Abstract
Somatostatin is an inhibitory peptide present in abundance in the gastrointestinal (GI) tract. The effects of somatostatin are mediated through its interaction with a family of G-protein-coupled receptors, namely sst1–5. Previous evidence suggested that the sst2 receptor mediates an inhibitory role of somatostatin on GI afferent nerve sensitivity. In the present study we further evaluated mechanical and chemical sensitivity of mesenteric afferents in mice deficient in the sst2 receptor. Multi-unit recordings were made from mesenteric afferents from mouse jejunal segments perfused in vitro. Ramp distension of the jejunum up to 60 mmHg induced biphasic increases in afferent activity in both wild-type (WT) and sst2 gene knock-out (KO) mice. However, the level of afferent activity was significantly higher in the KO (n = 15) compared to the WT (n = 16) mice across the entire pressure range. The mesenteric afferent sensitivity to acid was evaluated by intraluminal infusion of hydrochloric acid (HCl 20 mm) for 2 min. Peak afferent discharge rate following acid infusion was significantly greater in KO (36.76 ± 6.47 impulses s−1, n = 7) than in WT preparations (16.53 ± 3.91 impulses s−1, n = 5, P < 0.01). The response to bath-applied bradykinin (1 μm, 3 ml) was not significantly different in the KO and the WT preparations. It is interesting that in the WT preparations, octreotide inhibited both low- and high-threshold mechanosensory responses, whereas in the sst2 KO group it appeared to inhibit the low-threshold responses preferentially and failed to affect the high-threshold responses. The results of the present investigation demonstrate that sst2 deficiency is associated with exaggerated jejunal afferent sensitivity to both mechanical and chemical stimulations, suggesting that somatostatin plays an important inhibitory role in the control of visceral sensitivity by interacting with the sst2 receptor.
Somatostatin (SST) is a 14 or 28 amino acid neuropeptide that was originally discovered as a neuroendocrine hormone in the hypothalamus (Brazeau et al. 1973) and was subsequently detected throughout the body where it inhibits various physiological processes (Patel, 1999). The importance of SST in the gastrointestinal (GI) tract is indicated by the observation that the GI tract contains about 65% of the SST present in the whole body (McIntosh & Arnold, 1978). SST is present in various cell types in the gut wall such as the endocrine D cells of the gut mucosa, Islets of Langerhans and enteric neurons (Hokfelt et al. 1975; Track et al. 1979; Larsson, 1985). The peptide inhibits gastric and pancreatic secretion, gut motility, absorption of nutrients, blood flow and proliferation and differentiation of epithelial cells (Krejs, 1986; Tulassay, 1998).
The actions of SST are mediated by five different G-protein-coupled receptors referred to as sst1, sst2, sst3, sst4 and sst5 (Barnett, 2003; Guillermet-Guibert et al. 2005). They are linked to multiple cellular effector systems such as inhibiting adenylate cyclase activity, reducing the conductance of voltage-dependent Ca2+ channels and activating K+ channels (Barnett, 2003).
The various sst subtypes are thought to serve distinct functions, yet their precise biological roles have not been identified. In clinical studies, the synthetic SST analogue, octreotide, was found to increase discomfort and pain thresholds in patients with IBS (Kuiken et al. 2005). In a previous study, we confirmed that octreotide inhibits populations of rat mesenteric afferents that are likely to be involved in nociceptive transmission (Booth et al. 2001). Because this effect was mimicked by a selective sst2 agonist (BIM23027) and was blocked by a selective sst2 antagonist (Cyanamid154806), it was proposed that sst2 mediates the important modulatory role of SST on gut sensation. Moreover, the sst2 receptor antagonist alone caused augmentation of the afferent response to bradykinin, suggesting a role for endogenous SST in the regulation of mesenteric afferent sensitivity (Booth et al. 2001). We therefore hypothesized that sst2 receptors suppress afferent sensitivity and that in the absence of sst2 receptor activation the afferents would become hypersensitive.
In the present study, we examined this hypothesis using sst2 receptor-deficient mice. We conducted multi-unit recordings of the mesenteric afferent nerve supplying segments of the mouse jejunum in vitro and tested the afferent sensitivity to mechanical distension, acid and bradykinin in wild-type (WT) and sst2 gene knockout (KO) mice. We found that compared with WT mice, sst2-deficient mice had a significantly elevated sensitivity of the mesenteric afferent nerve to the mechanical and chemical stimulation, suggesting an important functional role for endogenous SST in the regulation of intestinal afferent sensitivity.
Methods
Animals
sst2 WT and KO mice with a genetic background of C57/BL6 were generated at GlaxoSmithKline (Harlow, UK). Methods for the generation of the mutant and the WT control mice have been described in detail elsewhere (Allen et al. 2003). Mating pairs of sst2−/− and sst2+/+ N1F1 littermates were obtained to generate separate colonies of sst2 WT and KO mice at the University of Sheffield according to the UK Animals Scientific Procedures Act (1986). In the course of the study, genotyping was performed on randomly selected animals (six WT and six KO mice) to confirm the absence of sst2 gene in the KO mice. A total of 16 WT and 15 KO mice of either sex (20–30 g, 8–12 weeks old) were used in this study. There were no overt differences in feeding behaviour, litter size, growth rate and body weight (36.8 ± 1.9 and 38.1 ± 1.7 g, respectively, P > 0.05) between WT and KO groups.
Tissue preparation and nerve recording
Mice were killed with an overdose of pentobarbitone sodium (80 mg kg−1) injected intraperitoneally. A mid-line laparotomy was performed and sections of jejunum (30–35 mm in length) were removed. The intestinal segments were immediately placed into oxygenated (95% O2–5% CO2) Krebs solution containing (mm): NaCl 113, KCl 5.9, NaH2PO4 1.2, MgSO4 1.2, NaHCO3 25, CaCl2 1.2 and glucose 11.5, at room temperature. One of the jejunal segments was placed into a purpose-built chamber (20 ml). Krebs solution was constantly perfused through the tissue chamber (37°C) at 10 ml min−1. The jejunum was cannulated at each end, and intraluminal pressure was recorded via a Neurolog pressure amplifier (NL 108, Digitimer, UK). Two syringe pumps were connected in parallel to the intraluminal inflow cannula via a T-piece connector, to allow intraluminal perfusion of Krebs solution or test solutions through the lumen. The mesenteric bundle was pinned out onto the Sylgard base of the recording chamber and a mesenteric nerve was carefully dissected from the bundle. Nerve activity was recorded using a suction electrode connected to a headstage (NL100), amplified (NL104) and filtered (NL 125, band pass 200–3000 Hz). Whole nerve activity was displayed on an oscilloscope (Tektronix TDS 210). The nerve signal was recorded (20 kHz sampling rate) to a computer through a Micro 1401 interface and Spike 2 software (Cambridge Electronic Design, UK). Intraluminal pressure was sampled at 100 Hz.
Experimental protocols
The preparation was left to stabilize for up to 60 min. Throughout the experiment, the gut segment was constantly perfused intraluminally with Krebs solution at 10 ml h−1 against a distal pressure head of 2 cmH2O. To distend the intestine, a three-way tap on the intraluminal outlet cannula was closed while perfusion with Krebs solution continued. In this manner, the gut segment was distended to 60 mmHg in approximately 120 s, before the three-way tap was opened to return intraluminal pressure to baseline. This was repeated at regular intervals of 15 min until the response became stable. The effects of sst2 activation on the afferent response to distension were observed by applying the SST analog, octreotide (25 μg in 3 ml Krebs solution), into the bath. Nerve responses to distension before and after the treatment were compared.
To test the afferent sensitivity to acid, the intraluminal perfusate was switched from Krebs solution alone to Krebs solution containing 20 mm hydrochloric acid (HCl) for 2 min before returning again to Krebs solution. A minimum interval of 20 min was left between each acid test. To test the effects of bradykinin (BK), the preparation was challenged with 3 ml 1 μm BK applied to the bath over 20 s.
Data analysis
Mesenteric afferent nerve activity was recorded as mean discharge rate (impulses s−1) with a time constant of 10 s. A script written for Spike2 program was used to measure the afferent discharge rate at different intraluminal pressures in order to plot the averaged pressure–response curve for multi-unit activity during ramp distensions. To illustrate the time course and amplitude of the responses to HCl or BK, rate histograms were plotted for 10 min after treatment commenced. Statistical analysis was performed using Graphpad Prism version 3.00 (Graphpad Software, San Diego, CA, USA). Where appropriate, data are expressed as means ±s.e.m. and compared using paired or unpaired Student's t tests. n refers to the number of preparations. A P value of less than 0.05 is considered statistically significant.
Results
Responses of mesenteric afferents to gut distension in sst2 WT and KO mice
In accord with previous studies (Booth et al. 2001; Rong et al. 2004), jejunal afferents displayed irregular spontaneous activity. The mean level of spontaneous multi-unit activity in sst2−/− preparations (27.6 ± 3.6 impulses s−1, n = 15) appeared to be higher than in the WT preparations (20.1 ± 2.8 impulses s−1, n = 16), although statistical significance was not reached (P > 0.05, Student's t test).
In both WT and sst2−/− mice, ramp distension of the jejunal segments evoked biphasic activation of the mesenteric afferent nerve as a result of activation of low- and high-threshold mechanosensitive fibres (Fig. 1A, also see Rong et al. 2004). The first phase of the response was a rapid increase in afferent activity during the first 5 mmHg rise in intraluminal pressure. The second phase of the response was a slower increase in afferent firing between 5 and 60 mmHg, although the rate of increase in afferent activity escalated when the pressure reached 20 mmHg. The magnitude of the afferent response to distension differed between the two groups of animals. Compared with the WT group (n = 16), the pressure–nerve response curve of the sst2 KO group (n = 15) was markedly shifted upwards (Fig. 1B). At each pressure point (i.e. 1, 2, 5 and 10–60 mmHg), the mean level of nerve activity of the KO group was significantly greater than that of the WT group.
Figure 1. Mechanosensory responses of jejunal afferent nerves in WT and sst2 KO mice.
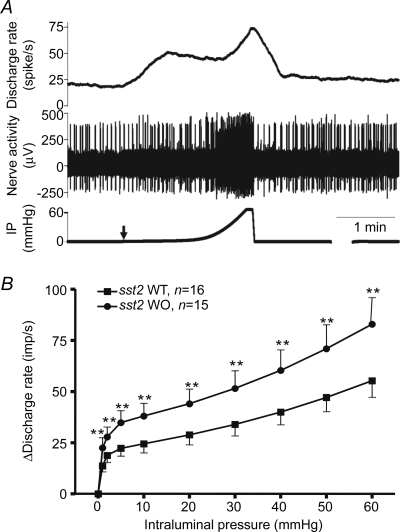
A, a representative multi-unit recording of distension-evoked biphasic activation of the jejunal afferent nerves from a WT preparation. Traces from top to bottom are mean discharge frequency, nerve signal and intraluminal pressure. Arrow indicates start of distension. B, the stimulus–response curves of the jejunal afferent nerves of WT and KO mice. **P < 0.01, Student's t test.
Responses of mesenteric afferents to intraluminal acid infusion
Nerve responses to intraluminal acid (20 mm HCl, 10 ml h−1 for 2 min) infusion were tested in five WT and seven KO preparations. The pattern of the nerve responses to HCl was similar for both groups. The afferent nerve discharge started to increase after a latency of about 60 s, reached the peak level approximately 120 s after the beginning of acid infusion and then declined slowly towards the baseline (Fig. 2A). However, as illustrated in the rate histograms in Fig. 2B, the mean level of the acid response of the sst2 KO group was significantly greater than that of the WT group. Peak afferent discharge rate following acid infusion was 36.76 ± 6.47 impulses s−1 in KO and 16.53 ± 3.91 impulses s−1 in WT preparations (P < 0.01).
Figure 2. Responses of jejunal afferent nerves to intraluminal acid infusion in WT and sst2 KO mice.
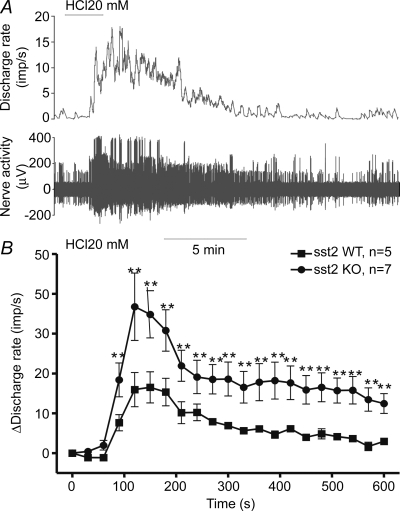
A, representative traces of the nerve activity and mean discharge frequency following intraluminal acid infusion (20 mm HCl, 0.1 ml min−1 for 2 min) in a jejunum preparation from a WT mouse. B, mean change in jejunal afferent activity following intraluminal acid infusion in WT and sst2 KO mice. **P < 0.01, Student's t test.
Responses of mesenteric afferents to BK
In both KO (n = 7) and WT (n = 8) animals, bath-applied BK (1 μm, 3 ml over 20 s) evoked a rapid increase in afferent discharge, which reached peak approximately 60 s after the start of BK infusion (Fig. 3). The mean level of the nerve response of the KO group was increased relative to that of the WT group; but the difference was not statistically significant. The peak increase in afferent discharge was 33.47 ± 6.96 impulses s−1 (n = 7) in the KO and 25.44 ± 6.06 impulses s−1 (n = 8) in the WT preparations, respectively (P > 0.05).
Figure 3. Responses of jejunal afferent nerves to bradykinin in WT and sst2 KO mice.
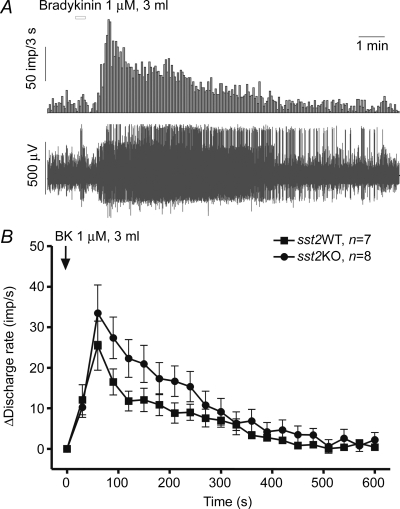
A, representative traces of the afferent nerve activity and mean discharge frequency following bath-application of bradykinin (1 μm, 3 ml) in a jejunum preparation from a WT mouse. B, averaged changes in jejunal afferent activity evoked by bath-applied bradykinin in WT and sst2 KO mice.
Effects of octreotide on mesenteric afferent nerve activity
The effects of sst2 agonist on the mechanosensitivity of mesenteric afferent nerves were examined in the sst2 KO and the WT preparations (n = 6 for each group) by applying octreotide (25 μg in 3 ml Krebs solution, perfused in 20 s) to the bathing fluid. In both groups, octreotide treatment resulted in a rapid decrease in the spontaneous nerve discharge and, moreover, inhibited the responses to jejunal distensions (Fig. 4). In the WT preparations, octreotide inhibited both low- and high-threshold mechanosensory responses; whereas in the sst2 KO group it inhibited the low-threshold responses and failed to affect the high-threshold responses. Thus in the WT group, when the intraluminal pressure was raised by 10 mmHg from the baseline, the mean discharge rate increased by 22.8 ± 4.8 impulses s−1 before and only by 11.4 ± 2.6 impulses s−1 (n = 7, P < 0.01, paired t test) after exposure to octreotide. In the sst2 KO group, mean nerve discharge rate increased by 40.1 ± 9.0 impulses s−1 before and only by 27.7 ± 5.1 impulses s−1 (n = 7, P < 0.01) for the above distension pressure range following octreotide exposure. In the WT group, when the intraluminal pressure was further raised from 10 to 60 mmHg, the mean discharge rate increased by 37.2 ± 5.8 impulses s−1 before and only by 26.5 ± 5.0 impulses s−1 (n = 7, P < 0.05, paired t test) after octreotide treatment; whereas in the KO group, the mean discharge rate increased by a similar degree over this pressure range before and after application of octreotide (50.8 ± 10.6 and 51.0 ± 10.9 impulses s−1, n = 7, P > 0.05).
Figure 4. Effects of octreotide on the mechanosensory responses of jejunal afferent nerves in WT and sst2 KO mice.
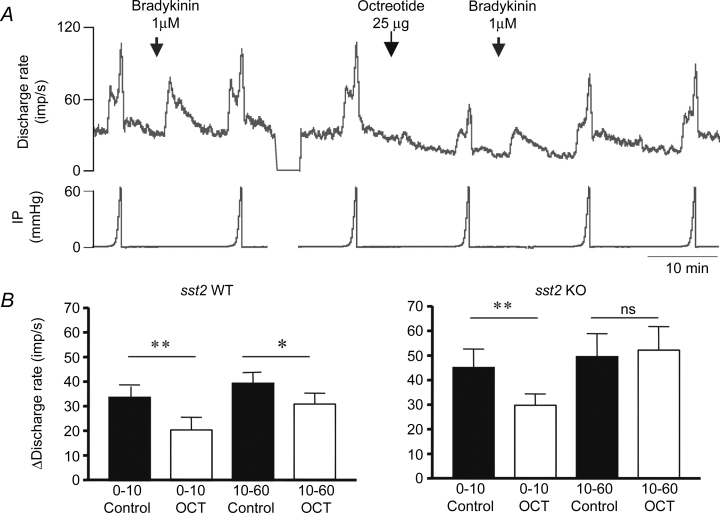
A, representative traces of mean discharge frequency and intraluminal pressure (IP) to illustrate the nerve responses to jejunal distension and bradykinin before and following bath-application of octreotide (25 μg in 3 ml Krebs solution superfused over 20 s). B, bar graph to illustrate the effects of octreotide on low- (pressure range, 0–10 mmHg) and high-threshold (pressure range, 10–60 mmHg) mechanosensory responses in WT and sst2 KO mice. Note that whereas octreotide inhibited both low- and high-threshold mechanosensory responses in WT mice, in the KO it only inhibited the low-threshold response. *P < 0.05; **P < 0.01, paired Student's t test, n = 7 for each group.
Discussion
Visceral pain remains a tremendous challenge within clinical and basic medical sciences. The emphasis of basic research in this field in general has been to identify algesic agents that directly or indirectly activate nociceptors and that facilitate nociceptive neurotransmission. However, as with other body systems, the normal functioning of the visceral sensory system requires a balance of various excitatory and inhibitory mechanisms. Opioids are the best example of a molecular mechanism for inhibition of pain with actions at both spinal and supra-spinal sites. There is increasing evidence that opioid receptors are expressed in the periphery to modulate pain at its source (Stein et al. 2003). The same may be true for SST. In this respect, the results of the present investigation provide several important additions to our understanding of the role of SST and in particular, its sst2 receptor subtype, in the control of GI afferent sensitivity. We have demonstrated that sst2 deficiency is associated with exaggerated jejunal afferent sensitivity to both mechanical and chemical stimulations, suggesting that endogenous SST plays an important inhibitory role in the control of visceral sensitivity via interaction with the sst2 receptor subtype.
Our results support previous reports which showed that peripheral sst receptor activation could inhibit somatosensory and visceral nociceptor excitability (Pinter et al. 2006; Carlton et al. 2001b; 2003; Helyes et al. 2004). In the periphery, SST is present in a subpopulation of small-diameter primary afferent neurons and in approximately one-third of fine cutaneous sensory axons (Carlton et al. 2003). Expression and activity-induced release of SST from both central and peripheral terminals of adult sensory neurons is modulated during inflammation (Malcangio, 2003). Intraplantar administration of SST reduced mechanical hyperalgesia in carrageenan-induced inflammation (Corsi et al. 1997). The sst receptor agonist octreotide injected intraplantarly inhibited nocifensive behaviours in phase 2 of the formalin test and nociceptor responses to thermal stimuli (Carlton et al. 2001a,b). Intraperitoneal application of another octapeptide analogue, vapreotide (RC-160), produced long-lasting anti-nociceptive effects in the tailflick and hot plate tests in rats and mice (Eschalier et al. 1991). Close arterial injection of octreotide in the rat inhibited mechanoreceptor activity in response to noxious joint rotation in the normal and inflamed knee joint (Heppelmann & Pawlak, 1997). By contrast, the sst receptor antagonist cyclosomatostatin enhanced nociceptor activity (Heppelmann & Pawlak, 1999).
Within the GI tract, SST is found in D-type endocrine cells of the gut mucosa, in enteric neurons and in extrinsic nerve terminals (Hokfelt et al. 1975; Track et al. 1979; Larsson, 1985), all of which may be a potential source of SST in these in vitro jejunal preparations. All five subtypes of SST receptors have been detected in the GI tract (Lewin, 1992; Sandvik et al. 1995; Krempels et al. 1997; Gugger et al. 2004). In clinical studies, the synthetic SST analogue, octreotide, was found to alleviate pain in patients with GI diseases (Kuiken et al. 2005). The analgesic effect of octreotide in visceral pain was suggested to involve activation of peripheral sst receptors. In support of this, we confirmed in a previous study that octreotide inhibited populations of rat mesenteric afferents that are likely to be involved in nociceptive transmission (Booth et al. 2001). However, Su et al. (2001) found that octreotide was ineffective in attenuating responses of pelvic afferents to colorectal distension in either normal or acetic acid-inflamed colon when administered intravenously but attenuated responses when given intrathecally in the rat. Administration of octreotide over a broad dose range (0.5 μg kg−1 to 2.4 mg kg−1) did not alter responses of colonic afferent fibres to noxious colorectal distension in untreated, acetic acid-treated or zymosan-treated colons. This might suggest that the inhibitory action of SST is restricted to certain subpopulations of visceral afferents and that pelvic afferents are insensitive whereas vagal and splanchnic afferents are under a tonic inhibitory control as discussed below.
Based on all these data, we can conclude that SST analogues are potent analgesic agents; however, the relative importance of peripheral and central actions and the sst receptor subtype(s) that mediates the analgesic effects remain to be determined. In this respect, the results of the present investigation provided important clues by showing that sst2 deficiency is associated with marked hypersensitivity of gut afferent nerves to mechanical distension, intraluminal acid and possibly BK. This suggests that sst2 receptor is a major player among the various sst receptor subtypes in mediating the inhibitory modulation of SST on visceral afferent sensitivity.
Although the precise molecular mechanisms of these effects are not yet known, Carlton et al. (2004) suggested that sst receptor activation might phasically and tonically inhibit the capsaicin transient receptor potential vanilloid 1 (TRPV1) ion channel. Their behavioural studies demonstrated that activation of peripheral sst receptors with octreotide significantly reduced capsaicin-induced nocifensive behaviour of rats in a dose-dependent manner. In vitro single-unit recordings using a skin–nerve preparation also revealed that activation of peripheral sst receptors on identified nociceptors diminished capsaicin-evoked responses. Furthermore, blockade of peripheral sst receptors dramatically enhanced capsaicin-induced nociceptor activity and nocifensive behaviours, suggesting that SST exerted a tonic inhibitor influence over TRPV1 receptor activation. TRPV1 ion channels are also involved in regulating GI afferent sensitivity. TRPV1 is abundantly expressed on GI afferents (Ward et al. 2003) and mesenteric afferent bundles show marked sensitivity to capsaicin which activates and then desensitizes GI afferents (Rong et al. 2004). Moreover, pharmacological blockade or genetic knockout of TRPV1 are associated with hyposensitivity of jejunal afferents (Rong et al. 2004) and with attenuated pain behaviour (Jones et al. 2005). This contrasts with the current observations in the sst2 receptor knockout animal in which the afferent responses to both mechanical and chemical stimulation are augmented. It is tempting to speculate therefore that afferent nerve terminals are exposed to a balance of pro-nociceptive and anti-nociceptive chemical mediators and that an imbalance might occur via altered release or altered receptor expression which could underline abnormal gut sensitivity. Certainly there is evidence for up-regulation of pro-nociceptive mechanisms in patients with GI disease associated with painful symptoms (Yiangou et al. 2001a,b,c).
The observation that octreotide caused an attenuated response to distension even in the sst2 receptor KO implicates other sst receptors in visceral afferent function. Octreotide binds with high affinity to both sst2 and sst5 receptor subtypes (Hannon et al. 2002). Thus the response to octreotide in the sst2 receptor KO is consistent with a role for sst5 receptors. Moreover, from the current observations it would appear that the functional expression of sst2 and sst5 receptors can be attributed to different subpopulations of mesenteric afferents. Sst2 receptors appear to be expressed on both low- and high-threshold mechanosensory fibres as both components of the response to distension are augmented in the sst2 receptor KO. However, sst5 appears to be selectively expressed in low-threshold fibres and not in the high-threshold population. This conclusion is based on the observation that octreotide inhibited spontaneous firing and low-threshold distension-evoked afferent nerve discharge in the sst2 KO mice, whereas the high-threshold component was unaffected. It appears therefore that sst2 receptors play a role in regulating the high-threshold firing of jejunal afferents whereas the low-threshold response is modulated by sst2 and sst5 receptor subtypes. In the rat, these two populations can be distinguished on the basis of the projection pathway to the CNS. Low-threshold afferents are absent in animals in which vagal afferents have been eliminated by prior chronic vagotomy, whereas high-threshold responses are preserved (Booth et al. 2001). One might speculate therefore that it is the vagal afferent pathway that expresses both sst2 and sst5 receptors whereas spinal afferents express only sst2 receptors.
In conclusion, these data demonstrate that the sst2 receptor mediates an important inhibitory modulation on afferent sensitivity to mechanical and chemical stimulations in the small intestinal. Moreover, it appears that high-threshold mechanosenitive fibres selectively express sst2, whereas low-threshold fibres may have multiple sst receptors.
References
- Allen JP, Hathway GJ, Clarke NJ, Jowet MI, Topps S, Kendrick KM, Humphrey PPA, Wilkinson LS, Emson PC. Somatostatin receptor 2 knockout/lacZ knockin mice show impared motor coordination and reveal sites of somatostatin action within the striatum. Eur J Neurosci. 2003;17:1881–1895. doi: 10.1046/j.1460-9568.2003.02629.x. [DOI] [PubMed] [Google Scholar]
- Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. 2003;20:255–264. doi: 10.1385/ENDO:20:3:255. [DOI] [PubMed] [Google Scholar]
- Booth CE, Kirkup AJ, Hicks GA, Humphrey PP, Grundy D. Somatostatin sst2 receptor-mediated inhibition of mesenteric afferent nerves of the jejunum in the anesthetized rat. Gastroenterology. 2001;121:358–369. doi: 10.1053/gast.2001.26335. [DOI] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Du J, Hargett GL, Ji G, Coggeshall RE. Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain. 2004;110:616–627. doi: 10.1016/j.pain.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Kraemer B, Coggeshall RE. A role for peripheral somatostatin receptors in counter-irritation-induced analgesia. Neuroscience. 2003;120:499–508. doi: 10.1016/s0306-4522(03)00337-3. [DOI] [PubMed] [Google Scholar]
- Corsi MM, Ticozzi C, Netti C, Fulgenzi A, Tiengo M, Gaja G, Guidobono F, Ferrero ME. The effect of somatostatin on experimental inflammation in rats. Anesth Analg. 1997;85:1112–1115. doi: 10.1097/00000539-199711000-00028. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Davidson E, Zhou S, Coggeshall RE. Somatostatin receptors on peripheral primary afferent terminals: inhibition of sensitized nociceptors. Pain. 2001a;90:233–244. doi: 10.1016/S0304-3959(00)00407-3. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Zhou S, Coggeshall RE. Tonic control of peripheral cutaneous nociceptors by somatostatin receptors. J Neurosci. 2001b;21:4042–4049. doi: 10.1523/JNEUROSCI.21-11-04042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschalier A, Aumaitre O, Ardid D, Fialip J, Duchene-Marullaz P. Long-lasting antinociceptive effect of RC-160, a somatostatin analog, in mice and rats. Eur J Pharmacol. 1991;199:119–121. doi: 10.1016/0014-2999(91)90646-8. [DOI] [PubMed] [Google Scholar]
- Gugger M, Waser B, Kappeler A, Schonbrunn A, Reubi JC. Cellular detection of sst2A receptors in human gastrointestinal tissue. Gut. 2004;53:1431–1436. doi: 10.1136/gut.2004.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermet-Guibert J, Lahlou H, Cordelier P, Bousquet C, Pyronnet S, Susini C. Physiology of somatostatin receptors. J Endocrinol Invest. 2005;28:5–9. [PubMed] [Google Scholar]
- Hannon JP, Nunn C, Stolz B, Bruns C, Weckbecker G, Lewis I, Troxler T, Hurth K, Hoyer D. Drug design at peptide receptors: somatostatin receptor ligands. J Mol Neurosci. 2002;18:15–27. doi: 10.1385/JMN:18:1-2:15. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Szabo A, Nemeth J, Jakab B, Pinter E, Banvolgyi A, Kereskai L, Keri G, Szolcsanyi J. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund's adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50:1677–1685. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Pawlak M. Inhibitory effect of somatostatin on the mechanosensitivity of articular afferents in normal and inflamed knee joints of the rat. Pain. 1997;73:377–382. doi: 10.1016/S0304-3959(97)00124-3. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Pawlak M. Peripheral application of cyclo-somatostatin, a somatostatin antagonist, increases the mechanosensitivity of rat knee joint afferents. Neurosci Lett. 1999;259:62–64. doi: 10.1016/s0304-3940(98)00912-4. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Efendic S, Hellerstrom C, Johansson O, Luft R, Arimura A. Cellular localization of somatostatin in endocrine-like cells and neurons of the rat with special references to the A1-cells of the pancreatic islets and to the hypothalamus. Acta Endoncrinol Suppl (Copenh) 1975;200:5–41. [PubMed] [Google Scholar]
- Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejs GJ. Physiological role of somatostatin in the digestive tract: gastric acid secretion, intestinal absorption, and motility. Scand J Gastroenterol Suppl. 1986;119:47–53. doi: 10.3109/00365528609087431. [DOI] [PubMed] [Google Scholar]
- Krempels K, Hunyady B, O'Carroll AM, Mezey E. Distribution of somatostatin receptor messenger RNAs in the rat gastrointestinal tract. Gastroenterology. 1997;112:1948–1960. doi: 10.1053/gast.1997.v112.pm9178687. [DOI] [PubMed] [Google Scholar]
- Kuiken SD, Tytgat GN, Boeckxstaens GE. Review article: drugs interfering with visceral sensitivity for the treatment of functional gastrointestinal disorders – the clinical evidence. Aliment Pharmacol Ther. 2005;21:633–651. doi: 10.1111/j.1365-2036.2005.02392.x. [DOI] [PubMed] [Google Scholar]
- Larsson LI. Distribution and morphology of somatostatin cells. Adv Exp Med. 1985;188:383–402. doi: 10.1007/978-1-4615-7886-4_21. [DOI] [PubMed] [Google Scholar]
- Lewin MJ. The somatostatin receptor in the GI tract. Annu Rev Physiol. 1992;54:455–468. doi: 10.1146/annurev.ph.54.030192.002323. [DOI] [PubMed] [Google Scholar]
- McIntosh C, Arnold R. The radioimmunoassay and physiology of somatostatin in the pancreas and gastrointestinal tract. Z Gastroenterol. 1978;16:330–340. [PubMed] [Google Scholar]
- Malcangio M. GDNF and somatostatin in sensory neurones. Curr Opin Pharmacol. 2003;3:41–45. doi: 10.1016/s1471-4892(02)00007-3. [DOI] [PubMed] [Google Scholar]
- Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- Pinter E, Helyes Z, Szolcsanyi J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther. 2006;112:440–456. doi: 10.1016/j.pharmthera.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvik AK, Dimaline R, Brenna E, Waldum HL. Differential expression and regulation of SSTR2 messenger RNA in rat gastric antrum and corpus. Am J Physiol Gastrointest Liver Physiol. 1995;269:G542–G547. doi: 10.1152/ajpgi.1995.269.4.G542. [DOI] [PubMed] [Google Scholar]
- Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- Su X, Burton MB, Gebhart GF. Effects of octreotide on responses to colorectal distension in the rat. Gut. 2001;48:676–682. doi: 10.1136/gut.48.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Track NS, Creutzfeldt C, Litzenberger J, Neuhoff C, Arnold R, Creutzfeldt W. Appearance of gastrin and somatostatin in the human fetal stomach, duodenum and pancreas. Digestion. 1979;19:292–306. doi: 10.1159/000198374. [DOI] [PubMed] [Google Scholar]
- Tulassay Z. Somatostatin and the gastrointestinal tract. Scand J Gastroenterol Suppl. 1998;228:115–121. doi: 10.1080/003655298750026642. [DOI] [PubMed] [Google Scholar]
- Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Baecker PA, Ford AP, Knowles CH, Chan CL, Williams NS, Anand P. ATP-gated ion channel P2X3 is increased in human inflammatory bowel disease. Neurogastroenterol Motil. 2001a;13:365–369. doi: 10.1046/j.1365-2982.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, Anand P. Vanilloid receptor 1 immuno-reactivity in inflamed human bowel. Lancet. 2001b;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Smith JA, Sangameswaran L, Eglen R, Birch R, Knowles C, Williams N, Anand P. Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur J Gastroenterol Hepatol. 2001c;13:891–896. doi: 10.1097/00042737-200108000-00003. [DOI] [PubMed] [Google Scholar]


