Abstract
In skeletal muscle, carnitine plays an essential role in the translocation of long-chain fatty-acids into the mitochondrial matrix for subsequent β-oxidation, and in the regulation of the mitochondrial acetyl-CoA/CoASH ratio. Interest in these vital metabolic roles of carnitine in skeletal muscle appears to have waned over the past 25 years. However, recent research has shed new light on the importance of carnitine as a regulator of muscle fuel selection. It has been established that muscle free carnitine availability may be limiting to fat oxidation during high intensity submaximal exercise. Furthermore, increasing muscle total carnitine content in resting healthy humans (via insulin-mediated stimulation of muscle carnitine transport) reduces muscle glycolysis, increases glycogen storage and is accompanied by an apparent increase in fat oxidation. By increasing muscle pyruvate dehydrogenase complex (PDC) activity and acetylcarnitine content at rest, it has also been established that PDC flux and acetyl group availability limits aerobic ATP re-synthesis at the onset of exercise (the acetyl group deficit). Thus, carnitine plays a vital role in the regulation of muscle fuel metabolism. The demonstration that its availability can be readily manipulated in humans, and impacts on physiological function, will result in renewed business and scientific interest in this compound.
Introduction
Carnitine, named from the Latin for ‘flesh’, was discovered in muscle tissue one hundred years ago (Gulewitsch & Krimberg, 1905) and subsequently identified as 3-hydroxy-4-N,N,N-trimethylaminobutyric acid, a water soluble quaternary amine, around 20 years later (Tomita & Sendju, 1927). More than 95% of the body's total carnitine store exists within skeletal muscle tissue (Brass, 1995), where it performs at least two known vital metabolic roles, the most well documented being the translocation of long-chain fatty acids (acyl groups) into the mitochondrial matrix for subsequent β-oxidation. Towards the end of the 20th century, a large amount of research was directed towards investigating the effects of l-carnitine supplementation on exercise performance, the main premise being that increasing carnitine availability would increase fat oxidation during prolonged exercise, spare glycogen stores and, thus, delay the onset of fatigue. However, scientific interest in l-carnitine as an ergogenic aid, and its metabolic roles, soon declined when it became apparent that l-carnitine feeding does not alter fuel metabolism during exercise or, more importantly, impact upon the muscle carnitine pool in humans. Despite this, l-carnitine feeding as a tool to apparently promote weight loss and improve exercise performance remains the foundation of a multimillion dollar dietary supplement industry in the present day. Over the past decade, however, there has been renewed interest in the different metabolic roles of carnitine in skeletal muscle, which has led to the realization that if the muscle carnitine pool can indeed be manipulated, it can have a significant impact on physiological function.
The purpose of the present review is to outline these important, but often under-recognized, roles of carnitine in skeletal muscle fuel metabolism and to highlight how manipulating the carnitine pool of skeletal muscle, both physiologically and pharmacologically, provides insight into the regulation of muscle energy metabolism and function.
Distinct roles for carnitine in skeletal muscle fuel metabolism
(i) As an effector of mitochondrial long-chain fatty acyl group translocation
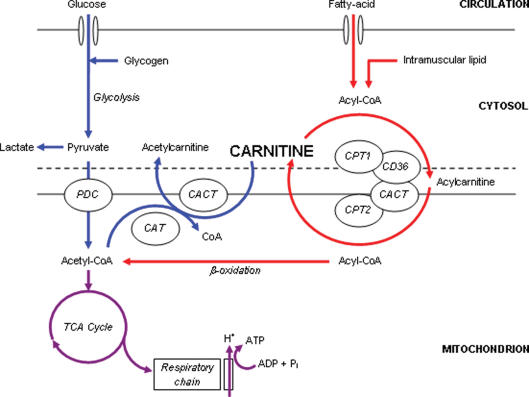
Interest in carnitine as a metabolically important compound arose in the 1950s when it was established as an essential growth factor for the Tenebrio molitor beetle larvae (Carter et al. 1952). The physiological significance of this finding became apparent over the next decade by the pioneering work of Irving Fritz, and others, which established that mitochondria in a variety of tissues are impermeable to fatty acyl-CoA, but not to fatty acylcarnitine, and that carnitine and carnitine palmitoyltransferase are essential for the translocation of long-chain fatty acids into skeletal muscle mitochondria for β-oxidation (Fritz, 1955; Fritz & McEwen, 1959; Bremer, 1962a,b; Fritz & Yue, 1963; Fritz & Marquis, 1965). Fritz and colleagues' original working hypothesis of the essential role of carnitine in the translocation of long-chain fatty acids into the mitochondrial matrix (Fritz & Yue, 1963) is represented schematically in Fig. 1, which has been modified to include more recent molecular research.
Figure 1. A schematic diagram of the metabolic roles of carnitine in skeletal muscle.
Carnitine's role in long-chain fatty acid (acyl group) translocation into the mitochondrial matrix, for subsequent β-oxidation is highlighted in red, whereas the role of carnitine as a buffer of excess acetyl-CoA production is highlighted in blue. PDC, pyruvate dehydrogenase complex; TCA, tricarboxylic acid cycle; CAT, carnitine acetyltransferase; CACT, carnitine acylcarnitine translocase; CPT, carnitine palmitoyltransferase; CD36, fatty acid translocase.
In order to take part in the β-oxidation pathway, cytosolic long-chain acyl-CoA must be transported across the otherwise impermeable inner mitochondrial membrane. Carnitine palmitoyltransferase 1 (CPT1), situated within the outer mitochondrial membrane (Murthy & Pande, 1987), catalyses the reversible esterification of carnitine with long-chain acyl-CoA to form long-chain acylcarnitine. Cytosolic acylcarnitine is then transported into the mitochondrial matrix in a simultaneous 1: 1 exchange with intramitochondrial free carnitine via the carnitine acylcarnitine translocase (CACT), which is situated within the mitochondrial inner membrane (Pande, 1975). Recent research involving isolated mitochondria from human skeletal muscle has suggested that the fatty acid translocase FAT/CD36, which is located in the outer mitochondrial membrane, translocates acylcarnitine from CPT1 to CACT (Bezaire et al. 2006). Once inside the mitochondrial matrix, acylcarnitine is transesterified back to free carnitine and long-chain acyl-CoA in a reaction catalysed by carnitine palmitoyltransferase 2 (CPT2), which is situated on the matrix side of the inner mitochondrial membrane (Woeltje et al. 1987). The intramitochondrial long-chain acyl-CoA is then oxidized and cleaved by the β-oxidation pathway. CPT1 is considered to be the rate-limiting enzyme for long-chain fatty acid entry into the mitochondria and oxidation (McGarry & Brown, 1997).
The significance of carnitine to long-chain fatty acid oxidation is highlighted by systemic carnitine deficiency (lipid storage myopathy), where an 85% reduction in skeletal muscle carnitine content has been associated with a 75% reduction in palmitate oxidation in muscle homogenates (Engel & Angelini, 1973). Furthermore, the importance of the carnitine acyltransferase reactions is highlighted by patients with inherited enzyme deficiencies. For example, patients with CPT2 (or CACT) deficiency typically experience muscle pain and weakness, myoglobinuria, hypoglycaemia, hyperamonaemia, hypoketosis, and cardiac disorders, usually prompted by fasting, high fat intake, or prolonged exercise (Ramsay et al. 2001; Bonnefont et al. 2004). Indeed, a recent study in patients with CPT2 deficiency demonstrated that long-chain fatty acid ([13C]palmitate) oxidation was normal at rest, but it was severely impaired during prolonged cycle exercise at 50% of maximal oxygen uptake (V˙O2,max; Orngreen et al. 2005). The fact that there have been no reported inherited defects in the muscle CPT1 isoform (M-CPT1), suggests that a loss of M-CPT1 might be incompatible with life.
(ii) As an acetyl group buffer at the onset of exercise when the rate of acetyl-CoA generation is greater than its rate of utilization by the TCA cycle
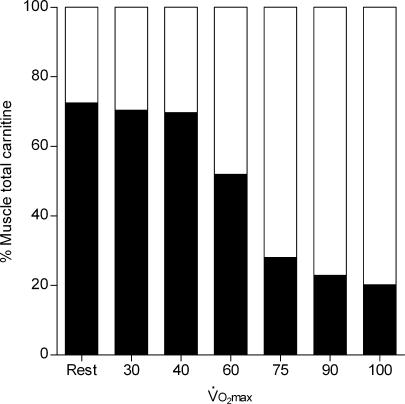
Another metabolic role of carnitine in muscle became apparent in 1966 when Childress and colleagues recognized that the blowfly flight muscle, which does not oxidize fatty acids during flight, was rich in carnitine and carnitine acetyltransferase (Childress et al. 1966). During the initial phase of flight, acetyl-CoA was generated faster than its utilization by the TCA cycle, with a corresponding 4-fold increase in acetylcarnitine. This led to the hypothesis that free carnitine buffers excess acetyl-CoA, which was later confirmed in contracting frog (Alkonyi et al. 1975) and rat (Carter et al. 1981) skeletal muscle, and has also been established in equine and human skeletal muscle during exercise. For example, following a few minutes of high intensity exercise, skeletal muscle free carnitine content is reduced from approximately 75% of the total muscle carnitine pool at rest (∼24 mmol (kg dry muscle)−1 in humans) to around 20%, with almost all of the reduction being attributed to formation of acetylcarnitine (Fig. 2; Foster & Harris, 1987; Harris et al. 1987; Carlin et al. 1990; Sahlin, 1990; Constantin-Teodosiu et al. 1991, 1992). The increase in acetylcarnitine formation during high intensity exercise, which occurs to a greater extent in Type I muscle fibres (Constantin-Teodosiu et al. 1996), is directly related to an increase in muscle acetyl-CoA (Carlin et al. 1990; Constantin-Teodosiu et al. 1991), suggesting that the rate of acetyl-CoA formation from pyruvate oxidation, catalysed by the pyruvate dehydrogenase complex (PDC), is in excess of its utilization by the TCA cycle (i.e. its rate of condensation with oxaloacetate is less than its rate of formation) leading to its subsequent accumulation. Accordingly, muscle acetylcarnitine content does not change at the onset of exercise at lower intensities (Fig. 2), suggesting that the rate of acetyl-CoA formation from pyruvate (and fatty acid oxidation) is well matched by its rate of utilization by the TCA cycle (Sahlin, 1990; Constantin-Teodosiu et al. 1991). Thus, during high intensity exercise (and other circumstances of increased PDC flux) carnitine buffers the excess acetyl groups formed, in a reaction catalysed by carnitine acetyltransferase (CAT), ensuring a viable pool of free CoASH is maintained for the continuation of the PDC and TCA cycle reactions (Fig. 1). Constantin-Teodosiu et al. (1991) calculated that if the activity of the PDC was maximal (approximately 20–25 μmol s−1 (kg wet muscle)−1) at high exercise intensities, and its rate of pyruvate oxidation were supported solely by the CoASH available in the muscle at rest (∼10 μmol (kg wm)−1), then the entire pool of muscle CoASH would become acetylated within 1 s of the initiation of contraction, hypothetically resulting in the immediate and complete inhibition of the PDC reaction and of the TCA cycle at the level of α-ketoglutarate dehydrogenase, underling the importance of carnitine and the rapid CAT reaction. Indeed, during the first 3 min of intense exercise (75%V˙O2,max), approximately 11 000 μmol (kg dm)−1 of acetyl groups are transferred from the small pool of CoASH (∼45 μmol (kg dm)−1) to carnitine (Constantin-Teodosiu et al. 1992).
Figure 2. The fraction of total carnitine (∼22 mmol (kg dry muscle)−1, not including long-chain acylcarnitine) represented as free (filled) or acetyl carnitine (open) in human vastus lateralis muscle at rest and following 4 min (or 10 min at 40, 55 and 75%V˙O2,max) of exercise on a cycle ergometer at various exercise intensities.
Values are a review of results taken from 4 papers (Harris et al. 1987; Sahlin, 1990; Constantin-Teodosiu et al. 1991, 1992).
In addition to maintaining a viable pool of CoASH, the accumulation of acetylcarnitine itself provides a store of acetyl groups, which is readily available for transacetylation back to acetyl-CoA for utilization by the TCA cycle. Indeed, in the latter half of 4 h of exercise at 55%V˙O2,max, muscle acetylcarnitine content returns to near resting values, which is paralleled by a decrease in PDC activity and therefore acetyl group delivery from pyruvate (Watt et al. 2002). Recent work by our group has focused on this acetyl group buffering role of carnitine in skeletal muscle by manipulating muscle PDC activation status and acetylcarnitine content, thereby providing a readily available pool of acetyl groups for utilization by the TCA cycle, which we believe is particularly important during the rest to exercise transition when inertia in mitochondrial ATP production is most evident, as will be discussed later.
Regulation of the integration of skeletal muscle fat and carbohydrate utilization
From the research described above, it is apparent that carnitine plays a central role in both fat and carbohydrate metabolism. However, the mechanisms that regulate the relative contributions of fat and carbohydrate oxidation to skeletal muscle energy production, particularly during exercise, have not been clearly elucidated. There is increasing evidence to suggest that fatty acid oxidation is indirectly regulated by PDC flux, both at rest and during exercise, and it is apparent that the CPT1 is central to this regulation. For example, increasing glucose availability at rest, via a hyperglycaemic-hyperinsulinaemic clamp, decreases long-chain fatty acid ([13C]oleate) oxidation, calculated indirectly from expired [13CO2, with no effect on medium-chain fatty acid ([13C]octanoate) oxidation (Sidossis & Wolfe., 1996). This would suggest an inhibition of fat oxidation at the level of CPT1 (medium-chain fatty acids are oxidized independent of CPT1), particularly as there was a decrease in muscle long-chain acylcarnitine content. The same inhibitory effect of carbohydrate metabolism on fat oxidation at the level of CPT1 is seen during exercise at 50%V˙O2,max. Hyperglycaemia (induced as a result of pre-exercise ingestion of glucose) increased glycolytic flux and reduced long-chain fatty acid ([1-13C]palmitate) oxidation (Coyle et al. 1997), whereas [1-13C]octanoate oxidation was unaffected. It should be noted that these responses might have been mediated, at least in part, via an insulin-induced inhibition of adipose tissue lipolysis, resulting in decreased plasma free fatty acid (FFA) availability. However, restoring plasma FFA concentration during exercise, via lipid and heparin infusion, increased the rate of fat oxidation from 3.1 to 4 μmol kg−1 min−1, but did not fully restore it compared to control (6.1 μmol kg−1 min−1), suggesting that the inhibitory effect of increased carbohydrate availability on fat oxidation does not reside at the level of FFA availability (Horowitz et al. 1997). In support of this theory, reducing pre-exercise muscle glycogen content increases fat oxidation rates 2-fold during exercise at 65%V˙O2,peak, but has no effect on the rate of muscle FFA uptake or FAT/CD36 (the protein responsible for FFA uptake into skeletal muscle) protein levels (Roepstorff et al. 2005). Moreover, simply increasing exercise intensity, from moderate to high, or increasing carbohydrate availability during exercise, increases PDC flux with a corresponding decrease in long-chain fatty acid oxidation rates (Romijn et al. 1995; van Loon et al. 2001; Roepstorff et al. 2005).
The two principal intracellular mechanisms that have been proposed to explain how increased PDC flux can lead to a down-regulation of long-chain fatty acid oxidation at the level of CPT1 are outlined below. However, it should be acknowledged that, in addition to this effect of carbohydrate flux on long-chain fatty acid oxidation, muscle pH has been proposed as a mechanism for directly regulating CPT1 activity, and thereby long-chain fatty acid oxidation during exercise. In vitro studies in isolated, intact mitochondria from resting human skeletal muscle have demonstrated that a decrease in pH, from 7.0 to 6.8 (which can be achieved in vivo during maximal exercise; Sahlin et al. 1976; Harris et al. 1977), resulted in a 50% decrease in the maximal activity of CPT1 (Starritt et al. 2000). However, whether a fall in muscle pH influences CPT1 activity, or fat oxidation rates, in vivo during exercise, particularly at submaximal exercise workloads when muscle pH is unlikely to decline, has not been determined.
(i) PDC flux dictates CPT1 activity via malonyl-CoA
Malonyl-CoA is a potent inhibitor of CPT1 activity in vitro and is therefore a likely candidate as the intracellular regulator of the rate of long-chain fatty acid oxidation in human skeletal muscle. Indeed, in resting human skeletal muscle, changes in malonyl-CoA concentration have occurred with opposite changes in fat oxidation (Bavenholm et al. 2000; Rasmussen et al. 2002). Skeletal muscle malonyl-CoA concentration is regulated by AMP-activated protein kinase (AMPK) and by the cytosolic concentration of citrate, which, respectively, inactivate and activate the enzyme responsible for malonyl-CoA synthesis from acetyl-CoA (acetyl-CoA carboxylase; ACC; Saha et al. 1995). Thus, an increase in the production of acetyl-CoA and citrate, due to increased glycolytic flux, would increase muscle malonyl-CoA concentration (via an increase in ACC activity) and therefore inhibit long-chain fatty acid oxidation via CPT1. In support of this theory, a significant negative correlation was obtained between muscle malonyl-CoA content and fat oxidation rates in healthy middle-aged men during a two-step euglycaemic–hyperinsulinaemic clamp (Bavenholm et al. 2000). Furthermore, hyperglycaemia with hyperinsulinaemia increased resting skeletal muscle malonyl-CoA content 3-fold (0.13–0.35 μmol (kg wet muscle)−1) in healthy humans, resulting in a functional decrease in CPT1 activity (suggested by an 80% inhibition of leg [13C]oleate oxidation, with no change in [13C]octanoate oxidation), and a shunting of long-chain fatty acids towards storage (Rasmussen et al. 2002). However, it appears that muscle malonyl-CoA concentration may not regulate long-chain fatty acid oxidation during exercise, despite high PDC flux during exercise, and therefore the marked accumulation of acetyl groups (Constantin-Teodosiu et al. 1991). It has recently been demonstrated that, despite a 122% increase in fat oxidation rates (due to depleted pre-exercise muscle glycogen content) and a 2-fold increase in α2-AMPK activity during exercise at 65%V˙O2,peak, there were no differences in muscle malonyl-CoA content, or ACCβ (muscle isoform) phosphorylation, compared to control (Roepstorff et al. 2005). Indeed, if anything, an increase in α2-AMPK activity would be expected to decrease muscle malonyl-CoA content. These findings are also supported by other studies where no association between malonyl-CoA content and fat oxidation rates was obtained in skeletal muscle during prolonged moderate-intensity exercise (Odland et al. 1996) or during graded-intensity exercise (Odland et al. 1998; Dean et al. 2000) in humans, or in rats where the time course of change in malonyl-CoA did not fit with the time course of change in substrate oxidation (Winder et al. 1990). Furthermore, recent evidence suggests that the sensitivity of CPT1 to malonyl-CoA paradoxically decreases in response to exercise-induced increases in whole-body fat oxidation in healthy human volunteers, and is associated with an increase in FAT/CD36 content in isolated skeletal muscle mitochondria (Holloway et al. 2006). It is also worth noting that there is evidence of a malonyl-CoA insensitive CPT1 isoform in rat skeletal muscle (Kim et al. 2002), and that phosphorylation increases the enzyme's activity and alters its sensitivity to malonyl-CoA (Kerner et al. 2004). Taking these findings together, it appears that malonyl-CoA does not play a major role in the regulation of CPT1 activity and therefore long-chain fatty acid oxidation in human skeletal muscle during exercise.
(ii) PDC flux dictates free carnitine availability
Carnitine is the principal substrate for CPT1, and therefore it may play a role in the regulation of fat oxidation during exercise. As discussed above, muscle free carnitine content declines during exercise at a high intensity (van Loon et al. 2001) and during moderate intensity exercise when muscle glycogen content is elevated (Roepstorff et al. 2005). We believe that the marked lowering of the muscle free carnitine pool during conditions of high PDC flux may limit the ability of CPT1 to transport long-chain acyl-CoA into the mitochondrial matrix and thus the rate of fat oxidation (van Loon et al. 2001; Stephens et al. 2006a). van Loon et al. (2001) demonstrated that a 35% decrease in the rate of long-chain fatty oxidation that occurred at an exercise intensity above 75%Wmax, measured using a [U-13C]palmitate tracer, was paralleled by a 65% decline in skeletal muscle free carnitine content (to 5.6 mmol (kg dm)−1). More recently, Roepstorff et al. (2005) showed a 2.5-fold decrease in the rate of fat oxidation, compared to control, during moderate intensity exercise (65%V˙O2,max) when free carnitine availability was reduced by 50% as a result of pyruvate, and therefore acetyl-CoA, production being increased as a result of pre-exercise muscle glycogen content being elevated. Also, indirect evidence has demonstrated that during bicycle exercise at 75%V˙O2,max to exhaustion, both muscle free carnitine content and fat oxidation rates were markedly higher when pre-exercise muscle glycogen content was lowered compared to control (Putman et al. 1993). We have hypothesized (van Loon et al. 2001; Stephens et al. 2006a) that muscle free carnitine availability becomes limiting to CPT1 at a concentration of about 6 mmol (kg dm)−1 or approximately 1.8 mm intracellular water (although partitioning of free carnitine between the cytosol and the mitochondrial matrix makes it very difficult to estimate the absolute carnitine concentration in the vicinity of CPT1). This seems plausible given the reported in vitro Km of CPT1 for free carnitine in human skeletal muscle is approximately 0.5 mm (McGarry et al. 1983). Furthermore, the catalytic site of CPT1 for carnitine is located within the contact sites of the outer mitochondrial membrane (Zammit, 1999), which may limit its exposure to the predominantly cytosolic store of free carnitine, i.e. ∼90% of the cellular carnitine pool (Idell-Wenger et al. 1978). In support of the hypothesis that free carnitine availability may limit fat oxidation, muscle free carnitine content has been shown to decrease from approximately 11 to below 5.5 mmol (kg dm)−1 (assuming a total carnitine content of 22 mmol (kg dm)−1) between the exercise intensities of 60 and ∼80%V˙O2,max (Fig. 2), and it has been calculated (from respiratory exchange measurements) that maximal and minimal fat oxidation rates during exercise are achieved at exercise intensities of ∼65 and > 80%V˙O2,max, respectively (Achten & Jeukendrup, 2004).
Manipulating the carnitine pool of skeletal muscle
(i) Insensitivity of skeletal muscle to increased carnitine delivery
In keeping with the hypothesis that muscle free carnitine availability can limit fat oxidation during conditions of high PDC flux, it is our premise that increasing muscle total carnitine content could potentially alleviate the decline in fat oxidation rates routinely observed during high intensity exercise, and concomitantly reduce muscle glycogen utilization. It is worth noting that trained humans, and equines, have a higher capacity to utilize fatty acids during exercise, and appear to have a higher total muscle carnitine content compared to untrained controls (Lennon et al. 1983; Foster & Harris, 1992). Furthermore, increasing skeletal muscle carnitine content has been reported to delay fatigue development by 25% during electrical stimulation in rat soleus muscle strips in vitro (Brass et al. 1993). In keeping with these observations, it is the foundation of a multimillion dollar dietary supplement industry that l-carnitine feeding can increase human skeletal muscle carnitine stores and fat oxidation at rest and promote weight loss.
For the latter statement to be even considered as viable, an increase in skeletal muscle carnitine content is clearly essential. The majority of the pertinent studies in healthy humans to date have, however, failed to increase skeletal muscle carnitine content via oral or intravenous l-carnitine administration (Brass, 2000; Stephens et al. 2006a). For example, neither feeding l-carnitine daily for up 3 months (Barnet et al. 1994; Vukovich et al. 1994; Wächter et al. 2002) nor intravenously infusing l-carnitine for up to 5 h (Brass et al. 1994; Stephens et al. 2006a) had an effect on muscle total carnitine content, or indeed net uptake of carnitine across the leg (Soop et al. 1988). Furthermore, feeding 2–5 g day−1 of l-carnitine for 1 week to 3 months prior to a bout of exercise had no effect on perceived exertion, exercise performance, V˙O2,max, or markers of muscle substrate metabolism such as RER, V˙O2, blood lactate, leg FFA turnover, and post exercise muscle glycogen content (Greig et al. 1987; Oyono-Enguelle et al. 1988; Soop et al. 1988; Gorostiaga et al. 1989; Wyss et al. 1990; Decombaz et al. 1993; Trappe et al. 1994; Barnett et al. 1994; Vukovich et al. 1994; Wächter et al. 2002). A primary reason for l-carnitine feeding failing to impact upon total muscle carnitine content, and therefore muscle energy metabolism, in humans is undoubtedly the poor bioavailability of orally administered l-carnitine (< 20% for a 2–6 g dose; Harper et al. 1988; Segre et al. 1988). However, the finding that intravenous l-carnitine administration also has no impact on muscle carnitine content suggests that muscle carnitine transport is most likely the limiting factor to muscle carnitine accumulation in healthy humans.
In contrast to the findings in humans, it appears that an increase in muscle carnitine content can be achieved following l-carnitine supplementation in animals. For example, daily intragastric administration of ∼5 mg l-carnitine for 4 weeks increased soleus muscle total carnitine content by 25% in sedentary rats and 50% in trained rats, and was associated with an increase in exercise (swimming) time to exhaustion by 15 and 30%, respectively (Bacurau et al. 2003). Also, feeding horses 10 g of l-carnitine daily for 5 weeks during training increased muscle carnitine content by 50% compared to control (Rivero et al. 2002). However, this 50% increase in muscle carnitine content (i.e. 14 mmol (kg dm)−1; Foster & Harris, 1992) equated to more than 40% of the total l-carnitine dose administered (assuming a 50% contribution of skeletal muscle to total body mass), which is highly unlikely to be achievable in humans given the poor bioavailability of orally administered l-carnitine (Harper et al. 1988; Segre et al. 1988).
(ii) Augmenting skeletal muscle carnitine availability in humans
Carnitine is transported into skeletal muscle against a considerable concentration gradient (> 100 fold) via a saturable, Na+-dependent, high affinity, active transport process (Rebouche, 1977). The protein responsible for carnitine transport into skeletal muscle has been identified as the novel organic cation transporter OCTN2 (Tamai et al. 1998). Confirmation of OCTN2 as the protein responsible for carnitine transport in vivo, and its physiological significance, was presented by Nezu et al. (1999), who were the first to demonstrate that patients with systemic carnitine deficiency (SCD) have a loss of OCTN2 carnitine transporter function. In some SCD patients, muscle carnitine content is less than 1% of normal values (Treem et al. 1988; Tein et al. 1990). In addition, the juvenile visceral steatosis (JVS) mouse, which has muscle carnitine deficiency and presents several symptoms of SCD, has also been shown to lack high-affinity carnitine transport activity in skeletal muscle and express a mutation in OCTN2 (Nezu et al. 1999; Tein, 2003).
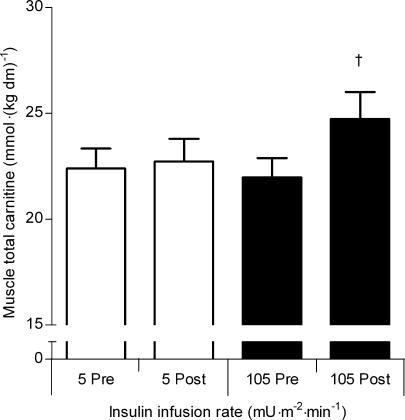
The Km for carnitine of OCTN2, in vitro, is 4.3 μmol l−1 (Tamai et al. 1998), which would suggest that in the basal state skeletal muscle carnitine uptake is saturated, and it is unlikely that plasma carnitine availability per se will be rate limiting to muscle carnitine transport and storage (plasma total carnitine concentration is ∼50 μmol l−1; Wachter et al. 2002; Stephens et al. 2006a,b). Indeed, maintaining a steady-state plasma carnitine concentration of ∼550 μmol l−1 for 5 h had no impact upon skeletal muscle total carnitine content in healthy male volunteer subjects (Stephens et al. 2006a; Fig. 3). However, hypercarnitinaemia (∼550 μmol l−1) combined with hyperinsulinaemia (∼160 mU l−1), achieved via intravenous infusion of insulin (105 mU m−2 min−1 insulin clamp) and l-carnitine, increased plasma l-carnitine clearance, and resulted in an increase in skeletal muscle total carnitine content of ∼15% in the same subjects (Stephens et al. 2006a,b; Fig. 3). Furthermore, by performing a similar experiment but with different insulin infusion rates (5, 30, 55 and 105 mU m−2 min−1 insulin clamps) during euglycaemic hypercarnitinaemia, it was demonstrated that plasma carnitine clearance (presumably into skeletal muscle as there was no change in urinary carnitine excretion) is increased at a steady-state serum insulin concentration of around 90 mU l−1, but not 50 mU l−1, suggesting that a threshold concentration exists for the stimulatory effect of insulin on skeletal muscle carnitine accumulation (Stephens et al. 2006c). These findings were in concordance with our hypothesis that insulin would augment Na+-dependent skeletal muscle carnitine transport via OCTN2, secondary to its action of increasing sarcolemmal Na+/K+-ATPase pump activity and therefore intracellular Na+ flux. For example, insulin increases Na+/K+-ATPase activity by increasing translocation of α2 and β1 pump subunits from an intracellular storage site to the plasma membrane (Sweeney & Klip, 1998), and via an increase in the sensitivity of the Na+/K+-ATPase to intracellular Na+ (Clausen, 1986, 2003; Ewart & Klip, 1995). Due to the 1: 1 stochiometry of Na+–carnitine cotransport via OCTN2 (Tamai et al. 1998), increasing Na+/K+-ATPase activity, via an increase in circulating insulin concentration, would lower the intracellular Na+ concentration, which would increase the electrochemical gradient for Na+ and therefore favour Na+–carnitine cotransport. Indeed, muscle carnitine transport is inhibited by the potent Na+/K+-ATPase inhibitor ouabain (Rebouche, 1977; Georges et al. 2000), and is increased in vesicles preloaded with K+ (Berardi et al. 2000). In support of this hypothesis, the Na+-dependent uptake of other nutrients, such as some amino acids (Zorzano et al. 2000) and creatine (Green et al. 1996; Steenge et al. 1998) by skeletal muscle, is augmented by insulin.
Figure 3. Muscle total carnitine content before and after 5 h of hypercarnitinaemia (550 μmol l−1) accompanied by intravenous insulin infusion at rates of 5 and 105 mU m−2 min−1.
Values are the mean ±s.e.m. (n = 8). †P < 0.05, significantly greater than pre infusion value. From Stephens et al. (2006a), reproduced with permission.
We have also recently demonstrated that oral feeding of l-carnitine (3 g) and carbohydrate (4 × 500 ml solutions each containing 94 g of simple sugars), which increased serum insulin concentration to ∼75 mU l−1, resulted in greater whole body retention of carnitine compared to ingestion of l-carnitine alone. The retention of carnitine was calculated from measurements of 24 h urinary carnitine excretion. Furthermore, this effect was maintained throughout 2 weeks of l-carnitine (3 g d−1) and carbohydrate feeding (2 × 500 ml solutions containing 94 g of simple sugars; Stephens et al. 2006d). Importantly, the physiologically high serum insulin concentration achieved in this experiment was in keeping with the predicted threshold, determined under insulin clamp conditions, at which an increase in muscle carnitine accumulation was thought to occur (i.e. between 50 and 90 mU l−1; Stephens et al. 2006c). Assuming that the greater whole-body retention of l-carnitine following daily l-carnitine and carbohydrate feeding resided within skeletal muscle (the major carnitine store within the body), this would suggest that an insulin mediated elevation of muscle total carnitine can be sustained in the long term. These findings are important, as they demonstrate that effective whole-body retention of carnitine can be achieved by l-carnitine feeding in a day-to-day setting, which is obviously a far more practical setting than the intravenous infusion of l-carnitine and insulin.
(iii) The effect of increasing skeletal muscle free carnitine availability on muscle fuel metabolism in humans
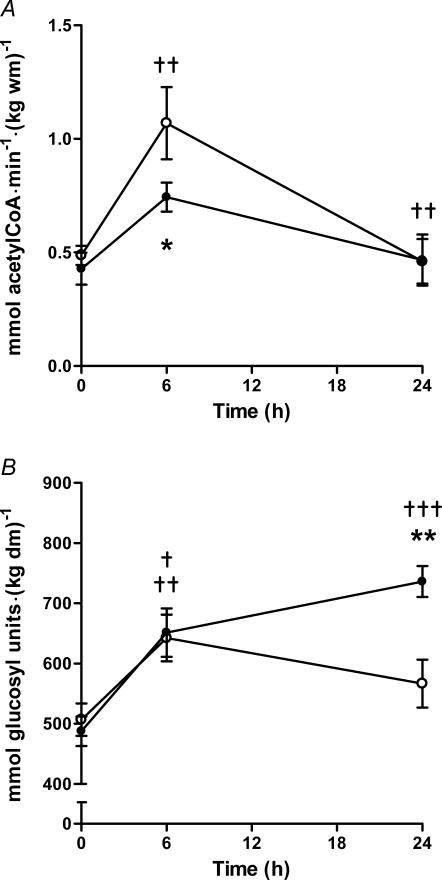
Our recent research (Stephens et al. 2006b) has demonstrated that a 15% increase in skeletal muscle carnitine content, achieved via 5 h of intravenous l-carnitine infusion during controlled hyperinsulinaemia (∼160 mU l−1), resulted in a significant alteration in muscle fuel metabolism compared to control (euglycaemic hyperinsulinaemia). As would be expected, muscle PDC activity increased during euglycaemic hyperinsulinaemia under control and l-carnitine infusion conditions (Fig. 4A). However, the increase in PDC activity during l-carnitine infusion was not as pronounced as recorded in the control visit, such that post-infusion PDC activity was 30% less than control. This blunting of PDC activity following l-carnitine infusion was paralleled by a 40% reduction in muscle lactate content. Twenty-four hours after the commencement of the insulin infusion, and following an overnight fast, PDC activity and lactate content had returned to their respective basal values during both visits. However, muscle glycogen and long-chain acyl-CoA content was 30 and 40% greater than control following l-carnitine administration, respectively, despite carbohydrate administration over the previous 24 h having been exactly the same in both treatment groups (Fig. 4B). This clear difference in muscle glycogen content clearly suggests that an increase in non-oxidative glucose disposal must have occurred following l-carnitine administration. Indeed, an increase in non-oxidative glucose disposal (30%), calculated indirectly as the difference between whole body glucose disposal and oxidation (measured by indirect calorimetry), during steady-state l-carnitine infusion in the presence of an elevated serum insulin concentration (∼75 mU l−1), has been previously reported in other human studies (Ferrannini et al. 1988; Angelini et al. 1993; Negro et al. 1994). Unfortunately, the fate of the glucose was not determined in these experiments, nor were the mechanisms involved elucidated. Due to carnitine's role in long-chain fatty acyl group translocation into the mitochondrial matrix, we believe it is entirely plausible that the apparent reduction in glycolytic flux and carbohydrate oxidation outlined above (decreased PDC activity and lactate content, and increased glycogen accumulation), in the face of high carbohydrate availability, could have been caused by a carnitine-mediated increase in skeletal muscle long-chain fatty acid oxidation, i.e. an increase in long-chain acyl-CoA translocation into the mitochondrial matrix via CPT1, resulting in an increase in β-oxidation. According to Randle's glucose–fatty acid cycle (Randle et al. 1963, 1964; Garland et al. 1963; Garland & Randle, 1963), a concept proposed in the 1960s from experiments involving rat heart and diaphragm muscle, an increase in β-oxidation would result in an elevation of muscle acetyl-CoA concentration and, consequently, an increase in muscle citrate and glucose-6-phosphate content. This, in turn, would result in the down-regulation of carbohydrate flux, due to product inhibition of PDC, phosphofructokinase and hexokinase, respectively. Indeed, the decrease in PDC activity observed in our study was paralleled by a reduction in muscle lactate content and resulted in an accumulation of muscle glycogen overnight, conditions which are both consistent with the premise that glycolytic flux, and therefore carbohydrate oxidation, was inhibited. In support of this theory, muscle long-chain acyl-CoA content returned to basal overnight during the l-carnitine infusion visit (whereas it remained suppressed during the control visit), which suggests that β-oxidation was indeed increased (assuming that the greater long-chain acyl-CoA content observed in our study was intramitochondrial). However, as already stated, we have hypothesized that muscle carnitine availability becomes limiting to CPT1 at a value of around 6 mmol (kg dm)−1, a content routinely observed in healthy humans during intense exercise. This raises the question as to why the increase in muscle carnitine content described above would increase fat oxidation in resting subjects, where free carnitine content is around 17 mmol (kg dm)−1 (∼5 mmol l−1 intracellular water), well above the reported Km of CPT1 for carnitine (0.5 mm). At present, this point requires further investigation but may be related to the compartmentalization of carnitine in skeletal muscle, which is not evident from measurements made on homogenized muscle biopsy samples. Nevertheless, taking our observations into account, it is not unreasonable to predict that increasing muscle total carnitine content would indeed alleviate the decline in skeletal muscle fat oxidation seen during incremental exercise in healthy individuals.
Figure 4.
A, muscle PDC activity before and after 5 h of intravenous saline (CON; ○) or l-carnitine (CARN; •) infusion accompanied by a 6 h euglycaemic hyperinsulinaemic clamp (the saline/l-carnitine infusion commenced after a 1 h equilibration period), and 18 h after the end of the respective infusion visits (24 h). Values represent means ±s.e.m. (n = 7). ††P < 0.01, significantly greater than pre CON and CARN infusion value, and significantly less than post CON and CARN infusion value. *P < 0.05, CARN significantly less than corresponding CON value. B, muscle glycogen content before and after 5 h of intravenous saline (CON; ○) or l-carnitine (CARN; •) infusion accompanied by a 6 h euglycaemic hyperinsulinaemic clamp (the saline/l-carnitine infusion commenced after a 1 h equilibration period), and 18 h after the end of the respective infusion visits (24 h). Values represent means ±s.e.m. (n = 7). †P < 0.05, ††P < 0.01, †††P < 0.001, significantly different than pre infusion value. **P < 0.01, CARN significantly greater than corresponding CON value. From Stephens et al. (2006b), reproduced with permission; © 2006, The Endocrine Society.
(iv) Increasing skeletal muscle PDC activity and acetylcarnitine availability
As outlined above, it is now well established that a lag in oxidative ATP delivery occurs at the onset of steady-state exercise (characterized by a rapid hydrolysis of phosphocreatine (PCr) and accumulation of lactate), which is attributable to inertia in mitochondrial ATP production (Yoshida et al. 1995; Grassi et al. 1998a, 1998b), rather than the classically accepted theory of a delay in muscle oxygen delivery (Margaria et al. 1965). However, it is currently unclear as to exactly where this mitochondrial inertia resides.
Work within our laboratory over the past decade has investigated the PDC as a potential site of limitation to mitochondrial energy production at the onset of muscular contraction. Our group was the first to demonstrate that by pharmacologically activating the PDC, using dichloroacetate (DCA; a systemic PDC kinase inhibitor), acetylcarnitine (and to a lesser extent acetyl-CoA) availability in resting skeletal muscle was markedly increased. Furthermore, PCr hydrolysis and lactate accumulation (collectively responsible for oxygen-independent ATP production) were reduced following 20 min of intense contraction under conditions of controlled muscle blood flow and oxygen delivery (Timmons et al. 1996). In line with this observation, it was shown that activation of the PDC at rest using DCA, which increased muscle acetylcarnitine content 10-fold, was accompanied by a 30% reduction in ATP re-synthesis from oxygen-independent routes after only 1 min of contraction (when muscle force production was identical to the control group), suggesting the existence of an inherent lag in the activation of oxygen-dependent (mitochondrial) ATP regeneration at the very onset of contraction (Timmons et al. 1997, 1998a,b). Following 6 min of contraction, the contribution from oxygen-independent routes to ATP re-synthesis had fallen to ∼50% of that observed in the control group, while tension development was greater (Timmons et al. 1997). It was also apparent from these studies that some of the acetylcarnitine that was stockpiled at rest following PDC activation was utilized during the subsequent contraction (Timmons et al. 1996, 1997). Taking these findings together, it was concluded that the activation, and thereby flux, through PDC must limit acetyl-CoA availability and consequently mitochondrial ATP re-synthesis at the onset of exercise. Moreover, that the activation of PDC and ‘priming’ of mitochondria with acetylcarnitine prior to exercise, by administering DCA, could significantly increase mitochondrial ATP re-synthesis at the onset of exercise. Another important finding from this series of studies was that the onset of fatigue development during contraction was substantially delayed following DCA administration, most probably due to the deleterious effects of PCr hydrolysis and Pi and H+ accumulation being reduced from the onset of contraction (Timmons et al. 1996; Timmons et al. 1997).
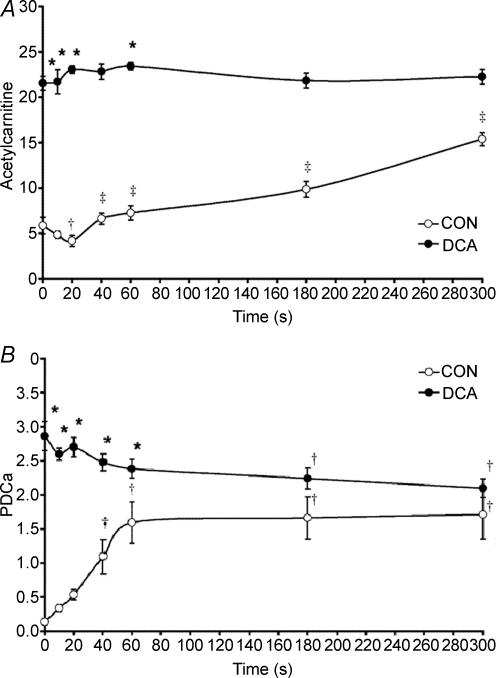
If there is indeed inertia in mitochondrial ATP re-synthesis at the onset of exercise at the level of PDC, then this would suggest that acetyl-CoA supply via the PDC will be insufficient to match the demands of the TCA cycle during this period, resulting in a reduction in the concentration of acetyl-CoA and acetylcarnitine. However, as outlined above, acetylcarnitine is thought to accumulate during moderate-to-intense muscular contraction (Childress et al. 1966; Harris et al. 1987; Sahlin, 1990; Constantin-Teodosiu et al. 1991, 1992). Thus, the hypothesis that metabolic inertia resides at the level of PDC at the onset of contraction is in contrast with the reports that acetyl-CoA production is in excess of the demands of the TCA cycle during this period. However, close scrutiny of the relevant literature reveals that most studies to date have failed to investigate the metabolic events occurring within the initial seconds of contraction, or indeed, at any time point during contraction prior to significant PDC activation. To address this issue, a study was performed in which five muscle biopsy samples were obtained from canine gracilis muscle over 1 min (rest, 10, 20, 40 and 60 s) of contraction. This study revealed that a lag in acetyl group provision (predominantly in the form of acetylcarnitine) did indeed occur during the initial 20 s of contraction (Fig. 5A), which resulted from, and was mirrored by, a lag in PDC activation (Fig. 5B). This study therefore unequivocally demonstrated the existence of a period of metabolic inertia (an ‘acetyl group deficit’) in skeletal muscle at the onset of contraction (Roberts et al. 2002).
Figure 5.
A, muscle acetylcarnitine concentration at rest and during 5 min of ischaemic contraction following pretreatment with saline (CON, open circles; n = 6) or DCA (closed circles; n = 6). Results are expressed as means ±s.e.m. with units of mmol (kg dry muscle)−1. *Significantly different from the corresponding CON value (P < 0.05); †significantly lower than rest within the same group (P = 0.08); ‡different from 20 s within same group (P < 0.05). B, muscle pyruvate dehydrogenase complex activation status (PDCa) at rest and during 5 min of ischaemic contraction following pretreatment with saline (CON, ○; n = 6) or DCA (•; n = 6). PDCa is expressed as mmol acetyl-CoA min−1 (kg wet muscle)−1 at 37°C. Results are expressed as means ±s.e.m.*Significantly different from corresponding CON value (P < 0.05); †significantly different from rest within the same trial (P < 0.05). From Roberts et al. (2002).
As DCA both activates the PDC and acetylates free-coenzyme A and carnitine pools at rest (Fig. 5), it was not possible to determine from any of the above studies whether the reduction in oxygen-independent ATP re-synthesis at the onset of contraction following DCA administration was directly attributable to acetyl-CoA delivery via the PDC being increased and/or was due to a large pool of acetyl groups being made available to the TCA cycle at the immediate onset of contraction. With this question in mind, it was demonstrated that administration of sodium acetate, which markedly increased the availability of acetyl-CoA and acetylcarnitine in resting skeletal muscle, independent of PDC activation, decreased the contribution from oxygen-independent ATP production during the first minute of muscle contraction (when the PDC was largely inactive) compared to control (Roberts et al. 2005). However, following this first minute, when near maximal activation of PDC had occurred in both control and sodium acetate groups, it appeared that acetyl-CoA derived from the PDC, rather than stockpiled acetyl groups per se, was the principal route of substrate delivery to the TCA cycle. It would appear therefore that both the increase in PDC activation status and the stockpiling of acetyl groups in resting muscle as a result of DCA administration contribute to reducing inertia in mitochondrial ATP production at the onset of subsequent contraction, but the former is quantitatively more important. Similarly, it was also demonstrated that if a bout of high intensity exercise (75%V˙O2,max) is performed 3 min following the performance of short duration low intensity exercise (55%V˙O2,max), which increased muscle acetylcarnitine content 2-fold greater than at rest but did not affect PDC activity, this was associated with a 40% reduction in non-oxygen-dependent ATP re-synthesis and an acceleration of V˙O2 on-kinetics in healthy male volunteers (Campbell-O'Sullivan et al. 2002). This latter observation may also partly explain the well reported ergogenic benefits of ‘warming up’ before intense exercise (which have classically been attributed to an exercise induced elevation of muscle temperature and/or the augmentation of local muscle blood flow).
Thus, by manipulating the muscle carnitine pool at rest, these investigations have collectively established the activation of the PDC as a rate-limiting step in the rate of rise in mitochondrial ATP re-synthesis in skeletal muscle at the onset of exercise, which in turn will dictate the magnitude of oxygen-independent ATP delivery, and thereby the rate of fatigue development during intense exercise.
Summary and conclusions
General scientific interest in the metabolic roles of carnitine in skeletal muscle appears to have declined over recent years. However, research over the past decade has shed new light on the importance of carnitine as a regulator of skeletal muscle fuel selection and physiological function. It has been established that free carnitine availability may be limiting to the CPT1 reaction, and thus the rate of fat oxidation, during high intensity submaximal exercise when the rate of acetyl-CoA production from the PDC reaction is in excess of its rate of utilization by the TCA cycle. Furthermore, increasing muscle total carnitine content in resting healthy humans (via insulin-mediated stimulation of Na+-dependent skeletal muscle carnitine transport) reduces muscle glycolysis, increases glycogen storage and is accompanied by an apparent increase in fat oxidation. It is important to acknowledge that an increase in human skeletal muscle carnitine content had previously not been achievable. By manipulating skeletal muscle PDC activity and acetylcarnitine availability, we have established that acetyl group delivery is limiting to mitochondrial ATP re-synthesis at the onset of exercise. Moreover, this ‘acetyl group deficit’ can be overcome by activating the PDC and/or stockpiling acetyl groups (mainly in the form of acetylcarnitine) at rest, which can then provide substrate to the TCA cycle from the immediate onset of intense exercise, resulting in a reduction in oxygen-independent ATP re-synthesis (PCr hydrolysis and lactate accumulation) and fatigue development.
In conclusion, manipulating the carnitine pool of skeletal muscle at rest, both physiologically and pharmacologically, has provided insight into the regulation of skeletal muscle fat and carbohydrate oxidation, both at rest and during exercise, and the interchange between anaerobic and oxidative energy provision at the onset of exercise. With the recent discovery that the carnitine content of human skeletal muscle can be increased, a wealth of possibilities will emerge to further investigate the important roles of carnitine in skeletal muscle energy metabolism.
References
- Achten J, Jeukendrup AE. Relation between plasma lactate concentration and fat oxidation rates over a wide range of exercise intensities. Int J Sports Med. 2004;25:32–37. doi: 10.1055/s-2003-45231. [DOI] [PubMed] [Google Scholar]
- Alkonyi I, Kerner J, Sandor A. The possible role of carnitine and carnitine acetyl-transferase in the contracting frog skeletal muscle. FEBS Lett. 1975;52:265–268. doi: 10.1016/0014-5793(75)80821-0. [DOI] [PubMed] [Google Scholar]
- Angelini A, Imparato L, Landi C, Porfido FA, Ciarimboli M, Marro A. Variation in levels of glycaemia and insulin after infusion of glucose solutions with or without added L-carnitine. Drug Exp Clin Res. 1993;19:219–222. [PubMed] [Google Scholar]
- Angelini C, Lucke S, Cantarutti F. Carnitine deficiency of skeletal muscle: report of a treated case. Neurology. 1976;26:633–637. doi: 10.1212/wnl.26.7.633. [DOI] [PubMed] [Google Scholar]
- Bacurau RF, Navarro F, Bassit RA, Meneguello MO, Santos RV, Almeida AL, Costa Rosa LF. Does exercise training interfere with the effects of L-carnitine supplementation? Nutrition. 2003;19:337–341. doi: 10.1016/s0899-9007(02)01015-8. [DOI] [PubMed] [Google Scholar]
- Barnett C, Costill DL, Vukovich MD, Cole KJ, Goodpaster BH, Trappe SW, Fink WJ. Effect of L-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling. Int J Sport Nutr. 1994;4:280–288. doi: 10.1123/ijsn.4.3.280. [DOI] [PubMed] [Google Scholar]
- Bavenholm PN, Pigon J, Saha AK, Ruderman NB, Efendic S. Fatty acid oxidation and the regulation of malonyl-CoA in human muscle. Diabetes. 2000;49:1078–1083. doi: 10.2337/diabetes.49.7.1078. [DOI] [PubMed] [Google Scholar]
- Berardi S, Stieger B, Hagenbuch B, Carafoli E, Krahenbuhl S. Characterization of L-carnitine transport into rat skeletal muscle plasma membrane vesicles. Eur J Biochem. 2000;267:1985–1994. doi: 10.1046/j.1432-1327.2000.01198.x. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Bruce CR, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am J Physiol Endocrinol Metab. 2006;290:E509–E515. doi: 10.1152/ajpendo.00312.2005. [DOI] [PubMed] [Google Scholar]
- Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Brass EP. Pharmacokinetic considerations for the therapeutic use of carnitine in haemodialysis patients. Clin Ther. 1995;17:176–185. doi: 10.1016/0149-2918(95)80017-4. [DOI] [PubMed] [Google Scholar]
- Brass EP. Supplemental carnitine and exercise. Am J Clin Nutr. 2000;72:618S–623S. doi: 10.1093/ajcn/72.2.618S. [DOI] [PubMed] [Google Scholar]
- Brass EP, Hoppel CL, Hiatt WR. Effect of intravenous L-carnitine on carnitine homeostasis and fuel metabolism during exercise in humans. Clin Pharmacol Ther. 1994;55:681–692. doi: 10.1038/clpt.1994.85. [DOI] [PubMed] [Google Scholar]
- Brass EP, Scarrow AM, Ruff LJ, Masterson KA, Van Lunteren E. Carnitine delays rat skeletal muscle fatigue in vitro. J Appl Physiol. 1993;75:1595–1600. doi: 10.1152/jappl.1993.75.4.1595. [DOI] [PubMed] [Google Scholar]
- Bremer J. Reversible acetylation of carnitine by mitochondria. J Biol Chem. 1962a;237:2228–2231. [PubMed] [Google Scholar]
- Bremer J. The metabolism of fatty acid esters by carnitine. J Biol Chem. 1962b;237:3628–3632. [PubMed] [Google Scholar]
- Campbell-O'Sullivan SP, Constantin-Teodosiu D, Peirce N, Greenhaff PL. Low intensity exercise in humans accelerates mitochondrial ATP production and pulmonary oxygen kinetics during subsequent more intense exercise. J Physiol. 2002;538:931–939. doi: 10.1113/jphysiol.2001.013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin JI, Harris RC, Cederblad G, Constantin-Teodosiu D, Snow DH, Hultman E. Association between muscle acetyl-CoA and acetylcarnitine levels in the exercising horse. J Appl Physiol. 1990;69:42–45. doi: 10.1152/jappl.1990.69.1.42. [DOI] [PubMed] [Google Scholar]
- Carter HE, Bhattacharyya PK, Weidman KR, Fraenkel G. Chemical studies on vitamin BT isolation and characterization as carnitine. Arch Biochem Biophys. 1952;38:405–416. doi: 10.1016/0003-9861(52)90047-7. [DOI] [PubMed] [Google Scholar]
- Carter AL, Lennon DL, Stratman FW. Increased acetyl carnitine in rat skeletal muscle as a result of high-intensity short-duration exercise. Implications in the control of pyruvate dehydrogenase activity. FEBS Lett. 1981;126:21–24. doi: 10.1016/0014-5793(81)81023-x. [DOI] [PubMed] [Google Scholar]
- Childress CC, Sacktor B, Travnor D. Function of carnitine in the fatty acid-oxidase deficient insect flight muscle. J Biol Chem. 1966;242:754–760. [PubMed] [Google Scholar]
- Clausen T. Regulation of active Na+/K+ transport in skeletal muscle. Physiol Rev. 1986;66:542–580. doi: 10.1152/physrev.1986.66.3.542. [DOI] [PubMed] [Google Scholar]
- Clausen T. Na+/K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Carlin JI, Cederblad G, Harris RC, Hultman E. Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta Physiol Scand. 1991;143:367–372. doi: 10.1111/j.1748-1716.1991.tb09247.x. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Cederblad G, Hultman E. PDC activity and acetyl group accumulation in skeletal muscle during prolonged exercise. J Appl Physiol. 1992;73:2403–2407. doi: 10.1152/jappl.1992.73.6.2403. [DOI] [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Tsintzas K, Williams C, Boobis L, Greenhaff PL. Carnitine metabolism in human muscle fibre types at the onset of sub-maximal exercise. J Physiol. 1996;523.P [Google Scholar]
- Coyle EF, Jeukendrup AE, Wagenmakers AJ, Saris WH. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am J Physiol Endocrinol Metab. 1997;273:E268–E275. doi: 10.1152/ajpendo.1997.273.2.E268. [DOI] [PubMed] [Google Scholar]
- Dean D, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim KH, Witters L, Richter EA, Ruderman N. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- Decombaz J, Deriaz O, Acheson K, Gmuender B, Jequier E. Effect of L-carnitine on submaximal exercise metabolism after depletion of muscle glycogen. Med Sci Sports Exerc. 1993;25:733–740. [PubMed] [Google Scholar]
- Engel AG, Angelini C. Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science. 1973;179:899–902. doi: 10.1126/science.179.4076.899. [DOI] [PubMed] [Google Scholar]
- Ewart HS, Klip A. Hormonal regulation of the Na+/K+ ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am J Physiol Cell Physiol. 1995;269:C295–C311. doi: 10.1152/ajpcell.1995.269.2.C295. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Buzzigoli G, Bevilacqua S, Boni C, Del Chiaro D, Oleggini M, Brandi L, Maccari F. Interaction of carnitine with insulin-stimulated glucose metabolism in humans. Am J Physiol Endocrinol Metab. 1988;255:E946–E952. doi: 10.1152/ajpendo.1988.255.6.E946. [DOI] [PubMed] [Google Scholar]
- Foster CV, Harris RC. Formation of acetylcarnitine in muscle of horse during high intensity exercise. Eur J Appl Physiol Occup Physiol. 1987;56:639–642. doi: 10.1007/BF00424803. [DOI] [PubMed] [Google Scholar]
- Foster CV, Harris RC. Total carnitine content of the middle gluteal muscle of thoroughbred horses: normal values, variability and effect of acute exercise. Equine Vet J. 1992;24:52–57. doi: 10.1111/j.2042-3306.1992.tb02779.x. [DOI] [PubMed] [Google Scholar]
- Fritz IB. The effects of muscle extracts on the oxidation of palmitic acid by liver slices and homogenates. Acta Physiol Scand. 1955;34:367–385. doi: 10.1111/j.1748-1716.1955.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Fritz IB, Marquis NR. The role of acylcarnitine esters and carnitine palmityltransferase in the transport of fatty acyl groups across mitochondrial membranes. Proc Natl Acad Sci U S A. 1965;54:1226–1233. doi: 10.1073/pnas.54.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz IB, McEwen B. Effects of carnitine on fatty-acid oxidation by muscle. Science. 1959;129:334–335. doi: 10.1126/science.129.3345.334. [DOI] [PubMed] [Google Scholar]
- Fritz IB, Yue KTN. Long-chain carnitine acyltransferase and the role of acylcarnitine derivatives in the catalytic increase of fatty acid oxidation induced by carnitine. J Lipid Res. 1963;4:279–288. [PubMed] [Google Scholar]
- Garland PB, Randle PJ. Effects of alloxan diabetes and adrenaline on concentrations of free fatty acids in rat heart and diaphragm muscles. Nature. 1963;199:381–382. doi: 10.1038/199381a0. [DOI] [PubMed] [Google Scholar]
- Garland PB, Randle PJ, Newsholme EA. Citrate as an intermediary in the inhibition of phosphofructokinase in rat heart muscle by fatty acids, ketone bodies, pyruvate, diabetes, and starvation. Nature. 1963;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- Georges B, Le Borgne F, Galland S, Isoir M, Ecosse D, Grand-Jean F, Demarquoy J. Carnitine transport into muscular cells. Inhibition of transport and cell growth by mildronate. Biochem Pharmacol. 2000;59:1357–1363. doi: 10.1016/s0006-2952(00)00265-3. [DOI] [PubMed] [Google Scholar]
- Gorostiaga EM, Maurer CA, Eclache JP. Decrease in respiratory quotient during exercise following L-carnitine supplementation. Int J Sports Med. 1989;10:169–174. doi: 10.1055/s-2007-1024895. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect Vo2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998a;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Stary CM, Wagner PD, Hogan MC. Peripheral O2 diffusion does not affect Vo2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998b;85:1404–1412. doi: 10.1152/jappl.1998.85.4.1404. [DOI] [PubMed] [Google Scholar]
- Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am J Physiol Endocrinol Metab. 1996;271:E821–E826. doi: 10.1152/ajpendo.1996.271.5.E821. [DOI] [PubMed] [Google Scholar]
- Greig C, Finch KM, Jones DA, Cooper M, Sargeant AJ, Forte CA. The effect of oral supplementation with L-carnitine on maximum and submaximum exercise capacity. Eur J Appl Physiol Occup Physiol. 1987;56:457–460. doi: 10.1007/BF00417775. [DOI] [PubMed] [Google Scholar]
- Gulewitsch W, Krimberg R. Physiol Chem. 1905;45:326–330. [Google Scholar]
- Harper P, Elwin CE, Cederblad G. Pharmacokinetics of intravenous and oral bolus doses of L-carnitine in healthy subjects. Eur J Clin Pharmacol. 1988;35:555–562. doi: 10.1007/BF00558253. [DOI] [PubMed] [Google Scholar]
- Harris RC, Foster CV, Hultman E. Acetylcarnitine formation during intense muscular contraction in humans. J Appl Physiol. 1987;63:440–442. doi: 10.1152/jappl.1987.63.1.440. [DOI] [PubMed] [Google Scholar]
- Harris RC, Sahlin K, Hultman E. Phosphagen and lactate contents of m. quadriceps femoris of man after exercise. J Appl Physiol. 1977;43:852–857. doi: 10.1152/jappl.1977.43.5.852. [DOI] [PubMed] [Google Scholar]
- Holloway GP, Bezaire V, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, Bonen A, Spriet LL. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol. 2006;571:201–210. doi: 10.1113/jphysiol.2005.102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JF, Mora-Rodriguez R, Byerley LO, Coyle EF. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am J Physiol Endocrinol Metab. 1997;273:E768–E775. doi: 10.1152/ajpendo.1997.273.4.E768. [DOI] [PubMed] [Google Scholar]
- Idell-Wenger JA, Grotyohann LW, Neely JR. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem. 1978;253:4310–4318. [PubMed] [Google Scholar]
- Kerner J, Distler AM, Minkler P, Parland W, Peterman SM, Hoppel CL. Phosphorylation of rat liver mitochondrial carnitine palmitoyltransferase-I: effect on the kinetic properties of the enzyme. Biol Chem. 2004;279:41104–41113. doi: 10.1074/jbc.M406570200. [DOI] [PubMed] [Google Scholar]
- Kim JY, Koves TR, Yu GS, Gulick T, Cortright RN, Dohm GL, Muoio DM. Evidence of a malonyl-CoA-insensitive carnitine palmitoyltransferase I activity in red skeletal muscle. Am J Physiol Endocrinol Metab. 2002;282:E1014–E1022. doi: 10.1152/ajpendo.00233.2001. [DOI] [PubMed] [Google Scholar]
- Lennon DL, Stratman FW, Shrago E, Nagle FJ, Madden M, Hanson P, Carter AL. Effects of acute moderate-intensity exercise on carnitine metabolism in men and women. J Appl Physiol. 1983;55:489–495. doi: 10.1152/jappl.1983.55.2.489. [DOI] [PubMed] [Google Scholar]
- Margaria R, Aghemo P, Rovelli E. Indirect determination of maximal O2 consumption in man. J Appl Physiol. 1965;20:1070–1073. doi: 10.1152/jappl.1965.20.5.1070. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Mills SE, Long CS, Foster DW. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983;214:21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy MS, Pande SV. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987;84:378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro P, Gossetti F, La Pinta M, Mariani P, Carboni M. The effect of L-carnitine, administered through intravenous infusion of glucose, on both glucose and insulin levels in healthy subjects. Drugs Exp Clin Res. 1994;20:257–262. [PubMed] [Google Scholar]
- Nezu J, Tamai I, Oku A, Ohashi R, Yabuuchi H, Hashimoto N, Nikaido H, Sai Y, Koizumi A, Shoji Y, Takada G, Matsuishi T, Yoshino M, Kato H, Ohura T, Tsujimoto G, Hayakawa J, Shimane M, Tsuji A. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat Genet. 1999;21:91–94. doi: 10.1038/5030. [DOI] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJ, Lopaschuk GD, Spriet LL. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am J Physiol Endocrinol Metab. 1996;270:E541–E544. doi: 10.1152/ajpendo.1996.270.3.E541. [DOI] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJ, Wong D, Hollidge-Horvat MG, Spriet LL. Effects of increased fat availability on fat–carbohydrate interaction during prolonged exercise in men. Am J Physiol Regul Integr Comp Physiol. 1998;274:R894–R902. doi: 10.1152/ajpregu.1998.274.4.R894. [DOI] [PubMed] [Google Scholar]
- Orngreen MC, Duno M, Ejstrup R, Christensen E, Schwartz M, Sacchetti M, Vissing J. Fuel utilization in subjects with carnitine palmitoyltransferase 2 gene mutations. Ann Neurol. 2005;57:60–66. doi: 10.1002/ana.20320. [DOI] [PubMed] [Google Scholar]
- Oyono-Enguelle S, Freund H, Ott C, Gartner M, Heitz A, Marbach J, Maccari F, Frey A, Bigot H, Bach AC. Prolonged submaximal exercise and L-carnitine in humans. Eur J Appl Physiol Occup Physiol. 1988;58:53–61. doi: 10.1007/BF00636603. [DOI] [PubMed] [Google Scholar]
- Pande SV. A mitochondrial carnitine acylcarnitine translocase system. Proc Natl Acad Sci U S A. 1975;72:883–887. doi: 10.1073/pnas.72.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Spriet LL, Hultman E, Lindinger MI, Lands LC, McKelvie RS, Cederblad G, Jones NL, Heigenhauser GJ. Pyruvate dehydrogenase activity and acetyl group accumulation during exercise after different diets. Am J Physiol Endocrinol Metab. 1993;265:E752–E760. doi: 10.1152/ajpendo.1993.265.5.E752. [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Gandour RD, van der Leij FR. Molecular enzymology of carnitine transfer and transport. Biochim Biophys Acta. 2001;1546:21–43. doi: 10.1016/s0167-4838(01)00147-9. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PJ, Hales CJ, Newsholme EJ. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Newsholme EJ, Garland PJ. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964;93:652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BB, Holmback UC, Volpi E, Morio-Liondore B, Paddon-Jones D, Wolfe RR. Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J Clin Invest. 2002;10:1687–1693. doi: 10.1172/JCI15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebouche CJ. Carnitine movement across muscle cell membranes. Studies in isolated rat muscle. Biochim Biophys Acta. 1977;471:145–155. doi: 10.1016/0005-2736(77)90402-3. [DOI] [PubMed] [Google Scholar]
- Rivero JL, Sporleder HP, Quiroz-Rothe E, Vervuert I, Coenen M, Harmeyer J. Oral L-carnitine combined with training promotes changes in skeletal muscle. Equine Vet J Suppl. 2002;34:269–274. doi: 10.1111/j.2042-3306.2002.tb05431.x. [DOI] [PubMed] [Google Scholar]
- Roberts PA, Loxham SJG, Poucher SM, Constantin-Teodosiu D, Greenhaff PL. The acetyl group deficit at the onset of ischaemic contraction in canine skeletal muscle. J Physiol. 2002;544:591–602. doi: 10.1113/jphysiol.2002.021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PA, Loxham SJ, Poucher SM, Constantin-Teodosiu D, Greenhaff PL. Acetyl-CoA provision and the acetyl group deficit at the onset of contraction in ischemic canine skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E327–E334. doi: 10.1152/ajpendo.00441.2003. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2005;288:E133–E142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Zhang XJ, Wolfe RR. Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. J Appl Physiol. 1995;79:1939–1945. doi: 10.1152/jappl.1995.79.6.1939. [DOI] [PubMed] [Google Scholar]
- Saha AK, Kurowski TG, Ruderman NB. A malonyl-CoA fuel-sensing mechanism in muscle: effects of insulin, glucose, and denervation. Am J Physiol Endocrinol Metab. 1995;269:E283–E289. doi: 10.1152/ajpendo.1995.269.2.E283. [DOI] [PubMed] [Google Scholar]
- Sahlin K. Muscle carnitine metabolism during incremental dynamic exercise in humans. Acta Physiol Scand. 1990;138:259–262. doi: 10.1111/j.1748-1716.1990.tb08845.x. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Segre G, Bianchi E, Corsi M, D'Iddio S, Ghirardi O, Maccari F. Plasma and urine pharmacokinetics of free and of short-chain carnitine after administration of carnitine in man. Arzneimittelforschung. 1988;38:1830–1834. [PubMed] [Google Scholar]
- Sidossis LS, Wolfe RR. Glucose and insulin-induced inhibition of fatty acid oxidation: the glucose-fatty acid cycle reversed. Am J Physiol Endocrinol Metab. 1996;270:E733–E738. doi: 10.1152/ajpendo.1996.270.4.E733. [DOI] [PubMed] [Google Scholar]
- Soop M, Bjorkman O, Cederblad G, Hagenfeldt L, Wahren J. Influence of carnitine supplementation on muscle substrate and carnitine metabolism during exercise. J Appl Physiol. 1988;64:2394–2399. doi: 10.1152/jappl.1988.64.6.2394. [DOI] [PubMed] [Google Scholar]
- Starritt EC, Howlett RA, Heigenhauser GJ, Spriet LL. Sensitivity of CPT I to malonyl-CoA in trained and untrained human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E462–E468. doi: 10.1152/ajpendo.2000.278.3.E462. [DOI] [PubMed] [Google Scholar]
- Steenge GR, Lambourne J, Casey A, Macdonald IA, Greenhaff PL. Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am J Physiol Endocrinol Metab. 1998;275:E974–E979. doi: 10.1152/ajpendo.1998.275.6.E974. [DOI] [PubMed] [Google Scholar]
- Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J. 2006a;20:377–379. doi: 10.1096/fj.05-4985fje. [DOI] [PubMed] [Google Scholar]
- Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Skeletal muscle carnitine accumulation alters fuel metabolism in resting human skeletal muscle. J Clin Endocrinol Metab. 2006b;91:5013–5018. doi: 10.1210/jc.2006-1584. [DOI] [PubMed] [Google Scholar]
- Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. A threshold exists or the stimulatory effect of insulin on plasma L-carnitine clearance in humans. Am J Physiol Endocrinol Metab. 2006c;292:E637–E641. doi: 10.1152/ajpendo.00508.2006. [DOI] [PubMed] [Google Scholar]
- Stephens FB, Evans CE, Constantin-Teodosiu D, Greenhaff PL. Carbohydrate ingestion augments L-carnitine retention in humans. J Appl Physiol. 2006d;102:1065–1070. doi: 10.1152/japplphysiol.01011.2006. [DOI] [PubMed] [Google Scholar]
- Sweeney G, Klip A. Regulation of the Na+/K+-ATPase by insulin: why and how? Mol Cell Biochem. 1998;182:121–133. [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- Tein I. Carnitine transport: pathophysiology and metabolism of known molecular defects. J Inherit Metab Dis. 2003;26:147–169. doi: 10.1023/a:1024481016187. [DOI] [PubMed] [Google Scholar]
- Tein I, De Vivo DC, Bierman F, Pulver P, De Meirleir LJ, Cvitanovic-Sojat L, Pagon RA, Bertini E, Dionisi-Vici C, Servidei S. Impaired skin fibroblast carnitine uptake in primary systemic carnitine deficiency manifested by childhood carnitine-responsive cardiomyopathy. Pediatr Res. 1990;28:247–255. doi: 10.1203/00006450-199009000-00020. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Greenhaff PL. Acetyl group availability is a major determinant of the oxygen deficit in human skeletal muscle during submaximal exercise. Am J Physiol Endocrinol Metab. 1998a;274:E377–E380. doi: 10.1152/ajpendo.1998.274.2.E377. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Hultman E, Kaijser L, Chwalbinska-Moneta J, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. Substrate availability limits human skeletal muscle oxidative ATP regeneration at the onset of ischemic exercise. J Clin Invest. 1998b;101:79–85. doi: 10.1172/JCI1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Poucher SM, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. The metabolic responses from rest to steady-state determine contractile function in ischemic canine skeletal muscle. Am J Physiol Endocrinol Metab. 1997;273:E233–E238. doi: 10.1152/ajpendo.1997.273.2.E233. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Poucher SM, Constantin-Teodosiu D, Worrall V, Macdonald IA, Greenhaff PL. Increased acetyl group availability enhances contractile function of canine skeletal muscle during ischemia. J Clin Invest. 1996;97:879–883. doi: 10.1172/JCI118490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Sendju Y. Über die Oxyaminverbindungen welche die Biuret Reaktionen zeigen. III. Spaltung der γ-amino-β-oxybuttersäure in die optisch-aktiven Komponente. Physiol Chem. 1927;169:263–277. [Google Scholar]
- Trappe SW, Costill DL, Goodpaster B, Vukovich MD, Fink WJ. The effects of L-carnitine supplementation on performance during interval swimming. Int J Sports Med. 1994;15:181–185. doi: 10.1055/s-2007-1021044. [DOI] [PubMed] [Google Scholar]
- Treem WR, Stanley CA, Finegold DN, Hale DE, Coates PM. Primary carnitine deficiency due to a failure of carnitine transport in kidney, muscle, and fibroblasts. N Engl J Med. 1988;319:1331–1336. doi: 10.1056/NEJM198811173192006. [DOI] [PubMed] [Google Scholar]
- van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovich MD, Costill DL, Fink WJ. Carnitine supplementation: effect on muscle carnitine and glycogen content during exercise. Med Sci Sports Exerc. 1994;26:1122–1129. [PubMed] [Google Scholar]
- Wächter S, Vogt M, Kreis R, Boesch C, Bigler P, Hoppeler H, Krähenbühl S. Long-term administration of L-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. Clin Chim Acta. 2002;318:51–61. doi: 10.1016/s0009-8981(01)00804-x. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJ, Dyck DJ, Spriet LL. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol. 2002;541:969–978. doi: 10.1113/jphysiol.2002.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Arogyasami J, Elayan IM, Cartmill D. Time course of exercise-induced decline in malonyl-CoA in different muscle types. Am J Physiol Endocrinol Metab. 1990;259:E266–E271. doi: 10.1152/ajpendo.1990.259.2.E266. [DOI] [PubMed] [Google Scholar]
- Woeltje KF, Kuwajima M, Foster DW, McGarry JD. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. II. Use of detergents and antibodies. J Biol Chem. 1987;262:9822–9827. [PubMed] [Google Scholar]
- Wyss V, Ganzit GP, Rienzi A. Effects of L-carnitine administration on VO2max and the aerobic-anaerobic threshold in normoxia and acute hypoxia. Eur J Appl Physiol Occup Physiol. 1990;60:1–6. doi: 10.1007/BF00572178. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kamiya J, Hishimoto K. Are oxygen uptake kinetics at the onset of exercise speeded up by local metabolic status in active muscles? Eur J Appl Physiol Occup Physiol. 1995;70:482–486. doi: 10.1007/BF00634376. [DOI] [PubMed] [Google Scholar]
- Zammit VA. Carnitine acyltransferases: functional significance of subcellular distribution and membrane topology. Prog Lipid Res. 1999;38:199–224. doi: 10.1016/s0163-7827(99)00002-8. [DOI] [PubMed] [Google Scholar]
- Zorzano A, Fandos C, Palacin M. Role of plasma membrane transporters in muscle metabolism. Biochem J. 2000;349:667–688. doi: 10.1042/bj3490667. [DOI] [PMC free article] [PubMed] [Google Scholar]