Abstract
The rapid onset of vasodilatation within seconds of a single contraction suggests that the vasodilators involved may be products of skeletal muscle activation, such as potassium (K+). To test the hypothesis that K+ was in part responsible for the rapid dilatation produced by muscle contraction we stimulated four to five skeletal muscle fibres in the anaesthetized hamster cremaster preparation in situ and measured the change in diameter of arterioles at a site of overlap with the stimulated muscle fibres before and after a single contraction stimulated over a range of stimulus frequencies (4, 10, 20, 30, 40, 60 and 80 Hz; 250 ms train duration). Muscle fibres were stimulated in the absence and presence of an inhibitor of a source of K+, the voltage dependent K+ channel inhibitor 3,4-diaminopyridine (DAP, 3 × 10−4m) and inhibitors of the K+ dilatory signal transduction pathway, either a Na+ K+-ATPase inhibitor (ouabain; 10−4m) or an inward rectifying K+ channel inhibitor (barium chloride, BaCl2; 5 × 10−5m). We observed significant inhibitions of the rapid dilatation at all stimulus frequencies with each inhibitor. The dilatory event at 4 s was significantly inhibited at all stimulus frequencies by an average of 65.7 ± 3.6%, 58.8 ± 6.1% and 64.4 ± 2.1% in the presence DAP, ouabain and BaCl2, respectively. These levels of inhibition did not correlate with non-specific changes in force generation by skeletal muscle measured in vitro. Therefore, our data support that K+ is involved in the rapid dilatation in response to a single muscle contraction across a wide range of stimulus frequencies.
Blood flow to active skeletal muscle increases rapidly at the onset of exercise. Rapid vasodilatation has been implicated as a component of this process (Tschakovsky et al. 1996; Shoemaker et al. 1998; Naik et al. 1999; Hamann et al. 2004; Saunders & Tschakovsky, 2004; Tschakovsky & Sheriff, 2004). Microvascular studies support this by showing that vasodilatation occurs within seconds in response to skeletal muscle contraction (Marshall & Tandon, 1984; Dodd & Johnson, 1991; Mihok & Murrant, 2004; VanTeeffelen & Segal, 2006). The identity of the vasodilator(s) responsible for the rapid dilatation remain unclear. Mihok & Murrant (2004) show that there may be multiple dilators responsible for the dilatation resulting from a single contraction and that dilator production is dependent on the stimulation parameters used to induce the skeletal muscle contraction. They observed that low stimulus frequencies (4 Hz) resulted in a single rapid dilatation peaking within 3–4 s of the contraction before returning to baseline by 10 s; higher stimulus frequencies (20, 30 and 40 Hz) resulted in a biphasic dilatation with two peaks, one peak at approximately 4 s and another at approximately 20 s. Even higher stimulus frequencies (60 and 80 Hz) resulted in a single larger dilatation peaking at approximately 4 s but lasting much longer than the dilatation at low frequencies. Although aspects of the pattern of dilatation differ depending on stimulus frequency, what remains constant is the presence of a very rapid dilatation at approximately 4 s. The rapid nature of the dilatation indicates that the vasodilators may be a product of skeletal muscle activation, ones that are produced even before the skeletal muscle cells themselves contract. Both acetylcholine (ACh), used for transmission of the action potential from the α motor neuron to the skeletal muscle cell at the neuromuscular junction, and potassium (K+), released from skeletal muscle cell as the action potential develops along the fibres, are vasodilatory products that fit this description. VanTeeffelen & Segal, 2006) have shown that the early component of the dilatation in response to a single contraction of the whole muscle was, in part, dependent on ACh. Whether K+ plays a role in this early dilatation is unknown.
K+ has been implicated in the vasodilatations that produce the changes in blood flow in response to exercise (Tominaga et al. 1973; Mohrman & Sparks, 1974; Radawski et al. 1975; Stowe et al. 1975; Hnik et al. 1976; Hirche et al. 1980; Wilkerson et al. 1982; Vyskocil et al. 1983; Kiens et al. 1989; Lott et al. 2001) although some studies question its significance (Mohrman, 1982; Lash & Bohlen, 1987; Wilson et al. 1994). K+ levels in both the plasma (Tominaga et al. 1973; Radawski et al. 1975; Stowe et al. 1975; Bockman et al. 1976; Wilkerson et al. 1982; Juel et al. 1990; Wilson et al. 1994) and the interstitial space (Hirche et al. 1980; Juel et al. 2000; Lott et al. 2001) increase in response to muscle contraction and in some cases the release has been shown to occur immediately upon the initiation of contraction (Scott et al. 1970; Mohrman & Sparks, 1974; Vyskocil et al. 1983; Kiens et al. 1989) and in response to a single contraction (Mohrman & Sparks, 1974; Hnik et al. 1976). A primary source of K+ resulting from skeletal muscle cell activation is the slow voltage dependent K+ (Kv) channels associated with the action potential being propagated down nerves and skeletal muscle cell membranes and thus K+ has the potential to be released directly into the interstitial space surrounding any vasculature overlapping the active skeletal muscle fibres. K+ is vasodilatory at lower concentrations, in the range of 1–15 mm (Dawes, 1941; Emanuel et al. 1959; Chen et al. 1972; Duling, 1975; Kiens et al. 1989; Wilson et al. 1994; Burns et al. 2004) and interstitial K+ levels produced by muscle contraction are at levels that promote vasodilatation (Vyskocil et al. 1983; Juel et al. 2000; Lott et al. 2001). The vasodilatatory response to K+ has been attributed to a transient hyperpolarization of vascular smooth muscle cells by stimulation of inward rectifying K+ (KIR) channels (Loeb et al. 2000; Burns et al. 2004) and the sodium–potassium pump (Na+ K+-ATPase) (Haddy, 1983; Burns et al. 2004). Hyperpolarization will close voltage gated Ca2+ channels, decrease intracellular Ca2+ and produce vasodilatation. Therefore, there is evidence that K+ may be involved in the events initiating the rapid dilatation in response to skeletal muscle contraction.
The goal of this study was to determine whether K+ is involved in producing the rapid dilatation in response to a single contraction. We tested the hypothesis that K+ was, in part, responsible for producing the initial, rapid vasodilatory peak observed at all stimulus frequencies. We used an anaesthetized hamster cremaster muscle preparation in situ to directly stimulate a small bundle of skeletal muscle fibres (4–5) for a single contraction (train duration 250 ms) over a range of stimulus frequencies (4, 10, 20, 30, 40, 60 and 80 Hz) and monitored the diameter of arterioles overlying the active skeletal muscle cells. The diameter of the overlying arterioles were measured in the absence and the presence of a blocker of source of K+, 3,4-diaminopyridine (DAP, 3 × 10−4m) to block Kv channels, or blockers of key components in the signalling pathway to K+, barium chloride (BaCl2, 5 × 10−5m) to block KIR channels or ouabain (10−4m) to block the Na+ K+-ATPase.
Methods
All experimental procedures were approved by the institutional animal care and use committee and were conducted in accordance with the guidelines of the Canadian Council on Animal Care as set out in the Guide to the Care and Use of Experimental Animals.
In situ protocol
Adult male Golden hamsters (100–130 g) were anaesthetized with sodium pentobarbital (70 mg kg−1i.p.) and tracheotomized. Polyethelyne catheters (outer tip diameter approximately 0.5 mm) were placed in the left femoral artery and vein to monitor mean arterial pressure and for supplemental pentobarbital administration, respectively. Supplemental pentobarbital was given as needed during surgery and constantly infused throughout the experimental protocol (10 mg ml−1 saline, 0.56 ml h−1). Hamster esophageal temperature was maintained at 37°C via convective heat from a coiled water-filled glass tube (42.0°C) secured under the hamster. The right cremaster was prepared for in situ microscopy as previously described (Baez, 1973; Murrant, 2005). Briefly, the cremaster was isolated, cut longitudinally, separated from the testis and epididymis and gently spread over a semicircular lucite platform. The edges of the tissue were secured with insect pins to maintain tension but not stretch the muscle. Once exposed, the cremaster muscle was constantly superfused with a bicarbonate-buffered salt solution containing (mmol l−1): NaCl 131.9, KCl 4.7, CaCl2 2.0, MgSO4 1.2, NaHCO3 30, and 0.3 mg l−1 tubarine (curare) equilibrated with gas containing 5% CO2–95% N2 (pH 7.35–7.45). Cremaster muscle temperature was maintained at 34.0°C. Following the surgery, all preparations were allowed to equilibrate for 45–60 min prior to data collection. The cremaster microvasculature was visualized by transillumination with a tungsten lamp and with an Olympus BX51WI microscope using a ×20 long working distance water immersion objective (n.a. 0.50) and a ×1.6 magnification changer. The microscope image was displayed via a video camera (Hitachi VK-C370) on a monitor and recorded on a videotape recorder (Sony, SVO-9600 MD). Final magnification of the site was ×2000. Diameter measurements were reproducible to within ± 0.3 μm.
Transverse arterioles of approximately 40 μm maximum diameter were observed. Transverse arterioles were identified as previously described (Mihok & Murrant, 2004). Our only selection criterion required that muscle fibres associated with the transverse arteriole run approximately perpendicular to the vessel. This architecture is common and can be found in all areas of the tissue preparation.
Muscle fibre bundles (3–5 fibres) were stimulated directly using a platinum wire microelectrode (tip diameter approximately 25 μm), which was placed in close proximity to, but not touching, muscle fibres running approximately perpendicular to the arteriole (observation site). The microelectrode was positioned at least 1000 μm away from the chosen site of the arteriole–stimulated muscle fibre intersection. Curare was added to the superfusate to ensure direct electrode stimulation of the skeletal muscle cells, but not nervous stimulation of the muscle fibres. Blocking nicotinic cholinergic membrane receptors on the skeletal muscle ensured that if a motor nerve was stimulated by the electrode, then the resulting acetylcholine release would not stimulate skeletal muscle cell contraction. Therefore the addition of curare isolated any muscle contraction to the control of the electrode only. The ground electrode was placed in the superfusate around the outer rim of the tissue support pedestal. Each stimulus was a square wave pulse of 0.4 ms duration and 8–15 V (Grass S48 stimulator, Quincy, MA, USA). The voltage was chosen to maximally stimulate four to five muscle fibres and then kept constant throughout the duration of the experiment.
After the equilibration period, arteriolar diameter at the observation site was continuously recorded for 1 min before muscle stimulation, and during and for 2 min after each single contraction. Muscle fibres were stimulated with a range of stimulus frequencies of 4, 10, 20, 30, 40, 60 and 80 Hz with a train duration of 250 ms. For each experiment, the order of stimulus frequencies was randomized and a 4–5 min rest period was allowed between each contraction. To test for a role of K+ in the dilatations to a single muscle contraction, the cremaster muscle was superfused with either 3 × 10−4m DAP (n = 12) for 10 min or 10−4m ouabain (n = 14) for 20 min or 5 × 10−5m BaCl2 (n = 14) for 30 min, and the stimulation protocol was repeated. Following each experiment, arteriolar diameters were recorded after 2 min of superfusion of the preparation with 10−4m sodium nitroprusside (considered to produce maximal dilatation).
Experiments showed that BaCl2 induced a significant decrease in baseline diameter (Table 1). In order to determine the effects of this change in baseline diameter on arteriolar vasodilatation in response to muscle contraction we contracted the muscle cells over the same range of stimulus frequencies used in situ in the absence and the presence of 10−8m phenylpherine (PHE), added to decrease the baseline diameter to approximately the same levels as with BaCl2.
Table 1.
Baseline and maximal arteriolar diameters for in situ experiments
| Baseline Diameter* (μm) | ||||
|---|---|---|---|---|
| Control | Experimental | Maximum diameter (μm) | n | |
| DAP | 22.0 ± 2.4 | 19.9 ± 2.7 | 41.8 ± 3.1 | 12 |
| BaCl2 | 21.7 ± 1.8 | 15.3 ± 1.5† | 40.1 ± 2.3 | 14 |
| Ouabain | 20.8 ± 1.8 | 17.2 ± 1.5 | 39.3 ± 2.3‡ | 14 |
| Phenylephrine | 22.5 ± 2.4 | 15.7 ± 1.3† | 46.5 ± 3.3 | 8 |
Baseline diameters were not significantly different across stimulus frequencies within each experiment therefore baseline diameters for 4 Hz were used as representative.
Significant difference from control values.
Significant difference from phenylephrine maximum diameter.
Time controls for the above experiments involving a drug treatment that followed an incubation time were conducted by stimulating the skeletal muscle cells over a range of stimulus frequencies and then waiting 30 min, with no treatment, and repeating the stimulation protocol. This was done in order to ensure that there was no change in responsiveness of the preparation over the longest drug incubation time used in this study.
In a separate set of experiments the effectiveness of the concentration and exposure duration for both ouabain and BaCl2 at blocking the dilatory response to K+ were tested by recording the arteriolar dilatory response to 15 mm KCl (equimolar NaCl removed to preserve the osmolarity of the superfusate) for 5 min in the absence of blockers and then again following exposure to 10−4m ouabain (n = 4) for 20 min or 5 × 10−5m BaCl2 (n = 4) for 30 min.
Following each experiment animals were killed with an overdose of sodium pentobarbital (70 mg kg−1i.v.).
In vitro protocol
In the in situ protocol, skeletal muscle cells were exposed to DAP, BaCl2 and ouabain each of which could alter skeletal muscle function, which may in turn result in an altered dilatory response to muscle contraction. To determine if there were systematic effects of the blockers on the force of contraction of the cremaster muscle fibres, following the in situ experiments the left cremaster muscle was exteriorized and cut into two strips, each of which was tied at the ends with 5.0-gauge suture silk and hung in a tissue chamber containing Krebs–Henseleit solution consisting of (mmol l−1): NaCl, 118; KCl, 4.75; CaCl2, 2.54; KH2PO4, 1.18; MgSO4, 1.18; NaHCO3, 24.8; glucose, 10. The solution contained 0.3 mg l−1 tubocurarine and 10 U insulin l−1 and was aerated continuously with 95% O2–5% CO2 at 27°C (pH 7.4). The cremaster strips were attached to a fixed point at one end and a force transducer on the other (either model FT03, Grass Medical Instruments, Quincy, MA, USA, or model 60-2995, Harvard Apparatus, Southmatic, MA, USA). Stimulating electrodes were fixed at each side of the muscle. The length of each muscle was adjusted to be optimal for force development. After an hour of equilibration, force of contraction was measured as muscles were stimulated over a range of stimulus frequencies (4, 10, 20, 30, 40, 60, 80 Hz), at a constant train duration of 250 ms with a supramaximal voltage (100 V). For each experiment, the order of stimulus frequencies was randomized. One of the pair of muscle strips (experimental) was then incubated with either 3 × 10−4m DAP (n = 6), 5 × 10−5m BaCl (n = 6), or 10−4m ouabain (n = 6) for 10, 20 and 30 min, respectively, the other of the pair was incubated in Krebs–Henseleit solution for the incubation time that corresponded with the experimental trial to act as an animal- and time-matched control. Following the incubation period the muscles were stimulated again over the above range of stimulus frequencies. Data were collected and analysed using the MP100WSW data acquisition system and Acqknowledge III software (Biopac Systems Inc., Goleta, CA, USA) on an IBM computer. Following each experiment muscle length and weight were measured.
Data analysis and statistics
All data are reported as means ± standard error of the mean (s.e.m.). Baseline diameter was defined as the diameter just prior to muscle stimulation. Only one arteriole per preparation was used to collect data, and n indicates the number of arterioles observed. All in situ experiments were videotaped and analysed off line. Images were digitized and arteriolar diameters measured via ImagePro Plus software. Arteriolar diameter was measured immediately following stimulation each second for 10 s, then every 5 s until 60 s and then at 90 and 120 s. Group means were compared with an ANOVA or a repeated measures ANOVA where data was analysed over time. When the ANOVAs identified significant differences, a protected LSD was used post hoc to determine if diameter changes were significantly different (Snedecor & Cochran, 1989). Differences were considered significant when P < 0.05.
Results
In situ protocol
Baseline and maximal diameters are shown in Table 1. Within each experiment the baseline diameters did not differ between stimulus frequencies so the baseline diameter prior to 4 Hz is shown as representative. Control baseline diameters did not significantly differ from experimental baseline diameters except following BaCl2 exposure. BaCl2 significantly decreased baseline diameter. PHE also significantly decreased baseline diameter (by design), to a level which was not significantly different from the baseline effects of BaCl2. The maximal diameters did not differ between groups except for maximum diameters between PHE and ouabain. Significant differences in baseline diameters may lead to an altered responsiveness of the blood vessels; significant differences in baseline diameter and maximal diameter may alter the dilatory potential (maximal diameter-baseline diameter) of the blood vessels. We found very low correlations between peak change in diameter and baseline diameter (average r2 value 0.2 ± 0.03) as well as very low correlations between the peak change in diameter and dilatory potential (average r2 value 0.1 ± 0.03) indicating that differences in baseline diameter and maximal diameter did not have a significant influence on the ability of the blood vessels to dilate.
Figure 1 shows the three patterns of dilatation typically observed for a single contraction over a range of stimulus frequencies. A single impulse (4 Hz for 250 ms), which produces a twitch contraction, produced a single, rapid dilatation that peaked at approximately 3 s and returned to baseline within 10 s. Stimulus frequencies of 20, 30 and 40 Hz produced a biphasic dilatation characterized by a single rapid dilatation peaking within 4 s followed by a secondary, longer lasting dilatation peaking at approximately 20 s before returning to baseline approximately 60 s later; 60 and 80 Hz produced a single, larger dilatation that peaked within 10 s and, in some cases, required over 120 s to recover. Contractions at each stimulus frequency produced significant dilatations within 1 s of the contraction (the first time period measured). Characteristic of all stimulus frequencies was an initial dilatory event that peaked at approximately 4 s. A second dilatory event occurred at approximately 20 s at stimulus frequencies of 20, 30 and 40 Hz.
Figure 1. The change in diameter over time in response to a single contraction at 4 Hz (A) 20 Hz (B) and 60 Hz (C).
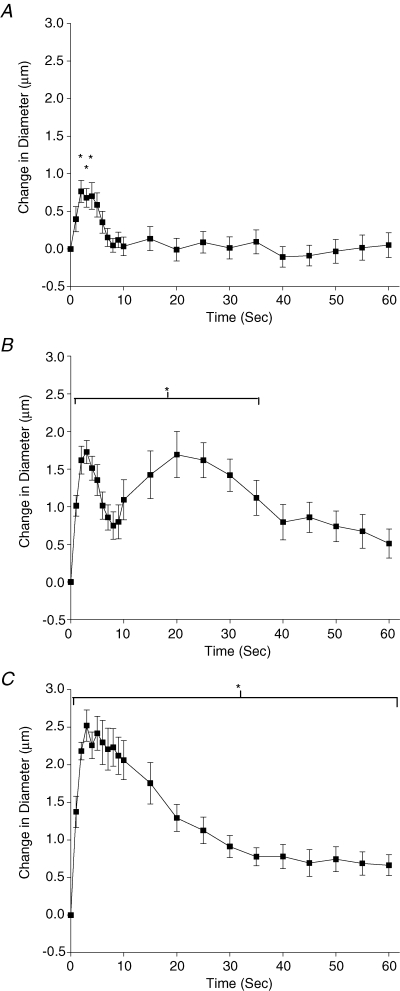
Representative data (control data from the BaCl2 experiment) showing the three typical patterns of dilatation observed over a range of stimulus frequencies. *Data or the range of data significantly different from 0.
Figure 2 shows the effect of 3 × 10−4m DAP on the magnitude of the dilatory event at 4 s and the magnitude of the dilatation at 20 s across all stimulus frequencies. DAP significantly inhibited the initial dilatory event at all stimulus frequencies but did not consistently inhibit the magnitude of the dilatation at 20 s. The dilatory event at 20 s was inhibited significantly by DAP at frequencies where there is a pronounced dilatory peak at 20 s, 20 and 40 Hz, but not consistently as there was no inhibition of the second dilatory 30 Hz. The dilatation at 20 s was not inhibited at higher stimulus frequencies, 60 and 80 Hz, but there is no pronounced dilatory peak at 20 s at these frequencies.
Figure 2. The effect of 3 × 10−4m DAP on the change in diameter at 4 s (A) and 20 s (B) across all stimulus frequencies tested.
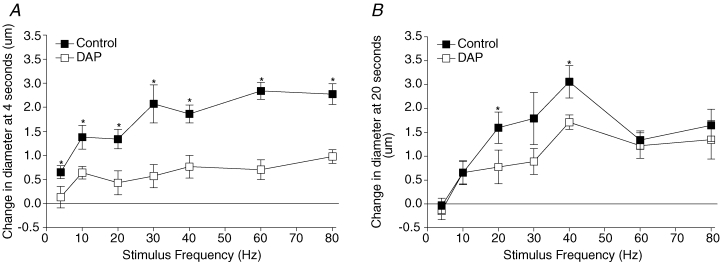
*Control change in diameter significantly different from diameter in the presence of DAP. At 4 s the P values for main and interactive effects were: drug < 0.0001, frequency < 0.0001, and drug–frequency interaction = 0.13, and at 20 s these were 0.0077, < 0.0001 and 0.53, respectively.
Ouabain significantly inhibited the initial dilatory event at 4 s across all stimulus frequencies as well as dilatory events at 20 s at most stimulus frequencies where a distinct second prolonged peak occurs (20–80 Hz) (Fig. 3). BaCl2 produced a significant decrease in baseline diameter. To test whether this change in baseline diameter influenced the ability of the blood vessel to respond to muscle contraction, we added 10−8m PHE in order to decrease baseline diameter similarly (Table 1). Figure 4 compares the change in diameter from control changes (control – experimental) induced by the range of stimulus frequencies in the presence of BaCl2 and PHE. We observed that BaCl2 significantly inhibited the initial dilatory event at 4 s across most stimulus frequencies (except 4 and 40 Hz) but did not consistently inhibit dilatations at 20 s when compared to PHE. Experiments where both ouabain and BaCl2 were added simultaneously, in order to determine if the initial dilatory response could be eliminated completely, resulted in spontaneous skeletal muscle activity (where the muscle appeared to be twitching), and thus these experiments were not continued.
Figure 3. The effect of 10−4m ouabain on the change in diameter at 4 s (A) and 20 s (B) across all stimulus frequencies tested.
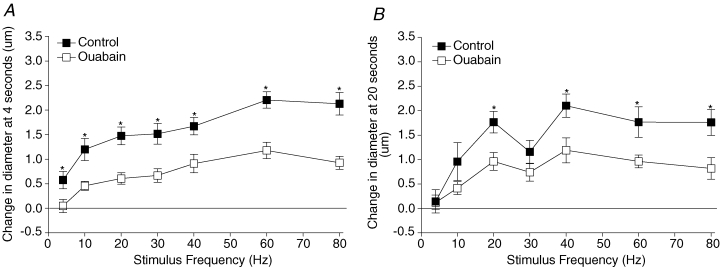
*Control change in diameter is significantly different from diameter in the presence of ouabain. At 4 s the P values for main and interactive effects were: drug < 0.0001, frequency < 0.0001, and drug–frequency interaction = 0.58, and at 20 s these were < 0.0001, < 0.0001, and 0.41, respectively.
Figure 4. The effect of 5 × 10−5m BaCl2 (□) and 10−8m PHE (▪) on the change in diameter at 4 s (A) and 20 s (B) across all stimulus frequencies tested.
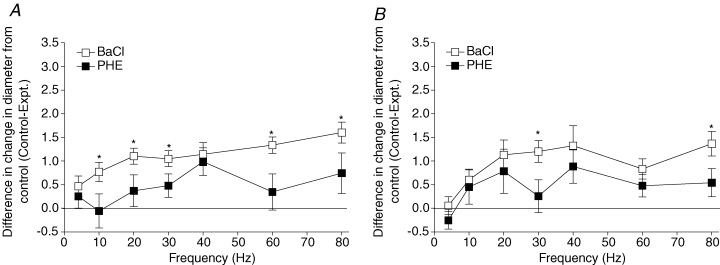
The change in diameter is represented by the change in diameter under control conditions minus the change in diameter under experimental conditions (Control–Expt.). *Control change in diameter significantly different from diameter in the presence of BaCl2. At 4 s the P values for main and interactive effects were: drug < 0.0001, frequency = 0.0095, and drug–frequency interaction = 0.58, and at 20 s these were 0.0044, 0.0045, 0.86, respectively.
Given that all experiments involved a 10–30 min blocker incubation period between when control and experimental data were taken, we undertook time control experiments to ensure that the reactivity of our preparation did not change over time. Figure 5 shows that the initial dilatory response to muscle contraction across all stimulus frequencies at 4 and 20 s did not systematically differ significantly from the dilatory response following 30 min, which encompasses our longest incubation time. Thus, the vasculature was equally reactive to skeletal muscle stimulation over time.
Figure 5. Time controls for the data in Figs 2–4.
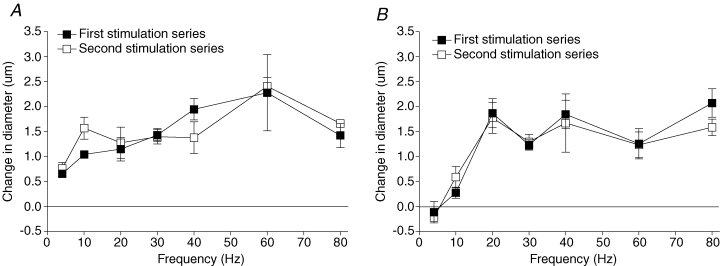
Skeletal muscle cells were stimulated over a range of stimulus frequencies (first stimulation series, ▪) and then again following 30 min (second stimulation series, □). A, effect of stimulating the preparation twice with no treatment on the change in diameter at 4 s. B, effect of stimulating the preparation twice with no treatment on the change in diameter at 20 s. At 4 s the P values for main and interactive effects were: time = 0.6143, frequency = < 0.0001 and time–frequency interaction = 0.6410, and at 20 s these were 0.6143, < 0.0001, and 0.8720, respectively.
In a separate set of experiments we tested the efficacy of 5 × 10−5m BaCl2 and 10−4m ouabain in their ability to inhibit the dilatory effects of K+. We found that the dilatation in response to 15 mm K+ was transient, peaking within 2 s before returning to baseline diameter by 7 s. BaCl2 significantly decreased the 15.0 ± 2.6 μm peak change in diameter induced by K+ to 7.9 ± 1.8 μm and ouabain significantly decreased the 10.5 ± 2.0 μm change in diameter induced by K+ to 3.4 ± 1.4 μm.
In vitro protocol
We tested the effects of DAP, BaCl2 and ouabain on force generation of cremaster strips in vitro to assess any systematic changes in skeletal muscle force generation in the presence of each of the blockers (Table 2). We found that BaCl2 exposure decreased force significantly at all tetanic stimulus frequencies by up to 54.8 ± 5.8% when compared to time matched controls and ouabain decreased force significantly at all tetanic stimulus frequencies by up to 46.7 ± 10.6%. Twitch contraction force development (4 Hz at 250 ms train duration) was not significantly inhibited by either BaCl2 or ouabain but there was a trend to decrease twitch force development. Conversely, DAP did not significantly inhibit force at any stimulus frequency; in fact there was a trend to amplify developed force in the presence of DAP and a significant increase in developed force at 4 and 10 Hz (Table 2).
Table 2.
In vitro force data (in grams) under control conditions and after BaCl2, ouabain or DAP exposure at each stimulus frequency tested
| Stimulus freq. (Hz) | DAP control‡ | DAP | BaCl2 control‡ | BaCl2 | Ouabain control‡ | Ouabain |
|---|---|---|---|---|---|---|
| 4 | 0.8 ± 0.1 | 1.2 ± 0.3* | 2.0 ± 0.2 | 1.5 ± 0.3 | 1.4 ± 0.3 | 0.9 ± 0.1 |
| 10 | 1.2 ± 0.2 | 1.9 ± 0.5* | 3.4 ± 0.4 | 2.0 ± 0.6* | 2.6 ± 0. 6 | 1.6 ± 0.3* |
| 20 | 2.8 ± 0.4 | 4.0 ± 1.2 | 5.9 ± 0.5 | 3.1 ± 0.8* | 3.9 ± 0.7 | 2.2 ± 0.4* |
| 30 | 3.6 ± 0.5 | 4.6 ± 1.3 | 6.6 ± 0.5 | 3.5 ± 1. 0* | 4.9 ± 0.9 | 2.6 ± 0.4* |
| 40 | 4.2 ± 0.5 | 4.9 ± 1.3 | 7.3 ± 0.4 | 4.5 ± 1.3* | 5.4 ± 1.0 | 3.3 ± 0.6* |
| 60 | 4.7 ± 0.6 | 5.2 ± 1.3 | 7.9 ± 0.6 | 4.7 ± 1.3* | 5.5 ± 1.0 | 2.9 ± 0.4* |
| 80 | 4.6 ± 0.7 | 4.9 ± 1.3 | 7.7 ± 0.5 | 4.8 ± 1.3* | 5.5 ± 1.1 | 3.4 ± 0.6* |
Time and animal matched controls.
Significant difference from the time and animal matched controls.
Discussion
The purpose of this study was to determine the role of K+ in the rapid vasodilatations in response to a single muscle contraction. We found that inhibiting either a source of K+, by blocking the KV channel on skeletal muscle cells, or by inhibiting mechanisms in the K+ dilatory signal transduction pathway, the KIR channel and Na+ K+-ATPase on cells of the vasculature (smooth muscle and/or endothelial cells), was successful in attenuating the rapid dilatation produced by a single muscle contraction at all stimulus frequencies. Therefore, there is a dilatory role for K+ in response to muscle contraction at all stimulus frequencies.
Experimental considerations
The hamster cremaster model was used to evaluate the dynamics between contracting skeletal muscle and the associated vasculature and processes initiating vasodilatation. The hamster cremaster muscle is a very thin muscle, approximately five to six skeletal muscle layers thick, consisting of mostly fast twitch fibres (∼13–20% type IIa and 60–75% type IIb, 15% type I; Sarelius et al. 1983), similar to extensor digitorum longus (EDL) of the hamster (Phipps & Elbrink, 1983) as well as other species (Crow & Kushmerick, 1982; Beaune et al. 1994; Faucher et al. 2005). The cremaster muscle has also been shown to be biochemically similar to EDL in terms of the relative amounts of eNOS and nNOS (Lau et al. 2000). Hamster cremaster muscle has contractile properties (Murrant, 2005) that are typical of other mixed muscles (Murrant & Barclay, 1995; McGuire et al. 2003; Singh et al. 2004). The cremaster muscle has a vasculature architecture that differs from other more three dimensional muscles, but the control of capillary recruitment (Sarelius, 1986; Sweeney & Sarelius, 1989) is similar to other muscles whose architecture and capillary recruitment mechanisms have been studied (tibialis anterior: Lund et al. 1987; Delashaw & Duling, 1988; and gastrocnemius: Hilton et al. 1978). Therefore, the fact that the cremaster is composed of muscle fibres similar to other fast twitch muscles (such as EDL) and that it has capillary recruitment control mechanisms similar to other skeletal muscles make this a good model for the study of active hyperaemia and blood flow control in skeletal muscle.
The advantages of using this preparation to study the events initiating the rapid dilatation in response to skeletal muscle contraction are (1) that the cremaster is thin and ideal for visualization of all levels of the microvasculature via microscopy and small fibre bundles associated with any level of the vasculature can be targeted; (2) unlike thicker preparations, a few muscle fibres, anywhere in the preparation, can be stimulated to contract such that any level of the vasculature can be studied; and (3) only a very small, localized region of a blood vessel can be affected, and therefore local dilatory events can be monitored in the absence of vascular compression and in the absence of interference of conducted dilatory responses from contracting muscles in other regions of the tissue (Berg et al. 1997; Murrant & Sarelius, 2000; Emerson & Segal, 2001) both of which can occur when contracting the whole muscle. This preparation is therefore ideal for studying the very early events of vasodilatation at the onset of muscle contraction and is ideal for identifying the initiators of active hyperaemia.
Significant differences were observed in the baseline diameter following BaCl2 application as well as differences in maximal diameter between the PHE and ouabain groups. Differences in baseline diameter could result in changes in smooth muscle reactivity as a result of differences in the smooth muscle cell length. Because of the length–tension relationship in smooth muscle, the length of the smooth muscle can influence its ability to constrict or dilate to vasoactive substances (Davis & Gore, 1989; Seow, 2000). Differences in baseline diameter, along with differences in the maximal diameter, also changes the dilatory potential of the vessel (dilatory potential = maximal diameter – baseline diameter). We could not identify that either differences in baseline diameter or maximal diameter affected the behaviour of the blood vessel response to muscle contraction. We could not demonstrate strong correlations between the peak change in diameter and the baseline diameters under control condition or correlations between peak change in diameter and dilatory potential in this study as well as others (Mihok & Murrant, 2004; Murrant, 2005). Thus, the difference in baseline diameter and maximal diameter does not significantly impact the response of the vessels to muscle contraction or our interpretation of the data.
Effects of developed force in vitro
Force could not be measured directly in the in situ preparation. The in vitro experiments using hamster cremaster strips allow us to obtain a force response relative to that in situ for correct data interpretation. The in vitro developed force is expressed in Table 2 in grams, not normalized to muscle mass or normalized to cross-sectional area as is usual for in vitro skeletal muscle preparations. These normalizations create a large variability in the data as both normalizations use the weight of the muscle. The cremaster muscle consists of five to six layers of muscle cells in multiple orientations, only one of which will be aligned parallel to the ties on the end of the muscle and contributing to force generation. Therefore only about one-sixth of the weight will be contributing to force generation. Muscle strip weight is also variable as the ends of the muscle strip are tied first then the muscle strip is cut out leaving tissue at the sides of each preparation that is not included in the ties, and therefore not contributing to force but contributing to weight. Therefore, normalizations were not used in the expression of the data in Table 2 as they are misleading. If we were to normalize force to mN mm−2 (based on muscle length at optimal length in mm, weight in mg, and density of 1.06 g cm−3) for BaCl2 controls at 80 Hz the result is 27.1 ± 5.2 mN m−2 (average length 11.2 ± 0.03 mm, average weight 53.3 ± 24.2 mg). This force represents only-one sixth of the force that could be generated by a muscle of this weight, and therefore the predicted force generated would be 6 times this or 162.6 mN mm−2, which is comparable to other in vitro skeletal muscle preparations (Brooks & Faulkner, 1988; Murrant & Barclay, 1995; McGuire et al. 2001; Mishima et al. 2005).
The dilatory responses did not correlate with force generated by the skeletal muscle cells themselves. We observed that both BaCl2 and ouabain significantly inhibited force generation while DAP had a tendency to amplify force. BaCl2 has been shown to inhibit K+ influx and efflux via KIR channels on skeletal muscle cells with a pronounced decrease in contractile force and decrease in M-wave (compound action potential) area (Clausen & Overgaard, 2000). Ouabain has been shown to increase the rate of force decline within seconds of exposure (Nielsen & Clausen, 1996; Harrison et al. 1997) and also decreases the M-wave area (Ferguson et al. 2001). The interference with excitability by both blockers is thought to be the cause of the decrease in force generation. DAP significantly increased force at 4 and 10 Hz and there was a non-significant trend to increase force at all other stimulus frequencies. DAP has been observed to increase contractile force (Van Lunteren & Moyer, 1996) possibly by prolonging the action potential. Both the delayed repolarization and a reduced accumulation of K+ in the t-tubules are thought to be the mechanisms by which DAP may amplify force generation. Regardless of the specifics of the direct effects of the blockers on skeletal muscle we show that both BaCl2 and ouabain significantly inhibited force generation, and therefore the reduced vascular reactivity in the presence of these blockers could be due to the reduced force production. Although we do demonstrate that BaCl2 and ouabain significantly decreased force production we also show that DAP did not have an effect on force generation where as all three blockers significantly inhibited the vasodilatations induced by muscle contraction. Thus, there was no systematic change in diameter that correlated with the effects of the inhibitors on force generation and the changes in vasodilatation in the presence of the blockers may not be attributable to changes in force generation.
Inhibition of a source of K+
We observed that the initial rapid dilatation at 4 s was inhibited across all stimulus frequencies with DAP and that the 20 s dilatation was not consistently inhibited. DAP was used to target inhibition of the skeletal muscle cells' Kv channels but both vascular smooth muscle cells and endothelial cells were exposed to DAP as well. At the vasculature level, DAP has been shown to cause depolarization and vasoconstriction of cerebral arterioles (Knot & Nelson, 1995), possibly through Kv, on smooth muscle themselves (Cheong et al. 2001a,b; Kerr et al. 2001; Thorneloe et al. 2001; Amberg et al. 2003). Kv channels have been identified on microvascular endothelial cells (Fan & Walsh, 1999; Cheong et al. 2001a,b; Hogg et al. 2002), but their physiological relevance has not been yet been determined. We did not observe a significant decrease in baseline diameter in the presence of DAP, and therefore the potential depolarization produced in our vessels with our concentration of DAP did not significantly depolarize our vessels. Thus the effect of depolarization with DAP via its effects on vascular smooth muscle or endothelial cells was considered minimal and the decrease in vasodilatation after DAP incubation was primarily attributed to blocking Kv channels on skeletal muscle cells.
Inhibition of the sites of action of K+
We observed that both BaCl2 and ouabain decreased contraction-induced vasodilatation at 4 s at all stimulus frequencies except 4 Hz and 40 Hz with BaCl2. Ouabain also decreased the amount of dilatation at 20 s where a significant second dilatory event occurs at stimulus frequencies of 20, 30 and 40 Hz and above. The presence of a delayed second dilatation that is longer lasting implies that there is a second dilator present, acting on a different time scale. Our data indicate that K+ may not be the primary dilator involved in the second dilatation, as KV and KIR channel blocking did not systematically inhibit the second dilatation. The dilator causing the 20 s dilatation may also be dependent on Na+ K+-ATPase as their inhibition systematically decreased the amount of dilatation as there may be overlap of signalling pathways when multiple dilators are involved. Determining the identity of the other dilators involved will be a necessary first step in determining the role of Na+ K+-ATPase in the second dilatation.
The primary target for BaCl2 and ouabain were the vascular cells, but which cell type, endothelial cells or vascular smooth muscles cells, was the primary site of inhibition is unknown. KIR channels and Na+K+-ATPase have been identified on both cell types (for review see Jackson, 2005) and both microvascular smooth muscle cells and endothelial cells are connected via gap junctions (Little et al. 1995); therefore changes in the membrane potential of one cell type can be reflected in changes in the other cell type (Beach et al. 1998; Emerson & Segal, 2000). Under these circumstances it is difficult to discern in which cell type the signals are initiated. Skeletal muscle cells were also exposed to the BaCl2 and ouabain in situ, and we observed in vitro a significant decrease in force generated by skeletal muscle cells exposed to both of these inhibitors, probably as a result of an interference with excitability as discussed previously. Decreasing the excitability of the skeletal muscle cells may decrease the amount of K+ efflux per action potential and therefore may contribute to the decreased dilatory response that we observed in the presence of these blockers. The effects of BaCl2 and ouabain are similar to DAP at 4 s but not at 20 s. Regardless, the potential decrease in K+ efflux from skeletal muscle as a result of ouabain and BaCl2 incubation would still implicate K+ in the dilatory response to muscle contraction.
Blocking KIR channels with BaCl2 produced a significant baseline vasoconstriction, probably the result of depolarization of smooth muscle cells (Edwards & Hirst, 1988; Hirst & Edwards, 1989). To ensure that the decrease in vasodilatory response was not strictly due to depolarization events, we used PHE to depolarize and constrict the vascular smooth muscle (Xia & Duling, 1995; Xia et al. 1995; Welsh & Segal, 1998) and mimic the depolarization and baseline constriction seen with BaCl2. The relationship between membrane potential and diameter change for PHE is similar to that of other constrictors (high KCl) (Welsh & Segal, 1998). Although there was a tendency for less dilatation to occur in the presence of PHE, there was greater inhibition with BaCl2. Therefore, although depolarization may explain some of the effects of BaCl2 it does not explain them all, and the totality of the data, from all blockers used in this study, support a role for K+ in initiation of the rapid dilatation in response to skeletal muscle contraction.
The role of K+ in active hyperaemia
Our data indicate that K+ is one of the dilators involved in the rapid dilatation observed at the onset of contraction and that a single contraction, at any stimulus frequency, may yield enough K+ to produce a vasodilatory response. We have identified a role for K+ in the initiation of the rapid dilatation induced by a single muscle contraction, but its precise role in active hyperaemia, especially beyond a single contraction, will need to be further defined. There are indications that its dilatory role may be limited and confined to very early events of initiation. (1) K+ has the ability promote a transient dilatation, and therefore depending on how long the contraction bout is the role for K+ may diminish over time. (2) The contribution of K+ to the overall amount of dilatation differs depending on the stimulus frequency used to stimulate the skeletal muscle cells. We have shown that the amount of overall vasodilatation attributable to K+ is dependent on the stimulus frequency. At low stimulus frequencies (4 Hz), where the dilatation in response to muscle contraction is composed of only a single rapid peak, the contribution of K+ will be much greater than the contribution of K+ at higher stimulus frequencies where there appear to be multiple dilators (60 Hz) and K+ plays less of a role in the total dilatation. Therefore, given the very transient ability of K+ to dilate, and the variable contribution of its affects depending on stimulus frequency, the physiological role for K+ in active hyperaemia, especially over time, is unknown. The literature does support a role for K+ in the initiation of vasodilatory processes and our data support this role, but, given the very transient nature of the effects of K+, its role may be confined to only the very early events of initiation.
We have shown that we could partially block the contraction induced dilation with the concentrations of DAP, BaCl2 and ouabain used in this study. We also show that the concentrations of ouabain and BaCl2 used in this study were effective at blocking approximately 50% of the dilatory response to 15 mm K+. Although a 100% block is not necessary to demonstrate a role for K+ in skeletal muscle contraction induced dilations, we do not know the full extent of the role of K+. Our inability to fully inhibit the rapid dilatation to muscle contraction may be due to the concentrations of the drugs used and it is possible that higher concentrations of the blockers would have yielded full inhibition. Our inability to inhibit the full response could also indicate that there are other vasodilators involved in the rapid dilatation induced by muscle contraction. Further studies are required to determine if more than one dilator is responsible for the rapid dilatation induced by skeletal muscle contraction.
In conclusion, we have shown that there is a role for K+ in the rapid response to a single contraction in cremaster muscle. The role is detectable within 4 s of the contraction at all stimulus frequencies, but less defined in a secondary dilatation, by 20 s. Our data indicate that there are potentially multiple dilators involved in response to a single contraction and that K+ is at least partially responsible for the very rapid component of this dilatation. What is yet to be determined is the role for K+ over time and the physiological relevance of such a dilator whose dilatory ability is transient.
Acknowledgments
We would like to thank Dr Jack K. Barclay, Sean Leonard and Jasna Junuzovic for their helpful insight and discussion regarding these experiments, data interpretation and manuscript preparation. This work was supported by NSERC and Premiers Research Excellence Award.
References
- Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol. 2003;284:C583–C595. doi: 10.1152/ajpcell.00301.2002. [DOI] [PubMed] [Google Scholar]
- Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 1973;5:384–394. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- Beach JM, McGahren ED, Duling BR. Capillaries and arterioles are electrically coupled in hamster cheek pouch. Am J Physiol Heart Circ Physiol. 1998;275:H1489–H1496. doi: 10.1152/ajpheart.1998.275.4.H1489. [DOI] [PubMed] [Google Scholar]
- Beaune B, Fellmann N, Villie F, Marcollet M, Coudert J. Effects of orchiectomy on EDL muscle ultrastructure and energy-supplying enzymes in growing male guinea pigs. Am J Physiol Cell Physiol. 1994;266:C143–C148. doi: 10.1152/ajpcell.1994.266.1.C143. [DOI] [PubMed] [Google Scholar]
- Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol Heart Circ Physiol. 1997;272:H2693–H2700. doi: 10.1152/ajpheart.1997.272.6.H2693. [DOI] [PubMed] [Google Scholar]
- Bockman EL, Berne RM, Rubio R. Adenosine and active hyperemia in dog skeletal muscle. Am J Physiol. 1976;230:1531–1537. doi: 10.1152/ajplegacy.1976.230.6.1531. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT, Brace RA, Scott JB, Anderson DK, Haddy FJ. The mechanism of the vasodilator action of potassium. Proc Soc Exp Biol Medical. 1972;140:820–824. doi: 10.3181/00379727-140-36560. [DOI] [PubMed] [Google Scholar]
- Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel [KVα1] subunits in terminal arterioles of rabbit. J Physiol. 2001a;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong A, Dedman AM, Xu SZ, Beech DJ. KVα1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001b;281:H1057–H1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- Clausen T, Overgaard K. The role of K+ channels in the force recovery elicited by Na+-K+ pump stimulation in Ba2+-paralysed rat skeletal muscle. J Physiol. 2000;527:325–332. doi: 10.1111/j.1469-7793.2000.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Gore RW. Length-tension relationship of vascular smooth muscle in single arterioles. Am J Physiol Heart Circ Physiol. 1989;256:H630–H640. doi: 10.1152/ajpheart.1989.256.3.H630. [DOI] [PubMed] [Google Scholar]
- Dawes GS. The vaso-dilator action of potassium. J Physiol. 1941;99:224–238. doi: 10.1113/jphysiol.1941.sp003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delashaw JB, Duling BR. A study of the functional elements regulating capillary perfusion in striated muscle. Microvasc Res. 1988;36:162–171. doi: 10.1016/0026-2862(88)90016-7. [DOI] [PubMed] [Google Scholar]
- Dodd LR, Johnson PC. Diameter changes in arteriolar networks of contracting skeletal muscle. Am J Physiol Heart Circ Physiol. 1991;260:H662–H670. doi: 10.1152/ajpheart.1991.260.3.H662. [DOI] [PubMed] [Google Scholar]
- Duling BR. Effects of potassium ion on the microcirculation of the hamster. Circ Res. 1975;37:325–332. doi: 10.1161/01.res.37.3.325. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD. Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol. 1988;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel DA, Scott JB, Haddy FJ. Effect of potassium on small and large blood vessels of the dog forelimb. Am J Physiol. 1959;197:637–642. doi: 10.1152/ajplegacy.1959.197.3.637. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol. 2001;280:H160–H167. doi: 10.1152/ajpheart.2001.280.1.H160. [DOI] [PubMed] [Google Scholar]
- Fan J, Walsh KB. Mechanical stimulation regulates voltage-gated potassium currents in cardiac microvascular endothelial cells. Circ Res. 1999;84:451–457. doi: 10.1161/01.res.84.4.451. [DOI] [PubMed] [Google Scholar]
- Faucher M, Guillot C, Marqueste T, Kipson N, Mayet-Sornay MH, Desplanches D, Jammes Y, Badier M. Matched adaptations of electrophysiological, physiological, and histological properties of skeletal muscles in response to chronic hypoxia. Pflugers Arch. 2005;450:45–52. doi: 10.1007/s00424-004-1370-6. [DOI] [PubMed] [Google Scholar]
- Ferguson RA, Ball D, Krustrup P, Aagaard P, Kjaer M, Sargeant AJ, Hellsten Y, Bangsbo J. Muscle oxygen uptake and energy turnover during dynamic exercise at different contraction frequencies in humans. J Physiol. 2001;536:261–271. doi: 10.1111/j.1469-7793.2001.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddy FJ. Potassium effects on contraction in arterial smooth muscle mediated by Na+,K+-ATPase. Fed Proc. 1983;42:239–245. [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol. 2004;557:1013–1020. doi: 10.1113/jphysiol.2004.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AP, Nielsen OB, Clausen T. Role of Na+-K+ pump and Na+ channel concentrations in the contractility of rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1402–R1408. doi: 10.1152/ajpregu.1997.272.5.R1402. [DOI] [PubMed] [Google Scholar]
- Hilton SM, Hudlicka O, Marshall JM. Possible mediators of functional hyperaemia in skeletal muscle. J Physiol. 1978;282:131–147. doi: 10.1113/jphysiol.1978.sp012453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirche H, Schumacher E, Hagemann H. Extracellular K+ concentration and K+ balance of the gastrocnemius muscle of the dog during exercise. Pflugers Arch. 1980;387:231–237. doi: 10.1007/BF00580975. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989;69:546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hnik P, Holas M, Krekule I, Kuriz N, Mejsnar J, Smiesko V, Ujec E, Vyskocil F. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflugers Arch. 1976;362:85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- Hogg DS, McMurray G, Kozlowski RZ. Endothelial cells freshly isolated from small pulmonary arteries of the rat possess multiple distinct K+ current profiles. Lung. 2002;180:203–214. doi: 10.1007/s004080000094. [DOI] [PubMed] [Google Scholar]
- Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Bangsbo J, Graham T, Saltin B. Lactate and potassium fluxes from human skeletal muscle during and after intense, dynamic, knee extensor exercise. Acta Physiol Scand. 1990;140:147–159. doi: 10.1111/j.1748-1716.1990.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1.2-Kv1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K+ current of rabbit vascular myocytes. Circ Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- Kiens B, Saltin B, Walloe L, Wesche J. Temporal relationship between blood flow changes and release of ions and metabolites from muscles upon single weak contractions. Acta Physiol Scand. 1989;136:551–559. doi: 10.1111/j.1748-1716.1989.tb08701.x. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol Heart Circ Physiol. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Lash JM, Bohlen HG. Perivascular and tissue PO2 in contracting rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. 1987;252:H1192–H1202. doi: 10.1152/ajpheart.1987.252.6.H1192. [DOI] [PubMed] [Google Scholar]
- Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics. 2000;2:21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Loeb AL, Godeny I, Longnecker DE. Functional evidence for inward-rectifier potassium channels in rat cremaster muscle arterioles. Microvasc Res. 2000;59:1–6. doi: 10.1006/mvre.1999.2187. [DOI] [PubMed] [Google Scholar]
- Lott ME, Hogeman CS, Vickery L, Kunselman AR, Sinoway LI, MacLean DA. Effects of dynamic exercise on mean blood velocity and muscle interstitial metabolite responses in humans. Am J Physiol Heart Circ Physiol. 2001;281:H1734–H1741. doi: 10.1152/ajpheart.2001.281.4.H1734. [DOI] [PubMed] [Google Scholar]
- Lund N, Damon DH, Damon DN, Duling BR. Capillary grouping in hamster tibials anterior muscles: flow patterns, and physiological significance. Int J Microcirc Clin Exp. 1987;5:359–372. [PubMed] [Google Scholar]
- Marshall JM, Tandon HC. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol. 1984;350:447–459. doi: 10.1113/jphysiol.1984.sp015211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Bradford A, MacDermott M. The effects of dietary creatine supplements on the contractile properties of rat soleus and extensor digitorum longus muscles. Exp Physiol. 2001;86:185–190. doi: 10.1113/eph8602131. [DOI] [PubMed] [Google Scholar]
- McGuire M, MacDermott M, Bradford A. Effects of chronic intermittent asphyxia on rat diaphragm and limb muscle contractility. Chest. 2003;123:875–881. doi: 10.1378/chest.123.3.875. [DOI] [PubMed] [Google Scholar]
- Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol. 2004;82:282–287. doi: 10.1139/y04-016. [DOI] [PubMed] [Google Scholar]
- Mishima T, Yamada T, Matsunaga S, Wada M. N-acetylcysteine fails to modulate the in vitro function of sarcoplasmic reticulum of diaphragm in the final phase of fatigue. Acta Physiol Scand. 2005;184:195–202. doi: 10.1111/j.1365-201X.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- Mohrman DE. Lack of influence of potassium or osmolality on steady-state exercise hyperemia. Am J Physiol Heart Circ Physiol. 1982;242:H949–H954. doi: 10.1152/ajpheart.1982.242.6.H949. [DOI] [PubMed] [Google Scholar]
- Mohrman DE, Sparks HV. Role of potassium ions in the vascular response to a brief tetanus. Circ Res. 1974;35:384–390. doi: 10.1161/01.res.35.3.384. [DOI] [PubMed] [Google Scholar]
- Murrant CL. Stimulation characteristics that determine arteriolar dilation in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R505–R513. doi: 10.1152/ajpregu.00571.2004. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Barclay JK. Endothelial cell products alter mammalian skeletal muscle function in vitro. Can J Physiol Pharmacol. 1995;73:736–741. doi: 10.1139/y95-096. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Coupling of muscle metabolism and muscle blood flow in capillary units during contraction. Acta Physiol Scand. 2000;168:531–541. doi: 10.1046/j.1365-201x.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- Naik JS, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol. 1999;87:1741–1746. doi: 10.1152/jappl.1999.87.5.1741. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, Clausen T. The significance of active Na+,K+ transport in the maintenance of contractility in rat skeletal muscle. Acta Physiol Scand. 1996;157:199–209. doi: 10.1046/j.1365-201X.1996.d01-748.x. [DOI] [PubMed] [Google Scholar]
- Phipps BA, Elbrink J. Monosaccharide transport and hexokinase activity in leg muscles from cardiomyopathic hamsters. Can J Physiol Pharmacol. 1983;61:369–375. doi: 10.1139/y83-056. [DOI] [PubMed] [Google Scholar]
- Radawski DP, Hoppe W, Haddy FJ. Role of vasoactive substances in active hyperemia in skeletal muscle (38520) Proc Soc Exp Biol Medical. 1975;148:270–276. doi: 10.3181/00379727-148-38520. [DOI] [PubMed] [Google Scholar]
- Sarelius IH. Cell flow path influences transit time through striated muscle capillaries. Am J Physiol Heart Circ Physiol. 1986;250:H899–H907. doi: 10.1152/ajpheart.1986.250.6.H899. [DOI] [PubMed] [Google Scholar]
- Sarelius IH, Maxwell LC, Gray SD, Duling BR. Capillarity and fiber types in the cremaster muscle of rat and hamster. Am J Physiol Heart Circ Physiol. 1983;245:H368–H374. doi: 10.1152/ajpheart.1983.245.2.H368. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Tschakovsky ME. Evidence for a rapid vasodilatory contribution to immediate hyperemia in rest-to-mild and mild-to-moderate forearm exercise transitions in humans. J Appl Physiol. 2004;97:1143–1151. doi: 10.1152/japplphysiol.01284.2003. [DOI] [PubMed] [Google Scholar]
- Scott JB, Rudko M, Radawski D, Haddy FJ. Role of osmolarity, K+, H+, Mg++, and O2 in local blood flow regulation. Am J Physiol. 1970;218:338–345. doi: 10.1152/ajplegacy.1970.218.2.338. [DOI] [PubMed] [Google Scholar]
- Seow CY. Response of arterial smooth muscle to length perturbation. J Appl Physiol. 2000;89:2065–2072. doi: 10.1152/jappl.2000.89.5.2065. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol. 1998;76:418–427. doi: 10.1139/cjpp-76-4-418. [DOI] [PubMed] [Google Scholar]
- Singh YN, Schlenker EH, Singh BN, Burbach JA. Consequences of thyroxine treatment on diaphragm and EDL of normal and dystrophic hamsters. Can J Physiol Pharmacol. 2004;82:345–352. doi: 10.1139/y04-029. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, IA, USA: Iowa State University Press; 1989. [Google Scholar]
- Stowe DF, Owen TL, Anderson DK, Haddy FJ, Scott JB. Interaction of O2 and CO2 in sustained exercise hyperemia of canine skeletal muscle. Am J Physiol. 1975;229:28–33. doi: 10.1152/ajplegacy.1975.229.1.28. [DOI] [PubMed] [Google Scholar]
- Sweeney TE, Sarelius IH. Arteriolar control of capillary cell flow in striated muscle. Circ Res. 1989;64:112–120. doi: 10.1161/01.res.64.1.112. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K+ channels of vascular smooth muscle. Circ Res. 2001;89:1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- Tominaga S, Suzuki T, Nakamura T. Evaluation of roles of potassium, inorganic phosphate, osmolarity, pH, pCO2, pO2, and adenosine or AMP in exercise and reactive hyperemias in canine hindlimb muscles. Tohoku J Exp Med. 1973;109:347–363. doi: 10.1620/tjem.109.347. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol. 2004;97:739–747. doi: 10.1152/japplphysiol.00185.2004. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol. 1996;271:H1697–H1701. doi: 10.1152/ajpheart.1996.271.4.H1697. [DOI] [PubMed] [Google Scholar]
- Van Lunteren E, Moyer M. Effects of DAP on diaphragm force and fatigue, including fatigue due to neurotransmission failure. J Appl Physiol. 1996;81:2214–2220. doi: 10.1152/jappl.1996.81.5.2214. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;290:H119–H127. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- Vyskocil F, Hnik P, Rehfeldt H, Vejsada R, Ujec E. The measurement of K+e concentration changes in human muscles during volitional contractions. Pflugers Arch. 1983;399:235–237. doi: 10.1007/BF00656721. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol Heart Circ Physiol. 1998;274:H178–H186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Horvath SM, Gutin B, Molnar S, Diaz FJ. Plasma electrolyte content and concentration during treadmill exercise in humans. J Appl Physiol. 1982;53:1529–1539. doi: 10.1152/jappl.1982.53.6.1529. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Kapoor SC, Krishna GG. Contribution of potassium to exercise-induced vasodilation in humans. J Appl Physiol. 1994;77:2552–2557. doi: 10.1152/jappl.1994.77.6.2552. [DOI] [PubMed] [Google Scholar]
- Xia J, Duling BR. Electromechanical coupling and the conducted vasomotor response. Am J Physiol Heart Circ Physiol. 1995;269:H2022–H2030. doi: 10.1152/ajpheart.1995.269.6.H2022. [DOI] [PubMed] [Google Scholar]
- Xia J, Little TL, Duling BR. Cellular pathways of the conducted electrical response in arterioles of hamster cheek pouch in vitro. Am J Physiol Heart Circ Physiol. 1995;269:H2031–H2038. doi: 10.1152/ajpheart.1995.269.6.H2031. [DOI] [PubMed] [Google Scholar]


