Abstract
Adenosine is an important signalling molecule involved in a large number of physiological functions. In the brain these processes are as diverse as sleep, memory, locomotion and neuroprotection during episodes of ischaemia and hypoxia. Although the actions of adenosine, through cell surface G-protein-coupled receptors, are well characterized, in many cases the sources of adenosine and mechanisms of release have not been defined. Here we demonstrate the activity-dependent release of adenosine in the cerebellum using a combination of electrophysiology and biosensors. Short trains of electrical stimuli delivered to the molecular layer in vitro, release adenosine via a process that is both TTX and Ca2+ sensitive. As ATP release cannot be detected, adenosine must either be released directly or rapidly produced by highly localized and efficient extracellular ATP breakdown. Since adenosine release can be modulated by receptors that act on parallel fibre–Purkinje cell synapses, we suggest that the parallel fibres release adenosine. This activity-dependent adenosine release exerts feedback inhibition of parallel fibre–Purkinje cell transmission. Spike-mediated adenosine release from parallel fibres will thus powerfully regulate cerebellar circuit output.
Adenosine is an important neuromodulator in the central nervous system, playing a role in a plethora of physiological and pathophysiological processes. The action of adenosine on cell surface receptors is well defined with A1, A2a, A2b and A3 receptors all cloned (Fredholm et al. 2000). Although extensively studied, the cellular source and mechanisms of adenosine release remain unclear (for review see Latini & Pedata, 2001). Adenosine can in principle gain access to the extracellular space by the breakdown of ATP, by translocation from cell cytoplasm via nucleoside transport proteins or possibly by the exocytosis of adenosine itself. There has been considerable investigation of adenosine release during pathological episodes such as hypoxia, ischaemia and hypercapnia as adenosine is neuroprotective (Rudolphi et al. 1992; Fredholm, 1997; Dale et al. 2000; Dulla et al. 2005). Release under these conditions is often Ca2+ independent, relatively insensitive to TTX and is not mediated via glutamate receptor activation. In contrast, little is known about the physiological release of adenosine with few examples where a role and cellular source of adenosine have been identified (but see Dale, 1998). In many cases, adenosine release is evoked with stimuli such as high K+, prolonged electrical stimulation and glutamate receptor activation (Latini & Pedata, 2001). The physiological relevance of these experiments is unclear.
The presence of adenosine, adenosine deaminase and A1 receptors in the cerebellar cortex (Braas et al. 1986; Geiger & Nagy, 1986; Rivkees et al. 1995) strongly suggests that adenosine plays an important role in cerebellar function. The activation of A1 receptors inhibits synaptic transmission between parallel fibres and Purkinje cells (Kocsis et al. 1984). These receptors are tonically activated by endogenous adenosine, since application of A1 receptor antagonists enhances synaptic transmission (Takahashi et al. 1995; Dittman & Regehr, 1996). The source of this adenosine has not been determined but could arise from the release of adenosine or the release of ATP and its subsequent metabolism. A recent report has suggested that ATP can be released from parallel fibres (Beierlein & Regehr, 2006).
To examine this issue we have used selective and sensitive microelectrode biosensors (Llaudet et al. 2003) to measure the release of adenosine from cerebellar slices in real time. These biosensors are small enough (25–50 μm diameter) to place either in or close to defined areas in cerebellar slices. Here we report that adenosine can be released from the molecular layer by using a physiological stimulus, short bursts (1–10 s) of focal electrical stimuli at the same voltage used to elicit synaptic transmission. The adenosine release is both TTX and Ca2+ sensitive and does not appear to arise from the extracellular metabolism of ATP. Modulation of parallel fibre–Purkinje cell synaptic transmission can increase or decrease adenosine release, strongly suggesting that parallel fibres are involved in adenosine release.
Methods
Slice preparation
Transverse slices of cerebellum (400 μm) were prepared from male Wistar rats, at postnatal days 21–28 (P21–28), with modified methods based on Llinas & Sugimori (1980). As previously described (Wall & Usowicz, 1997) and in accordance with the UK Animals (Scientific Procedures) Act 1986, male rats were killed by cervical dislocation and decapitated. The cerebellum was rapidly removed and transverse slices were cut on a Microm HM 650V microslicer (Carl Zeiss, Welwyn Garden City, UK) in cold (2–4°C) high Mg2+, low Ca2+ aCSF, composed of (mm): 127 NaCl, 1.9 KCl, 7 MgCl2, 0.5 CaCl2, 1.2 KH2PO4, 26 NaHCO3, 10 d-glucose (pH 7.4 when bubbled with 95% O2 and 5% CO2). Slices were stored in normal aCSF (1.3 mm MgCl2, 2.4 mm CaCl2) at room temperature for 1–6 h before recording.
Recording from slices
An individual slice was transferred to a recording chamber, submerged in aCSF and perfused at 6 ml min−1 (30–35°C). The slice was placed upon a suspended grid to allow perfusion of the slice from above and below and thus reduce the likelihood of hypoxia. All solutions were vigorously bubbled (95% O2 and 5% CO2) and all tubing had low gas permeability (Tygon; Fisher Scientific, Loughborough, UK). For the stimulation of purine release and parallel fibre–Purkinje cell (PF) EPSPs, square voltage pulses (2–8 V, 200 μs duration) were delivered by an isolated pulse stimulator (Model 2100 AM systems; Olympic Peninsula, Washington, DC, USA) via a concentric bipolar metal stimulating electrode (FHC) placed on the surface of the molecular layer. Purine biosensors were either positioned just above the surface of the slice (bent so their longitudinal surface was parallel to the stimulated molecular layer) or carefully inserted (at an angle of ∼70 deg) into the stimulated molecular layer. For the extracellular recording of PF EPSPs, an electrode (aCSF-filled microelectrode) was placed on the same track along which the parallel fibres travel (for example see Yuan & Atchison, 1999). A typical extracellular field potential consisted of an initial component which persisted in either 10 μm CNQX or 5 mm kynurenate but was blocked by 1 μm TTX (parallel fibre volley), followed by a component which could be blocked by 1 μm TTX and greatly reduced by either 10 μm CNQX or 5 mm kynurenate. This component is probably produced by parallel fibre-mediated glutamatergic excitatory synaptic currents and subsequent action potentials in Purkinje cells and interneurones (Clark & Barbour, 1997). Parallel fibre EPSP amplitude was estimated from the CNQX/kynurenate-sensitive potential, which was measured by subtracting what remained in CNQX/kynurenate from control potentials. Confirmation of PF EPSP identity was achieved by evoking pairs of EPSPs (interval 50 ms) and observing facilitation (20–30%) and by examining the pharmacological profile (inhibition by A1, GABAB and mGlu4R receptor agonists). Sensor signals were acquired at 1 kHz with either a Digidata 1322A (Axon) or a MiniDigi (Axon) using pCLAMP 9.2 (Axon) or Axoscope 9.2 (Axon). Extracellular recordings were made using an ISO-DAM extracellular amplifier (WPI, Stevenage, UK), filtered at 1 kHz and digitized on line (10 kHz) with a Digidata 1322A controlled by pCLAMP 9.2.
Biosensor characteristics
Biosensors were obtained from Sarissa Biomedical Ltd (Coventry, UK). In brief the adenosine biosensor consisted of three entrapped enzymes (adenosine deaminase, nucleoside phosphorylase and xanthine oxidase) within a matrix that was deposited around a Pt or Pt/Ir (90/10) wire etched to 25–50 μm (Llaudet et al. 2003). The biosensor had an exposed length of 500 μm that was coated with enzymes and thus capable of detecting purines. Biosensors had an additional screening layer, which greatly reduced the responses to non-specific electro active interferents (such as 5-HT, dopamine, noradrenaline and ascorbate). Screened null sensors, possessing the matrix but no enzymes, were used to control for the release of any non-specific electro active interferents. The ATP biosensor consisted of the entrapped enzymes glycerol kinase and glycerol-3-phosphate oxidase (Llaudet et al. 2005). Glycerol (2 mm) was included in solutions as glycerol is a co-substrate required for ATP detection.
Biosensors were calibrated with known concentrations of adenosine and ATP (10 μm typically giving responses of 2–3 nA for adenosine and 1.5 nA for ATP). Calibration was performed before the slice was present in the perfusion chamber and after the experiment (following slice removal), this allowing quantification of any run-down in sensitivity during the experiment. Previous reports have detailed the properties of adenosine and ATP biosensors (Llaudet et al. 2003, 2005): selectivity, linear responses to increasing analyte concentration and rapid response.
Determination of ATP breakdown inhibition
To determine which compounds were the most effective at blocking the extracellular metabolism of ATP, the breakdown of etheno-ATP (ɛ-ATP) was measured using HPLC. Individual 400 μm cerebellar slices were incubated (on a shaker, at room temperature) in 400 μl of aCSF containing either 5 or 50 μmɛ-ATP (2 or 20 nmol of ɛ-ATP) with or without inhibitors. At 0, 10, 25 and 45 min, 50 μl samples were taken and snap frozen with dry ice.
Samples were thawed out and diluted (1 in 10) in distilled water. HPLC analysis was performed with a Luna C8 (2) reverse phase column and a Thermo Separation Products HPLC gradient pump (P2000) and fluorescence detector (FL3000). The mobile phase consisted of 20 mm potassium phosphate pH 6.0 (solution A) and 75% potassium phosphate pH 6.0 and 25% methanol (solution B). A concave gradient was run going from 100% solution A to 100% solution B in 10 min. ɛ-ATP typically eluted around 3.5 min, ɛ-ADP around 4 min, ɛ-AMP 5 min and ɛ-adenosine around 9 min. The column was re-equilibrated with solution A for 10 min between runs. To quantify ɛ-ATP breakdown, the relative proportions of the breakdown products was obtained from peak areas.
Drugs
All drugs were made up as 10–100 mm stock solutions, stored frozen and then thawed and diluted with aCSF on the day of use. R(+)-baclofen hydrochloride, muscimol Evans Blue, α,β-methylene-ADP, adenosine, inosine, 6-[ (4-nitrobenzyl)thiol]-9-β-d-ribofuranosylpurine (NBTI) 8-cyclopentyltheophylline (8CPT) and dipyridamole were purchased from Sigma. Erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), ARL67165, l-AP4, d(–)-2- amino-5-phosphonopentanoic acid (AP5) were purchased from Tocris-Cookson. ATP was purchased from Roche and etheno-ATP (ɛ-ATP) was purchased as a 5 mm solution from Invitrogen.
Results
Trains of electrical stimuli release adenosine in the molecular layer
To investigate the release of purines in the cerebellum, trains (1–10 s, 2–20 Hz) of electrical stimuli were applied to areas of the cerebellum and purine release measured in real time using specific and selective microelectrode biosensors (Llaudet et al. 2003). Stimuli applied to the molecular layer of transverse slices, consistently produced a slow rising current on adenosine biosensors, positioned on the surface of the molecular layer (Fig. 1A). Movement of the stimulator and sensor to the adjacent granule cell layer caused a loss of current, suggesting the detected analyte was released in the molecular layer. The sensor-current (amplitude 20–200 pA) had a 10–90% rise time of 14.4 ± 2.2 s and a 10–90% decay of 131.6 ± 14.2 s (n = 30). The current was due to purine detection, as no current was produced on a null sensor (identical to an adenosine sensor but lacking the purine detection enzyme cascade) placed close to the adenosine biosensor (n = 8, Fig. 1A). The current waveform was not determined by sensor-response kinetics, as it was much slower than the sensor response to purine application (Llaudet et al. 2003). Since the adenosine biosensors can detect inosine and hypoxanthine as well as adenosine, EHNA, a specific inhibitor of adenosine deaminase (the first enzyme in the biosensor enzyme cascade), was used to determine the purine detected (Agarwal et al. 1977; Safiulina et al. 2005). EHNA (20 μm) greatly reduced (85 ± 5%, n = 3) the response of adenosine biosensors to exogenous adenosine (10 μm) but had no effect on the detection of inosine (and by inference hypoxanthine, Fig. 1B). In five slices, application of 20 μm EHNA greatly reduced the biosensor current following stimulation (mean inhibition 75 ± 3%, Fig. 1C). Thus, most of the biosensor current results from adenosine detection. The remaining current is probably a combination of adenosine (as EHNA only partial blocks the adenosine sensor response) and inosine either directly released or as a consequence of adenosine breakdown (due to incomplete inhibition of adenosine deaminase in the slice).
Figure 1. Electrical stimulation in the molecular layer releases adenosine.
A, superimposed current traces from adenosine (Ado) and null biosensors following electrical stimulation of the molecular layer (5 V for 5 s at arrow). The lack of signal on the null sensor indicates that the current on the adenosine biosensor is due to purine detection and is not non-specific. B, application of 10 μm adenosine (Ado) and then 10 μm inosine (Ino) produced current responses on an adenosine biosensor. Addition of 20 μm EHNA (to block adenosine deaminase) almost abolished the response to adenosine but had little effect on the response to inosine. C, superimposed traces from an adenosine biosensor in control and in the presence of 20 μm EHNA (to block adenosine deaminase) following electrical stimulation of the molecular layer (8 V for 8 s at arrow). EHNA reduced the current by ∼75% demonstrating that most of the signal is due to adenosine detection. All stimulations were at a frequency of 20 Hz.
The amount of adenosine released was dependent on both the frequency and duration of stimulation. With 100 stimuli, no adenosine release could be detected at a frequency of 2 Hz but was detected at 5 Hz. Maximal adenosine release occurred around 20 Hz with little increase at higher frequencies (40–80 Hz, Fig. 2A). Increasing the stimulus train length released more adenosine (Fig. 2B). At 20 Hz, the shortest train to release detectable adenosine was ∼1 s (20 stimuli) but in most slices 5–8 s (100–160 stimuli) were required. With an interval between trains of stimuli of 5 min, adenosine release was reliable and did not decrement. However, with shorter intervals the amount of adenosine released diminished. If it is assumed that all the sensor-current results from adenosine detection, then it is possible to calculate the adenosine concentration at the sensor. In 15 slices, an 8 s (20 Hz) stimulation produced concentrations ranging from 130 to 1650 nm (mean 492 ± 89 nm). This range of concentrations may stem from different distances between the adenosine release sites and the biosensor.
Figure 2. Properties of adenosine release.
A, graph summarizing how adenosine release varies with stimulation frequency (data from 4 slices). The number of stimuli was kept constant (100) and the stimulation frequency was varied between 2 and 80 Hz. The data are normalized to the concentration of adenosine detected at a stimulus frequency of 20 Hz. B, superimposed traces from an adenosine biosensor following 20, 100 and 160 stimuli. The stimulus frequency was kept constant at 20 Hz. The stimulation strength was between 3 and 8 V (A) and 5 V (B).
Mechanisms of adenosine release
We next examined the TTX-, Ca2+- and receptor-sensitivity of adenosine release. Application of the sodium channel blocker TTX (0.5–1 μm) abolished adenosine release (n = 11, Fig. 3A) demonstrating that adenosine release is action potential dependent. In seven slices, adenosine release was also abolished by substitution of normal aCSF for Ca2+-free aCSF (Mg2+ increased to 3.7 mm, Fig. 3B). To measure the Ca2+ dependence of adenosine release, extracellular Ca2+ concentration was changed between 0.5 and 5 mm with Mg2+ kept at 1.3 mm. Adenosine release was examined in the presence of 1 μm 8CPT to block A1 receptors (see later). The Ca2+ dependence was approximately linear (1.1 ± 0.1, n = 6, Fig. 3B inset) which is lower than that measured for classical neurotransmitters (Dodge & Rahamimoff, 1967; Mintz et al. 1995; Reid et al. 1998).
Figure 3. Mechanisms of adenosine release.
A, superimposed current traces from an adenosine biosensor placed on the surface of the molecular layer in control, in 10 μm CNQX + 50 μm AP5 and in 0.5 μm TTX. The adenosine release following electrical stimulation (5 V, 8 s at arrow) was abolished by TTX but was insensitive to the block of glutamate receptors. B, superimposed traces from an adenosine biosensor placed on the surface of the molecular layer in control, Ca2+-free aCSF (3.7 mm Mg2+) and following reintroduction of Ca2+ (wash). The adenosine release following electrical stimulation (7 V, 8 s at arrow) was abolished by removal of Ca2+. Inset, graph plotting normalized adenosine release against external Ca2+ concentration. Adenosine release was normalized to what occurred at 2.4 mm Ca2+ and summarizes data from 6 slices. C, superimposed traces from an adenosine biosensor placed on the surface of the molecular layer in control and in the presence of the GABAA receptor agonist muscimol (30 μm). Adenosine was released by a 5 V, 7 s stimulus at the arrow. Inset, superimposed averages of EPSPs in control and in 30 μm muscimol (*). Muscimol reduced EPSP amplitude but had little effect on the volley. D, superimposed traces from an adenosine biosensor placed on the surface of the molecular layer in control and in the presence of 5 μm NBTI and 10 μm dipyridamole to block equilibrative transport. Adenosine was released by a 5 V, 7 s stimulus at the arrow. All stimuli were at a frequency of 20 Hz.
It is possible that the action potential-dependent opening of Ca2+ channels and subsequent entry of Ca2+ directly causes the release of adenosine. However, it is also possible that the stimulation indirectly evokes adenosine release through the downstream actions of another neurotransmitter. We have taken two approaches to address this possibility. Firstly, we have tested the most likely neurotransmitter candidates that could perform this role (mean current in control versus mean current with antagonist present). Adenosine release was not sensitive to 10 μm CNQX and 50 μm AP5 (AMPA and NMDA glutamate receptor antagonists, 53.5 ± 10 versus 51.8 ± 11 pA, n = 5, Fig. 3A), 100 μm CPCCOET or 300 μm AIDA (Group 1 mGluR antagonists, 44.2 ± 13 versus 52 ± 18.5 pA, n = 3), 10 μm PPADs (P2 receptor antagonist, 34.6 ± 5 versus 32.8 ± 4.3 pA, n = 3), 300 μm l-NMMA (NO synthase inhibitor, n = 4, 25.3 ± 6.3 versus 21.6 ± 5 pA) and prazosin (α1 adrenoceptor antagonist, 103 ± 13 versus 99.6 ± 14 pA, n = 3). Furthermore application of 10 μm 5-HT or 10 μm noradrenaline did not induce adenosine release (n = 3). Secondly, we have applied the GABAA receptor agonist muscimol (30 μm) and measured adenosine release. The rationale for this approach is that the activation of GABAA receptors will hyperpolarize and reduce the input resistance of neurones. The actions of muscimol on glia are less certain, but Bergmann glia express GABAA receptors on their processes and therefore may be inhibited (Riquelme et al. 2002). Thus, if adenosine is released as a consequence of the downstream actions of another transmitter, inhibition of the target cells should greatly reduce or abolish adenosine release. Furthermore, if GABAA receptors are not present on parallel fibres and parallel fibres are the source of adenosine then muscimol should have no effect on adenosine release. The effectiveness of muscimol (30 μm) was confirmed by two observations: (1) the prolonged loss of spontaneous action potential firing in Purkinje cells following muscimol application (n = 3) and (2) muscimol (30 μm) reduced the amplitude of parallel fibre EPSPs by 56 ± 6.2% (n = 6) with little effect upon the parallel fibre volley (8.1 ± 7.7% reduction, Fig. 3C inset, n = 6). The lack of effect of muscimol on the parallel fibre volley suggests few GABAA receptors are expressed by parallel fibres and thus the reduction in EPSP amplitude results from a reduction in the resistance of Purkinje cells. Since muscimol (30 μm) had no significant effect on the magnitude of adenosine release (55.3 ± 8.5 versus 58.1 ± 8.1 pA, Fig. 3C, n = 5) it seems very unlikely that adenosine is released as a result of the downstream actions of another transmitter. Furthermore it is unlikely to come from Purkinje cells or interneurones as they will be shunted by GABAA receptor activation.
Direct release of adenosine could occur via the equilibrative nucleoside transporters ENT1 and ENT2 translocating adenosine from the cytoplasm to the extracellular space. However, blockade of these molecules by a combination of 5 μm NBTI and 10 μm dipyridamole (for review see Noji et al. 2004) had no significant (P = 0.71) effect on the amount of adenosine released (n = 5, Fig. 3D, 41.6 ± 7.8 versus 38.3 ± 10.7 pA, 1 μm 8CPT was present to prevent A1 receptor activation).
Adenosine was not released as a result of electroporation and consequent release of cytoplasmic ATP (for example, see Hamann & Attwell, 1996) (with subsequent conversion to adenosine by ecto-ATPases) since electroporation is insensitive to TTX and only occurred at much higher stimulus strengths (see later and Fig. 4C).
Figure 4. ATP release is not detected.
A, superimposed traces from an adenosine biosensor placed on the surface of the molecular layer and an ATP biosensor placed within the molecular layer. To maximize the likelihood of detecting ATP, adenosine was first detected (by an adenosine biosensor) then the ATP biosensor was placed in the molecular layer where the adenosine was detected. Following stimulation (arrow, 10 s) although adenosine was detected, no ATP release was measured. B, superimposed traces from an adenosine biosensor placed on the surface of the molecular layer and an ATP biosensor placed within the molecular layer. Following the application of the ecto-ATPase inhibitor Evans Blue (100 μm) the adenosine current was not diminished and there was still no ATP detection. The traces in B are from the same slice as in A. C, superimposed traces from an adenosine biosensor placed on the surface of the molecular layer and an ATP biosensor placed within the layer as in A and B. The biosensors were positioned at an approximately equal distance from the stimulating electrode. TTX (0.5 μm) was present to block TTX-dependent adenosine release. Stimulation (30 V, 10 s) caused cell damage and resulted in the electroporation of ATP, which produced a large current on the ATP biosensor. A small proportion of the released ATP (∼20%) was broken down to adenosine and was detected by the adenosine biosensor. The traces in C have been scaled by sensor calibration. All stimulations were at 20 Hz.
ATP release cannot be detected
An important potential source of adenosine is the extracellular metabolism of ATP. To investigate whether adenosine is released in the form of ATP, a number of approaches were taken. Firstly, ATP biosensors were used to test directly for ATP release (Llaudet et al. 2005). These biosensors have a detection limit of ∼60 nm (producing ∼10 pA of current). In eight slices an ATP biosensor was positioned close to the adenosine biosensor on the surface of the molecular layer. Following stimulation, adenosine was detected but no signal was observed on the ATP biosensor. In three further slices, after measuring adenosine release, the adenosine biosensor was carefully removed and replaced with an ATP sensor (thus potential ATP release was measured in exactly the same place where adenosine was detected). Again there was no signal following stimulation. It is possible that ATP is released deep within the slice and is then metabolized to adenosine before it reaches the sensor on the surface of the slice. To investigate this, ATP biosensors were inserted into the molecular layer, in the region where adenosine was detected. Again no signal was observed on the ATP sensor following stimulation, although adenosine could still be detected on the slice surface (n = 8, Fig. 4A). To reduce the possible extracellular ATP breakdown we have made use of three agents: Evans Blue and ARL67156 (which inhibit ecto-ATPases, Crack et al. 1995; Bultmann et al. 1999) and α,β-methylene-ADP (which inhibits ecto-5′-nucleotidase, for review see Zimmerman, 1996). In the presence of Evans Blue (100 μm, n = 5) or ARL67156 (100 μm, n = 3) no signal was detected on the inserted ATP sensor and the adenosine signal was not significantly different from the control (P = 0.53, Fig. 4B). There was also no significant difference between the amount of adenosine detected in control, following application of 100 μmα,β-methylene-ADP (47 ± 19 versus 54 ± 17 pA, P = 0.06) and after wash (P = 0.22).
We have exploited the electroporation-induced release of ATP (Hamann & Attwell, 1996) to measure the kinetics of ATP breakdown. An ATP biosensor was inserted into the molecular layer and an adenosine sensor was placed very close to the ATP sensor on the surface of the molecular layer. TTX (0.5 μm) was present to prevent TTX-sensitive adenosine release. Following 30–35 V stimulation, ATP was released and the resultant breakdown to adenosine was measured by the adenosine biosensor (Fig. 4C). Although ATP breakdown was rapid, only 15 ± 5% of the ATP was converted to adenosine (n = 4). If adenosine were to arise from ATP breakdown, this suggests that to give the observed signal of ∼0.5 μm adenosine, around 2.5–5 μm ATP would have to be released. This quantity of ATP would give an easily measurable signal on the ATP sensor. Therefore it seems unlikely that adenosine arises from the extracellular breakdown of ATP.
Inhibitors slow ATP metabolism
The inability to either directly detect ATP or reduce the adenosine signal could reflect an inability to block the ecto-ATPases and 5′-nucleotidases which convert ATP to adenosine. Thus, we have assayed the effectiveness of Evans Blue, ARL67156 and α,β-methylene-ADP in preventing either ATP breakdown or conversion of ATP to adenosine using HPLC (Fig. 5). We added ɛ-ATP (5 and 50 μm) to cerebellar slices and observed the metabolism and resultant build up of ɛ-adenosine (Fig. 5A and D). Although the ecto-ATPase inhibitors ARL67156 and Evans Blue (100 μm) did not abolish ɛ-ATP breakdown they did slow its rate of metabolism (Fig. 5B and C). At both 5 and 50 μmɛ-ATP, Evans Blue was a more effective inhibitor than ARL67156. For example, after 25 min only 8.6% of 5 μmɛ-ATP remained in control, compared with 39.3% in Evans Blue and 26.4% in ARL67156. Both inhibitors appeared more effective at lower substrate concentrations, suggesting a competitive mode of action. Other ecto-ATPase inhibitors, such as α,β-methylene-ADP, suramin and PPADs, were less effective than Evans Blue (not illustrated).
Figure 5. Metabolism of ɛ-ATP by cerebellar slices at room temperature.
A, graph plotting percentage composition against time for the breakdown of 50 μmɛ-ATP. At time zero there was ∼90% ATP (▪) and 10% ADP (▴). Following incubation there was a fall in the proportion of ATP and a subsequent increase in AMP (□) and adenosine (^) with little change in ADP. B, graph plotting percentage of 50 μmɛ-ATP remaining against time in control and in the presence of inhibitors (Evans Blue and ARL67156). C, graph plotting percentage of adenosine against time in control and in the presence of inhibitors during metabolism of 50 μmɛ-ATP. α,β-Methylene-ADP (100 μm) was the most effective in slowing the build-up of adenosine. D, graph plotting percentage composition against time for the breakdown of 5 μmɛ-ATP. There was a more rapid breakdown of 5 μm ATP compared with 50 μmɛ-ATP (compare with A). E, graph plotting percentage of ɛ-ATP (5 μm) remaining against time in control and in the presence of inhibitors. Note that both Evans Blue and ARL67156 (100 μm) were more effective at slowing the metabolism of 5 μmversus 50 μmɛ-ATP (compare E and B). F, graph plotting percentage of adenosine against time in control and in the presence of inhibitors during metabolism of 5 μmɛ-ATP. Again inhibitors are more effective at slowing build up of adenosine with lower concentrations of substrate. Graphs A, B and C summarize data from 4 experiments; graphs D, E and F are from 3 experiments.
Although the ecto-5′-nucelotidase inhibitor, α,β-methylene-ADP (100 μm), had little effect on ATP breakdown, it was the most effective in slowing adenosine formation (Fig. 5C and F). α,β-Methylene-ADP (100 μm) appeared to slow conversion to adenosine by reducing the breakdown of ADP and reducing the conversion of AMP to adenosine. Again, inhibition of adenosine formation was more effective at lower substrate concentrations. These enzyme inhibitors would be expected to slow (but not block) the breakdown of ATP/formation of adenosine, with their effectiveness dependent on substrate concentration. As they had no effect on the adenosine signal and did not reveal an ATP signal, we conclude that ATP breakdown is most unlikely to contribute to the observed adenosine release.
Adenosine release can be decreased by inhibiting parallel fibre transmission
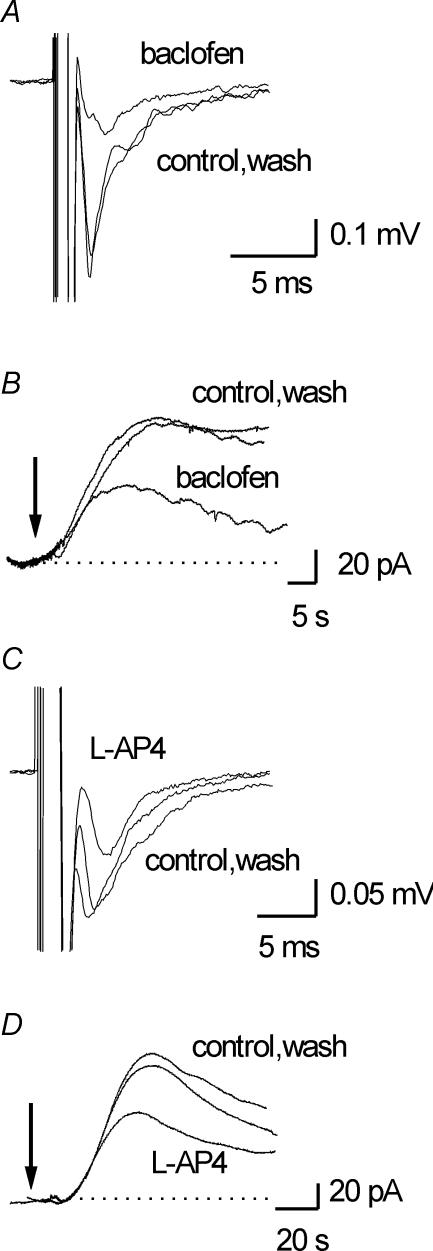
Our data suggest that the most likely source of adenosine is the parallel fibres (granule cell axons) which run along the molecular layer making glutamatergic synapses onto Purkinje cells. These parallel fibre–Purkinje cell synapses are inhibited by presynaptic GABAB and mGlu4 receptors (Batchelor & Garthwaite, 1992; Neale et al. 2001). If adenosine is released from parallel fibres (or parallel fibres release a transmitter required for adenosine release) then adenosine release should also be modulated by these presynaptic receptors. Thus, adenosine release was measured before and after the addition of the GABAB receptor agonist baclofen (10–50 μm). Baclofen reversibly reduced parallel fibre (PF) EPSP amplitude by 67.8 ± 4.9% (n = 6, Fig. 6A) and also significantly reduced adenosine release by 66.5 ± 8% (n = 5, Fig. 6B). Parallel fibres are also modulated by mGluR4 receptors: application of the mGluR4 receptor agonist l-AP4 (50 μm) caused a reversible decrease in PF EPSP amplitude (41.3 ± 8%, n = 4, Fig. 6C). Application of l-AP4 (50 μm) caused a significant (P < 0.05) reversible decrease in the amplitude of adenosine signals (44.2%, 60 ± 17.7 versus 33.5 ± 10.6 pA, n = 4, Fig. 6D) demonstrating that adenosine release is also modulated by mGluR4 receptors. The matching of the pharmacological profile of parallel fibre–Purkinje cell synaptic transmission with that of adenosine release strongly suggests that activation of parallel fibres is essential for the activity-dependent adenosine release and this release can be modulated by endogenous transmitters.
Figure 6. Adenosine release is modulated by parallel fibre receptor agonists.
Parallel fibre (PF) EPSPs were evoked every 10 s by a stimulating electrode placed on the surface of the molecular layer and recorded with an extracellular electrode. A, superimposed averages of EPSPs in control, in 25 μm baclofen and following wash. Baclofen reversibly reduced EPSP amplitude by ∼60%. B, superimposed traces from an adenosine biosensor in control, baclofen (25 μm) and in wash. Adenosine release was evoked by a 10 s stimulus at the arrow. Baclofen reversibly reduced adenosine release by ∼50%. C, superimposed averages of EPSPs in control, in 50 μm l-AP4 and following wash. l-AP4 reversibly reduced EPSP amplitude by ∼40%. D, superimposed traces from an adenosine biosensor placed on the surface of the molecular layer in control, 50 μm l-AP4 and in wash. Following l-AP4 application adenosine release was decreased by ∼40%. Adenosine release was evoked by a 5 s stimulus (20 Hz) at the arrow.
Endogenous adenosine release modulates information flow in the cerebellum
Parallel fibre to Purkinje cell synaptic transmission is inhibited by presynaptic A1 receptors (Kocsis et al. 1984; Rivkees et al. 1995) which raises two possibilities: firstly adenosine release from parallel fibres should be modulated by endogenous adenosine and secondly, the released adenosine could inhibit parallel fibre–Purkinje cell synaptic transmission and inhibit adenosine release (negative feedback). To test the first possibility, the effects of an A1 receptor antagonist on adenosine release were investigated. Block of A1 receptors (8CPT, 1 μm) caused an increase in PF EPSP amplitude (45.8 ± 10%, n = 10, Fig. 7A) demonstrating continual adenosine-mediated inhibition and the presence of an extracellular adenosine tone (Takahashi et al. 1995; Dittman & Regehr, 1996). Application of 8CPT (1 μm) also caused a significant increase in the amplitude of adenosine signals (89 ± 15%, n = 8, Fig. 7B) demonstrating that adenosine release is also modulated by the level of extracellular adenosine. The much larger effect of 8CPT on adenosine release compared with PF EPSPs may stem from the block of adenosine auto-inhibition (released adenosine binds to A1 receptors and inhibits its own release). We investigated the feasibility of this by examining modulation of parallel fibre transmitter release by released adenosine. If the concentration of adenosine released reaches a sufficient magnitude, it should inhibit glutamate release from parallel fibres (via the activation of A1 receptors). PF EPSPs were evoked every 10 s (to measure baseline transmission) and then a 10 s train (20 Hz) of stimuli was delivered to release adenosine. Following the train of stimuli, the amplitude of PF EPSPs was diminished but then slowly recovered back to the baseline (mean recovery time 122 ± 6.5 s, n = 6) with a time course very similar to the decay of adenosine release (decay ∼130 s, Fig. 7C and D). Application of 1 μm 8 CPT, to block the A1 receptors, significantly increased the speed of recovery following the train (recovery time reduced from 122 ± 6.5 to 44 ± 8.7 s, Fig. 7C and D). Thus, parallel fibre-dependent adenosine release is functionally important and acts to auto inhibit both the parallel fibre–Purkinje cell synapse and indeed adenosine release itself.
Figure 7. The adenosine released by electrical stimulation modulates parallel fibre–Purkinje cell synaptic transmission.
Parallel fibre (PF) EPSPs were evoked every 10 s by a stimulating electrode placed on the surface of the molecular layer and recorded with an extracellular electrode. A, graph plotting PF EPSP amplitude against time. Application of 1 μm 8CPT, to block A1 receptors, increased EPSP amplitude by ∼30%. B, superimposed traces from an adenosine biosensor in control and in the presence of 1 μm 8CPT. Adenosine release was evoked by a 7 s stimulus at the arrow. Application of 8CPT increased adenosine release by 43%. C, graph plotting PF EPSP (evoked every 10 s) amplitude against time. At the asterisk a train of stimuli was delivered (10 s, 20 Hz) to cause adenosine release. Following the train there was a marked reduction in PF EPSP amplitude followed by a slow recovery back to control amplitude (PF EPSP amplitude during the train is not plotted). The time course of PF EPSP amplitude recovery was ∼150 s which is very similar to the time course of adenosine release. Application of the A1 receptor antagonist 8CPT increased PF EPSP amplitude by ∼25% and also markedly speeded recovery following a train of stimuli (∼50 s). Thus, the slow component of recovery results from the released adenosine activating A1 receptors. D, graph summarizing data from 6 recordings. Following blockade of A1 receptors there was significant speeding of EPSP recovery.
Discussion
We have demonstrated that adenosine can be released from the molecular layer of cerebellar slices by electrical stimulation. This release of adenosine was via a process that is both TTX and Ca2+ sensitive and as ATP release cannot be detected, adenosine is either released directly or rapidly produced by efficient extracellular ATP breakdown. Since adenosine release was modulated by receptors that act on parallel fibre–Purkinje cell synapses, parallel fibres are the most likely source of adenosine. No previous study has measured adenosine release from cerebellar slices, although adenosine can be released from cultured granule cells (Schousboe et al. 1989; Philibert & Dutton, 1989; Sweeney, 1996). Prolonged electrical stimulation (1–5 min) can release adenosine in other brain regions (the cortex, hippocampus and striatum, Pedata et al. 1990; Lloyd et al. 1993). However, we have characterized the properties of adenosine release evoked by a plausibly physiological stimulus: short duration trains (1–10 s), localized to a small area of the slice and at the same minimal strength that evokes transmitter release.
What is the source of adenosine?
We have provided strong evidence that parallel fibre activity is required for adenosine release. Firstly, the stimulating electrode and biosensors were arranged along a beam of parallel fibres; thus, parallel fibres are activated by the stimulus and the sensors are in the correct place to measure what is released. Secondly, activation or inhibition of the G-protein coupled receptors (GABAB, A1 and mGluR4) present on parallel fibre terminals modulates adenosine release by a similar magnitude as synaptic transmission. This proportionality in the reduction of EPSP amplitude and adenosine release suggests a common mechanism for adenosine and glutamate release. However, there are apparent differences. Our results suggest that the dependence of adenosine release on extracellular Ca2+ is ∼1 whereas previous studies have shown that the Ca2+ dependence of glutamate release at parallel fibre synapses is ∼3 (Mintz et al. 1995; Brown et al. 2004). This difference in Ca2+ dependence may be more apparent than real as different stimuli were used to elicit glutamate and adenosine release. Single stimuli were used to evoke glutamate release (Mintz et al. 1995; Brown et al. 2004) whereas in this study, trains of stimuli were required to release adenosine. The Ca2+ dynamics during a train of stimuli will be more complex than for a single stimulus and may result in intracellular Ca2+ accumulation thus reducing the apparent dependence on extracellular Ca2+. Thirdly, it is physiologically plausible that parallel fibres maintain transmitter release at frequencies up to and above the stimulation frequency used in this study (Kreitzer & Regehr, 2000). Thus, parallel fibre-dependent adenosine release could occur physiologically and act to limit parallel fibre-dependent excitation of Purkinje cells. Finally, moving the biosensor further away (along the same beam of parallel fibres) resulted in only a small reduction in current amplitude with little change in rise time.
Other possible sources of adenosine include Purkinje cells, interneurones and glia. Stimulation in the molecular layer will activate all these cell types but the TTX sensitivity of adenosine release makes Bergmann glia an unlikely source of adenosine as they do not fire action potentials (Clark & Barbour, 1997). Purkinje cells are strongly immunoreactive for adenosine (Braas et al. 1986) and express GABAB receptors in their dendrites (Lujan & Shigemoto, 2006) but there is little evidence that Purkinje cells express either A1 adenosine receptors or mGluR4 receptors. The restricted expression of mGluR4 receptors also means adenosine release from molecular layer interneurones is unlikely (Mateos et al. 1998). We can also exclude glutamate released from parallel fibres activating receptors on neurones and glia, since adenosine release was not blocked by the glutamate receptor antagonists CNQX and AP5. Furthermore Purkinje cells, interneurones and (possibly) Bergmann glia will be shunted by application of the GABAA receptor agonist muscimol, which had no effect on adenosine release. Thus, the most likely source of adenosine is the parallel fibres. However, on several occasions it has been possible to stimulate parallel fibres and record EPSPs without observing release of detectable amounts of adenosine. Thus, perhaps only a subset of parallel fibres release adenosine.
Properties of adenosine release
Some of the characteristics of adenosine release appear different from parallel fibre–Purkinje cell synaptic transmission. Firstly, a single stimulus is sufficient to evoke glutamate release from parallel fibres, yet a train of such stimuli is required to produce an adenosine signal on the biosensor. This could simply reflect that enough adenosine has to be released to diffuse through the tissue to reach the sensor on the slice surface. Clearly the distance between synaptic glutamate receptors and the release sites is much smaller than that between the adenosine biosensor and release sites. Thus, the requirement for trains of stimuli could be an artefact of the recording system. However, similar stimulation protocols were required when the sensors were pushed into the molecular layer (presumably the sensor will be closer to the release sites). At the calyx of Held, trains of stimuli (10 Hz) release adenosine, which can be detected indirectly through the activation of A1 receptors and the consequent inhibition of transmitter release. This inhibition only reaches significance after the first 20 stimuli in a train, suggesting that multiple stimuli are required to release adenosine (Kimura et al. 2003; Wong et al. 2006). In the hippocampus, 10 Hz but not 3 Hz stimulation released adenosine (measured by inhibition of EPSCs, Brager & Thompson, 2003). Secondly, once released the time course of adenosine was very slow. The decay of adenosine takes around 130 s which is vastly longer than that for classical fast transmitters like glutamate. This is similar to neuropeptides, which have a long duration of action and act in a paracrine manner affecting many neurones. This may reflect the large number of parallel fibres that are stimulated resulting in the spill over of adenosine and diffusion over a large area.
Mechanism of adenosine release?
The inhibition of ENT1 and ENT2 had no effect on adenosine release and thus adenosine was not transported across the cell membrane by these equilibrative transporters. NBTI and dipyridamole do inhibit adenosine transport leading to greater synaptic inhibition in the cerebellum (M. J. Wall, personal observation) and in the hippocampus (Frenguelli et al. 2007). However, it is possible that other transporters, which are insensitive to NBTI/dipyridamole, are involved in adenosine release. Adenosine release was blocked by TTX and by removal of Ca2+. The calcium dependence of adenosine release was lower than that reported for classical neurotransmitters, but was similar to that reported for the release of neuropeptides (for example see Peng & Zucker, 1993). The adenosine release thus has the characteristics associated with conventional synaptic release. Nevertheless there are three alternate interpretations of our data. Firstly, an intermediate transmitter could in principle cause the downstream release of adenosine. This seems unlikely as we have eliminated the obvious candidates for such an intermediate transmitter and have shown that inhibiting neurones and glia (through GABAA receptor activation) has little effect on adenosine release. Secondly, action potential activity in parallel fibres could release potassium which depolarizes other cells (including glia) resulting in adenosine release. This seems unlikely as activation of parallel fibre receptors (GABAB, A1 and mGlu4R) inhibits adenosine release but would not affect the amount of potassium released during action potentials. Furthermore any cells depolarized by the potassium efflux would probably be shunted by GABAA receptor activation suggesting that this treatment should diminish release if that were the case.
Thirdly, adenosine could arise from the breakdown of exocytotically released ATP. Beierlein & Regehr (2006) have recently described parallel fibre-mediated Ca2+ rises in Bergmann glia that are blocked by PPADs (50 μm) and may thus depend on parallel fibre-mediated ATP release. However, we have been unable to measure any ATP release (either with or without ecto-ATPase inhibition). Thus, the ATP released from parallel fibres, as reported by Beierlein & Regehr (2006), may be too small to detect by our methods. This contrasts with studies in the retina where glial cells release ATP which is rapidly converted by ectoenzymes into adenosine. In this case, the ATP can be detected using luciferin–luciferase chemiluminescence and blockers of ATP breakdown reduce the production of adenosine (reviewed by Newman, 2004). In the hippocampus, it has been reported that exogenous ATP is converted to adenosine in less than a second (Dunwiddie et al. 1997). More recent experiments demonstrated that only a small proportion (∼7%) of bath-applied ATP is converted, albeit rapidly to adenosine by hippocampal slices (Frenguelli et al. 2007). Our experiments suggest that in the cerebellum, only 10–20% of ATP is rapidly converted to adenosine. Furthermore biosensor measurements of the breakdown of bath-applied ATP show that less than 10% of the applied ATP is converted to adenosine (Wall MJ & Dale N, personal observations). For the complete conversion of parallel fibre ATP to occur, high densities of ectonucleotidases would have to be localized at parallel fibre synapses around the site of release such that no ATP escaped the confines of the synapse. Although an ecto-ATPase (CD39) is present on the soma and dendrites of Purkinje cells (Wang & Guidotti, 1998) and 5′-nucleotidase activity is present on Bergmann glia membranes (Schoen et al. 1987), the density of these enzymes is unclear and this explanation seems to us unlikely. A direct mechanism of adenosine release is supported by Wong et al. (2006) who demonstrated that trains of stimuli release adenosine at the calyx of Held (detected by A1 receptor mediated-inhibition of EPSCs) with no detectable release of ATP (measured by means of the luciferin–luciferase method). In the hippocampus, the adenosine released by trains of stimuli (detected by A1 receptor-mediated inhibition of EPSCs) could not be reduced by blocking 5′-ectonucleotidases (Mitchell et al. 1993; Brager & Thompson, 2003). Frenguelli et al. (2007) have also shown that adenosine release during ischaemia does not arise from the extracellular metabolism of ATP.
The functional significance
Irrespective of the detailed mechanism of adenosine release, its dependency on parallel fibre activity is potentially very significant. During periods of high parallel fibre activity adenosine will be released resulting in an increased extracellular concentration of adenosine and greater inhibition of parallel fibre–Purkinje cell synapses. Although the increased synaptic inhibition will reduce the amplitude of EPSPs, the effects on high-frequency synaptic transmission will probably be more important. During high-frequency parallel fibre activity the increased synaptic inhibition will reduce glutamate depletion and thus maintain transmission to Purkinje cells (Kimura et al. 2003; Wong et al. 2006). Activation of A1 receptors by endogenous adenosine at the calyx of Held causes a reduction in the relative amplitude of EPSCs at the end of the train compared with the EPSCs at the beginning. This is because there is no ambient level of adenosine at the calyx of Held and adenosine is released and accumulates during the stimulation, reaching a significant concentration late in the train. In contrast, Billups et al. (2005) have shown that activation of presynaptic group III mGluRs has no net effect on the amplitude of EPSCs. However, there is an increase in the size of the readily releasable vesicle pool which is balanced by a reduction in the probability of release. Thus, the synaptic state of the synapse has changed and the metabolic demand has been reduced. We have not measured the actions of adenosine during a train of EPSPs but have investigated recovery from depression following a train. We found that blocking A1 receptors greatly speeds the recovery of EPSP depression. Similar observations have been observed following trains of stimuli at the calyx of Held, where blocking group III mGluRs increased the speed of recovery (Billups et al. 2005) while GABAB receptor activation slowed recovery (Sakaba & Neher, 2003). The action of GABAB receptors appears to be through retardation of synaptic vesicle recruitment during sustained activity.
The dynamics of adenosine signalling are likely to be complex as release of adenosine auto-inhibits its own release. An intriguing question is whether adenosine released from active parallel fibres could laterally inhibit neighbouring but inactive parallel fibres. Interestingly adenosine is also vaso-active and there is evidence that periods of activity release adenosine resulting in the dilatation of cerebellar blood vessels (Li & Iadecola, 1994).
Acknowledgments
We thank Dr Enrique Llaudet and Shakila Bibi of Sarissa Biomedical Ltd for biosensor manufacture and Dr Robert Eason and Matthew Simpson for assistance with the HPLC experiments. This work was supported by the Wellcome Trust (M.J.W. and N.D.).
References
- Agarwal RP, Spector T, Parks RE. Tight-binding inhibitors-IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmac. 1977;26:359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Garthwaite J. GABAB receptors in the parallel fibre pathway of rat cerebellum. Eur J Neurosci. 1992;4:1059–1064. doi: 10.1111/j.1460-9568.1992.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Regehr WG. Brief bursts of parallel fiber activity trigger calcium signals in Bergmann glia. J Neurosci. 2006;26:6958–6967. doi: 10.1523/JNEUROSCI.0613-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups B, Graham BP, Wong AYC, Forsythe ID. Unmasking group III metabotropic glutamate autoreceptor function at excitatory synapses in the rat CNS. J Physiol. 2005;565:885–896. doi: 10.1113/jphysiol.2005.086736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, Newby AC, Wilson VS, Snyder SH. Adenosine-containing neurons in the brain localized by immunocytochemistry. J Neurosci. 1986;6:1952–1961. doi: 10.1523/JNEUROSCI.06-07-01952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Thompson SM. Activity-dependent release of adenosine contributes to short-term depression at CA3-CA1 synapses in rat hippocampus. J Neurophysiol. 2003;89:22–26. doi: 10.1152/jn.00554.2002. [DOI] [PubMed] [Google Scholar]
- Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultmann R, Trendelenburg M, Tuluc F, Wittenburg H, Starke K. Concomitant blockade of P2X-receptors and ecto-nucleotidases by P2-receptor antagonists: functional consequences in rat vas deferens. Naunyn Schmiedebers Arch Pharmacol. 1999;359:339–344. doi: 10.1007/pl00005360. [DOI] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol. 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack BE, Pollard CE, Buekers MW, Roberts SM, Hunt SF, Ingall AH, McKechnie KCW, Ijerzman AP, Leff P. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br J Pharm. 1995;114:475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N. Delayed production of adenosine underlies temporal modulation of swimming in frog embryo. J Physiol. 1998;511:265–272. doi: 10.1111/j.1469-7793.1998.265bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol. 2000;526:143–155. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine and neuroprotection. Int Rev Neurobiol. 1997;40:259–280. [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull N, Shulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmeidebers Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischemia in the mammalian hippocampus. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04425.x. DOI: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JD, Nagy JI. Distribution of adenosine deaminase activity in rat brain and spinal cord. J Neurosci. 1986;6:2707–2714. doi: 10.1523/JNEUROSCI.06-09-02707.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Attwell D. Non-synaptic release of ATP by electrical stimulation in slices of rat hippocampus, cerebellum and habenula. Eur J Neurosci. 1996;8:150–1515. doi: 10.1111/j.1460-9568.1996.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Kimura M, Saitoh N, Takahashi T. Adenosine A1 receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. J Physiol. 2003;553:415–426. doi: 10.1113/jphysiol.2003.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Eng DL, Bhisitkul RB. Adenosine selectively blocks parallel-fiber-mediated synaptic potentials in rat cerebellar cortex. Proc Natl Acad Sci U S A. 1984;81:6531–6534. doi: 10.1073/pnas.81.20.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Modulation of transmission during trains at a cerebellar synapse. J Neurosci. 2000;20:1348–1357. doi: 10.1523/JNEUROSCI.20-04-01348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Li J, Iadecola C. Nitric oxide and adenosine mediate vasodilation during functional activation in cerebellar cortex. Neuropharm. 1994;33:1453–1461. doi: 10.1016/0028-3908(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Llaudet E, Botting NP, Crayston JA, Dale N. A three-enzyme microelectrode sensor for detecting purine release from central nervous system. Biosens Bioelectron. 2003;18:43–52. doi: 10.1016/s0956-5663(02)00106-9. [DOI] [PubMed] [Google Scholar]
- Llaudet E, Hatz S, Droniou M, Dale N. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem. 2005;77:3267–3273. doi: 10.1021/ac048106q. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd HGE, Lindstom K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- Lujan R, Shigemoto R. Localization of metabotropic GABA receptor subunits GABAB1 and GABAB2 relative to synaptic sites in the rat developing cerebellum. Eur J Neurosci. 2006;23:1479–1490. doi: 10.1111/j.1460-9568.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- Mateos JM, Azkue J, Sarria R, Kuhn R, Grandes P, Knopfel T. Localization of the mGlu4a metabotropic glutamate receptor in rat cerebellar cortex. Histochem Cell Biol. 1998;109:135–139. doi: 10.1007/s004180050211. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Lupica CR, Dunwiddie TV. Activity-dependent release of endogenous adenosine modulates synaptic responses in the rat hippocampus. J Neurosci. 1993;13:3439–3447. doi: 10.1523/JNEUROSCI.13-08-03439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale SA, Garthwaite J, Batchelor AM. Metabotropic glutamate receptor subtypes modulating neurotransmission at parallel fibre-Purkinje cell synapses in rat cerebellum. Neuropharm. 2001;41:42–49. doi: 10.1016/s0028-3908(01)00046-6. [DOI] [PubMed] [Google Scholar]
- Newman EA. Glial modulation of synaptic transmission in the retina. Glia. 2004;47:268–274. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji T, Karaswa A, Kusaka H. Adenosine uptake inhibitors. Eur J Pharm. 2004;495:1–16. doi: 10.1016/j.ejphar.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Pedata F, Pazzagli M, Tilli S, Pepeu G. Regional differences in the electrically stimulated release of endogenous and radioactive adenosine and purine derivatives from rat brain slices. Naunyn Schmiedebers Arch Pharmacol. 1990;342:447–453. doi: 10.1007/BF00169463. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zucker RS. Release of LHRH is linearly related to the time integral of presynaptic Ca2+ elevation above a threshold level in bullfrog sympathetic ganglia. Neuron. 1993;10:465–473. doi: 10.1016/0896-6273(93)90334-n. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Dutton GR. Dihydropyridines modulate K+-evoked amino acid and adenosine release from cerebellar neuronal cultures. Neurosci Lett. 1989;102:97–102. doi: 10.1016/0304-3940(89)90314-5. [DOI] [PubMed] [Google Scholar]
- Reid CA, Bekkers JM, Clements JD. N- and P/Q-type Ca2+ channels mediate transmitter release with a similar cooperativity at rat hippocampal autapses. J Neurosci. 1998;18:2849–2855. doi: 10.1523/JNEUROSCI.18-08-02849.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABAA receptors concentrate on the glial processes that wrap inhibitory synapses. J Neurosci. 2002;22:10720–10730. doi: 10.1523/JNEUROSCI.22-24-10720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkees SA, Price SL, Zhou FC. Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum, and basal ganglia. Brain Res. 1995;677:193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Neuroprotective role of adenosine in cerebral ischemia. Trends Pharm Sci. 1992;13:439–445. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- Safiulina VF, Kasyanov AM, Giniatullin R, Cherubini E. Adenosine down regulates giant depolarizing potentials in the developing rat hippocampus by exerting a negative control on glutamatergic inputs. J Neurophysiol. 2005;94:2797–2804. doi: 10.1152/jn.00445.2005. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABAB receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- Schoen SW, Graeber MB, Reddington M, Kreutzberg GW. Light and electron microscopical immunocytochemistry of 5′-nucleotidase in rat cerebellum. Histochem. 1987;87:107–113. doi: 10.1007/BF00533394. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Frandsen A, Drejer J. Evidence for evoked release of adenosine and glutamate from cultured cerebellar granule cells. Neurochem Res. 1989;14:871–875. doi: 10.1007/BF00964817. [DOI] [PubMed] [Google Scholar]
- Sweeney MI. Adenosine release and uptake in cerebellar granule neurons both occur via an equilibrative nucleoside carrier that is modulated by G proteins. J Neurochem. 1996;67:81–88. doi: 10.1046/j.1471-4159.1996.67010081.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kovalchuk Y, Atwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J Neurosci. 1995;15:5693–5702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Wang TF, Guidotti G. Widespread expression of ecto-apyrase (CD39) in the central nervous system. Brain Res. 1998;790:318–321. doi: 10.1016/s0006-8993(97)01562-x. [DOI] [PubMed] [Google Scholar]
- Wong AYC, Billups B, Johnston J, Evans RJ, Forsythe ID. Endogenous activation of adenosine A1 receptors, but not P2X receptors, during high-frequency synaptic transmission at the calyx of Held. J Neurophysiol. 2006;95:3336–3342. doi: 10.1152/jn.00694.2005. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Comparative effects of methylmercury on parallel-fiber and climbing-fiber responses of rat cerebellar slices. J Pharmacol Exp Ther. 1999;288:1015–1025. [PubMed] [Google Scholar]
- Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]