Abstract
AMPA receptors (AMPARs) mediate the bulk of fast synaptic excitation in the CNS. We have recently shown that AMPAR-dependent synaptic transmission in immature neocortical pyramidal neurons is mediated by GluR2-deficient receptors that can be modulated by intra- or extracellular polyamines (PAs). Phosphorylation of AMPARs, e.g. by PKC, can lead to enhanced excitation, and PAs are known to modulate PKC activity. Therefore, PAs and PKC might interact to influence AMPAR function. To test this hypothesis, we made whole cell recordings from immature (P12–14) layer V pyramidal neurons and assayed two measures of PA influence on synaptic AMPAR function – inward rectification and use-dependent unblock (UDU), with the latter assayed by differences in rectification between a pair of EPSCs evoked at short (50 ms) latencies. We have previously shown that EPSCs in immature pyramidal neurons displayed inward rectification, which was enhanced by intracellular spermine, as was UDU. Staurosporin (ST), a PKC inhibitor, reversed the effect of PA on rectification and UDU, suggesting that PKC modulates postsynaptic activation of AMPARs. Similarly, polyamine-dependent rectification of spontaneous EPSCs was reversed by treatment with ST or GFX109203X, a specific PKC inhibitor. Chelating intracellular Ca2+ with BAPTA reproduced the effects of ST. In addition, PA immunoreactivity in layer V pyramidal neurons was reduced by PKC inhibition indicating that PKC activity influences PA metabolism. Taken together, these data support the involvement of postsynaptic PKC activation in both the inward rectification and UDU of EPSCs in immature rat cortex, and suggest an important mechanism by which excitatory synaptic transmission can be dynamically modulated by changes in either [Ca2+]i or [PA]i.
AMPARs are a class of heteromeric ionotropic glutamate receptors, named for their preferred agonist (α-amino-3-hydroxy-5-methyl-4-isoxazole propionate), and consist of assemblies of GluR1–4 subunits. AMPAR heterogeneity results from differential expression of the subunits and is further increased by post-transcriptional modification such as alternative splicing and RNA editing (Tallaksen-Greene & Albin, 1996; Paschen et al. 1996; Tsuzuki et al. 2000; Carlson et al. 2000; Kortenbruck et al. 2001). In one subunit in this family, GluR2, a critical pore-lining residue is arginine rather than glutamine. The positive charge resulting from the arginine residue in even a single GluR2 subunit in the mulitmeric channel alters electrostatic interactions between the channel and positively charged polyvalent cations such polyamines and Ca2+, and thus renders the channel impermeable to Ca2+ and non-rectifying, two common features of GluR2-containing AMPARs (Hollmann et al. 1991; Keller et al. 1992; Brorson et al. 1999). Spermine, a PA highly expressed in the CNS (Pellegrini-Giampietro, 2003) and elsewhere, blocks Ca2+-permeable AMPARs and causes inward rectification (Isa et al. 1995; Bowie & Mayer, 1995; Koh et al. 1995; Kamboj et al. 1995; Washburn et al. 1997). Binding of spermine to GluR2-deficient AMPARs occurs within the pore region of the channel and is use and voltage dependent. Repetitive activation of these receptors results in facilitation due to polyamine unblocking, first discovered in interneurons with low GluR2 expression (Rozov & Burnashev, 1999; Armstrong & MacVicar, 2001). This use-dependent unblock (UDU) leads to a postsynaptic form of short-term potentiation.
Polyamines (PAs) are positively charged molecules, consisting of putrescine, cadaverine, spermidine and spermine (Coffino, 2001; Wallace et al. 2003). PAs are present in almost all cells and are implicated in physiological roles such as regulation of cell division and protein synthesis (Gilad & Gilad, 1992; Song et al. 1998; Wallace et al. 2003). Proliferating and differentiating cells express high PA levels, and PAs have specific functions in the nervous system (Gilad et al. 1995). An important role for PAs in early neocortical circuit function is suggested by the findings that in immature rats (< P15) layer V pyramidal neurons express PA-sensitive AMPA receptors lacking the GluR2 subunit (Kumar et al. 2002; see below) and that this developmental period corresponds to a stage at which neuronal spermine content is higher than in more mature neocortex (P16–P20) when pyramidal neurons express mainly PA-insensitive AMPARs (Shin et al. 2005). Early expression of PA-sensitive, GluR2-lacking AMPARs appears to be a common theme in development of the nervous system (Pickard et al. 2000; Eybalin et al. 2004; Balland et al. 2006).
Protein kinase C (PKC) is a ubiquitous Ca2+-dependent kinase known to phosphorylate serine residues of the intracellular carboxy terminal domains of AMPAR subunits GluR1 (Ser831), GluR2 (Ser880) and GluR4 (Ser842) and increase AMPAR activation (Lee et al. 2000; Kim et al. 2001; McDonald et al. 2001). In general, AMPAR phosphorylation increases channel conductance, peak open probability and Ca2+ permeability, alters interaction with PDZ (PSD-95, Disc large, Z0-1) domain-containing proteins, and increases clustering and synaptic delivery of the receptors (Xia et al. 2000; Chung et al. 2000; Hirai, 2001). AMPAR-interacting proteins include PDZ domain-containing proteins, like glutamate receptor-interacting proteins (GRIP or AMPA-binding protein, PICK1). Overexpression of PICK1 in hippocampal neurons results in a PKC- and CaMKII-dependent decrease in functional GluR2 expression and an increased sensitivity to philanthotoxin (PhTx), a polyamine site ligand (Perez et al. 2001; Terashima et al. 2004). The detailed mechanisms by which phosphorylation directly or indirectly influence synaptic AMPAR function remain unknown. In particular, the relationships between PKC and PAs and AMPARs have each been studied independently, but a dynamic role of PKC in PA-dependent modulation of AMPAR has not been investigated.
We hypothesized that a mechanism through which PKC could influence AMPAR-mediated synaptic function would involve an altered metabolism or binding of PAs and thus a modified functional interaction with AMPARs. This study explores the interactions between PKC and spermine on excitatory neurotransmission mediated by AMPARs in immature cortical pyramidal neurons.
Methods
In vitro slice preparation and electrophysiology
Slice preparation and electrophysiology have been previously described (Kumar & Huguenard, 2001, Shin et al. 2005). Briefly, immature Sprague–Dawley rats (P12–P14) were anaesthetized with 50 mg kg−1 pentobarbital sodium and decapitated. The brain was removed and then sectioned using a vibratome. Slices (300 μm) were cut in a chilled (4°C) low-Ca2+, low-Na+ solution containing (mm): 234 sucrose, 11 glucose, 24 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2 equilibrated with a 95% O2–5% CO2. The slices were incubated in oxygenated artificial CSF (ACSF; mm: 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 MgCl2, 2 CaCl2, and 10 glucose, pH 7.4) first at 32°C for 1 h and subsequently for 0.5–5 h at room temperature before being transferred to a recording chamber maintained at room temperature (23–25°C).
Recording electrodes (1.2–2 μm tip diameters, 3–6 MΩ) were filled with (mm): 120 caesium gluconate, 1 MgCl2, 1 CaCl2, 11 KCl, 10 Hepes, 2 NaATP, 0.3 NaGTP, 1 N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide, 11 EGTA, pH 7.3 (corrected with CsOH), 290 mosmol l−1, and 0.05 spermine (in experiments indicated). Drugs and chemicals were applied through the perfusate (2 ml min−1).
Whole cell recordings were made from layer V pyramidal neurons of the frontal cortex (1.4 mm to 2.2 mm anteroposterior to bregma; Paxinos & Watson, 1986) at a developmental stage characterized by low GluR2 expression (P12–P14; Kumar et al. 2002). EPSCs were evoked by stimulating intralaminar connections via concentric bipolar electrodes (CB-XRC75; Frederick Haer & Co, Bowdoin, ME, USA) with 75 μm tip diameters that were positioned intracortically at a distance of 100–300 μm lateral to the recorded neuron. Paired constant current pulses (50 ms separation, 20–100 μs duration, 100–500 μA intensity) were applied at low frequencies (0.1–0.3 Hz). Threshold was determined by stepwise increments in current strength until all-or-none postsynaptic responses intermingled with failures were obtained (Dobrunz & Stevens, 1997). Stimulus intensity was then fixed at ∼1.2 times the threshold throughout the remainder of the experiment. EPSCs were recorded with an Axopatch-1D amplifier (Axon Instruments, Union City, CA, USA) and pCLAMP software (Axon Instruments), filtered at 1–2 kHz and digitized at 10 kHz. Series resistance was typically 8–10 MΩ and was monitored continuously. Those experiments in which this parameter changed by > 20% were rejected. Given the small amplitude of the evoked responses and holding currents (typically < 100 pA), no series resistance compensation was employed in this study.
To isolate AMPAR responses, a cocktail solution containing 50 μm PTX, 100 μm APV and 0.1 μm NBQX was applied via bath exchange (Kumar et al. 2002). Rectification index (RI) was determined as the slope of I–V curve at positive potential (+40 to 0 mV) divided by the slope of I–V curve at negative potential (0 to −50 mV). Paired pulse ratio (PPR) was calculated as the 2nd EPSC/1st EPSC.
The PKC activator phorbol-12 myristate-13-acetate (PMA, 100 nm) and inhibitor staurosporin (1 μm) were applied in separate experiments via local perfusion, which allowed for fast exchange of media at the level of the synapse (Kumar et al. 2002). The following compounds were bath applied as required for specific protocols: d(–)-2-amino-5-phosphonopentanoic acid (d-APV), 2,3-dihydro-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX, diluted in dimethylsulfoxide, < 0.1% final concentration), picrotoxin (PTX) (all from Research Biochemicals/Sigma, St Louis, MO, USA), spermine trihydrochloride (spermine; Tocris Cookson; made fresh on the day of use). Inhibitors of the protein kinase C (PKC), staurosporin (ST) and GFX109203X (GFX), and of protein kinase A (PKA), H89 as well as 1,2-bis(2-aminophenoxy)ethano-N,N,N,N-tetraacetic acid tetrakis acetoxymethyl ester (BAPTA), and PMA were purchased from Sigma.
Immunocytochemistry
Brain slices were prepared with the same protocol as used for recording. Medial cortex extending ± 5 mm from midline was dissected from coronal sections of frontal cortex taken at the same anterior–posterior positions as those used for physiology, and divided at midline. Sections from one hemisphere (ipsilateral) were treated with 1 μm ST for 10 min, while the other (contralateral) was incubated in vehicle for the same time. The prepared slices were washed with PBS and followed by an overnight treatment in fixative composed of 4% paraformaldehyde and 0.5% glutaraldehyde. Slices were cryoprotected, by immersion in 30% sucrose until sunk, and then resectioned at 35 μm with a freezing microtome (Microm, HM 400; Microm, Kalamazoo, MI, USA).
Immunocytochemical labelling for spermine was obtained via standard diaminobenzidine (DAB) immunoperoxidase protocols (Laube & Veh, 1997; Laube et al. 2002). Briefly, tagged and matched free-floating control and ST-treated sections were placed together in pairs in individual incubation wells for the entire experiment to insure the same treatment. Sections were then exposed to polyclonal spermine antibody (Chemicon International, Temecula, CA, USA) for 48 h (1: 1000, 4°C). After 2 × 10 min rinses in PBS, the sections were exposed to a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Inc., Burlingame, CA, USA) followed by ABC reagent employed for the avidin–biotin staining technique (Vectastain Elite Kit, Vector Laboratories) and visualized with DAB (Sigma) as the chromagen. Paired sections were then mounted on gelatin-coated slides, air-dried, dehydrated with ascending series of ethanol and coverslipped with DPX (Aldrich Chemical Company, Inc., Milwaukee, WI, USA).
Digital images were obtained in layer V at the same corresponding distance from midline from the control and ST-treated slices by a light microscope (Nickon Eclipse E800) equipped with AxioCam digital colour camera (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA). Positive and negative spermine immunoreactive cells were counted from a 350 × 270 μm region in at least three slices per animal, and total of three animals were used in the analysis.
Statistical analysis
All data are represented means ±s.e.m. Statistical significance was assessed using Student's t test.
Results
Polyamine dependent rectification of AMPAR currents is reversed by staurosporin
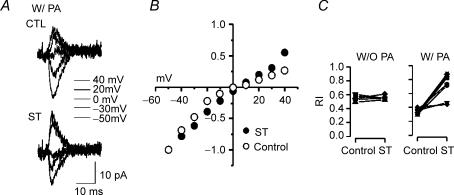
As previously reported, synaptic AMPAR responses from immature layer V pyramidal neurons showed significant inward rectification, especially when intracellular PA levels were increased by inclusion of exogenous spermine in the recording pipette (Kumar et al. 2002; Shin et al. 2005). Evoked AMPAR EPSCs at positive membrane potentials were proportionally smaller than those recorded at negative potentials. Inhibition of PKC by staurosporin (ST, 1 μm) surprisingly reduced the rectification induced by exogenous spermine (Fig. 1C right). With 50 μm spermine included in the patch pipette, rectification indices (RI) in control (vehicle treated) and ST conditions in the same neurons (n = 7) were 0.33 ± 0.04 and 0.73 ± 0.18 (n = 7, t test P < 0.05), respectively. By contrast, in the absence of pipette spermine the RI was not affected by ST (Control: 0.55 ± 0.04, ST: 0.55 ± 0.04, n = 5, t test P > 0.05, Fig. 1C left). Thus cortically evoked EPSCs were enhanced by PKC inhibition, but only in the presence of intracellular PA. This suggests that in this system inhibition of PKC does not directly affect AMPAR function through, for example, alterations in AMPAR gating or surface expression. Instead blockade of PKC appears to alter the interactions between polyamines and AMPARs, such that functional effects on EPSCs are dependent on high intracellular levels of polyamines.
Figure 1. Staurosporin (ST) reverses PA-induced inward rectification of synaptic AMPA receptors of immature layer V pyramidal neurons.
Representative minimally evoked EPSCs recorded from a P12 rat neocortical pyramidal neuron in the presence of 50 μm intracellular spermine at potentials of –50, −30, 0, +20 and +40 mV. A, traces recorded from the same neuron during vehicle perfusion (upper traces, CTL) and perfusion with ST (lower traces, ST, 1 μm). B, population I–V curves for synaptic currents obtained with intracellular PA and normalized to peak amplitude at –50 mV in two groups of neurons (i.e. either with or without ST) at various holding potentials. I–V curves show significant rectification, which is decreased by ST. Each point on the plots (○: control, •: ST) represents the average of ≥ 5 recordings. For clarity, standard error bars in B are not shown, but ranged from 0.01 to 0.25. C, each line and distinct symbol represents RIs calculated in a neuron in control conditions, and then later in the same cell after exposure to ST. ST significantly increased rectification index (i.e. reduced rectification), when spermine was included in the patch pipette (W/PA, right), but not when spermine was excluded (W/O PA, left; n = 5, 7).
PKC/PA modulation of spontaneous AMPAR EPSCs
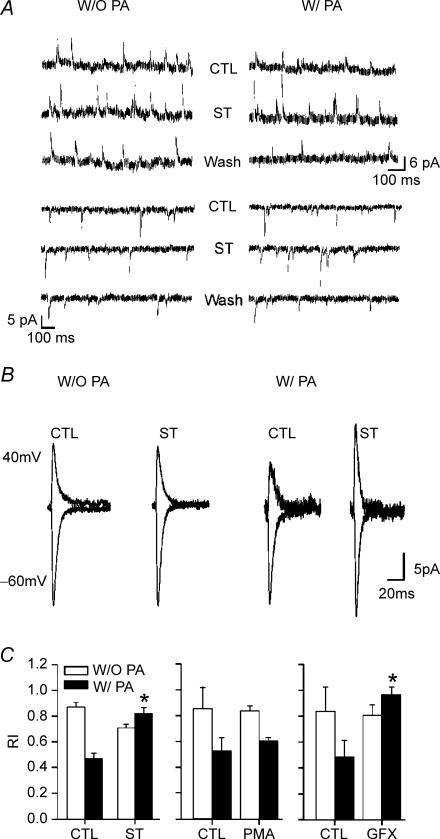
To examine the generality of the ST effect on synaptic AMPARs, spontaneous EPSCs (sEPSCs), presumably arising from a variety of presynaptic terminals, were recorded in ST-treated slices. Outward currents were recorded at +40 mV and inward currents at −60 mV (Fig. 2B) to evaluate the effect of PKC blockade on rectification of AMPAR sEPSCs. As with locally evoked responses, ST decreased inward rectification of sEPSCS, but only in the presence of exogenous polyamines (Fig. 2).
Figure 2. Rectification of spontaneous EPSCs (sEPSCs) in immature rats.
A, traces show continuous voltage clamp recordings with sEPSCs in control (CTL), ST-perfused (ST) and washout (Wash) conditions in an individual P14 layer V pyramidal neuron. Upper three traces represent sEPSCs recorded at 40 mV and lower three traces at −60 mV. B, averaged sEPSCs at 40 mV (upper traces) and −60 mV (lower traces) obtained from the same two neurons in A. Each trace is the averages of all the successfully isolated sEPSCs occurring at holding potentials of 40 mV or −60 mV. Note increased sIPSC amplitude at both holding potentials, but especially at +40 mV, when the recording pipette contained spermine (W/PA, right traces CTL versus ST). C, effects of PKC and PKA inhibitors on rectification index (RI) in the absence (W/O PA, white bars) or presence (W/PA, black bars) of intracellular spermine (ST: staurosporin, PMA: phorbol-12-myristate-13-acetate, GFX: GFX109203X). Exogenous PAs increased RI (decreased rectification) in neurons in every case, and this effect was reversed by ST and GFX (*P < 0.05). Each averaged EPSC was obtained from at least 50 individual sEPSCs. RI was calculated as the ratio of conductance at 40 mV divided by that measured at −60 mV.
ST is a broad-spectrum protein kinase inhibitor affecting both PKC and PKA (Tamaoki et al. 1986). To exclusively block PKC, the more selective PKC inhibitor GFX109203X (GFX, 1 μm) (Marano et al. 1995), was tested. Application of GFX reproduced the ST-induced effects on inward rectification of AMPAR-dependent EPSCs (Fig. 2C), while H-89, an inhibitor of PKA (Chijiwa et al. 1990) exhibited little or no effect (data not shown). The rectification indices measured during perfusion of GFX-free ACSF and GFX-containing ACSF in the same PA-treated neurons were 0.49 ± 0.13 and 0.93 ± 0.07 (n = 5), respectively. These results confirmed that the ST-induced effect is dependent on PKC inhibition, and excludes the possibility that PKA influences the spermine–AMPAR interactions.
Following the findings that PKC inhibition by both ST and GFX reduced PA-dependent rectification, we hypothesized that PKC activation should increase the interaction between spermine and GluR2-deficient AMPARs. In this case the rectification should be enhanced by phorbol 12-myristate 13-acetate (PMA), a PKC activator (Liu & Heckman, 1998). However, rectification indices in PMA treated neurons were not significantly smaller with spermine (RI = 0.52 ± 0.11, n = 4) than the control (RI = 0.61 ± 0.02, n = 5) (Fig. 2C). These results suggest that synaptic AMPARs in immature neocortical pyramidal neurons are constitutively phosphorylated and that PKC potentiation by PMA produced little further functional phosphorylation of sites relevant to PA interaction.
The results presented thus far clearly demonstrate that PA-induced rectification of both evoked (eEPSC) and spontaneous EPSCs (sEPSC) were decreased by PKC inhibition in immature, GluR2-deficient, pyramidal neurons (Figs 1 and 2).
Use-dependent polyamine unblock of synaptic AMPARs is affected by ST
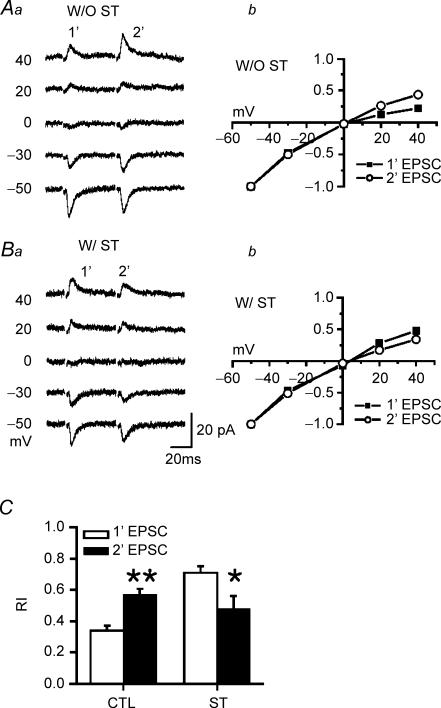
To provide further evidence for a postsynaptic locus of ST action, we evaluated rectification indices of synaptic AMPAR mediated responses using a paired-pulse protocol with postsynaptic holding potentials ranging between −50 mV and +40 mV. RIs of paired AMPAR-dependent EPSCs were obtained from I–V curves in individual neurons recorded either with or without PA in the patch pipette. As expected for use-dependent PA unblocking (Rozov & Burnashev, 1999; Shin et al. 2005), RI was larger for the second response of the pair (RI: 0.57 ± 0.04, n = 6, P < 0.01, Figs 3Aa, Ab and C) compared to the first response (RI: 0.34 ± 0.03), indicating reduced rectification. This effect was most robust in the presence of exogenous PAs (Shin et al. 2005).
Figure 3. ST counteracts the postsynaptic plasticity (facilitation) mediated by PA unblock.
A, representative traces are averages of > 3 consecutive trials at holding potentials of 40, 20, 0, −30, and –50 mV. Paired responses (50 ms interpulse interval) in a young (P13) neuron obtained in the absence (Aa: W/ST) or presence of 1 μm staurosporin (Ba: W/O ST) but always in the presence of 50 μm spermine (PA). ST was applied by local perfusion and control was equivalent local perfusion with vehicle. Ab and Bb, I–V curves derived from responses in Aa and Ba, respectively, for the first (▪) and second (○) EPSC within each pair. C, RIs of paired AMPAR-dependent EPSCs obtained from I–V curves (Ab and Bb) in pyramidal neurons (n = 6) from P12–14 rats either with ST (CTL) or without (ST) through the local perfusion. 1′-EPSC (white bar) indicates the first EPSC and 2′-EPSC (black bar) the second EPSC. Exogenous intracellular PA increased the first pulse rectification, but this is relieved in the second pulse (paired t test **P < 0.01). By contrast, ST increased rectification of the second response compared to that of the first response (paired t test *P < 0.05).
PKC inhibition, via 1 μm ST, produced complex effects on use-dependent unblocking and rectification. ST suppressed rectification in the first response of the pair (0.73 ± 0.04, n = 6), and surprisingly enhanced rectification of the second response (0.48 ± 0.08, Fig. 3Ba and b). This effect only occurred in the presence of intracellular PA. Thus ST produced a switch of the normal PA-dependent postsynaptic paired-pulse facilitation (Rozov & Burnashev, 1999), such that it was replaced by postsynaptic paired pulse depression, possibly resulting from altered PA blockade of the second response. Although analysis of paired pulse ratios is complicated by the fact that they may be influenced by both pre- and postsynaptic factors (see below), this result of increased rectification in the second of a pair of responses is consistent with the switch from paired pulse facilitation (PPF) to paired pulse depression (PPD) produced by ST perfusion in the same cells (Fig. 4), and supports PKC-dependent modulation of the interaction of PAs with AMPARs.
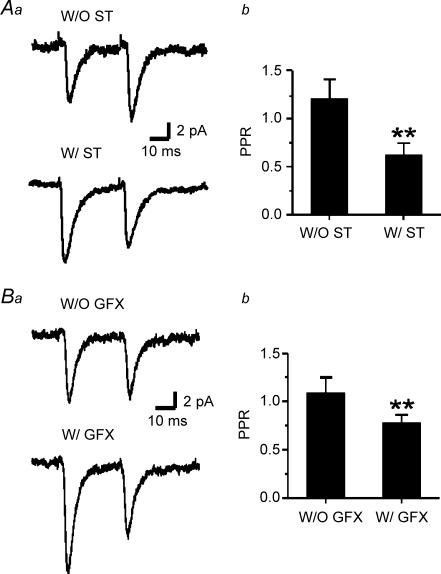
Figure 4. ST and GFX induce paired-pulse depression.
Aa, representative traces are averages of > 50 consecutive responses. Paired stimuli separated by 50 ms (20 Hz) in PA-including neurons. Responses to paired stimuli (upper trace without ST (W/O ST) and lower trace with ST perfusion (W/ST)) in a neuron from a P12 rat. b, paired-pulse ratio (PPR, the value obtained by dividing the average amplitude of the second response by that of the first) was decreased by ST (n = 12, **P < 0.01) in P12–P14 rats. Ba and b, under the same conditions PPR was also decreased by the specific PKC inhibitor GFX (n = 4, **P < 0.01). In all cases, holding potential was −60 mV.
By contrast, in the absence of spermine in the pipette ST produced little change in RI for either the first or second EPSC (0.53 ± 0.10, n = 4 and 0.55 ± 0.13, not shown). At this point it is not clear why the ST effect depends on exogenous PA. Our previous results have shown that AMPAR response rectification is progressively reduced during a prolonged recording (Shin et al. 2005), consistent with the existence of a functionally relevant, endogenous level of synaptic PAs that is progressively reduced during whole cell recording as a result of intracellular dialysis via the patch pipette. One potential explanation for the dependence of the ST effect on exogenous PAs is that endogenous PAs that interact with synaptic receptors are different from spermine.
ST-induced PPD
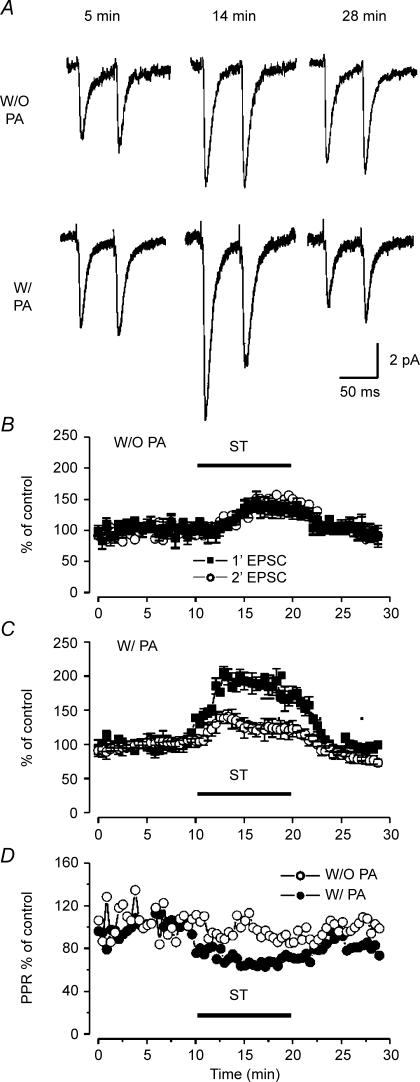
Similar to the results obtained from inhibitory neocortical neurons with GluR2-deficient receptors (Rozov & Burnashev, 1999), immature pyramidal neurons lacking GluR2 at synaptic sites exhibit a postsynaptic form of PPF when recorded with PA-containing intracellular solution (Shin et al. 2005). The paired pulse ratios (PPRs) of recordings obtained from PA-free and PA-containing electrodes were 1.2 and 1.7, respectively (Shin et al. 2005). Notably, ST treatment resulted in PPD such that in the presence of exogenous PAs the first EPSC became greater than the second EPSC (Fig. 4A). The PPRs obtained from control and ST-treated groups were 1.25 ± 0.24 (n = 7) and 0.62 ± 0.12, respectively. The apparent PPD resulted from an increase in the first response rather than a decrease in the second one (Fig. 5A lower panel and Fig. 5C). To exclude a non-specific block of both PKC and PKA from ST, 1 μm GFX or 10 μm H-89 were applied in separate experiments by local perfusion to test paired pulse response. As a result, GFX reproduced the ST-induced effects on paired pulse depression of AMPAR-dependent EPSCs (Fig. 4B), while H-89, a specific inhibitor of PKA, exhibited little or no effect (data not shown). This differential effect on first versus second response is likely to be a postsynaptic phenomenon, as it was only observed when spermine was included in the postsynaptic cell. By contrast, in the absence of pipette spermine, ST increased the amplitude of each of the responses in the pair by an equal amount (Fig. 5A upper panel and Fig. 5B). The increased PSC amplitude that occurred in PA free conditions, likely through a presynaptic action, also presumably occurred in recordings with PA-containing pipettes and may explain the increased amplitude of the first of the paired responses. Local perfusion of ST into the slice produced a time-dependent and reversible increase in intracortically evoked EPSC in P12–P14 neurons that was greater in the presence of intracellular polyamines. Compared to control, ST increased the amplitude of the first EPSC in the pair by 1.9 ± 0.2-fold, measured 4 min after the onset of ST perfusion. This was accompanied by a lesser enhancement of the second response (1.2 ± 0.1-fold increased amplitude, n = 7). Note that the PPR was decreased by ∼40% by ST in the presence but not absence of PAs (Fig. 5D).
Figure 5. ST modifies AMPAR-mediated EPSCs in a time- and PA-dependent manner.
A, representative traces are averages of > 40 consecutive responses in the absence (upper) or presence (lower) of PA at a holding potential of –60 mV. Staurosporin was applied for a 10 min period beginning 10 min after the recording began. Paired pulse responses were measured at 5 min (control), 14 min (ST) and 28 min (wash) after establishing the whole cell patch clamp. B and C, normalized amplitudes of first and second paired responses recorded at –60 mV in PA-free (B, W/O PA, n = 5) and PA-containing conditions (C, W/PA, open symbols, n = 7). Each symbol represents mean ±s.e.m.D, paired pulse ratios from experiments in panels B and C. In the presence but not absence of intracellular PA, ST produced a reversible ∼40% reduction of control PPR.
The findings of increased amplitude of evoked EPSCs are consistent with the finding that local perfusion of ST increased spontaneous EPSC amplitudes at a negative potential, −60 mV (Fig. 2). The increased amplitudes of both sEPSC and eEPSC obtained in the presence of exogenous PAs might be due to a cooperative action of PKC inhibition and intracellular (postsynaptic) PA to augment AMPAR activation. The dependence of this effect on intracellular PAs is demonstrated in Fig. 5. Though both the first and second of the paired responses were increased by ST, the degree of increase of both the first and second response was much larger when PAs were included in the recording pipette.
Short-term presynaptic changes such as depression or facilitation within a pair of responses should be at least partially dependent on the vesicular release associated with the first response (Debanne et al. 1996). Thus the ratio between the mean amplitudes of the second EPSC and the first EPSC (paired-pulse ratio, PPR) is inversely proportional to the initial release probability (Dobrunz et al. 1997). Therefore, a ST-dependent change in release probability should alter PPR. The lack of any ST-dependent change in PPR (Fig. 5D) obtained in the absence of exogenous PAs suggests that there is little presynaptic contribution to ST effects in this system, and that the change in PPR seen when spermine is included in the recording pipette results mainly from postsynaptic actions.
Effect of intracellular Ca2+ manipulation
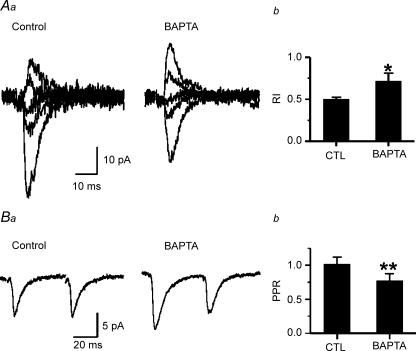
Because PKC activation requires elevated [Ca2+]i (Gustavsson et al. 1994) and given our results suggesting constituitive phosporylation of either AMPARs themselves or of modulatory proteins, we predicted that chelation of intracellular [Ca2+] would have similar effects as the putative ST-dependent inhibition of PKC. We added 1 mm BAPTA, a fast Ca2+ buffer, to the internal solution and obtained EPSC I–V relationships. BAPTA in the recording pipette caused a decrease in the inward rectification of AMPAR-mediated EPSC (Fig. 6A) and produced PPD (Fig. 6B) in younger rats. These results are consistent with the reversal of rectification (Fig. 1) and PPD (Fig. 4) in AMPAR-mediated EPSC in ST-perfused slices. Therefore, we suggest that lowered [Ca2+]i in the postsynaptic neuron reduces a constituitive PKC dependent suppression of synaptic AMPARs.
Figure 6. BAPTA inhibits inward rectification and paired pulse facilitation.
Aa, representative EPSCs recorded from a P12 rat neocortical pyramidal neuron at potentials of −50, −30, 0, +20 and +40 mV. Recording were made with either BAPTA (1 mm) containing- or BAPTA-free intracellular solution in the pipette (Control). Each trace is the average of > 3 consecutive responses. b, alterations in RI by BAPTA (**P < 0.01, n = 4, 3). Ba, representative EPSCs recorded at −60 mV during paired stimuli separated by 50 ms in a layer V pyramidal neuron from a P12 rat. b, paired pulse ratio calculated as 2′-EPSC/1′-EPSC (**P < 0.01, n = 5, 4).
Depletion of PA by ST
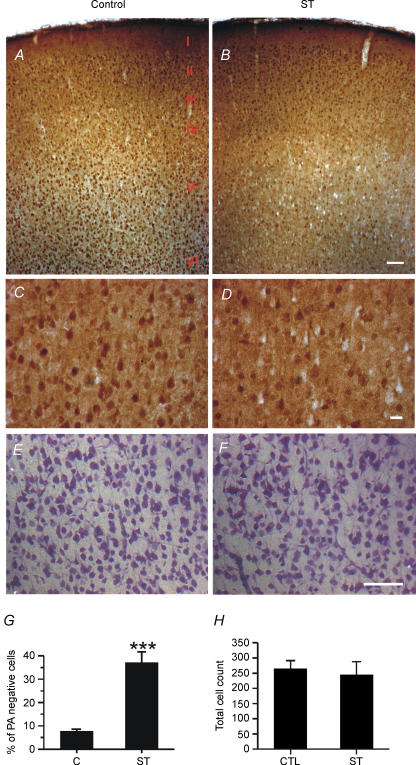
Based on finding that ST significantly reversed the effects of exogenous polyamines on AMPAR synaptic function, including inward rectification and PPF, we hypothesized that there may be a relationship between ST-dependent inhibition of PKC activity and the cellular PA levels in pyramidal neurons. An analysis of PA immunoreactivity was performed in ST-treated and control slices to directly test whether PKC inhibition would alter PA levels. We found that a brief (10 min) ST treatment depleted spermine in many V pyramidal neocortical neurons (Figs 7A and C versus B and D). Immunostaining results showed that ST-treated slices exhibited a 10-fold increase in the number of spermine-negative neurons compared to control slices (Fig. 7G). Nissl staining of adjacent sections showed that ST had no obvious effect on cell morphology or neuronal numbers (Figs 7E, F and H), suggesting that the reduced spermine immunoreactivity was not a result of cell death or apoptosis. Consistent with previous studies showing that PAs induce strong inward rectification and block synaptic AMPARs in developing neurons (Isa et al. 1995, 1996; Koh et al. 1995; Washburn et al. 1997; Itazawa et al. 1997), this finding suggests that at least part of the effect of ST on synaptic AMPAR responses is a result of depletion of functional PAs at synapses.
Figure 7. ST depletes spermine in layer V pyramidal neurons.
A and B, spermine immunoreactivity in rat neocortical layer I, II, II, IV, V and VI in Control (A) and ST-treated (B) P13 rat brain slices. Note the increased number of spermine immunonegative cells in layer V from ST-treated brain slices. Scale bars, 100 μm. C and D, enlargement of layer V region from A and B, respectively. Several non-immunoreactive (white) pyramdial cells were observed in ST-treated slices. Scale bars, 25 μm. E and F, Nissl staining of control (E) and ST-treated (F) brain slices from P13 rat. Scale bars, 100 μm. G, percentage of non-immunoreactive (PA negative) cells in equivalent regions of control and staurosporin (ST) treated slices (n = 4 rats each, 3 slices from each rat, ***P < 0.001). H, total numbers of cells counted in the same area as those from C and D. No significant differences in cell count were observed between the control and ST groups.
Discussion
In this report, we have shown four new findings supporting polyamine and Ca2+/PKC interactions with synaptic GluR2-deficient AMPA receptors in immature rat neocortical neurons. The first is that PKC inhibition reduces PA-dependent inward rectification. Second, when PAs are included in the patch pipette, ST causes paired pulse depression associated with changes in RI, supporting postsynaptic actions. Third, buffering intracellular Ca2+ with BAPTA reproduced the effects of PKC inhibition. Fourth, ST induces a depletion of spermine in pyramidal neurons indicating a possible role of PKC in PA metabolism that might contribute to the observed effects on AMPAR-dependent synaptic responses.
PKC and PAs interact to modify AMPAR function
Evoked and spontaneous AMPAR-dependent EPSCs were both increased in amplitude by PKC blockade, and these effects were most prominent when exogenous spermine was included in the recording pipette. Similar effects were seen with Ca2+ chelation indicating that elevation of [Ca2+]i is required, which indirectly supports PKC involvement. PMA did not enhance the PA-induced rectification or PA unblocking, suggesting constitutive PKC activation at synaptic sites in immature pyramidal neurons. These results suggest there is persistent activation of PKC, perhaps augmented by exogenous intracellular polyamines, that in turn facilitates a PA/AMPAR interaction and promotes rectification. In this scenario PKC blockade then reduces the PA/AMPAR interaction, unblocks the PA site, and enhances current flow through the AMPARs.
However, ST treatment results in enhanced rectification observed in the second of a pair of synaptic AMPAR responses compared to the first (Fig. 3Ba and b). A potential mechanism underlying this switch in the use-dependence of the receptors from use-dependent unblocking to use-dependent blocking, is a phosphatase-dependent reduced affinity of spermine for the PA site on GluR2-deficient AMPARs in the closed (resting) state, combined with a use-dependent change in spermine affinity. In other words, ion flux through the channel pore, perhaps Ca2+ ions, would trigger, through an as yet unidentified mechanism, an increased affinity of spermine for the PA site. Local elevation of Ca2+ might overcome PKC blockade leading to re-phosphorylation of the relevant PA binding site. Based on the lack of ST effects in the absence of exogenous PAs, we postulate that this mechanism is especially prominent with the exogenous PA we used, spermine, which is the largest of the endogenous polyamines with a large number of amide groups, and thus would have high affinity for AMPARs (Tikhonov et al. 2000). Thus, supplementation of endogenous PAs with high levels of spermine via the patch pipette allows for expression of the PKC effect.
Interaction of PA with PKC and Ca2+
Spermine has been shown to interfere with the phosphoinositide/Ca2+ signalling pathway, since it competes with Ca2+ (Moruzzi et al. 1987, 1990, 1995). Ca2+ entry through GluR2-deficient AMPARs will lead to synaptic increases in [Ca2+]i which could locally promote PKC activity. Consistent with this idea, we found that chelating intracellular Ca2+ reversed AMPAR-dependent rectification and PPF, suggesting that elevated Ca2+ in the vicinity of the synapse plays an essential role in PKC activation and thus regulation of synaptic transmission. Thus PKC might alter affinity of PAs for AMPARs and increase inward rectification as well as PPF (Fig. 6). Locally elevated [Ca2+]i arising from Ca2+ permeable AMPAR activation should compete with spermine for binding to and activation of PKC. Elevated spermine levels may thus increase the threshold for PKC activation through antagonism of Ca2+ binding. Given that Ca2+ permeability of AMPARs is independent of intracellular PAs (Gilbertson et al. 1991; Jonas et al. 1994; Kamboj et al. 1995; Otis et al. 1995), then even PA-blocked channels will flux Ca2+ entry to some extent and potentially promote local PKC activation, suggesting that microdomains exist within which AMPAR function is rapidly and dynamically modulated via postsynaptic actions
PKC and AMPARs
The interaction between GluR2 and GRIP/ABP by S880 phosphorylation is essential for function of synaptic AMPA receptors and activity-dependent synaptic depression (Xia et al. 2000; Chung et al. 2003). However, PKC may alter AMPA channel gating or open probability (Daw et al. 2000; McDonald et al. 2001; Hirai, 2001; Perez et al. 2001; Seidenman et al. 2003; Terashima et al. 2004) and thereby influence ion flux and PA unblock. There might also be a reduced expression of functional AMPARs on the postsynaptic neuronal surface. Phosphorylation of AMPARs leads to receptor internalization (Chung et al. 2000, 2003; Xia et al. 2000; McDonald et al. 2001; Hirai, 2001), and blockade of PKC-dependent phosphorylation might thereby increase AMPAR retention at synapses and augment EPSCs.
PICK1 is colocalized with PKCα and AMPARs at excitatory synapses and was shown to homo-oligomerize through its PDZ domain (Nakazawa et al. 1997; Dev et al. 1999, 2004; Hirbec et al. 2003; Leitges et al. 2004). Viral infection of PICK1 in the hippocampal CA1 region resulted in an increased AMPAR rectification and reduced amount of surface AMPAR (Chung et al. 2000; Perez et al. 2001; Terashima et al. 2004). The PICK-induced alteration of AMPAR function was dependent on increased PKC activity. Consistent with this, our results have shown that PKC inhibition produced the opposite effect – a decreased AMPAR rectification and a decreased sensitivity of AMPAR-mediated EPSCs to PAs.
PKC and PA metabolism
The activity of ornithine decarboxylase (ODC, the key synthetic enzyme in polyamine metabolism; Gilad et al. 1995) can be decreased by PKC depletion and increased by PKCɛ overexpression (Ostrowski et al. 1992; Wheeler et al. 2003). PKA plays a role in increasing ODC expression at the transcriptional level (Abrahamsen et al. 1992). However, any change in ODC activity in our experiment was likely to be coupled to the activation of PKC rather than PKA as PKC activators can cause a rapid increase in the ODC activity (Kapoor et al. 2001). Thus PKC activation may be required to maintain physiological levels of intracellular PAs, perhaps through stimulation of PA synthetic pathways. The decrease in inward rectification by ST could thus be explained by PKC-dependent regulation of PA metabolism. Then two possible pathways could be suggested. On the one hand, PA levels are highly dependent on the activity of ODC, which is phosphorylated by PKC leading to an increase in activity (Hsieh & Verma, 1988; Verma et al. 1988; Mills & Smart, 1989; Butler et al. 1991; Otieno & Kensler, 2000). The prominent depletion of spermine in ST-treated neurons (Fig. 7) suggests a different action that may occur alone or in addition to direct channel/receptor modification. On the other hand, PKC might activate specific PA transporters (Gilad & Gilad, 1991; Dot et al. 2000) and accelerate import of extracellular PAs.
Overall, we proposed two separate mechanisms by which PAs regulate AMPARs: (1) through a well established process through which PAs bind to and impede current flow through GluR2-deficient AMPARs (Bowie & Mayer, 1995; Isa et al. 1995; Kamboj et al. 1995), and (2) through interacting with PKC to influence ion flux via a distinct mechanism that indirectly alters PA–AMPAR interactions. Of interest is the finding that PKC inhibition only influenced AMPAR function under conditions of excess PAs, suggesting PKC is activated under these conditions and that its activation either directly or indirectly inhibits AMPAR function. We speculate that during periods of increased neuronal activity, such as those occurring during seizures, there may be Ca2+-dependent activation of PKC which would increase ODC activity and lead to increased PA levels. If present, such a mechanism would provide a dynamic negative feedback to decrease synaptic excitation, and potentially suppress or terminate seizures.
Acknowledgments
This work was supported by the NINDS and a fellowship from the Epilepsy Foundation of America.
References
- Abrahamsen MS, Li RS, Dietrich-Goetz W, Morris DR. Multiple DNA elements responsible for transcriptional regulation of the ornithine decarboxylase gene by protein kinase A. J Biol Chem. 1992;267:18866–18873. [PubMed] [Google Scholar]
- Armstrong JN, Macvicar BA. Theta-frequency facilitation of AMPA receptor-mediated synaptic currents in the principal cells of the AMPA medical septum. J Neurophysiol. 2001;85:1709–1718. doi: 10.1152/jn.2001.85.4.1709. [DOI] [PubMed] [Google Scholar]
- Balland B, Lachamp P, Strube C, Kessler JP, Tell F. Glutamatergic synapses in the rat nucleus tractus solitarii develop by direct insertion of calcium-impermeable AMPA receptors and without activation of NMDA receptors. J Physiol. 2006;574:245–261. doi: 10.1113/jphysiol.2006.108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Brorson JR, Zhang Z, Vandenberghe W. Ca2+ permeation of AMPA receptors in cerebellar neurons expressing Glu receptor 2. J Neurosci. 1999;19:9149–9159. doi: 10.1523/JNEUROSCI.19-21-09149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AP, Mar PK, McDonald FF, Ramsay RL. Involvement of protein kinase C in the regulation of ornithine decarboxylase mRNA by phorbol esters in rat hepatoma cells. Exp Cell Res. 1991;194:56–61. doi: 10.1016/0014-4827(91)90129-i. [DOI] [PubMed] [Google Scholar]
- Carlson NG, Howard J, Gahring LC, Rogers SW. RNA editing (Q/R site) and flop/flip splicing of AMPA receptor transcripts in young and old brains. Neurobiol Aging. 2000;21:599–606. doi: 10.1016/s0197-4580(00)00127-5. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nakanishi S, Henley JM. The PDZ domain of PICK1 differentially accepts protein kinase C-α and GluR2 as interacting ligands. J Biol Chem. 2004;279:41393–41397. doi: 10.1074/jbc.M404499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase C α binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Huang EP, Stevens CF. Very short-term plasticity in hippocampal synapses. Proc Natl Acad Sci U S A. 1997;94:14843–14847. doi: 10.1073/pnas.94.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dot J, Lluch M, Blanco I, Rodriguez-Alvarez J. Polyamine uptake in cultured astrocytes: characterization and modulation by protein kinases. J Neurochem. 2000;75:1917–1926. doi: 10.1046/j.1471-4159.2000.0751917.x. [DOI] [PubMed] [Google Scholar]
- Eybalin M, Caicedo A, Renard N, Ruel J, Puel JL. Transient Ca2+-permeable AMPA receptors in postnatal rat primary auditory neurons. Eur J Neurosci. 2004;20:2981–2989. doi: 10.1111/j.1460-9568.2004.03772.x. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH. Polyamine uptake, binding and release in rat brain. Eur J Pharmacol. 1991;193:41–46. doi: 10.1016/0014-2999(91)90198-y. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH. Polyamines in neurotrauma. Ubiquitous molecules in search of a function. Biochem Pharmacol. 1992;44:401–407. doi: 10.1016/0006-2952(92)90428-l. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH, Casanova MF, Casero RA., Jr Polyamines and their metabolizing enzymes in human frontal cortex and hippocampus: preliminary measurements in affective disorders. Biol Psychiatry. 1995;38:227–234. doi: 10.1016/0006-3223(94)00256-3. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Scobey R, Wilson M. Permeation of calcium ions through non-NMDA glutamate channels in retinal bipolar cells. Science. 1991;251:1613–1615. doi: 10.1126/science.1849316. [DOI] [PubMed] [Google Scholar]
- Gustavsson L, Moehren G, Torres-Marquez ME, Benistant C, Rubin R, Hoek JB. The role of cytosolic Ca2+, protein kinase C, and protein kinase A in hormonal stimulation of phospholipase D in rat hepatocytes. J Biol Chem. 1994;269:849–859. [PubMed] [Google Scholar]
- Hirai H. Modification of AMPA receptor clustering regulates cerebellar synaptic plasticity. Neurosci Res. 2001;39:261–267. doi: 10.1016/s0168-0102(00)00237-6. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, Collingridge GL, Henley JM. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hsieh JT, Verma AK. Involvement of protein kinase C in the transcriptional regulation of ornithine decarboxylase gene expression by 12-O-tetradecanoylphorbol-13-acetate in T24 human bladder carcinoma cells. Arch Biochem Biophys. 1988;262:326–336. doi: 10.1016/0003-9861(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Isa T, Iino M, Itazawa S, Ozawa S. Spermine mediates inward rectification of Ca2+-permeable AMPA receptor channels. Neuroreport. 1995;6:2045–2048. doi: 10.1097/00001756-199510010-00022. [DOI] [PubMed] [Google Scholar]
- Itazawa SI, Isa T, Ozawa S. Inwardly rectifying and Ca2+-permeable AMPA-type glutamate receptor channels in rat neocortical neurons. J Neurophysiol. 1997;78:2592–2601. doi: 10.1152/jn.1997.78.5.2592. [DOI] [PubMed] [Google Scholar]
- Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor P, Raj VS, Saxena S, Balaraman S, Madhubala R. Effect of Leishmania donovani lipophosphoglycan on ornithine decarboxylase activity in macrophages. J Parasitol. 2001;87:1071–1076. doi: 10.1645/0022-3395(2001)087[1071:EOLDLO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Keller BU, Hollmann M, Heinemann S, Konnerth A. Calcium influx through subunits GluR1/GluR3 of kainate/AMPA receptor channels is regulated by cAMP dependent protein kinase. EMBO J. 1992;11:891–896. doi: 10.1002/j.1460-2075.1992.tb05127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenbruck G, Berger E, Speckmann EJ, Musshoff U. RNA editing at the Q/R site for the glutamate receptor subunits GLUR2, GLUR5, and GLUR6 in hippocampus and temporal cortex from epileptic patients. Neurobiol Dis. 2001;8:459–468. doi: 10.1006/nbdi.2001.0394. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Huguenard JR. Properties of excitatory synaptic connections mediated by the corpus callosum in the developing rat neocortex. J Neurophysiol. 2001;86:2973–2985. doi: 10.1152/jn.2001.86.6.2973. [DOI] [PubMed] [Google Scholar]
- Laube G, Bernstein HG, Wolf G, Veh RW. Differential distribution of spermidine/spermine-like immunoreactivity in neurons of the adult rat brain. J Comp Neurol. 2002;444:369–386. doi: 10.1002/cne.10157. [DOI] [PubMed] [Google Scholar]
- Laube G, Veh RW. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia. 1997;19:171–179. doi: 10.1002/(sici)1098-1136(199702)19:2<171::aid-glia8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Leitges M, Kovac J, Plomann M, Linden DJ. A unique PDZ ligand in PKCα confers induction of cerebellar long-term synaptic depression. Neuron. 2004;44:585–594. doi: 10.1016/j.neuron.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Marano CW, Laughlin KV, Russo LM, Mullin JM. The protein kinase C inhibitor, bisindolylmaleimide, inhibits the TPA-induced but not the TNF-induced increase in LLC-PK1 transepithelial permeability. Biochem Biophys Res Commun. 1995;209:669–676. doi: 10.1006/bbrc.1995.1551. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Chung HJ, Huganir RL. Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology. 2001;41:672–679. doi: 10.1016/s0028-3908(01)00129-0. [DOI] [PubMed] [Google Scholar]
- Mills KJ, Smart RC. Comparison of epidermal protein kinase C activity, ornithine decarboxylase induction and DNA synthesis stimulated by TPA or dioctanoylglycerol in mouse strains with differing susceptibility to TPA-induced tumor promotion. Carcinogenesis. 1989;10:833–838. doi: 10.1093/carcin/10.5.833. [DOI] [PubMed] [Google Scholar]
- Moruzzi M, Barbiroli B, Monti MG, Tadolini B, Hakim G, Mezzetti G. Inhibitory action of polyamines on protein kinase C association to membranes. Biochem J. 1987;247:175–180. doi: 10.1042/bj2470175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi MS, Marverti G, Piccinini G, Frassineti C, Monti MG. The effect of spermine on calcium requirement for protein kinase C association with phospholipid vesicles. Int J Biochem Cell Biol. 1995;27:783–788. doi: 10.1016/1357-2725(95)00054-s. [DOI] [PubMed] [Google Scholar]
- Moruzzi MS, Monti MG, Piccinini G, Marverti G, Tadolini B. Effect of spermine on association of protein kinase C with phospholipid vesicles. Life Sci. 1990;47:1475–1482. doi: 10.1016/0024-3205(90)90527-x. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Mikawa S, Ito M. Persistent phosphorylation parallels long-term desensitization of cerebellar purkinje cell AMPA-type glutamate receptors. Learn Mem. 1997;3:578–591. doi: 10.1101/lm.3.6.578. [DOI] [PubMed] [Google Scholar]
- Ostrowski J, Szczepiorkowski Z, Trzeciak L, Rochowska M, Skurzak H, Butruk E. Induction of ornithine decarboxylase in normal and protein kinase C-depleted human colon carcinoma cells. J Physiol Pharmacol. 1992;43:373–382. [PubMed] [Google Scholar]
- Otieno MA, Kensler TW. A role for protein kinase C-δ in the regulation of ornithine decarboxylase expression by oxidative stress. Cancer Res. 2000;60:4391–4396. [PubMed] [Google Scholar]
- Otis TS, Raman IM, Trussell LO. AMPA receptors with high Ca2+ permeability mediate synaptic transmission in the avian auditory pathway. J Physiol. 1995;482:309–315. doi: 10.1113/jphysiol.1995.sp020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Schmitt J, Uto A. RNA editing of glutamate receptor subunits GluR2, GluR5 and GluR6 in transient cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1996;16:548–556. doi: 10.1097/00004647-199607000-00004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Pellegrini-Giampietro DE. An. activity-dependent spermine-mediated mechanism that modulates glutamate transmission. Trends Neurosci. 2003;26:9–11. doi: 10.1016/s0166-2236(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Cα to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard L, Noël J, Henley JM, Collingridge GL, Molnar E. Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J Neurosci. 2000;20:7922–7931. doi: 10.1523/JNEUROSCI.20-21-07922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Shen F, Huguenard JR. Polyamines modulate AMPA receptor dependent synaptic responses in immature layer V pyramidal neurons. J Neurophysiol. 2005;93:2634–2643. doi: 10.1152/jn.01054.2004. [DOI] [PubMed] [Google Scholar]
- Song HJ, Kim TH, Cho CK, Yoo SY, Park KS, Lee YS. Increased expression of ornithine decarboxylase by gamma-ray in mouse epidermal cells: relationship with protein kinase C signaling pathway. J Radiat Res (Tokyo) 1998;39:175–184. doi: 10.1269/jrr.39.175. [DOI] [PubMed] [Google Scholar]
- Tallaksen-Greene SJ, Albin RL. Splice variants of glutamate receptor subunits 2 and 3 in striatal projection neurons. Neuroscience. 1996;75:1057–1064. doi: 10.1016/0306-4522(96)00337-5. [DOI] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem Biophys Res Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov DB, Magazanik LG, Mellor IR, Usherwood PN. Possible influence of intramolecular hydrogen bonds on the three-dimensional structure of polyamine amides and their interaction with ionotropic glutamate receptors. Receptors Channels. 2000;7:227–236. [PubMed] [Google Scholar]
- Tsuzuki K, Isa T, Ozawa S. Subunit composition of AMPA receptors expressed by single hippocampal neurons. Neuroreport. 2000;11:3583–3587. doi: 10.1097/00001756-200011090-00036. [DOI] [PubMed] [Google Scholar]
- Verma AK, Hsieh JT, Pong RC. Mechanisms involved in ornithine decarboxylase induction by 12-O-tetradecanoylphorbol-13-acetate, a potent mouse skin tumor promoter and an activator of protein kinase C. Adv Exp Med Biol. 1988;250:273–290. doi: 10.1007/978-1-4684-5637-0_25. [DOI] [PubMed] [Google Scholar]
- Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Reddig PJ, Dreckschmidt NE, Leitges M, Verma AK. Protein kinase Cδ-mediated signal to ornithine decarboxylase induction is independent of skin tumor suppression. Oncogene. 2002;21:3620–3630. doi: 10.1038/sj.onc.1205451. [DOI] [PubMed] [Google Scholar]