Abstract
Homeostatic regulation, i.e. the ability of neurons and neuronal networks to adjust their output in response to chronic alterations in electrical activity is a prerequisite for the pronounced functional plasticity in the developing brain. Cellular mechanisms of homeostatic plasticity have mainly been studied in cultured preparations. To understand the developmental time frame and properties of homeostatic plasticity under more physiological conditions, we have here compared the effects of activity deprivation on synaptic transmission in acutely isolated and cultured hippocampal slices at different stages of development. We find that transmission at both glutamatergic and GABAergic synapses is strongly and rapidly (15 h) regulated in the opposite directions in response to inactivity during narrow, separated time windows early in development. Following this critical period of synaptic development, induction of the homeostatic response requires longer periods (40 h) of inactivity. At glutamatergic synapses, activity blockade led to an increase in the amplitude and frequency of mEPSCs, and the threshold for induction of this response was increased during development. In contrast, homeostatic regulation at GABAergic synapses was expressed in a qualitatively distinct manner at different developmental stages. Immature neurons responded rapidly to inactivity by regulating mIPSC frequency, while longer activity blockade led to a decrease in the mIPSC amplitude independent of the neuronal maturation. The susceptibility of immature networks to homeostatic regulation may serve as a safety mechanism against rapid runaway destability during the time of intense remodelling of the synaptic circuitry.
Homeostatic plasticity is thought to complement the synapse-specific Hebbian forms of plasticity, in order to regulate the overall excitability of the neuronal networks in response to long-term changes in the activity levels (Turrigiano et al. 1998; Davis & Bezprozvanny, 2001; Burrone & Murthy, 2003; Thiagarajan et al. 2006). Chronic blockade of neuronal activity triggers a variety of cellular and synaptic mechanisms that appear to compensate for the diminished activity of the network. In the hippocampal culture, activity blockade by tetrodotoxin (TTX) has been reported to increase cellular and network excitability (Aptowicz et al. 2004; Karmarkar & Buonomano, 2006), and to increase glutamatergic transmission via presynaptic (Murthy et al. 2001; Burrone et al. 2002; Nakayama et al. 2005) and/or postsynaptic mechanisms (Ju et al. 2004; Stellwagen & Malenka, 2006). In parallel, there is a decrease in GABAergic synaptic drive (Hartman et al. 2006; Swanwick et al. 2006).
Previous studies have acknowledged that the response to inactivity depends on the preparation used; the properties and integrity of the network as well as the developmental stage of the cultures appear critical in determining the form of homeostatic plasticity expressed (Burrone et al. 2002; Kirov et al. 2004; Hartman et al. 2006; Karmarkar & Buonomano, 2006; Wierenga et al. 2006). However, the majority of studies addressing homeostatic plasticity have been carried out in neuronal cultures, where the development of the network is inevitability compromised in the absence of epigenetic factors critical for neuronal maturation. Thus, the knowledge on the developmental profile of the homeostatic response under more physiological conditions is still sparse. This is particularly true when considering plasticity at the immature hippocampal networks, where the mechanisms of synaptic transmission differ from that in the mature systems in several important aspects, including for example depolarizing GABAergic transmission (Ben-Ari, 2001), low density of AMPA-receptor containing synapses (Tyzio et al. 1999) and the mechanisms of glutamate release (e.g. Hanse & Gustafsson, 2001; Lauri et al. 2006).
To understand the developmental mechanisms and regulation of the homeostatic plasticity, we have here studied the effects of activity deprivation on the properties of glutamatergic and GABAergic synapses in acutely isolated as well as cultured hippocampal slices at different stages of development. This approach allowed us to study the developmental regulation of homeostatic plasticity in two different synapse types and preparations in parallel. We find that both glutamatergic and GABAergic transmission is strongly and rapidly (15 h) regulated in the opposite directions in response to inactivity in the neonate CA3. However, this rapid synaptic response to activity deprivation is developmentally down-regulated in a temporally and mechanistically distinct manner, depending on the synapse type. The rapid functional adaptation of immature synapses to altered levels of neuronal activity may protect the circuitry during the time of formation of the synaptic connectivity, when the network is particularly vulnerable to instability.
Methods
Postnatal day (P)0–P8 Wistar rats (P0 is the day of birth) were rapidly killed by decapitation without anaesthesia in accordance with the University of Helsinki animal welfare guidelines. Hippocampal slices (400 μm) were cut with a vibratome using standard methods (e.g. Lauri et al. 2006). The slices were used 1–4 h after cutting (‘acute slices’) or prepared for incubation. In these experiments the slices were washed with 1 ml incubation solution containing (mm): 105 NaCl, 3 KCl, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 15 d-glucose and 25 Hepes, and placed on Millicell CM 0.4 μm membrane inserts (Millipore, Bedford, MA, USA) with 1 ml of the above solution with or without 1 μm TTX and transferred to an incubator (35°C, 5% CO2 in air). In some experiments, chloramphenicol (2.5 μg ml−1) was included in the incubation solution to prevent contamination.
For preparation of organotypic cultures, hippocampi were dissected from P6–P7 rats under sterile conditions and cut transversally into 350 μm thick slices using a McIllwain tissue-chopper (Mickle Laboratory, Surrey, UK). Slices were washed with culture medium containing: 50% minimal essential medium with Hepes, 24% heat-inactivated horse serum, 24% Earle's balanced salt solution (all from Gibco BRL), 1%l-glutamine, 1% chloramphenicol, and placed on Millicell membrane inserts with 1 ml of the above medium. During the cultivation (at 35°C, 5% CO2 in air), the medium was changed 1 day after plating, and every third day thereafter. TTX was added to the culture medium 15–22 h (TTX15) or 45–50 h (TTX48) before electrophysiological recordings. Recordings were done at 7–8 or 14–16 days in vitro (DIV).
For electrophysiological recordings, the slices were placed in a submerged chamber and superfused with artificial cerebrospinal fluid (ACSF) containing (mm): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 15 d-glucose and 2 CaCl2; 5% CO2–95% O2, at a rate of 2–3 ml min−1 (32°C). Whole-cell recordings were obtained from CA3 pyramidal neurons by using the Axopatch 200B amplifier (Axon Instruments). Cells were voltage-clamped at −70 mV with 3–5 MΩ pipettes filled with a solution containing (mm): 130 CsMeSO4, 10 Hepes, 0.5 EGTA, 4 Mg-ATP, 0.3 Na-GTP, 5 N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium chloride and 8 NaCl (285 mOsm), pH 7.2. For recordings of mIPSCs, the NaCl concentration of the filling solution was increased to 30 mm. To isolate AMPA- receptor-mediated mEPSCs 1 μm TTX and 100 μm picrotoxin (PiTX) were added to perfusion solution. GABAA-mediated mIPSCs were recorded in the presence of 1 μm TTX, 10 μm 2.3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (NBQX) and 1 μm 3-N[1-(S)-(3,4-dichlorophenyl)ethyl,]amino-2-(S)-hydroxypropyl-P- benzyl-phosphinic acid (CGP55845). All compounds were from Tocris (Bristol, UK).
WinEDR program (version 2.3.3, Strathclyde Electrophysiology Software, UK) or Axoscope 9.2 (Axon Laboratories, USA) was used for data acquisition. Offline analysis was done using MiniAnalysis 6.0.3 program (Synaptosoft, USA). Spontaneous events were detected using peak detector algorithm, and all events were confirmed visually. Rise and decay times were measured between 20 and 80% and 90–37% of the peak amplitude, respectively. The histograms and cumulative distributions were constructed from at least 10 min of recording (at least 50 events) from each cell, using a bin width of 100 ms for inter-event interval and 1 pA for amplitude. The pooled data are given as mean ±s.e.m. for the number of cells indicated. Due to large differences in the baseline frequency of spontaneous events between the slice culture batches, the data from organotypic cultures have been normalized to the control level, obtained from four to seven cells recorded from control cultures from the same batch. Student's two-tailed t test, variance analysis (ANOVA) followed by the post hoc Tukey test and Pearson's correlation test was used for statistical analysis. The level of significance was set as P < 0.05. Membrane capacitances (Cm) were estimated by exponential fitting of the decay of the transient current in response to a 5 mV pulse step using pCLAMP software (Clampfit 9.2, Axon instruments).
Results
Brief activity deprivation leads to increase in glutamatergic transmission at P4 but not at P8
In order to study the effect of activity deprivation on synaptic transmission in acutely isolated tissue, neonatal (P0–P8) hippocampal slices were incubated in semi-permeable filters with a buffered ACSF alone or supplemented with TTX (1 μm). The incubation for 15–17 h under control conditions had no detectable effects on the excitability of the CA3 network, indicated by preservation of the typical spontaneous network activity characteristic for the immature hippocampus (e.g. Ben-Ari, 2001; Palva et al. 2000; see online Supplementary Fig. 1). Further, incubation procedure had no effect on the viability of the CA3 pyramidal neurons in terms of their passive membrane properties (input resistance, capacitance and holding current under voltage clamp; see online Supplementary Fig. 2).
Figure 1. Developmentally regulated effect of activity blockade on mEPSCs in CA3 pyramidal neurons in acute slices.
A, 15–17 h TTX treatment leads to an increase in mEPSC amplitude and frequency at P4. Aa, example recordings of mEPSCs from CA3 pyramidal cell in control and TTX-incubated slice at P4. Ab, examples of single and averaged (10) events on an expanded scale. Ac, cumulative distributions and average values of the mEPSC interval in acute (n = 7), control (n = 11) and TTX-incubated (n = 6) slices. Ad, cumulative distributions and average values of the mEPSC amplitude from the same recordings. B, 15–17 h TTX treatment has no effect on mEPSCs at P8. Cumulative distributions and average values on the mEPSC interval (a) and amplitude (b) from acute (n = 12), control-incubated (n = 11) and TTX-incubated (n = 12) slices. C, time course of the effect at P4. Pooled data on the effect of 2–5 h (n = 10 for both groups), 7–10 h (n = 6) and 15–17 h TTX treatment on mEPSCs at P4. *P < 0.05, **P < 0.01 and ***P < 0.001.
Figure 2. Developmentally regulated effect of 15–17 h TTX treatment on mIPSCs frequency.
A, 15–17 h TTX treatment leads to decrease in mIPSC frequency only at immature neurons at P0. Aa, example recordings of mIPSCs from CA3 pyramidal neurons (Cm < 30 pF) from control and TTX-treated slices, cut at P0 and incubated for 15–17 h. Ab, single and averaged + events on an expanded time scale.Ac, cumulative distributions of the inter-event interval of mIPSCs at acute (P1, n = 6), control (n = 5) and TTX-incubated (n = 4) slices, from neurons with capacitance < 30 pF. Inset: plot of the mIPSC intervals as a function of the cell membrane capacitance (Cm) from acute, control and TTX- incubated slices (n = 4–9 for each group). *P < 0.02. Ad, equivalent data as in Ac, for the effect of TTX incubation on the amplitude of mIPSCs. B, 15–17 h TTX treatment had no effect on mIPSCs at P4. Ba, cumulative distributions of the inter-event intervals of mIPSCs from acute (n = 10), control (n = 11) and TTX-incubated (n = 18) slices at P4. Inset: average mIPSC intervals from the same data. Bb, equivalent data for the effect of TTX incubation on the amplitude of mIPSCs.
In order to investigate the properties of functional glutamatergic synapses after activity deprivation we recorded action-potential-independent glutamatergic events (mEPSCs) from CA3 pyramidal cells before and after 15–17 h incubation under control conditions or in the presence of TTX. The incubation procedure alone had no effect on mEPSCs since there were no significant differences in the occurrence or amplitude of mEPSCs between acute and incubated slices at P4 (n = 7–11 cells for each group; Fig. 1A). However, in TTX incubated slices, the mean interval between mEPSCs was significantly shorter than in the control slices (P < 0.01; n = 5–11; Fig. 1A). Further, the average mEPSC amplitude was remarkably larger after TTX treatment at P4 (P < 0.001; Fig. 1A). No significant changes in the kinetics of mEPSCs were observed (rise time20–80% 1.6 ± 0.2 and 1.7 ± 0.2 ms, decay time90–37% 6.9 ± 0.5 and 8.6 ± 1.2 ms, control and TTX, respectively). Surprisingly, at P8, no differences in mEPSCs between acute, control incubated and TTX-treated slices were detected (n = 11–12; Fig. 1B). These data show that rapid homeostatic regulation of glutamatergic inputs to CA3 pyramidal neurons occurs readily during early postnatal development but the mechanism is developmentally down-regulated and no longer observed at the beginning of the second postnatal week.
To find out the minimum time of activity deprivation leading to the regulation of mEPSCs at P4, we tested the effect of shorter periods of TTX incubation. There was no difference in mEPSCs between control and TTX-treated slices after 2–5 h incubation (n = 10 for both groups). However, the mean interval between mEPSCs was significantly shorter in the TTX slices after 7–10 h treatment (P < 0.05, n = 6 for both groups), without associated difference in the mEPSC amplitude (Fig. 1C). Thus, activity deprivation affected mEPSC frequency and amplitude in a distinct time course, the effect on frequency being detectable after 7–10 h TTX treatment, while effects on mEPSC amplitude required 15–17 h activity blockade.
Brief activity deprivation has no effect on GABAergic transmission at P4 but leads to decrease in transmission in immature P0 neurons
Having established the relevance of the neuronal developmental stage for rapid homeostatic regulation of mEPSCs, we next asked whether similar regulation was seen at GABAergic synapses. GABAergic synapses develop earlier than glutamatergic ones and their functional density in the newborn hippocampus has been shown to depend on the neuronal size, estimated by the cell membrane capacitance (Cm; Colin Le-Brun et al. 2004). In line with this, we found a strong correlation of the mean mIPSC interval with the Cm at P0 (r = 0.63, P < 0.02, n = 14). At P4, however, there was no significant correlation between capacitance and the occurrence of either mIPSC or mEPSCs (mIPSCs: r = 0.59, P > 0.2, n = 6; mEPSCs: r = 0.11, P > 0.7, n = 11). Thus, functional GABAergic synapses are formed in parallel with the development of the dendritic tree in the CA3 pyramidal neurons around at the time of birth (P0–P1).
We found that 15–17 h activity deprivation affected mIPSCs only at the most immature P0 neurons with capacitances below 30 pF. At these neurons, the average mIPSC interval in TTX incubated slices was considerably longer than in control slices (P < 0.05; n = 4–5; Fig. 2A). However, there were no significant differences in mIPSC intervals between control and TTX-incubated slices in P0 neurons with capacitance larger than 30 pF (n = 4–7 for each group; Fig. 2A) or at P4 (n = 13–18; Fig. 2B). TTX treatment had no effect on the amplitude or kinetics of mIPSCs in any group of cells at P0 (Fig. 2A) or at P4 (Fig. 2B). We also checked that incubation under control conditions had no effect on mIPSCs compared with acute slices at a corresponding developmental stage (Fig. 2A and B).
These results indicate that mIPSC frequency can be regulated in response to brief activity deprivation in the immature neurons. However, the effect disappears rapidly after birth.
The threshold for induction of homeostatic plasticity at glutamatergic synapses is increased during development
Homeostatic regulation of both glutamatergic and GABAergic transmission has been shown in mature hippocampal cultures in response to longer time periods (> 48 h) of activity deprivation (e.g. Murthy et al. 2001; Burrone et al. 2002; Nakayama et al. 2005; Stellwagen & Malenka, 2006; Hartman et al. 2006; Swanwick et al. 2006). Thus, one possible mechanism to explain the developmental differences in the homeostatic response is that the threshold for its induction is up-regulated during maturation of the circuitry. Since acute slices older than P0–P1 do not allow long incubations in vitro and since the occurrence of glutamatergic activity at P0 is rare (e.g. Tyzio et al. 1999), we tested this hypothesis using cultured hippocampal slices. The cultures were studied at two developmental stages, corresponding to time of active formation of glutamatergic synapses (7–8 days in vitro (DIV)) and after the majority of the connections are established (14–16 DIV) (De Simoni et al. 2003). TTX (1 μm) was added 15–22 h (TTX15) or 45–50 h (TTX48) before recording mEPSCs from CA3 pyramidal neurons. At 7–8 DIV, both the amplitude and frequency of mEPSCs were significantly higher following brief TTX treatment (TTX15) as compared with controls (n = 11–16; P < 0.05, interval; P < 0.001, amplitude; Fig. 3A). The effect of long TTX treatment (TTX48) on mEPSCs was not different from the 15 h incubation at this developmental stage. In contrast, at 14–16 DIV, brief TTX treatment had no significant effects on mEPSC as compared with controls (n = 14–19), while the 48 h TTX treatment led to an increase in both the frequency and amplitude of mEPSCs (n = 14–19, P < 0.05 interval; P < 0.001, amplitude compared with TTX15; Fig. 3B) as described before (Murthy et al. 2001; Burrone et al. 2002; Wierenga et al. 2006). The finding that induction of homeostatic plasticity at glutamatergic synapses requires longer time periods of activity deprivation in mature slice cultures compared with young ones suggests that threshold for its induction is up-regulated during maturation of the circuitry.
Figure 3. Developmentally regulated effect of activity blockade on mEPSCs in organotypic cultures.
A, effect of 15 h and 48 h TTX treatment on mEPSCs at 7–8 DIV. Aa, examples of mEPSC recordings from CA3 pyramidal neurons from control and TTX-treated cultures at 8 DIV. Ab, cumulative distributions of the mEPSC intervals in control (n = 6) and TTX-treated cultures (TTX15, n = 5; TTX48, n = 5) from one batch of slices at 8 DIV. Inset: pooled data from recordings at 7–8 DIV, showing the average inter-event interval of mEPSCs at the three groups normalized to the control level (control, n = 16; TTX15, n = 12; TTX48, n = 11). Ac, analysis of the amplitude of mEPSCs from the same neurons as in b. B, equivalent data for cultures at 14–16 DIV. Example traces (a) and cumulative distributions (b and c) from one batch of slices at 16 DIV (control, n = 6; TTX15 n = 5; TTX48 n = 6). Insets show the pooled data from all recordings at 14–16 DIV, normalized to the control level (control, n = 19; TTX15 n = 10; TTX48 n = 18).
The effect of long activity deprivation on mIPSC amplitude is independent of neuronal maturation stage
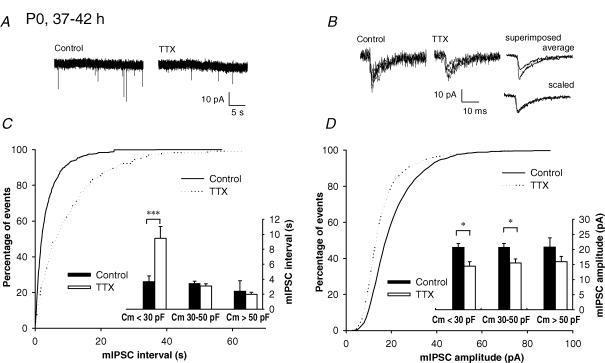
Prolonged TTX treatment in hippocampal cultures leads to a decrease in the mIPSC amplitude (Hartman et al. 2006; Swanwick et al. 2006). Therefore, we next tested the effect of long (37–42 h) TTX treatment on mIPSCs at immature slices. Acute slices at P0 survived < 42 h incubation in vitro without significant alterations in the passive membrane properties (Supplementary Fig. 2) or mIPSCs at CA3 pyramidal neurons (Fig. 4). As expected, the mIPSC interval was significantly longer in TTX-treated slices as compared with controls in the cells with capacitance less than 30 pF (P < 0.005, n = 5–8, Fig. 4A–C). However, no differences in mIPSC frequency were observed at neurons with Cm > 30 pF, suggesting that this mechanism is restricted to the immature neurons only. In contrast, long TTX treatment led to a decrease in the mIPSC amplitude in all neurons independent of their membrane capacitance (n = 13–20, P < 0.005, Fig. 4D). These data indicate that only immature neurons respond to inactivity by regulating the frequency of mIPSCs. Further, scaling of mISPC amplitude requires long periods (40 h) of inactivity and appears independent of the neuronal maturation.
Figure 4. Effect of 37–42 h TTX treatment on mIPSCs amplitude at P0.
A, example recordings of mIPSCs from CA3 pyramidal neurons (Cm < 30 pF) from control and TTX-treated slices, cut at P0. B, single and averaged (10) events on an expanded time scale. C, cumulative distributions of the inter-event interval of mIPSCs at control (n = 8) and TTX-incubated (n = 5) slices, from P0 neurons with capacitance < 30 pF. Inset: plot of the mIPSC intervals as a function of the cell membrane capacitance (Cm) from control and TTX-incubated slices (n = 4–9 for each group). *P < 0.05 and ***P < 0.001. D, equivalent data as in C, for the effect of TTX incubation on the mIPSC amplitude.
Discussion
In the neonatal hippocampus, both the GABAergic and glutamatergic synapses respond rapidly to inactivity (Lauri et al. 2003; Colin-Le Brun et al. 2004). We show here that this process is age dependent and disappears during maturation of the circuitry in a temporally and mechanistically distinct manner depending on the synapse type.
Blocking TTX-sensitive neuronal activity in the developing circuitry had opposing effects on GABAergic and glutamatergic transmission, which is consistent with data obtained previously using neuronal cultures (Turrigiano & Nelson, 2004; but see Buckby et al. 2006; Gonzalez-Islas & Wenner, 2006). Activity blockade led to an increase in the amplitude and frequency of mEPSCs at both immature and mature glutamatergic synapses, but the time course of this response was dependent on the developmental stage. At P4, the effect on mEPSC frequency appeared already within 7–10 h of TTX treatment, while a significant increase in mEPSC amplitude was observed after 15 h of activity blockade. At more mature synapses, a longer period of inactivity (48 h) was required for homeostatic regulation of both mEPSC frequency and amplitude. These data support the idea that the threshold for induction of homeostatic plasticity at glutamatergic synapses is increased in parallel with maturation of the circuitry.
At GABAergic synapses, brief activity deprivation led to rapid reduction of mIPSC frequency. This response, however, was restricted to the immature neurons only. In contrast, reduction in mISPC amplitude was only observed in response to longer periods of inactivity (40 h) and appeared independent of the maturational stage of the neurons. These data are consistent with previous findings in hippocampal neuronal cultures, where activity deprivation (> 48 h) affected mIPSC frequency only in young cultures, while scaling of mIPSC amplitude was observed at all stages of development (e.g. Hartman et al. 2006). The decrease in the mIPSC frequency in response to activity blockade only at immature neurons might indicate that activity is necessary for the formation and/or stabilization of GABAergic synapses (Colin-Le Brun et al. 2004; Hartman et al. 2006), while regulation of mIPSC amplitude represents the classical homeostatic compensatory response to inactivity. Interestingly, activity deprivation caused down-regulation of GABAergic transmission at P0, even though at this developmental stage the GABAergic synaptic responses are depolarizing (Ben-Ari, 2001). This result is not contradictory to the hypothesis that homeostatic response to activity deprivation acts to maintain the network excitability, considering that the shunting-type inhibitory action of GABAergic transmission might represent the prevailing functional effect in terms of network excitability already at P0 (e.g. Lamsa et al. 2000).
Age-dependent decrease in the homeostatic response has been previously demonstrated in layer 4 of the visual cortex in vivo (Desai et al. 2002), and in embryonic spinal cord in ovo (Gonzalez-Islas & Wenner, 2006). In addition, developmental effects on homeostatic response have been described for both glutamatergic and GABAergic synapses in culture (Burrone et al. 2002; Hartman et al. 2006; Karmarkar & Buonomano, 2006; Wierenga et al. 2006). Spontaneous activity of the neuronal networks is altered during development, which might in part influence the synaptic response to TTX treatment. In the immature hippocampus, spontaneous activity is characterized by network-driven bursts of activity, which are gradually down-regulated during development and disappear at around P12 (Ben-Ari, 2001). On the other hand, an increase in neuronal connectivity during development leads to an increase in overall levels of spontaneous synaptic activity, which is particularly evident in neuronal cultures (De Simoni et al. 2003). Understanding the exact role of various patterns of spontaneous activity in the induction of homeostatic plasticity awaits further studies.
Our data show that the critical developmental period for rapid homeostatic regulation of GABAergic connectivity in the hippocampus occurs earlier than that of glutamatergic, which is consistent with the earlier development of the GABAergic system (Tyzio et al. 1999; Colin-Le Brun et al. 2004). Thus, the fast compensatory response to inactivity appears to correspond to the time of intense morphological development of the given synapse type.
What could be the physiological significance of the different time windows for fast homeostatic scaling at inhibitory and excitatory synapses at the network level? During early postnatal development, glutamatergic connectivity is sparse and characterized by synaptic mechanisms that enable rapid changes in the weight of inputs to a certain cell. For example, rapid activation of silent synapses (Isaac et al. 1995; Durand et al. 1996) as well as an increase in the glutamate release probability (Lauri et al. 2006) occurs in response to high frequency activity that is spontaneously generated in the immature circuitry (Palva et al. 2000). These mechanisms, which are thought to be critical for the fine tuning of the connectivity during development, also render the network vulnerable for instability. Therefore, the need for physiological mechanisms that balance the network excitability is evident. At the time when glutamatergic synapses are only weakly transmitting, the most efficient way to compensate for a brief activity deprivation is to down-regulate inhibitory transmission. Later in development, when glutamatergic circuitry is already established, fine-tuning of the circuit activity could be done by regulation of both excitatory and inhibitory synapses.
Compared with the large amount of data on the mechanisms by which homeostatic changes in response to inactivity are expressed, surprisingly little is known on the signals necessary for its induction. Our present data indicate that the induction rules of homeostatic plasticity are developmentally regulated, and that there are different critical periods for regulation of inhibitory and excitatory transmission thus adding new levels of complexity to the cellular mechanisms underlying slow forms of homeostatic adaptation to inactivity.
Acknowledgments
This work was supported by the Academy of Finland (T.T. and S.E.L.), The Sigrid Juselius foundation (T.T. and S.E.L.), and The University of Helsinki research funds (S.E.L. and J.H.).
Supplemental material
Online supplemental material for this paper can be accessed at:http://jp.physoc.org/cgi/content/full/jphysiol.2007.130062/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.130062
References
- Aptowicz CO, Kunkler PE, Kraig RP. Homeostatic plasticity in hippocampal slice cultures involves changes in voltage-gated Na+ channel expression. Brain Res. 2004;998:155–163. doi: 10.1016/j.brainres.2003.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Buckby LE, Jensen TP, Smith PJ, Empson RM. Network stability through homeostatic scaling of excitatory and inhibitory synapses following inactivity in CA3 of rat organotypic hippocampal slices cultures. Mol Cell Neurosci. 2006;31:805–816. doi: 10.1016/j.mcn.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Colin-Le Brun I, Ferrand N, Caillard O, Tosetti P, Ben-Ari Y, Gaiarsa JL. Spontaneous synaptic activity is required for the formation of functional GABAergic synapses in the developing rat hippocampus. J Physiol. 2004;559:129–139. doi: 10.1113/jphysiol.2004.065060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol. 2001;63:847–869. doi: 10.1146/annurev.physiol.63.1.847. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Wenner P. Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron. 2006;49:563–575. doi: 10.1016/j.neuron.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. Quantal variability at glutamatergic synapses in area CA1 of the rat neonatal hippocampus. J Physiol. 2001;531:467–480. doi: 10.1111/j.1469-7793.2001.0467i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. Different forms of homeostatic plasticity are engaged with distinct temporal profiles. Eur J Neurosci. 2006;23:1575–1584. doi: 10.1111/j.1460-9568.2006.04692.x. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Goddard CA, Harris KM. Age-dependence in the homeostatic upregulation of hippocampal dendritic spine number during blocked synaptic transmission. Neuropharmacology. 2004;47:640–648. doi: 10.1016/j.neuropharm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Palva JM, Ruusuvuori E, Kaila K, Taira T. Synaptic GABAA activation inhibits AMPA-kainate receptor-mediated bursting in the newborn (P0–P2) rat hippocampus. J Neurophysiol. 2000;83:359–366. doi: 10.1152/jn.2000.83.1.359. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Lamsa K, Pavlov I, Riekki R, Johnson BE, Molnar E, Rauvala H, Taira T. Activity blockade increases the number of functional synapses in the hippocampus of newborn rats. Mol Cell Neurosci. 2003;22:107–117. doi: 10.1016/s1044-7431(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa A, Segerstråle M, Collingridge GL, Isaac JTR, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kiyosue K, Taguchi T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J Neurosci. 2005;25:4040–4051. doi: 10.1523/JNEUROSCI.4115-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva JM, Lamsa K, Lauri SE, Rauvala H, Kaila K, Taira T. Fast network oscillations in the newborn rat hippocampus in vitro. J Neurosci. 2000;20:170–1178. doi: 10.1523/JNEUROSCI.20-03-01170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Swanwick CC, Murthy NR, Kapur J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain derived neurotrophic factor. Mol Cell Neurosci. 2006;31:481–492. doi: 10.1016/j.mcn.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: Overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2006;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Walsh MF, Turrigiano GG. Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol. 2006;96:2127–2133. doi: 10.1152/jn.00107.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.