection of 8-Br-cGMP significantly increased MAP (P < 0.05) at doses of 1, 3, 10, 30 and 100 μm. There were no significant changes in MAP after intrathecal injection of the vehicle (PBS: P = 0.78) only. A dose–response relationship was fitted to the data using the variable-slope Hill equation, and the EC50 calculated was 6.03 μm (Fig. 1). Analysis of the portion of the curve between the lowest dose and the EC50 indicated that the slope of the curve became significantly more steep at 10−5.59m, suggesting the use of a 2.5 μm dose in the haemorrhage studies.
Figure 1. The dose–response relationship of 8-Br-cGMP (1 μm to 100 μm).
The response is expressed as a change in MAP (mmHg) from baseline as a result of each injection of 8-Br-cGMP (1, 3, 10, 30, 100 μm, 10 μl). The dose–response (Hill) equation has been fitted to the data points ±s.e.m. (▪). EC50= 6.03 μm.
At 1 μm 8-Br-cGMP, HR was transiently maintained and then HR decreased slightly (11.7 beats min−1, P < 0.05). The 3 μm injection of 8-Br-cGMP caused a rise in HR (4.8 beats min−1, P < 0.05) followed by a return to baseline HR. No significant changes in HR occurred at the 10, 30 and 100 μm doses of 8-Br-cGMP. HR did not change as a result of PBS injection.
Baseline MAP values in the ODQ-treated group (98 ± 4 mmHg) were not statistically different from baseline values recorded in the DMSO-treated group (98 ± 4.5 mmHg). Intrathecal ODQ injection resulted in no significant change (P = 0.40) in MAP. Injection of its vehicle only (DMSO) also did not produce any significant MAP change (P = 0.97). HR did not change as a result of ODQ or DMSO administration (P > 0.05).
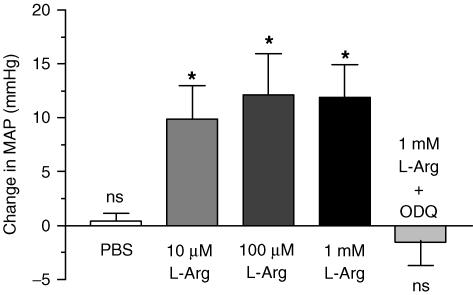
Baseline MAP in the l-arginine-treated group was 91 ± 6.4 mmHg, in the l-arginine control group; 93 ± 2.8 mmHg and in the l-arginine + ODQ-treated group; 102 ± 11.7 mmHg. These were not statistically different. MAP increased as a result of all three doses of l-arginine (9–12 mmHg, P < 0.05). PBS did not produce a change in MAP or HR in the l-arginine control group. HR increased slightly as a result of 10 μm and 100 μm l-arginine; 5.7 beats min−1 and 10.4 beats min−1, respectively (P < 0.05). No change occurred when 1 mm l-arginine was administered (P > 0.05).
Effects of intrathecal 8-Br-cGMP on the cardiovascular response to haemorrhage
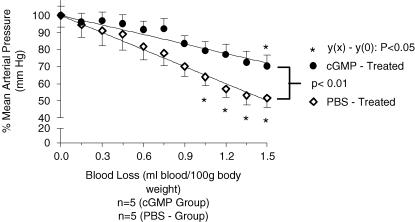
Baseline MAP values in the cGMP-treated group were 91 ± 3.5 mmHg and in the PBS-treated group, 90 ± 3.8 mmHg. MAP values were not statistically different, across the groups (P > 0.05). MAP decrease during loss of blood in the cGMP-treated group was at a rate of 17 ± 1.3 mmHg ml−1 (100 g)−1, whereas in the PBS-treated group the rate of decrease was higher; 30 ± 1.1 mmHg ml−1 (100 g)−1. These values, calculated as slopes of the lines of regression, were statistically different (P < 0.0001). MAP in the cGMP-treated group was maintained until 1.35 ml blood (100 g body weight)−1 had been removed, whereas MAP in the PBS-treated group was maintained only until 0.9 ml blood (100 g body weight)−1 had been removed (Fig. 2). HR was maintained throughout the course of haemorrhage in the cGMP-treated group, whereas HR at the onset of haemorrhage and at the end of it was significantly different for the PBS-treated group. Thus the administration of 8-Br-cGMP significantly (P < 0.05) increased the volume of blood loss necessary to elicit the decompensatory phase of haemorrhage.
Figure 2. The effect of intrathecal 8-Br-cGMP (2.5 μm, 10 μl) and vehicle (PBS, 10 μl intrathecal) injected prior to haemorrhage (1.5% body weight) on MAP (mmHg ± S.E.M.) measured throughout the removal of blood.
MAP during haemorrhage is expressed as a percentage of the initial MAP. *, a significantly different MAP value at x ml blood (100 g body weight)−1 (P < 0.05, Dunnett's post test), compared to the MAP value prior to the onset of haemorrhage; 0 ml blood (100 g body weight)−1. MAP maintenance was observed in cGMP-treated rats till 1.35 ml blood (100 g body weight)−1 had been removed as opposed to maintenance of MAP till 0.90 ml blood (100 g body weight)−1 was removed in the control group. The lines of regression formed by the decrease of MAP over the course of haemorrhage are statistically different (P < 0.01); the control group exhibits a steeper gradient than the group treated with cGMP.
Effects of intrathecal ODQ on the cardiovascular response to haemorrhage
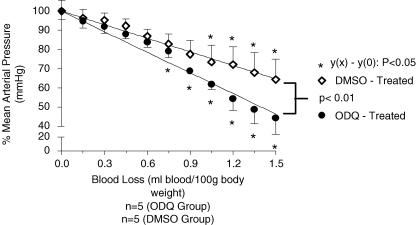
Baseline cardiovascular values in the ODQ-treated group were 99 ± 2.2 mmHg and in the DMSO-treated group, 97 ± 1.8 mmHg. MAP values were not statistically different, across the groups (P > 0.05). The rate of MAP decrease during loss of blood in the ODQ-treated group was 43 ± 2.0 mmHg ml−1 (100 g)−1, whereas in the DMSO-treated group the rate of decrease was significantly lower; 27 ± 0.8 mmHg ml−1 (100 g)−1 (P < 0.0001). The ODQ-treated group maintained MAP until 0.6 ml blood (100 g body weight)−1 had been removed from the animal, whereas MAP was maintained for longer in the DMSO-treated group; until 0.9 ml blood (100 g body weight)−1 had been removed. (Fig. 3).
Figure 3. The effect of intrathecal ODQ (100 mm, 10 μl) and vehicle (DMSO, 10 μl intrathecal) injected prior to haemorrhage (1.5% body weight) on MAP (mmHg ±s.e.m.) measured throughout the removal of blood.
MAP during haemorrhage is expressed as a percentage of the initial MAP. *, a significantly different MAP value at x ml blood (100 g body weight)−1 (P < 0.05, Dunnett's post test), compared to the MAP value prior to the onset of haemorrhage; 0 ml blood (100 g body weight)−1. MAP maintenance was observed in ODQ-treated rats till 0.60 ml blood (100 g body weight)−1 had been removed as opposed to maintenance of MAP till 0.90 ml ml blood (100 g body weight)−1 had been removed in the control group. The lines of regression formed by the decrease of MAP over the course of haemorrhage are statistically different (P < 0.01); the ODQ-treated group exhibits a steeper gradient than the group treated with cGMP.
Results summary
Baseline values across all groups were not statistically significant (one-way ANOVA, P > 0.05). Resting MAP values increased significantly after the intrathecal injection of 8-Br-cGMP (1,3, 10, 30, 100 μm, 10 μl), whereas no effect was observed after intrathecal injection of ODQ. At 1 μm, HR fell after being transiently maintained. At 3 μm, HR rose in response to 8-Br-cGMP and then returned to baseline. HR did not change at 10, 30 and 100 μm 8-Br-cGMP. The dose–response curve (Fig. 1), demonstrates a positive correlation between the dose of 8-Br-cGMP given and the change in MAP produced by the drug. Administration of 2.5 μm 8-Br-cGMP maintained MAP (Fig. 2) and HR (Fig. 4) for longer during haemorrhage than in the control group. Administration of ODQ had the reverse effect; it resulted in a shorter period of MAP maintenance and a greater rate of fall in MAP than in the control group (Fig. 3). Finally, l-arginine administration (10, 100, 1000 μm, 10 μl) resulted in a statistically significant increase in MAP at all doses. ODQ pre-administration blocked the pressor effect noted above (Fig. 5). HR rose in response to l-arginine at 10 μm and 100 μm, but remained unchanged at the highest dose (1 mm) used.
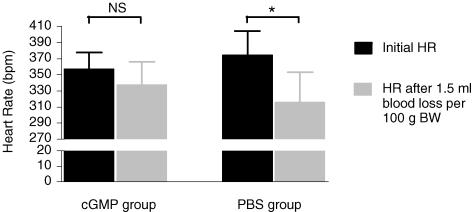
Figure 4. The effect of intrathecal 8-Br-cGMP/PBS on HR changes (initial HR versus HR post-haemorrhage).
The cGMP-treated group appears to maintain HR (P > 0.05), whereas in the PBS-treated group, a statistically significant decline in HR is observed (P < 0.05).
Figure 5. The effect of intrathecal l-arginine (l-Arg, middle three bars; 10, 100, 1000 μm, 10 μl injection volume) and vehicle (PBS, leftmost bar; 10 μl volume).
l-Arginine elicits an increase in MAP at all doses (P < 0.05), denoted by an asterisk (*). No changes (ns, P > 0.05), were observed when PBS was given or when l-Arg was injected in ODQ-pretreated rats (rightmost bar; 10 μl of 100 mm ODQ followed by 10 μl of 1 mm l-Arg).
Discussion
We have conducted a series of experiments to investigate the contribution of the NO–cGMP signalling system to spinal mechanisms controlling blood pressure. The NO precursor l-arginine and the soluble cGMP analogue, 8-Br-cGMP, each elicited an increase in blood pressure when administered intrathecally. Blockade of soluble guanylate cyclase with ODQ did not alter resting blood pressure, but blocked the increase in pressure elicited by intrathecal l-arginine. Intrathecal 8-Br-cGMP attenuated and ODQ augmented the fall in blood pressure elicited by haemorrhage.
Yaksh & Rudy (1976) demonstrated the procedure described above for the insertion of the intrathecal cannula in rats. They documented the spread of drug in the spinal cord after intrathecal injection. According to the study, drug injected at the level of T12 (where the tip of the cannula is placed) flows rostrally further than it flows caudally. They also measured the spread of 10 μl injected from this point and found that this fluid is mostly restricted to the thoracic region of the spinal cord where the IML, containing the SPN that provide cardiac and vascular sympathetic innervation, are found. Injecting the drug at a level higher than this may result in the distribution of the drug into the midbrain, which might affect the responses currently being studied by an action on brainstem cardiovascular centres.
Another issue that should be addressed is the choice of doses of ODQ and cGMP used for the haemorrhage study. First, ODQ was used at a dose of 100 mm to conform with the doses used in other previously published studies, for example in the study of Hoheisel et al. (2005) where localized superfusion of the intact spinal cord with ODQ was done at a concentration of 100 mm. In the present study it was found that 100 mm ODQ does not change arterial pressure, thus this dose was deemed appropriate for this study. In the case of cGMP, it was found that cGMP at doses between 1 and 100 μm elevated arterial pressure, a potentially confounding effect in the haemorrhage study. Two things were done to minimize this effect. First, to determine a dose of cGMP that elicited a small but consistently detectable increase in arterial pressure, a computer program for determination of ‘breakpoints’ or significant changes in the slope of a line (Jones & Dey, 1995) was used to analyse the dose–response curve between the lowest dose tested and the calculated EC50 of the curve. The inflection point was determined, where the slope of the curve changed from the relatively flat portion of the low-dose end of the curve to the steep rising phase passing through the EC50 (Fig. 1). This corresponded closely to the 2.5 μm dose of cGMP used. Second, 2.5 μm cGMP was administered and the change in arterial pressure from baseline was noted, then the haemorrhage experiment proceeded only after arterial pressure had returned to baseline values so that haemorrhage proceeded from the baseline level of arterial pressure. While a higher concentration of cGMP might have produced even more dramatic results, we believe that the decision to have arterial pressure at baseline to conduct the haemorrhage experiment would have meant that the effective concentration of cGMP within the spinal cord would probably have been very similar between a large dose and a long wait for return to baseline, compared to a modest arterial pressure increase and the rapid return to baseline at the time of the experiment.
In the present study, we intrathecally injected an exogenous, membrane permeable analogue of cGMP, 8-Br-cGMP and a selective inhibitor of soluble GC (ODQ), in order to observe the effects of the NO–cGMP signalling pathway on blood pressure. An increase in resting MAP as a result of 8-Br-cGMP (1, 3, 10, 30, 100 μm) is probably explained by an increase in sympathetic nervous activity arising in the spinal cord, either by a direct effect on sympathetic preganglionic neurons (SPN) or on other neuronal inputs to the SPN. The dose–response relationship (Fig. 1) demonstrates that MAP increases in response to the second messenger in a dose-dependent manner. Since 8-Br-cGMP is membrane permeable, it is likely to have been distributed uniformly through the spinal cord, as well as other neurons and nerve terminals. This study was not designed to explore the site of action of the NO-cGMP signalling pathway within the spinal cord. However, based on studies that have located cGMP, NOS and NO in the SPN of the IML (the only other known location of NOS is in the dorsal horn, where, presumably, it modulates pain pathways), and the observation in our own study that MAP/HR trends during the sympathoexcitatory phase of haemorrhage are changed by the presence of 8-Br-cGMP/ODQ, we can speculate that the sympathetic outflow from the spinal cord is probably involved in these responses.
Analysis of resting HR changes as a result of 8-Br-cGMP demonstrated that HR changes were different for different doses of 8-Br-cGMP. At a low dose of cGMP (1 μm), HR was maintained transiently and then it decreased. This is presumably due to the effect of the baroreceptor reflex in response to the small rise in MAP. At 3 μm, HR rose. This is likely to be associated with the higher dose of the drug directly affecting the sympathetic outflow to the heart (T1/T2). HR subsequently returned to baseline more rapidly than MAP, probably due to the buffering influence of the baroreceptor reflex. At the higher doses of cGMP, changes in HR were not detectable despite the larger changes in MAP. This may be explained by the competition between the direct effect of cGMP on chronotrophic sympathetic outflow to the heart, with the HR decreases elicited by the baroreceptor reflex responding to larger increases in MAP produced at these doses. In addition, analysis of HR data from the haemorrhage studies suggests that at 2.5 μm 8-Br-cGMP (similar to the dose that produced an increase in resting HR), HR was maintained throughout haemorrhage (Fig. 4). These data further augment the possibility that the MAP responses observed as a result of exogenous cGMP or inhibiting endogenous cGMP are likely to have resulted from an overall increase in SNA.
The result of intrathecal injection of 8-Br-cGMP followed by haemorrhage showed that the compensatory (sympathoexcitatory) phase was maintained as a result of the injection, as MAP was maintained during the course of haemorrhage (Fig. 2). Reports suggest that the compensatory phase of haemorrhage in rats is short, and that MAP decreases from the start of haemorrhage (Schadt & Ludbrook, 1991). This phenomenon was observed in the present study in vehicle-treated animals.
In the present study, we also observed the effects of a soluble GC-selective inhibitor; ODQ. This is quite a specific test for the production of endogenous cGMP by NO, as NO is the only known physiological activator of soluble GC. Intrathecal injection of the drug did not result in any observeable effect on resting MAP. During haemorrhage, however, it appears that intrathecal ODQ may have effects on MAP that are the opposite of those observed as a result of 8-Br-cGMP. MAP in ODQ pretreated animals decreased at a higher rate than in control animals, whereas 8-Br-cGMP pretreatment resulted in a lower rate of MAP decrease during haemorrhage. Taken together, these results suggest that during the resting state, the pathways (e.g. NO pathway) that activate GC are not functioning at a level that would produce any observable effect (very little formation of endogenous cGMP), whereas this pathway is upregulated at the onset of haemorrhage. A response to cGMP was observed at the resting level because injecting exogenous cGMP does not rely on the endogenous NO–cGMP pathway, it simply tests its downstream effects. The response to the soluble GC inhibitor suggests that the NO–cGMP signalling pathway is involved in the cardiovascular response to haemorrhage because blockade of endogenous cGMP formation diminished the ability to maintain MAP during the course of blood loss. These results conform to the hypothesis that the NO–cGMP signalling cascade is an important modulator of cardiovascular function, rather than it functioning directly in impulse transmission. More work will be needed to determine the importance of natriuretic peptides in these responses, as natriuretic peptides also elicit raised concentrations of cGMP via activation of the membrane-bound form of GC.
l-Arginine, with and without ODQ pre-administration, was studied in order to provide additional data regarding the role of the NO–cGMP signalling mechanism in the responses that have been described in this study. We chose to use the NO precursor, l-arginine, rather than a NO donor, because the former relies on endogenous NOS to produce NO, ensuring that NO is formed only at sites where there exists a capacity to form NO. In contrast, a NO donor would release NO throughout the spinal cord. l-Arginine elicited a pressor response at all doses of l-arginine (as did its downstream effector, cGMP). ODQ, the sGC-specific blocker, inhibited the pressor effect of l-arginine. This supports the hypothesis that the pressor and sympathoexcitatory responses observed in this study are mediated by the NO–cGMP mechanism. Furthermore, HR data were also similar to those obtained with 8-Br-cGMP. While the lower doses of l-arginine produced a rise in HR, no change in HR occurred when the highest dose was used. This, as was discussed for 8-Br-cGMP, may be attributed to the competing influences of the chronotropic sympathetic drive and the baroreceptor reflex stimulated by the increase in MAP.
The downstream pathways of cGMP within the CNS are quite diverse (Siegel et al. 1999). Cyclic GMP is known to exert its effects via activation of protein kinase G (PKG), regulation of cyclic nucleotide-gated ion channels (CNGC) and via activation of phosphodiesterases (Ahern et al. 2002). PKG regulates a vast number of proteins and almost all types of ion channels (White, 1999). It is therefore plausible that an increase of MAP may be the result of activation of CNGC and ion channels regulated by PKG, resulting in depolarization and sympathoexcitation of SPN, in turn resulting in an increase in SNA, eliciting an increase in arterial pressure by increasing the firing rate of sympathetic nerve fibres to blood vessels and the heart.
Cyclic GMP, NOS and NO have been localized within SPN of the thoracic spinal cord using antibodies, immunoflouroscent staining and confocal miscroscopy (Anderson, 1992; Blottner & Baumgarten, 1992; Saito et al. 1994; Phillips et al. 2002). The presence of cGMP within SPN is attributed to the stimulation of the soluble and particulate isoforms of GC by NO and natriuretic peptides, respectively.
The NO–cGMP signalling pathway is considered to function as a modulator of SNA, rather than as a direct step in the signalling pathway of SPN (Wu & Dun, 1995, 1996; Arnolda et al. 2000). However, reports on the effects of intrathecal injections of NO donor substances and NOS inhibitors on MAP in rats have been inconsistent (Lee et al. 1996; Garcia et al. 1997; Koga et al. 1999). Experiments that have involved measurements of electrical activity of SPN in prepared slices of the thoracolumbar spinal cord of rats have shown that endogenous NO appears to enhance both excitatory and inhibitory postsynaptic potentials in SPN (Wu & Dun, 1995; Wu & Dun, 1996).
NO appears to have diverse signalling pathways. It is able to act independently of cGMP by modifying protein side chains (S-nitrosylation; Ahern et al. 2002). For example, NO has been found to block depolarization of the NMDA receptor by nitrosylation of a particular cysteine residue on the NR2A subunit of that receptor. (Manzoni et al. 1992). This supported the conclusion of Arnolda et al. (2000) that a NO donor, 3-morpholinylsydnoneimine chloride (SIN-1), attenuates the pressor responses elicited by NMDA in the thoracic spinal cord of rats. The NMDA receptor, in response to glutamate at the synaptic cleft, induces an influx of calcium that activates NOS; thus this blockade may be considered to be acute negative feedback inhibition. The cGMP-dependent pathways of NO signal transduction, when physiologically activated, might then be considered to be a chronic excitatory influence in surrounding SPN.
We have only considered the cGMP-mediated effects of NO in this study. As mentioned above NO may have important effects outside the NO/cGMP pathway. Also, as mentioned above, the effects of exogenous cGMP do not rely on the NO/cGMP pathway but rather directly test cGMP effects on its downstream targets. Thus, 8-Br- cGMP might exert effects in cells that are not ordinarily exposed to cGMP and don't possess functional membrane-bound particulate or soluble guanylyl cyclase, but do possess the downstream mechanisms to respond to exogenous cGMP. In this case the responses we have reported here would also include contributions from cells that are not part of the normal physiological mechanism of action of the NO/cGMP pathway. Although additional studies with specific guanylyl cyclase activators and NO donors with guanylyl cyclase inhibitors would be required to address these possibilities, the data obtained when l-arginine was administered (on its own and with ODQ) augment our hypothesis that the NO–cGMP pathway is involved in the pressor responses observed when cGMP (the downstream second messenger) was studied.
In conclusion, this study demonstrates a dose-dependent increase in resting MAP when exogenous cGMP is injected into the spinal cord, and it also demonstrates MAP and HR maintenance in the presence of exogenous cGMP during haemorrhage. No effect on resting MAP is observed when the formation of endogenous cGMP is inhibited. Endogenous cGMP, however, appears to be involved in the maintenance of MAP during haemorrhage. Taken together, these data suggest the possibility that there is little formation of endogenous cGMP in the resting state, but, in response to an external stimulus (such as haemorrhage) that seeks to decrease blood pressure, endogenous cGMP acts to increase MAP. Finally, l-arginine results in a pressor response and it is blocked by a selective sGC inhibitor. Thus the results of this study study support the overall hypothesis that the NO–cGMP pathway modulates the neural control of cardiovascular function.
References
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO: review. Trends Neurosci. 2002;25:510–517. doi: 10.1016/s0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- Anderson CR. NADPH diaphorase- positive neurons in the rat spinal cord include a subpopulation of autonomic preganglionic neurons. Neurosci Lett. 1992;139:280–284. doi: 10.1016/0304-3940(92)90571-n. [DOI] [PubMed] [Google Scholar]
- Ang KK, McRitchie RJ, Minson JB, Llewellyn-Smith IJ, Pilowsky PM, Chalmers JP, Arnolda LF. Activation of spinal opioid receptors contributes to hypotension after haemorrhage in conscious rats. Am J Physiol Heart Circ Physiol. 1999;276:H1552–H1558. doi: 10.1152/ajpheart.1999.276.5.H1552. [DOI] [PubMed] [Google Scholar]
- Arnolda LF, Mckitrick DJ, Llewellyn-Smith IJ, Minson JB. Nitric oxide limits pressor responses to sympathetic activation in rat spinal cord. Hypertension. 2000;36:1089–1092. doi: 10.1161/01.hyp.36.6.1089. [DOI] [PubMed] [Google Scholar]
- Barcroft H, Edholm O, McMichael J, Sharpey-Shafer E. Posthaemorrhagic fainting; study by cardiac output and forearm flow. Lancet. 1944;1:489–491. [Google Scholar]
- Blottner D, Baumgarten H. Nitric oxide synthetase (NOS)-containing sympathoadrenal cholinergic neurons of the rat IML-cell column: evidence from histochemistry, immunohistochemistry and retrograde labelling. J Comp Neurol. 1992;316:45–55. doi: 10.1002/cne.903160105. [DOI] [PubMed] [Google Scholar]
- Calaresu FR, McKitrick DJ. Interaction of putative transmitters in central pathways involved in the control of heart rate and arterial pressure. Cardioscience. 1991;2:147–154. [PubMed] [Google Scholar]
- Dampney RAL. Functional organisation of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–351. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Celuch SM, Adler-Graschinsky E. Possible participation of spinal nitric oxide in the control of the blood pressure in anaesthetized rats. Brain Res. 1997;764:67–74. doi: 10.1016/s0006-8993(97)00421-6. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system: review. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Unger T, Mense S. The possible role of the NO-cGMP pathway in nociception: different spinal and supraspinal action of enzyme blockers on rat dorsal horn neurons. Pain. 2005;117:358–367. doi: 10.1016/j.pain.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Holobotvskyy VV, Arnolda LF, McKitrick DJ. Effect of anaesthetic and rat strain on heart rate responses to simulated haemorrhage. Acta Physiol Scand. 2004;180:29–38. doi: 10.1046/j.0001-6772.2003.01218.x. [DOI] [PubMed] [Google Scholar]
- Jones R, Dey I. Determining one or more change points. Chem Phys Lipids. 1995;76:1–6. doi: 10.1016/0009-3084(94)02422-2. [DOI] [PubMed] [Google Scholar]
- Koga N, Takano Y, Honda K, Saito R, Kamiya H. Roles of nitric oxide in the spinal cord in cardiovascular regulation in rats. Neurosci Lett. 1999;267:173–176. doi: 10.1016/s0304-3940(99)00358-4. [DOI] [PubMed] [Google Scholar]
- Lee SB, Koh HC, Kim ON, et al. Intrathecal administration of sodium nitroprusside, a nitric oxide donor, increases blood pressure in anaesthetised rats. Neurosci Lett. 1996;203:53–56. doi: 10.1016/0304-3940(95)12269-9. [DOI] [PubMed] [Google Scholar]
- Ludbrook J, Potocnik SJ, Woods RL. Simulation of acute haemorrhage in unanaesthetised rabbits. Clin Exp Pharmacol Physiol. 1988;15:575–584. doi: 10.1111/j.1440-1681.1988.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Manzoni O, Prezeau L, Marin P, Deshager S, Bockaert J, Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992;8:653–662. doi: 10.1016/0896-6273(92)90087-t. [DOI] [PubMed] [Google Scholar]
- Phillips JK, Powers K, Holobotovskyy VV, McKitrick DJ, Arnolda LF. Expression of nitric oxide and cGMP in sympathetic neurons of the rat RVLM and spinal cord. Proceedings of the Australian Health and Medical Research Congress. 2002 NHMRC, Melbourne, 24–29 November, abstract, 2825. [Google Scholar]
- Reuss MH, Reuss S. Nitric oxide synthase neurons in the rodent spinal cord: distribution, relation to substance P fibers, and effects of dorsal rhizotomy. J Chem Neuroanat. 2001;21:181–196. doi: 10.1016/s0891-0618(01)00091-6. [DOI] [PubMed] [Google Scholar]
- Saito S, Kidd GJ, Trapp BD, Dawson TM, Bredt DS, Wilson DA, Traystman RJ, Snyder SH, Hanley DF. Rat spinal cord neurons contain nitric oxide synthase. Neuroscience. 1994;59:447–456. doi: 10.1016/0306-4522(94)90608-4. [DOI] [PubMed] [Google Scholar]
- Schadt J, Gladdis R. Cardiovascular responses to haemorrhage and nalaxone in conscious barodenervated rabbits. Am J Physiol Regul Integr Comp Physiol. 1986;251:R909–R915. doi: 10.1152/ajpregu.1986.251.5.R909. [DOI] [PubMed] [Google Scholar]
- Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals: review. Am J Physiol Heart Circ Physiol. 1991;260:H305–H318. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- Shen Y-T, Knight D, Thomas J, Vatner S. Relative roles of cardiac receptors and arterial baroreceptors during haemorrhage in conscious dogs. Circ Res. 1990;66:397–405. doi: 10.1161/01.res.66.2.397. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Agranoff BW, Albers RW, et al. Basic Neurochemistry. 6. Philadelphia, USA: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- Warren J, Brannon E, Stead E, Merrill A. The effect of venesection on and the pooling of blood in the extremities on atrial pressure and cardiac output in normal subjects with observations on acute circulatory collapse in three instances. J Clin Invest. 1945;24:337–344. doi: 10.1172/JCI101611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE. Cyclic GMP and ion channel regulation. Adv Second Messenger Phosphoprotein Res. 1999;33:251–277. doi: 10.1016/s1040-7952(99)80013-5. [DOI] [PubMed] [Google Scholar]
- Wu SY, Dun NJ. Calcium-activated release of nitric oxide potentiates excitatory synaptic potentials in immature rat sympathetic preganglionic neurons. J Neurophysiol. 1995;74:2600–2603. doi: 10.1152/jn.1995.74.6.2600. [DOI] [PubMed] [Google Scholar]
- Wu SY, Dun NJ. Potentiation of IPSCs by nitric oxide in immature rats sympathetic preganglionic neurones in vitro. J Physiol. 1996;495:479–490. doi: 10.1113/jphysiol.1996.sp021608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zanzinger J. Role of nitric oxide in the neural control of cardiovascular function: review. Cardiovasc Res. 1999;43:639–649. doi: 10.1016/s0008-6363(99)00085-1. [DOI] [PubMed] [Google Scholar]